Abstract
Introduction
Guidelines based on the Barcelona Clinic Liver Cancer (BCLC) classification system recommend that hepatic resection should be performed only in patients in BCLC stage A. Patients with stage B or stage C should receive palliative or no treatment. However, actual clinical practice varies, and a recent analysis of hepatocellular carcinoma (HCC) surgery outcomes in high volume surgical centers throughout the world concluded that hepatectomy can provide survival benefit for selected patients in all three BCLC stages. The aim of this study is to evaluate the efficacy and tolerability of adjuvant sorafenib after hepatic resection in patients with intermediate-stage and advanced HCC.
Material and methods
In a retrospective case-control study involving 81 patients with intermediate/advanced HCC, 27 who received sorafenib 400 mg BID (median duration 7.33 months) following hepatic resection were compared with a matched group of 54 patients who received hepatic resection only. Overall survival (OS) and time to recurrence (TTR) were evaluated over a median follow-up time of 14.5 months.
Results
The median OS was significantly longer in the surgery+sorafenib group than in the surgery-only group (18.6 vs. 11.9 months, respectively; p = 0.014). However, the median TTR did not differ significantly between the 2 groups (p = 0.291).
Conclusions
Sorafenib is effective as adjuvant therapy after liver resection in intermediate-stage and advanced HCC, and can be considered a viable treatment option following surgery in such patients.
Keywords: carcinoma, hepatocellular, hepatectomy, sorafenib
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide, and the third most common cause of cancer-related deaths [1]. Because of the high prevalence of hepatitis B virus (HBV) infection in China, HCC has a particularly high incidence in the Chinese population [2]. Treatment of HCC is complex because of the need to destroy tumor cells while at the same time preserving the often compromised liver function.
Guidelines based on the Barcelona Clinic Liver Cancer (BCLC) classification system [3–5] recommend that hepatic resection should be performed only in patients in BCLC stage A, that is, only in those with small, single tumors and no signs or symptoms of liver dysfunction. Patients with multiple or large tumors (stage B) or those with symptomatic or invasive tumors (stage C) should receive palliative or no treatment [6, 7]. However, actual clinical practice varies, and a recent analysis of HCC surgery outcomes in high volume surgical centers throughout the world concluded that hepatectomy can provide survival benefit for selected patients in all three BCLC stages [8].
Current BCLC-recommended treatments are not those currently applied in Asia-Pacific region countries such as China, Korea and Japan [9–12]. In the treatment algorithms used in the Asia-Pacific region, where radical resection is impractical, palliative resection combined with comprehensive therapy can be considered as a treatment option in patients with intermediate-stage and advanced HCC (BCLC stage B and stage C), even in the presence of poor prognostic factors such as venous and/or bile duct tumor thrombi or hilar lymph node metastasis. Survival benefits for this type of therapy have been reported in a broad group of such patients, although their outcome is inferior to the outcome of patients who do not have portal hypertension or have only single tumors [8, 13–17].
Sorafenib is an oral multikinase inhibitor that blocks tumor cell proliferation and angiogenesis by inhibiting the serine/threonine kinases Raf-1/B-Raf, and the tyrosine kinases of vascular endothelial growth factor receptor (VEGFR-2/-3) and platelet-derived growth factor receptor (PDGFR) [18]. In patients with advanced HCC, sorafenib therapy has produced significant improvement in the time to progression (TTP) and overall survival (OS) [19, 20].
As treatment outcomes with sorafenib therapy following hepatic resection in patients with intermediate-stage and advanced HCC are not yet known, we performed a retrospective, case-control study in HCC patients who were treated with or without adjuvant sorafenib therapy following surgery. The aim of the study was to evaluate the effectiveness and safety of adjuvant sorafenib in patients receiving surgery + sorafenib by comparing them with a matching group of patients treated with surgery alone.
Material and methods
Study design and patients
This single-center, retrospective, case-control study was performed at West China Hospital, Sichuan, China. Relevant data were retrieved from the patients’ clinical records, including medical history, laboratory results, radiologic findings, histology results, treatments and survival data, as well as the dosage and adverse events of adjuvant sorafenib therapy administered between January 2010 and June 2012. Patient status data (alive vs. deceased vs. progression) were collected periodically until the last follow-up day of the study, which was September 30, 2013.
Surgery + sorafenib group
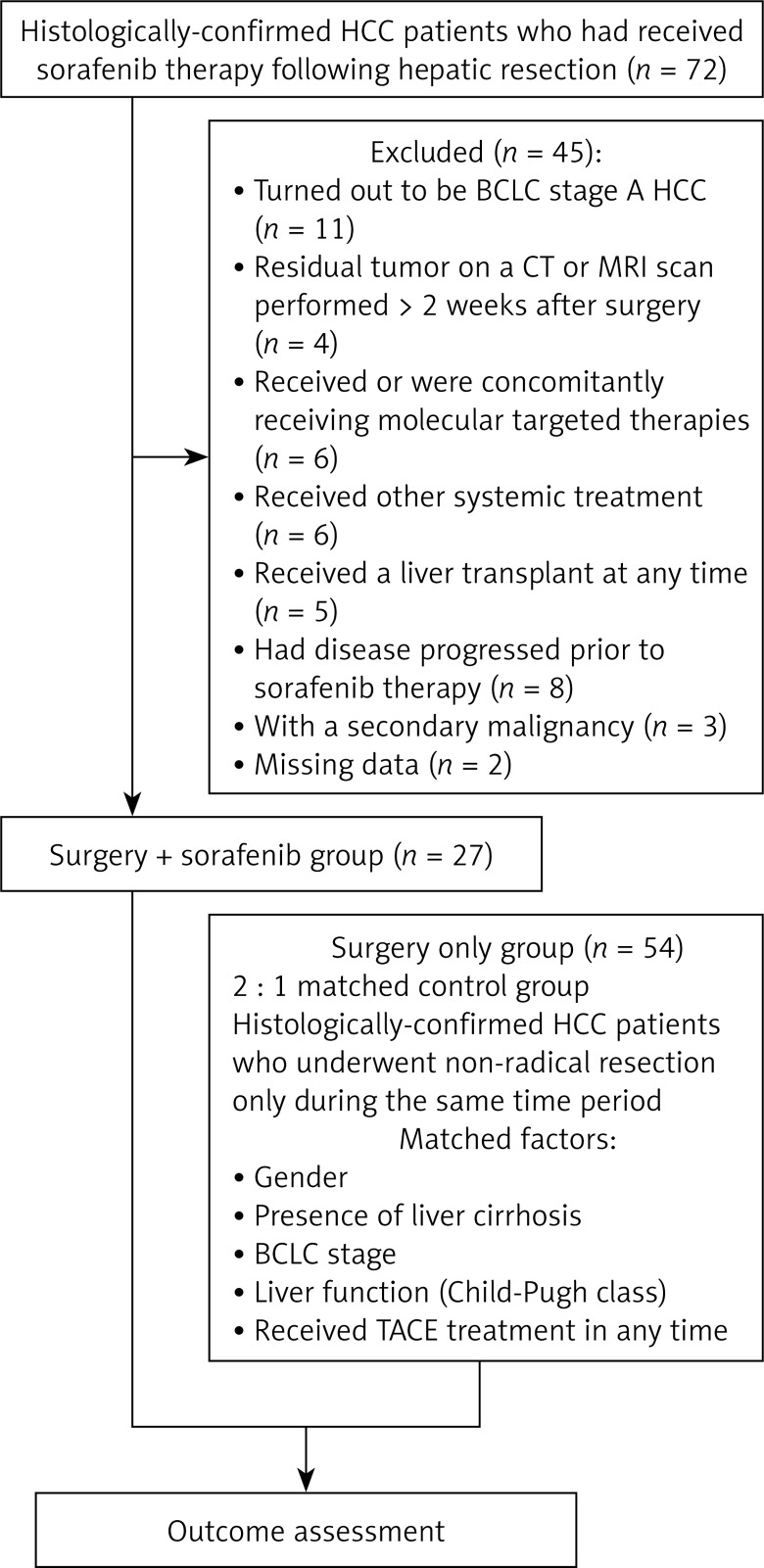
We identified a total of 72 patients with histologically confirmed HCC who had received adjuvant sorafenib therapy following hepatic resection. Patients meeting the following criteria were eligible for inclusion in the study: liver tumor(s) classified as BCLC stage B or C, as confirmed by intraoperative ultrasound or postoperative pathology; no visible residual tumor on a computed tomography (CT) or magnetic resonance imaging (MRI) scan (as assessed by local radiological review) performed > 2 weeks after surgery; and no documented tumor recurrences evident on a CT or MRI scan performed prior to commencement of sorafenib therapy.
Patients who had previously received or were concomitantly receiving molecular targeted therapies or any other systemic treatment, those who had received a liver transplant at any time, those whose disease had progressed prior to sorafenib therapy, those with a secondary malignancy, and those with missing data were excluded from the analysis. In the event of drug-related adverse events, treatment interruptions or dosage reductions of sorafenib from 400 mg BID to 200 mg BID or to 200 mg BID, every alternate day was permitted. If further dosage reductions were required, patients were excluded from the study. The number of patients excluded on the basis of each exclusion criterion is shown in the flow chart in Figure 1.
Figure 1.
Flow chart of protocol
Surgery only group
A 2 : 1 matched control group was created from patients who had undergone non-radical resection without sorafenib therapy during the same time period (Figure 1). The control group was matched for gender, presence of liver cirrhosis, BCLC stage, liver function (Child-Pugh class), transarterial chemoembolization (TACE) treatment, and diagnosis.
Treatment
Surgery
The extent of hepatic resection was defined according to Couinaud’s classification of liver anatomy. Major hepatectomy was defined as resection of 3 or more segments, while minor hepatectomy was the resection of fewer than 3 segments.
Sorafenib treatment
An initial sorafenib dosage of 400 mg was administered orally twice daily. Subsequently, discontinuations and dosage reductions of sorafenib were based on tolerance. Treatment was continued until clinical disease progression or unacceptable drug-related toxicity occurred.
Patients in both the sorafenib + surgery and surgery-only groups did not receive any other therapy after surgery except TACE.
BCLC classification
The following categories were used to classify patients:
Stage B HCC: presence of 2 to 3 tumors, at least 1 of which was more than 3 cm in diameter; or more than 3 tumors of any diameter.
Stage C HCC: any tumor with radiologically evident and histologically proven macrovascular invasion (portal vein, hepatic vein, inferior vena cava).
Outcome assessment
The patients’ clinical, laboratory, and radiologic records were reviewed independently by several investigators. The primary endpoints of the study were OS and time to recurrence (TTR). The patients’ survival duration was calculated from the date of surgery to death or study closure, while TTR was calculated from the date of surgery to radiologic recurrence or metastasis. Outcomes were also assessed in BCLC stage B and C subgroups of the 2 patient groups.
The secondary endpoint of the study was safety. Adverse events (AEs) of sorafenib were classified according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0. [21].
Statistical analysis
Continuous data are presented as means and standard deviations, and categorical data as counts and percentages. Because matched data were used, the linear mixed model or generalized estimating equations (GEE) were used to compare the differences between the surgery + sorafenib and surgery-only groups. Kaplan-Meier curves with log-rank tests were performed to compare OS and TTR between the two treatment groups. Univariate and multivariate Cox proportional hazard models were performed to detect factors affecting survival and recurrence. Factors significantly affecting overall survival in univariate analyses were included in multivariate analyses. Adverse events were compared using Fisher’s exact test. A two-tailed p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.2 statistics software (SAS Inc., Cary, NC, USA).
Results
Patients
Demographic and clinicopathological characteristics of patients are shown in Table I. Twenty-seven patients were included in the surgery + sorafenib group and 54 matched patients were included in the surgery-only group. None of the patient characteristics showed any statistically significant difference between the 2 groups. The predominant cause of the underlying liver disease was HBV infection, which was found in 23 (85.2%) patients in the surgery + sorafenib group and 48 (88.9%) patients in the surgery-only group. No patients in either group had HCV infection. All patients were rated as Child-Pugh class A at baseline, a classification reflecting well-preserved liver function. There were no significant differences in mean tumor size, number of tumors, serum α-fetoprotein (AFP) concentrations measured before surgery, Eastern Cooperative Oncology Group (ECOG) performance status, distribution of cancer stages, or anti-virus therapy (all p > 0.05).
Table I.
Demographic and clinicopathological characteristics of patients
| Variable | Surgery + sorafenib group (n = 27) | Surgery-only group (n = 54) | P-value | |
|---|---|---|---|---|
| Age, mean ± SD [years] | 48.2 ±9.7 | 49.4 ±9.4 | 0.450 | |
| Gender: | ||||
| Male | 25 (92.6%) | 50 (92.6%) | 1.000 | |
| Female | 2 (7.4%) | 4 (7.4%) | ||
| Viral hepatitis status: | ||||
| HBV | 23 (85.2%) | 48 (88.9%) | 0.206 | |
| Unknown | 4 (14.8%) | 6 (11.1%) | ||
| Preoperative AFP: | ||||
| < 400 ng/ml | 12 (44.4%) | 15 (27.8%) | 0.195 | |
| ≥ 400 ng/ml | 15 (55.6%) | 39 (72.2%) | ||
| Liver cirrhosis: | ||||
| No | 6 (22.2%) | 12 (22.2%) | 1.000 | |
| Yes | 21 (77.8%) | 42 (77.8%) | ||
| ECOG performance status score: | ||||
| 0 | 12 (44.4%) | 35 (64.8%) | 0.076 | |
| 1 | 15 (55.56%) | 19 (35.19%) | ||
| BCLC stage: | ||||
| B | 12 (44.4%) | 24 (44.4%) | 1.000 | |
| C | 15 (55.6%) | 30 (55.6%) | ||
| Number of tumors: | ||||
| < 3 | 15 (55.6%) | 33 (61.1%) | 0.739 | |
| ≥ 3 | 12 (44.4%) | 21 (38.9%) | ||
| Tumor size, mean ± SD [cm] | 7.8 ±3.9 | 8.4 ±3.5 | ||
| Resection extent: | 0.788 | |||
| Minor | 8 (29.6%) | 18 (33.3%) | ||
| Major | 19 (70.7%) | 36 (66.7%) | ||
| Tumor differentiation: | ||||
| Poor | 19 (70.4%) | 36 (66.7%) | 0.839 | |
| Moderate | 8 (29.6%) | 18 (33.3%) | ||
| Cause of death: | 0.812 | |||
| Cancer recurrence | 17 (62.96%) | 44 (81.48%) | ||
| Miscellaneous – cancer recurrence and liver failure | 1 (3.7%) | 1 (1.85%) | ||
| Hepatic failure or multiple systemic organ dysfunction syndrome during the 90 days after surgery | 0 (0%) | 1 (1.85%) | ||
| Anti-virus therapy: | 0.744 | |||
| Yes | 24 (88.89%) | 46 (85.19%) | ||
| No | 3 (11.11%) | 8 (14.81%) | ||
AFP – α-fetoprotein, BCLC – Barcelona Clinic Liver Cancer staging classification, ECOG – Eastern Cooperative Oncology Group, HCC – hepatocellular carcinoma, HBV – hepatitis B virus, SD – standard deviation.
The median duration of sorafenib treatment in the surgery + sorafenib group was 7.3 months (95% CI: 5.8–8.9 months). No patient died within 30 days of resection, although 1 patient in the surgery-only group died within 90 days of resection.
During the 90 days after surgery, 17 patients in the surgery + sorafenib group died due to cancer recurrence, 1 due to miscellaneous cancer recurrence and liver failure, and 1 due to hepatic failure or multiple systemic organ dysfunction syndrome. No significant differences in cause of death were found between the surgery + sorafenib group and surgery-only group. Twenty-four patients received anti-viral therapy in the surgery + sorafenib group, and no significant difference was found between the two groups in this parameter (Table I).
Comparisons between treatments in overall survival and time to recurrence
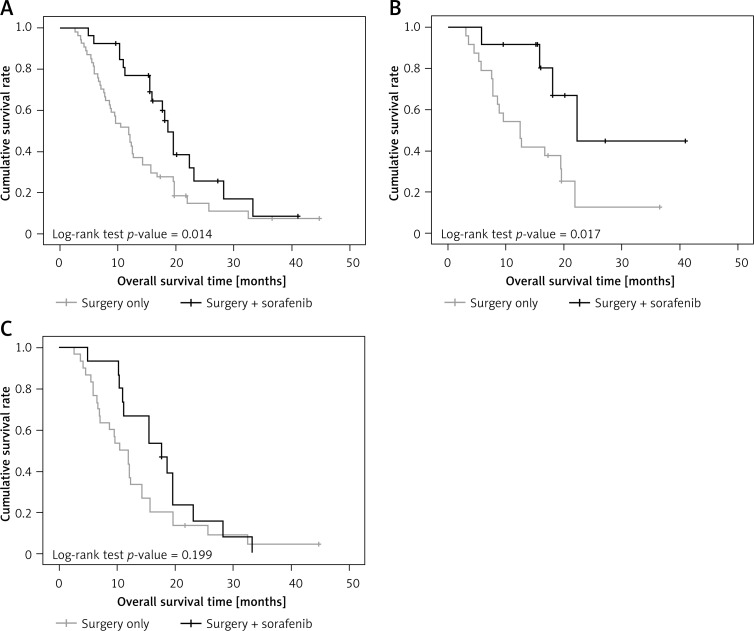
The median follow-up duration in the total patient population was 14.5 months (range: 2.6–44.7 months). During this period, 63 deaths occurred (45 in the surgery-only group and 18 in the surgery + sorafenib group). Overall survival rates were significantly higher in the surgery + sorafenib group than in the surgery-only group (median survival 18.6 and 11.9 months respectively, p = 0.014, Figure 2 A).
Figure 2.
A – Overall survival of patients in the surgery + sorafenib group and surgery only group. B – Overall survival of patients with BCLC stage B disease in the surgery + sorafenib group and surgery only group. C – Overall survival of patients with BCLC stage C disease in the surgery + sorafenib group and surgery group
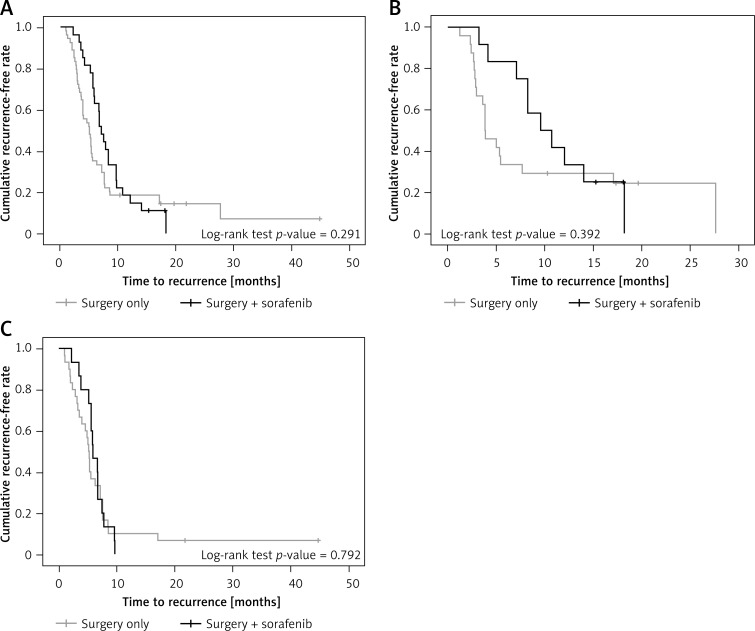
Time to recurrence for the 2 treatments is shown in Figure 3. There was no significant difference between the sorafenib + surgery and the surgery-only groups in time to recurrence (p = 0.291).
Figure 3.
A – Time to recurrence of patients in surgery + sorafenib group and surgery-only group. B – Time to recurrence of patients with BCLC stage B in surgery + sorafenib group and surgery-only group. C – Time to recurrence of patients with BCLC stage C in surgery + sorafenib group and surgery-only group
Subgroup analysis of overall survival
In univariate analyses, age and treatment group were found to be significantly related to overall survival (Table II). The risk of death was significantly decreased in the surgery + sorafenib group compared with the surgery-only group (HR = 0.51, p = 0.016) and was slightly, but significantly, decreased as age increased (HR = 0.97, p = 0.035). When factors significantly related to overall survival in the univariate analyses were included in the multivariate analysis (Table II), after adjustment for age, the risk of death was again significantly decreased in the surgery + sorafenib group compared with the surgery-only group (HR = 0.52, p = 0.019).
Table II.
Univariate and multivariate analyses to detect factors associated with overall survival
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age [years] | 0.97 (0.95–1) | 0.035* | 0.97(0.95–1) | 0.042* |
| Gender (ref: female) | 0.7 (0.3–1.64) | 0.415 | ||
| Viral hepatitis status (ref: unknown) | 0.74 (0.38–1.47) | 0.393 | ||
| Preoperative AFP (ref: < 400 ng/ml) | 0.96 (0.57–1.6) | 0.866 | ||
| Liver cirrhosis (ref: no) | 1.1 (0.59–2.03) | 0.773 | ||
| ECOG performance status score (ref: 0) | 0.85 (0.51–1.43) | 0.546 | ||
| BCLC stage (ref: B) | 1.66 (0.99–2.79) | 0.054 | ||
| Number of tumors (ref: < 3) | 1.28 (0.77–2.13) | 0.351 | ||
| Tumor size [cm] | 1 (0.94–1.06) | 0.897 | ||
| Resection extent (ref: minor) | 0.74 (0.44–1.25) | 0.261 | ||
| Tumor differentiation (ref: poor) | 0.75 (0.44–1.29) | 0.299 | ||
| TACE therapy (ref: no) | 1.12 (0.69–1.84) | 0.644 | ||
| Group (ref: surgery only) | 0.51 (0.3–0.88) | 0.016* | 0.52 (0.3–0.9) | 0.019* |
P < 0.05 represents significant association with overall survival.
Overall survival was also related to BCLC stage. For patients in BCLC stage B, overall survival rates were significantly higher in the surgery + sorafenib group than in the surgery-only group (median survival 22.3 and 12.5 months, respectively, p = 0.017, Figure 2 B). For patients in BCLC stage C, no significant difference was found between the two groups in overall survival rate (p = 0.199, Figure 2 C).
Subgroup analysis of time to recurrence
In univariate analyses, only BCLC stage was found to be significantly related to recurrence. The risk of recurrence was significantly increased in BCLC stage C compared to BCLC stage B (HR = 1.64, p = 0.045). No other factors were significantly related to recurrence in univariate analysis; therefore multivariate analysis was not performed (Table III).
Table III.
Univariate analyses to detect factors associated with recurrence
| Parameter | Univariate | |
|---|---|---|
| HR (95% CI) | P-value | |
| Age [years] | 0.98 (0.95–1) | 0.102 |
| Gender (ref: female) | 0.73 (0.31–1.7) | 0.468 |
| Viral hepatitis status (ref: unknown) | 0.46 (0.19–1.09) | 0.079 |
| Preoperative AFP (ref: < 400 ng/ml) | 1.02 (0.59–1.74) | 0.957 |
| Liver cirrhosis (ref: no) | 0.85 (0.48–1.49) | 0.558 |
| ECOG performance status score (ref: 0) | 1.08 (0.66–1.78) | 0.765 |
| BCLC stage (ref: B) | 1.64 (1.01–2.67) | 0.045* |
| Number of tumors (ref: < 3) | 0.9 (0.56–1.44) | 0.654 |
| Tumor size [cm] | 0.995 (0.94–1.05) | 0.872 |
| Resection extent (ref: minor) | 0.87 (0.53–1.43) | 0.581 |
| Tumor differentiation (ref: poor) | 0.65 (0.39–1.08) | 0.095 |
| TACE therapy (ref: no) | 1.21 (0.75–1.94) | 0.437 |
| Group (ref: surgery only) | 0.77 (0.47–1.26) | 0.295 |
Relationship between sorafenib and TACE
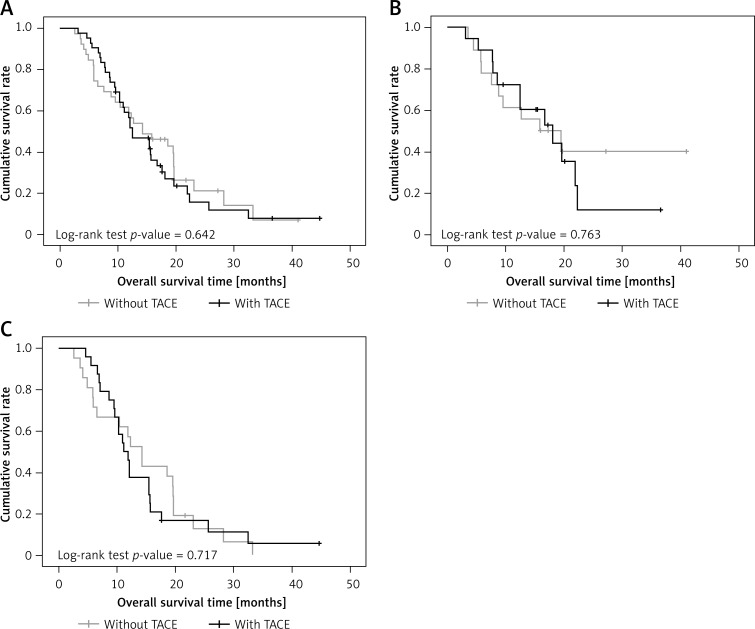
Equal percentages of patients in the surgery + sorafenib and surgery-only groups received TACE after surgery, 14 (51.9%) in the surgery + sorafenib group and 28 (51.9%) in the surgery only group. Overall survival was similar in those with TACE and those without TACE, both in the patient population as a whole and in patients with either BCLC stage B or stage C (p = 0.642 in overall survival for the total patient population; p = 0.763 in overall survival for BCLC stage B; p = 0.717 in overall survival for BCLC stage C; Figures 4 A–C).
Figure 4.
A – Overall survival rate between patients with and without TACE. B – Overall survival rate between patients with and without TACE in patients with BCLC stage B. C – Overall survival rate between patients with and without TACE in patients with BCLC stage C
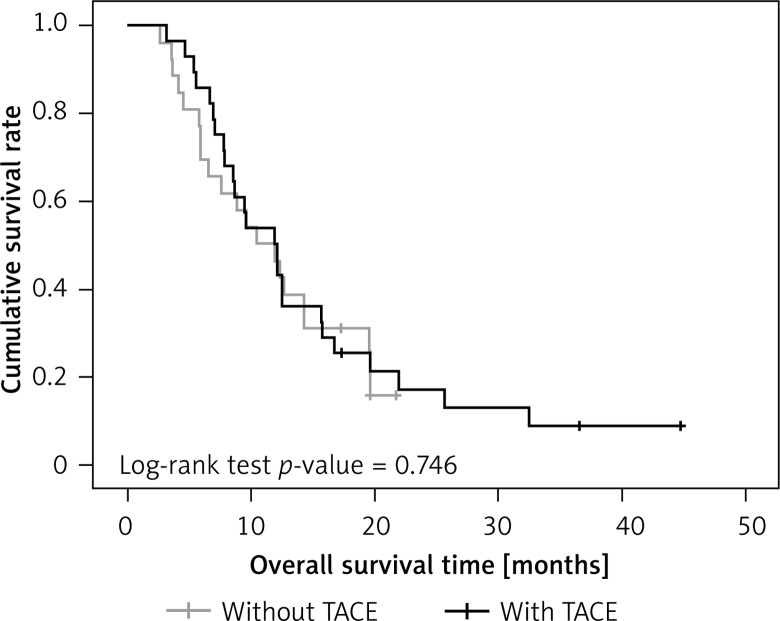
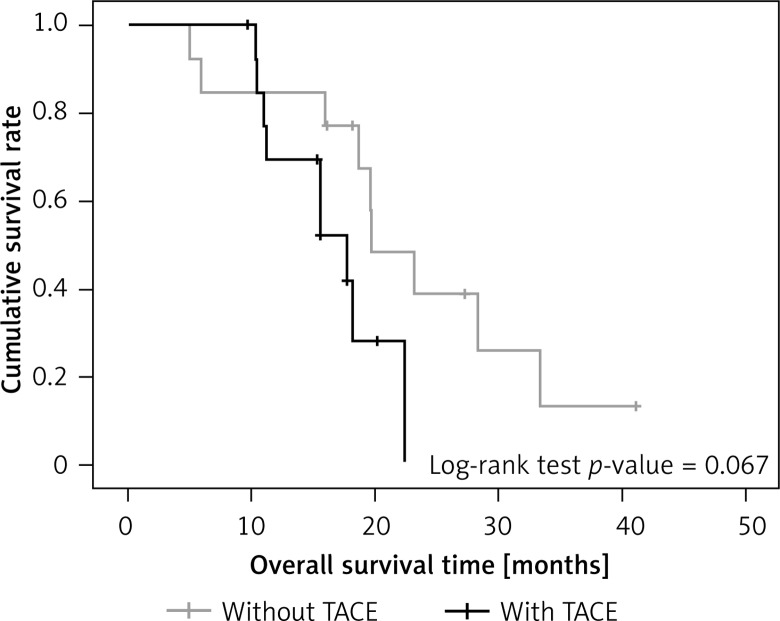
When outcomes for those with and without TACE were examined separately in the 2 treatment groups, there was also no significant difference in overall survival rate (surgery-only group, p = 0.746 in overall survival; surgery + sorafenib group p = 0.067 in overall survival) (Figures 5 and 6).
Figure 5.
Overall survival rate between surgery-only patients with and without TACE
Figure 6.
Overall survival between surgery + sorafenib patients with and without TACE
Tolerability of sorafenib
The overall incidence of treatment-related adverse events was 96.3% in the surgery + sorafenib group and 9.3% in the surgery-only group (Table IV). Significantly higher percentages of patients had hand-foot skin reaction and diarrhea in the surgery + sorafenib group compared with those in the surgery-only group (p < 0.001 for hand-foot skin reaction and p = 0.035 for diarrhea). There were no significant differences between the two treatments for adverse events of alopecia, rash, hypertension, anorexia, vomiting, nausea, or fatigue (all p > 0.05).
Table IV.
Adverse events between different treatments
| Adverse event | Surgery + sorafenib group (n = 27) | Surgery-only group (n = 54) | P-value | ||||
|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 or 4 | Grade 1 | Grade 2 | Grade 3 or 4 | ||
| HFSR | 7 (25.9%) | 7 (25.9%) | 2 (7.4%) | 0 (0%) | 0 (0%) | 0 (0%) | < 0.001 |
| Diarrhea | 4 (14.8%) | 1 (3.7%) | 1 (3.7%) | 2 (3.7%) | 0 (0%) | 0 (0%) | 0.035 |
| Alopecia | 2 (7.4%) | 1 (3.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.085 |
| Rash | 4 (14.8%) | 1 (3.7%) | 0 (0%) | 2 (3.7%) | 0 (0%) | 0 (0%) | 0.069 |
| Hypertension | 1 (3.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.308 |
| Anorexia | 3 (11.1%) | 0 (0%) | 0 (0%) | 1 (1.85%) | 0 (0%) | 0 (0%) | 0.139 |
| Vomiting | 1 (3.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.308 |
| Nausea | 2 (7.4%) | 0 (0%) | 0 (0%) | 1 (1.85%) | 0 (0%) | 0 (0%) | 0.290 |
| Fatigue | 3 (11.1%) | 0 (0%) | 0 (0%) | 2 (3.7%) | 0 (0%) | 0 (0%) | 0.227 |
HFSR – hand-foot skin reaction.
Adverse events in patients who received sorafenib were predominantly grade 1 or 2 in severity. Grade 3 adverse events occurred only in those who received sorafenib, and included diarrhea in 1 (3.7%) patient and hand-foot skin reactions in 2 (7.4%) patients. No grade 4 treatment-related adverse events were recorded in either group. Dosage reductions were required in 5 (18.5%) patients in the surgery + sorafenib group due to adverse events, but no patient on sorafenib required treatment discontinuation because of adverse events that caused them to be excluded from the study.
Discussion
In the current study, sorafenib significantly increased overall survival after hepatectomy in patients with intermediate or advanced HCC, but when survival was examined according to cancer stage, this increase reached significance in BCLC B, but not stage C, patients. Almost all patients receiving sorafenib experienced mild adverse effects, the most common being hand-foot skin syndrome. Approximately 50% of patients in both the surgery-only and the surgery + sorafenib group received post-surgery TACE, but the inclusion of TACE did not increase either overall survival or time to recurrence.
Although guidelines recommend hepatectomy only for BCLC stage A patients, current clinical practice is to use hepatectomy also in stage B and C patients [8, 16]. Liver resection is considered to be the best treatment for selected patients with large, solitary, multinodular, or macrovascular invasion HCC in some regions [9–12, 22], and in China liver resection may be considered a first-line treatment in patients with intermediate-stage or advanced HCC who have good preoperative liver function and adequate postoperative residual liver volume [8]. The safety of liver resection has been well established for HCC [23–27], and a number of studies have reported promising results in intermediate-stage and advanced cases [8, 13–17, 26–28].
However, even after curative hepatectomy, microvascular invasion and microscopic satellite nodules, indicators of microscopic intrahepatic metastases, may remain [29]. Therefore, the antiangiogenic, proapoptotic, and antiproliferative effects of sorafenib [18] make it a logical drug to use after hepatectomy in terms of theory.
Although the benefits of HCC treatments should be assessed via randomized, controlled trials, a randomized controlled trial is not always practical. Few papers have compared the effectiveness of the use of TACE or sorafenib after surgery in intermediate/advanced stage HCC. In general, therefore, the optimal treatment strategy for intermediate/advanced stage HCC cases is still controversial.
The present retrospective case-control study was conducted in an attempt to prolong the long-term survival of patients with intermediate-stage and advanced HCC. To the best of our knowledge, this is the first study to evaluate the effectiveness and safety of sorafenib therapy following surgery in such patients. Our results provide evidence of the superiority of sorafenib treatment after surgery compared to surgery alone in terms of the OS of patients with intermediate-stage and advanced HCC. Furthermore, subgroup analysis showed that the median OS of patients with BCLC stage B disease was longer with sorafenib treatment than without it (p = 0.017), although sorafenib did not significantly increase the median TTR. Because most published studies of the combination of sorafenib and surgery have focused on early-stage patients, it is not feasible to compare our results with those of others.
Currently, sorafenib, which blocks tumor cell proliferation and angiogenesis, is the only treatment recommended for advanced HCC on the basis of 2 phase III clinical trials [19, 20], although another substance, Brucea javanica extract, has been found to inhibit cell proliferation and increase apoptosis in animal and cellular studies of HCC [30]. It prolongs survival in advanced HCC patients by about 3 months [19, 20] and is equally effective in younger and older (> 75 years) patients [31]. In our study, sorafenib increased median overall survival time by about 7 months in the total patient group, and about 10 months in stage B patients alone. One might speculate that sorafenib did not increase overall survival in stage C patients because these patients often have a greater degree of cirrhosis and poorer remaining liver function than patients in earlier stages of HCC, and that increased deaths caused by the worsening liver disease might mask any potential effect of sorafenib on OS.
Sorafenib was well tolerated in our study, and drug-related adverse events reported by patients receiving sorafenib were predominantly only grade 1 or 2 in severity. The most frequent sorafenib-related adverse events in the surgery + sorafenib group were hand-foot skin reactions, which occurred in 17 (63.0%) patients, and diarrhea, which occurred in 6 (22.2%). The incidence of hand-foot skin reactions in our patients was in the middle of the wide range (7% to 100%) reported by others [19, 20, 29, 32, 33]. The incidence of this adverse effect varies with ethnicity, and Asian patients have been reported to have a higher incidence of this syndrome than other groups [20]. It is thought that this is a genetic predisposition due partly to changes in the genes for tumor necrosis factor α (TNF-α) and vascular endothelial growth factor (VEGF) [34]. Also, an increase in the incidence of hand-foot syndrome may be beneficial, since early expression of this and other dermatologic adverse effects has been significantly related to better survival [32, 35–37].
The TACE is currently recommended as the standard treatment for intermediate-stage HCC [38], and has been shown to be superior to supportive treatment in these patients [39–42]. However, TACE has been shown to be inferior to hepatectomy in intermediate-stage HCC [39, 43]. Whether TACE can cause significant improvement when used after hepatectomy is unresolved [44, 45], as is the question of whether it causes significant improvement when added to sorafenib treatment [32, 46–48]. Recently, several investigators have reported that the combination of sorafenib and TACE may be an effective and tolerable treatment strategy for intermediate-stage and advanced HCC. A number of studies, including a systematic review [32, 49–54] of 11 related studies involving 1000 patients, have reported that the sorafenib + TACE combination showed promise as an effective and tolerable treatment strategy. However, these studies did not examine the effects of this combination when used after surgery, and our results show TACE to provide no additional effect on OS or on TTR in either surgery + sorafenib or surgery-only patients.
The current study has several limitations. Firstly, it was a retrospective study and had only a small sample size from a single institute. Most patients in our study had HBV-related disease, a background that is quite different from that seen in patients in western countries. Another limitation is that the use of TACE may have complicated the efficacy comparisons between the 2 patient groups. However, the TACE/sorafenib combination has previously been found to be an effective treatment, and we matched the use of TACE when selecting patients for the control group.
In conclusion, the present study is the first to demonstrate the efficacy and tolerability of sorafenib therapy following hepatic resection in patients with intermediate-stage and advanced HCC. Our results suggest that adjuvant therapy with sorafenib following liver resection is effective in such patients, especially for intermediate-stage patients. Sorafenib treatment after surgery could therefore be a viable treatment option for these patients. Prospective, multicenter, randomized, controlled studies involving substantially larger patient populations are necessary to confirm our findings.
Acknowledgments
The authors acknowledge the contributions of the other investigators in this trial and Content Ed Net, Shanghai Co. Ltd for editorial assistance during manuscript preparation.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.International Agency for Research on Cancer (IARC) Available at: http://www-dep.iarc.fr/Accessed 1 Nov, 2011.
- 2.Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–85. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 4.EASL-EORTC Clinical Practice Guidelines Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M, Practice Guidelines Committee, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225–35. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115–20. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 8.Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? An observational study of the East-West HCC Study Group. Ann Surg. 2013;257:929–37. doi: 10.1097/SLA.0b013e31828329b8. [DOI] [PubMed] [Google Scholar]
- 9.Chinese Anti-Cancer Association Society of Liver Cancer, Chinese Society of Clinical Oncology, Chinese Society of Hepatology Liver Cancer Study Group [The expert consensus on the treatment standards for hepatocellular carcinoma (in Chinese) Dig Dis and Endosc. 2009;3:40–51. [Google Scholar]
- 10.Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–64. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 11.Korean Liver Cancer Study Group. National Cancer Center, Korea Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 12.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–74. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin DX, Zhang QY, Li X, Ye QW, Lin F, Li LL. An aggressive approach leads to improved survival in hepatocellular carcinoma patients with portal vein tumor thrombus. J Cancer Res Clin Oncol. 2011;137:139–49. doi: 10.1007/s00432-010-0868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris-Stiff G, Gomez D, de Liguori Carino N, Prasad KR. Surgical management of hepatocellular carcinoma: is the jury still out? Surg Oncol. 2009;18:298–321. doi: 10.1016/j.suronc.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Chirica M, Scatton O, Massault PP, et al. Treatment of stage IVA hepatocellular carcinoma: should we reappraise the role of surgery? Arch Surg. 2008;143:538–43. doi: 10.1001/archsurg.143.6.538. [DOI] [PubMed] [Google Scholar]
- 16.Torzilli G, Donadon M, Marconi M, et al. Hepatectomy for stage B and stage C hepatocellular carcinoma in the Barcelona Clinic Liver Cancer classification: results of a prospective analysis. Arch Surg. 2008;143:1082–90. doi: 10.1001/archsurg.143.11.1082. [DOI] [PubMed] [Google Scholar]
- 17.Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–16. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–44. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 20.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute Common Terminology Criteria for Adverse Events, v3.0. 2006. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 22.Jarnagin W, Chapman WC, Curley S, et al. Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford) 2010;12:302–10. doi: 10.1111/j.1477-2574.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 24.Torzilli G, Makuuchi M, Inoue K, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984–92. doi: 10.1001/archsurg.134.9.984. [DOI] [PubMed] [Google Scholar]
- 25.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–30. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng KK, Vauthey JN, Pawlik TM, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005;12:364–73. doi: 10.1245/ASO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Pawlik TM, Poon RT, Abdalla EK, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005;140:450–7. doi: 10.1001/archsurg.140.5.450. [DOI] [PubMed] [Google Scholar]
- 28.Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329–40. doi: 10.1097/SLA.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 29.Wang JH, Changchien CS, Hu TH, et al. The efficacy of treatment schedules according to Barcelona Clinic Liver Cancer staging for hepatocellular carcinoma – survival analysis of 3892 patients. Eur J Cancer. 2008;44:1000–6. doi: 10.1016/j.ejca.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Yue Y, Yang Y, Shi L, Wang Z. Suppression of human hepatocellular cancer cell proliferation by Brucea javanica oil-loaded liposomes via induction of apoptosis. Arch Med Sci. 2015;11:856–62. doi: 10.5114/aoms.2015.53306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishikawa H, Takeda H, Tsuchiya K, et al. Japanese Red Cross Study Group Sorafenib therapy for BCLC stage B/C hepatocellular carcinoma. Clinical outcome and safety in aged patients: a multicenter study in Japan. J Cancer. 2014;5:499–509. doi: 10.7150/jca.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–27. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Reig M, Torres F, Rodriguez-Lope C, et al. Early dermatoligic adverse events predict better outcomes in HCC patients treated with sorafenib. J Hepatol. 2014;61:318–24. doi: 10.1016/j.jhep.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Chung YH, Kim JA, et al. Genetic predisposition to hand-foot skin reaction after sorafenib therapy in patients with hepatocellular carcinoma. Cancer. 2013;119:136–42. doi: 10.1002/cncr.27705. [DOI] [PubMed] [Google Scholar]
- 35.Vincenzi B, Santini D, Russo A, et al. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist. 2010;15:85–92. doi: 10.1634/theoncologist.2009-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otsuka T, Eguchi Y, Kawazoe S, et al. Skin toxicities and survival in advanced hepatocellular carcinoma patients treated with soarfenib. Hepatol Res. 2012;42:879–86. doi: 10.1111/j.1872-034X.2012.00991.x. [DOI] [PubMed] [Google Scholar]
- 37.Di Costanzo GG, Tortora R, Iodice L, et al. Safety and effectiveness of sorafenib in patients with hepatocellular carcinoma in clinical practice. Dig Liver Dis. 2012;44:788–92. doi: 10.1016/j.dld.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 39.Roayaie S. TACE vs. surgical resection for BCLC stage B HCC. J Hepatol. 2014;61:3–4. doi: 10.1016/j.jhep.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolization versus supportive treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 41.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 42.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 43.Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: a RCT. J Hepatol. 2014;61:82–8. doi: 10.1016/j.jhep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Zhong JH, Ma L, Li LQ. Postoperative therapy options for hepatocellular carcinoma. Scand J Gastroenterol. 2014;49:449–61. doi: 10.3109/00365521.2014.905626. [DOI] [PubMed] [Google Scholar]
- 45.Zhong J, Xiang B, Ma L, Li L. Conventional oral systemic therapy for postoperative hepatocellular carcinoma: a systematic review. Mol Clin Oncol. 2014;2:1091–6. doi: 10.3892/mco.2014.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist. 2012;202:359–66. doi: 10.1634/theoncologist.2011-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dufour JF, Hoppe H, Heim MH, et al. Continuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: results of a phase I study. Oncologist. 2010;15:1198–204. doi: 10.1634/theoncologist.2010-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung YH, Han G, Yoon JH, et al. Interim analysis of START: Study in Asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma. Int J Cancer. 2013;132:2448–58. doi: 10.1002/ijc.27925. [DOI] [PubMed] [Google Scholar]
- 49.Abdel-Rahman O, Elsayed ZA. Combination trans arterial chemoembolization (TACE) plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review of the literature. Dig Dis Sci. 2013;58:3389–96. doi: 10.1007/s10620-013-2872-x. [DOI] [PubMed] [Google Scholar]
- 50.Qu XD, Chen CS, Wang JH, et al. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer. 2012;12:263. doi: 10.1186/1471-2407-12-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Y, Wang WJ, Guan S, et al. Sorafenib combined with transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma: a large-scale multicenter study of 222 patients. Ann Oncol. 2013;24:1786–92. doi: 10.1093/annonc/mdt072. [DOI] [PubMed] [Google Scholar]
- 52.Cabrera R, Pannu DS, Caridi J, et al. The combination of sorafenib with transarterial chemoembolisation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2011;34:205–13. doi: 10.1111/j.1365-2036.2011.04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bai W, Wang YJ, Zhao Y, et al. Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study. J Dig Dis. 2013;14:181–90. doi: 10.1111/1751-2980.12038. [DOI] [PubMed] [Google Scholar]
- 54.Chao Y, Chung YH, Han G, et al. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular cancer: final results of the START trial. Int J Cancer. 2015;136:1458–67. doi: 10.1002/ijc.29126. [DOI] [PubMed] [Google Scholar]