Abstract
Marjolin’s ulcer is a rare, aggressive skin cancer developing in scar tissue, chronic ulcers and areas affected by inflammations. Its incidence is estimated to range from 1% to 2% of all burn scars. It most frequently takes the form of squamous cell carcinoma which sometimes is diagnosed during examination of lesions developing in scars and hard-to-heal chronic wounds (pressure sores, leg ulcers). Therapeutic management of Marjolin’s ulcer requires well-designed treatment plan to ensure optimal medical care and good quality of life for the patient. The high risk of metastases and damage to the structure of vitally important organs determines the need for early diagnosis and prompt surgical intervention with supplementary therapy.
The purpose of the study was to examine etiopathogenesis of Marjolin’s ulcer and principles of its treatment. The authors focused on the aspect of malignant degeneration in chronic wounds (leg ulcers, pressure sores) as a very rare, aggressive form of Marjolin’s ulcer. A review of the available literature on the issue of Marjolin ulcers was conducted using the key words; Marjolin ulcers, pressure sore, chronic wound. Malignant degeneration in chronic wounds is a very rare aggressive form of Marjolin ulcer.
Increased oncological alertness should be displayed by nursing and medical personnel taking care of patients with chronic wounds.
Keywords: Marjolin’s ulcer, chronic wounds, diagnosis, treatment
History
Aurelius Cornelius Celsus is an author of the first systematic medical treaty, in which he described malignant lesions within the epithelium of burn scars [1]. Yet, the history related to neoplastic wounds is much younger as it goes back to the late 19th and early 20th century. Jean Nicolas Marjolin, a French physician, was a co-author of the first edition of “Dictionnaire de Me´decine” from 1828, to which he contributed a total of 32 articles [2]. In the section related to ulceration he described two groups of skin damage of this type: ulcers resulting from internal factors, and ulcers caused by local factors. In the latter group he distinguished local fistula, as well as scrofulous, varicose, fungal, verrucous, verminous, ulcerous venereal, and cancerous origins. The causes of internal ulcers were classified by him as: venereal, scrofulous, contractile, psoriatic, scorbutic, cancerous, and cachectic. The scholar described chronic ulcers arising in scar tissue, but he did not identify their relationship with malignancy or thermal injury. The description most closely resembling the eponym can be found in the following fragment: […] Verrucous ulcer. Their surface is formed by a large number of conical villi of dense consistency, tightly concentrated extremely close to one another, like thick woolly velvet; these growths seem to originate from skin; the cuticle around the ulcer is thick […] a liquid secreted in small amount is highly viscous, almost colourless, with foul smell; They cause hardly any pain, they slowly grow, and can extend sideways… [2, 3].
Recognition of specific associations related to malignant ulcers is credited to Caesar Hawkins, an English surgeon, who described skin cancer developing in burn wounds and lacerations caused by flogging in his article entitled ‘‘Warty Tumours of Cicatrices’’, from 1833, where he wrote: […] The tumour…makes its appearance in some old scar, many years after the injury which has produced it has been healed… from a flogging or a scald…. In this stage it gives no pain nor inconvenience… the growth of the tumour becomes more rapid, the warty appearance being in some measure lost, a more solid substance projecting from the diseased skin, which bears much resemblance to the fungus of fungus hćmatodes. […] the tumour ulcerates and sloughs alternately …a foul excavated ulcer, except in its circumference, where the skin is raised, thickened, and everted… [2, 4].
In 1839 Guillaume Dupuytren described a case of a 62-year-old woman who had fallen on burning coal at the age of nine months. At a later stage of her life, an ulcer covered the distal end of her cicatrised forearm and formed a fungoid mass that surrounded her arm like a bracelet. Contrary to Marjolin, Dupuytren did not hesitate to use the term malignant ulcer to describe the lesion [5]. In 1850, the year of Marjolin’s death, Robert William Smith, Professor of Surgery at Trinity College in Dublin, used the term “warty ulcer of Marjolin” for ulceration in burn wounds [6, 7]. J. Da Costa also mentioned Marjolin’s ulcer in ‘Modern Surgery’ from 1910. He defined it as […] epithelioma extending from epithelial margin of chronic ulcer, scar or sinus [8]. Following these two references, the term Marjolin’s ulcer became generally accepted and continues to be used in the literature even today. At present the name is used for all neoplasms growing in scar tissues, chronic ulcers, and areas affected by inflammation [9, 10].
Etiopathogenesis
The pathophysiology of Marjolin’s ulcer has been discussed for over 100 years. Various aetiological factors are responsible for malignant transformation. These include areas of chronic scar tissues that may lose cells of the immune system constituting part of skin physiology. Due to this, malignant cells may avoid immunological detection and may become more aggressive and prone to metastasis [11, 12]. Chronic irritation and repeated attempts to treat the wound over time may stimulate cell proliferation and increase the speed of spontaneous mutations. Toxins released by necrotic tissue may produce direct mutagenic effects in cells [13]. Mutations in genes responsible for cell division and apoptosis are the cause of increased incidence of carcinoma. Mutations of this type have been reported in patients with Marjolin’s ulcer [14, 15]. Analysis by Sinha et al. provides a new perspective of gene expression in squamous cells conditioning the immune mechanisms that modulate the microenvironment of chronic wounds. Researchers have confirmed the reduction in the activity of matrix metalloproteinases and collagen, which suggests a chronic disorder of the extracellular matrix rotation leading to fibrosis. The loss of epithelial function (inhibition of claudins, cadherin proteins) with a concomitant increase in the mesenchymal markers (fibronectin, vimentin, laminin-4) was also observed. Clear differences in gene expression in squamous cancer cells (SCC) and Marjolin’s ulcers compared to physiological cells confirm the genetic diversity of these histologically similar neoplasms [16]. No specific factor has been identified. It is highly likely that the pathogenesis is linked with multiple factors of environmental, immunological, and genetic nature (Table 1).
Table 1.
| Theory | Mechanism |
|---|---|
| Toxins theory | Toxins released over a long period of time by damaged tissues lead to cellular mutations |
| Chronic irritation theory | Chronic irritation with repeated reepithelialisation and recurring tissue damage (healing – damage) contribute to irritation of carcinogenic nature |
| Theory of traumatic epithelial grafting | Epithelial elements grafted into the dermis lead to immunological response and impair the regeneration process |
| Cocarcinogen theory | Chemical and/or physical injury stimulates proliferation of already existing yet latent malignant cells |
| Initiation and promotion theory | Two-stage process of malignant transformation of healthy cells. During the initiation stage healthy cells turn into latent malignant cells, which later during promotion stage may be activated by a cocarcinogen, e.g. infection |
| Theory of immunologically privileged locations | Cicatrisation in burn wounds definitively obliterates lymphatic vessels in the place of injury hindering physiological immunological control and increasing the risk of neoplastic growth. Initially skin changes develop slowly, yet they soon impair the immune system and spread as remote metastases, increasing the risk of death |
| Inheritance theory | HLA DL4 is associated with cancer growth and anomalies in the p53 gene, identified in patients with Marjolin’s ulcer. This group of patients was also found with mutations in FAS in the region of apoptosis, predisposing for malignant degeneration of scars |
| Theory of ultraviolet radiation | UV radiation leads to a decrease in the number of Langerhans cells, which results in impaired cutaneous immunological detection; it also leads to changes in the p53 gene inhibiting tumour growth |
Marjolin’s ulcer is a rare, frequently aggressive skin cancer that develops in previously damaged areas or those affected by chronic inflammation. It most often develops in deep burn wounds where the healing process is slow and of secondary nature [17]. As for its incidence rates, Marjolin’s ulcer affects from 1% to 2% of all burn scars. It may also develop in scar tissues of other origins and as a result of chronic tissue injury associated for instance with Chronic osteomyelitis in sinuses, post-traumatic wounds, decubitus ulcers, and chronic fistulas. It was also found in the area of genital organs, as a complication resulting from Fournier gangrene [18].
Classification of Marjolin’s ulcer based on the criterion of time distinguishes acute and chronic forms of the condition. The term acute ulcer refers to malignant transformation occurring within 12 months from skin injury. Yet, the average duration of the cancerous change growth, from the time of skin damage to malignant transformation, is in excess of 30 years. The reported threshold values ranged from four weeks to 75 years [1]. Squamous cell carcinoma (SCC) is the most frequent histological type identified during diagnoses of scars and chronic wounds; however, in acute conditions basal cell carcinoma (BCC) is more common. A meta-analysis carried out by A. Koval-Vern and B.K. Criswell reviewed 412 cases of skin burns described in 146 articles published between 1923 and 2004 and confirmed that 71% of the cases represented (SCC), 12% were (BCC), 6% of the cases represented melanoma, 5% sarcoma, and 4% other neoplasms [19].
The cancer most frequently affects patients in the fifth decade of life, and men are three times more susceptible to the condition than women. In terms of location, the skin changes most frequently affect lower extremities (53.3%), upper extremities (18.7%), torso (12.4%), and face and nape (5.8%) [20–22]. SCC is the second most frequent skin cancer. It is characterised by infiltrative growth, and it frequently spreads to lymph nodes. If it develops within scars or chronic skin injuries, it is more aggressive than SCC of different aetiology [25, 26]. According to Hahn et al., 32% of patients with malignant ulceration, at the time of diagnosis are found with a diffuse process [23]. Metastases are rather frequent and are found in more than 27% of patients [1, 12]. Metastases to regional lymph nodes negatively impact the prognosis and as a rule lead to death within 2–3 years [20].
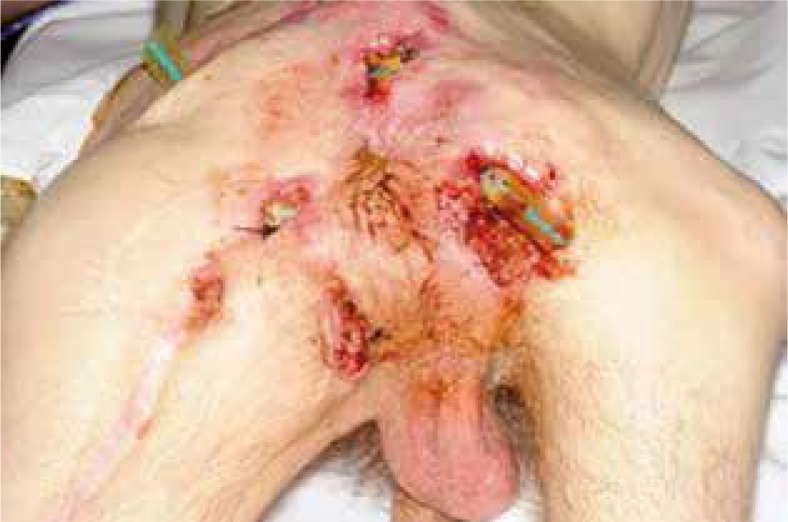
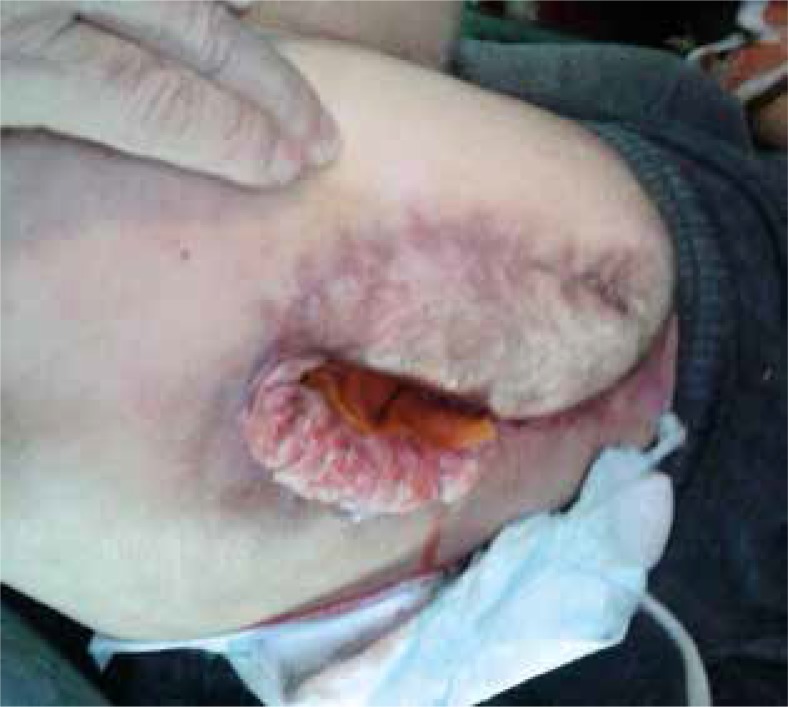
The risk of cancerous transformation leading to Marjolin’s ulcer definitely increases in the case of scars resulting from skin burn (76.5%), chronic non-healing traumatic wounds (8.1%), venous leg ulcers (6.3%), and fistulae in the course of purulent-inflammatory diseases of bones (2.6%) [24–27]. According to Mustoe et al., in the case of pressure sores the risk does not exceed 0.5% [28]. Even though the term “pressure ulcers” matches the concept of Marjolin’s ulcer, some authors argue that it is a separate, more aggressive clinical entity [29]. The period of malignant transformation is long and takes over 30 years, although in the literature there are reports of more rapid transformations [30–32]. Fairbairn describes a pressure sore healing and opening for approximately 10 years before the cancer diagnosis (Fig. 1) [32]. Bazaliński et al. described fulminant malignant ulceration in a scar following removal of a pressure sore in the area of right trochanter (Fig. 2) [31]. In the literature we can encounter numerous studies presenting single cases of pressure ulcer carcinomas. In most clinical cases the diagnosis was formulated too late, which led to serious surgical intervention and less favourable recovery prognosis.
Fig. 1.
Pressure sore with malignant transformation
Fig. 2.
Fulminant malignant in a scar following removal of ptessure score
Diagnosis and treatment
Due to their greater aggressiveness in comparison to other skin neoplasms, Marjolin’s ulcers require well designed treatment plans in order to optimise the patient’s medical care and his/her chances for survival (Tables 2 and 3) [21, 22]. Metastases are the most important prognostic factor; regional may affect 20-66% of cases, distant – 14% (lungs, brain) [19, 32, 33]. The most frequently applied local treatment methods include wide local excision, en block excision of local lymph nodes, or, if it is impossible to retain recommended surgical margins, amputation of large neurovascular structures of the limbs in the location of the advanced lesion. Additional treatment (neoadjuvant or adjuvant therapy), such as radio and/or chemotherapy, is recommended in patients with unfavourable prognostic factors or remote metastases [10, 25–27, 30]. Local radiation may be used as a supplementary therapy or as a method of choice if the size or location of the tumour makes complete resection impossible or if the patient does not agree to surgical treatment. Due to the fact that radiation may lead to radionecrosis of the skin, the use of this method may negatively affect the conditions for tissue repair [10, 25, 26]. Criteria for using radiotherapy in the treatment of Marjolin’s ulcers, proposed by E. Aydogdu et al., are mainly based on such factors as malignancy degree and growth stage, as well as tumour location [21, 34].
Table 2.
| Better prognosis | Worse prognosis | |
|---|---|---|
| Time from injury to malignancy development | < 5 years | > 5 years |
| Location | head, nape, upper limbs | torso, lower limbs |
| Clinical picture | exophytic formation | infiltrative formation |
| Degree of differentiation | G1 | G2 and G3 |
| Intensity of T lymphocyte infiltration around tumour | dense infiltrations | trace infiltrations |
| Regional and remote metastases (at the time of diagnosis) | absent | present |
Table 3.
General rules for proceeding if Marjolin’s ulcer is suspected or diagnosed [21]
| 1. Excise and, as far as possible, provide primary dressing for chronic, non-healing wounds |
| 2. Regularly inspect burn scars as well as chronic non-healing wounds, and inform patients at risk about the possible development of Marjolin’s ulcer |
| 3. Prevent and treat infections of chronic wounds |
| 4. If suspicious-looking changes are present, always collect specimens from the centre and edges of the ulcer to perform histological examination |
| 5. Venous ulcers which do not heal during three-month conservative treatment sh ould be qualified for specimens collection |
| 6. Pay attention to the condition of regional lymph nodes (the risk of metastases into regional lymph nodes is greater in Marjolin’s ulcer than in typical skin cancer) |
| 7. During resection of Marjolin’s ulcer maintain surgical margin of 2 cm in width and remove the tumour with fascia |
| 8. Regional lymph nodes that are clinically suspicious or have been verified by mi croscopy examination should be qualified for surgery |
| 9. Amputation of limbs should be applied only if infiltrations extend to bones, main vascular and nerve trunks and if poor functional effects are predicted |
| 10. Recommendations for chemotherapy and radiotherapy are defined on a case-by-case basis |
| 11. Following treatment, the patients should be systematically monitored by specialists |
Most pressure ulcer carcinomas are located in sacral and iliac areas. These regions have extensive lymphatic drainage into the pelvis, which explains the frequent local and remote metastases [34, 35]. Diagnosis of carcinogenic nature of pressure sores in iliac and ischial areas is difficult due to the rapid progression of damage and tissue necrosis towards the skeletal system, with secondary osteomyelitis and advancing systemic infection. In the case of suspicions (verrucous wound, ulceration failing to respond to local therapy for 3–6 months) tissue specimens should be collected from various places of the ulcer and its margin. This way it is possible to minimise false negative results of histopathological examination. By adopting biopsy procedures it may be possible to increase the rate of cancer diagnosis, yet it may also prove necessary to perform a more focused examination, i.e. magnetic resonance imaging (MRI) to assess the level and extent of destruction as well as inflammation of tissues [36, 37].
Sentinel lymph node biopsy is highly sensitive and is recommended to identify latent condition in lymph nodes. Lymphadenectomy is an inevitable element of radical surgery if cancer progression is confirmed [10, 26].
In many cases pressure sores, particularly in the iliac, ischial, and trochanter regions, require surgical removal of large areas of soft tissue and bones. In order to avoid local recurrence, it is necessary to perform wide local excision with a 2–5 cm margin of healthy tissue, with primary or delayed skin graft [21, 25, 38]. The patient’s clinical condition deteriorates once malignant transformation occurs in the pelvic area. The damage frequently is too big to allow conventional reconstruction with soft-tissue flaps [39]. In rare cases the recommended surgery involves hemicorporectomy (amputation of lower limbs and sex organs). The procedure is associated with numerous complications and radically affects the patient’s quality of life [39, 40]. A study by Grotting et al., which involved 10 patients with cancer originating from pressure sores, reported 80% of deaths due to recurrences within 18 months following resection and surgical reconstruction [32, 41].
Leg ulcers of vascular origin are common pathologies found in individuals with advanced venous insufficiency. The risk of neoplastic growth in this type of wound is rather low. The change develops over a period of more than 25 years [42]. The incidence rate varies across populations; the authors point to developing countries, difficult access to medical specialists, and individual interactions resulting from the level of health-related behaviours [25, 27]. According to Ciesielczyk et al., the condition accounts for less than 0.5% of all skin cancers [25]. Poccia et al. claimed that 2.4% of venous ulcers may undergo neoplastic transformation [43]. Senet et al. examined 155 chronic leg ulcers in 145 patients and identified Marjolin’s ulcer in 10.4% of the cases (9 cases of SSC, 5 of BCC) [44]. Factors predetermining development of cancer in venous ulcers include: advanced varicose veins, venous thromboembolism, chronic skin damage (old wounds that do not heal), chronic infections, and ulcers. Furthermore, it was observed that exposition to sunrays is more likely to result in development of BCC than SCC [45]. Reich Schupke et al. draw attention to the fact that the wounds of atypical morphology, pain, foul-smelling exudate, as well as resistance to treatment despite optimal care may suggest a cancerous change within the wound [46]. Delayed diagnosis may directly lead to less favourable prognosis, loss of limb, and/or metastases [31]. Therefore, patients with chronic leg ulcers should be monitored by multidisciplinary teams of specialists responsible for treatment, medical care, and rehabilitation [42, 47].
To comply with the current standards of medical care it is necessary to ensure well-designed early preventive operations linked with oncological alertness. Early surgical intervention, protective vaccinations, and efforts of medical personnel aimed at education of patients with extensive burn scars and hard-to-heal wounds will minimise the percentage of malignant ulcers diagnosed too late.
In conclusions
Malignant degeneration in chronic wounds is a very rare, aggressive form of Marjolin’s ulcer.
Increased oncological alertness should be displayed by nursing and medical personnel taking care of patients with chronic wounds.
Early diagnosis of wounds developing in damaged skin, including histopathological examination, decreases the risk of tissue destruction and extensive surgical resection.
Footnotes
The authors declare no conflict of interest.
References
- 1.Novick M, Gard DA, Hardy SB. Burn scar carcinoma: a review and analysis of 46 cases. J Trauma. 1977;17:809–17. [PubMed] [Google Scholar]
- 2.Sharma A, Schwartz RA, Swan KG. Marjolin’s Warty Ulcer. J Surg Oncol. 2011;103:193–5. doi: 10.1002/jso.21783. [DOI] [PubMed] [Google Scholar]
- 3.Marjolin J. In: Dictionnaire de Medicine. Adelon N, editor. Bechet: Paris; 1828. pp. 31–50. [Google Scholar]
- 4.Hawkins C. On warty tumors in cicatrices. Lond Med Gaz. 1833;13:481–2. [Google Scholar]
- 5.Dupuytren B. Lecons orales de clinique chirurgicale faites a L’hotel-dieu de Paris. Paris: Chez Germer Baillie`re; 1839. [Google Scholar]
- 6.Smith RW. Observations upon the warty ulcer of Marjolin. Dublin Quart J Med Sci. 1850;9:257–75. [Google Scholar]
- 7.Fordyce JA. Malignant diseases in scars and ulcers – Marjolin’s ulcer. In: Keen WW, editor. Surgery, its Principles and Practice. Vol. 2. Philadelphia: W B Saunders; 1911. pp. 631–2. [Google Scholar]
- 8.Da Costa JC. Carcinomatous changes in an area of chronic ulceration, or Marjolin’s ulcer. Ann Surg. 1903;37:496. [PMC free article] [PubMed] [Google Scholar]
- 9.Kózka M, Spałkowska M, Balawander R, Sroga J, Dobosz J. Rak kolczystokomórkowy w bliźnie poparzeniowej – opis przypadku owrzodzenia Marjolina [Squamous cell carcinoma in burn scar – case study of Marjolin’s ulcer] Leczenie Ran. 2013;10:71–5. [Google Scholar]
- 10.Choi JY, Bae YC, Nam SB, Bae SH. Impact of Disturbed Wound Healing after Surgery on the Prognosis of Marjolin’s Ulcer. Arch Plast Surg. 2013;40:198–202. doi: 10.5999/aps.2013.40.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bostwick J, 3rd, Pendergrast WJ, Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66–9. [PubMed] [Google Scholar]
- 12.Kerr-Valentic M, Samimi K, Rohlen B, et al. Marjolin’s ulcer: Modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184–91. doi: 10.1097/PRS.0b013e3181904d86. [DOI] [PubMed] [Google Scholar]
- 13.Treves N, Pack GT. The development of cancer in burn scar: an analysis and report of thirty-four cases. Surg Gynecol Obstet. 1930;58:749–51. [Google Scholar]
- 14.Harland DL, Robinson WA, Franklin WA. Deletion of the p53 gene in a patient with aggressive burn scar carcinoma. J Trauma. 1997;42:104–7. doi: 10.1097/00005373-199701000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Shin MS, Kim HS. Somatic mutations of Fas (Apo-1/CD95) gene in cutaneous cell carcinomas arising from a burn scar. J Invest Dermatol. 1999;114:122–26. doi: 10.1046/j.1523-1747.2000.00819.x. [DOI] [PubMed] [Google Scholar]
- 16.Sinha S, Su S, Workentine M, Agabalyan N, Cheng M, Gabriel V, Biernaskie J. Transcriptional analysis reveals evidence of chronically impeded ecm turnover and epithelium to mesenchyme transition in scar tissue giving rise to Marjolin’s ulcer. J Burn Care Res. 2017;38:14–22. doi: 10.1097/BCR.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 17.Nthumba PM. Marjolin’s ulcers: theories, prognostic factors and their peculiarities in spina bifida patients. World J Surg Oncol. 2010;8:108. doi: 10.1186/1477-7819-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copcu E. Marjolin’s ulcer: A preventable complication of burns? Plast Reconstr Surg. 2009;124:156–164. doi: 10.1097/PRS.0b013e3181a8082e. [DOI] [PubMed] [Google Scholar]
- 19.Koval-Vern A, Criswell BK. Burn scar neoplasm: A literature review and statistical analysis. Burns. 2005;31:403–13. doi: 10.1016/j.burns.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Zieliński T, Lewandowska M. Owrzodzenie Marjolina – nowotwór złośliwy rozwijający się na podłożu przewlekłych owrzodzeń i blizn. Analiza 8 przypadków [Marjolin’s ulcer – malignancy developing in chronic ulcers and scars. Analysis of 8 cases] Przegl Dermatol. 2010;97:38–42. [Google Scholar]
- 21.Wojewoda T, Wysocki MW, Mituś J. Wrzód Marjolina – opis przypadku i przegląd piśmiennictwa [Marjolin’s ulcer – case study and literature review] Pol Przegl Chir. 2009;81:766–73. [Google Scholar]
- 22.Philips TJ, Salman SM, Bhawan J, et al. Burn scar carcinoma. Dermatol Surg. 1998;24:561–5. [PubMed] [Google Scholar]
- 23.Hahn SB, Kim DJ, Jeon CH. Clinical study of Marjolins ulcer. Yonsei Med J. 1999;31:234–41. doi: 10.3349/ymj.1990.31.3.234. [DOI] [PubMed] [Google Scholar]
- 24.Trent JT, Kirsner RS. Wounds and malignancy. Adv Skin Wound Care. 2003;16:31–4. doi: 10.1097/00129334-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Ciesielczyk B, Murawa D, Nowaczyk P. Owrzodzenia Marjolina jako skutek przewlekłych owrzodzeń żylnych oraz przewlekłego urazu skóry – opisy przypadków [Marjolin’s ulcer as a result of chronic venous ulcers and chronic skin injury – case studies] Pol Przegl Chir. 2007;79:1198–206. [Google Scholar]
- 26.Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2014-208176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalya FL, Mabula JB, Rambau P, et al. Marjolin’s ulcers at a university teaching hospital in Northwestern Tanzania: a retrospective review of 56 cases. World J Surg Onkol. 2012;10:38. doi: 10.1186/1477-7819-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mustoe T, Upton J, Marcellino V, et al. Carcinoma in Chronic pressure sores: a fulminant disease process. Plast Reconstr Surg. 1986;77:116–21. doi: 10.1097/00006534-198601000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Stancard CE, Cruse CW, Wells KE, Karl R. Chronic pressure ulcer carcinomas. Ann Plast Surg. 1993;30:274–7. doi: 10.1097/00000637-199303000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Thio D, Clarkson JH, Misra A, Srivastava S. Malignant change after 18 months in a lower limb ulcer: acute Marjolin’s revisited. Br J Plast Surg. 2003;56:825–8. doi: 10.1016/j.bjps.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Bazaliński D, Guzik G, Barańska B. Owrzodzenie Marjolina w przebiegu nieskutecznie leczonej odleżyny okolicy krętarzowej uda prawego. Opis przypadku [Marjolin’s ulcer in unsuccessfully treated pressure sore in the area of right thigh trochanter. Case study] Leczenie Ran. 2015;4:155–8. [Google Scholar]
- 32.Fairbairn NG, Hamilton SA. Management of Marjolin’s ulcer in a chronic pressure sore secondary to paraplegia: a radical surgical solution. Int Wound J. 2011;8:533–6. doi: 10.1111/j.1742-481X.2011.00831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan K, Giannone AL, Mehrabi E, Khan A, Giannone RE. Marjolin’s ulcer complicating a pressure sore: the clock is ticking. Am J Case Rep. 2016;17:111–4. doi: 10.12659/AJCR.896352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aydogdu E, Yildrim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin ulcer? Burns. 2005;31:421–31. doi: 10.1016/j.burns.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Tobin Ch, Sanger JR. Marjolin’s Ulcers: A Case Series and Literature Review. Wounds. 2014;26:248–54. [PubMed] [Google Scholar]
- 36.Bozkurt M, Kapi E, Kuvat SV, Ozekinci S. Current concepts in the management of Marjolin’s ulcers: outcomes from a standardized treatment protocol in 16 cases. J Burn Care Res. 2010;31:776–80. doi: 10.1097/BCR.0b013e3181eed210. [DOI] [PubMed] [Google Scholar]
- 37.Bloemsma GC, Lapid O. Marjolin’s ulcer in an amputation stump. J Burn Care Res. 2008;29:1001–3. doi: 10.1097/BCR.0b013e31818ba0bf. [DOI] [PubMed] [Google Scholar]
- 38.Malheiro E, Pinto A, Choupina M. Marjolin’s ulcer of the scalp: case report and literature review. Ann Burns Fire Disasters. 2001;14:39–42. [Google Scholar]
- 39.Knudsen MA, Biering-Sørensen F. Development of Marjolin’s ulcer following successful surgical treatment of chronic sacral pressure sore. Spinal Cord. 2008;46:239–40. doi: 10.1038/sj.sc.3102090. [DOI] [PubMed] [Google Scholar]
- 40.Peterson R, Sardi A. Hemicorporectomy for chronic pressure ulcer carcinoma: 7 years of follow-up. Am Surg. 2004;70:507–11. [PubMed] [Google Scholar]
- 41.Grottig JC, Bunkis J, Vasconez LO. Pressure sore carcinoma. Ann Plast Surg. 1987;18:527–32. doi: 10.1097/00000637-198706000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Combemale P, Bousquet M, Kanitakis J, Bernard P. Angiodermatology Group, French Society of Dermatology. Malignant transformation of leg ulcers: a retrospective study of 85 cases. J Eur Acad Dermatol Venereol. 2007;21:935–41. doi: 10.1111/j.1468-3083.2006.02118.x. [DOI] [PubMed] [Google Scholar]
- 43.Poccia I, Persichetti P, Marangi GF, et al. Basal Cell Carcinoma Arising in a Chronic Venous Ulcer: Two Cases and a Review of the Literature. Wounds. 2014;26:30–5. [Google Scholar]
- 44.Senet P, Combemale P, Debure C, et al. Angio-Dermatology Group of The French Society of Dermatology. Malignancy and chronic leg ulcers: the value of systematic wound biopsies: a prospective, multicenter, cross-sectional study. Arch Dermatol. 2012;148:704–8. doi: 10.1001/archdermatol.2011.3362. [DOI] [PubMed] [Google Scholar]
- 45.Blank AA, Schnyder UW. Squamous cell carcinoma and basal cell carcinoma within the clinical picture of a chronic venous insufficiency in the third stage. Dermatologica. 1990;181:248–50. doi: 10.1159/000247946. [DOI] [PubMed] [Google Scholar]
- 46.Reich-Schupke S, Doerler M, Wollina U, Dissemond J, et al. Squamous cell carcinomas in chronic venous leg ulcers. Data of the German Marjolin Registry and review. Journal of the German Society of Dermatology. 2015;13:1006–14. doi: 10.1111/ddg.12649. [DOI] [PubMed] [Google Scholar]
- 47.Szewczyk MT, Jawień A. Zalecenia specjalistycznej opieki pielęgniarskiej nad chorym z żylnymi owrzodzeniami podudzi [Guidelines for specialist nursing care of patients with venous leg ulcers] Pielęg Chir Angiol. 2007;3:1–44. [Google Scholar]