Synopsis
Recent advances in genomic profiling and sequencing of melanoma have provided new insights into the development of the basis for molecular biology to more accurately subgroup melanoma patients. The development of novel mutation-targeted and immunomodulation therapy as a major component of precision oncology, have revolutionized the management and outcome of patients with metastatic melanoma. Positron Emission Tomography (PET) imaging plays an important role in noninvasively assessing the tumor biologic behavior, complementary to traditional tissue biomarkers, with the goal of guiding individualized treatment, and assessing response to therapy. This review summarizes the recent genomic discoveries in melanoma in the era of targeted therapy, and their implications for functional PET imaging
Keywords: melanoma, molecular imaging, targeted therapy, immunotherapy, biomarker, precision oncology
Introduction
Melanoma of the skin represents the sixth most common cancer in the United States, and its incidence has continued to rise over the past few decades 1. Although many patients with early-stage melanoma have favorable outcome following complete surgical resection, treatment continue to be challenged for patients with advanced metastatic disease 2. Furthermore, accurate restaging and follow up therapy assessment becomes crucial for the appropriate management of patients with melanoma, since about 50 to 80% of melanoma patients with loco-regional disease and nearly all patients with distant metastases will experience tumor recurrence after treatment 3.
Recent interest in understanding the biology and pathogenesis of melanoma has led to the discovery of vital signaling pathways and the development of mutation-driven therapy, immunotherapy and targeted therapies, which have revolutionized the clinical history of this disease by dramatically improved the outcomes of patients with metastatic disease. Immunotherapy is mostly based on immune checkpoint inhibitors targeting CTLA4, and more recently PD1/PDL1 interaction 4. Targeted therapies with MAPK pathway kinase inhibitors (KIs) have also been developed due to the discovery that BRAF and NRAS mutations, which are among the major oncogenic drivers of melanoma proliferation and survival 5. As new therapies become available, there is a need to identify biomarkers to guide patient selection, and monitor treatment response. The use of molecular imaging using Positron Emission Tomography (PET) offers unique insights in the field of oncology, helping early tumor detection, characterization, real time monitoring of treatment response, and identification of tumor recurrence. This review summarizes the recent genomic and therapeutic discoveries in melanoma and their implications for imaging.
Molecular genetics and immunomodulation in melanoma
The concept of targeting genomic alterations has experienced significant success in oncology, especially in patients with melanoma. Oncogenic targets are genes that are mutated and/or expressed in tumor tissue, contributing to tumor growth and dissemination, and have the potential to be pharmacologically targetable 6. Investigators at The Cancer Genome Atlas (TCGA) 7 Research Network identified molecular subtypes of melanoma that could potentially guide clinicians to identify the more aggressive tumors, and the ones more likely to respond to certain therapies. More than 90% of melanoma tumors harbor activating mutations in oncogenes within the mitogen-activated protein kinase (MAPK) pathway, which plays a major role in coordinating the balance between melanocyte differentiation and proliferation 8. Approximately 50% of patients with melanoma are displaying mutations in the harbor v-raf murine sarcoma viral oncogene homolog B1 (BRAF) V600 mutations 4, about 20% in the NARS and up to 14% in the NF1 gene, and 3–5% harbor an activating KIT mutation 9, 10. These findings have led to the development of BRAF and MEK inhibitors whose applications in the clinic has shown unprecedented survival responses. BRAF blockade therapy with the FDA- approved vemurafenib or dabrafenib, and also in combination of MEK inhibitor, trametinib, have successfully lead to an improved progression free survival (PFS) and overall survival (OS) in patients with the typical BRAF V600-mutant, compared with conventional chemotherapy 11–13. However, even when treated with the combination, most patients develop mechanisms of drug resistance, without achieving a complete tumor regression. Furthermore, the remaining subset of 50-60% of patients with advanced melanoma without a BRAF-V600 mutation, also called BRAF wild-type (BRAF WT), do not benefit from treatment with BRAF inhibitors.
Along with the development of BRAF and MEK inhibitors, immunotherapy have made an important step forward, and specifically the immune checkpoint inhibitors. Immunomodulatory strategies use monoclonal antibodies to target key regulators of T-lymphocyte activation and thereby inhibit immunotolerance toward tumor cells 14. Ipilimumab, a fully human monoclonal antibody that targets cytotoxic T-lymphocyte antigen 4 (anti-CTLA-4), was approved by the FDA in 2011 for first and second line treatment of patients with unresectable or advanced melanoma 15, resulting in significant OS benefit 16 [Figures 1–3]. The successes of ipilimumab were quickly followed by trials targeting the immune inhibitor interaction between programmed cell death protein 1 (PD-1), found on T-cells, and its ligand, (PD-L1), found on tumor cells [Figure 4]; pembrolizumab and nivolumab were the first anti-PD-1 pathway family of check point inhibitors to gain accelerated approval from FDA for the treatment of ipilimumab-refractory melanoma [Figure 5], which demonstrated objective anti-tumor response rates in up to 45% of treatment-naïve patients with advanced melanoma, with durable long-term survival 17, 18. Furthermore, combined checkpoint blockade with nivolumab plus ipilimumab has demonstrated even greater objective response rates and survival than anti–PD-1 and anti–CTLA-4 monotherapy alone strategies, albeit with significantly more adverse events 19–21.
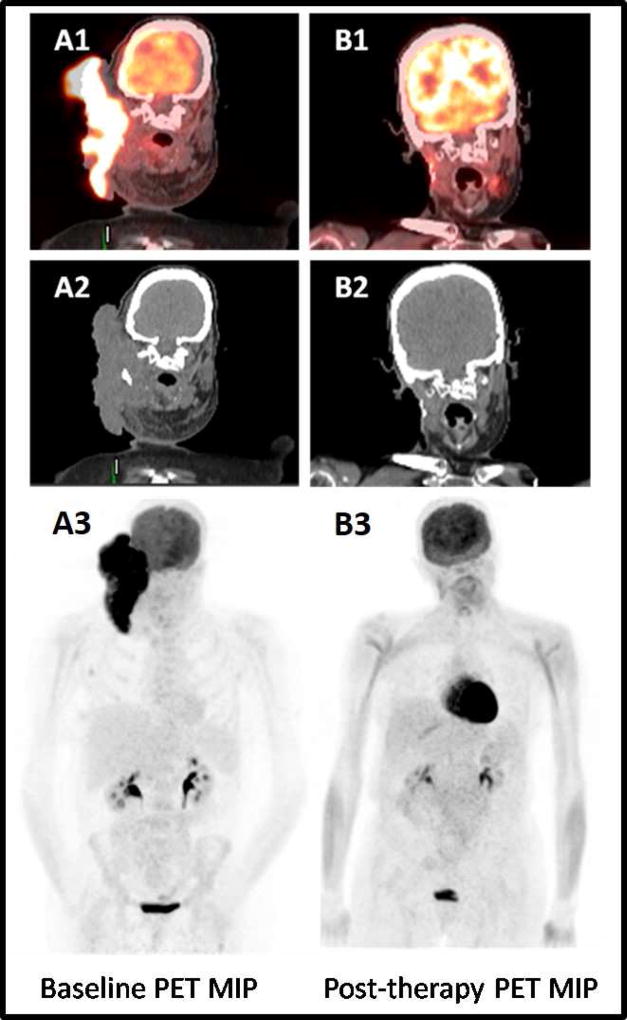
Figure 1. Complete metabolic tumor response after combined radiation and Ipilimumab treatment in a patient with locally advanced metastatic melanoma.
72-year-old woman diagnosed with locally advanced metastatic malignant melanoma, who underwent combined therapy with radiation plus an immune checkpoint inhibitor, ipilimumab. Baseline fused coronal PET/CT (A1), CT (A2) and MIP (A3) images demonstrate a large intense FDG-avid right neck mass. After radiotherapy and 4 cycles of ipilimumab, the fused coronal PET/CT (B1), CT (B2) and MIP PET (B3) images show complete metabolic response.
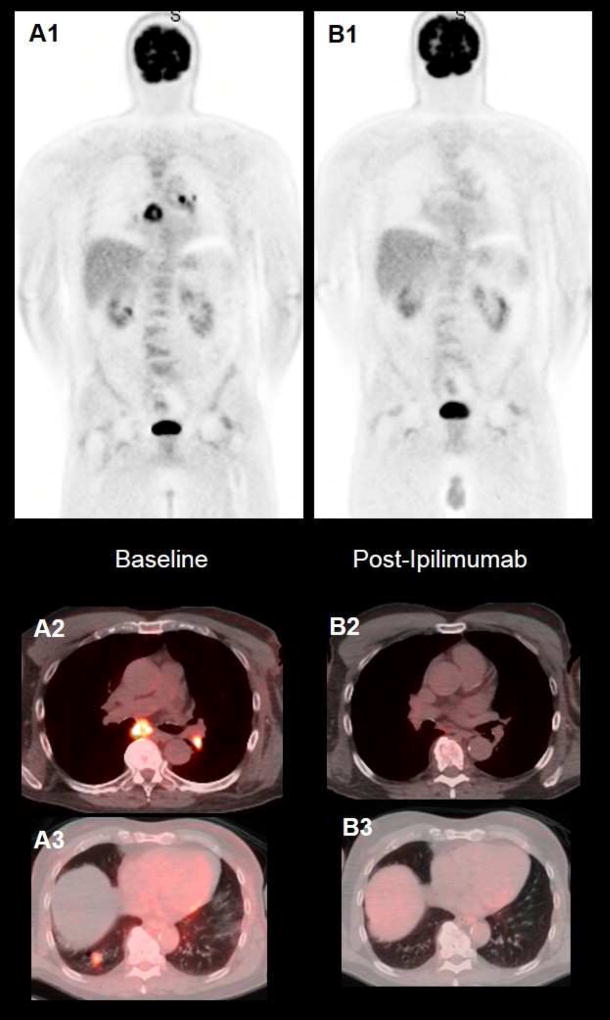
Figure 3. Complete metabolic tumor response after treatment with Ipilimumab.
65-year-old man with stage IV metastatic melanoma. Baseline axial CT (A1), fused PET/CT (B1) and MIP (A3) images demonstrate an intense FDG-avid left upper lobe pulmonary metastatic lesion. After CyberKnife stereotactic radiotherapy, and concurrent treatment with ipilimumab, the axial CT (B1), fused PET/CT (B2) and MIP (B3) images demonstrate complete metabolic resolution of the lung nodule, with mild FDG-avid radiotherapy inflammatory related lung changes.
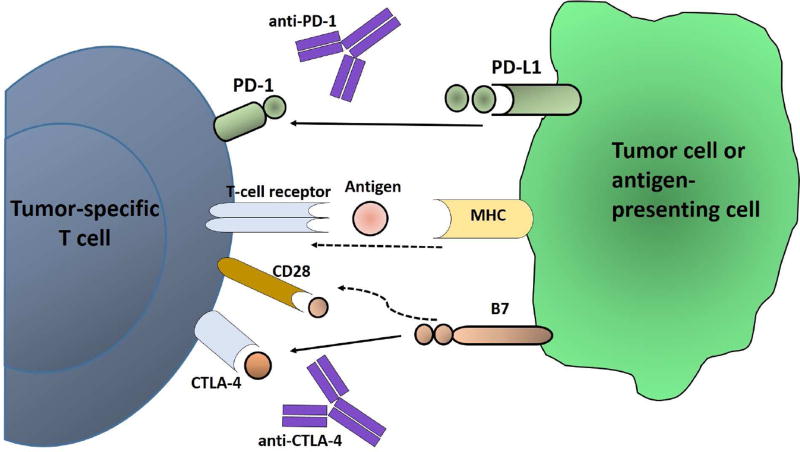
Figure 4. Immune Checkpoint Blockage.
Schema of the immunotherapy exemplified by antibodies directed against CTLA-4 (ipilimumab, tremilimumab), which block the immunosupression mediated by the interaction between CTLA-4 (located on the CD8+ and CD4+ T cells) and the B7 family members (located on the antigen-presenting cells). The other second major checkpoint is mediated by the interaction between PD-1 (located on T cells) and its ligand PD-L1 (located on either antigen-presenting cells or on the tumor cells).
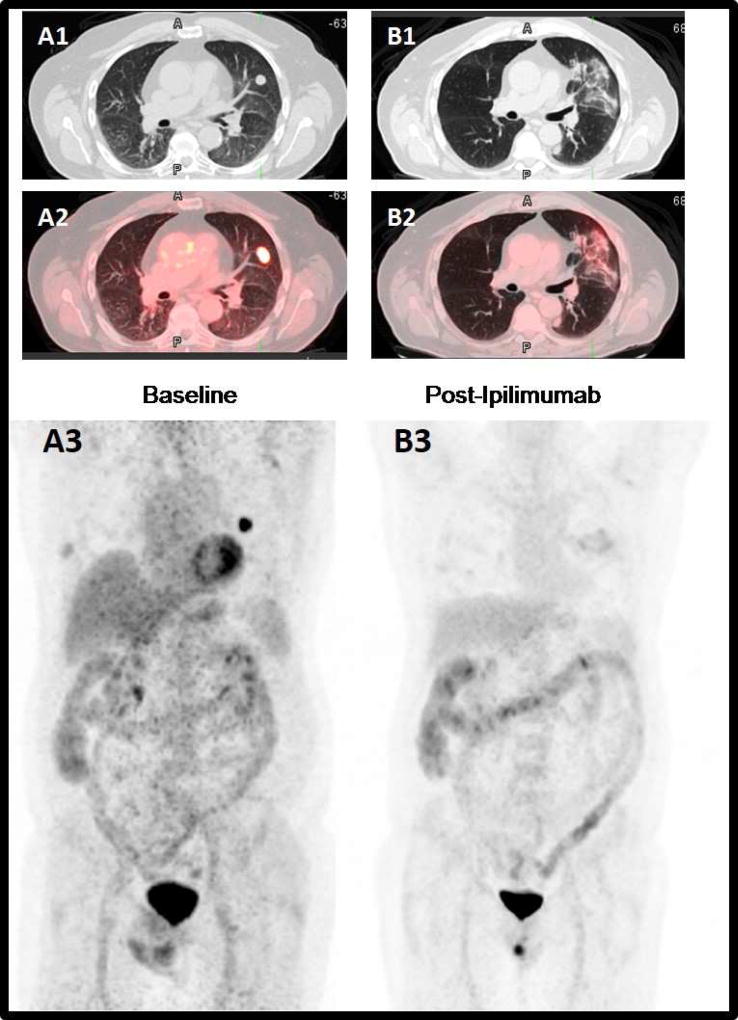
Figure 5. Ipilimumab-refractory melanoma in a patient with stage IV disease.
72-year-old gentleman diagnosed with stage IV melanoma. Baseline PET MIP image (A1) and axial fused PET/CT images (A2, A3) demonstrate multiple FDG-avid metastatic lesions. After 4 cycles of Ipilimumab, MIP PET image (B1) show increase in size and FDG-avidity of the prior lesions and development of new FDG-avid lesions (B2, B3) indicating progression metabolic disease.
All these novel treatment strategies showed promising results for patients with advanced metastatic melanoma, however they also hold significant challenges since there is no a robust biomarker to identify what subpopulation of patients with melanoma will most likely benefit from a type treatment or another; without a predictive biomarker, a considerable number of patients may receive treatment without benefit. Besides, monitoring the effects of these treatments may be also a challenge because when the immune system is stimulated, immune cells infiltrate the tumor that lead to the cytotoxic effects of the therapy, and these immune infiltrates may cause imaging misinterpretations in the response assessment. The following sections outline the current ways in which PET imaging can be used as a biomarker in melanoma patients undergoing immune or targeted therapies, and how to adapt the imaging interpretation to respond to the unique challenges of immunotherapy.
PET Imaging in Assessing Response to Therapy in the Era of Personalized Treatment
As the treatment of cancer evolves to include new agents, attention has been given to how a specific treatment might impact imaging findings. Fluorine-18 fluorodeoxyglucose (18F-FDG) Positron PET/computed tomography (CT) is a powerful imaging tool widely used to evaluate treatment response in multiple malignancies, including melanoma. In the setting of assessing response to mutation-drive therapy, BRAF kinase mutation has been shown to affect multiple signaling pathway, which up-regulate glycolysis and glucose transport, facilitating tumor growth 22, 23. Thus, the resultant change in glucose metabolism from targeting mutated BRAF kinase agents could theoretically be visualized using FDG PET/CT. In this context, in a phase I study, including 27 patients with stage IV BRAFv600 mutation, McArthur et al. 24 were able to show homogeneously, dose-dependent decreased 18F-FDG uptake in metastases 15 days after starting treatment with vemurafenib, confirming the utility of FDG PET imaging in documenting treatment response early on in the course of therapy. The investigators demonstrated that a median reduction in SUVmax of 82% resulted in significantly different median times of progression-free survival (PFS); however, no definitive relationship was found between reduction in target lesion SUVmax and best response according to RECIST (Response Evaluation Criteria in Solid Tumors) 24. Similarly, Carlino et al. 25 reported that FDG PET/CT registered responses in all 26 patients with BRAF-mutated metastatic melanoma treated with dabrafenib and that 26% of cases exhibited homogeneous PET responses, while 74% showed heterogeneous PET responses; defining a homogeneous response as >90% of lesions responded with no progressive metabolic lesions, and up to 10% stable lesions allowed, whereas a heterogeneous response was defined when lesions responded alongside with progressing or new lesions 25. Although these early data are promising, additional studies are required to characterize the PET response characteristics of the mutation-drive melanoma therapies.
To assess if a patient is responding to treatment, the response evaluation criteria in solid tumour (RECIST) guideline is currently widely used to evaluate antitumor activity of traditional cytotoxic agents. It helps clinicians to objectively determine whether the tumors have progressed (> 20% increase in target lesion size), have responded (> 30% decrease in target tumor size) or remained stable (based on a set of radiological measurement criteria). However, in the setting of assessing response to immunomodulation, antitumor responses to immunotherapies are unique in that lesions may progressed before a documented response 26, response can be seen in spite of the presence of new lesions and lesions may remain stable or slowly regress over time, which may take longer than cytotoxic therapies. Immune modulation therapy response can also be confounded by the presence of inflammatory cells, which can mimic FDG-avid tumor 27, further complicating assessments of therapy response. Another consideration to have in mind when performing FDG PET/CT imaging following immunotherapy is that it potentiates T lymphocytes, producing a high rate (up to 70%) of adverse immune- related adverse reactions, including colitis, dermatitis, hypophysitis, arthritis, and thyroiditis, which can also lead to false findings on FDG PET imaging 28–35. These effects raise concerns about the use of existing response interpretation criteria including WHO, RECIST or PERCIST criteria 26, and there is a necessity for defining new standards response criteria to assess response to these novel immunotherapies. In a series of workshop in 2004 and 2005, the immune related response criteria (irRC) was introduced. The novelty of the IrRC is the incorporation of measurable new lesions into a new concept of “total tumor burden” and comparison of this variable with baseline measurements 26. Using IrRC criteria, Kong and collaborators 36 recently aimed to described the patterns of residual metabolic activity in patients following prolonged treatment with anti-CTLA-4 or anti-PD-1 antibodies in 27 patients with unresectable stage IIIC or IV melanoma. The data suggested that patients with residual metastases may have metabolically inactive lesions on FDG PET imaging, whereas isolated metabolically active lesions in clinically well patients may reveal immune cell infiltrates rather than melanoma 36. Moreover, Sachpekidis et al. 37 showed that FDG PET/CT imaging may have a role in the early detection of ipilimumab immune-related response in 22 patients with unresectable metastatic melanoma; FDG PET/CT after two cycles of ipilimumab was highly predictive of the final treatment outcome in patients with progression and stable metabolic disease 37. Further prospective studies and long-term follow-up are in need to clarify whether or not FDG-PET may be useful as a biomarker of duration of treatment response, early response assessment, and to what extent immune activation can lead to false-positive results.
PET as a predictive biomarker of prognosis in the new generation therapies
MAP-Kinase inhibitors and immunologic checkpoint blockade antibodies have achieved improved OS in patients with metastatic melanoma 38. There is also evidence that FDG PET/CT findings can be used to prognosticate clinical outcomes after the new generation mutation- driven therapy and immunomodulation therapy. In the above mentioned phase I study, including 27 patients with BRAF V600-mutated metastatic melanoma treated with vemurafenid, McArthur et al 24 reported that, although PET response was unrelated to OS, subjects who experienced a reduction in the SUVmax of less than 82% had a mean PFS of 183 days, which was substantially shorter than the 484 days of PFS for those with a greater than 82% reduction in SUVmax. Similarly, Carlino et al. 25 in a phase I study using dabrafenib therapy in BRAF V600- mutated metastatic melanomas, found that patients with heterogeneous FDG PET responses had a significantly shorter PFS than homogeneous FDG PET responders (mean, 3.0 vs 7.4 months, respectively). In the context of combined BRAF and MEK inhibitors therapy, Schmitt et al 39 investigated the association between survival and early changes on FDG PET/CT imaging in 22 patients with BRAF-mutant melanoma. Investigators found that for the least metabolically active tumor, change in SUVmax was associated with PFS, but not OS; for the least metabolically active tumor, no association was seen between changes in SUVmax and PFS or OS39.
In the setting of immunomodulation therapy agents, there is no published data assessing the prognostic benefits of using FDG PET, other than case reports illustrating radiologic features of the immune-related adverse reactions, highlighting the challenges of monitoring immunomodulation therapy with FDG PET. Thus, Bronstein et al. 33 reviewed case reports of 119 stage IV melanoma patients treated with anti-CTLA-4, concluding that there was a significant association between the incidence of radiologic manifestations of immune-related adverse events and clinical responses to anti-CTLA-4 therapy; interestingly, 25% of patients with radiologic manifestation due to immune-related adverse events experienced complete response compared with 3% of patients who did not exhibit radiologic evidence of immune- related adverse events 33.
FDG PET/CT has also been recently proposed as a potential marker to aid predicting the patient’s prognosis in different tumors, by using the various PET metabolic parameters such as the maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV) and total lesion glycolysis (TLG). SUVmax is a semi quantitative measure of tumor FDG uptake, whereas MTV refers to volumetric measurement of tumor cells with high glycolytic activity, and TLG is the sum of SUVs within the tumor, calculated as MTV×SUVmean. In the setting of melanoma, Kang et al. 40 reported that the SUVmax from FDG PET/CT can provide important information for predicting recurrence. Using PET volumetric parameters, Son et al. 41 retrospectively conducted a review study including 41 patients with a histological diagnosis of cutaneous melanoma, who underwent pretreatment FDG PET/CT scans; SUVmax and TLG were found to be significantly higher in patients with recurrence than in patients without, and SUVmax and TLG were also found to be significantly higher in non-survivors than in survivors. Investigators concluded that pretreatment MTV and TLG may be useful in stratifying the likelihood of recurrence and melanoma-specific death, and that TLG was found to be the best predictive marker 41. Further additional studies are necessary for assessing whether pre or post-treatment FDG PET parameters can predict immunomodulation treatment success.
Summary/Discussion
The molecular classification of cancer through next generation sequencing has transformed drug development towards the molecularly guided era of precision oncology. With the advances and successes of the mutation-driven, and immunomodulation therapy, the ultimate goal is to tailor treatment for specific melanoma subtypes and patients. Treatment response criteria should be chosen based on the subtype of tumor and treatment delivered to the patient and should evolve in parallel with the advances in novel targeted agent. As a result, it is crucial that radiologists are aware that the major advancements in cancer immunotherapy is challenging the current imaging approach in evaluating treatment response and early recognizing immune-related adverse events for a successful patient management. Further prospective studies and long-term follow-up are needed to determine the role of FDG PET/CT imaging as a potential prognostic biomarker to identify patients suitable for targeted therapy, predict early response assessment, duration of treatment response, monitor secondary resistances, and eventually predicting patient outcome.
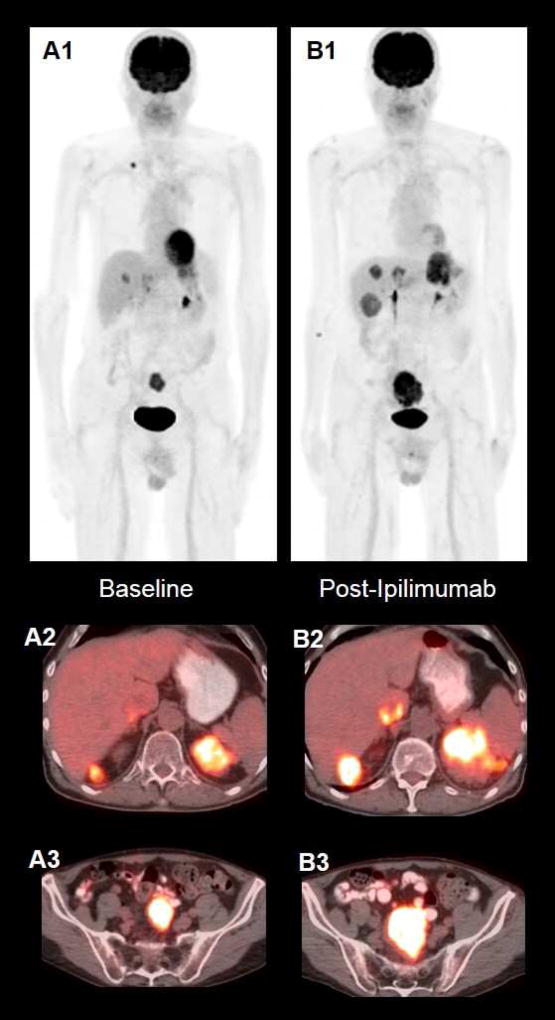
Figure 2. Complete metabolic tumor response after Ipilimumab treatment in a stage IV metastatic melanoma patient.
68-year-old woman diagnosed with stage IV melanoma. Baseline PET MIP image (A1) and axial fused PET/CT images (A2, A3) demonstrate multiple FDG- avid mediastinal nodal lesions (A2) and a metastatic pulmonary nodule in the right lung base (A3). After 4 cycles of Ipilimumab, MIP PET image (B1) and axial fused PET/CT images (B2. B3) show complete metabolic response.
Key Points.
-
-
Understanding the molecular and genetic alterations in the pathogenesis of melanoma will elucidate the mechanisms involved in tumor growth, and aid for the development of potential targeted drugs.
-
-
Future research directions will involve incorporation of molecular characteristics and next generation probes into new strategies to improve early tumor detection.
-
-
Novel targeted therapies modulating immune inhibitory pathways have revolutionized the field of melanoma, bringing a new exciting approach in the response assessment of melanoma.
-
-
In the context of new targeted therapy, next steps involve strategies for using PET imaging as prognostic biomarker to identify patients who could benefit of targeted therapy, predict early identification of responders/non-responders, monitor secondary resistances, ultimately leading to improve clinical management, individualizing therapy decision and eventually predicting patient outcome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leiter U, Meier F, Schittek B, Garbe C. The natural course of cutaneous melanoma. J Surg Oncol. 2004;86(4):172–178. doi: 10.1002/jso.20079. [DOI] [PubMed] [Google Scholar]
- 4.Ascierto PA, Marincola FM. 2015: The Year of Anti-PD-1/PD-L1s Against Melanoma and Beyond. EBioMedicine. 2015;2(2):92–93. doi: 10.1016/j.ebiom.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzies AM, Long GV. Systemic treatment for BRAF-mutant melanoma: where do we go next? Lancet Oncol. 2014;15(9):e371–381. doi: 10.1016/S1470-2045(14)70072-5. [DOI] [PubMed] [Google Scholar]
- 6.Iams WT, Sosman JA, Chandra S. Novel Targeted Therapies for Metastatic Melanoma. Cancer J. 2017;23(1):54–58. doi: 10.1097/PPO.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas N. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161(7):1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inamdar GS, Madhunapantula SV, Robertson GP. Targeting the MAPK pathway in melanoma: why some approaches succeed and other fail. Biochem Pharmacol. 2010;80(5):624–637. doi: 10.1016/j.bcp.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44(9):1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 13.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 14.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19(19):5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 15.Chmielowski B. Ipilimumab: A First-in-Class T-Cell Potentiator for Metastatic Melanoma. J Skin Cancer. 2013;2013:423829. doi: 10.1155/2013/423829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribas A, Hamid O, Daud A, et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA. 2016;315(15):1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 19.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17(11):1558–1568. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng B, Jeong JH, Asara JM, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33(2):237–247. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kao YS, Fong JC. A novel cross-talk between endothelin-1 and cyclic AMP signaling pathways in the regulation of GLUT1 transcription in 3T3-L1 adipocytes. Cell Signal. 2011;23(5):901–910. doi: 10.1016/j.cellsig.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 24.McArthur GA, Puzanov I, Amaravadi R, et al. Marked, homogeneous, and early [18F]fluorodeoxyglucose-positron emission tomography responses to vemurafenib in BRAF-mutant advanced melanoma. J Clin Oncol. 2012;30(14):1628–1634. doi: 10.1200/JCO.2011.39.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlino MS, Saunders CA, Haydu LE, et al. (18)F-labelled fluorodeoxyglucose-positron emission tomography (FDG-PET) heterogeneity of response is prognostic in dabrafenib treated BRAF mutant metastatic melanoma. Eur J Cancer. 2013;49(2):395–402. doi: 10.1016/j.ejca.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 27.Shozushima M, Tsutsumi R, Terasaki K, Sato S, Nakamura R, Sakamaki K. Augmentation effects of lymphocyte activation by antigen-presenting macrophages on FDG uptake. Ann Nucl Med. 2003;17(7):555–560. doi: 10.1007/BF03006668. [DOI] [PubMed] [Google Scholar]
- 28.Kahler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9(4):277–286. doi: 10.1111/j.1610-0387.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- 29.Lammert A, Schneider HJ, Bergmann T, et al. Hypophysitis caused by ipilimumab in cancer patients: hormone replacement or immunosuppressive therapy. Exp Clin Endocrinol Diabetes. 2013;121(10):581–587. doi: 10.1055/s-0033-1355337. [DOI] [PubMed] [Google Scholar]
- 30.Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckert A, Schoeffler A, Dalle S, Phan A, Kiakouama L, Thomas L. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology. 2009;218(1):69–70. doi: 10.1159/000161122. [DOI] [PubMed] [Google Scholar]
- 32.Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11. [PMC free article] [PubMed] [Google Scholar]
- 33.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol. 2011;197(6):W992–W1000. doi: 10.2214/AJR.10.6198. [DOI] [PubMed] [Google Scholar]
- 34.Koo PJ, Klingensmith WC, Lewis KD, Bagrosky BM, Gonzalez R. Anti-CTLA4 antibody therapy related complications on FDG PET/CT. Clin Nucl Med. 2014;39(1):e93–96. doi: 10.1097/RLU.0b013e318292a775. [DOI] [PubMed] [Google Scholar]
- 35.Gilardi L, Colandrea M, Vassallo S, Travaini LL, Paganelli G. Ipilimumab-induced immunomediated adverse events: possible pitfalls in (18)F-FDG PET/CT interpretation. Clin Nucl Med. 2014;39(5):472–474. doi: 10.1097/RLU.0b013e31828da691. [DOI] [PubMed] [Google Scholar]
- 36.Kong BY, Menzies AM, Saunders CA, et al. Residual FDG-PET metabolic activity in metastatic melanoma patients with prolonged response to anti-PD-1 therapy. Pigment Cell Melanoma Res. 2016;29(5):572–577. doi: 10.1111/pcmr.12503. [DOI] [PubMed] [Google Scholar]
- 37.Sachpekidis C, Larribere L, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A, Hassel JC. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging. 2015;42(3):386–396. doi: 10.1007/s00259-014-2944-y. [DOI] [PubMed] [Google Scholar]
- 38.Spagnolo F, Picasso V, Lambertini M, Ottaviano V, Dozin B, Queirolo P. Survival of patients with metastatic melanoma and brain metastases in the era of MAP-kinase inhibitors and immunologic checkpoint blockade antibodies: A systematic review. Cancer Treat Rev. 2016;45:38–45. doi: 10.1016/j.ctrv.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt RJ, Kreidler SM, Glueck DH, et al. Correlation between early 18F-FDG PET/CT response to BRAF and MEK inhibition and survival in patients with BRAF-mutant metastatic melanoma. Nucl Med Commun. 2016;37(2):122–128. doi: 10.1097/MNM.0000000000000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang S, Ahn BC, Hong CM, et al. Can (18)F-FDG PET/CT predict recurrence in patients with cutaneous malignant melanoma? Nuklearmedizin. 2011;50(3):116–121. doi: 10.3413/Nukmed-0356-10-09. [DOI] [PubMed] [Google Scholar]
- 41.Son SH, Kang SM, Jeong SY, et al. Prognostic Value of Volumetric Parameters Measured by Pretreatment 18F FDG PET/CT in Patients With Cutaneous Malignant Melanoma. Clin Nucl Med. 2016;41(6):e266–273. doi: 10.1097/RLU.0000000000001205. [DOI] [PubMed] [Google Scholar]