SUMMARY
Oxytocin is a hormone with various actions. Oxytocin-containing parvocellular neurons project to the brainstem and spinal cord. Oxytocin release from these neurons suppresses nociception of inflammatory pain, the molecular mechanism of which remains unclear. Here, we report that the noxious stimulus receptor, TRPV1, is an ionotropic oxytocin receptor. Oxytocin elicits TRPV1 activity in native and heterologous expression systems, irrespective of the presence of the classical oxytocin receptor. In TRPV1 knockout mice, DRG neurons exhibit reduced oxytocin sensitivity relative to controls, and oxytocin injections significantly attenuate capsaicin-induced nociception in in vivo experiments. Furthermore, oxytocin potentiates TRPV1 in planar lipid bilayers, supporting a direct agonistic action. Molecular modeling and simulation experiments provide insight into oxytocin-TRPV1 interactions, which resemble RTX/DkTx. Together our findings suggest the existence of endogenous regulatory pathways that modulate nociception via direct action of oxytocin on TRPV1, implying its analgesic effect via channel desensitization.
Keywords: Transient Receptor Potential Vanilloid 1 – TRPV1, Oxytocin, Nociception, Oxytocin Receptor
eTOC Blurb
Oxytocin is known to suppress painful stimuli of inflammatory origin. Nersesyan et al. now find that oxytocin attenuates pain via the pain-sensing receptor TRPV1.
INTRODUCTION
Oxytocin is a versatile hormone with various physiological actions. The wide range of oxytocin-elicited effects include stimulation of uterine smooth muscle contraction to facilitate labor, induction of lactation, and expression of complex social and bonding behaviors related to reproduction and care for offspring (Carter, 2014). Oxytocin is produced in the brain in the hypothalamic paraventricular (PVN), supraoptic (SON), and intermediate accessory nuclei (Sawchenko and Swanson, 1983). Magnocellular neurons release oxytocin into the blood stream (Scharrer, 1940) and also innervate various regions of the brain, including the nucleus accumbens (Ross et al., 2009) and the central nucleus of the amygdala (Knobloch et al., 2012). The brainstem and various regions of the spinal cord (Swanson, 1983) are innervated by oxytocin-containing parvocellular neurons, and the secreted neuropeptide modulates nociception via targeting C-type fibers in the dorsal root ganglion (DRG) (Juif and Poisbeau, 2013).
Many activities of oxytocin are accomplished through the canonical oxytocin receptor (OXTR), a G-protein-coupled receptor that is present in neural tissues, uterus, and breast (Gimpl and Fahrenholz, 2001). However, not all oxytocin-provoked actions have been linked to its cognate receptor. For example, oxytocin is present at distinctly high levels in brain regions that lack OXTR (Gimpl and Fahrenholz, 2001). Among oxytocin actions is the regulation of Ca2+ fluxes. These responses can involve (i) OXTR mediated signaling via Gαq/11, that stimulate release of Ca2+ from internal stores, and (ii) the influx of extracellular Ca2+. The latter mechanism is dependent upon the extracellular Ca2+ concentrations, which suggested the participation of voltage-gated or ligand-coupled channels (Sanborn et al., 1998).
Here, we aimed to identify the receptor responsible for an ionotropic oxytocin effect. Our data indicate that oxytocin directly targets the pain receptor, TRPV1, a non-selective Ca2+-permeable cation channel. TRPV1 is expressed in afferent somatosensory neurons and along the spinal cord. The original discovery of the TRPV1 channel as a heat and capsaicin receptor validated the existence of nociceptive neural circuitry that mitigates awareness of noxious stimulus exposure (Caterina et al., 1997; Julius, 2013).
In this work, using native and heterologous expression systems, we find that oxytocin-elicited Ca2+ responses are mediated through TRPV1 channels. This activity is consistently observed in TRPV1 expressing cells, irrespective of the presence of OXTR. Furthermore, DRG neurons isolated from the TRPV1-deficient mice displayed reduced oxytocin sensitivity in comparison to the wild type and other controls. Planar lipid bilayer experiments confirmed that oxytocin can directly activate the TRPV1 channel. Molecular modeling indicated that, similarly to resiniferatoxin (RTX) and the tarantula Double Knot Toxin (DkTx), oxytocin interacts with TRPV1 at the extracellular domain leading to channel gating. Together, our results provide solid evidence that oxytocin is a direct TRPV1 agonist. These results imply that oxytocin-induced suppression of nociception might be achieved directly through potentiation of the pain receptor TRPV1, causing analgesia upon the desensitization of the channel.
RESULTS
Oxytocin elicits Ca2+ influx through TRPV1 channels
To assess the mechanism of oxytocin-induced anti-nociception, we tested whether it exerts any action on pain-sensing TRPV1. To test the effects of oxytocin on TRPV1 channels, initial experiments used a heterologous expression system, Human Embryonic Kidney (HEK)-293 cells, to stably express the channel. Oxytocin concentrations used were in the low micromolar range, which matches some physiological conditions. In the neurosecretory granules of the posterior pituitary, oxytocin is present at high concentrations (>100 μM) and is complexed to its carrier protein neurophysin (Gimpl and Fahrenholz, 2001; Ludwig and Leng, 2006). Upon secretion, oxytocin concentrations can range from tens to hundreds micromolar (Gimpl and Fahrenholz, 2001; Ludwig and Leng, 2006). In contrast, in cerebrospinal fluid, oxytocin is present in low picomolar concentrations, while plasma oxytocin can reach hundreds picomolar (Kendrick et al., 1991; Winslow et al., 2003).
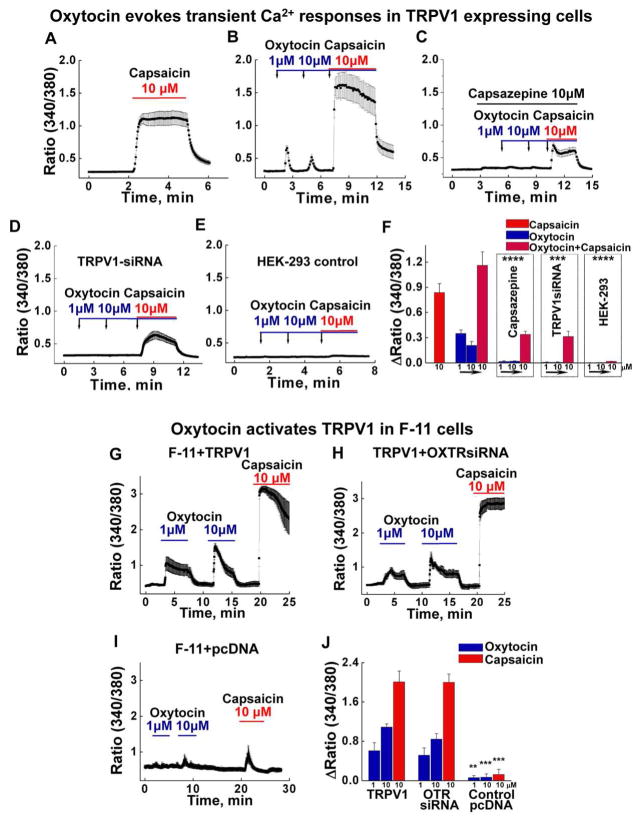
Under these experimental conditions, oxytocin induced rapid Ca2+ influx in cells stably expressing TRPV1 (Figure 1A, B), albeit modest in comparison to capsaicin. This effect was not observed in control HEK-293 cells, used as a control (Figure 1B, E). The observed TRPV1 responses displayed a rapid desensitization, which was noticeable during the individual and sequential oxytocin applications (Figure 1B). Co-application of capsaicin with oxytocin elicited a stronger Ca2+ response, indicating a cumulative effect of both compounds (Figure 1A, B). However, the co-application also exhibited a more rapid “run-down” in comparison to the control (Figure 1B and A). Importantly, the oxytocin-evoked activity was abolished with (i) the pre-application of TRPV1 antagonist capsazepine or (ii) upon introduction of TRPV1-specific siRNA (Figure 1C, D, and S1). The Ca2+-imaging performed on the HEK-293 cells is summarized in Figure 1F.
Figure 1. Oxytocin evokes TRPV1 activity in HEK-293 cells stably expressing the channel.
A, Control experiment with10 μM capsaicin-induced TRPV1 activation (n exp. = 6, n cells = 81). B, Subsequent applications of 1 and 10 μM of oxytocin, followed by 10 μM capsaicin elicited transient Ca2+ responses with oxytocin, but cumulative effect in the presence of both compounds (n exp. = 9, n cells = 96). C, Capsazepine significantly inhibited both the oxytocin and oxytocin/capsaicin-induced responses (n exp. = 6, n cells = 84, p values in comparison to the control in panel B are the following: oxytocin 1 μM p = 1.6E-7, 10 μM p = 9.3E-7, oxytocin/capsaicin p = 4.25E-7). D, Co-expression of TRPV1-specific siRNA (0.75 μg), and GFP (0.25 μg), significantly inhibited all the responses (n exp. = 6, n cells = 66, p values in comparison to the control in panel B are the following: oxytocin 1 μM p = 1.24E-4, 10 μM p = 3.58E-4, oxytocin/capsaicin p = 8.19E-4). E, Control HEK-293 cells non-expressing TRPV1 channels show no responses to any of the agonists (n exp. = 4, n cells = 100, p values in comparison to the control in panel B are the following: oxytocin 1 μM p = 1.04E-11, 10 μM p = 1.16E-9, oxytocin/capsaicin p = 1.88E-13). F, The summary presents the mean under all the conditions. All error bars stand for ±s.e.m.
Oxytocin elicits TRPV1 responses in F-11 neuronal cells transiently expressing the channel.
G, Oxytocin and capsaicin-induced responses on F-11 cells with transiently expressed TRPV1 (0.5 μg) and GFP (0.15 μg) (n exp. = 6, n cells = 71). H, Oxytocin and capsaicin-induced responses on F-11 cells with transiently expressed TRPV1 (0.5 μg), GFP (0.15 μg), and OXTRsiRNA (0.4 μg) (n exp. = 6, n cells = 60). I, Oxytocin and capsaicin-induced responses on F-11 cells with transiently expressed empty pcDNA vector (0.5 μg) and GFP (0.15 μg) (n exp. = 6, n cells = 48, p values in comparison to TRPV1/GFP expressing cells are the following: oxytocin 1 μM p = 0.009, 10 μM p = 2.1E-6, oxytocin/capsaicin p = 2.5E-9). J, The summary presents the mean of Ca2+ responses obtained under all the conditions. All error bars stand for ±s.e.m.
Interesting, pre-activation of TRPV1 using capsaicin provoked stronger responses to oxytocin (Figure S2). Under these experimental conditions, oxytocin elicited TRPV1 responses beginning at low picomolar concentrations (Figure S2). Similar channel behavior was observed when oxytocin was applied after potentiating TRPV1 with a low-pH pulse (pH 4.5 or 5.5, data not shown). These results indicated that oxytocin potency to TRPV1 increases when the channel is present in its active/open state. It is tempting to speculate that these conditions are physiologically relevant, reflecting increased oxytocin affinity to the channel following TRPV1 potentiation by inflammatory stimuli.
To determine whether oxytocin-evoked Ca2+ responses are mediated through the OXTR or vasopressin 1a receptors, we tested these responses using G protein-coupled receptor inhibitors. Cells treated with the PLC inhibitor U73122, its negative control U73343 (following the protocol described previously (Horowitz et al., 2005; Thyagarajan et al., 2009)), or the oxytocin receptor (OXTR) inhibitor atosiban displayed no differences in the oxytocin-induced responses (Figure S3).
Next, we tested TRPV1 responses using neuronal F-11 cells. Transiently expressing TRPV1 F-11 cells promptly responded to oxytocin (Figure 1). Unlike HEK-293, TRPV1-F-11 cells demonstrated more sustained activity and less desensitization (Figure 1G, H). Since the neuronal F-11 cells could also endogenously express the canonical oxytocin receptor, we again tested whether or not OXTR mediates the oxytocin-elicited Ca2+ signals using OXTR-specific siRNA. No difference occurred in the magnitude or kinetics of oxytocin-induced responses (Figure 1G, H, S1), whereas F-11 cells transfected with control pcDNA vector were essentially unresponsive to all the stimuli (Figure 1I). The summary of Ca2+ influx in F-11 cells is presented in Figure 1J.
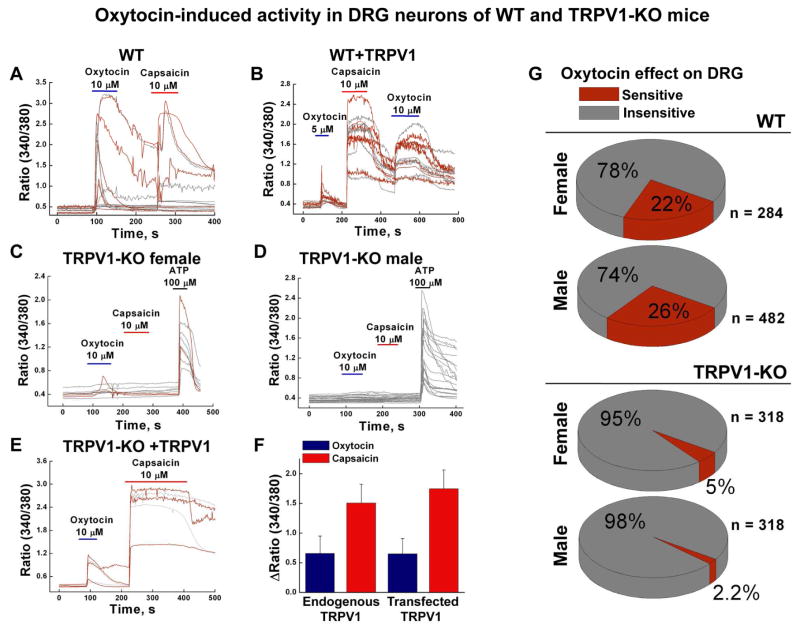
We next examined oxytocin-evoked activity using dorsal root ganglion (DRG) neurons isolated from the wild type (WT) and TRPV1 knockout (TRPV1-KO, referred to TRPV1−/−) mice (Figure 2 A–G). In the majority of experiments, DRG neurons that were sensitive to oxytocin were also sensitized by capsaicin. A few exceptions were noted when the initial oxytocin-elicited responses were of higher magnitude and were further desensitized to subsequent applications of capsaicin or oxytocin (Figure 2A). DRG neurons transiently expressing TRPV1 channels were sensitive to both agonists (Figure 2B).
Figure 2. Oxytocin sensitivity is diminished in DRG neurons isolated from TRPV1-deficient mice.
A, Oxytocin- and capsaicin-elicited Ca2+ responses in DRG neurons obtained from the WT mice (nexp. = 33). B, Oxytocin- and capsaicin-elicited Ca2+ responses in the WT DRG neurons transiently expressing TRPV1 (3 μg) together with GFP (0.5 μg), (nexp. = 6). C and D panels show oxytocin- and capsaicin-sensitivity of DRG neurons obtained from TRPV1-KO female (nexp.= 21) and male (nexp. = 21) mice, respectively. E, Oxytocin- and capsaicin-induced responses obtained from the TRPV1-KO (−/−) DRG neurons transiently expressing TRPV1 (0.375 μg) along with GFP (0.5 μg), (nexp. = 6). F, Summary of oxytocin- and capsaicin-induced responses obtained from the WT and WT with transiently expressed TRPV1 channels. G, Pie graphs present distribution of the oxytocin-sensitive and oxytocin-insensitive DRG neurons isolated from the WT-female (ncells = 284), WT-male (ncells = 482), TRPV1-KO-female (ncells = 318), and TRPV1-KO-male mice (ncells = 318). All error bars stand for ±s.e.m.
On average, we found no essential differences between genders. In WT female mice, oxytocin-sensitive DRGs comprised ~22%, and male neurons ~26% (Figure 2G). In contrast, only a few DRG neurons isolated from TRPV1-KO animals exhibited oxytocin-sensitivity, comprising ~5% responsive neurons obtained from female mice and ~2.2% from the males (Figure 2C, D, G). We hypothesized that these residual Ca2+ signals were mediated through the other oxytocin receptors present in DRG neurons, including OXTR, vasopressin 1a receptors (V1aR), or some other receptors. Notably, these residual Ca2+ responses were kinetically slower compared to the WT mice, which could reflect G protein mediated Ca2+ release from intracellular stores (Figure 2A, C). Rescuing TRPV1 expression in TRPV1-KO DRG neurons by transiently expressing the channel recovered their responses to both agonists (Figure 2E).
Alternatively, we tested whether other pain receptors such as TRPA1 or TRPM8 are implicated in oxytocin-elicited activity. However, DRG neurons from TRPA1-KO and TRPM8-KO mice displayed similar sensitivity to oxytocin as those obtained from the WT (Figure S4).
Together, these results suggest that the DRG sensitivity to oxytocin is in large part mediated through TRPV1 channels.
Oxytocin evokes TRPV1 channel activation in whole-cell patch clamp
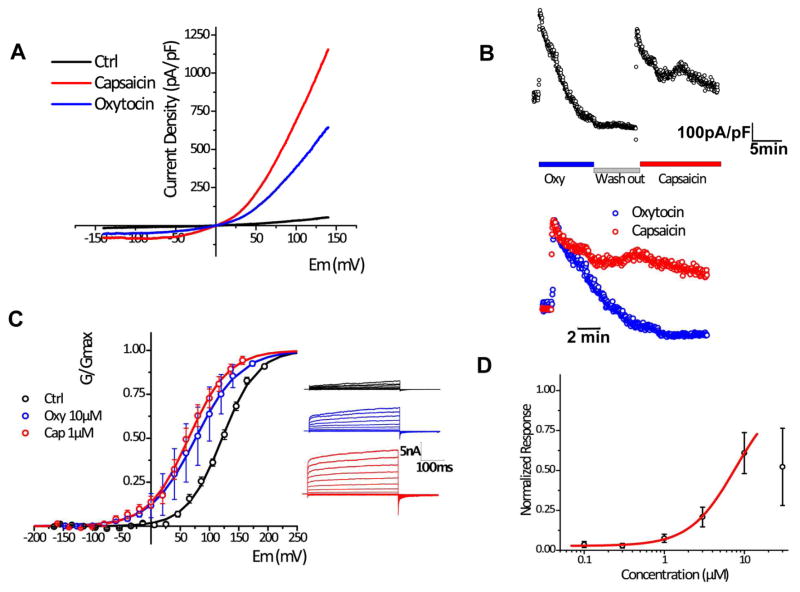
Next, we assessed the effect of oxytocin on TRPV1 activity using an electrophysiological approach. We found that oxytocin application elicited current potentiation in whole-cell patch clamp recordings performed on HEK-293 cells expressing TRPV1 (Figure 3 A–D). The channel current showed an outward rectification similar to that of capsaicin-evoked currents. However, the maximal response against oxytocin (10 μM) was modest compared to that of capsaicin (1 μM) (Figure 3A). The conductance versus voltage relation was similar under both conditions (GV curves), suggesting that differences in the current density are associated with differences in the desensitization induced by these agonists. In fact, oxytocin strongly desensitized the channel while the observed desensitization for capsaicin was only partial (Figure 3B). Desensitization observed in the context of the intact cells impeded our ability to obtain the full dose response curve, as concentrations over 30 μM rapidly inactivated the elicited current (Figure 3D).
Figure 3. Oxytocin potentiates TRPV1 currents in whole-cell patch clamp recordings.
A, Current-voltage (I–V) relations obtained from voltage-ramps from −140 to +140mV in presence of vehicle (black), 10 μM Oxytocin (blue) or 1 μM Capsaicin (red). B, (Upper) time course of representative 10 μM Oxytocin and 1 μM Capsaicin responses; (bottom) the overlay of normalized responses showing differences in the desensitization kinetics. C, (left) G–V curves were obtained by plotting peak tail currents at −100 mV in response to voltage-activated steady state currents from −160 to +160 mV in 20 mV increments: Vm values for control 121.66 ± 14.29 (n = 12), for oxytocin 64.22 ± 8.96 (n = 12), and for capsaicin 76.25 ± 21.03 (n = 4); charge z for control 0.85 ± 0.07 (n = 12), for oxytocin 0.75 ± 0.06 (n = 12), and for capsaicin 0.67 ± 0.108 (n = 4); (Right) Representative traces obtained in transfected cells subjected to voltage steps in control conditions (black), or in presence of 10 μM Oxytocin (blue) or 1 μM Capsaicin (red). D, Dose-response curve fitted to concentrations <30 μM. EC50=7.74 ±8.5, h=1.5 ±0.6.
Oxytocin directly activates TRPV1 in planar lipid bilayers
The use of cellular systems to decipher direct agonistic actions of hormones can be complicated by the presence of various signaling pathways that give rise to intermediate effects. Thus, to validate direct actions of oxytocin on TRPV1, we used planar lipid bilayers to characterize oxytocin-induced channel activity.
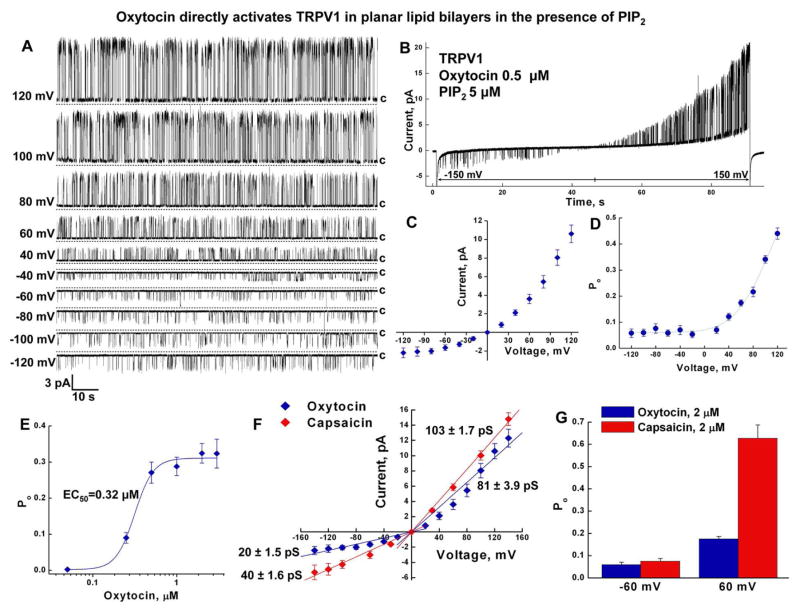
For these experiments, the TRPV1 protein was purified and incorporated into bilayers as described previously (Lukacs et al., 2013; Sun and Zakharian, 2015). Protein purity was validated using liquid chromatography mass spectrometry (LC-MS/MS). Distinct TRPV1 peptides were identified at high abundance (Figure S5 and Table S1). After incorporating TRPV1 in the bilayers, we found that oxytocin activates the channel directly, similar to the other known agonists, heat (Sun and Zakharian, 2015) and capsaicin (Lukacs et al., 2013). Likewise, TRPV1 opening required the presence of phosphatidylinositol-4,5-bisphosphate (PIP2) (Figure 4, and S6).
Figure 4. Oxytocin directly activates TRPV1 in planar lipid bilayers in the presence of PIP2.
A, Representative single channel current traces of oxytocin-induced (0.5 μM) TRPV1 obtained in the presence of PIP2 (5 μM), voltages are indicated on the left. B, Representative ramp recording obtained at −150 to +150 mV. C, D, Graphs demonstrate current voltage relationship (C) and voltage dependence (D) of oxytocin-induced TRPV1, n = 12. E, Dose response of oxytocin-TRPV1 activation, EC50 = 0.316 ± 0.02 μM. F, Current voltage relationship of oxytocin- and capsaicin-induced TRPV1 channels. G, Capsaicin induces higher open probability of TRPV1 outward channel activity, as indicated by open probability differences of oxytocin (2 μM) and capsaicin (2 μM) elicited openings at −60 and +60 mV.
Oxytocin-induced TRPV1 opening demonstrated distinct current rectification and profound voltage dependence. These features were evident in the single channel conductance and the open probability (Figure 4A–D). The channel exhibited the outward current with the mean slope conductance of ~81 pS, while the inward current was ~20 pS (Figure 4A, C). In addition, oxytocin-evoked TRPV1 openings showed strong voltage dependence, with marked low open probability obtained at negative voltages and an exponentially increasing open probability at positive potentials (Figure 4A, B, D). In planar lipid bilayers, oxytocin activated TRPV1 with an EC50 of ~0.32 μM (Figure 4E).
The comparison of oxytocin-evoked TRPV1 activity to that of capsaicin demonstrated that both conductance values of capsaicin-induced inward and outward currents were greater than that of oxytocin by about 20 and 22 pS, respectively (Figure 4F). The open probability of the channel was similar at negative voltages with both agonists, whereas the open probability was greater at positive voltages for capsaicin-evoked TRPV1 activity (Figure 4G).
In summary, the planar lipid bilayer experiments confirmed that oxytocin is a direct TRPV1 agonist, and this activity can occur independent of any other intermediate signaling pathway.
Identification of an oxytocin-binding site
To investigate the interaction between oxytocin and the TRPV1 ion channel at the atomic level, computational studies were performed on the TRPV1 homotetramer. To that end, oxytocin molecules were docked onto the pore loop region of structures representing the open and closed states of the channel, and MD simulations of the resulting complexes were performed (see methods). Negligible binding to the closed state was observed.
In contrast, the open state (denoted simulation Pep1) interacted with a single oxytocin molecule via its extracellular pore loop. The interaction persisted for approximately 200 ns, with the most prevalent binding pose (clustered using a 3Å RMSD criterion) observed for 80 ns. In a further simulation (denoted simulation Pep2) with four oxytocin molecules initiated from this binding pose, interaction with a single oxytocin peptide persisted for the duration of the 200 ns trajectory, with a stable binding pose observed for 150 ns (Figure 5 A–E).
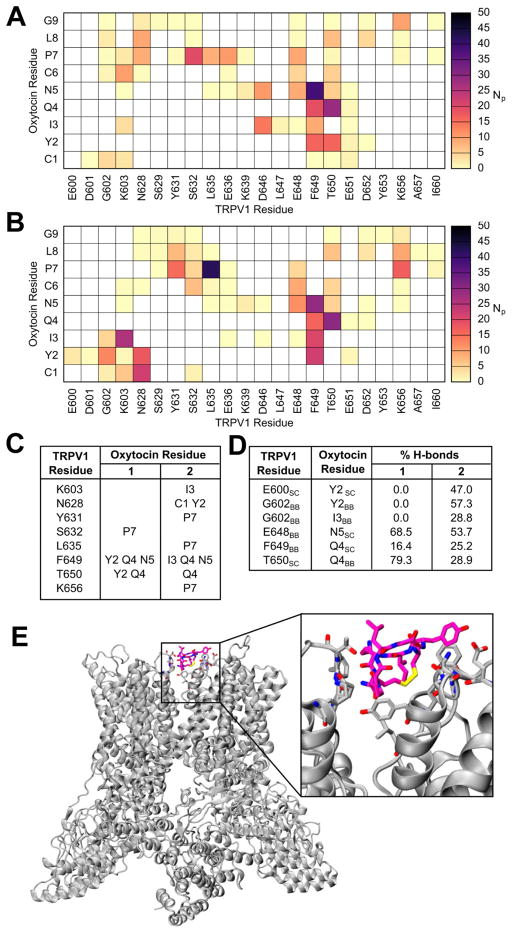
Figure 5. Modeling and molecular dynamics stimulations display oxytocin-TRPV1 interacting site.
Contact map displaying the number of interacting atom pairs (NP) between TRPV1 and oxytocin in simulation Pep1 (A) and Pep2 (B), averaged over the simulation length, using a 4Å cut-off. The prominent interactions (defined as NP >15) are summarized in panel C. The occurrence of hydrogen bonds is displayed in D. In E new cartoon representation of the TRPV1 system, in complex with oxytocin is illustrated; oxytocin and interacting TRPV1 residues are displayed in licorice representation.
The identified binding site lies on the external surface of the transmembrane domain, in the interfacial region between two adjacent subunits. The predicted binding site is illustrated in Figure 5E. The extension of this computational analysis and further discussion of the influence on the TRPV1 activation state are presented in the supplemental information (SI discussion and Figure S7).
Oxytocin interaction with TRPV1: similarity with other agonists
Next, we compared the TRPV1 activation of oxytocin to that of the tarantula Double Knot Toxin (DkTx). The latter has been studied extensively by both experimental and computational approaches (Bae et al., 2016; Bohlen et al., 2010). Mutations I599A, F649A, A657P and F659A inhibit channel activation by DkTx, whereas V595A and T695A attenuate channel activation to a lesser extent (Bohlen et al., 2010). Conversely, Y631A enhanced activation by DkTx (Bohlen et al., 2010). The atomic model of TRPV1 in complex with DkTx, determined by Bae et al, advocated that some of the residues, Y631, F649, A657, and T650, were in direct contact with the toxin (Bae et al., 2016). Remarkably, such interactions are replicated in the model of oxytocin-bound TRPV1, thus suggesting that oxytocin may activate TRPV1 via a similar pathway as DkTx. Furthermore, our simulations revealed direct engagement between oxytocin and A649 in both apo and holo TRPV1 structures, identifying an explicit pathway by which oxytocin could modulate the behavior of this region, and potentiate TRPV1.
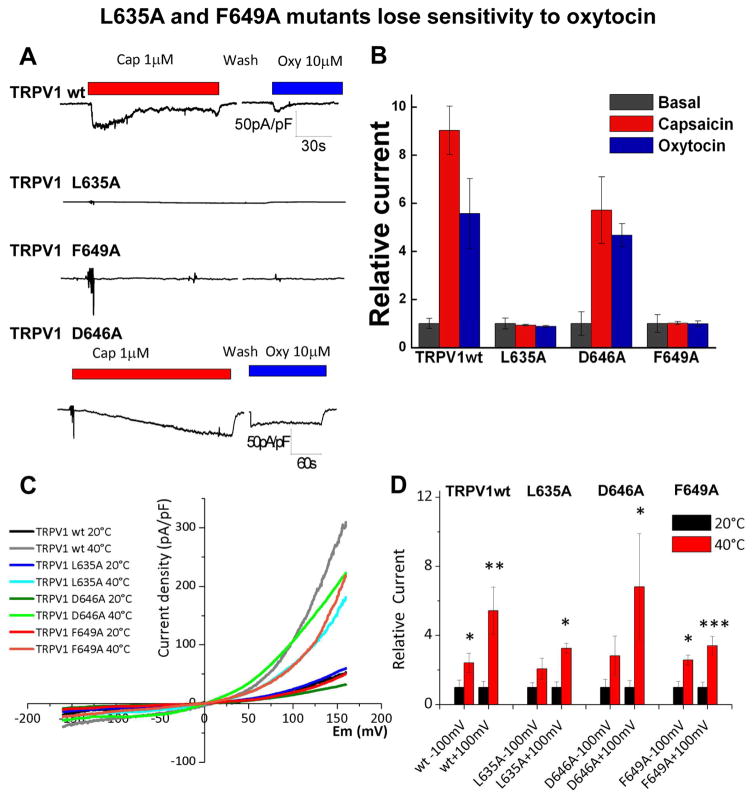
To validate the TRPV1-oxytocin binding site derived from computational analysis, we altered the most promising target residues by mutagenesis and tested their function. Patch clamp recordings showed that L635A and F649A TRPV1 mutants completely lose their sensitivity to oxytocin. Surprisingly, they were also insensitive to capsaicin (Figure 6A, B) but displayed potentiation by heat (Figure 6C, D, and S8). Conversely, D646A mutation did not alter sensitivity of TRPV1 to oxytocin (Figure 6A). Together, these experiments are consistent with the hypothesis that oxytocin interacts with TRPV1 via L635 and F649.
Figure 6. L635A and F649A mutants lose sensitivity to oxytocin.
Patch clamp recording show the current obtained from the wild type TRPV1 and mutants. Panel A demonstrates representative whole cell currents recorded by using gap-free protocol with a holding potential at −70 mV. Traces are shown in current densities (pA/pF). Panel B presents the summary of the relative to control current of TRPV1 wt, L635A, D646A, and F649A constructs obtained upon application of 1 μM capsaicin and 10 μM oxytocin, n = 5 for each construct. Panels C and D display heat-induced potentiation of TRPV1 and the mutants upon exposure to 40 °C pulses.
Oxytocin reduces capsaicin-induced aversive behavior in mice
In conclusion, our findings suggest that oxytocin suppresses nociception via activation and desensitization of the polymodal TRPV1 channels in nociceptors. To test this hypothesis, we performed in vivo experiments to quantify nocifensive behavior in mice subsequent to oxytocin and capsaicin application. Oxytocin alone did not provoke any noticeable aversive response in mice. However, co-application of oxytocin with capsaicin significantly induced analgesia over the capsaicin-evoked pain stimuli (Figure 7). Together, these results support a direct link between oxytocin and antinociception via TRPV1 activity modulation.
Figure 7. Oxytocin reduces capsaicin-induced aversive behavior in mice.
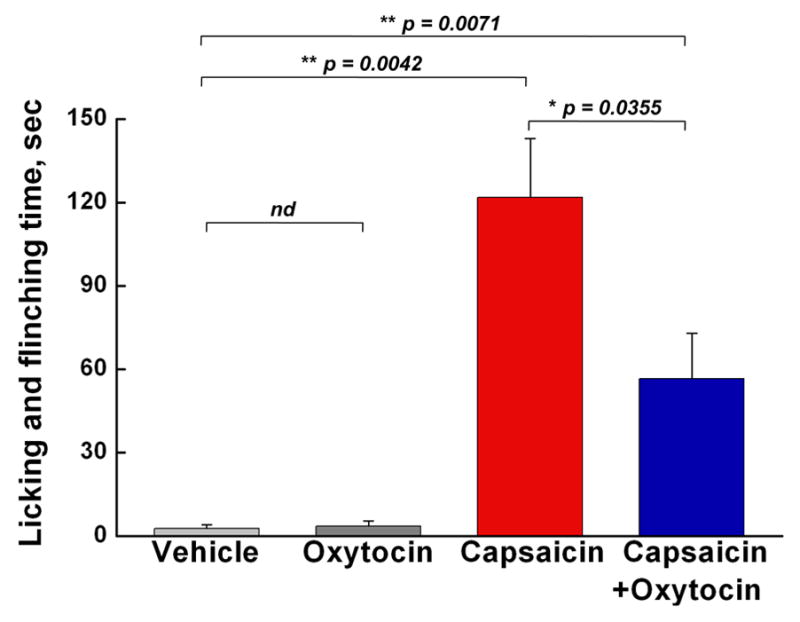
Mouse hindpaws were intracutaneously injected under light sevoflurane anesthesia with the vehicle (n = 3), oxytocin (4 μg/paw, n = 3), capsaicin (1.6 μg/paw, n = 6), or a combination of capsaicin and oxytocin (4 μg/paw, n = 6) and nocifensive behavior was quantified as the time spent licking and flinching. Columns represent the amount of time (sec) animals spent licking and flinching the injected paw during the 30 min observation period following the injection (mean ± SEM, p value according to Student’s t-test).
DISCUSSION
Physiological implication of oxytocin in pain modulation via TRPV1
Oxytocin is a highly abundant neurohypophysial peptide. Its prevalent site of expression is localized to the magnocellular neurons of the hypothalamic paraventricular and supraoptic nuclei (see for review (Gimpl and Fahrenholz, 2001)). Magnocellular oxytocin neurons of these nuclei innervate the forebrain and intensely release the hormone to the systemic circulation from the posterior pituitary in response to a variety of stimuli. The parvocellular oxytocin neurons further project to the brainstem and spinal cord where the hormone was shown to modulate inflammatory pain processing (Eliava et al., 2016). Furthermore, this neuropeptide is also synthesized in peripheral tissues, such as uterus, placenta, amnion, corpus luteum, testis, and heart (Gimpl and Fahrenholz, 2001).
Physiologic roles of oxytocin range from various modalities of neuroendocrine reflexes to the complex social and bonding behaviors related to reproduction and care of offspring. It is now well established that oxytocin facilitates reproduction in all vertebrates (Gimpl and Fahrenholz, 2001). A well-known action of the hormone is the stimulation of milk ejection that was confirmed on the model of oxytocin-deficient mice. The role of oxytocin in parturition is also established, although it involves more complex regulatory mechanisms. Some evidence indicates that from the paraventricular nuclei of the hypothalamus oxytocin can reach the central amygdala, where the hormone modulates emotion-related centers of the amygdala and brain stem (Stoop, 2012). Many of oxytocin actions have been well characterized by the function of its classical receptor OXTR, class I G protein-coupled receptor that is differentially expressed in various tissues (Gimpl and Fahrenholz, 2001). The expression of the oxytocin receptor in uterus and hypothalamus is strongly correlative with the presence of sex hormones, particularly estrogens. The estrogen receptors were shown to play a role of transcription factors of OXTR and are required for its expression mediated by estrogen binding (Young et al., 1998). Some of oxytocin actions, however, imply OXTR-independent mechanisms.
Among its many activities, oxytocin is known to be involved in nociception and pain responses. These involve both peripheral (Juif and Poisbeau, 2013) and central components (Gonzalez-Hernandez et al., 2014; Juif et al., 2013). Importantly, oxytocin neurons project directly into the C-type fibers of DRG (Juif and Poisbeau, 2013). In an animal model, oxytocin release suppresses nociception and induces analgesia by specifically affecting inflammatory pain pathways (Eliava et al., 2016). The oxytocin neuronal circuitry appears to occur via a dual process, by (a) releasing oxytocin from axons onto sensory spinal cord neurons and inhibiting their activity, and (b) indirectly stimulating oxytocin release from supraoptic neurons into the periphery (Eliava et al., 2016). Thus, oxytocin modulates pain by reaching the spinal cord through fast neuronal projections, and slower peripheral pathways (Eliava et al., 2016). Likewise, analgesic actions of oxytocin have been documented for migraine patients treated with intranasal oxytocin administration (Wang et al., 2013). One aspect of oxytocin-promoted pain regulation involves endogenous analgesia (Gonzalez-Hernandez et al., 2014). However, it is still debatable as to whether this effect is mediated through the OXTR, V1aR, or some other receptor, as the majority of these studies have been based upon pharmacological approaches using either OXTR or V1aR antagonists (for review see (Gonzalez-Hernandez et al., 2014)).
Also of importance is the effect of oxytocin on thermoregulation. Similarly to capsaicin (Kobayashi et al., 1998), peripheral administration of oxytocin induces strong hypothermia (Hicks et al., 2014). Hence, in addition to analgesia, our finding suggests a possible molecular mechanism for oxytocin-induced hypothermia.
The results reported here suggest that several oxytocin effects may be mediated by interaction with the TRPV1 pain receptor. TRPV1 is a well-investigated receptor in the pain pathway. The original discovery of TRPV1’s role in nociception inspired the development of novel classes of analgesic drugs (Caterina et al., 1997; Julius, 2013). Furthermore, TRPV1 serves as a molecular marker for the nociceptive neuronal circuitry that enables sensation of noxious heat, protons, neurogenic inflammation, and thermal hyperalgesia (Julius, 2013). Profound TRPV1 expression in C-fibers contributes to the signaling associated with acute and chronic pain conditions and could be implicated in various pathophysiological conditions such as arthritis, pancreatitis, migraine headache, and others.
Indeed, the expression of TRPV1 in trigeminal afferent neurons of dura mater suggested a plausible linkage of the channel actions to migraine (Huang et al., 2012; Shimizu et al., 2007). More evidence for this relationship was suggested by the effect of capsaicin on dilation of dural vessels (Akerman et al., 2003), and induction of trigeminal ganglion neuronal activity (Iwashita et al., 2013). However, the mechanisms by which TRPV1 contributes to initiating or propagating the neuronal signaling related to migraine are poorly understood (for review see (Dussor et al., 2014)). Our discovery of oxytocin as an endogenous agonist for the TRPV1 channel may shed more light onto the regulatory mechanisms that underlie physiology and pathology related to headaches and chronic migraine development.
The ability of oxytocin to modulate nociception is most likely achieved by activation of TRPV1 followed by its marked desensitization. Such mechanism is evident in nocifensive mice behavior, where oxytocin attenuates capsaicin-evoked nociceptive response (Figure 7). This is consistent with the fact that therapeutic targeting of TRPV1 has been primarily advanced via exogenous agonists that lead to prompt channel desensitization. These treatment options are traditionally based on capsaicin-containing remedies, such as capsaicin cream or high concentration capsaicin patches (Dussor et al., 2014). Intranasal capsaicin administration has also been tested in treating migraine; similar to another TRPV1 agonist civanide, capsaicin reduced the frequency of cluster headache attacks (Diamond et al., 2000; Fusco et al., 2003). In contrast, attempts to identify effective and safe TRPV1 antagonists have been stymied by numerous complications, including dysregulation of internal body temperature. In this light, identifying the endogenous mechanisms of TRPV1 potentiation and desensitization by oxytocin along with the patterns of expression and their functional relationship within the neuronal network offers a valuable asset to pain regulation.
METHODS
For detailed methods, see the Supplementary Information (SI).
Cell culture
HEK-293 cells were maintained in minimal essential medium (MEM) as previously described (Zakharian et al., 2009). F-11 cells were cultured in DMEM/F12 medium as previously described (Zakharian et al., 2009). Mouse DRG neurons were cultured in primary neuron basal medium (PNBM), (Lonza Inc., Allendale, NJ).
Whole-cell patch clamp recordings
The whole-cell patch clamp experiments were performed as previously described (Yudin et al., 2011; Zakharian et al., 2009). HEK-293T cells were cotransfected with rTRPV1 and eGFP with Lipofectamine 2000 according to manufacturer’s protocol. Whole-cell patch clamp recordings were performed 48–72h post transfection at room temperature.
Intracellular Ca2+ measurements
Ca2+ measurements were performed as previously described (Zakharian et al., 2009).
Preparation of the TRPV1 protein from HEK cells
TRPV1 protein isolation was performed as previously described (Sun and Zakharian, 2015; Zakharian et al., 2010). TRPV1 was purified by immunoprecipitation with anti-Myc-IgG conjugated to A/G protein magnetic beads (Pierce, Thermo Scientific, Milwaukee, WI). For the planar lipid bilayer experiments, the protein was eluted with Myc-peptide (150 μg/ml).
Planar lipid bilayer measurements
Planar lipid bilayer measurements and temperature studies were performed as previously described (Sun and Zakharian, 2015; Zakharian, 2013; Zakharian et al., 2010).
Animal studies
Age-matched male and female wild type and TRPV1−/−, TRPA−/−, and TRPM8−/− mice were purchased from Jackson Laboratory (Maine, USA).
Supplementary Material
Highlights.
TRPV1 acts as an ionotropic oxytocin receptor in cells
Oxytocin potentiates TRPV1 in cells and lipid bilayers
Oxytocin interacts with TRPV1 at the extracellular pore loop region
Oxytocin attenuates capsaicin-induced nociception via TRPV1 desensitization
Acknowledgments
We deeply appreciate the commitment of Dr. Liskin Swint-Kruse and her immense help in critical reading of the manuscript and fruitful suggestions.
We are grateful to Angela Daniels for her exceptional help with the animal experiments.
This work was initially supported by the National Institutes of Health through grant R01GM098052 to E.Z. CD and VO acknowledge ARCHER, the UK National Supercomputing Service (http://www.archer.ac.uk), the Hartree Centre, and the National Service for Computational Chemistry Software for providing computational resources. VO is supported by the Biotechnology and Biological Sciences Research Council (BBSRC) and Pfizer Neusentis. S.B. is thankful to MiNICAD, a Millennium Nucleus supported by Iniciativa Científica Milenio, Ministry of Economy, Development and Tourism, Chile. SB was supported by Anillo Científico ACT-140.
Footnotes
Author contributions:
Y.N., L.D., D.C.-B., V.O., R.K., X.S., C.C., A.C., and E.Z. conducted experiments. Y.N., L.D., and E.Z. conducted imaging experiments. D.C.-B. conducted electrophysiological experiments, using whole-cell patch clamp. L.D., Y.N., T.D., and X.S. conducted electrophysiological experiments, using lipid bilayers. V.O. performed computational studies and analysis. L.D. conducted Western blotting experiments. R.K. performed mouse behavioral experiments. E.Z. conducted experiments on protein isolation for mass spectrometry, and A.C. performed mass spectrometry experiments and their analysis. B.C. helped with the mouse tissue-isolation experiments. C.C. performed molecular biology studies. K.K.V. contributed reagents and material. Y.N., L.D., D.C.-B., V.O., R.K., X.S., A.C., K.Z., C.D., S.B., and E.Z. analyzed data. K.Z. designed and supervised mouse behavioral experiments. C.D. designed and supervised computational studies and simulation analysis. S.B. designed and supervised patch-clamp experiments. Y.N. and E.Z. designed the study, and E.Z. supervised the experiments. E.Z. wrote the manuscript, with contribution of Y.N. C.D. K.Z. and S.B. All authors critically read and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akerman S, Kaube H, Goadsby PJ. Vanilloid type 1 receptors (VR1) ontrigeminal sensory nerve fibres play a minor role in neurogenic dural vasodilatation, and are involved in capsaicin-induced dural dilation. Br J Pharmacol. 2003;140:718–724. doi: 10.1038/sj.bjp.0705486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae C, Anselmi C, Kalia J, Jara-Oseguera A, Schwieters CD, Krepkiy D, Won Lee C, Kim EH, Kim JI, Faraldo-Gómez JD, et al. Structural insights into the mechanism of activation of the TRPV1 channel by a membrane-bound tarantula toxin. eLife. 2016;5:e11273. doi: 10.7554/eLife.11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell. 2010;141:834–845. doi: 10.1016/j.cell.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Oxytocin pathways and the evolution of human behavior. Annual review of psychology. 2014;65:17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Diamond S, Freitag F, Phillips SB, Bernstein JE, Saper JR. Intranasal civamide for the acute treatment of migraine headache. Cephalalgia: an international journal of headache. 2000;20:597–602. doi: 10.1046/j.1468-2982.2000.00088.x. [DOI] [PubMed] [Google Scholar]
- Dussor G, Yan J, Xie JY, Ossipov MH, Dodick DW, Porreca F. Targeting TRP channels for novel migraine therapeutics. ACS chemical neuroscience. 2014;5:1085–1096. doi: 10.1021/cn500083e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva Gouveia M, Tang Y, Ciobanu AC, Triana del Rio R, Roth LC, Althammer F, et al. A New Population of Parvocellular Oxytocin Neurons Controlling Magnocellular Neuron Activity and Inflammatory Pain Processing. Neuron. 2016;89:1291–1304. doi: 10.1016/j.neuron.2016.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco BM, Barzoi G, Agro F. Repeated intranasal capsaicin applications to treat chronic migraine. Br J Anaesth. 2003;90:812. doi: 10.1093/bja/aeg572. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez A, Rojas-Piloni G, Condes-Lara M. Oxytocin and analgesia: future trends. Trends in pharmacological sciences. 2014;35:549–551. doi: 10.1016/j.tips.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol. 2005;126:243–262. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Li S, Dhaka A, Story GM, Cao YQ. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Molecular pain. 2012;8:66. doi: 10.1186/1744-8069-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita T, Shimizu T, Shibata M, Toriumi H, Ebine T, Funakubo M, Suzuki N. Activation of extracellular signal-regulated kinase in the trigeminal ganglion following both treatment of the dura mater with capsaicin and cortical spreading depression. Neuroscience research. 2013;77:110–119. doi: 10.1016/j.neures.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Juif PE, Breton JD, Rajalu M, Charlet A, Goumon Y, Poisbeau P. Long-lasting spinal oxytocin analgesia is ensured by the stimulation of allopregnanolone synthesis which potentiates GABA(A) receptor-mediated synaptic inhibition. J Neurosci. 2013;33:16617–16626. doi: 10.1523/JNEUROSCI.3084-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juif PE, Poisbeau P. Neurohormonal effects of oxytocin and vasopressin receptor agonists on spinal pain processing in male rats. Pain. 2013;154:1449–1456. doi: 10.1016/j.pain.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Julius D. TRP channels and pain. Annual review of cell and developmental biology. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Hinton MR, Goode JA. Cerebrospinal fluid and plasma concentrations of oxytocin and vasopressin during parturition and vaginocervical stimulation in the sheep. Brain Res Bull. 1991;26:803–807. doi: 10.1016/0361-9230(91)90178-m. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature reviews Neuroscience. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Lukacs V, Rives JM, Sun X, Zakharian E, Rohacs T. Promiscuous activation of transient receptor potential vanilloid 1 (TRPV1) channels by negatively charged intracellular lipids: the key role of endogenous phosphoinositides in maintaining channel activity. J Biol Chem. 2013;288:35003–35013. doi: 10.1074/jbc.M113.520288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn BM, Dodge K, Monga M, Qian A, Wang W, Yue C. Molecular mechanisms regulating the effects of oxytocin on myometrial intracellular calcium. Adv Exp Med Biol. 1998;449:277–286. doi: 10.1007/978-1-4615-4871-3_35. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol. 1983;218:121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- Scharrer E, Scharrer B. Secretory cells within the hypothalamus. Res Publ Assoc Res Nerv Ment Dis. 1940;20:170–194. [Google Scholar]
- Shimizu T, Toriumi H, Sato H, Shibata M, Nagata E, Gotoh K, Suzuki N. Distribution and origin of TRPV1 receptor-containing nerve fibers in the dura mater of rat. Brain Res. 2007;1173:84–91. doi: 10.1016/j.brainres.2007.07.068. [DOI] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Sun X, Zakharian E. Regulation of the temperature-dependent activation of transient receptor potential vanilloid 1 (TRPV1) by phospholipids in planar lipid bilayers. J Biol Chem. 2015;290:4741–4747. doi: 10.1074/jbc.M114.611459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Thyagarajan B, Benn BS, Christakos S, Rohacs T. Phospholipase C-mediated regulation of transient receptor potential vanilloid 6 channels: implications in active intestinal Ca2+ transport. Mol Pharmacol. 2009;75:608–616. doi: 10.1124/mol.108.052449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL, Yuan Y, Yang J, Wang CH, Pan YJ, Lu L, Wu YQ, Wang DX, Lv LX, Li RR, et al. The interaction between the oxytocin and pain modulation in headache patients. Neuropeptides. 2013;47:93–97. doi: 10.1016/j.npep.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Donaldson R, Rissman EF. Estrogen receptor alpha is essential for induction of oxytocin receptor by estrogen. Neuroreport. 1998;9:933–936. doi: 10.1097/00001756-199803300-00031. [DOI] [PubMed] [Google Scholar]
- Yudin Y, Lukacs V, Cao C, Rohacs T. Decrease in phosphatidylinositol 4,5- bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels. The Journal of physiology. 2011;589:6007–6027. doi: 10.1113/jphysiol.2011.220228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharian E. Recording of ion channel activity in planar lipid bilayer experiments. Methods in molecular biology. 2013;998:109–118. doi: 10.1007/978-1-62703-351-0_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharian E, Cao C, Rohacs T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J Neurosci. 2010;30:12526–12534. doi: 10.1523/JNEUROSCI.3189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharian E, Thyagarajan B, French RJ, Pavlov E, Rohacs T. Inorganic polyphosphate modulates TRPM8 channels. PLoS One. 2009;4:e5404. doi: 10.1371/journal.pone.0005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.