Abstract
Background
Phthalates are a class of chemicals that may be associated with obesity in non-pregnant populations. Little is known about the association between pregnancy phthalate exposure and maternal obesity.
Objective
We evaluated the association between early-pregnancy urinary concentrations of specific phthalate metabolites and the distribution of body mass index (BMI, cross-sectional), and early gestational weight gain (GWG, prospective).
Methods
We measured 1st trimester urinary phthalate metabolite concentrations (median 9.9 weeks gestation) in 347 women from the LIFECODES pregnancy cohort (Boston, MA), who delivered term births. All measures were adjusted for specific-gravity and log-transformed. We used quantile regression to evaluate shifts in the entire outcome distributions, calculating multivariable-adjusted differences in the associations between these phthalate metabolites and BMI and GWG at the 25th, 50th, and 75th percentiles of these anthropometric outcomes.
Results
Higher concentrations of mono-ethyl phthalate (MEP) were associated with a rightward shift of 2.8 kg/m2 at the 75th percentiles of BMI (lowest vs highest quartile, 95% CI: 0.2–5.4) and 1.3 kg at the 75th percentiles of early GWG (lowest vs second quartiles, 95% CI: 0.3–2.4). A significant right-shift in the upper tail of BMI was also observed at higher concentrations of mono-benzyl (MBzP), mono-3-carboxypropyl (MCPP), and a summary measure of di-(2-ethylhexyl) phthalate metabolites (ΣDEHP). ΣDEHP was also associated with lower GWG.
Conclusions
Certain phthalates may be associated with shifts in maternal obesity measures, with MEP, MBzP, MCPP, and ΣDEHP being cross-sectionally associated with 1st trimester BMI and MEP and ΣDEHP being positively and inversely associated with early GWG, respectively.
Keywords: phthalates, pregnancy, maternal obesity, quantile regression
Introduction
Maternal obesity is an increasingly common condition and is associated with a large number of adverse pregnancy outcomes (Leddy et al. 2008; Sebire et al. 2001; Guelinckx et al. 2008). Specifically, obese women have higher risk of preeclampsia (Salihu et al 2012), gestational diabetes mellitus (GDM) (Chu et al. 2007), and cesarean delivery (Weiss et al. 2004), as well as stillbirth and congenital anomalies (Chu et al. 2007). In addition, maternal obesity can have a long-term impact on the future health of both the mother and the offspring, especially in terms of heart disease, hypertension, and diabetes (Sridhar et al. 2014; Freeman 2010; Gilmore et al. 2015). Together with the standard obesity measure of body mass index (BMI), another important measure for maternal obesity is gestational weight gain (GWG) (Ferraro et al. 2015). Excessive gestational weight gain is an established predictor of pregnancy and post-pregnancy complications, as well as postpartum weight retention, which is known to influence the future risk of obesity (Gunderson 2009; Kirkegaard et al. 2015; Krukowski et al. 2016). Several studies have demonstrated that risks associated with excessive GWG are higher in early pregnancy, suggesting that early GWG may be an important and clinically relevant time period with respect to adverse health outcomes (Fontaine et al. 2012; Ferraro et al. 2015; Hedderson et al. 2010; Hedderson et al. 2014; Carreno et al. 2012).
In addition to nutritional and behavioral factors, a substantial body of literature suggests that exposures to endocrine disrupting chemicals (EDCs), a class of chemicals capable of interfering with the normal processes of endocrine systems, may increase the risk of obesity (Heindel et al. 2015). Phthalates are a class of EDCs that are used as plasticizers in a variety of consumer products, including food packaging, personal care products, floor tiles, and industrial solvents (Hauser and Calafat 2005). Phthalates interfere with the endocrine system through different pathways such as by activating peroxisome proliferator-activated receptors (PPARs), which can up-regulate adipogenesis (Desvergne et al. 2009). Animal and non-pregnant population studies have suggested a potential association between obesity and specific phthalate metabolites such as mono-ethyl phthalate (MEP), mono-benzyl phthalate (MBzP), monoethylhexyl- phthalate (MEHP), and mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP) (Hao et al. 2012; Hao et al. 2013; Hatch et al. 2008; Stahluht et al. 2007), but have provided inconsistent results, which could in part be due to differences in outcome assessments and inclusion of covariates (Thayer et al. 2012; Tang-Péronard et al. 2011). On the other hand, in pregnant populations, the evidence of an association between phthalate exposure and maternal obesity are limited to a single study (James-Todd et al. 2016).
Epidemiological studies focusing on phthalate exposure and obesity have applied standard statistical methods to report shifts in the mean of BMI as a function of phthalate metabolite concentrations (Hao et al. 2012; Hao et al. 2013; Hatch et al. 2008; Stahluht et al. 2007). Focusing on the mean alone, however, assumes that the exposure-outcome association is constant over the entire outcome distribution, and does not capture effects that primarily occur at the tails of the distribution (Beyerlein 2014). In environmental health, due to the complexity of biological mechanisms through which environmental chemicals may affect the human body, it may happen that certain chemical exposures could have differential effects based on differing levels of the outcome of interest (Bind et al. 2015). For example, a recent study from Bind et al found air pollution to be associated with a left shift in gene-specific methylation only in the lower tail of their distribution, suggesting heterogeneity between study participants with respect to the potential epigenetic effects of air pollution exposure (Bind et al. 2015). In the context of phthalates and obesity, a positive association between BMI and PPAR gamma mRNA expression has been observed (Redonnet et al. 2002), thus suggesting that the mechanism by which overexpression of PPAR gamma target genes might be induced by higher phthalate exposure could vary across the distribution of body mass index (BMI). As such, it could be hypothesized that women in the right tail of the BMI distribution might be more susceptible to the potentially obesogenic effects of higher phthalate exposure during pregnancy.
Therefore, the objective of this study was to evaluate, in a prospective cohort of pregnant women, early pregnancy distribution shifts in body size measures commonly used as indicators of maternal obesity (i.e. first trimester BMI and early GWG), as well as weight trajectories over the entire pregnancy, as a function of first-trimester urinary concentrations of specific phthalate metabolites.
Methods
Study population
We used data from the LIFECODES pregnancy cohort, an ongoing prospective study of pregnant women that was started in 2006. LIFECODES enrolls women at the first prenatal visit <15 gestation weeks (median: 9.9 gestation weeks). Eligible women include those who are: 1) planning to deliver at Brigham and Women’s Hospital (Boston, MA); and 2) not pregnant with more than 3 fetuses. All study participants completed a self-administered questionnaire to provide information on socio-demographic and lifestyle factors. Urine and blood samples, together with anthropometric measures, were collected at four time points that coincided with standard prenatal care visits (median: 9.9, 17.3, 26.1, and 35.3 gestation weeks). For the present study, which focuses on early markers of anthropometry in pregnancy, we focus on 1st trimester measures.
Among LIFECODES study participants enrolled between 2006 and 2008, a nested case-control study was conducted, described elsewhere (Ferguson et al. 2014). Our study population included the controls from this case-control study (i.e. those who delivered at term defined as delivery >37 weeks gestation). Furthermore, women in the present study population were recruited exclusively during the first trimester, as these were women with available information on urinary phthalate metabolite concentrations at the first study visit (n=347). All women gave their informed consent. The study was approved by the Partners Human Subject Committee at Brigham and Women’s Hospital.
Phthalates concentration assessment
Spot urine samples collected at the first study visit were stored at −80°C and analyzed by NSF International, Inc. (Ann Arbor, MI) following a protocol from the Center for Disease Control and Prevention, described in details elsewhere (Centers for Disease Control and Prevention 2005). In brief, solid phase extraction and high performance liquid chromatography were used, along with tandem mass spectrometry ( Centers for Disease Control and Prevention 2005). When detection limits were low, samples with levels below the limit were assigned by dividing the limit of detection by the square root of two (Hornung and Reed 1990).
Nine urinary phthalate metabolites were measured: mono-ethyl phthalate (MEP, metabolite of diethyl phthalate); metabolite of dibutyl phthalate (MnBP, metabolite of di-n-butyl phthalate); mono-isobutyl phthalate (MiBP, metabolite of diisobutyl phthalate); metabolite of benzyl butyl phthalate (MBzP, metabolite of butylbenzyl phthalate); mono-(3-carboxypropyl) phthalate (MCPP, metabolite of di-n-octyl phthalate), which is a nonspecific metabolite of several high molecular weight phthalates and a minor metabolite of DBP (Calafat et al. 2006); as well as 4 metabolites of di(2-ethylhexyl) phthalate (DEHP): mono-ethylhexyl-phthalate (MEHP); mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP); mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP); and mono-2-ethyl-5-oxohexyl phthalate (MEOHP). Due to the high degree of correlation between these four urinary phthalate metabolites (r>0.95), we created a summary measure of these four metabolites (ΣDEHP) by adding their molar concentrations.
Since individuals differ in their urinary dilution, we adjusted all phthalate metabolite concentrations for specific gravity (SG) of the individual sample. SG-adjusted urinary concentrations were calculated with the formula: Pc=P[(1.015-1)/SG-1], where P was the urinary concentration and 1.015 was the median SG over all samples (Boeniger et al. 1993). We further excluded from the study n=2 urine samples with SG outside of the normal range (SG>1.04).
For this study we only used phthalate metabolite concentrations measured at the first medical visit (9.9 median gestation weeks).
Outcomes
First trimester body mass index. BMI was calculated as weight (kg) divided by squared height (meters2) based on weight and height taken as a part of standard clinical work-up by trained medical staff at the time of the first prenatal visit. BMI was assessed continuously and categorically. For categorized BMI, the National Heart Lung and Blood Institute’s criteria were used (BMI <25, 25–30, >30 kg/m2).
Early pregnancy gestational weight gain. GWG was calculated as the difference in weight in kg between the second and the first prenatal visits as an indicator of early gestational weight gain (median time period between 1st and 2nd trimester weight measurements: 7.4 gestational weeks). For early pregnancy GWG, we further excluded n=2 women with unlikely extreme values of GWG (i.e. > or < than 3-sd unit). In addition to continuous GWG, early GWG was divided into inadequate, adequate, and excess GWG, using the criteria of the Institute of Medicine (Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines 2009; Fontaine et al. 2012). The entire trajectory of gestational weight (i.e. also including weight measurements taken at the 3rd and 4th medical visits) was investigated in a secondary analysis.
Covariates
All analyses were adjusted for established risk factors of maternal obesity potentially associated with urinary phthalate metabolite concentrations. These included maternal age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian, other), alcohol consumption (yes/no), smoking (yes/no), and educational level (college or more vs <college). Statistical models for GWG were additionally adjusted for baseline BMI (continuous). Proportion of missing data was negligible (<5% for all covariates), and all multivariable analyses were conducted as complete-case.
Statistical analysis
We calculated baseline characteristics of the study population, as well as the SG-adjusted geometric means and 25th and 75th percentiles of each phthalate metabolite, for the overall population and stratified by 1st trimester BMI and early GWG categories. Analyses focusing on phthalates and BMI were cross-sectional as these were assessed at the same time point. On the other hand, analyses of GWG were prospective.
In addition to the classical mean regression, we used quantile regression to investigate the associations between urinary phthalate metabolite concentrations and the entire distribution of 1st trimester BMI and early GWG. By focusing on the percentiles of the outcome, quantile regression evaluates shifts in the shape of the outcome distribution, rather than its location, and allows for detection of associations that primarily occur at the tails of the distributions (Koenker and Hallock 2001). To evaluate distribution shifts in the lower tail, median, and higher tail of 1st trimester BMI and early GWG, we specifically focused on the 25th, 50th, and 75th percentiles, respectively. For each of these percentiles we built a linear quantile regression model and presented multivariable-adjusted statistical associations with 95% confidence intervals (CI).
All exposure measures of SG-adjusted phthalate metabolites were log-transformed due to right-skewedness. To flexibly evaluate the exposure-outcome associations we adopted two approaches that allowed for detection of non-linear associations. First, we calculated shifts in the mean and percentiles of BMI and early GWG across quartiles of phthalate metabolite concentrations, using the lowest quartiles as referent groups. This model was replicated in two sensitivity analyses, where 1) baseline BMI was not included in the model; and 2) the outcome was investigated in terms of gestational weight adequacy (Bodnar et al 2011). Next, we evaluated urinary phthalate metabolite concentrations as continuous predictors by using restricted cubic splines transformation (Durrleman and Simon 1989). Splines models were graphically presented for only those metabolites showing significant results in the categorical models, and using the lowest reported concentration as referent value. Linearity of the dose-responses was evaluated by calculating p-values for linearity as reported in previous studies (Orsini and Greenland 2011).
As a secondary analysis we incorporated information on maternal weight at the third and fourth study visits to evaluate weight trajectories over the entire pregnancy as a function of first trimester phthalate metabolite concentration. Because of the high correlation between individual weight measures we used linear mixed models, to evaluate the trajectory over time of mean weight, and mixed quantile regression, to evaluate trajectories over time of the 25th, 50th, and 75th percentiles of weight (Geraci and Bottai 2014).
All analyses were performed in Stata, version 14, with the exception of the mixed quantile regression models that were evaluated with the R package lqmm (Geraci 2016).
Results
Baseline characteristics of the study population are presented in Table 1. The proportion of obese and overweight women was higher among non-Hispanic Black and Asian women. First trimester BMI and early GWG were higher among less educated women. Distributions of age, smoking, and alcohol consumption were similar across levels of 1st trimester BMI and early GWG. Urinary phthalate metabolite concentrations of MEP, MnBP, MBzP, and MCPP were higher among obese women. MiBP, and MBzP were lower among those with excess GWG (Table 2).
Table 1.
Baseline characteristics of the study population overall and by levels of 1st trimester BMI and early gestational weight gain (GWG)
| BMI | GWG | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Overall | <25 | 25–30 | >30 | Inadequate | Adequate | Excess | ||
| N (%)* | 347 (100) | 187 (54) | 92 (27) | 64 (19) | 120 (39) | 166 (53) | 25 (8) | ||
| Maternal age, mean (sd) | 32.0 (5.5) | 32.2 (4.9) | 32.0 (6.3) | 31.3 (5.7) | 30.8 (5.5) | 32.7 (5.3) | 31.9 (5.5) | ||
| Race/ethnicity, n (%) | |||||||||
| Caucasian | 205 (59) | 130 (70) | 46 (50) | 27 (42) | 66 (55) | 106 (64) | 14 (54) | ||
| Non-Hispanic black | 54 (16) | 19 (10) | 17 (18) | 18 (28) | 18 (15) | 25 (15) | 3 (12) | ||
| Hispanic | 19 (5) | 10 (5) | 9 (10) | 0 (0) | 9 (8) | 7 (4) | 3 (12) | ||
| Asians | 50 (14) | 19 (10) | 14 (15) | 16 (25) | 21 (17) | 20 (12) | 4 (16) | ||
| Unknown/other | 19 (5) | 9 (5) | 6 (6) | 3 (5) | 6 (5) | 8 (5) | 1 (4) | ||
| College or higher education, n (%) | 141 (41) | 92 (51) | 38 (41) | 11 (18) | 46 (39) | 79 (48) | 7 (27) | ||
| Alcohol use during pregnancy, n (%) | 19 (6) | 11 (6) | 6 (6) | 2 (3) | 6 (5) | 12 (7) | 1 (4) | ||
| Smoking status, n(%) | 8 (2) | 0 (0) | 3 (3) | 4 (6) | 2 (2) | 4 (2) | 1 (4) |
Numbers may not sum up to 347 because of missing values of BMI or GWG
Table 2.
Urinary phthalate metabolite levels at baseline by levels of 1st trimester BMI and early gestational weight gain (GWG)
| Phthalates metabolite, geometric mean (25th–75th percentile) |
Overall Population |
BMI <25 | 25<BMI<30 | BMI>30 | Inadequate GWG |
Adequate GWG |
Excess GWG |
|---|---|---|---|---|---|---|---|
| MEP, μg/l | 137.8 (48.6–345) | 113.0 (42.5–295.7) | 138.1 (46.6–290.5) | 236.3 (81.0–560.8) | 135.2 (44–363.8) | 131.8 (46.6–295.7) | 148.9 (53.4–263.7) |
| MnBP, μg/l | 17.3 (10.8–26.2) | 16.7 (10.6–25.1) | 15.6 (10.8–23.8 | 22.2 (12.2–31.1) | 19.0 (11.0–32.8) | 15.5 (10.7–22.6) | 20.4 (9.7–31.5) |
| MiBP, μg/l | 7.3 (4.5–11.1) | 7.0 (4.4–10.5) | 7.9 (4.6–13.9) | 7.5 (5.6–11.7) | 7.5 (4.5–11.1) | 7.3 (4.3–11.1) | 5.9 (3.3–11.9) |
| MBzP, μg/l | 7.0 (3.5–13.4) | 6.3 (3.1–13.2) | 6.7 (3.2–10.4) | 9.9 (5.2–16.7) | 8.1 (3.6–19.7) | 6.5 (3.3–12.4) | 5.3 (2.5–7.3) |
| MCPP, μg/l | 2.1 (1.0–3.1) | 1.9 (0.9–2.7) | 2.1 (1.1–3.2) | 2.8 (1.2–4.0) | 2.2 (1.1–3.5) | 2.2 (1.1–3.1) | 2.3 (1.0–4.8) |
| ΣDEHP, μmol/l | 0.4 (0.2–0.8) | 0.4 (0.2–0.8) | 0.5 (0.2–1.2) | 0.5 (0.2–0.7) | 0.4 (0.2–0.8) | 0.4 (0.2–1.0) | 0.3 (0.2–0.5) |
The 25th and 75th percentiles of BMI were 22 and 28 kg/m2 and for early GWG, 0.9 and 3.6 kg, respectively. Compared to the first quartiles, significantly higher BMI was observed at higher quartiles of MEP, MBzP, MCPP, and ΣDEHP metabolites (Table 3). The right shift in BMI at higher concentrations of MEP, MBzP, and MCPP, was only statistically significant in the right tail of the distribution, while no significant differences were detected at the 25th percentile. For instance, when comparing women in the highest and lowest MEP quartiles, the 75th percentile of BMI was 2.81 kg/m2 (95% CI: 0.20–5.42) higher, but only a smaller non-significant increase was observed at the 25th percentiles (β: 0.82 kg/m2; 95% CI: −0.91, 2.54). In contrast, higher levels of ΣDEHP were associated with a significant right shift in the entire BMI distribution.
Table 3.
1st trimester urinary phthalate metabolite concentrations and distribution shifts in 1st trimester BMI
| Multivariable adjusted* | ||||
|---|---|---|---|---|
| Log- transformed SG-adjusted phthalate metabolites |
Mean BMI | 25th Percentile | Median BMI | 75th percentile |
| MEP | ||||
| Q1 | Ref | |||
| Q2 | 1.83 (0.19, 3.47) | 0.04 (−1.62, 1.70) | 0.87 (−0.92, 2.65) | 1.61 (−0.90, 4.12) |
| Q3 | 1.41 (−0.28, 3.11) | 0.13 (−1.59, 1.84) | 0.72 (−1.13, 2.57) | 2.06 (−0.53, 4.66) |
| Q4 | 1.60 (−0.10, 3.31) | 0.82 (−0.91, 2.54) | 1.44 (−0.41, 3.30) | 2.81 (0.20, 5.42) |
| MnBP | ||||
| Q1 | Ref | |||
| Q2 | −0.3 (−1.93, 1.24) | −0.73 (−2.23, 0.77) | −0.21 (−1.96, 1.55) | −0.55 (−3.03, 1.92) |
| Q3 | 0.94 (−0.76, 2.63) | −0.15 (−1.76, 1.46) | −0.06 (−1.94, 1.81) | 0.88 (−1.76, 3.53) |
| Q4 | 0.09 (−1.64, 1.82) | 0.33 (−1.31, 1.97) | −0.04 (−1.96, 1.87) | 0.16 (−2.54, 2.85) |
| MiBP | ||||
| Q1 | Ref | |||
| Q2 | 0.76 (−0.88, 2.4) | 0.19 (−1.63, 2.01) | 1.27 (−0.41, 2.94) | 1.39 (−1.4, 4.19) |
| Q3 | 0.92 (−0.78, 2.62) | −0.05 (−1.95, 1.84) | 0.28 (−1.46, 2.02) | 1.83 (−1.07, 4.73) |
| Q4 | 0.91 (−0.79, 2.61) | −0.07 (−1.97, 1.82) | 0.86 (−0.88, 2.6) | 1.07 (−1.83, 3.97) |
| MBzP | ||||
| Q1 | Ref | |||
| Q2 | 1.24 (−0.4, 2.88) | 0.23 (−1.4, 1.86) | 1.12 (−0.41, 2.64) | 1.45 (−1.24, 4.14) |
| Q3 | 2.26 (0.63, 3.89) | 1.05 (−0.57, 2.67) | 1.94 (0.43, 3.46) | 3.61 (0.94, 6.28) |
| Q4 | 1.62 (−0.06, 3.3) | 1.04 (−0.64, 2.71) | 0.31 (−1.26, 1.87) | 1.73 (−1.02, 4.49) |
| MCPP | ||||
| Q1 | Ref | |||
| Q2 | 1 (−0.59, 2.59) | 0.33 (−1.14, 1.80) | 1.06 (−0.89, 3.00) | 2.52 (0.11, 4.94) |
| Q3 | 1.79 (0.18, 3.39) | 0.96 (−0.52, 2.45) | 1.17 (−0.79, 3.14) | 3.46 (1.01, 5.91) |
| Q4 | 1.6 (−0.04, 3.25) | 1.37 (−0.16, 2.89) | 1.2 (−0.81, 3.22) | 3.28 (0.77, 5.79) |
| ΣDEHP | ||||
| Q1 | Ref | |||
| Q2 | 1.93 (0.34, 3.52) | 1.4 (0.12, 2.68) | 1.03 (−0.98, 3.03) | 3.29 (1.09, 5.49) |
| Q3 | 1.2 (−0.41, 2.8) | 1.23 (−0.06, 2.52) | 1.08 (−0.95, 3.11) | 2.68 (0.45, 4.9) |
| Q4 | 1.5 (−0.17, 3.18) | 2.32 (0.97, 3.67) | 1.51 (−0.61, 3.63) | 1.68 (−0.64, 4.01) |
Adjusted for maternal age, race/ethnicity, education, smoking, alcohol
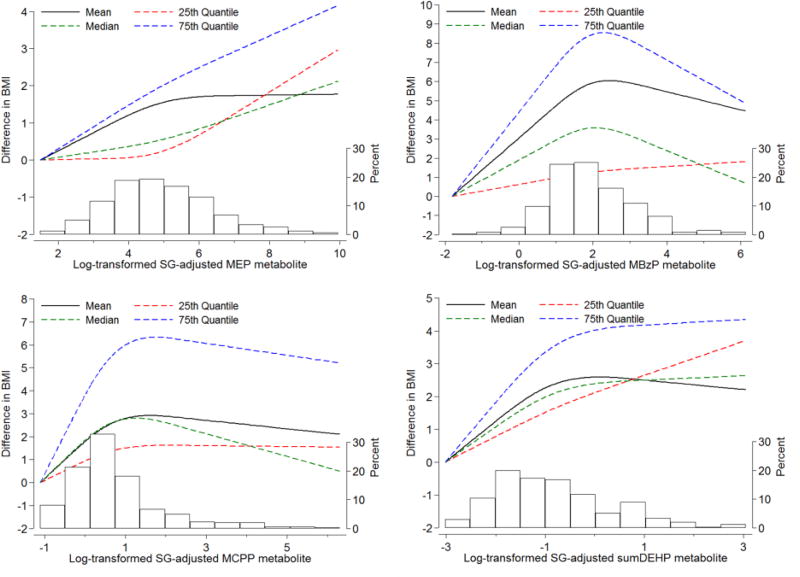
Figure 1 depicts the associations between the BMI distribution (i.e. mean, 25th, 50th, and 75th percentile) and urinary concentrations of MEP, MBzP, MCPP, and ΣDEHP, evaluated with restricted cubic splines models. This analysis confirmed that the shifts in BMI distributions were substantially larger in the right tail of BMI. A linear dose-response was only observed in the association between MEP concentrations and the 75th percentile of BMI. Non-linear associations were observed in all other scenarios, with a right shift in the BMI distribution, especially in the right tail, between the lowest and average urinary concentration, and no additional associations at higher levels.
Figure 1. Multivariable-adjusted distribution shifts of BMI as a function of baseline urinary phthalates levels.
Phthalates metabolites were flexibly modeled with restricted cubic splines with the lowest concentration serving as referent value. The histograms depict the distribution of phthalate levels in the study population. All models are adjusted for maternal age, race/ethnicity, education, smoking, alcohol. Shifts are at the mean, 25th, 50th, and 75th percentile of the outcome distribution.
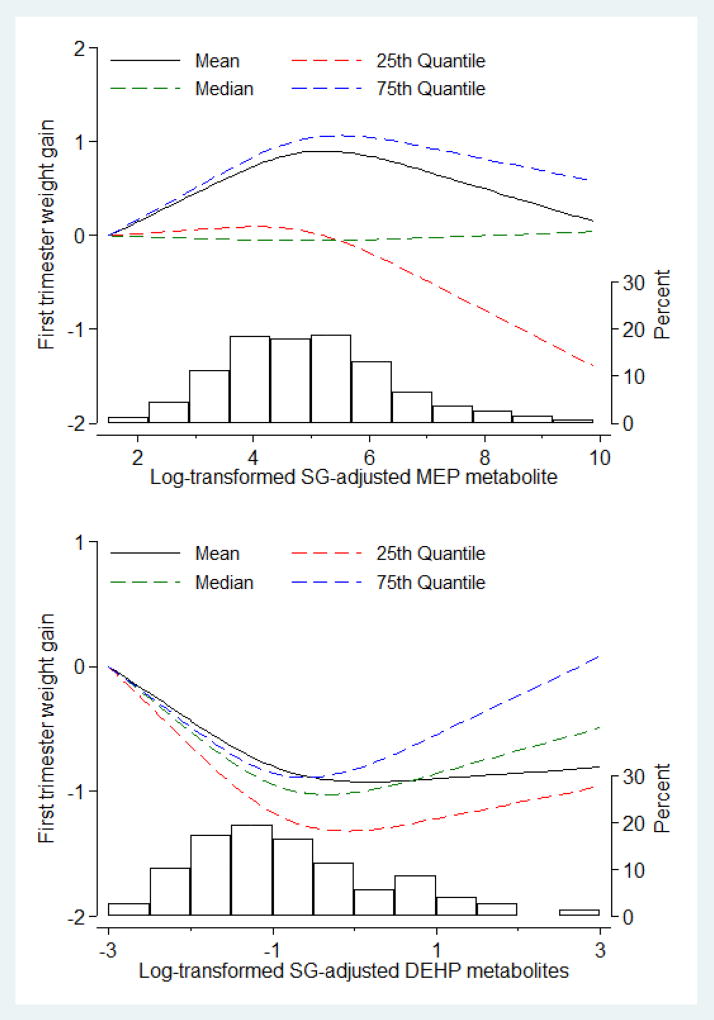
Significant shifts in the distribution of early GWG were detected at high levels of MEP, with the largest difference observed in the right tail of the distribution. While comparing women in the lowest and second quartile of MEP, the difference in early GWG was 1.30 kg (95% CI: 0.26–2.35) at the 75th percentile of the distribution, and 0.82 kg (95% CI: −0.09–1.62) at the 25th percentile (Table 4). On the other hand, lower GWG was observed when comparing participants in the third and first quartiles of ΣDEHP for both the 25th percentile (−0.94 kg; 95% CI: −1.67, −0.22), and 50th percentile (−0.98 kg; 95% CI: −1.74, −0.21) of the outcome distribution. Results were similar when excluding baseline BMI from the potential confounders and when investigating gestational weight adequacy (data not shown). The spline analysis showed that the associations between MEP and the 75th percentile of GWG followed a U-shape, with increasing GWG at low levels of the MEP distribution, and no additional increase at higher concentrations (Figure 2). The dose-response association between ΣDEHP and GWG was described by an inverse U-shape, with similar results throughout the evaluated percentiles.
Table 4.
1st trimester urinary phthalate metabolite concentrations and distribution shifts for early gestational weight gain (GWG, kg)
| Log- transformed SG-adjusted phthalate metabolites |
Mean GWG | 25th Percentile | Median GWG | 75th percentile |
|---|---|---|---|---|
| MEP | ||||
| Q1 | Ref | |||
| Q2 | 0.94 (0.24, 1.64) | 0.82 (−0.09, 1.72) | 0.84 (0.07, 1.62) | 1.3 (0.26, 2.35) |
| Q3 | −0.06 (−0.78, 0.65) | −0.18 (−1.1, 0.74) | 0.01 (−0.78, 0.8) | 0.39 (−0.68, 1.45) |
| Q4 | −0.1 (−0.83, 0.63) | −0.8 (−1.73, 0.14) | 0.16 (−0.65, 0.96) | 0.4 (−0.69, 1.48) |
| MnBP | ||||
| Q1 | Ref | |||
| Q2 | 0.27 (−0.41, 0.94) | 0.26 (−0.6, 1.13) | 0.45 (−0.25, 1.15) | 0.62 (−0.45, 1.7) |
| Q3 | −0.14 (−0.86, 0.58) | 0 (−0.93, 0.92) | 0.05 (−0.69, 0.8) | 0.21 (−0.94, 1.36) |
| Q4 | −0.3 (−1.03, 0.44) | −0.6 (−1.53, 0.34) | −0.51 (−1.27, 0.25) | −0.16 (−1.33, 1.01) |
| MiBP | ||||
| Q1 | Ref | |||
| Q2 | 0.12 (−0.58, 0.82) | 0.64 (−0.19, 1.46) | −0.01 (−0.81, 0.78) | 0.16 (−0.9, 1.22) |
| Q3 | 0.42 (−0.31, 1.15) | 0.39 (−0.47, 1.25) | 0.07 (−0.77, 0.9) | 0.87 (−0.23, 1.97) |
| Q4 | 0.35 (−0.37, 1.07) | 0.72 (−0.13, 1.57) | −0.02 (−0.84, 0.8) | 0.08 (−1.01, 1.17) |
| MBzP | ||||
| Q1 | Ref | |||
| Q2 | 0.17 (−0.54, 0.88) | 0.39 (−0.57, 1.35) | 0.28 (−0.5, 1.06) | 0.5 (−0.52, 1.51) |
| Q3 | −0.21 (−0.93, 0.5) | −0.05 (−1.02, 0.91) | −0.12 (−0.91, 0.66) | 0.02 (−1, 1.04) |
| Q4 | −0.28 (−1.01, 0.44) | −0.23 (−1.21, 0.75) | −0.3 (−1.1, 0.5) | −0.38 (−1.42, 0.66) |
| MCPP | ||||
| Q1 | Ref | |||
| Q2 | 0.08 (−0.61, 0.77) | 0.01 (−0.83, 0.84) | −0.16 (−0.91, 0.6) | 0.2 (−0.84, 1.24) |
| Q3 | −0.46 (−1.17, 0.26) | −0.38 (−1.24, 0.48) | −0.68 (−1.46, 0.09) | −0.26 (−1.33, 0.81) |
| Q4 | 0.22 (−0.49, 0.93) | 0.14 (−0.71, 0.99) | −0.04 (−0.81, 0.73) | 0.46 (−0.6, 1.52) |
| ΣDEHP | ||||
| Q1 | Ref | |||
| Q2 | 0.49 (−0.18, 1.16) | 0.33 (−0.38, 1.04) | 0.23 (−0.53, 0.98) | 0.3 (−0.79, 1.39) |
| Q3 | −0.58 (−1.26, 0.1) | −0.94 (−1.67, – 0.22) | −0.98 (−1.74, – 0.21) | −0.67 (−1.77, 0.44) |
| Q4 | −0.42 (−1.14, 0.3) | −0.41 (−1.17, 0.35) | −0.42 (−1.22, 0.39) | −0.27 (−1.43, 0.9) |
Adjusted for maternal age, race/ethnicity, education, smoking, alcohol, baseline BMI
Figure 2. Multivariable-adjusted distribution shifts of early gestational weight gain (GWG, kg) as a function of 1st trimester urinary MEP and ΣDEHP phthalate metabolites levels. Shifts are at the mean, 25th, 50th, and 75th percentile of the outcome distribution.
MEP and ΣDEHP concentration were flexibly modeled with restricted cubic splines with the lowest concentrations serving as referent value. The histograms depict the distribution of the metabolite in the study population. Models are adjusted for maternal age, race/ethnicity, education, smoking, alcohol, baseline BMI
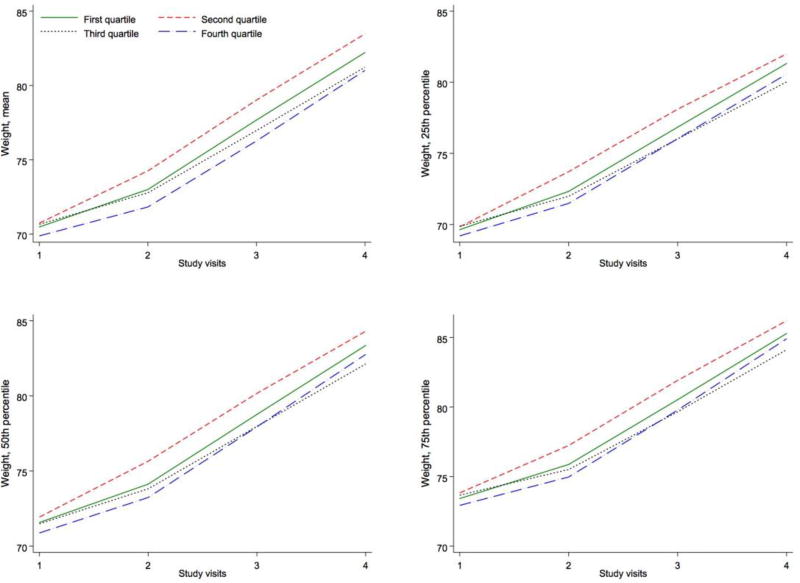
In a secondary analysis we incorporated all pregnancy measures of gestational weight. This analysis was only performed for MEP, where significant and consistent results with regards of BMI and GWG were observed. Figure 3 presents the longitudinal change in weight over pregnancy across quartiles of baseline MEP concentration. The difference in GWG between women in the lowest and second quartile of MEP largely occurred during the first trimester and remained constant at subsequent prenatal care visits.
Figure 3. Multivariable-adjusted trajectories of the distribution of gestational weight (kg) across pregnancy over quartiles of log-transformed SG-adjusted MEP metabolite concentration.
Mean trajectory estimated with linear mixed models. Trajectories of the 25th, 50th, and 75th percentiles estimated with mixed quantile regression. All models adjusted for maternal age, race/ethnicity, education, smoking, alcohol, baseline BMI. Median gestation weeks, Visit 1: 9.9; Visit 2: 17.3; Visit 3: 26.1; Visit 4: 35.3.
Discussion
In a prospective study of pregnant women, we found higher concentrations of MEP to be associated with a right-shift in the higher tails of maternal obesity measures (i.e. first trimester BMI and GWG). Positive cross-sectional associations were also observed between MBzP, MCPP, and ΣDEHP and 1st trimester BMI, while a negative prospective association was detected between ΣDEHP and early GWG. All associations were non-linear and only observed at specific quartiles of the exposures. These findings may provide evidence to suggest an adverse impact of higher phthalate exposure on maternal adiposity measures (i.e. 1st trimester BMI and early GWG and may suggest that higher exposure to phthalate parent compounds during early pregnancy might impact overweight/obese women to a greater extent than their normal weight counterparts.
The association between urinary phthalate metabolite concentrations and obesity has been mainly investigated in animal studies, with positive associations seen for DEHP metabolites and weight gain (Hao et al. 2013; Hao et al. 2012; Schmidt et al. 2012; Biemann et al. 2014). While several epidemiological studies have focused on childhood phthalate exposure and obesity measures (Deierlein et al. 2016; Trasande et al. 2013; Boas et al. 2010), few observational studies have investigated phthalates and BMI/weight gain in adult women. Of the studies that have evaluated this question, one study found positive associations with MnBP, and MBzP (Song et al. 2014), while another found inverse cross-sectional associations with MEHP (Hatch et al. 2008). Recent studies have investigated phthalate exposure during pregnancy as it relates with the risk of major pregnancy complications such as gestational diabetes and preterm birth (James-Todd et al. 2016; Huang et al. 2016; Philips et al. 2016; Ferguson et al. 2014; Ferguson et al. 2015; Werner et al. 2015). While studies have evaluated pregnancy phthalate metabolite concentrations, to our knowledge, this is among the first study to focus on maternal obesity measures as a primary outcome.
Interestingly, our results indicate that all observed associations are not constant over the entire outcome distributions, but are consistently stronger in the right tails. These differences suggest that women in the higher tails of the outcome distribution (i.e. with higher baseline values of obesity measures) may be more susceptible to pregnancy exposure with respect to certain phthalate metabolites. To detect such shifts occurring in the tails of the outcome distributions, we used quantile regression, which is becoming increasingly popular in the epidemiological literature (Beyerlein 2014; Marrie et al. 2009). Compared to alternative methods to evaluate tails of the distributions, such as categorization or dichotomization of continuous outcome, quantile regression provides important additional properties and modeling advantages, which include not requiring any distributional assumption, as well as not being sensitive to the presence of outliers. In addition, the use of quantile regression has been recommended to fully capture the effects of environmental exposures (Bind et al. 2015; Bind et al. 2016).
The biological mechanism through which phthalates may affect obesity is complex (Heindel, Newbold and Schug 2015). Phthalates are known to induce the expression of PPAR gamma, modifying the expression of its target genes and the differentiation of these cells into adipocytes (Feige et al. 2007; Hurst and Waxman 2003; Biemann et al. 2012). The association between PPAR polymorphisms and obesity (Yao et al. 2015; Redonnet et al 2002) may partly explain the larger associations observed in the right tail of first trimester BMI and early GWG, as the over-expression of PPAR gamma target genes induced by high levels of pregnancy phthalate exposure would more likely occur and have larger effects in those women with higher obesity levels before pregnancy. Of particular interest, we observed a positive association between BMI and ΣDEHP, but an inverse association when looking at ΣDEHP and GWG. DEHP metabolites are high molecular weight phthalates, which may have different effects from lower molecular weight metabolites such as MEP.
Most associations were non-linear. While different studies have presented suggestive non-linear associations when evaluating phthalate metabolites, these have generally been documented through categorical analyses. Categorizing continuous exposures presents some limitations and often relies on specific assumptions (e.g. step dose-response function, difficulty in detecting within-category differences, subjective choice of cut-off) (Greenland 1995b; Royston et al.2006). In the present study, we used spline transformations, a common tool to model a continuous covariate relaxing the assumption of linearity and also avoiding the loss of information implied by the use of categories (Greenland 1995a). In agreement with some other studies we observed non-monotonic dose responses, suggesting that EDCs at low-dose exposures might have a greater impact on adverse outcomes as compared to mid-to-higher dose exposures.
This study has several limitations. First, our study used single spot urines to measure early pregnancy urinary phthalate metabolite concentrations with respect to early maternal adiposity measures. However, single spot urines may not accurately classify long-term exposures, particularly pre-conception exposures. Moreover, both exposure and outcome measures are possibly subject to measurement error. Second, by only focusing on early pregnancy phthalate exposure and early markers of maternal adiposity, our study does not provide information on the potential effects of late-pregnancy exposures. Nevertheless, changes occurring during the first trimester of pregnancy are generally regarded as important predictors of maternal measures (Fontaine et al. 2012; Ferraro et al. 2015; Hedderson et al. 2010; Hedderson et al. 2014; Carreno et al. 2012). Third, the analysis of early pregnancy phthalate exposure and BMI (assessed at the first prenatal visit) was cross-sectional. As such, there is the possibility of reverse causation. Specifically, women of higher BMI may also have a higher surface area, potentially leading to greater exposure to topical products containing phthalates. In fact, obese women had significantly higher concentrations of MEP compared to normal BMI women. Fourth, other factors that could influence early GWG and BMI, such as hyperemesis, nausea, and vomiting, or dietary factors, were not available in our data. In particular, future studies should further investigate the role of dietary factors in the observed associations and whether low and high molecular weight metabolites have different effects on maternal obesity. Food is a principal source of certain phthalate metabolites, especially DEHP metabolites. Primary sources of DEHP metabolites such as diet, and of non-DEHP phthalate metabolites such as personal care products, should be investigated as potential confounders as well as effect modifiers of the association. Finally, we only included women with term births, in whom lower concentrations of specific phthalate metabolites, especially DEHP, have been observed (Ferguson et al. 2014). While this allows for the ability to evaluate risk factors independent of preterm birth status, it could also attenuate the associations as women with the highest DEHP exposure would be more likely to be excluded. We were also underpowered to examine possible effect modifications by race/ethnicity and age, and by first trimester BMI in the GWG model.
Despite these limitations, this study had several strengths. First, to our knowledge, the present study is among the first to evaluate the association between urinary phthalate metabolite concentrations and maternal obesity. Second, the use of quantile regression, combined with the application of flexible statistical techniques such as splines, allowed for the evaluation of urinary phthalate metabolite concentrations and distribution shifts in first trimester BMI and early GWG, outcomes that are important to adverse pregnancy outcomes (Marrie et al. 2009). By focusing on the entire distribution of the outcomes we observed associations that would not have been detectable using classical regression methods. Third, this study evaluated two body size measures commonly used as indicators of maternal obesity, which could provide insight into obesity status, as well as weight gain in pregnancy. Fourth, we were able to evaluate associations between a number of phthalates and a prospective measure of GWG in early pregnancy.
Conclusion
In a pregnancy cohort, we found higher early-pregnancy urinary concentrations of MEP, MBzP, MCPP, and ΣDEHP to be cross-sectionally associated with a right shift in the distribution of first trimester BMI in a non-linear fashion. Suggestive prospective associations between MEP (positive), DEHP metabolites (inverse), and early GWG distribution were also detected. Future studies should further investigate the role of baseline BMI in the prospective association between non-DEHP phthalate exposures and maternal obesity (e.g. as an effect modifier), as well as explore the potential mechanism involved in the obesogenic effects of certain phthalates in pregnant women. If replicated, the present study may suggest that reducing exposure to certain non-DEHP phthalate compounds could be particularly beneficial to women with higher BMI and GWG, with implications for potential reductions in related adverse maternal and child health outcomes. Even the inverse association between DEHP and GWG may suggest important effects that these EDCs could have on weight gain during the potentially sensitive time period of pregnancy.
Acknowledgments
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12HD051959), the National Institute of Environmental Health Sciences (R01ES018872, P30ES017885, R01ES026166), and the National Heart Lung and Blood Institute (K24RR018613). Funding support for KF was provided by the Intramural Research Program of NIEHS, NIH (ZIAES103321). The authors have no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest.
References
- Beyerlein A. Quantile Regression—opportunities and Challenges from a User’s Perspective. American Journal of Epidemiology. 2014;180(3):330–331. doi: 10.1093/aje/kwu178. [DOI] [PubMed] [Google Scholar]
- Biemann R, Fischer B, Santos AN. Adipogenic Effects of a Combination of the Endocrine-Disrupting Compounds Bisphenol A, Diethylhexylphthalate, and Tributyltin. Obesity Facts. 2014;7(1):48–56. doi: 10.1159/000358913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemann R, Santos AN, Santos AN, Riemann D, Knelangen J, Bluher M, et al. Endocrine Disrupting Chemicals Affect the Adipogenic Differentiation of Mesenchymal Stem Cells in Distinct Ontogenetic Windows. Biochemical and Biophysical Research Communications. 2012;417(2):747–752. doi: 10.1016/j.bbrc.2011.12.028. [DOI] [PubMed] [Google Scholar]
- Bind MA, Coull BA, Baccarelli AA, Tarantini L, Cantone L, Vokonas P, Schwartz J. Distributional Changes in Gene-Specific Methylation Associated with Temperature. Environmental Research. 2016;150:38–46. doi: 10.1016/j.envres.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bind MA, Coull BA, Peters A, Baccarelli AA, Tarantini L, Cantone L, et al. Beyond the Mean: Quantile Regression to Explore the Association of Air Pollution with Gene-Specific Methylation in the Normative Aging Study. Environmental Health Perspectives. 2015;123(8):759–765. doi: 10.1289/ehp.1307824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebæk NE, Hegedus L, Hilsted L, Juul A, Main KM. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, growth. Environ Health Perspect. 2010 Oct 1;118(10):1458–64. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Hutcheon JA, Platt RW, Himes KP, Simhan HN, Abrams B. Should gestational weight gain recommendations be tailored by maternal characteristics? American journal of epidemiology. 2011 Jun 1; doi: 10.1093/aje/kwr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. Interpretation of Urine Results Used to Assess Chemical Exposure with Emphasis on Creatinine Adjustments: A Review. American Industrial Hygiene Association Journal. 1993;54(10):615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental health perspectives. 2008 Jan 1;:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno CA, Clifton RG, Hauth JC, Myatt L, Roberts JM, Spong CY, Varner MW, Thorp JM, Jr, Mercer BM, Peaceman AM, Ramin SM. Excessive early gestational weight gain and risk of gestational diabetes mellitus in nulliparous women. Obstetrics and gynecology. 2012 Jun;119(6):1227. doi: 10.1097/AOG.0b013e318256cf1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (Centers for Disease Control and Prevention) Third National Report on Human Exposure to Environmental Chemicals 2005 [Google Scholar]
- Chu SY, Callaghan WM, Kim SY, Kim SY, Schmid CH, Lau J, et al. Maternal Obesity and Risk of Gestational Diabetes Mellitus. Diabetes Care. 2007;30(8):2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- Chu SY, Kim SY, Lau J, Schmid CH, Dietz PM, Callaghan WM, et al. Maternal Obesity and Risk of Stillbirth: A Metaanalysis. American Journal of Obstetrics and Gynecology. 2007;197(3):223–228. doi: 10.1016/j.ajog.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Deierlein AL, Wolff MS, Pajak A, Pinney SM, Windham GC, Galvez MP, Silva MJ, Calafat AM, Kushi LH, Biro FM, Teitelbaum SL. Longitudinal associations of phthalate exposures during childhood and body size measurements in young girls. Epidemiology. 2016 Jul 1;27(4):492–9. doi: 10.1097/EDE.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvergne B, Feige JN, Casals-Casas C. PPAR-Mediated Activity of Phthalates: A Link to the Obesity Epidemic? Molecular and Cellular Endocrinology. 2009;304(1–2):43–48. doi: 10.1016/j.mce.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Durrleman S, Simon R. Flexible Regression Models with Cubic Splines. Statistics in Medicine. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Rossi D, Zoete V, Métivier R, Tudor C, et al. The Endocrine Disruptor Monoethyl-Hexyl-Phthalate Is a Selective Peroxisome Proliferator-Activated Receptor Gamma Modulator That Promotes Adipogenesis. The Journal of Biological Chemistry. 2007;282(26):19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Phthalate Metabolites and Bisphenol-A in Association with Circulating Angiogenic Biomarkers across Pregnancy. Placenta. 2015;36(6):699–703. doi: 10.1016/j.placenta.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Yi-An Ko, Mukherjee B, Meeker JD. Variability in Urinary Phthalate Metabolite Levels across Pregnancy and Sensitive Windows of Exposure for the Risk of Preterm Birth. Environment International. 2014;70:118–124. doi: 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, J Meeker JD. Environmental Phthalate Exposure and Preterm Birth. JAMA Pediatrics. 2014;168(1):61–67. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro ZM, Contador F, Tawfiq A, Adamo KB, Gaudet L. Gestational Weight Gain and Medical Outcomes of Pregnancy. Obstetric Medicine. 2015;8(3):133–137. doi: 10.1177/1753495X15591320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine PL, Hellerstedt WL, Dayman CE, Wall MM, Sherwood NE. Evaluating BMISpecific Trimester Weight Gain Recommendations: Differences between Black and White Women. Journal of Midwifery & Women’s Health. 2012;57(4):327–335. doi: 10.1111/j.1542-2011.2011.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DJ. Effects of Maternal Obesity on Fetal Growth and Body Composition: Implications for Programming and Future Health. Seminars in Fetal & Neonatal Medicine. 2010;15(2):113–118. doi: 10.1016/j.siny.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Geraci M, Bottai M. Linear Quantile Mixed Models. Statistics and Computing. 2014;24(3):461–479. [Google Scholar]
- Geraci M. Linear Quantile Mixed Models: The Lqmm Package for Laplace Quantile Regression. [accessed October 10, 2016];Journal of Statistical Software. 2016 https://www.jstatsoft.org/article/view/v057i13.
- Gilmore LA, Klempel-Donchenko M, Redman LM. Pregnancy as a Window to Future Health: Excessive Gestational Weight Gain and Obesity. Seminars in Perinatology. 2015;39(4):296–303. doi: 10.1053/j.semperi.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S. Dose-Response and Trend Analysis in Epidemiology: Alternatives to Categorical Analysis. Epidemiology. 1995a;6(4):356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- Greenland S. Problems in the Average-Risk Interpretation of Categorical Dose-Response Analyses. Epidemiology. 1995b;6(5):563–565. doi: 10.1097/00001648-199509000-00020. [DOI] [PubMed] [Google Scholar]
- Gunderson EP. Childbearing and Obesity in Women: Weight Before, During, and after Pregnancy. Obstetrics and Gynecology Clinics of North America. 2009;36(2):317–332. doi: 10.1016/j.ogc.2009.04.001. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obesity reviews. 2008;9(2):140–150. doi: 10.1111/j.1467-789X.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- Hao C, Cheng X, Guo J, Xia H, Ma X. Perinatal Exposure to Diethyl-Hexyl-Phthalate Induces Obesity in Mice. Frontiers in Bioscience (Elite Edition) 2013;5:725–733. doi: 10.2741/e653. [DOI] [PubMed] [Google Scholar]
- Hao C, Cheng X, Xia H, Ma X. The Endocrine Disruptor Mono-(2-Ethylhexyl) Phthalate Promotes Adipocyte Differentiation and Induces Obesity in Mice. Bioscience Reports. 2012;32(6):619–629. doi: 10.1042/BSR20120042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, et al. Association of Urinary Phthalate Metabolite Concentrations with Body Mass Index and Waist Circumference: A Cross-Sectional Study of NHANES Data, 1999–2002. Environmental Health: A Global Access Science Source. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Calafat AM. Phthalates and Human Health. Occupational and Environmental Medicine. 2005;62(11):806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstetrics and gynecology. 2010 Mar;115(3):597. doi: 10.1097/AOG.0b013e3181cfce4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedderson MM, Xu F, Darbinian JA, Quesenberry CP, Sridhar S, Kim C, Gunderson EP, Ferrara A. Prepregnancy SHBG concentrations and risk for subsequently developing gestational diabetes mellitus. Diabetes care. 2014 May 1;37(5):1296–303. doi: 10.2337/dc13-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Newbold R, Schug TT. Endocrine Disruptors and Obesity. Nature Reviews. Endocrinology. 2015;11(11):653–661. doi: 10.1038/nrendo.2015.163. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene. 1990;5(1):46–51. [Google Scholar]
- Huang PC, Tsai CH, Liang WY, Li SS, Huang HB, Kuo PL. Early Phthalates Exposure in Pregnant Women Is Associated with Alteration of Thyroid Hormones. PloS One. 2016;11(7):e0159398. doi: 10.1371/journal.pone.0159398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by Environmental Phthalate Monoesters. Toxicological Sciences: An Official Journal of the Society of Toxicology. 2003;74(2):297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines 2009 [Google Scholar]
- James-Todd TM, Meeker JD, Huang T, Hauser R, Ferguson KK, Rich-Edwards JW, et al. Pregnancy Urinary Phthalate Metabolite Concentrations and Gestational Diabetes Risk Factors. Environment International. 2016;96:118–126. doi: 10.1016/j.envint.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard H, Nohr EA, Rasmussen KM, Stovring H, Sørensen TI, Lewis CE, et al. Maternal Prepregnancy Waist Circumference and BMI in Relation to Gestational Weight Gain and Breastfeeding Behavior: The CARDIA Study. The American Journal of Clinical Nutrition. 2015;102(2):393–401. doi: 10.3945/ajcn.114.099184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenker R, Hallock KF. Quantile Regression. Journal of Economic Perspectives. 2001;15(4):143–156. [Google Scholar]
- Krukowski RA, West DS, DiCarlo M, Shankar K, Cleves MA, Saylors ME, et al. Are Early First Trimester Weights Valid Proxies for Preconception Weight? BMC Pregnancy and Childbirth. 2016;16(1):357. doi: 10.1186/s12884-016-1159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leddy MA, Power ML, Schulkin J. The Impact of Maternal Obesity on Maternal and Fetal Health. Reviews in Obstetrics & Gynecology. 2008;1(4):170–178. [PMC free article] [PubMed] [Google Scholar]
- Marrie RA, Dawson NV, Garland A. Quantile Regression and Restricted Cubic Splines Are Useful for Exploring Relationships between Continuous Variables. Journal of Clinical Epidemiology. 2009;62(5):511–517. e1. doi: 10.1016/j.jclinepi.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Orsini N, Greenland S. A Procedure to Tabulate and Plot Results after Flexible Modeling of a Quantitative Covariate. Stata Journal. 2011;11(1):1. [Google Scholar]
- Philips EM, Jaddoe VWV, Trasande L. Reproductive Toxicology. (Elmsford, N.Y.): 2016. Effects of Early Exposure to Phthalates and Bisphenols on Cardiometabolic Outcomes in Pregnancy and Childhood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redonnet A, Bonilla S, Noel-Suberville C, Pallet V, Dabadie H, Gin H, Higueret P. Relationship between peroxisome proliferator-activated receptor gamma and retinoic acid receptor alpha gene expression in obese human adipose tissue. International journal of obesity. 2002 Jul 1;26(7):920. doi: 10.1038/sj.ijo.0802025. [DOI] [PubMed] [Google Scholar]
- Royston P, Altman DG, Sauerbrei W. Dichotomizing Continuous Predictors in Multiple Regression: A Bad Idea. Stat Med. 2006;25(1):127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- Salihu HM, De La Cruz C, Rahman S, August EM. Does Maternal Obesity Cause Preeclampsia? A Systematic Review of the Evidence. Minerva Ginecologica. 2012;64(4):259–280. [PubMed] [Google Scholar]
- Schmidt JS, Schaedlich K, Fiandanese N, Pocar P, Fischer B. Effects of di(2-Ethylhexyl) Phthalate (DEHP) on Female Fertility and Adipogenesis in C3H/N Mice. Environmental Health Perspectives. 2012;120(8):1123–1129. doi: 10.1289/ehp.1104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, et al. Maternal obesity and pregnancy outcome: a study of 287 213 pregnancies in London. International journal of obesity. 2001;25(8):1175. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- Song Y, Hauser R, Hu FB, Franke AA, Liu S, Sun Q. Urinary Concentrations of Bisphenol A and Phthalate Metabolites and Weight Change: A Prospective Investigation in US Women. International Journal of Obesity (2005) 2014;38(12):1532–1537. doi: 10.1038/ijo.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar SB, Darbinian J, Ehrlich SF, Markman MA, Gunderson EP, Ferrara A, et al. Maternal Gestational Weight Gain and Offspring Risk for Childhood Overweight or Obesity. American Journal of Obstetrics and Gynecology. 2014;211(3):259, e1–8. doi: 10.1016/j.ajog.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of Urinary Phthalate Metabolites are Associated with Increased Waist Circumference and Insulin Resistance in Adult U.S. Males. Environ Health Perspect. 2007;115:876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang?Péronard JL, Andersen HR, Jensen TK, Heitmann BL. Endocrine?disrupting chemicals and obesity development in humans: A review. obesity reviews. 2011 Aug 1;12(8):622–36. doi: 10.1111/j.1467-789X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environmental health perspectives. 2012 Jun 1;120(6):779. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Attina TM, Sathyanarayana S, Spanier AJ, Blustein J. Race/ethnicity-specific associations of urinary phthalates with childhood body mass in a nationally representative sample. Environmental Health Perspectives (Online) 2013 Apr 1;121(4):501. doi: 10.1289/ehp.1205526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, et al. Obesity, Obstetric Complications and Cesarean Delivery Rate--a Population-Based Screening Study. American Journal of Obstetrics and Gynecology. 2004;190(4):1091–1097. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP. The Association between Maternal Urinary Phthalate Concentrations and Blood Pressure in Pregnancy: The HOME Study. Environmental Health: A Global Access Science Source. 2015;14:75. doi: 10.1186/s12940-015-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YS, Li L, Jin YL, Chen Y, He LP. Association between PPAR-γ2 Pro12Ala Polymorphism and Obesity: A Meta-Analysis. Molecular Biology Reports. 2015;42(6):1029–1038. doi: 10.1007/s11033-014-3838-6. [DOI] [PubMed] [Google Scholar]