Abstract
Introduction
The role of Wnt/ β -catenin signaling pathway in HCV-associated hepatocellular carcinogenesis is still unknown.
Material and methods
This study aimed to perform quantitative analysis of immuno- and hybridocytochemical expression of β -catenin, E- and N-cadherins and HCV proteins (C, NS3, NS5A) in long-lasting (≥ 20 years) chronic hepatitis C (CH-C) (n = 54), hepatocellular carcinoma (HCC) (n = 61), and control liver samples (n = 8).
Results
Typical membranous expression of β -catenin in the control liver was higher than in the CH-C and HCC (p = 0.06). The mean β -catenin tissue expression in CH-C was similar to controls, and significantly higher than that of HCC (p = 0.005). E-cadherin expression was lower in CH-C than in the control (p = 0.045) and HCC (p < 0.001). In HCC both β -catenin and E-cadherin expressions were significantly lower in comparison to controls (p = 0.02, p = 0.001, respectively). Positive correlations were found between β -catenin and E-cadherin (in CH-C and HCC), β -catenin and N-cadherin (HCC), E- and N-cadherins expressions (HCC) (p < 0.05 in all cases). In CH-C the positive correlation was demonstrated between NS5A protein and β -catenin, and between the all HCV proteins (C, NS3, NS5A) and E-cadherin expression (p < 0.05 in all cases).
Conclusions
Alterations in cellular locations of β -catenin and E-cadherin in CH-C and HCC pointed to structural disturbances in intercellular junctions in the livers and presence of the transcriptionally inactive form of β -catenin. The reduced expression of E-cadherin in long-lasting CH-C may represent an early indicator of the epithelial-mesenchymal transition. The most important role in modulation of the Wnt/ β -catenin pathway in vivo is probably played by the NS5A viral protein.
Keywords: chronic hepatitis C, hepatocellular carcinoma, cadherin/catenin complex, NS3, NS5A and C protein, immuno- and hybridocytochemistry
Introduction
Hepatitis C virus (HCV) infection leads to development of chronic hepatitis C (CH-C) and liver cirrhosis and represents the main risk factor of primary hepatocellular carcinoma (HCC) [1].
The role of the Wnt/ β -catenin signalling pathway in hepatocellular carcinogenesis was demonstrated [2]. The studies on tissue expression of β -catenin allowed genetically distinct subsets of HCC to be distinguished [3, 4]. Mutations in the CTNNB1 gene seem to be manifested more frequently in HCC due to HCV than HBV infection [3], even if it was not confirmed by an extensive meta-analysis [5]. Most of the clinical data were correlated with nuclear localization and/or overexpression of β -catenin in HCC [5–7].
Reduced tissue expression of E-cadherin was described in HCC, as compared to the control [8], and correlations were demonstrated between the lowered expression of E-cadherin and clinical data [9].
Studies on involvement of HCV in hepatic carcinogenesis due to modulation of the Wnt/ β -catenin signalling pathway have been conducted since the 1990s [10]. It was not until studies on an in vitro model were performed that interactions of HCV proteins and Wnt/ β -catenin became evident [2, 11].
The clinical significance of hepatic expression involving HCV proteins in association with expression of E-cadherin-catenin unit (ECCU) complex proteins in long-term HCV infection in vivo remains unclear. This study aimed at analysis of tissue expression of β -catenin (at the levels of mRNA and protein) and by two cadherins (E and N) in livers of patients with prolonged HCV infection (over 20 years) and HCC. In cases of CH-C the results were related to cellular expression of hepatitis C virus proteins (core, NS3 and NS5A).
Material and methods
Patients and specimens
Chronic HCV infection group (CH-C; n = 54)
The group included 54 patients with chronic hepatitis and/or liver cirrhosis due to chronic HCV infection, diagnosed in the Department of Infectious Diseases, University of Medical Sciences in Poznan in the years 2007–2010. The group comprised 27 men and 27 women, 18 to 72 years of age (mean age: 37 ±14 years).
Patients with chronic renal insufficiency, active neoplastic diseases or diabetics and individuals manifesting excessive alcohol consumption were excluded. Other cases of liver damage (e.g. α 1-antitrypsin deficiency, Wilson’s disease) and other hepatic infections (HBV, HCMV, EBV, HIV) were also excluded.
In every patient of the CH-C group presence of anti-HCV antibodies was tested by chemiluminescence using the test of Architect Anti-HCV (Abbott, Wiesbaden, Germany) and the Architect i2000 analyser (Abbott). The HCV infection was also confirmed by detection of serum HCV-RNA with the GeneProof Hepatitis C Virus HCV test (GeneProof a.s., Brno, Czech Republic) manifesting sensitivity of 50 IU/ml. In all patients genotype of the HCV virus was examined (Versant HCV Genotype 2.0 Assay, LiPA). The biochemical tests for alanine aminotrasferease (ALT) and aspartate amino-trasferease (AST), γ-glutamyltransferase (GGTP), total cholesterol, and total protein with electrophoresis were performed using the MURA 200 analyser (Pointe Scientific, Italy), reagents and calibrators of the company. Alpha fetoprotein (AFP) concentration in the serum was examined by chemiluminescence in the Architect i2000 analyser, using the test of Architect AFP (Abbott, Sligo, Ireland).
None of the patients included in the studies underwent any antiviral therapy in the past. In all patients liver biopsy was performed within qualification procedures for antiviral treatment. Informed consent was obtained from every subject, and the institutional review committee approved this study (no. 182/12).
Hepatocellular carcinoma group (HCC; n = 61)
This group comprised archival paraffin blocks with HCC confirmed clinically and by histopathological examination (n = 4, age of patients 67–72 years). The remaining part of the group involved the commercially available tissue panel with HCC (Tissue Microarray Panel, Cybrdi Inc., Maryland, USA) (n = 57). The entire group of HCC included 48 men and 13 women with the average age of 51 ±13 years. HCV-associated HCC was confirmed only in 1 patient.
Control group (C; n = 8)
This group involved archival fragments of the normal liver of HCV-negative patients selected for transplantation (n = 2; 35 and 60 years old), liver around an excised focal lesion in the organ (n = 4, age of 20–72 years), and the negative control liver samples present in the tissue panel with HCC (Tissue Microarray Panel, Cybrdi Inc., Maryland, USA) (n = 2, 55 and 66 years old). The group included 6 men and 2 women with the average age of 51 ±20 years.
Histology and immunohistochemistry (IHC)
The histopathological examination of liver biopsies was conducted by two independent pathologists (SŁ, WB). In the assessment of inflammatory activity (grading, G) and fibrosis (staging, S) scores the point scale of the METAVIR Cooperative Study was applied [12]. In evaluation of the liver steatosis the percentage of hepatocytes with visible fatty (fine and/or large droplet) degeneration was taken into account. The final result involved the mean from 5 visual fields under the 20× microscope objective.
Detection and studies on cellular localization of β -catenin, E- and N-cadherins, as well as three HCV proteins – core (C, capsid) and nonstructural proteins 3 and 5 (NS3 and NS5A) – in liver biopsies took advantage of the new polymer-based IHC method [13, 14]. In the case of β -catenin, NS5A and C proteins the studies followed the ImmunoMax technique. In the latter one key reaction involved 8 min incubation with biotinylated tyramine (PerkinElmer Life Sciences, Inc., 1 : 50) at room temperature (RT). Mouse anti-human monoclonal antibodies (mAbs) were employed, directed against human beta-catenin (Clone 196618; Code M 7052) (in dilution 1 : 50) (R&D Systems, UK), against E-cadherin (clone NCH-38) (in dilution 1 : 100) (DakoCytomation, Gdynia, PL) and against N-cadherin (in dilution 1 : 50) (clone IAR06) (Novocastra Labs. Ltd, Newcastle upon Tyne, UK). The HCV proteins were detected using antibodies against human HCV core (capsid) protein (in dilution 1 : 50) (Chemicon International, Inc.), NS3 protein (clone MMM33) (in dilution 1 : 50) (Novocastra Labs.) and NS5A protein (in dilution 1 : 50) (Chemicon International, Inc.). The sections were incubated with these primary mAbs at night at 4°C, and afterwards were incubated with dextran backbone to which horseradish peroxidase (HRP) was attached and with secondary biotinylated link anti-rabbit and anti-mouse IgG (Dako REAL EnVision Detection System peroxidase/DAB+, Rabbit/Mouse, Dako). In negative controls specific antibodies were omitted and supplemented by normal serum of a respective species in 0.05 M Tris-HCl, pH ~7.6, supplemented with 0.1% bovine serum albumin (BSA) and 15 mM sodium azide. All the steps of the IHC technique were previously described [14].
Histological slides with IHC expression were examined under the optical Olympus BH-2 microscope coupled to a digital camera. Colour microscope images were recorded and archived using a 40× objective (at least 10 fields in every microscope slide with an IHC positive reaction), using LUCIA Image 5.0 computer software.
Hybridisation in situ (ISH)
On the basis of literature data [15], for detection of mRNA specific for human β -catenin single stranded oligonucleotide (DNA) probes were used, 5 ′ terminally labelled with digoxygenin, manifesting the following sequences: (1) 5 ′ -Dig-ATG GAA CCA GAC AGA AAA G-3 ′ ; (2) 5 ′ -Dig-TAC AGG ACT TGG GAG GTA TC-3 ′ . The probes were obtained by chemical synthesis (oligo.pl, IBB, PAN, Warszawa, Poland). The probes were used in concentration of 1000 ng/ml and detected with sheep anti-digoxygenin mAbs (Fab fragments), labelled with HRP (Roche, Mannheim, Germany). In ISH studies, both classical and amplified by the ImmunoMax technique, sequential paraffin sections of the tissue material pre-tested by IHC techniques were used. The protocol of R&D Systems was employed, with our own modification and ImmunoMax amplification of the signal, as previously described [16]. Negative controls included (a) hybridisation without addition of the molecular probe and (b) incubations of slides in an RNase A solution (R&D Systems), 20 mg/ml in 2xSSC/10 mM MgCl2, pH 8, for 30 min at RT before hybridization.
Examination of expression at the level of mRNA involved exclusively β -catenin, which in pilot experiments manifested the most pronounced IHC representation. Examination of the transcript supplemented IHC technique and aimed at confirmation or exclusion of β -catenin production (mRNA level) in the liver. Expression of mRNA for β -catenin was not evaluated quantitatively. The ISH studies for detection of the β -catenin transcript were conducted at least twice. Subsequently the sections were analysed under an Olympus BH-2 light microscope.
Microscopy image analysis
Expression of adhesion proteins ( β -catenin, E- and N-cadherins)
The images with positive IHC reaction, 2560 × 1920 pixels in size recorded in the LUCIA Image 5.0 software, were subjected to morphometric analysis, using the Filtr HSV software, originally developed in the Department of Bioinformatics and Computational Biology, Poznan University of Medical Sciences, according to the following formula: (area of positive IHC reaction/area studied) × 100%.
In the Results section values of average IHC expression of the three adhesion proteins ( β -catenin, E-and N-cadherins) are presented, expressed in percentages manifested by the IHC reactions per field of hepatic area.
Expression of viral proteins (C, NS3 and NS5A)
Immunoexpression of all three HCV proteins (core, NS3 and NS5A) was calculated using the 12-point modified IRS scale [16, 17]. Percentage of positive cells (PP) and staining intensity of the IHC reaction (SI) in at least ten visual fields of the Olympus BH-2 light microscope at the objective magnification of 40× were evaluated. The product of PP and SI yielded the final value of the evaluated reaction. The final score was obtained in the form of the average score from 10 visual fields and was presented as median values, with the minimum (min), maximum (max), average values and respective values of SD.
Statistical analysis
At the first stage of statistical analysis, compatibility of all the obtained results with the Gaussian distribution was verified using the Shapiro-Wilk test. Subsequently parameters of descriptive statistics (arithmetic means, standard deviations, median, minimum and maximum values) were calculated. The results were compared among three groups: CH-C, HCC and controls. Data on immunoexpression in the CH-C group were compared with the data obtained for HCC and the control (independent variables) using the Mann-Whitney test (the non-parametric test for independent variables in two groups). In the case of paired variables the Wilcoxon test was applied. The Kruskal-Wallis test was also used to compare expression of cell-adhesive proteins as related to cellular localization, grading/staging scores in CH-C and grade in HCC, and in cases when differences were manifested, additionally the multiple comparisons test (Dunn’s test) was applied. Expressions of HCV proteins evaluated in a semiquantitative scale were compared using Friedman’s test and in turn Dunn’s test. The proportions of cellular localization of β -catenin, E-cadherin and N-cadherin (membranous, cytoplasmic, mixed) were evaluated using the test of differences between two structural indices. Correlations between data rows were determined using Spearman’s rank correlation index. The results were accepted as significant at the significance level of p < 0.05. The statistical analysis was conducted using Statistica PL v. 9.0 software (StatSoft Inc., Tulsa, OK, USA).
Results
The clinicopathological data of patients with CH-C and HCC and the control group are listed in Table I.
Table I.
Clinicopathological characteristics of patients with chronic hepatitis C (CH-C), hepatocellular carcinoma (HCC) and normal liver (control)
| Characteristics | Group | Mean | Median | Min. | Max. | SD |
|---|---|---|---|---|---|---|
| Age [years] | CH-C | 37.48 | 32.00 | 18.00 | 72.00 | 14.51 |
| HCC | 51.13 | 49.00 | 20.00 | 98.00 | 13.40 | |
| Control | 51.25 | 57.50 | 20.00 | 72.00 | 19.93 | |
| Grade of tumour* | HCC | 2.05 | 2.00 | 1.00 | 3.00 | 0.57 |
| Duration of infection [years] | CH-C | 23.79 | 22.00 | 7.00 | 44.00 | 9.21 |
| BMI | 24.25 | 24.22 | 17.80 | 37.61 | 3.86 | |
| ALT [U/l] | 81.15 | 63.00 | 18.00 | 292.00 | 60.07 | |
| AST [U/l] | 58.25 | 42.00 | 15.70 | 182.00 | 38.74 | |
| AFP [ng/ml] | 5.97 | 3.61 | 1.30 | 67.04 | 9.54 | |
| HCV RNA [IU/ml] | 976419.35 | 88300.00 | 941.00 | 21500000.00 | 3051531.16 | |
| Total protein [g/dl] | 7.22 | 7.21 | 6.02 | 9.60 | 0.68 | |
| Albumin [g/dl] | 3.87 | 3.84 | 2.79 | 5.66 | 0.46 | |
| Gamma globulin [g/dl] | 1.44 | 1.48 | 0.84 | 2.26 | 0.34 | |
| Platelets [G/l] | 215.26 | 208.00 | 77.00 | 432.00 | 70.10 | |
| Cholesterol [mg/dl] | 177.21 | 178.65 | 99.00 | 274.00 | 39.08 | |
| GGTP [U/l] | 52.69 | 47.00 | 6.00 | 140.00 | 35.97 | |
| Grading (G)* | 1.78 | 2.00 | 1.00 | 3.00 | 0.75 | |
| Staging (S)* | 1.70 | 1.00 | 0.00 | 4.00 | 1.07 | |
| Steatosis (%) | 10.75 | 3.00 | 0.00 | 70.00 | 16.89 |
AFP – α-fetoprotein, ALT – alanine aminotransferase, AST – aspartate aminotransferase, BMI – body mass index, GGTP – γ-glutamyl- transpeptidase. Grade of tumour – differentiation of HCC as determined by pathologist, G – grading, necro-inflammatory activity in liver, S – staging, fibrosis scores;
parameters evaluated in a semi-quantitative scale METAVIR (see Material and methods), SD – standard deviation.
Immunoexpression of β -catenin, E-cadherin and N-cadherin
β -catenin
No significant differences in frequency of β -catenin detection were found between the examined groups (100%, 96%, and 92%, respectively) (Table II).
Table II.
Detection of β-catenin, E- and N-cadherins and the cellular localization of the proteins in chronic hepatitis C (CH-C), hepatocellular carcinoma (HCC) and normal liver (control)
| Variable | Group | No. of patients | % of positive samples | M (%) | C (%) | M + C (%) | N (%) |
|---|---|---|---|---|---|---|---|
| β-catenin | C | 8 | 100 | 75 | 25 | 0 | 0 |
| CH-C | 54 | 96 | 31 | 44 | 25 | 0 | |
| HCC | 61 | 92 | 60 | 13 | 22 | 5 | |
| E-cadherin | C | 7 | 100** | 86 | 0 | 14 | 0 |
| CH-C | 41 | 83* | 56 | 18 | 26 | 0 | |
| HCC | 60 | 58 | 77 | 14 | 9 | 0 | |
| N-cadherin | C | 8 | 50$,$$ | 100 | 0 | 0 | 0 |
| CH-C | 28 | 7 | 0 | 100 | 0 | 0 | |
| HCC | 59 | 17 | 40 | 0 | 60 | 0 |
M – membranous, C – cytoplasmic, M + C – membranous/cytoplasmic, N – nuclear pattern of IHC reaction;
p < 0.05 between E-cadherin expression in CH-C and HCC
p < 0.01 between E-cadherin expression in control and HCC;
p < 0.05 between N-cadherin expression in control and HCC
p < 0.01 between N-cadherin expression in control and CH-C.
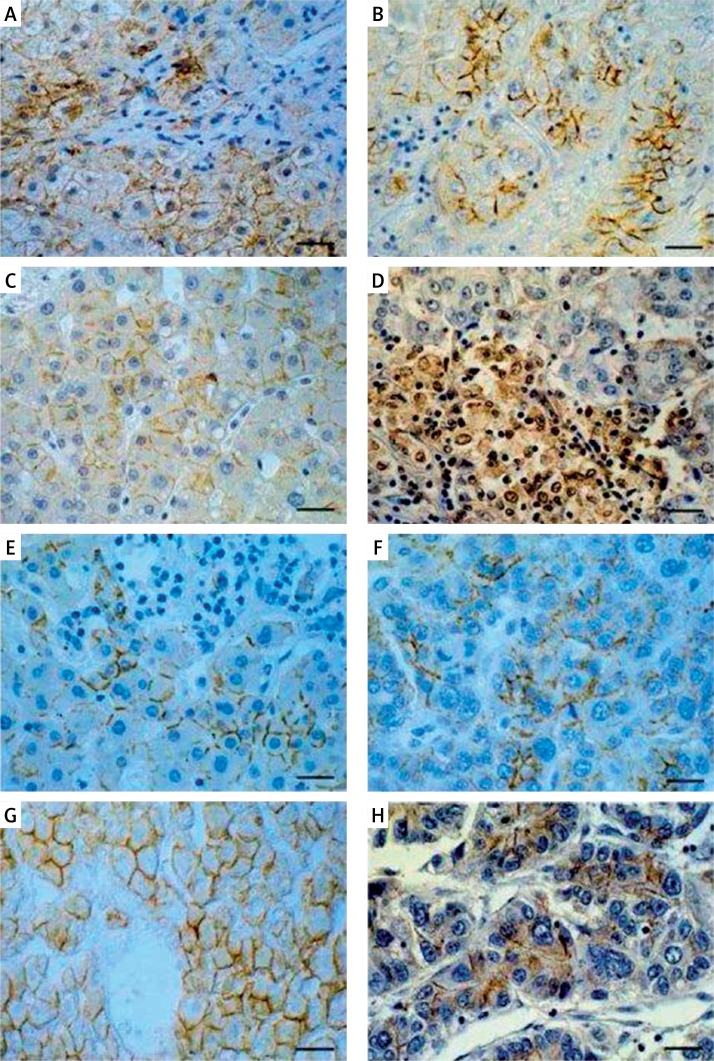
The cellular localization of β -catenin in the CH-C group involved mostly cell membranes and cytoplasm of hepatocytes (Figure 1 A, Table II). No significant quantitative differences were detected in β -catenin immunoexpression between the CH-C and the control groups (Table III).
Figure 1.
Immunohistochemical (A–C) and hybridocytochemical localization of β-catenin (D) in liver. Membranous/ cytoplasmic localization of β-catenin in liver with chronic hepatitis C (A), membranous localization of β-catenin in hepatocellular carcinoma (B) and in control liver (C); mRNA for β-catenin in cytoplasm and cell nuclei in HCC fragment (D). Immunocytochemical detection of E-cadherin (E–F) and N-cadherin (H) in liver. Predominantly membranous localization of E-cadherin in liver with chronic hepatitis C (E), hepatocellular carcinoma (F) and control liver (G). Membrano-cytoplasmic localization of N-cadherin in fragment of hepatocellular carcinoma (H). Immunohistochemistry (A–C, E–H) technique and hybridization in situ method (D). Hematoxylin counterstained. Bar = 40 μm
Table III.
Comparison of quantitatively assessed β-catenin, E-cadherin and N-cadherin immunoexpression (% of the IHC reaction area in analysed area of liver parenchyma) in chronic hepatitis C (CH-C), hepatocellular carcinoma (HCC) and normal liver (C)
| Variable | Group | No. | Mean | Median | Min. | Max. | SD | P-value |
|---|---|---|---|---|---|---|---|---|
| β-catenin | C | 8 | 25.37 | 19.80 | 0.43 | 69.64 | 26.97 | C vs. CH-C: NS C vs. HCC: 0.020 CH-C vs. HCC: 0.005 |
| CH-C | 54 | 7.00 | 3.69 | 0.00 | 28.75 | 7.41 | ||
| HCC | 61 | 5.71 | 1.63 | 0.00 | 44.95 | 9.44 | ||
| E-cadherin | C | 7 | 9.47 | 8.23 | 0.03 | 24.73 | 9.93 | C vs. CH-C: 0.045 C vs. HCC: < 0.001 CH-C vs. HCC: < 0.001 |
| CH-C | 41 | 1.78 | 0.42 | 0.00 | 8.61 | 2.67 | ||
| HCC | 60 | 1.95 | 0.01 | 0.00 | 78.80 | 10.49 | ||
| N-cadherin | C | 8 | 1.22 | 0.69 | 0.00 | 4.04 | 1.54 | C vs. CH-C: 0.064 C vs. HCC: NS CH-C vs. HCC: NS |
| CH-C | 28 | 0.19 | 0.00 | 0.00 | 3.92 | 0.78 | ||
| HCC | 59 | 0.55 | 0.00 | 0.00 | 14.35 | 2.16 |
Min. and max. – minimal and maximal values, respectively: NS – statistically non-significant. IHC – immunohistochemical, p – level of significance, SD – standard deviation, vs. – versus.
Expression of β -catenin in the HCC group was extremely variable and ranged from individual cells to numerous, intensely immunostained neoplastic liver cells. The membranous pattern of IHC reaction prevailed (Figure 1 B, Table II). Nuclear expression of β -catenin was observed in a few HCC cells in individual patients (data not shown).
Quantitatively, significantly lower β -catenin expression was detected in HCC as compared to both the control and the CH-C groups (Table III).
In the control group the membranous localization of the protein prevailed in hepatocytes (Figure 1 C, Table II). Quantitatively evaluated expression was significantly higher as compared with the HCC group (Table III). Negative control samples yielded negative immunoexpression (data not shown).
Presence of mRNA for β -catenin using the hybridization in situ method was confirmed in all examined liver samples with a positive IHC signal, independently of the group (CH-C, HCC and control). Both in the liver with chronic HCV infection and HCC mainly nuclear-cytoplasmic localization of the β -catenin transcript was demonstrated (Figure 1 D).
In the control liver mainly nuclear expression of β -catenin mRNA was detected, present both in hepatocytes and in cholangiocytes (data not shown). Negative control samples yielded a negative ISH reaction (data not shown).
E-cadherin
No significant differences in frequency of E-cadherin detection were found between the CH-C and control group (83% and 100%, respectively), but detectability of the protein in HCC was significantly lower (58%) as compared with the other groups (Table II).
Cellular localization of E-cadherin in the CH-C group involved mostly cell membranes of liver cells (Figure 1 E, Table II). A quantitative analysis demonstrated significantly lower expression in CH-C as compared with the control (Table III).
In the HCC group the membranous pattern of E-cadherin immunoexpression prevailed (Figure 1 F, Table II). Intensity of IHC expression was significantly lower than that in the control but higher than in the CH-C group (Table III).
The positive IHC reaction for E-cadherin in control liver was evident mainly in cell membranes of hepatocytes (Figure 1 G, Table II) and in quantitative analysis prevailed over that in the group with CH-C, and that with HCC (Table III).
N-cadherin
The frequency of N-cadherin detection was significantly higher in control samples (50%) as compared with CH-C (7%) and HCC (17%) groups (Table II), while no significant differences were found in quantitative expression of the protein between the analysed groups (Table III). A cytoplasmic signal was detected in a few hepatocytes in 2/28 patients with CH-C (Table II). Quantitative analysis failed to demonstrate significant differences in expression of the protein between CH-C patients and control samples (Table III).
In HCC samples N-cadherin expression showed mostly a mixed pattern of IHC reaction (membranous-cytoplasmic) (Figure 1 H, Table II). Quantitative analysis demonstrated no significant differences in expression of the protein between HCC and the control or CH-C groups (Table III). Only membranous expression of the protein was observed in control liver hepatocytes (Table II).
Comparison of IHC expression of β -catenin, E-cadherin and N-cadherin in individual groups
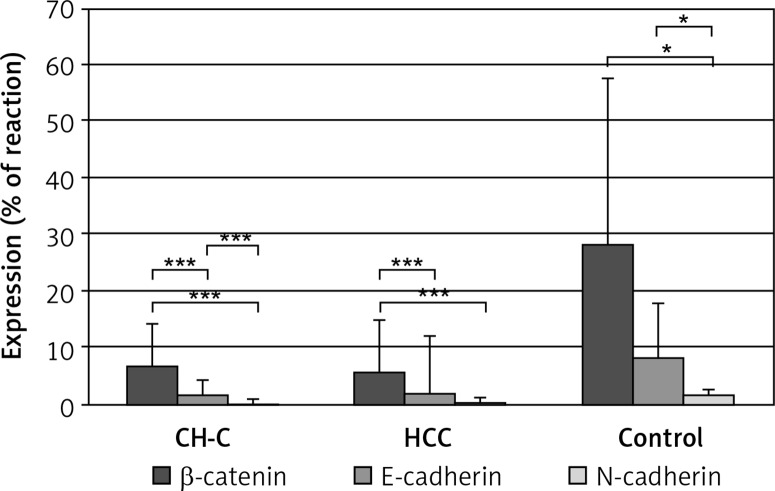
In CH-C and HCC groups significantly higher expression of β -catenin was detected as compared to expression of E-cadherin and N-cadherin. Comparison of expression manifested by two cadherins demonstrated higher expression of E-cadherin than N-cadherin in the CH-C and control groups (p < 0.001, p < 0.05, respectively), and the HCC group (p = 0.05, at the threshold of significance). In the control group expression of β -catenin and E-cadherin proved to be higher than expression of N-cadherin (p < 0.05 in both cases) (Figure 2).
Figure 2.
Comparative immunoexpression of β-catenin, E-cadherin and N-cadherin in liver with chronic hepatitis C (CH-C), hepatocellular carcinoma (HCC) and normal organ (control)
***p (level of significance) value < 0.001, *p < 0.05.
Reciprocal correlations between immunoexpression of β -catenin, E-cadherin and N-cadherin
In CH-C and HCC groups a positive correlation was detected only between expression of β -catenin and E-cadherin (r = 0.400; r = 0.514, respectively; p < 0.05 in both cases). In the HCC and control groups, positive correlations were detected between reciprocal expression of the two cadherins (r = 0.350, r = 0.867, respectively; p < 0.05 in both cases). In the control group a positive relationship was detected between expression of β -catenin and N-cadherin (r = 0.761, p < 0.05).
Tissue expression of β -catenin, E-cadherin, N-cadherin as related to histopathological data (grading, staging, grade)
No significant differences were found in expression of all proteins in HCV-infected livers of variably advanced histopathological lesions (grading and staging) (p > 0.05 in all cases) (Table IV). Also no significant differences were detected in expression of the examined proteins as related to histological malignancy (grade) of studied HCC (p > 0.05 in all cases). Similarly, expression of the proteins manifested no differences reflecting age and sex of the HCC patients (p > 0.05 in all cases) (data not shown).
Table IV.
Tissue expression of β-catenin, E-cadherin and N-cadherin (mean % of IHC reaction area ± SD) as related to grading and staging in chronic hepatitis C (CH-C) group
| Scoring system scale | No. | β-catenin | No. | E-cadherin | No. | N-cadherin | |
|---|---|---|---|---|---|---|---|
| Grading (G): | |||||||
| 1 | 22 | 6.99 ±6.65 | 18 | 1.73 ±2.97 | 14 | 0.10 ±0.36 | |
| 2 | 21 | 6.45 ±6.67 | 18 | 1.55 ±2.04 | 12 | 0.33 ±1.13 | |
| 3 | 11 | 8.05 ±10.34 | 5 | 2.77 ±3.83 | 2 | –1 | |
| Staging (S): | |||||||
| 0 | 2 | –1 | 1 | –1 | 1 | –1 | |
| 1 | 28 | 7.83 ±7.39 | 23 | 2.08 ±2.98 | 17 | 0.08 ±0.33 | |
| 2 | 13 | 5.34 ±4.14 | 10 | 1.01 ±1.16 | 7 | 0.56 ±1.48 | |
| 3 | 6 | 3.85 ±4.52 | 5 | 1.28 ±2.15 | 2 | –1 | |
| 4 | 5 | 12.90 ±13.78 | 2 | –1 | 1 | –1 | |
IHC – immunohistochemical, No. – number of patients
insufficient data for statistical evaluation, SD – standard deviation, p = 0.842 for expression of β-catenin and p = 0.702 for expression of E-cadherin between G1, G2 and G3 (Kruskal-Wallis test); p = 0.940 for expression of N-cadherin between G1 and G2 (Mann-Whitney test). In the case of β-catenin expression as related to staging 1 (S1), S2, S3, S4 – p = 0.473; for expression of E-cadherin as related to S1, S2 and S3 – p = 0.795 (Kruskal-Wallis test); for expression of N-cadherin in S1 and S2 – p = 0.756 (Mann-Whitney test).
Tissue expression of β -catenin, E-cadherin and N-cadherin vs. clinical data and laboratory results in CH-C group
No statistically significant relationships were detected between tissue expression of studied proteins and clinical data of patients (Table V). Expression of proteins depending on HCV genotype could be statistically compared only in the case of β -catenin. In patients with HCV genotype 1 (1b, 1a, 1a+b) the mean tissue expression of β -catenin was higher (6.70 ±6.69%) than in the group with the remaining genotypes of HCV (3a, 4a/4c/4d) (3.42 ±5.13%), although the difference was statistically insignificant (p = 0.09) (data not shown).
Table V.
Values of Spearman’s rank coefficient between expression of β-catenin, E-cadherin, N-cadherin (% IHC reaction area per area of hepatic parenchyma) and clinicopathological data in CH-C group
| Parameter | β-catenin | E-cadherin | N-cadherin |
|---|---|---|---|
| Age [years] | 0.004 | 0.086 | –0.162 |
| Duration of infection [years] | 0.122 | 0.194 | 1– |
| BMI | –0.055 | 0.210 | –0.075 |
| Grading* | –0.080 | 0.096 | –0.010 |
| Staging* | –0.004 | –0.003 | 0.067 |
| Fatty degeneration (%) | –0.028 | 0.015 | 0.100 |
| ALT [U/l] | –0.015 | 0.246 | 0.210 |
| AST [U/l] | 0.004 | 0.257 | 0.176 |
| AFP [ng/ml] | 0.173 | 0.202 | –0.034 |
| HCV RNA [IU/ml] | 0.037 | 0.134 | –0.228 |
| Total protein [g/dl] | 0.067 | –0.035 | 0.216 |
| Albumins [g/dl] | 0.052 | –0.010 | 0.063 |
| Gamma-globulins [g/dl] | –0.010 | –0.015 | 0.166 |
| Platelets [G/l] | 0.218 | 0.050 | –0.065 |
| Cholesterol [mg/dl] | 0.014 | 0.003 | 0.015 |
| GGTP [U/l] | 0.007 | 0.273 | 0.123 |
Parameters evaluated semiquantitatively (see Material and methods)
insufficient data for statistical analysis, AFP – α-fetoprotein, ALT – alanine aminotransferase, AST – aspartate aminotransferase, BMI – body mass index, CH-C – chronic hepatitis C, GGTP – γ-glutamyl- transpeptidase, IHC – immunohistochemistry.
IHC expression of β -catenin, E-cadherin and N-cadherin vs. cellular localization
β -catenin
Typical membranous (M) expression of β -catenin in the control liver (22.15 ±22.12%) was significantly higher than in the remaining groups: CH-C (6.81 ±8.08%) and HCC (6.78 ±11.19%) (Kruskal-Wallis test; p = 0.06, at the threshold of significance). Ectopic cytoplasmic (C) expression was significantly higher in CH-C (6.43 ±5.44%) than in HCC (2.78 ±3.78%) (Mann-Whitney test; p = 0.03). The mixed ectopic expression (M + C) was similar in CH-C (9.32 ±9.73%) and HCC (6.85 ±7.22%) (p = 0.57). No significant differences were detected upon comparisons of three patterns of cellular localizations (M, C, M + C) within CH-C and HCC groups (p > 0.05 in both groups) (data not shown).
E-cadherin
The membranous expression of E-cadherin in HCC (1.32 ±4.21%) was significantly lower compared with the control (9.67 ±10.86%), while in CH-C higher expression of the protein was demonstrated (1.50 ±2.21%) compared to HCC (1.32 ±4.21%) (p < 0.05 in both cases). No differences were found in cytoplasmic expression of the protein between groups (p > 0.05). The mixed expression (M + C) was present in an insufficient number of cases to allow comparison of the results. No significant differences were found comparing every form of cellular localization (M, C, M + C) within every group (CH-C, HCC, control) (p > 0.05 in all cases) (data not shown).
N-cadherin
In cases of expression involving this protein, due to the low number of samples with a positive IHC reaction of a specific localization (M, C, M + C), quantitative comparisons proved to be impossible between the groups or within each group.
Immunohistochemical expression of HCV proteins in CH-C group
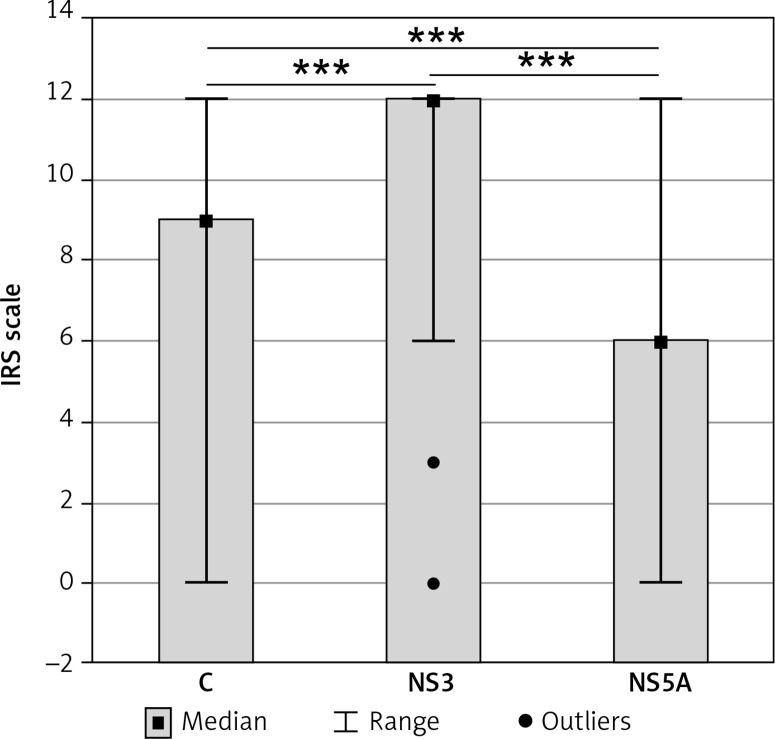
HCV proteins C (capsid, core), NS3 and NS5A were demonstrated in all patients of the CH-C group. The cytoplasmic localization of the proteins prevailed (data not shown). Quantitatively, expression of NS3 protein was significantly higher than that of C and NS5A proteins. Significantly higher expression was documented for C protein as compared to NS5A (Figure 3). Positive correlations were detected between expression of the capsid and both non-structural proteins (NS3 and NS5A) (r = 0.414, r = 0.362, respectively; p < 0.05 in both cases).
Figure 3.
Tissue immunoexpression (median values) of hepatitis C virus (HCV) proteins (C, NS3, NS5A) (12-point IRS scale) in liver with chronic hepatitis C
***p (level of significance) value < 0.001.
Correlations between HCV proteins and β -catenin, E-cadherin and N-cadherin expression
Statistically significant positive correlations were found between expression of all the examined HCV proteins (C, NS3 and NS5A) and of E-cadherin (r = 0.587, r = 0.327, r = 0.581; p < 0.05 in all cases). In addition, NS5A expression was highly positively correlated with expression of β -catenin (r = 0.412; p < 0.05). No correlations were observed between expression of any HCV proteins and of N-cadherin (data not shown).
Discussion
The studies related to expression of β -catenin in CH-C and HCC demonstrated striking changes in cellular localization of the protein, involving first of all lowered expression with the typical membranous localization of the protein as compared to such expression in the control. Intensity of the immunoexpression manifesting the cytoplasmic pattern was significantly higher in CH-C than in HCC. Transcripts for β -catenin in all studied groups were observed in cell nuclei and cytoplasm, which generally corresponds with data of other authors [7, 18]. In prolonged CH-C (> 20 years) no nuclear localization of β -catenin was observed, although the altered localization of β -catenin may indicate structural disturbances in intercellular junctions in the pathologically altered organ. Also in HCC the non-nuclear localization of the protein prevailed. The results are in line with those authors who have also observed a high proportion of HCC with non-nuclear β -catenin localization [6, 19, 20] or the complete absence of nuclear pattern of β -catenin expression [21]. In contrast to expectations, no significant differences were detected in intensity of the total β -catenin expression (M, C, M + C) in the CH-C vs. control and HCC groups. The IHC intensity of β -catenin in HCC was even significantly lowered as compared to the control, which may indicate the non-mutated form of β -catenin in the studied tissue material and is consistent with literature data [8, 22]. Some investigators demonstrated significant correlations between just reduced expression of β -catenin and HCC progression [8]. Others have described the association between nuclear localization of β -catenin and more intense proliferative activity of hepatocytes, and shorter survival of patients [20, 23] or lower invasiveness of HCC and longer 5-year survival [3, 22]. In order to draw conclusions related to the prognostic significance of β -catenin in CH-C and HCC, in our opinion the methods of quantitative evaluation of the protein in tissues should be made more precise and, in particular, the terms of normal and abnormal tissue expression of the adhesion protein should be defined. In CH-C it would be interesting to extend the studies to other components of the β -catenin-ubiquitinating complex (APC, axin and kinases), e.g. GSK-3 β or Akt, which are decisive for accessibility of “free” catenin and its accumulation in the cytoplasm or translocation to the nucleus.
Our in vivo studies showed a strong positive correlation between β -catenin and NS5A viral protein expression and confirmed the observations made on in vitro models, which suggested that NS5A may be responsible for elevated transcription of β -catenin-dependent genes. These interactions may facilitate neoplastic transformation of HCV-infected hepatocytes through the Wnt/ β -catenin pathway or c-Myc promoter activation [2, 11, 24, 25].
Current studies on tissue expression of E-cadherin and N-cadherin indicate that in CH-C, even in the course of prolonged course of the infection (> 20 years), no evident tissue exponents of cadherin switching are noted. Nevertheless, the lowered expression of E-cadherin in livers of patients with CH-C and HCC, as compared to the control, suggests the tendency to the epithelial-mesenchymal transition (EMT), even in the absence of the expected changes in expression of N-cadherin. The results are consistent with the literature data [8, 26]. This lowered expression of E-cadherin was not accompanied by the expected overexpression of β -catenin, although the two proteins manifested positive reciprocal correlations in both the CHC and HCC group. No significant relationships were documented between E- and N-cadherin expression and clinicopathological data; however, many other authors have demonstrated positive correlations between the lowered expression of E-cadherin and clinicopathological data [8, 26–28]. Similarly to our study, no relationship could be documented between expression of E-cadherin (and other cell-adhesive proteins) and age, sex, HCV genotype, development of liver cirrhosis, concentration of AFP or tumour diameter [27].
In patients with CH-C significant positive correlations were demonstrated between expression of E-cadherin and expression of all HCV proteins (C, NS3 and NS5A), and the core protein and NS5A in particular. No data on the topic have been identified in the literature related to chronic HCV infection. However, in HCC involvement of core protein in repression of E-cadherin transcription was demonstrated through hypermethylation of CpG islands in the CDH1 gene promoter [29, 30]. Decreased expression of E-cadherin was demonstrated in cultured HCC cells infected with HCV of 1b or 2a genotype. This results in activation of the Wnt signalling pathway and, through the induction of EMT, augments aggressiveness of HCC [31]. Bose et al. reported that HCV-infected hepatocytes changed their phenotype and manifested a significantly higher level of phosphorylation of SER552, which would be responsible for nuclear translocation of β -catenin [32]. Recently, it was reported that HCV-infected hepatocytes manifested reduced expression of E-cadherin as well as induction of mesenchymal marker production, e.g. N-cadherin [33]. Accurate evaluation of the HCV infection and Wnt/ β -catenin signalling pathway relationships in vivo would require further studies in more advanced lesions of chronic hepatitis C (liver cirrhosis, HCV-associated HCC). It is well known that an effective therapy for treatment of already hepatic fibrosis in a HCV-infected organ is urgently needed. It was recently proved in a mouse in vivo model of fibrosis that Wnt-inducible signalling pathway protein-1 (WISP-1 or CCN4) may represent a novel therapeutic target for liver injury and fibrosis. CCN4 inhibition by CCN4 mAb significantly attenuated liver injury and the progression of liver fibrosis [34].
In conclusion, changes in cellular localization of β -catenin and E-cadherin in livers of patients with chronic hepatitis C (CH-C) and hepatocellular carcinoma (HCC) point to structural disturbances in intercellular junctions in the pathologically altered organ. Prevalence of non-nuclear localization of β -catenin and E- and N-cadherins associated with the absence of β -catenin overexpression in the liver affected by long-lasting CH-C and HCC suggests the presence of a transcriptionally inactive form of β -catenin in the tissue material. The lowered expression of E-cadherin in long-lasting CH-C may provide an early indicator of the EMT process. Out of the three HCV proteins, the most important role in modulating the Wnt/ β -catenin pathway in vivo is probably played by the NS5A viral protein.
Acknowledgments
The work was supported in part by grant no. 2PO5A 00829 and no. N N401009437 from the Minister of Education and Science, Warsaw, Poland.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Selimovic D, El-Khattouti A, Ghozlan H, Haikel Y, Abdelkader O, Hassan M. Hepatitis C virus-related hepatocellular carcinoma: an insight into molecular mechanisms and theraupeutic strategies. World J Hepatol. 2012;4:342–55. doi: 10.4254/wjh.v4.i12.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgs MR, Lerat H, Pawlotsky JM. Hepatitis C virus-induced activation of beta-catenin promotes c-Myc expression and a cascade of pro-carcinogenetic events. Oncogene. 2013;32:4683–93. doi: 10.1038/onc.2012.484. [DOI] [PubMed] [Google Scholar]
- 3.Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY. Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol. 2000;157:763–70. doi: 10.1016/s0002-9440(10)64590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoshida Y, Nijman SM, Kobayashi M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–92. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Liu J, Jin R, et al. Cytoplasmic and/or nuclear expression of beta-catenin correlate with poor prognosis and unfavorable clinicopathological factors in hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9:e111885. doi: 10.1371/journal.pone.0111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong CM, Fan ST, Ng IO. Beta-catenin mutation and overexpression in hepatocellular carcinoma: clinicopathological and prognostic significance. Cancer. 2001;92:136–45. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 7.Cui J, Zhou XD, Liu YK, Tang ZY, Zile MH. Abnormal beta-catenin gene expression with invasiveness of primary hepatocellular carcinoma in China. World J Gastroenterol. 2001;7:542–6. doi: 10.3748/wjg.v7.i4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhai B, Yan HX, Liu SQ, Chen L, Wu MC, Wang HY. Reduced expression of E-cadherin/catenin complex in hepatocellular carcinoma. World J Gastroenterol. 2008;14:56665–73. doi: 10.3748/wjg.14.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho SB, Lee KH, Lee JH, et al. Expression of E-and N-cadherin and clinicopathology in hepatocellular carcinoma. Pathol Int. 2008;58:635–42. doi: 10.1111/j.1440-1827.2008.02282.x. [DOI] [PubMed] [Google Scholar]
- 10.Rogacki K, Kasprzak A, Stępiński A. Alterations of WNT/beta-catenin signaling pathway in hepatocellular carcinomas associated with hepatitis C virus. Pol J Pathol. 2015;66:9–21. doi: 10.5114/pjp.2015.51148. [DOI] [PubMed] [Google Scholar]
- 11.Street A, Macdonald A, Crowder K, Harris M. The hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-depended survival signaling cascade. J Biol Chem. 2004;279:12232–41. doi: 10.1074/jbc.M312245200. [DOI] [PubMed] [Google Scholar]
- 12.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 13.Sabattini E, Bisgaard K, Ascani S, et al. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol. 1998;51:506–11. doi: 10.1136/jcp.51.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurgul E, Kasprzak A, Bluszczyk A, et al. Ghrelin and obestatin in thyroid gland – immunohistochemical expression in nodular goiter, papillary and medullary cancer. Folia Histochem Cytobiol. 2015;53:19–25. doi: 10.5603/FHC.a2015.0004. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, Fujii H, Sankila A, et al. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol. 1999;155:1795–801. doi: 10.1016/s0002-9440(10)65496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasprzak A, Spachacz R, Wachowiak J, Stefańska K, Kaczmarek E, Zabel M. Tissue expression of interleukin 2 (IL-2) and IL-2 receptor (IL-2Ralpha/CD25) in non-Hodgkin’s B-cell lymphomas in children: correlation with clinical data. J Pediatr Hematol Oncol. 2010;32:462–71. doi: 10.1097/MPH.0b013e3181e33f9c. [DOI] [PubMed] [Google Scholar]
- 17.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–40. [PubMed] [Google Scholar]
- 18.Cui J, Zhou X, Liu Y, Tang Z. Mutation and overexpression of beta-catenin gene may play an important role in primary hepatocellular carcinoma among Chinese people. J Cancer Res Clin Oncol. 2001;127:577–81. doi: 10.1007/s004320100259. [DOI] [PubMed] [Google Scholar]
- 19.Tien LT, Ito M, Nakao M, et al. Expression of beta-catenin in hepatocellular carcinoma. World J Gastroenterol. 2005;11:2398–401. doi: 10.3748/wjg.v11.i16.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joo M, Lee HK, Kang YK. Expression of beta-catenin in hepatocellular carcinoma in relation to tumor cell proliferation and cyclin D1 expression. J Korean Med Sci. 2003;18:211–7. doi: 10.3346/jkms.2003.18.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo Y, Kanai Y, Sakamoto M, et al. Beta-catenin accumulation and mutation of exon 3 of beta-catenin gene in hepatocellular carcinoma. Jpn J Cancer Res. 1999;90:1301–9. doi: 10.1111/j.1349-7006.1999.tb00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao TL, Chu JS, Jeng MY, Lai PL, Hsu HC. Expression of mutant nuclear beta-catenin correlates with non-invasive hepatocellular carcinoma, absence of portal vein spread, and good prognosis. J Pathol. 2001;193:95–101. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH720>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Inagawa S, Itabashi M, Adachi S, et al. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin Cancer Res. 2002;8:450–6. [PubMed] [Google Scholar]
- 24.Milward A, Mankouri J, Harris M. Hepatitis C virus NS5A protein interacts with beta-catenin and stimulates its transcriptional activity in phosphoinositide-3 kinase-dependent fashion. J Gen Virol. 2010;91:373–81. doi: 10.1099/vir.0.015305-0. [DOI] [PubMed] [Google Scholar]
- 25.Park JY, Park WS, Nam SW, et al. Mutations of beta-catenin and AXIN 1 genes are a late event in human hepatocellular carcinogenesis. Liver Int. 2005;25:70–6. doi: 10.1111/j.1478-3231.2004.0995.x. [DOI] [PubMed] [Google Scholar]
- 26.Ihara A, Koizumi H, Hashizume R, Uchikoshi T. Expression of epithelial cadherin and alpha and beta-catenins in nontumoral livers and hepatocellular carcinomas. Hepatology. 1996;23:1441–7. doi: 10.1053/jhep.1996.v23.pm0008675162. [DOI] [PubMed] [Google Scholar]
- 27.Guo C, Liu QG, Yang W, Zhang ZL, Yao YM. Relation among p130Cas, E-cadherin and beta-catenin expression, clinicopathologic significance and prognosis in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2008;7:490–6. [PubMed] [Google Scholar]
- 28.Mima K, Hayashi H, Kuroki H, et al. Epithelial-mesenchymal transcition expression profiles as a prognostic factor for disease-free survival in hepatocellular carcinoma: clinical significance of transforming growth factor-beta-signaling. Oncology Lett. 2013;5:149–54. doi: 10.3892/ol.2012.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arora P, Kim EO, Jung JK, Jang KL. Hepatitis C virus core protein downregulates E-cadherin expression via activation of DNA methyltransferase 1 and 3b. Cancer Lett. 2008;261:244–52. doi: 10.1016/j.canlet.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 30.Ripoli M, Barbano R, Balsamo T, et al. Hypermethylated levels of E-cadherin promoter in Huh-7 cells expressing the HCV core protein. Virus Res. 2011;160:74–81. doi: 10.1016/j.virusres.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Quan H, Zhou F, Nie D, et al. Hepatitis C virus core protein epigenetically silences SFRP1 and enhances HCC aggressiveness by inducing epithelial-mesenchymal transition. Oncogene. 2014;33:2826–35. doi: 10.1038/onc.2013.225. [DOI] [PubMed] [Google Scholar]
- 32.Bose SK, Meyer K, Di Bisceglie AM, Ray RB, Ray R. Hepatitis C virus induces epithelial-mesenchymal transition in primary human hepatocytes. J Virol. 2012;86:13621–8. doi: 10.1128/JVI.02016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iqbal J, McRae S, Mai T, Banaudha K, Sarkar-Dutta M, Waris G. Role of hepatitis C virus induced osteopontin in epithelial to mesenchymal transition, migration and invasion of hepatocytes. PLoS One. 2014;9:e87464. doi: 10.1371/journal.pone.0087464. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Li X, Chen Y, Ye W, et al. Blockade of CCN4 attenuates CCl4-induced liver fibrosis. Arch Med Sci. 2015;3:647–53. doi: 10.5114/aoms.2015.52371. [DOI] [PMC free article] [PubMed] [Google Scholar]