ABSTRACT
Fungal species display an extraordinarily diverse range of lifestyles. Nevertheless, the survival of each species depends on its ability to sense and respond to changes in its natural environment. Environmental changes such as fluctuations in temperature, water balance or pH, or exposure to chemical insults such as reactive oxygen and nitrogen species exert stresses that perturb cellular homeostasis and cause molecular damage to the fungal cell. Consequently, fungi have evolved mechanisms to repair this damage, detoxify chemical insults, and restore cellular homeostasis. Most stresses are fundamental in nature, and consequently, there has been significant evolutionary conservation in the nature of the resultant responses across the fungal kingdom and beyond. For example, heat shock generally induces the synthesis of chaperones that promote protein refolding, antioxidants are generally synthesized in response to an oxidative stress, and osmolyte levels are generally increased following a hyperosmotic shock. In this article we summarize the current understanding of these and other stress responses as well as the signaling pathways that regulate them in the fungi. Model yeasts such as Saccharomyces cerevisiae are compared with filamentous fungi, as well as with pathogens of plants and humans. We also discuss current challenges associated with defining the dynamics of stress responses and with the elaboration of fungal stress adaptation under conditions that reflect natural environments in which fungal cells may be exposed to different types of stresses, either sequentially or simultaneously.
INTRODUCTION
Planet Earth plays host to an extravagantly diverse range of fungal species. Recent estimates suggest the probable existence of as many as 3 million fungal species (1), and the circa 75,000 of these that have been characterized to date display a wide range of lifestyles. Many fungi occupy specific niches within natural environments, playing essential roles in nutrient scavenging and recycling. Some thrive in close harmony with species from other kingdoms, a superb example being the mycorrhizal fungi, which display mutualistic interactions with plants. Other fungi are pathogenic, causing devastating infections of plants or animals. Indeed, the global threats that fungi pose to human health and food security are being increasingly recognized (2). Fortunately, a relatively small number of fungal species cause infections in humans (circa 400 species are described in the Atlas of Clinical Fungi [3]). Some of these fungi normally occupy environmental niches but are capable of colonizing and damaging human (or animal) tissues, whereas other fungi appear to be obligately associated with their host.
These diverse fungal niches are dynamic in that they display fluctuations in local parameters such as temperature, water balance, pH, or the levels of particular compounds such as nutrients and reactive oxygen and nitrogen species. These fluctuations are often capable of perturbing cellular homeostasis and causing molecular damage, thereby imposing stress on the fungal cell. Consequently, fungal cells must be able to adapt to these dynamic changes if they are to survive, grow, and colonize any niche. This stress adaptation is dependent on three fundamental principles. The first is the ability to detect environmental signals, i.e., the changing inputs from the local environment. The second is the ability to transduce these signals to regulate the cellular processes that mediate the stress adaptation. The third represents the adaptive responses themselves that allow cells to survive the stress. These adaptive processes either counteract or detoxify the initial stress and repair or remove the molecular damage caused by that stress. These fundamental principles must apply to all fungi.
Given the elemental nature of environmental stresses, it is not surprising that there are fundamental similarities across the fungal kingdom (and beyond) with regard to the basic cellular processes that mediate adaptation to specific stresses. For example, evolutionarily divergent ascomycetes and basidiomycetes induce protein refolding mechanisms in response to changes in temperature (4, 5) and the synthesis of antioxidants following exposure to oxidative stresses (6, 7). However, different niches exert different evolutionary pressures, and this has led to considerable diversity between fungal species with regard to the robustness of specific stress responses. For example, the yeast Debaryomyces hansenii, which is found in hypersaline waters, can tolerate higher levels of salt than Saccharomyces cerevisiae (8), which seems to have evolved to grow on fruit and to be disseminated by wasps (9). Also, Candida glabrata, which is a fungal pathogen of humans that is relatively resistant to phagocytic killing (10), displays extremely high levels of oxidative stress resistance compared to other yeasts (11). This evolutionary tuning of stress resistance to the local niche has led to some divergence between fungal species in the regulation of the cellular processes that mediate adaptation to some stresses.
This chapter summarizes our current understanding of the mechanisms underlying fungal stress adaptation and the regulation of these responses. We focus on those stresses that have been perceived to be the most relevant and hence have been most studied to date and the experimentally tractable model fungi in which stress adaptation mechanisms are best characterized. Also, we compare and contrast these outlooks on stress sensing to those from other fungi which have provided fascinating insights into niche-dependent stress adaptation.
ADAPTING TO INDIVIDUAL STRESSES
Heat Shock
Temperature modulates diverse facets of biology and disease (Fig. 1). Organisms across the tree of life must contend with changes in temperature that can manifest across a multitude of scales, from global climate change, to seasonal environmental change, to abrupt change associated with transitions in environmental niches. For microbial pathogens, temperature can signal the successful infection of a host and serves as a central cue governing proliferation, developmental programs, and virulence (12). As an example, fever is a ubiquitous response to infection, with elevated febrile temperatures thought to serve as an adaptive host response to restrict microbial proliferation. In a broader context, mammalian endothermy may have evolved as a strategy to minimize infections caused by fungal species, most of which have a diminished capacity to proliferate at elevated temperatures (13). Of the ∼3 million fungal species estimated to exist, less than 0.1% are able to cause disease in mammals, and this can largely be attributed to most rapidly losing their capacity for growth above ambient temperature (14).
FIGURE 1.

Cartoon summarizing stress pathways in the model fungus S. cerevisiae. See text. This figure summarizes some, but not all, of the known components of these signaling pathways. Components of MAPK signaling modules are highlighted in blue, transcription factors in pink, components of the calmodulin-calcineurin pathway in cyan, Rim pathway components in green, and the molecular chaperone Hsp90 in yellow. Note that the C. albicans Cek1 MAPK pathway, which contributes to cell wall remodeling in this fungus, is included (dark blue ovals with white lettering).
Thermal transitions have a profound impact on fungal development and virulence. For example, virulence of the dimorphic fungal pathogens is controlled by a temperature-dependent change in morphology (15). Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum are key species that exemplify the characteristic response to temperature of the dimorphic fungi: these species grow as filamentous molds in the soil in response to ambient temperature and convert to growth as yeast cells in response to host temperature upon inhalation of spores into mammalian lungs. The polymorphic fungus Candida albicans is another fungal pathogen for which temperature induces a dramatic morphological change (16). In contrast to the dimorphic fungi, C. albicans proliferates in the yeast form at ambient temperatures, and elevated temperature promotes a transition to filamentous growth. A temperature of 37°C is required to enable filamentation in response to diverse cues such as serum, and a further increase in temperature to 39°C is sufficient to induce filamentation in the absence of other cues (17). Beyond morphogenesis, temperature exerts a profound influence on diverse aspects of C. albicans biology, including mating, phenotypic switching, and drug resistance (17). In addition to the phenotypic consequences of growth at sustained elevated temperatures, fungi also have a profound response to acute but temporary increases in temperature that is referred to as a heat shock response. Strikingly, a transient heat shock can activate a C. albicans transcriptional program that is associated with increased host cell adhesion, host cell damage, and virulence (18).
Fungi have evolved complex molecular machinery and regulatory circuits to respond to the stress induced by sustained or transient responses to elevated temperature, with the heat shock response being one of the most evolutionarily conserved stress responses in nature. Core to the heat shock response is a global arrest of translation elongation and transcriptional activation of genes encoding heat shock proteins, which include molecular chaperones that promote folding and refolding (19). In C. albicans and the model yeast S. cerevisiae, ∼10 to 20% of genes in the genome are induced in response to heat shock (20, 21). This transcriptional response is orchestrated in large part by the heat shock transcription factor Hsf1. In C. albicans, Hsf1 binds to 49 targets constitutively and an additional 55 targets in response to heat shock, with targets enriched for functions in protein folding and entry into the host (18). Hsf1 typically binds at nucleosome-depleted regions in the promoters of target genes and recognizes three motifs with distinct binding affinities (18). Hsf1 is activated by phosphorylation, and this activation is required for virulence in C. albicans (19, 21).
Complex functional relationships influence mobilization of cellular responses to thermal stress. Hsf1 promotes the basal expression and thermal induction of genes encoding the molecular chaperone Hsp90 (Fig. 1). Hsp90 in turn physically interacts with Hsf1, thereby exerting a repressive effect on activation of the heat shock response (19). As a consequence, perturbation of Hsp90 function by small molecules, mutations, or elevated temperature causes activation of Hsf1 and induction of the heat shock response in the absence of thermal stress. In contrast, Hsp90 is required to mobilize a rapid transcriptional response to thermal stress, such that depletion of Hsp90 causes delayed induction of the transcriptional program induced by heat shock (18). Hsp90 influences transcriptional programs not only via effects on Hsf1, but also by modulating chromatin remodeling, nucleosome removal, and RNA polymerase II stalling (22–24).
As a molecular chaperone, Hsp90 regulates the stability and function of diverse client proteins, which include many core cellular regulators beyond Hsf1. For many client proteins, Hsp90 stabilizes otherwise metastable regulators, thereby enabling their activation in response to stress or other cues (25, 26). In the context of thermal adaptation, Hsp90 stabilizes the Hog1 stress-activated protein kinase, as well as the mitogen-activated protein kinases Mkc1 and Cek1 (27–29). In this context, Hsp90 enables signaling required for cell wall remodeling and adaptation to heat shock. Classical genetic screens and chemical genomic approaches to identify mutants that are hypersensitive to Hsp90 inhibition under distinct stress conditions have provided powerful strategies to identify novel Hsp90 client proteins and key regulators of thermal adaptation (27, 29, 30).
The circuitry underpinning thermal adaptation can be activated by a remarkable diversity of temperature-sensing mechanisms. For example, Hsp90 function is exquisitely sensitive to elevated temperature because the global problems in protein folding that arise for thermal stress create an elevated cellular demand for Hsp90 that exceeds its functional capacity to engage with client proteins (30). In the broader context, DNA, RNA, proteins, and lipids can all serve as thermosensors that sense changes in temperature to initiate crucial cellular responses and developmental programs (12).
Osmotic Stress
Managing changes in water balance is another fundamental challenge for fungi in most environments. The classic experimental model for this has been the imposition of hyperosmotic shock through addition of sorbitol or salts such as NaCl (31). This results in a sudden decrease in the intracellular turgor pressure that is required for fungal growth. The fungus must restore its turgor pressure before it can resume growth, and to achieve this, it activates the synthesis and accumulation of intracellular osmolytes such as glycerol (Fig. 1).
S. cerevisiae responds to osmotic stress by increasing the flux from glycolysis to glycerol. This is achieved by inducing the genes encoding glycerol-3-phosphate dehydrogenase (GPD1) and glycerol-3-phosphate phosphatase (GPP1). S. cerevisiae can also assimilate glycerol from the growth medium, although this uptake is repressed by glucose (32), which is in contrast to the response of some other yeasts (33, 34). However, the intracellular accumulation of glycerol is also dependent on restricting glycerol efflux from the cell through the plasma membrane-based aquaglyceroporin, Fps1 (35). Following the imposition of a hyperosmotic stress, the intracellular accumulation of glycerol and the restoration of turgor pressure take about 30 minutes (36), after which growth may resume.
This type of response, which involves signaling, gene regulation, and subsequent changes in metabolism, is too slow to protect the fungal cell against hypo-osmotic shock. This stress causes the immediate accumulation of water and rapid increases in cell volume which, if not countered quickly, would cause a yeast cell to burst (37). In S. cerevisiae, this rapid rise in turgor pressure is alleviated by swift opening of the Fps1 aquaglyceroporin, the activity of which is regulated by protein phosphorylation. Mutations that inactivate Fps1, and hence block rapid glycerol efflux, confer hypo-osmotic stress sensitivity on the yeast cell (38). Clearly, responses to hyper- and hypo-osmotic shocks occur over differing timescales that reflect the relative imminence of potentially irreversible damage to the fungal cell.
Regarding the regulation of osmotic stress responses, the activation of Hog1 in S. cerevisiae has formed the paradigm of osmotic stress activation of fungal stress-activated protein kinases (SAPKs) (39). Like all mitogen-activated protein kinase (MAPK) modules, the Hog1 MAPK module comprises three tiers of kinases; the MAPKKK(s) at the top of the pathway phosphorylates and activates a downstream MAPKK, which then phosphorylates and activates the terminal MAPK. The central importance of this MAPK module in fungal stress sensing is illustrated by its involvement in diverse environmental responses in diverse fungi, which even include light sensing in Aspergillus nidulans (40). In S. cerevisiae, two functionally redundant pathways converge at the MAPKK Pbs2 to relay osmotic stress signals to Hog1. These are the Sln1 two-component signaling pathway and a pathway that contains the SH3-domain-containing Sho1 transmembrane protein. In the first pathway, loss of turgor pressure, induced by high osmolarity, inactivates the transmembrane histidine kinase Sln1 and thus halts phosphorelay through the phosphorelay protein Ypd1, leading to a rapid dephosphorylation of the Ssk1 response regulator (41). Dephosphorylated Ssk1 activates the MAPKKKs Ssk2/22 in a two-step mechanism (42), leading to phosphorylation and activation of the Pbs2 MAPKK. In the second pathway, the Ste11 MAPKKK phosphorylates Pbs2 when stimulated by osmotic stress signals received from the Sho1 branch of the Hog1 pathway (43). Many proteins are involved in the osmotic stress signaling from Sho1 to Ste11-Pbs2, including Cdc42, Ste20, Cla4, Ste50, and Opy2 (43, 44). Sho1 was originally thought to function as the osmosensor at the top of the pathway (45). However, two functionally redundant osmosensensors have since been identified: the mucins, Msb2 and Hkr1 (46). As different signaling mechanisms are employed by Msb2 and Hkr2 (47, 48), the Sho1 branch is now divided into the Hkr1 and Msb2 subbranches. While Msb2 and other Sho1 branch components also participate in the filamentous growth MAPK pathway, Hkr1 plays a specific role in Hog1 signaling (49), and this is mediated through the newly discovered scaffold protein Ahk1 (50).
Because the SAPKs are among the most evolutionarily conserved stress signaling proteins in fungi (11), it is remarkable that the mechanisms underlying the osmotic stress regulation of S. cerevisiae Hog1 have significantly diverged. For example, in the distantly related model yeast Schizosaccharomyces pombe, two-component signaling functions to relay H2O2, but not osmotic, stress signals to the Sty1 SAPK (51), and an orthologue of Sho1 is seemingly absent from the fission yeast genome (11). In C. albicans, although Sho1 pathway components have been identified and characterized, they are not required for the relay of osmotic stress signals to Hog1 (52, 53). Consistent with this, Hog1 in C. albicans is regulated by a single MAKKK, Ssk2, with the Ste11 MAPKKK—predicted to function downstream of Sho1—having no obvious role (54). In contrast, the available evidence does support the involvement of two-component signaling in the activation of C. albicans Hog1 by osmotic stress, because close homologues of the Sln1-Ypd1-Ssk1 pathway are present in C. albicans, and Hog1 is hyperactivated in cells lacking Sln1 (53). However, the observation that osmotic stress-induced Hog1 activation is not notably impaired in cells lacking the Ssk1 response regulator (55) suggests the presence of a novel osmotic stress-sensing pathway in this fungal pathogen. In certain C. glabrata isolates, only the Sho1 branch functions to relay osmotic stress signals to Hog1. This is due to a truncated ssk2 allele which prevents signaling through the Sln1 branch (56). Intriguingly, gain of function mutations have been identified in the related Ssk2 MAPKKK in Cryptococcus neoformans (57), which are responsible for high basal levels of Hog1 activation in serotype A strains (58).
Hog1 has been shown to play a central role in the regulation of osmoadaptation in S. cerevisiae. This MAPK regulates accumulation of glycerol via transcriptional activation of GPD1 and GPP1 in response to osmotic stress via the transcription factors Hot1, Msn2, and Msn4 (59) and by controlling the activity of the Fps1 acquaglyceroporin (60). It should be noted that additional TORC2/Ypk1-dependent signaling mechanisms do contribute to the regulation of Fps1 and hence to survival in the face of hyperosmotic stress (38). However, Hog1 also mediates the transient delay in cell cycle progression following hyperosmotic shock by phosphorylation of Sic1 and Hsl1, and by downregulating G1 and G2 cyclins (61). Once osmo-adaptation is achieved, the yeast cell has essentially achieved a new homeostatic state in which turgor pressure has been restored in the face of the external osmotic conditions (62). Consequently, the input signal has been dampened, Hog1 becomes deactivated, the block to cell cycle progression is released, and growth can resume.
Oxidative Stress
Reactive oxygen species (ROS) are highly damaging, reduced forms of oxygen, which include the superoxide anion O2·, hydrogen peroxide (H2O2), and the hydroxyl radical (·OH). These reactive molecules damage proteins, DNA, and lipids and can trigger programmed cell death (63). All fungi that grow aerobically are exposed to superoxide anions generated as a by-product of aerobic respiration in the mitochondria (64). Environmental fungi are also exposed to ROS generated following exposure to UV light or to drugs/xenobiotics found in the environment (63). In addition, pathogenic fungi are exposed to superoxide and hydrogen peroxide ROS, which are generated by plant (65) or animal (66) host defense systems as a major antimicrobial defense mechanism. Significantly, other toxic chemicals are subsequently derived from the host-generated ROS (66).
Oxidative stress occurs when the levels of ROS exceed the antioxidant capacity of the cell which functions to maintain the intracellular redox environment in a reduced state. In response to oxidative stress, fungal cells mount a wide range of defense and repair strategies. One well-characterized and conserved response involves the rapid induction of mRNAs that encode oxidative stress detoxification and repair proteins (Fig. 1). Transcript profiling studies indicate that a set of core antioxidant genes are induced in fungi following exposure to H2O2 (20, 67–71). These include catalase (CAT1), glutathione peroxidase (GPX), and superoxide dismutase (SOD) antioxidant encoding genes, in addition to genes encoding components of the glutathione/glutaredoxin (GSH1, TTR1) and thioredoxin (TSA1, TRX1, TRR1) systems. An additional and very rapid response to oxidative stress, which precedes transcriptional activation, is the dynamic redirection of the metabolic flux from glycolysis to the pentose phosphate pathway. This metabolic switch is triggered by the oxidation and inactivation of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase, which functions to promote the generation of reducing power in the form of NADPH (72). Yeast cells also transiently delay cell cycle progression following exposure to ROS to allow DNA damage repair. For example, H2O2 causes a G2 cell cycle arrest in S. cerevisiae by activating the Rad53 DNA damage checkpoint (73). For more details on activation of DNA damage checkpoints in S. cerevisiae, readers are directed to the recent excellent review in reference 74. In the pathogenic fungus C. albicans, exposure to H2O2 also triggers Rad53-mediated cell cycle arrest. Interestingly, in this fungus, such genotoxic-induced cell cycle arrest promotes the formation of a filamentous hyperpolarized bud growth form (75, 76).
Orthologues of transcription factors that are vital for oxidative stress-responsive gene induction in S. cerevisiae, the AP-1-like bZip factor Yap1 and the Skn7 response regulator, have now been studied in many fungi (66). The elegant mechanism underlying activation of S. cerevisiae Yap1 is well characterized. Following exposure to H2O2, specific cysteine residues located within two distinct cysteine-rich domains become rapidly oxidized (77). This oxidation event, which requires the thiol peroxidase Gpx3 (78) and the Yap1 binding protein Ybp1 (79), triggers a conformational change within Yap1 that masks a nuclear export sequence (80). Consequently, Yap1 accumulates in the nucleus, resulting in the induction of Yap1-dependent genes (78). In the human fungal pathogen C. albicans, Cap1 oxidation is similarly regulated by Gpx3 and Ybp1 (81). However, in the model yeast S. pombe, Pap1 oxidation depends instead on the 2-Cys peroxiredoxin Tpx1 (82), and in the fungal symbiont Epichloe festucae, redox regulation of the analogous YapA factor is independent of both Gpx3 and Tpx1 (83). Thus, multiple mechanisms may exist to regulate the oxidation of fungal AP-1-like factors. Interestingly, AP-1-like factors have been found to be dispensable for the virulence of the human fungal pathogens Aspergillus fumigatus (84), C. neoformans (85), and C. albicans (81, 86) but are required for the virulence of a number of plant pathogens (87–89).
In S. cerevisiae, Yap1 collaborates with Skn7 to regulate many oxidative stress-response genes, and this may be conserved, because similar findings have been reported in C. glabrata (90) and S. pombe (91). Little is known about Skn7 activation following oxidative stress, but a study has reported a DNA-independent interaction between Yap1 and Skn7 in S. cerevisiae (92), and in C. glabrata, Yap1 and Skn7 cooperatively bind to the upstream region of core oxidative stress genes (90). In S. pombe, a role for two-component mediated phosphorylation of the Skn7 homologue in responses to high levels of H2O2 stress has been uncovered (93). A recent review summarizes the functions of Skn7 and its roles in fungal virulence (94).
In addition to Yap1 and Skn7 are the bZip factors of the ATF/CREB family. The best characterized is the Atf1 transcription factor in the model yeast S. pombe. In response to oxidative stress, Atf1 is hyperphosphorylated by the Sty1 SAPK (95). This stabilizes this transcription factor, which is vital for its function in oxidative stress-induced gene expression (96, 97). Analogous transcription factors shown to play roles in oxidative stress-mediated gene expression include Atf1 in C. neoformans (98), Moatf1 in Magnaporthe oryzae (99), and AtfA in A. nidulans and A. fumigatus (100, 101). Moreover, there is emerging evidence to support the general concept that such transcription factors are targets of fungal SAPK pathways (101, 102).
Although the Hog1 SAPK in S. cerevisiae is dispensable for oxidative stress responses (20, 103), homologues play important roles in oxidative stress tolerance in many other fungi. These include the model yeast S. pombe (104); the filamentous fungus A. nidulans (101); a number of human pathogenic fungi including C. albicans (105), C. neoformans (58), and A. fumigatus (106); and the plant pathogens Bipolaris oryzae (107) and Fusarium graminearum (108). The Sty1 pathway in the model yeast S. pombe has provided key insight into the oxidative stress-mediated activation of such pathways. Sty1 is robustly phosphorylated in response to oxidative stress and plays a major role in the regulation of the oxidative-stress-induced transcriptome (91). In S. pombe, a two-component signaling system operates to relay H2O2, and not osmotic, stress signals to the Sty1 SAPK module. The Mak2 and Mak3 histidine kinases contain redox-sensing PAS and GAF domains, which are essential for the relay of H2O2 signals to Sty1 (93). In addition, protein oxidation also regulates the H2O2-induced activation of Sty1.
The redox-sensitive peroxiredoxin enzyme Tpx1 is essential for H2O2-mediated Sty1 activation and, because intermolecular disulfide bonds are formed between conserved cysteine residues in Sty1 and Tpx1 following H2O2 stress, it appears that Tpx1 may regulate Sty1 function directly (109). Mechanisms underlying H2O2-mediated SAPK activation have also been explored in C. albicans. C. albicans Hog1 is robustly activated following exposure to H2O2 (70), and hog1Δ cells are sensitive to a range of ROS (105, 110), despite a limited transcription role (70). Similar to that in S. pombe, oxidative stress-induced Hog1 phosphorylation is drastically reduced in cells lacking the Ssk1 response regulator (55), although no similar roles have been found for the upstream histidine kinases (53, 111). Both the redox-sensitive peroxiredoxin Tsa1 and thioredoxin Trx1 enzymes are vital for H2O2-induced Hog1 activation in C. albicans (75). Thus, protein oxidation appears to be an important mediator of both C. albicans and S. pombe SAPK activation following oxidative stress. Mechanistic details regarding oxidative-stress-mediated SAPK activation in other fungi are lacking, although in A. nidulans it has been shown that the SskA response regulator is essential for oxidative stress-induced activation of the SakA SAPK (112).
Nitrosative Stress
Fungal cells experience nitrosative stress when they are exposed to relatively high levels of reactive nitrogen species (RNS). These RNS include nitric oxide (·NO) and its derivatives peroxynitrite (ONOO−, which is formed by the reaction of nitric oxide with superoxide, O2·−), nitrite (NO2–), nitrogen dioxide (·NO2–), and nitrate (NO3–). Fungi are confronted by RNS in the soil, as well as in mammalian hosts, where these reactive molecules are components of the phagocytic armory used to combat microbial infection (113–115). Therefore, the protective responses of fungal pathogens against nitrosative stress are important for pathogenicity and have been examined in some detail, for example, in C. albicans and C. neoformans (116, 117). In contrast, for plant pathogens such as Blumeria graminis, Botrytis cinerea, and M. oryzae, NO has been shown to promote the spread of infection in plant hosts. These effects might be mediated by the action of NO as a developmental signal (118–120) but could also be due to the influence of fungus-derived NO on the behavior of the plant host (121).
Excess RNS damage proteins by reacting with thiols, metal centers, and tyrosine residues. Fungi protect themselves by buffering RNS with antioxidants such as glutathione and by inducing mechanisms to detoxify the RNS and repair the damage they cause. NO reacts with glutathione (GSH) to generate S-nitrosoglutathione (GSNO). To restore redox homeostasis, fungi recycle this GSNO to GSH via glutathione disulfide. This is achieved through the evolutionarily conserved enzymes GSNO reductase (GSNOR: Fdh3) and glutathione reductase (122–124). NO detoxification to nitrate is mediated by nitric oxide oxidoreductases (e.g., S. cerevisiae Yhb1), which are conserved enzymes that are members of the flavohemoglobin family (125).
While the processes that protect against RNS are evolutionarily conserved, the regulatory mechanisms that induce these processes in response to RNS appear to have diverged. For example, while RNS exposure triggers global changes in the expression profiles of S. cerevisiae and C. albicans that include the induction of Yhb1 and glutathione synthesis (116, 126), seemingly unrelated transcription factors drive these changes. In S. cerevisiae, YHB1 expression is induced by the transcription factor Fzf1 (126) (Fig. 1), whereas Cta4 activates YHB1 in C. albicans (127). Nevertheless, the outcomes are similar: these transcription factors both promote Yhb1 and glutathione expression and, hence, nitrosative stress resistance in these fungi. For the fungal pathogen C. albicans the deletion of YHB1 or its activator CTA4 attenuates virulence slightly (116, 127, 128). Similarly, inactivation of the nitric oxide oxidoreductase (Fhb1) or the GSNOR (Gno1) in C. neoformans attenuates the virulence of this pathogen (129). NO production by the host does not seem to be a major factor in limiting fungal virulence (116). Nevertheless, the available data suggest that RNS detoxification does contribute to fungal virulence.
Cell Wall Stress
The fungal cell wall is a dynamic structure that is continually remodeled during cell growth and division. The cell wall represents ∼25% of the yeast cell dry weight, underscoring the extensive metabolic commitment to support this elaborate structure, which provides the key interface for mediating interactions with the environment (130). Fungal cell wall architecture involves layers of polysaccharides and glycoproteins, although the specific composition varies across species. Typically, a matrix of chitin, β-1,3-glucan, and β-1,6-glucan constitutes the core inner layer, with mannans and other mixed glycans and glycoproteins prevalent in the outer layer. Cell walls provide crucial protection against changes in external osmotic potential and can confer resistance to infection and to degradation by soil predators such as amoebae and protists. These adaptive advantages may contribute to the emergence and maintenance of cell walls in the fungal kingdom (131). Cell walls serve not only as a protective shell, but also as a means to modulate immune recognition. Fungal cell wall glycans, glycolipids, and proteins that are absent from mammals activate a wealth of immune recognition mechanisms, and the dynamic exposure of such pathogen-associated molecular patterns can modulate immune recognition (132). As a consequence, perturbation of fungal cell wall architecture can potentiate immune responses and induce lethal cell wall stress. Molecules that potently inhibit fungal cell wall biosynthesis have been elaborated in nature as with the echinocandins and exploited in modern medicine, with semisynthetic derivatives now a front-line treatment for fungal infections (133).
Fungi have evolved complex cellular circuitry to sense and respond to cell wall stress. Although details vary among fungi, the core architecture is broadly conserved. Cell wall stress is typically sensed at the plasma membrane via cell surface sensors, which include Wsc1, Wsc2, Wsc3, Mid2, and Mtl1 in S. cerevisiae (130). This stimulates nucleotide exchange on the small G-protein Rho1, which orchestrates cell wall integrity signaling. Downstream effectors of Rho1 coordinate synthesis of β-glucans, transcriptional control of cell wall genes, polarization of the actin cytoskeleton, and targeting of secretory vesicles. The most well-established pathway through which cell wall integrity signals are transduced from Rho1 is the MAPK cascade that includes Pkc1, Bck1, Mkk1/2, and Mpk1/Slt2 in S. cerevisiae (17). Although this cascade provides a powerful mechanism to amplify cell surface signals and coordinate highly sensitive responses, additional robustness is integral to ensure maintenance of cell wall physiology and is achieved by complex genetic interaction networks. These networks can enable compensatory responses to cell wall perturbations. For example, activation of chitin synthesis suppresses the antifungal activity of echinocandins (134–136), which inhibit biosynthesis of β-1,3-glucan. The highly connected genetic networks that control cell wall stress response circuitry can be activated by diverse environmental stresses, providing a powerful strategy to rapidly mobilize protective mechanisms.
Environmentally contingent hubs of cellular signaling are crucial for orchestrating cell wall stress responses. An excellent example is the molecular chaperone Hsp90. Hsp90 modulates the stability and function of diverse regulators of cellular signaling, thereby enabling responses to a myriad of stresses, including perturbation of the cell wall (29, 137, 138) (Fig. 1). Hsp90 enables responses to cell wall stress at least in part by modulating cell wall integrity signaling. Hsp90 stabilizes the terminal MAPK in the cell wall integrity pathway, Slt2 in S. cerevisiae, and Mkc1 in C. albicans (139–141). Hsp90 also stabilizes an additional MAPK that is implicated in cell wall remodeling: Cek1 (29). Compromise of Hsp90 function leads to depletion of these kinases and hypersensitivity to cell wall stress. Additional Hsp90 client proteins important for cell wall remodeling are the protein phosphatase calcineurin and kinase Hog1. Signaling through the cell wall integrity pathway, Hog1, and calcineurin coordinately regulates the synthesis of chitin in response to stress induced by perturbation of the cell wall or cell membrane (142). Chitin levels are dramatically increased in response to echinocandins, which provides protection that enables cells to cope with cell wall damage (134). Cell damage induced by various genetic or environmental insults can also be buffered by activation of other core cellular signaling pathways such as the cyclic AMP protein kinase A cascade (17). Although the molecular details have been explored in the greatest depths in S. cerevisiae, many conserved principles of coordinate control of cell wall stress response are emerging from studies in diverse fungi (143–146).
There is broad therapeutic potential of targeting core regulators of cell wall stress responses as a strategy to enhance the efficacy of antifungal drugs that target the cell wall, as with the echinocandins. This potential is illustrated by the discovery that inhibition of Hsp90 of calcineurin enhances the efficacy of echinocandins against diverse fungal pathogens in multiple models of infection (137, 144, 147). The therapeutic challenge of exploiting these conserved eukaryotic cellular regulators as targets for antifungal drug development lies in the development of molecules that can distinguish the pathogen from the host. Achieving this goal can be facilitated by structure-guided drug design (148). As a complementary approach to targeting regulators of cell wall stress response, systematic screens for molecules that potentiate the activity of echinocandins provide a powerful strategy to enhance the efficacy of our limited arsenal of antifungal drugs and thwart the emergence of drug resistance (149).
pH Stress
Alkaline pH imposes several stresses on fungi. One of the most significant relates to nutrient acquisition. At high extracellular pH, the establishment of electrochemical gradients across the plasma membrane for nutrient transport and ATP synthesis is more difficult (150). Moreover, the solubility and biological availability of essential elements such as iron are dramatically reduced at high pH. The finding that the addition of micromolar concentrations of copper or iron ions significantly improves the growth of S. cerevisiae at high pH suggests that these two elements are limiting factors for growth under alkaline pH conditions (151).
An important aspect of pH regulation is the ability to regulate gene expression in response to ambient pH, which allows fungi to synthesize the environmentally appropriate gene products, particularly secreted proteins. This ability has practical implications, for example, in the production of secondary metabolites or for fungal pathogenicity on plant and animal hosts (152, 153). Environmental pH also has profound effects on fungal development. For example, in C. albicans a shift from acidic to neutral-alkaline pH promotes the transition from yeast to filamentous growth (152).
The best-known alkaline pH-responsive signal transduction mechanism in fungi is the Pal/Rim pathway (Fig. 1). This pathway has been extensively studied in A. nidulans (Pal) and S. cerevisiae (Rim). In A. nidulans, the Pal pathway sequentially involves the proteins PalH, PalI, PalF, PalC, PalA, and PalB. Moreover, the pathway encompasses a number of co-opted components of the multivesicular body and endosomal sorting complexes required for transport (153). Activation of the pathway by alkaline pH leads to proteolytic activation of the zinc finger transcription factor PacC/Rim101 (154, 155). PacC undergoes two successive C-terminal proteolytic cleavages from the full-length 72-kDa to the processed 27-kDa active form, the first of which is pH-dependent and carried out by the signaling protease PalB (153).
PalH/Rim21, a seven-transmembrane-domain plasma membrane protein, is thought to function as a pH receptor. PalH forms a complex with the arrestin-like protein PalF/Rim8, which is ubiquitinated in an alkaline pH-dependent manner and recruits endosomal sorting complexes required for transport-I Vps23, thereby creating multiple docking sites for the downstream signaling components (156–159).
An unanswered question is how PalH/Rim21 senses alkaline ambient pH. The membrane potential of the yeast plasma membrane is mainly generated by differences in proton concentration between the inside and outside of the cell. External alkalization therefore leads to impaired phospholipid flipping and plasma membrane depolarization by collapsing the proton electrochemical gradient. Interestingly, it was shown that the Rim101 pathway in yeast can be activated in a pH-independent manner by either protonophore treatment or depletion of phosphatidylserine in the inner leaflet of the plasma membrane, both of which cause plasma membrane depolarization similar to external alkalization. This activation is dependent on Rim21, suggesting that plasma membrane depolarization is a key signal sensed by Rim21 (159). Moreover, a recent study suggests that alterations in lipid asymmetry cause changes in lipid composition and local charge on the inner leaflet, leading to dissociation of the Rim21 complex from the plasma membrane and recruitment of downstream proteins (160). Thus, Rim21 senses external alkalization, as well as altered lipid asymmetry. It was proposed that Rim21 uses its flexible C-terminal cytosolic tail like an antenna to monitor the status of membrane lipid asymmetry (160).
To achieve infection, pathogenic fungi must adapt to wide variations in the ambient pH of host tissues, which in humans, can vary from 2 to >8 depending on the niche. Early studies of C. albicans revealed that genes encoding two functionally redundant cell wall β-glycosidases, PHR1 and PHR2, display divergent pH-dependent expression patterns and virulence functions in cell wall remodeling. While PHR1 is expressed preferentially at neutral-alkaline pH and is required for systemic infection, PHR2 is expressed preferentially at acidic pH and is required for vaginal infection (161). These pH-dependent expression patterns are dependent on PacC/Rim signaling (162). More recently, multiple roles for the PacC/Rim pathway during human colonization and infection have been established in C. albicans, including filamentation, adhesion to host cells, tissue invasion, iron acquisition, and protease secretion (152). The role of PacC/Rim101 in mammalian infection is conserved in filamentous ascomycetes such as Fusarium oxysporum and A. fumigatus (163, 164). In the basidiomycete human pathogen C. neoformans the RIM pathway is also involved in pathogenicity (165), although the function of PalH/Rim21 appears to be carried out by a distinct membrane protein (166).
Alkaline pH signaling is also relevant in other fungus-host interactions. For example, deletion of PacC in the nematophagous fungus Clonostachys rosea and the insect pathogen Metarhizium robertsii results in attenuated virulence (167, 168). In plant pathogens, the role of PacC in virulence varies depending on the pathogen-host system. While it contributes to virulence in the rice blast fungus M. oryzae and the fruit pathogen Penicillium expansum, it appears to function as a negative virulence regulator in the vascular wilt fungus F. oxysporum (169–171).
Besides the Pal/Rim pathway, additional cell signaling pathways function in fungal adaptation to neutral/alkaline pH. The calcium-dependent protein phosphatase calcineurin and its downstream transcription factor Crz1 are required for growth at alkaline pH and for fungal virulence on animal and plant hosts (172–176). Crz1 also mediates tolerance to high cation concentrations (177). Mds3, a negative regulator of the TOR pathway, promotes adaptation to neutral/alkaline pH as well as virulence-related morphogenesis in C. albicans (178). Moreover, mutations in the cell wall integrity MAPK cascade confer sensitivity to alkaline stress, and alkalinization results in rapid and transient phosphorylation of the Slt2 MAPK in S. cerevisiae (179). This suggests that alkaline pH stress profoundly affects the composition of the fungal cell wall.
Recently, a novel signaling pathway was identified which is required for resistance to alkaline pH and cation stress in A. nidulans (176). This pathway, which appears to be specific to filamentous fungi, is defined by the transcription factor SltA and the serine protease SltB. Activation of SltA requires proteolytic cleavage and removal of the N-terminal domain by SltB and phosphorylation of the functional C-terminal moiety (180). Interestingly, while the SltA pathway is conserved in filamentous fungal pathogens, its role in infection remains to be determined.
Weak Acid Stress Response
The weak acid stress response has been largely studied in the context of food spoilage, but it is likely to be highly relevant to environmental and pathogenic fungi that occupy acidic niches or that are exposed to phagocytic attack. Weak organic acids such as acetic, propionic, sorbic, and benzoic acids impose stress on fungal cells when the environmental pH lies below the pKa of the acid in question (i.e., below pH 4.8 for acetic acid). Below its pKa, a weak acid is in its associated nonpolar form and is better able to cross the plasma membrane. Acetic acid enters the S. cerevisiae cell via the aquaglyceroporin Fps1, whereas propionic, sorbic, and benzoic acids appear to diffuse passively across the plasma membrane (181). Once they enter the higher pH of the cytoplasm, they dissociate into the free acid anion and proton (H+). The equilibrium of this reaction drives the accumulation of the acid anion and protons and, consequently, acidification of the cytoplasm. Weak acid stress is imposed partly by this cytoplasmic acidification and partly by the accumulation of the organic anion, which can impose toxic effects on yeasts and molds (182, 183). For example, sorbic acid appears to exert membrane-active antimicrobial effects in S. cerevisiae (182).
Fungal cells respond to weak acid stress by attempting to maintain their intracellular pH and by exporting the organic anion. In S. cerevisiae these tasks are executed by Pma1 and Pdr12, respectively (184, 185). Pma1 is the plasma membrane H+-ATPase proton pump. This pump is essential for growth and for the restoration of cytoplasmic pH, which is an energy-demanding process (186). Pdr12 is an ATP-binding cassette transporter that mediates efflux of the organic anion from the yeast cell. Pdr12 is also important for the restoration of cytoplasmic pH during sorbic and benzoic acid stress (187).
In S. cerevisiae, resistance to acetic acid stress is dependent on Hog1 signaling. Hog1 downregulates the Fps1 aquaglyceroporin, thereby reducing acetate accumulation (181), and activates the Haa1 transcriptional regulon, which includes genes encoding membrane stress proteins (188). Meanwhile, resistance to propionic, sorbic, and benzoic acids depends on the induction of PDR12 (184) via the transcription factor War1 (189, 190). War1 is potentially regulated by direct binding of the organic anion, thereby precluding a requirement for upstream signaling.
Analogous regulatory mechanisms appear to exist in some yeasts. For example, Hog1 mediates sorbic acid resistance in C. glabrata (191), and resistance to this weak acid in C. albicans is dependent on a War1 orthologue (192) as well as the Msn2/4-like transcription factor Mnl1 (193). However, alternative mechanisms seem to mediate weak acid stress resistance in other yeasts, most notably the food spoilage organism Zygosaccharomyces bailii, which displays high levels of weak acid stress resistance. In this species, survival in the face of weak acid stress appears to be dependent on population heterogeneity: a proportion of cells that display low cytoplasmic pH, and therefore reduced weak acid accumulation, give rise to a population of resistant cells (194). Rather than inducing a Pdr12-like weak acid exporter, Z. bailii degrades sorbate and benzoate, exploiting them as a carbon source (184).
Core Stress Response
In the preceding sections we discussed fungal responses to individual stresses. Here we describe how exposure to different types of stress can lead to similar responses via what has been termed the core stress response (CSR). Genome-wide transcript profiling studies first revealed the existence of CSRs in the model yeasts S. cerevisiae (20, 67) and S. pombe (68), in the pathogenic fungus C. glabrata (71), and to a lesser extent in C. albicans (69, 70). Formally, the CSR describes a set of genes that are commonly regulated in response to diverse types of stress (Fig. 2).
FIGURE 2.
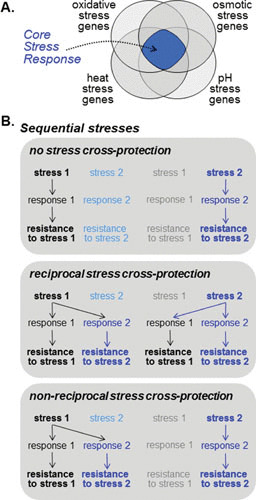
The CSR can lead to stress cross-protection. (A) CSRs, which have been defined by genome-wide transcriptional profiling, represent the set of genes that is commonly up- or downregulated by different types of stress (see text). This Venn diagram illustrates the conceptual overlap between these sets of genes, highlighting the core stress genes. (B) A CSR can lead to stress cross-protection during exposure to sequential stresses; i.e., cells that are exposed to one type of stress can then display elevated resistance to a subsequent stress of a different type (see text). In some cases no cross-protection is observed. In other cases it is observed, but this cross-protection can be reciprocal or nonreciprocal. This can depend on the nature and dose of the initial and subsequent stress.
In S. cerevisiae, between 200 and 300 genes were found to be upregulated in response to diverse stresses including heat shock, osmotic stress, oxidative stresses, increases or decreases in pH, and amino acid starvation (20, 67). In addition, between 300 and 600 genes were commonly downregulated following exposure to these diverse stress treatments (20, 67). Thus, in S. cerevisiae, the CSR involves approximately 10 to 14% of the yeast genome. Similar numbers of genes were reported to be regulated in the closely related, but pathogenic, yeast C. glabrata following exposure to glucose starvation and osmotic, oxidative, and heat stresses (71). Furthermore, in the divergent yeast S. pombe, approximately 140 genes are commonly upregulated and 100 downregulated in response to a range of stresses including osmotic, oxidative, heavy metal, DNA damage, and heat stress (68). Common processes were represented in the induced core stress gene sets such as carbohydrate metabolism, protein folding and degradation, redox regulation, and DNA repair. In contrast, repressed genes were associated with energy-consuming and growth-related processes, including RNA processing, transcription and translation, and biosynthesis of ribosomes and nucleotides. Interestingly, the pathogenic fungus C. albicans mounts a significantly smaller CSR than those described above, because only 24 and 37 genes were commonly induced or repressed, respectively, by osmotic, oxidative, and heavy metal stress (70). Despite this, the CSR genes in C. albicans do belong to some of the same functional categories as those in S. cerevisiae, S. pombe, and C. glabrata, suggesting that some of the processes involved in the CSR are evolutionarily conserved.
In S. cerevisiae, different signaling pathways and transcription factors converge to control a common set of stress genes, although the functionally redundant Msn2 and Msn4 (C2H2)2 zinc finger transcription factors play a major role (20, 67). Consistent with this, many of the CSR genes carry a stress response element within their promoters, to which Msn2 and Msn4 bind (20, 67). This Msn2/4-mediated CSR forms the basis of the previously characterized general stress response in S. cerevisiae, which is composed of an Msn2/4-stress response element regulon that is activated in response to diverse stresses (195). Msn2/4 rapidly accumulate in the nucleus following a range of nutrient and stress conditions (196, 197) and are subject to complex regulation by several pathways (198). However, a number of other factors also regulate the CSR in S. cerevisiae. In response to osmotic stress, the Hog1 SAPK contributes to the regulation of CSR genes (20), likely through the Hot1 transcription factor (59), whereas the Yap1 AP-1-like transcription factor contributes to CSR gene induction postoxidative stress (20). In addition, the Mec1 DNA-damage specific pathway contributes to CSR gene regulation in response to DNA damaging agents (199).
Recently, it has been shown that the Msn2 transcription factor also makes a major contribution to the regulation of CSR genes in C. glabrata (71). Consistent with this, in this pathogenic yeast, Msn2 rapidly localizes to the nucleus following glucose starvation and in response to osmotic, oxidative, or heat stress (71). In stark contrast, homologues of Msn2 and Msn4 do not have the same broad stress-protective roles in C. albicans (193, 200), which may contribute to the relatively small CSR seen in this fungus (70). Moreover, in S. pombe, which lacks close homologues of Msn2/4, the CSR is regulated by a different mechanism. In this yeast, the Sty1 SAPK is activated in response to diverse stress conditions including osmotic, oxidative, and heat stress; nutrient limitation; UV light; and cold stress (201). Following activation, Sty1 accumulates in the nucleus, where it phosphorylates the Atf1 transcription factor, leading to its activation and stabilization (95–97). Sty1 and, to a lesser extent, Atf1 are the major regulators of the CSR in S. pombe (68). It is interesting that CSR genes implicated in stress defense are dependent on Sty1 and Atf1, whereas CSR genes with regulatory functions are induced by Sty1 independently of Atf1 (68).
Similar to the Sty1 SAPK in S. pombe, the Hog1 SAPK in the fungal pathogen C. albicans is activated in response to diverse stresses (110). Significantly, a CSR was only observed after treating cells with three stress conditions—osmotic, oxidative, and heavy metal stress—that activate Hog1 (69, 70). Intriguingly, however, Hog1 regulated the transcriptional responses to osmotic and heavy metal stresses, but not to oxidative stress, and this was reflected in the role of Hog1 in the regulation of C. albicans CSR genes. Instead, the Cap1 AP-1-like transcription factor regulated the C. albicans CSR following oxidative stress (70). Thus, the C. albicans SAPK pathway functions in parallel with other pathways to regulate the core transcriptional response to stress. Hence, although aspects of a CSR are conserved across the fungal kingdom, the mechanisms underlying its regulation have diverged significantly.
What is the physiological role of a CSR? Earlier studies in S. cerevisiae revealed a phenomenon called “stress cross-protection,” in which exposure to a nonlethal dose of one stress provided significant protection from the subsequent exposure of a potentially lethal dose of a second unrelated stress (202) (Fig. 2). Such stress cross-protection was impaired in the presence of cycloheximide, illustrating a role for new protein synthesis (202). The Msn2/4-mediated general stress response in S. cerevisiae, together with the identification of the CSR, probably accounts for the observed stress cross-protection in several fungi (68, 71, 110). Interestingly, subsequent studies have revealed that the CSR triggered by the initial low stress dose is not required to survive this stress but instead provides protection against the second stress (203). Furthermore, the actual genes and processes necessary to acquire resistance to the same severe stress are different depending on the nature of the initial mild stressor. In other words, the mechanism of stress cross-protection is determined by the initial stress (204). This probably underlies previous findings that stress cross-protection is context dependent and not always reciprocal (203) (Fig. 2).
But why might stress cross-protection have evolved? Some microbes occupy reasonably predictable niches in which one environmental input is generally followed by a second input. In such cases, fungi that have evolved to anticipate the second input following exposure to the first would have a fitness advantage (205). Domesticated brewing yeasts provide an excellent example of this “adaptive prediction” because, as they ferment sugars, they become exposed to increasing ethanol concentrations (input 1) and then, when the sugars are exhausted, they switch to respiratory metabolism and become exposed to oxidative stress (input 2). Presumably, as a consequence of this environmental predictability, S. cerevisiae has evolved to activate oxidative stress genes following exposure to ethanol (206). Asymmetric adaptive prediction appears to have evolved in other fungi such as C. albicans (207, 208). Unlike S. cerevisiae, this pathogen displays increased resistance to acute oxidative stress following exposure to glucose (209), which possibly reflects anticipation of phagocytic attack after entry to the bloodstream. Therefore, it is conceivable that CSRs have evolved as a result of adaptive prediction.
ADAPTING TO STRESS IN NATURAL ENVIRONMENTS
Combinatorial Stress Responses
Our understanding of stress responses in yeast has been shaped largely by the study of individual stresses or, as discussed in the previous section, how the prior exposure to one stress can provide stress cross-protection against a second unrelated stress. However, the diverse environments that fungi occupy are complex and dynamic, and it is conceivable that fungi will often be exposed to multiple stresses. At times these stresses may be imposed sequentially, in which case stress cross-protection may facilitate survival. However, at other times, a fungus may be exposed simultaneously to multiple stresses, termed “combinatorial stress.” Recent studies have revealed that certain combinations of stresses are particularly potent in terms of their ability to kill functionally divergent model (S. cerevisiae, S. pombe) and pathogenic (C. albicans, C. glabrata) yeasts (210, 211). Notably, all of these species are acutely sensitive to combinations of cationic and oxidative stresses (210). Pathogenic fungi encounter this combination of stresses following phagocytosis; microbes are exposed to high levels of ROS generated by the respiratory burst (212), and the resulting accumulation of anionic charge is compensated for by a rush of potassium (K+) ions into the endocytic vacuole, which simultaneously imposes a cationic stress (212).
Strikingly, studies investigating the mechanistic basis underlying the exquisite sensitivity of C. albicans to such combinations of stress have revealed that exposure to cationic stress prevents this pathogen from mounting an oxidative stress response. The oxidative stress regulon in C. albicans is largely regulated by the Cap1 AP-1-like transcription factor (213), and Cap1 fails to be activated following exposure to combinatorial oxidative and cationic stress (210, 214). This phenomenon, which has been termed “stress pathway interference” (210) (Fig. 3), contrasts starkly with that of stress cross-protection in which exposure to one stress protects against the subsequent exposure to a different stress (103). Exposure of C. albicans to H2O2 triggers the rapid oxidation of Cap1, and this masks the nuclear export sequence, resulting in the rapid nuclear accumulation of Cap1 and the induction of Cap1-dependent genes. However, cationic stress inhibits this Cap1-mediated oxidative stress response in two ways. First, cations inhibit catalase activity, which triggers significant increases in intracellular ROS levels following combinations of cationic and oxidative stresses (210). Such high levels of ROS trap Cap1 in a partially oxidized form that fails to induce target antioxidant-encoding genes (214). Second, cations stimulate the interaction of Cap1 with the nuclear export factor Crm1, which results in significant delays in the H2O2-induced nuclear accumulation of this transcription factor. Importantly, the cationic stress-mediated inhibition of oxidative stress responses contributes to the fungicidal potency of human neutrophils, because effective killing of C. albicans is dependent on the combinatorial effects of the oxidative burst and cationic fluxes (210). These findings may also explain the lack of expression of C. albicans oxidative stress genes in certain host niches, such as during systemic infections of the kidney, despite the presence of neutrophil infiltrates (215).
FIGURE 3.

Exposure to combinatorial stresses can yield nonadditive outputs. Simultaneous exposure to some combinations of stress (i.e., certain combinatorial stresses) can yield additive outputs if there are no significant interactions between the stress pathways that mediate these responses. However, for some combinatorial stresses (see text), stress pathway interference can block the normal response to one of the imposed stresses, leading to combinatorial stress sensitivity. We are unaware of any examples of the opposite effect, where stress pathway enhancement might lead to elevated levels of combinatorial stress resistance.
Although to date, studies have focused on combinatorial oxidative plus cationic stress, it is feasible that other stress combinations will influence stress outputs (207). Indeed, we have found that pH has drastic effects on the oxidative stress tolerance of a number of fungi (J.Q., A.J.P.B., unpublished). This is an exciting new area in the field of stress responses that is likely to be of broad relevance across the fungal kingdom due to the complexity of natural environments.
Dynamics of Stress Responses
Our perspectives of fungal stress responses and stress resistance have been shaped largely by our experimental approaches. For example, plate assays, which are widely used to examine stress resistance, often do not differentiate between the ability of a strain to survive immediately following exposure to an acute stress and its ability to adapt and resume growth in the longer term. Also, the availability of powerful molecular genetics and genomics approaches has led to major advances in our understanding of fungal stress responses at the gene and protein levels, but the metabolic changes that underlie stress adaptation have received less attention. Yet these changes play vital roles in fungal stress resistance.
The resistance of yeast cells to stress is enhanced by increases in metabolic flux toward the generation of antioxidants such as glutathione and stress protectants such as trehalose (122, 216–219). Like the accumulation of glycerol in response to hyperosmotic stress (36), these increases in glutathione and trehalose levels are mediated in large part by changes in gene expression and enzyme synthesis and hence are slow. Other metabolic responses are much faster. For example, there is a rapid shift in metabolic flux from lower glycolysis toward the pentose phosphate pathway upon exposure to oxidative stress (72). This metabolic shift, which is mediated through the sensitivity of glyceraldehyde-3-phosphate dehydrogenase to oxidative inactivation (220), increases the NADPH synthesis and hence the availability of protective reducing equivalents (72). This metabolic response to oxidative stress occurs within seconds, preceding transcriptional responses to oxidative stress (221).
Clearly, different aspects of a fungal adaptive response take place over different timescales (36) (Fig. 4). Initial metabolic and biophysical responses can occur within seconds to minutes (37, 221). Signal transduction is activated rapidly, within minutes, and often remains active for tens of minutes (36). This triggers changes in gene expression: transcript levels often rise within 5 minutes and, depending on the stability of the mRNA, can remain elevated for tens of minutes. Resultant changes in enzyme levels are often observed in tens of minutes and can last for hours, depending on the stability of these proteins. Consequently, the accumulation metabolites such as glycerol can take tens of minutes to an hour.
FIGURE 4.

Different aspects of stress adaptation occur over different timescales. This generic figure summarizes this principle of an environmental insult such as osmotic stress (see text). However, some stresses may include adaptation mechanisms that occur over other timescales.
While these general principles hold, the dynamics of a stress response are strongly influenced by the dose. Large single doses are often applied to experimentally dissect a stress response in vitro. However, in reality, that stress response might have evolved to maintain cellular homeostasis in the face of less acute but multiple challenges. For example, researchers often use large acute heat shocks to study thermal adaptation, although many fungi encounter less dramatic thermal fluctuations in the wild. Also, researchers generally examine the impact of a single dose, and yet fungi can face repetitive doses of certain stresses in some habitats (e.g., repetitive hypo-osmotic shock during rainfall). The mathematical modeling of stress responses permits the analysis of the vast theoretical space represented by variations in the dose, exposure time, and frequency of a stress. Indeed, mathematical modeling is already improving our appreciation of the dynamics of stress responses, the influence of stress dose, and their impact on stress adaptation in fungi (36, 62, 222, 223). These dynamics are significant because they influence the length of time for which the molecular memory of a stress is retained and hence the period over which stress adaptation provides protection against a subsequent stress (224) (see “Core Stress Response,” above).
Impact of Growth Conditions on Stress Resistance
Historically, the dissection of fungal stress responses has largely been performed under a relatively small set of experimental conditions to facilitate comparison between studies. This has influenced our perceptions of stress adaptation. For example, it is well known that changes in carbon source exert dramatic effects on stress resistance in S. cerevisiae. Yet stress adaptation in C. albicans has largely been examined using glucose-rich media, although this fungus generally inhabits glucose-poor niches. Temperature also affects stress responses (29).
A shift from glucose to a nonfermentative carbon source increases stress resistance in S. cerevisiae, partly through activation of the CSR. Glucose inhibits the CSR through Msn2/4 phosphorylation, which is mediated by Ras-cAMP-protein kinase A signaling (225). Phosphorylation of Msn2/4 prevents the nuclear accumulation of these transcription factors, thereby blocking their activation of core stress genes (196). Glucose also regulates YAP1 (the AP1-like transcription factor central to the transcriptional response to oxidative stress) and ENA1 (a P-type ATPase Na+ pump required for cationic stress resistance) (226, 227).
Changes in carbon source also affect stress resistance in C. albicans. Exposure to glucose increases oxidative stress resistance by upregulating oxidative stress genes in this yeast (209). In contrast, growth on glucose decreases the resistance of C. albicans to osmotic and cell wall stresses, in large part through carbon source-dependent changes in the preadapted state of the cell wall (37, 228). Consequently, as different host niches contain different carbon sources, the nature of a niche must determine the ability of C. albicans to counteract stresses in that niche. Not surprisingly, the virulence of this pathogen is influenced by the carbon source (228).
CONCLUSIONS AND PERSPECTIVES
It is clear that major advances have been made in our understanding of fungal stress adaptation over the past decades. However, much remains to be learned with regard to how cells respond to stress in their natural environments. Several issues should be addressed.
First, many gaps remain in our understanding of stress signaling pathways and of stress responses themselves, even for individual stresses in model fungi. Just one example is our understanding of how nitrosative stress responses are regulated, which is rudimentary compared to oxidative and osmotic stress signaling even in S. cerevisiae. The situation is worse for model ascomycete and basidiomycete pathogens such as C. albicans and C. neoformans. Their lifestyles differ markedly from S. cerevisiae and S. pombe, and differential evolutionary pressures appear to have driven regulatory rewiring and niche-specific tuning of stress responses, yielding different stress sensitivities and different patterns of stress cross-protection.
Second, our understanding of the dynamics of stress adaptation needs to improve, incorporating the immediate and long-term contributions of metabolic responses and biophysical changes alongside those driven by transcriptional and posttranslational gene regulation. This needs to be considered alongside the issue of population heterogeneity. The molecular basis for the differential stress sensitivity of genetically identical cells experiencing the same environmental conditions needs to be better understood. To achieve this we require experimental approaches that provide dynamic views of cell-to-cell variation at high resolution.
Third, more consideration needs to be given to the nature of the stresses that are encountered by a fungus in its natural habitat and the nature of the microenvironment(s) in which it must respond to these stresses. How acute is the stress, how long is the exposure, and how frequently is it encountered? This is significant because some fungal stress responses may have evolved to maintain cellular homeostasis in the face of modest but repetitive challenges rather than single acute doses (see “Dynamics of Stress Responses,” above). Is the stress imposed in combination with other environmental insults? This should be considered because certain combinations of stresses can yield unexpected stress responses (see “Combinatorial Stress Responses,” above). What is the temperature of the niche, and what nutrients are available? These factors are important because they strongly influence the preadapted state of the fungus and hence its ability to counteract the stress (see “Impact of Growth Conditions on Stress Resistance,” above).
If these issues are addressed, there will be a paradigm shift in our understanding of fungal stress responses and their relevance to survival in natural environments.
ACKNOWLEDGMENTS
We thank our numerous friends and colleagues for stimulating discussions about stress adaptation. We are also grateful to the following institutions for generously supporting our research. A.J.P.B was funded by the European Research Council (STRIFE, ERC-2009-AdG-249793), the UK Medical Research Council (MR/M026663/1 and MR/N006364/1), the UK Biotechnology and Biological Research Council (BB/K017365/1), and the Wellcome Trust (080088; 097377). L.E.C. is supported by the Canadian Institutes of Health Research Operating Grants (MOP-86452 and MOP-119520), the Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grants (06261 and 462167), an NSERC E.W.R. Steacie Memorial Fellowship (477598), a National Institutes of Health R01 Grant (R01AI120958), and a Canada Research Chair in Microbial Genomics and Infectious Disease. Work in the A.D.P. laboratory is funded by grants from the Spanish Ministerio de Innovación y Competitividad (BIO2013-47870-R), the European Commission (Marie Curie ITN FUNGIBRAIN; FP7-PEOPLE-ITN-607963), and the Junta de Andalucia (BIO296). J.Q. is funded by the UK Biotechnology and Biological Research Council (BB/K016939/1) and the Wellcome Trust (097377).
REFERENCES
- 1.Hawksworth DL. 2012. Global species numbers of fungi: are tropical studies and molecular approaches contributing to a more robust estimate? Biodivers Conserv 21:2425–2433 10.1007/s10531-012-0335-x. [DOI] [Google Scholar]
- 2.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186–194 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Hoog GS, Guarro J, Gene J, Figueras MJ. 2000. Atlas of Clinical Fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands/Universitat Rovira i Virgili, Reus, Spain. [Google Scholar]
- 4.Lindquist S, Craig EA. 1988. The heat-shock proteins. Annu Rev Genet 22:631–677 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 5.Steen BR, Lian T, Zuyderduyn S, MacDonald WK, Marra M, Jones SJ, Kronstad JW. 2002. Temperature-regulated transcription in the pathogenic fungus Cryptococcus neoformans. Genome Res 12:1386–1400 10.1101/gr.80202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown SM, Campbell LT, Lodge JK. 2007. Cryptococcus neoformans, a fungus under stress. Curr Opin Microbiol 10:320–325 10.1016/j.mib.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pócsi I, Miskei M, Karányi Z, Emri T, Ayoubi P, Pusztahelyi T, Balla G, Prade RA. 2005. Comparison of gene expression signatures of diamide, H2O2 and menadione exposed Aspergillus nidulans cultures: linking genome-wide transcriptional changes to cellular physiology. BMC Genomics 6:182 10.1186/1471-2164-6-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breuer U, Harms H. 2006. Debaryomyces hanseniian extremophilic yeast with biotechnological potential. Yeast 23:415–437 10.1002/yea.1374. [DOI] [PubMed] [Google Scholar]
- 9.Stefanini I, Dapporto L, Legras JL, Calabretta A, Di Paola M, De Filippo C, Viola R, Capretti P, Polsinelli M, Turillazzi S, Cavalieri D. 2012. Role of social wasps in Saccharomyces cerevisiae ecology and evolution. Proc Natl Acad Sci USA 109:13398–13403 10.1073/pnas.1208362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roetzer A, Gratz N, Kovarik P, Schüller C. 2010. Autophagy supports Candida glabrata survival during phagocytosis. Cell Microbiol 12:199–216 10.1111/j.1462-5822.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikolaou E, Agrafioti I, Stumpf M, Quinn J, Stansfield I, Brown AJP. 2009. Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol Biol 9:44 10.1186/1471-2148-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro RS, Cowen LE. 2012. Thermal control of microbial development and virulence: molecular mechanisms of microbial temperature sensing. MBio 3:00238-12 10.1128/mBio.00238-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergman A, Casadevall A. 2010. Mammalian endothermy optimally restricts fungi and metabolic costs. MBio 1:00212-10 10.1128/mBio.00212-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Solache MA, Casadevall A. 2010. Global warming will bring new fungal diseases for mammals. MBio 1:00061-10 10.1128/mBio.00061-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein BS, Tebbets B. 2007. Dimorphism and virulence in fungi. Curr Opin Microbiol 10:314–319 10.1016/j.mib.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. 2011. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol 10:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro RS, Robbins N, Cowen LE. 2011. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol Mol Biol Rev 75:213–267 10.1128/MMBR.00045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leach MD, Farrer RA, Tan K, Miao Z, Walker LA, Cuomo CA, Wheeler RT, Brown AJ, Wong KH, Cowen LE. 2016. Hsf1 and Hsp90 orchestrate temperature-dependent global transcriptional remodelling and chromatin architecture in Candida albicans. Nat Commun 7:11704 10.1038/ncomms11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leach MD, Klipp E, Cowen LE, Brown AJ. 2012. Fungal Hsp90: a biological transistor that tunes cellular outputs to thermal inputs. Nat Rev Microbiol 10:693–704 10.1038/nrmicro2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell 11:4241–4257 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholls S, Leach MD, Priest CL, Brown AJ. 2009. Role of the heat shock transcription factor, Hsf1, in a major fungal pathogen that is obligately associated with warm-blooded animals. Mol Microbiol 74:844–861 10.1111/j.1365-2958.2009.06883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campos EI, Fillingham J, Li G, Zheng H, Voigt P, Kuo WH, Seepany H, Gao Z, Day LA, Greenblatt JF, Reinberg D. 2010. The program for processing newly synthesized histones H3.1 and H4. Nat Struct Mol Biol 17:1343–1351 10.1038/nsmb.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawarkar R, Sievers C, Paro R. 2012. Hsp90 globally targets paused RNA polymerase to regulate gene expression in response to environmental stimuli. Cell 149:807–818 10.1016/j.cell.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 24.Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, Boone C, Emili A, Houry WA. 2005. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120:715–727 10.1016/j.cell.2004.12.024. [PubMed] [DOI] [PubMed] [Google Scholar]
- 25.Taipale M, Jarosz DF, Lindquist S. 2010. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 11:515–528 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 26.Taipale M, Tucker G, Peng J, Krykbaeva I, Lin ZY, Larsen B, Choi H, Berger B, Gingras AC, Lindquist S. 2014. A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways. Cell 158:434–448 10.1016/j.cell.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diezmann S, Michaut M, Shapiro RS, Bader GD, Cowen LE. 2012. Mapping the Hsp90 genetic interaction network in Candida albicans reveals environmental contingency and rewired circuitry. PLoS Genet 8:e1002562 10.1371/journal.pgen.1002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawle P, Horst D, Bebelman JP, Yang XX, Siderius M, van der Vies SM. 2007. Cdc37p is required for stress-induced high-osmolarity glycerol and protein kinase C mitogen-activated protein kinase pathway functionality by interaction with Hog1p and Slt2p (Mpk1p). Eukaryot Cell 6:521–532 10.1128/EC.00343-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leach MD, Budge S, Walker L, Munro C, Cowen LE, Brown AJ. 2012. Hsp90 orchestrates transcriptional regulation by Hsf1 and cell wall remodelling by MAPK signalling during thermal adaptation in a pathogenic yeast. PLoS Pathog 8:e1003069 10.1371/journal.ppat.1003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Meara TR, Veri AO, Polvi EJ, Li X, Valaei SF, Diezmann S, Cowen LE. 2016. Mapping the Hsp90 genetic network reveals ergosterol biosynthesis and phosphatidylinositol-4-kinase signaling as core circuitry governing cellular stress. PLoS Genet 12:e1006142 10.1371/journal.pgen.1006142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hohmann S. 2002. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66:300–372 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira C, van Voorst F, Martins A, Neves L, Oliveira R, Kielland-Brandt MC, Lucas C, Brandt A. 2005. A member of the sugar transporter family, Stl1p is the glycerol/H+ symporter in Saccharomyces cerevisiae. Mol Biol Cell 16:2068–2076 10.1091/mbc.E04-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dušková M, Ferreira C, Lucas C, Sychrová H. 2015. Two glycerol uptake systems contribute to the high osmotolerance of Zygosaccharomyces rouxii. Mol Microbiol 97:541–559 10.1111/mmi.13048. [DOI] [PubMed] [Google Scholar]
- 34.Kayingo G, Martins A, Andrie R, Neves L, Lucas C, Wong B. 2009. A permease encoded by STL1 is required for active glycerol uptake by Candida albicans. Microbiology 155:1547–1557 10.1099/mic.0.023457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luyten K, Albertyn J, Skibbe WF, Prior BA, Ramos J, Thevelein JM, Hohmann S. 1995. Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J 14:1360–1371. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klipp E, Nordlander B, Krüger R, Gennemark P, Hohmann S. 2005. Integrative model of the response of yeast to osmotic shock. Nat Biotechnol 23:975–982 10.1038/nbt1114. [DOI] [PubMed] [Google Scholar]
- 37.Ene IV, Walker LA, Schiavone M, Lee KK, Martin-Yken H, Dague E, Gow NA, Munro CA, Brown AJ. 2015. Cell wall remodeling enzymes modulate fungal cell wall elasticity and osmotic stress resistance. MBio 6:e00986 10.1128/mBio.00986-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muir A, Roelants FM, Timmons G, Leskoske KL, Thorner J. 2015. Down-regulation of TORC2-Ypk1 signaling promotes MAPK-independent survival under hyperosmotic stress. eLife 4:09336 10.7554/eLife.09336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hohmann S, Krantz M, Nordlander B. 2007. Yeast osmoregulation. Methods Enzymol 428:29–45 10.1016/S0076-6879(07)28002-4. [DOI] [PubMed] [Google Scholar]
- 40.Yu Z, Armant O, Fischer R. 2016. Fungi use the SakA (HogA) pathway for phytochrome-dependent light signalling. Nat Microbiol 1:16019 10.1038/nmicrobiol.2016.19. [DOI] [PubMed] [Google Scholar]
- 41.Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865–875 10.1016/S0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 42.Posas F, Saito H. 1998. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J 17:1385–1394 10.1093/emboj/17.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tatebayashi K, Yamamoto K, Tanaka K, Tomida T, Maruoka T, Kasukawa E, Saito H. 2006. Adaptor functions of Cdc42, Ste50, and Sho1 in the yeast osmoregulatory HOG MAPK pathway. EMBO J 25:3033–3044 10.1038/sj.emboj.7601192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu C, Jansen G, Zhang J, Thomas DY, Whiteway M. 2006. Adaptor protein Ste50p links the Ste11p MEKK to the HOG pathway through plasma membrane association. Genes Dev 20:734–746 10.1101/gad.1375706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maeda T, Takekawa M, Saito H. 1995. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science 269:554–558 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- 46.Tatebayashi K, Tanaka K, Yang HY, Yamamoto K, Matsushita Y, Tomida T, Imai M, Saito H. 2007. Transmembrane mucins Hkr1 and Msb2 are putative osmosensors in the SHO1 branch of yeast HOG pathway. EMBO J 26:3521–3533 10.1038/sj.emboj.7601796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka K, Tatebayashi K, Nishimura A, Yamamoto K, Yang HY, Saito H. 2014. Yeast osmosensors Hkr1 and Msb2 activate the Hog1 MAPK cascade by different mechanisms. Sci Signal 7:ra21 10.1126/scisignal.2004780. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto K, Tatebayashi K, Saito H. 2016. Binding of the extracellular eight-cysteine motif of Opy2 to the putative osmosensor Msb2 is essential for activation of the yeast high-osmolarity glycerol pathway. Mol Cell Biol 36:475–487 10.1128/MCB.00853-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pitoniak A, Birkaya B, Dionne HM, Vadaie N, Cullen PJ. 2009. The signaling mucins Msb2 and Hkr1 differentially regulate the filamentation mitogen-activated protein kinase pathway and contribute to a multimodal response. Mol Biol Cell 20:3101–3114 10.1091/mbc.E08-07-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimura A, Yamamoto K, Oyama M, Kozuka-Hata H, Saito H, Tatebayashi K. 2016. Scaffold protein Ahk1, which associates with Hkr1, Sho1, Ste11, and Pbs2, inhibits cross talk signaling from the Hkr1 osmosensor to the Kss1 mitogen-activated protein kinase. Mol Cell Biol 36:1109–1123 10.1128/MCB.01017-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buck V, Quinn J, Soto Pino T, Martin H, Saldanha J, Makino K, Morgan BA, Millar JB. 2001. Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol Biol Cell 12:407–419 10.1091/mbc.12.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Román E, Cottier F, Ernst JF, Pla J. 2009. Msb2 signaling mucin controls activation of Cek1 mitogen-activated protein kinase in Candida albicans. Eukaryot Cell 8:1235–1249 10.1128/EC.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Román E, Nombela C, Pla J. 2005. The Sho1 adaptor protein links oxidative stress to morphogenesis and cell wall biosynthesis in the fungal pathogen Candida albicans. Mol Cell Biol 25:10611–10627 10.1128/MCB.25.23.10611-10627.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheetham J, Smith DA, da Silva Dantas A, Doris KS, Patterson MJ, Bruce CR, Quinn J. 2007. A single MAPKKK regulates the Hog1 MAPK pathway in the pathogenic fungus Candida albicans. Mol Biol Cell 18:4603–4614 10.1091/mbc.E07-06-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chauhan N, Inglis D, Roman E, Pla J, Li D, Calera JA, Calderone R. 2003. Candida albicans response regulator gene SSK1 regulates a subset of genes whose functions are associated with cell wall biosynthesis and adaptation to oxidative stress. Eukaryot Cell 2:1018–1024 10.1128/EC.2.5.1018-1024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gregori C, Schüller C, Roetzer A, Schwarzmüller T, Ammerer G, Kuchler K. 2007. The high-osmolarity glycerol response pathway in the human fungal pathogen Candida glabrata strain ATCC 2001 lacks a signaling branch that operates in baker’s yeast. Eukaryot Cell 6:1635–1645 10.1128/EC.00106-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bahn YS, Geunes-Boyer S, Heitman J. 2007. Ssk2 mitogen-activated protein kinase kinase kinase governs divergent patterns of the stress-activated Hog1 signaling pathway in Cryptococcus neoformans. Eukaryot Cell 6:2278–2289 10.1128/EC.00349-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bahn YS, Kojima K, Cox GM, Heitman J. 2005. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol Biol Cell 16:2285–2300 10.1091/mbc.E04-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rep M, Krantz M, Thevelein JM, Hohmann S. 2000. The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J Biol Chem 275:8290–8300 10.1074/jbc.275.12.8290. [DOI] [PubMed] [Google Scholar]
- 60.Lee J, Reiter W, Dohnal I, Gregori C, Beese-Sims S, Kuchler K, Ammerer G, Levin DE. 2013. MAPK Hog1 closes the S. cerevisiae glycerol channel Fps1 by phosphorylating and displacing its positive regulators. Genes Dev 27:2590–2601 10.1101/gad.229310.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yaakov G, Duch A, García-Rubio M, Clotet J, Jimenez J, Aguilera A, Posas F. 2009. The stress-activated protein kinase Hog1 mediates S phase delay in response to osmostress. Mol Biol Cell 20:3572–3582 10.1091/mbc.E09-02-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muzzey D, Gómez-Uribe CA, Mettetal JT, van Oudenaarden A. 2009. A systems-level analysis of perfect adaptation in yeast osmoregulation. Cell 138:160–171 10.1016/j.cell.2009.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halliwell B. 2006. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cadenas E, Davies KJ. 2000. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230 10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 65.O’Brien JA, Daudi A, Butt VS, Bolwell GP. 2012. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236:765–779 10.1007/s00425-012-1696-9. [DOI] [PubMed] [Google Scholar]
- 66.Brown AJ, Haynes K, Quinn J. 2009. Nitrosative and oxidative stress responses in fungal pathogenicity. Curr Opin Microbiol 12:384–391 10.1016/j.mib.2009.06.007. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Causton HC, Ren B, Koh SS, Harbison CT, Kanin E, Jennings EG, Lee TI, True HL, Lander ES, Young RA. 2001. Remodeling of yeast genome expression in response to environmental changes. Mol Biol Cell 12:323–337 10.1091/mbc.12.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen D, Toone WM, Mata J, Lyne R, Burns G, Kivinen K, Brazma A, Jones N, Bähler J. 2003. Global transcriptional responses of fission yeast to environmental stress. Mol Biol Cell 14:214–229 10.1091/mbc.E02-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Enjalbert B, Nantel A, Whiteway M. 2003. Stress-induced gene expression in Candida albicans: absence of a general stress response. Mol Biol Cell 14:1460–1467 10.1091/mbc.E02-08-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Enjalbert B, Smith DA, Cornell MJ, Alam I, Nicholls S, Brown AJ, Quinn J. 2006. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol Biol Cell 17:1018–1032 10.1091/mbc.E05-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roetzer A, Gregori C, Jennings AM, Quintin J, Ferrandon D, Butler G, Kuchler K, Ammerer G, Schüller C. 2008. Candida glabrata environmental stress response involves Saccharomyces cerevisiae Msn2/4 orthologous transcription factors. Mol Microbiol 69:603–620 10.1111/j.1365-2958.2008.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ralser M, Wamelink MM, Kowald A, Gerisch B, Heeren G, Struys EA, Klipp E, Jakobs C, Breitenbach M, Lehrach H, Krobitsch S. 2007. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J Biol 6:10 10.1186/jbiol61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flattery-O’Brien JA, Dawes IW. 1998. Hydrogen peroxide causes RAD9-dependent cell cycle arrest in G2 in Saccharomyces cerevisiae whereas menadione causes G1 arrest independent of RAD9 function. J Biol Chem 273:8564–8571 10.1074/jbc.273.15.8564. [DOI] [PubMed] [Google Scholar]
- 74.Finn K, Lowndes NF, Grenon M. 2012. Eukaryotic DNA damage checkpoint activation in response to double-strand breaks. Cell Mol Life Sci 69:1447–1473 10.1007/s00018-011-0875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.da Silva Dantas A, Patterson MJ, Smith DA, Maccallum DM, Erwig LP, Morgan BA, Quinn J. 2010. Thioredoxin regulates multiple hydrogen peroxide-induced signaling pathways in Candida albicans. Mol Cell Biol 30:4550–4563 10.1128/MCB.00313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi QM, Wang YM, Zheng XD, Lee RT, Wang Y. 2007. Critical role of DNA checkpoints in mediating genotoxic-stress-induced filamentous growth in Candida albicans. Mol Biol Cell 18:815–826 10.1091/mbc.E06-05-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Delaunay A, Isnard AD, Toledano MB. 2000. H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J 19:5157–5166 10.1093/emboj/19.19.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB. 2002. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111:471–481 10.1016/S0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 79.Veal EA, Ross SJ, Malakasi P, Peacock E, Morgan BA. 2003. Ybp1 is required for the hydrogen peroxide-induced oxidation of the Yap1 transcription factor. J Biol Chem 278:30896–30904 10.1074/jbc.M303542200. [DOI] [PubMed] [Google Scholar]
- 80.Wood MJ, Storz G, Tjandra N. 2004. Structural basis for redox regulation of Yap1 transcription factor localization. Nature 430:917–921 10.1038/nature02790. [DOI] [PubMed] [Google Scholar]
- 81.Patterson MJ, McKenzie CG, Smith DA, da Silva Dantas A, Sherston S, Veal EA, Morgan BA, MacCallum DM, Erwig LP, Quinn J. 2013. Ybp1 and Gpx3 signaling in Candida albicans govern hydrogen peroxide-induced oxidation of the Cap1 transcription factor and macrophage escape. Antioxid Redox Signal 19:2244–2260 10.1089/ars.2013.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bozonet SM, Findlay VJ, Day AM, Cameron J, Veal EA, Morgan BA. 2005. Oxidation of a eukaryotic 2-Cys peroxiredoxin is a molecular switch controlling the transcriptional response to increasing levels of hydrogen peroxide. J Biol Chem 280:23319–23327 10.1074/jbc.M502757200. [DOI] [PubMed] [Google Scholar]
- 83.Cartwright GM, Scott B. 2013. Redox regulation of an AP-1-like transcription factor, YapA, in the fungal symbiont Epichloe festucae. Eukaryot Cell 12:1335–1348 10.1128/EC.00129-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lessing F, Kniemeyer O, Wozniok I, Loeffler J, Kurzai O, Haertl A, Brakhage AA. 2007. The Aspergillus fumigatus transcriptional regulator AfYap1 represents the major regulator for defense against reactive oxygen intermediates but is dispensable for pathogenicity in an intranasal mouse infection model. Eukaryot Cell 6:2290–2302 10.1128/EC.00267-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paul S, Doering TL, Moye-Rowley WS. 2015. Cryptococcus neoformans Yap1 is required for normal fluconazole and oxidative stress resistance. Fungal Genet Biol 74:1–9 10.1016/j.fgb.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jain C, Pastor K, Gonzalez AY, Lorenz MC, Rao RP. 2013. The role of Candida albicans AP-1 protein against host derived ROS in in vivo models of infection. Virulence 4:67–76 10.4161/viru.22700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo M, Chen Y, Du Y, Dong Y, Guo W, Zhai S, Zhang H, Dong S, Zhang Z, Wang Y, Wang P, Zheng X. 2011. The bZIP transcription factor MoAP1 mediates the oxidative stress response and is critical for pathogenicity of the rice blast fungus Magnaporthe oryzae. PLoS Pathog 7:e1001302 10.1371/journal.ppat.1001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Molina L, Kahmann R. 2007. An Ustilago maydis gene involved in H2O2 detoxification is required for virulence. Plant Cell 19:2293–2309 10.1105/tpc.107.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu PL, Wang CL, Chen PY, Lee MH. 2016. The YAP1 homolog-mediated redox sensing is crucial for a successful infection by Monilinia fructicola. Mol Plant Pathol 30:12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roetzer A, Klopf E, Gratz N, Marcet-Houben M, Hiller E, Rupp S, Gabaldón T, Kovarik P, Schüller C. 2011. Regulation of Candida glabrata oxidative stress resistance is adapted to host environment. FEBS Lett 585:319–327 10.1016/j.febslet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen D, Wilkinson CR, Watt S, Penkett CJ, Toone WM, Jones N, Bähler J. 2008. Multiple pathways differentially regulate global oxidative stress responses in fission yeast. Mol Biol Cell 19:308–317 10.1091/mbc.E07-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mulford KE, Fassler JS. 2011. Association of the Skn7 and Yap1 transcription factors in the Saccharomyces cerevisiae oxidative stress response. Eukaryot Cell 10:761–769 10.1128/EC.00328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quinn J, Malakasi P, Smith DA, Cheetham J, Buck V, Millar JB, Morgan BA. 2011. Two-component mediated peroxide sensing and signal transduction in fission yeast. Antioxid Redox Signal 15:153–165 10.1089/ars.2010.3345. [DOI] [PubMed] [Google Scholar]
- 94.Fassler JS, West AH. 2011. Fungal Skn7 stress responses and their relationship to virulence. Eukaryot Cell 10:156–167 10.1128/EC.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilkinson MG, Samuels M, Takeda T, Toone WM, Shieh JC, Toda T, Millar JB, Jones N. 1996. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev 10:2289–2301 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- 96.Lawrence CL, Jones N, Wilkinson CR. 2009. Stress-induced phosphorylation of S. pombe Atf1 abrogates its interaction with F box protein Fbh1. Curr Biol 19:1907–1911 10.1016/j.cub.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 97.Lawrence CL, Maekawa H, Worthington JL, Reiter W, Wilkinson CR, Jones N. 2007. Regulation of Schizosaccharomyces pombe Atf1 protein levels by Sty1-mediated phosphorylation and heterodimerization with Pcr1. J Biol Chem 282:5160–5170 10.1074/jbc.M608526200. [DOI] [PubMed] [Google Scholar]
- 98.Missall TA, Lodge JK. 2005. Function of the thioredoxin proteins in Cryptococcus neoformans during stress or virulence and regulation by putative transcriptional modulators. Mol Microbiol 57:847–858 10.1111/j.1365-2958.2005.04735.x. [DOI] [PubMed] [Google Scholar]
- 99.Guo M, Guo W, Chen Y, Dong S, Zhang X, Zhang H, Song W, Wang W, Wang Q, Lv R, Zhang Z, Wang Y, Zheng X. 2010. The basic leucine zipper transcription factor Moatf1 mediates oxidative stress responses and is necessary for full virulence of the rice blast fungus Magnaporthe oryzae. Mol Plant Microbe Interact 23:1053–1068 10.1094/MPMI-23-8-1053. [DOI] [PubMed] [Google Scholar]
- 100.Hagiwara D, Suzuki S, Kamei K, Gonoi T, Kawamoto S. 2014. The role of AtfA and HOG MAPK pathway in stress tolerance in conidia of Aspergillus fumigatus. Fungal Genet Biol 73:138–149 10.1016/j.fgb.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 101.Lara-Rojas F, Sánchez O, Kawasaki L, Aguirre J. 2011. Aspergillus nidulans transcription factor AtfA interacts with the MAPK SakA to regulate general stress responses, development and spore functions. Mol Microbiol 80:436–454 10.1111/j.1365-2958.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bahn YS, Jung KW. 2013. Stress signaling pathways for the pathogenicity of Cryptococcus. Eukaryot Cell 12:1564–1577 10.1128/EC.00218-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schüller C, Brewster JL, Alexander MR, Gustin MC, Ruis H. 1994. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J 13:4382–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Degols G, Shiozaki K, Russell P. 1996. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol 16:2870–2877 10.1128/MCB.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alonso-Monge R, Navarro-García F, Román E, Negredo AI, Eisman B, Nombela C, Pla J. 2003. The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans. Eukaryot Cell 2:351–361 10.1128/EC.2.2.351-361.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Du C, Sarfati J, Latge JP, Calderone R. 2006. The role of the sakA (Hog1) and tcsB (sln1) genes in the oxidant adaptation of Aspergillus fumigatus. Med Mycol 44:211–218 10.1080/13693780500338886. [DOI] [PubMed] [Google Scholar]
- 107.Moriwaki A, Kubo E, Arase S, Kihara J. 2006. Disruption of SRM1, a mitogen-activated protein kinase gene, affects sensitivity to osmotic and ultraviolet stressors in the phytopathogenic fungus Bipolaris oryzae. FEMS Microbiol Lett 257:253–261 10.1111/j.1574-6968.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 108.Zheng D, Zhang S, Zhou X, Wang C, Xiang P, Zheng Q, Xu JR. 2012. The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum. PLoS One 7:e49495 10.1371/journal.pone.0049495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Veal EA, Findlay VJ, Day AM, Bozonet SM, Evans JM, Quinn J, Morgan BA. 2004. A 2-Cys peroxiredoxin regulates peroxide-induced oxidation and activation of a stress-activated MAP kinase. Mol Cell 15:129–139 10.1016/j.molcel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 110.Smith DA, Nicholls S, Morgan BA, Brown AJ, Quinn J. 2004. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol Biol Cell 15:4179–4190 10.1091/mbc.E04-03-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li D, Gurkovska V, Sheridan M, Calderone R, Chauhan N. 2004. Studies on the regulation of the two-component histidine kinase gene CHK1 in Candida albicans using the heterologous lacZ reporter gene. Microbiology 150:3305–3313 10.1099/mic.0.27237-0. [DOI] [PubMed] [Google Scholar]
- 112.Furukawa K, Hoshi Y, Maeda T, Nakajima T, Abe K. 2005. Aspergillus nidulans HOG pathway is activated only by two-component signalling pathway in response to osmotic stress. Mol Microbiol 56:1246–1261 10.1111/j.1365-2958.2005.04605.x. [DOI] [PubMed] [Google Scholar]
- 113.Fang FC. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2:820–832 10.1038/nrmicro1004. [PubMed] [DOI] [PubMed] [Google Scholar]
- 114.Gross NT, Nessa K, Camner P, Jarstrand C. 1999. Production of nitric oxide by rat alveolar macrophages stimulated by Cryptococcus neoformans or Aspergillus fumigatus. Med Mycol 37:151–157 10.1080/j.1365-280X.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- 115.Nathan C, Shiloh MU. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA 97:8841–8848 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hromatka BS, Noble SM, Johnson AD. 2005. Transcriptional response of Candida albicans to nitric oxide and the role of the YHB1 gene in nitrosative stress and virulence. Mol Biol Cell 16:4814–4826 10.1091/mbc.E05-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Missall TA, Pusateri ME, Donlin MJ, Chambers KT, Corbett JA, Lodge JK. 2006. Posttranslational, translational, and transcriptional responses to nitric oxide stress in Cryptococcus neoformans: implications for virulence. Eukaryot Cell 5:518–529 10.1128/EC.5.3.518-529.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baidya S, Cary JW, Grayburn WS, Calvo AM. 2011. Role of nitric oxide and flavohemoglobin homolog genes in Aspergillus nidulans sexual development and mycotoxin production. Appl Environ Microbiol 77:5524–5528 10.1128/AEM.00638-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prats E, Carver TL, Mur LA. 2008. Pathogen-derived nitric oxide influences formation of the appressorium infection structure in the phytopathogenic fungus Blumeria graminis. Res Microbiol 159:476–480 10.1016/j.resmic.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Z, Wang J, Chai R, Qiu H, Jiang H, Mao X, Wang Y, Liu F, Sun G. 2015. An S-(hydroxymethyl)glutathione dehydrogenase is involved in conidiation and full virulence in the rice blast fungus Magnaporthe oryzae. PLoS One 10:e0120627 10.1371/journal.pone.0120627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arasimowicz-Jelonek M, Floryszak-Wieczorek J. 2016. Nitric oxide in the offensive strategy of fungal and oomycete plant pathogens. Front Plant Sci 7:252 10.3389/fpls.2016.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grant CM, Collinson LP, Roe JH, Dawes IW. 1996. Yeast glutathione reductase is required for protection against oxidative stress and is a target gene for yAP-1 transcriptional regulation. Mol Microbiol 21:171–179 10.1046/j.1365-2958.1996.6351340.x. [DOI] [PubMed] [Google Scholar]
- 123.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. 2001. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410:490–494 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 124.Tillmann AT, Strijbis K, Cameron G, Radmaneshfar E, Thiel M, Munro CA, MacCallum DM, Distel B, Gow NA, Brown AJ. 2015. Contribution of Fdh3 and Glr1 to glutathione redox state, stress adaptation and virulence in Candida albicans. PLoS One 10:e0126940 10.1371/journal.pone.0126940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tillmann A, Gow NA, Brown AJ. 2011. Nitric oxide and nitrosative stress tolerance in yeast. Biochem Soc Trans 39:219–223 10.1042/BST0390219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sarver A, DeRisi J. 2005. Fzf1p regulates an inducible response to nitrosative stress in Saccharomyces cerevisiae. Mol Biol Cell 16:4781–4791 10.1091/mbc.E05-05-0436. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chiranand W, McLeod I, Zhou H, Lynn JJ, Vega LA, Myers H, Yates JR III, Lorenz MC, Gustin MC. 2008. CTA4 transcription factor mediates induction of nitrosative stress response in Candida albicans. Eukaryot Cell 7:268–278 10.1128/EC.00240-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ullmann BD, Myers H, Chiranand W, Lazzell AL, Zhao Q, Vega LA, Lopez-Ribot JL, Gardner PR, Gustin MC. 2004. Inducible defense mechanism against nitric oxide in Candida albicans. Eukaryot Cell 3:715–723 10.1128/EC.3.3.715-723.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.de Jesús-Berríos M, Liu L, Nussbaum JC, Cox GM, Stamler JS, Heitman J. 2003. Enzymes that counteract nitrosative stress promote fungal virulence. Curr Biol 13:1963–1968 10.1016/j.cub.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 130.Levin DE. 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 69:262–291 10.1128/MMBR.69.2.262-291.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xie X, Lipke PN. 2010. On the evolution of fungal and yeast cell walls. Yeast 27:479–488 10.1002/yea.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Erwig LP, Gow NA. 2016. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol 14:163–176 10.1038/nrmicro.2015.21. [PubMed] [DOI] [PubMed] [Google Scholar]
- 133.Roemer T, Krysan DJ. 2014. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 4:a019703 10.1101/cshperspect.a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Munro CA. 2013. Chitin and glucan, the yin and yang of the fungal cell wall, implications for antifungal drug discovery and therapy. Adv Appl Microbiol 83:145–172 10.1016/B978-0-12-407678-5.00004-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 135.Walker LA, Gow NA, Munro CA. 2013. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob Agents Chemother 57:146–154 10.1128/AAC.01486-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Walker LA, Munro CA, de Bruijn I, Lenardon MD, McKinnon A, Gow NA. 2008. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog 4:e1000040 10.1371/journal.ppat.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cowen LE. 2013. The fungal Achilles’ heel: targeting Hsp90 to cripple fungal pathogens. Curr Opin Microbiol 16:377–384 10.1016/j.mib.2013.03.005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 138.Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. 2009. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog 5:e1000532 10.1371/journal.ppat.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.LaFayette SL, Collins C, Zaas AK, Schell WA, Betancourt-Quiroz M, Gunatilaka AA, Perfect JR, Cowen LE. 2010. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog 6:e1001069 10.1371/journal.ppat.1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Millson SH, Truman AW, King V, Prodromou C, Pearl LH, Piper PW. 2005. A two-hybrid screen of the yeast proteome for Hsp90 interactors uncovers a novel Hsp90 chaperone requirement in the activity of a stress-activated mitogen-activated protein kinase, Slt2p (Mpk1p). Eukaryot Cell 4:849–860 10.1128/EC.4.5.849-860.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Truman AW, Millson SH, Nuttall JM, Mollapour M, Prodromou C, Piper PW. 2007. In the yeast heat shock response, Hsf1-directed induction of Hsp90 facilitates the activation of the Slt2 (Mpk1) mitogen-activated protein kinase required for cell integrity. Eukaryot Cell 6:744–752 10.1128/EC.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Munro CA, Selvaggini S, de Bruijn I, Walker L, Lenardon MD, Gerssen B, Milne S, Brown AJ, Gow NA. 2007. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol Microbiol 63:1399–1413 10.1111/j.1365-2958.2007.05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dichtl K, Samantaray S, Wagener J. 2016. Cell wall integrity signalling in human pathogenic fungi. Cell Microbiol 18:1228–1238 10.1111/cmi.12612. [DOI] [PubMed] [Google Scholar]
- 144.Lamoth F, Juvvadi PR, Steinbach WJ. 2016. Heat shock protein 90 (Hsp90): a novel antifungal target against Aspergillus fumigatus. Crit Rev Microbiol 42:310–321. [DOI] [PubMed] [Google Scholar]
- 145.Ortiz-Urquiza A, Keyhani NO. 2015. Stress response signaling and virulence: insights from entomopathogenic fungi. Curr Genet 61:239–249 10.1007/s00294-014-0439-9. (Erratum, 61:251. doi:10.1007/s00294-015-0485-y.) [DOI] [PubMed] [Google Scholar]
- 146.Valiante V, Macheleidt J, Föge M, Brakhage AA. 2015. The Aspergillus fumigatus cell wall integrity signaling pathway: drug target, compensatory pathways, and virulence. Front Microbiol 6:325 10.3389/fmicb.2015.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Juvvadi PR, Lee SC, Heitman J, Steinbach WJ. 2017. Calcineurin in fungal virulence and drug resistance: prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence 8:186–197 10.1080/21505594.2016.1257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hast MA, Nichols CB, Armstrong SM, Kelly SM, Hellinga HW, Alspaugh JA, Beese LS. 2011. Structures of Cryptococcus neoformans protein farnesyltransferase reveal strategies for developing inhibitors that target fungal pathogens. J Biol Chem 286:35149–35162 10.1074/jbc.M111.250506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Robbins N, Spitzer M, Yu T, Cerone RP, Averette AK, Bahn YS, Heitman J, Sheppard DC, Tyers M, Wright GD. 2015. An antifungal combination matrix identifies a rich pool of adjuvant molecules that enhance drug activity against diverse fungal pathogens. Cell Rep 13:1481–1492 10.1016/j.celrep.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cyert MS, Philpott CC. 2013. Regulation of cation balance in Saccharomyces cerevisiae. Genetics 193:677–713 10.1534/genetics.112.147207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Serrano R, Bernal D, Simón E, Ariño J. 2004. Copper and iron are the limiting factors for growth of the yeast Saccharomyces cerevisiae in an alkaline environment. J Biol Chem 279:19698–19704 10.1074/jbc.M313746200. [DOI] [PubMed] [Google Scholar]
- 152.Davis DA. 2009. How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr Opin Microbiol 12:365–370 10.1016/j.mib.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 153.Peñalva MA, Lucena-Agell D, Arst HN Jr. 2014. Liaison alcaline: pals entice non-endosomal ESCRTs to the plasma membrane for pH signaling. Curr Opin Microbiol 22:49–59 10.1016/j.mib.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 154.Li W, Mitchell AP. 1997. Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics 145:63–73. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tilburn J, Sarkar S, Widdick DA, Espeso EA, Orejas M, Mungroo J, Peñalva MA, Arst HN Jr. 1995. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J 14:779–790. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Galindo A, Calcagno-Pizarelli AM, Arst HN Jr, Peñalva MA. 2012. An ordered pathway for the assembly of fungal ESCRT-containing ambient pH signalling complexes at the plasma membrane. J Cell Sci 125:1784–1795 10.1242/jcs.098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Herrador A, Herranz S, Lara D, Vincent O. 2010. Recruitment of the ESCRT machinery to a putative seven-transmembrane-domain receptor is mediated by an arrestin-related protein. Mol Cell Biol 30:897–907 10.1128/MCB.00132-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hervás-Aguilar A, Galindo A, Peñalva MA. 2010. Receptor-independent ambient pH signaling by ubiquitin attachment to fungal arrestin-like PalF. J Biol Chem 285:18095–18102 10.1074/jbc.M110.114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Obara K, Yamamoto H, Kihara A. 2012. Membrane protein Rim21 plays a central role in sensing ambient pH in Saccharomyces cerevisiae. J Biol Chem 287:38473–38481 10.1074/jbc.M112.394205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nishino K, Obara K, Kihara A. 2015. The C-terminal cytosolic region of Rim21 senses alterations in plasma membrane lipid composition: insights into sensing mechanisms for plasma membrane lipid asymmetry. J Biol Chem 290:30797–30805 10.1074/jbc.M115.674382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.De Bernardis F, Mühlschlegel FA, Cassone A, Fonzi WA. 1998. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun 66:3317–3325. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.El Barkani A, Kurzai O, Fonzi WA, Ramon A, Porta A, Frosch M, Mühlschlegel FA. 2000. Dominant active alleles of RIM101 (PRR2) bypass the pH restriction on filamentation of Candida albicans. Mol Cell Biol 20:4635–4647 10.1128/MCB.20.13.4635-4647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bertuzzi M, Schrettl M, Alcazar-Fuoli L, Cairns TC, Muñoz A, Walker LA, Herbst S, Safari M, Cheverton AM, Chen D, Liu H, Saijo S, Fedorova ND, Armstrong-James D, Munro CA, Read ND, Filler SG, Espeso EA, Nierman WC, Haas H, Bignell EM. 2014. The pH-responsive PacC transcription factor of Aspergillus fumigatus governs epithelial entry and tissue invasion during pulmonary aspergillosis. PLoS Pathog 10:e1004413 10.1371/journal.ppat.1004413. (Erratum, 11:e1004802. doi:10.1371/journal.ppat.1004802.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ortoneda M, Guarro J, Madrid MP, Caracuel Z, Roncero MI, Mayayo E, Di Pietro A. 2004. Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infect Immun 72:1760–1766 10.1128/IAI.72.3.1760-1766.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.O’Meara TR, Norton D, Price MS, Hay C, Clements MF, Nichols CB, Alspaugh JA. 2010. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog 6:e1000776 10.1371/journal.ppat.1000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ost KS, O’Meara TR, Huda N, Esher SK, Alspaugh JA. 2015. The Cryptococcus neoformans alkaline response pathway: identification of a novel rim pathway activator. PLoS Genet 11:e1005159 10.1371/journal.pgen.1005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang W, Shang Y, Chen P, Gao Q, Wang C. 2015. MrpacC regulates sporulation, insect cuticle penetration and immune evasion in Metarhizium robertsii. Environ Microbiol 17:994–1008 10.1111/1462-2920.12451. [DOI] [PubMed] [Google Scholar]
- 168.Zou CG, Tu HH, Liu XY, Tao N, Zhang KQ. 2010. PacC in the nematophagous fungus Clonostachys rosea controls virulence to nematodes. Environ Microbiol 12:1868–1877 10.1111/j.1462-2920.2010.02191.x. [DOI] [PubMed] [Google Scholar]
- 169.Caracuel Z, Roncero MI, Espeso EA, González-Verdejo CI, García-Maceira FI, Di Pietro A. 2003. The pH signalling transcription factor PacC controls virulence in the plant pathogen Fusarium oxysporum. Mol Microbiol 48:765–779 10.1046/j.1365-2958.2003.03465.x. [DOI] [PubMed] [Google Scholar]
- 170.Landraud P, Chuzeville S, Billon-Grande G, Poussereau N, Bruel C. 2013. Adaptation to pH and role of PacC in the rice blast fungus Magnaporthe oryzae. PLoS One 8:e69236 10.1371/journal.pone.0069236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang T, Sun X, Xu Q, Candelas LG, Li H. 2013. The pH signaling transcription factor PacC is required for full virulence in Penicillium digitatum. Appl Microbiol Biotechnol 97:9087–9098 10.1007/s00253-013-5129-x. [DOI] [PubMed] [Google Scholar]
- 172.Juvvadi PR, Gehrke C, Fortwendel JR, Lamoth F, Soderblom EJ, Cook EC, Hast MA, Asfaw YG, Moseley MA, Creamer TP, Steinbach WJ. 2013. Phosphorylation of calcineurin at a novel serine-proline rich region orchestrates hyphal growth and virulence in Aspergillus fumigatus. PLoS Pathog 9:e1003564 10.1371/journal.ppat.1003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J 16:2576–2589 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schumacher J, Viaud M, Simon A, Tudzynski B. 2008. The Galpha subunit BCG1, the phospholipase C (BcPLC1) and the calcineurin phosphatase co-ordinately regulate gene expression in the grey mould fungus Botrytis cinerea. Mol Microbiol 67:1027–1050 10.1111/j.1365-2958.2008.06105.x. [DOI] [PubMed] [Google Scholar]
- 175.Serrano R, Ruiz A, Bernal D, Chambers JR, Ariño J. 2002. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol Microbiol 46:1319–1333 10.1046/j.1365-2958.2002.03246.x. [DOI] [PubMed] [Google Scholar]
- 176.Spielvogel A, Findon H, Arst HN Jr, Araújo-Bazán L, Hernández-Ortíz P, Stahl U, Meyer V, Espeso EA. 2008. Two zinc finger transcription factors, CrzA and SltA, are involved in cation homoeostasis and detoxification in Aspergillus nidulans. Biochem J 414:419–429 10.1042/BJ20080344. [DOI] [PubMed] [Google Scholar]
- 177.Stathopoulos AM, Cyert MS. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev 11:3432–3444 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zacchi LF, Gomez-Raja J, Davis DA. 2010. Mds3 regulates morphogenesis in Candida albicans through the TOR pathway. Mol Cell Biol 30:3695–3710 10.1128/MCB.01540-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Serrano R, Martín H, Casamayor A, Ariño J. 2006. Signaling alkaline pH stress in the yeast Saccharomyces cerevisiae through the Wsc1 cell surface sensor and the Slt2 MAPK pathway. J Biol Chem 281:39785–39795 10.1074/jbc.M604497200. [DOI] [PubMed] [Google Scholar]
- 180.Mellado L, Arst HN Jr, Espeso EA. 2016. Proteolytic activation of both components of the cation stress-responsive Slt pathway in Aspergillus nidulans. Mol Biol Cell 27:2598–2612 10.1091/mbc.E16-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mollapour M, Piper PW. 2007. Hog1 mitogen-activated protein kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol Cell Biol 27:6446–6456 10.1128/MCB.02205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stratford M, Nebe-von-Caron G, Steels H, Novodvorska M, Ueckert J, Archer DB. 2013. Weak-acid preservatives: pH and proton movements in the yeast Saccharomyces cerevisiae. Int J Food Microbiol 161:164–171 10.1016/j.ijfoodmicro.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 183.Stratford M, Plumridge A, Nebe-von-Caron G, Archer DB. 2009. Inhibition of spoilage mould conidia by acetic acid and sorbic acid involves different modes of action, requiring modification of the classical weak-acid theory. Int J Food Microbiol 136:37–43 10.1016/j.ijfoodmicro.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 184.Piper P, Calderon CO, Hatzixanthis K, Mollapour M. 2001. Weak acid adaptation: the stress response that confers yeasts with resistance to organic acid food preservatives. Microbiology 147:2635–2642 10.1099/00221287-147-10-2635. [DOI] [PubMed] [Google Scholar]
- 185.Piper P, Mahé Y, Thompson S, Pandjaitan R, Holyoak C, Egner R, Mühlbauer M, Coote P, Kuchler K. 1998. The pdr12 ABC transporter is required for the development of weak organic acid resistance in yeast. EMBO J 17:4257–4265 10.1093/emboj/17.15.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Holyoak CD, Stratford M, McMullin Z, Cole MB, Crimmins K, Brown AJ, Coote PJ. 1996. Activity of the plasma membrane H(+)-ATPase and optimal glycolytic flux are required for rapid adaptation and growth of Saccharomyces cerevisiae in the presence of the weak-acid preservative sorbic acid. Appl Environ Microbiol 62:3158–3164. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ullah A, Orij R, Brul S, Smits GJ. 2012. Quantitative analysis of the modes of growth inhibition by weak organic acids in Saccharomyces cerevisiae. Appl Environ Microbiol 78:8377–8387 10.1128/AEM.02126-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mira NP, Palma M, Guerreiro JF, Sá-Correia I. 2010. Genome-wide identification of Saccharomyces cerevisiae genes required for tolerance to acetic acid. Microb Cell Fact 9:79 10.1186/1475-2859-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kren A, Mamnun YM, Bauer BE, Schüller C, Wolfger H, Hatzixanthis K, Mollapour M, Gregori C, Piper P, Kuchler K. 2003. War1p, a novel transcription factor controlling weak acid stress response in yeast. Mol Cell Biol 23:1775–1785 10.1128/MCB.23.5.1775-1785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schüller C, Mamnun YM, Mollapour M, Krapf G, Schuster M, Bauer BE, Piper PW, Kuchler K. 2004. Global phenotypic analysis and transcriptional profiling defines the weak acid stress response regulon in Saccharomyces cerevisiae. Mol Biol Cell 15:706–720 10.1091/mbc.E03-05-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jandric Z, Gregori C, Klopf E, Radolf M, Schüller C. 2013. Sorbic acid stress activates the Candida glabrata high osmolarity glycerol MAP kinase pathway. Front Microbiol 4:350 10.3389/fmicb.2013.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lebel K, MacPherson S, Turcotte B. 2006. New tools for phenotypic analysis in Candida albicans: the WAR1 gene confers resistance to sorbate. Yeast 23:249–259 10.1002/yea.1346. [DOI] [PubMed] [Google Scholar]
- 193.Ramsdale M, Selway L, Stead D, Walker J, Yin Z, Nicholls SM, Crowe J, Sheils EM, Brown AJ. 2008. MNL1 regulates weak acid-induced stress responses of the fungal pathogen Candida albicans. Mol Biol Cell 19:4393–4403 10.1091/mbc.E07-09-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stratford M, Steels H, Nebe-von-Caron G, Novodvorska M, Hayer K, Archer DB. 2013. Extreme resistance to weak-acid preservatives in the spoilage yeast Zygosaccharomyces bailii. Int J Food Microbiol 166:126–134 10.1016/j.ijfoodmicro.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mager WH, De Kruijff AJ. 1995. Stress-induced transcriptional activation. Microbiol Rev 59:506–531. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Görner W, Durchschlag E, Martinez-Pastor MT, Estruch F, Ammerer G, Hamilton B, Ruis H, Schüller C. 1998. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev 12:586–597 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Görner W, Durchschlag E, Wolf J, Brown EL, Ammerer G, Ruis H, Schüller C. 2002. Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO J 21:135–144 10.1093/emboj/21.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Petrenko N, Chereji RV, McClean MN, Morozov AV, Broach JR. 2013. Noise and interlocking signaling pathways promote distinct transcription factor dynamics in response to different stresses. Mol Biol Cell 24:2045–2057 10.1091/mbc.E12-12-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gasch AP, Huang M, Metzner S, Botstein D, Elledge SJ, Brown PO. 2001. Genomic expression responses to DNA-damaging agents and the regulatory role of the yeast ATR homolog Mec1p. Mol Biol Cell 12:2987–3003 10.1091/mbc.12.10.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nicholls S, Straffon M, Enjalbert B, Nantel A, Macaskill S, Whiteway M, Brown AJ. 2004. Msn2- and Msn4-like transcription factors play no obvious roles in the stress responses of the fungal pathogen Candida albicans. Eukaryot Cell 3:1111–1123 10.1128/EC.3.5.1111-1123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Smith DA, Morgan BA, Quinn J. 2010. Stress signalling to fungal stress-activated protein kinase pathways. FEMS Microbiol Lett 306:1–8 10.1111/j.1574-6968.2010.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lewis JG, Learmonth RP, Watson K. 1995. Induction of heat, freezing and salt tolerance by heat and salt shock in Saccharomyces cerevisiae. Microbiology 141:687–694 10.1099/13500872-141-3-687. [DOI] [PubMed] [Google Scholar]
- 203.Berry DB, Gasch AP. 2008. Stress-activated genomic expression changes serve a preparative role for impending stress in yeast. Mol Biol Cell 19:4580–4587 10.1091/mbc.E07-07-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Berry DB, Guan Q, Hose J, Haroon S, Gebbia M, Heisler LE, Nislow C, Giaever G, Gasch AP. 2011. Multiple means to the same end: the genetic basis of acquired stress resistance in yeast. PLoS Genet 7:e1002353 10.1371/journal.pgen.1002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mitchell A, Pilpel Y. 2011. A mathematical model for adaptive prediction of environmental changes by microorganisms. Proc Natl Acad Sci USA 108:7271–7276 10.1073/pnas.1019754108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mitchell A, Romano GH, Groisman B, Yona A, Dekel E, Kupiec M, Dahan O, Pilpel Y. 2009. Adaptive prediction of environmental changes by microorganisms. Nature 460:220–224 10.1038/nature08112. [DOI] [PubMed] [Google Scholar]
- 207.Brown AJ, Budge S, Kaloriti D, Tillmann A, Jacobsen MD, Yin Z, Ene IV, Bohovych I, Sandai D, Kastora S, Potrykus J, Ballou ER, Childers DS, Shahana S, Leach MD. 2014. Stress adaptation in a pathogenic fungus. J Exp Biol 217:144–155 10.1242/jeb.088930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Brunke S, Hube B. 2014. Adaptive prediction as a strategy in microbial infections. PLoS Pathog 10:e1004356 10.1371/journal.ppat.1004356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rodaki A, Bohovych IM, Enjalbert B, Young T, Odds FC, Gow NA, Brown AJ. 2009. Glucose promotes stress resistance in the fungal pathogen Candida albicans. Mol Biol Cell 20:4845–4855 10.1091/mbc.E09-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kaloriti D, Jacobsen M, Yin Z, Patterson M, Tillmann A, Smith DA, Cook E, You T, Grimm MJ, Bohovych I, Grebogi C, Segal BH, Gow NA, Haynes K, Quinn J, Brown AJ. 2014. Mechanisms underlying the exquisite sensitivity of Candida albicans to combinatorial cationic and oxidative stress that enhances the potent fungicidal activity of phagocytes. MBio 5:e01334-14 10.1128/mBio.01334-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kaloriti D, Tillmann A, Cook E, Jacobsen M, You T, Lenardon M, Ames L, Barahona M, Chandrasekaran K, Coghill G, Goodman D, Gow NA, Grebogi C, Ho HL, Ingram P, McDonagh A, de Moura AP, Pang W, Puttnam M, Radmaneshfar E, Romano MC, Silk D, Stark J, Stumpf M, Thiel M, Thorne T, Usher J, Yin Z, Haynes K, Brown AJ. 2012. Combinatorial stresses kill pathogenic Candida species. Med Mycol 50:699–709 10.3109/13693786.2012.672770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291–297 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 213.Wang Y, Cao YY, Jia XM, Cao YB, Gao PH, Fu XP, Ying K, Chen WS, Jiang YY. 2006. Cap1p is involved in multiple pathways of oxidative stress response in Candida albicans. Free Radic Biol Med 40:1201–1209 10.1016/j.freeradbiomed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 214.Kos I, Patterson MJ, Znaidi S, Kaloriti D, da Silva Dantas A, Herrero-de-Dios CM, d’Enfert C, Brown AJ, Quinn J. 2016. Mechanisms underlying the delayed activation of the Cap1 transcription factor in Candida albicans following combinatorial oxidative and cationic stress important for phagocytic potency. MBio 7:e00331-16 10.1128/mBio.00331-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Enjalbert B, MacCallum DM, Odds FC, Brown AJ. 2007. Niche-specific activation of the oxidative stress response by the pathogenic fungus Candida albicans. Infect Immun 75:2143–2151 10.1128/IAI.01680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.François J, Parrou JL. 2001. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 25:125–145 10.1111/j.1574-6976.2001.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 217.Grant CM. 2001. Role of the glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol Microbiol 39:533–541 10.1046/j.1365-2958.2001.02283.x. [DOI] [PubMed] [Google Scholar]
- 218.Nwaka S, Holzer H. 1997. Molecular biology of trehalose and the trehalases in the yeast Saccharomyces cerevisiae. Prog Nucleic Acid Res Mol Biol 58:197–237 10.1016/S0079-6603(08)60037-9. [DOI] [PubMed] [Google Scholar]
- 219.Wiemken A. 1990. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie van Leeuwenhoek 58:209–217 10.1007/BF00548935. [DOI] [PubMed] [Google Scholar]
- 220.Peralta D, Bronowska AK, Morgan B, Dóka É, Van Laer K, Nagy P, Gräter F, Dick TP. 2015. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat Chem Biol 11:156–163 10.1038/nchembio.1720. [DOI] [PubMed] [Google Scholar]
- 221.Ralser M, Wamelink MM, Latkolik S, Jansen EE, Lehrach H, Jakobs C. 2009. Metabolic reconfiguration precedes transcriptional regulation in the antioxidant response. Nat Biotechnol 27:604–605 10.1038/nbt0709-604. [DOI] [PubMed] [Google Scholar]
- 222.Komalapriya C, Kaloriti D, Tillmann AT, Yin Z, Herrero-de-Dios C, Jacobsen MD, Belmonte RC, Cameron G, Haynes K, Grebogi C, de Moura AP, Gow NA, Thiel M, Quinn J, Brown AJ, Romano MC. 2015. Integrative model of oxidative stress adaptation in the fungal pathogen Candida albicans. PLoS One 10:e0137750 10.1371/journal.pone.0137750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schaber J, Adrover MA, Eriksson E, Pelet S, Petelenz-Kurdziel E, Klein D, Posas F, Goksör M, Peter M, Hohmann S, Klipp E. 2010. Biophysical properties of Saccharomyces cerevisiae and their relationship with HOG pathway activation. Eur Biophys J 39:1547–1556 10.1007/s00249-010-0612-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Leach MD, Tyc KM, Brown AJ, Klipp E. 2012. Modelling the regulation of thermal adaptation in Candida albicans, a major fungal pathogen of humans. PLoS One 7:e32467 10.1371/journal.pone.0032467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Garreau H, Hasan RN, Renault G, Estruch F, Boy-Marcotte E, Jacquet M. 2000. Hyperphosphorylation of Msn2p and Msn4p in response to heat shock and the diauxic shift is inhibited by cAMP in Saccharomyces cerevisiae. Microbiology 146:2113–2120 10.1099/00221287-146-9-2113. [DOI] [PubMed] [Google Scholar]
- 226.Alepuz PM, Cunningham KW, Estruch F. 1997. Glucose repression affects ion homeostasis in yeast through the regulation of the stress-activated ENA1 gene. Mol Microbiol 26:91–98 10.1046/j.1365-2958.1997.5531917.x. [DOI] [PubMed] [Google Scholar]
- 227.Stanhill A, Schick N, Engelberg D. 1999. The yeast ras/cyclic AMP pathway induces invasive growth by suppressing the cellular stress response. Mol Cell Biol 19:7529–7538 10.1128/MCB.19.11.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ene IV, Adya AK, Wehmeier S, Brand AC, MacCallum DM, Gow NA, Brown AJ. 2012. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol 14:1319–1335 10.1111/j.1462-5822.2012.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


