Abstract
The US Preventive Services Task Force (USPSTF) makes recommendations to primary care providers regarding preventive services for asymptomatic patients. Recommendations are based on the scientific evidence that the delivery of the preventive service leads to improvements in meaningful patient outcomes. After a review of the available evidence, the USPSTF found insufficient evidence to recommend routine iron supplementation for pregnant women or routine screening for iron deficiency anemia in pregnant women or young children. The USPSTF identified a critical evidence gap that is related to whether changing hematologic indexes in otherwise asymptomatic pregnant women or in infants within populations who are reflective of the United States leads to an improvement in maternal or child health outcomes. Future research opportunities are described to address these important evidence gaps.
Keywords: iron, pregnant women, screening, supplementation, young children
INTRODUCTION
In 2015, the US Preventive Services Task Force (USPSTF) released its findings that the evidence is insufficient to assess the balance of benefits and harms of routine iron supplementation during pregnancy or of routinely screening pregnant women for iron deficiency anemia (IDA) to prevent adverse maternal or child health outcomes (1). The USPSTF also reported that the evidence is insufficient to assess the balance of benefits and harms of screening for IDA in children aged 6–24 mo (2). Because of the insufficient scientific evidence, the USPSTF neither recommends for nor against these preventive services but does highlight the scientific gaps that impede a recommendation. In contrast, the American College of Obstetricians and Gynecologists recommends that pregnant women routinely be screened for anemia (3), and the American Academy of Pediatrics recommends that children be screened for anemia at ∼12 mo of age (4).
This article, the findings of which were presented at the outset of an NIH workshop that was held in September 2016, summarizes the approaches that were used by the USPSTF in developing recommendations, the evidence considered by the USPSTF, and the specific gaps that are related to IDA in pregnant women and young children. The 2015 USPSTF iron-screening and -supplementation recommendations helped frame the need for the workshop by identifying and exploring the critical gaps in knowledge that are related to pregnant women and young children.
USPSTF
The USPSTF works to improve the health of all Americans by making evidence-based recommendations for clinical preventive-care services, including preventive medications, screening tests, and counseling, that are delivered by clinicians to asymptomatic patients receiving primary care. The USPSTF is a 16-member panel of volunteers who are experts in primary care and evidence-based medicine. Members are appointed by the director of the Agency for Healthcare Research and Quality, and each member serves a 4-y term. The methods and all recommendations of the USPSTF are available on its website (5). The USPSTF also presents research plans and draft recommendations on its website for public comment.
The USPSTF recommendations are developed for asymptomatic patients who are receiving primary care in the United States. The USPSTF primarily considers evidence that is reflective of this population. When developing recommendations, the USPSTF relies on previously published scientific evidence that has been identified through systematic evidence reviews. Expert opinion cannot be used to fill in gaps in the scientific literature even when there is a consensus of clinical experts.
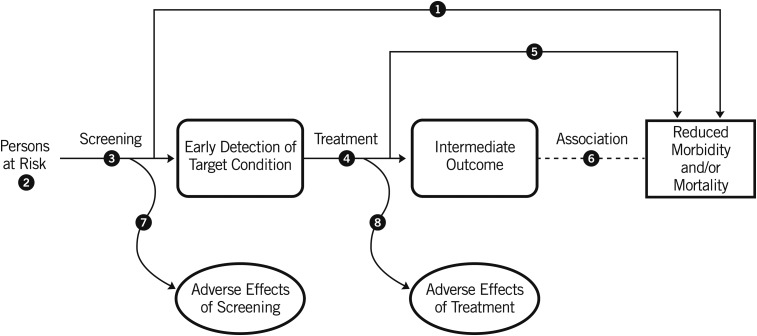
The USPSTF recommendations are based on the certainty of a net benefit for patients that results from the specific preventive service. The USPSTF begins the process by developing analytic frameworks to understand the linkage between the preventive service under consideration and its potential outcomes. Figure 1 presents the generic framework of an analytic framework for a screening preventive service. These analytic frameworks include an assessment of the direct linkage between the preventive service and health outcomes that patients can experience (e.g., patient-centered health outcomes), which are referred to in the USPSTF reports as final health outcomes. However, because this type of direct evidence (e.g., randomized trials of the preventive service) is often unavailable, the analytic frameworks also consider an indirect pathway in which the linkage between the preventive service and intermediate outcomes is first assessed, and second, the linkage between changes in intermediate outcomes and final health outcomes is assessed.
FIGURE 1.
Generic analytic framework for a screening preventive service. The numbered circles identify key questions for the systematic evidence review process. Reproduced from reference 5 with permission.
Recommendations are based on the strength between the preventive service and patient-centered health outcomes. For example, changes in a biometric measure (e.g., blood pressure or lipid concentration) are only relevant if these changes can be directly linked to a relevant patient health outcome (e.g., stroke, myocardial infarction, or death). The USPSTF also considers benefits and harms for all aspects of the preventive service across a long time horizon. For example, the evaluation of a screening service would include consideration of the screening test outcomes, the clinical actions that take place after screening test results, and the long-term impact of treatment that is initiated on the basis of the results of the screening test. This evaluation is particularly important for children and adolescents for whom the benefit of a clinical service may not be experienced for decades but the harms may be experienced earlier.
The USPSTF assesses the adequacy of the evidence on the benefits, and then the harms, as being convincing, adequate, or inadequate. If the USPSTF shows that there is at least adequate evidence, it estimates the magnitude of each of the benefits and harms (substantial, moderate, small, or zero). If the USPSTF finds that there is inadequate evidence, it is unable to determine the magnitude. The net benefit is defined as the benefits minus the harms of a preventive service. At the final step, the USPSTF evaluates whether the overall evidence base is sufficient to have a high or moderate level of certainty that there is a net benefit to providing the service or whether the evidence base is insufficient to determine whether there is a net benefit. To ensure transparency and that all available evidence is considered, the USPSTF invites experts and the public to comment on research plans and draft evidence reports and recommendations before they are finalized.
The USPSTF communicates recommendations in several ways. To help clinicians, policy makers, and the public fully understand its recommendations, each recommendation includes the rationale for the recommendation and a discussion of the clinical considerations including how primary care providers and patients should use the recommendation.
Each recommendation is assigned a letter (Table 1). However, these letter assignments only provide a high-level summary of the USPSTF recommendation. An A or B grade indicates that the USPSTF recommends the preventive service. A D grade indicates that the USPSTF recommends against providing the preventive service because there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. A C grade indicates that the USPSTF recommends selectively offering or providing the preventive service on the basis of professional judgment and patient preference because there is at least moderate certainty that the net benefit is small. For this workshop, an I statement that is issued by the USPSTF indicates that the evidence base is insufficient to assess the balance of benefits and harms. I statements are sometimes incorrectly interpreted as implying that the USPSTF recommends that the preventive service should not be offered. Rather, the statement indicates that the USPSTF simply cannot issue a more specific recommendation on the basis of the available scientific evidence. When an I is assigned, the statement includes a detailed description of the gaps in evidence that precluded the USPSTF from making a more specific recommendation and a discussion of the suggestions for practice on the basis of the insufficient evidence.
TABLE 1.
Description of letter grades assigned to each recommendation statement by the USPSTF1
| Grade | Definition |
| A | The USPSTF recommends the service. There is high certainty that the net benefit is substantial. |
| B | The USPSTF recommends the service. There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial. |
| C | The USPSTF recommends selectively offering or providing the service to individual patients on the basis of professional judgment and patient preferences. There is at least moderate certainty that the net benefit is small. |
| D | The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that harms outweigh the benefits. |
| I | The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
USPSTF, US Preventive Services Task Force.
IDA
Elsewhere in these proceedings, Gupta et al. (6) report on iron status with the use of NHANES data for the 2003–2010 cycles. It has been estimated that 2.2% of children aged 12–24 mo and 2.6% of pregnant women in the United States have IDA. Risk factors for IDA in pregnant women include having a diet that is low in iron-rich foods and a short interpregnancy interval. In children aged 1–5 y, ∼1–2% have IDA (4). Risk factors that may increase the risk of IDA in young children include prematurity or low birth weight, the use of a non–iron-fortified formula or the introduction to cow milk in the first year of life, and exclusive breastfeeding without regular intake of iron-fortified food after age 6 mo (7). Demographic factors that are associated with an increased risk of IDA include low socioeconomic status and having parents who are migrant workers or recent immigrants. Despite these recognized risk factors, the evidence on the accuracy of risk-prediction tools to identify pregnant women or young children at increased risk of IDA has been limited (7, 8).
IRON SUPPLEMENTATION IN PREGNANT WOMEN
The USPSTF showed that, although routine iron supplementation to pregnant women could increase hematologic indexes [e.g., serum ferritin (SF) and hemoglobin concentrations], no study was identified to assess the degree to which changing iron status led to improved maternal or infant health outcomes. The USPSTF identified this area as a critical evidence gap. Furthermore, trials that studied the effect of iron supplementation on maternal and infant health outcomes have reported inconsistent or inconclusive outcomes. Studies that reported on maternal health outcomes such as cesarean delivery and quality of life were sparse and reported inconsistent findings. Although there were more studies that reported on infant health outcomes in general, the number of studies that reported on common infant health outcome (including preterm delivery, low birth weight, small for gestational age, and infant mortality) were few. These studies were frequently underpowered or reported inconsistent findings. For example, although some studies reported a significantly higher mean birth weight in infants born to women who were receiving supplementation during pregnancy, the finding that the mean birth weight that was reported in both groups fell within normal limits did not suggest a benefit of iron supplementation. Only one study showed a significant reduction in infants who were born with low birth weight, but the remaining studies may have been underpowered to detect a change.
Overall, the USPSTF established adequate evidence that the harm of routine iron supplementation in pregnant women is small to none on the basis of 10 trials that reported on the harms of routine iron supplementation during pregnancy. Most of the trials reported that the harms, including nausea, constipation, and diarrhea, were transient and not serious. In general, no significant difference was shown between supplemented and control groups. The evidence regarding the effect of supplementation on maternal hypertension was inconsistent.
The I statement for routine iron supplementation for pregnant women was consistent with USPSTF findings in 2006 (9). Gaps remain regarding the health benefit of changing measures of iron status in otherwise asymptomatic pregnant women.
SCREENING FOR IDA IN PREGNANT WOMEN
In 2006, the USPSTF recommended the screening of pregnant women for anemia (B grade) (9). Scientific evidence that led to this recommendation was largely based on studies that included subjects who were not reflective of the nutritional status of Americans. As the evidence and methods of considering evidence for guidelines has evolved, the USPSTF has more recently focused on studies with findings that are applicable to the population of the United States. In this context, studies from other countries may be less applicable on the basis of a woman’s baseline nutrition and other risk factors for IDA (e.g., parasite burden). The updated 2015 evidence review did not show any good- or fair-quality studies that were applicable to typical patients seen in the United States across the analytic framework including the accuracy of screening tests or risk-prediction tools and the benefits and harms of treating screen-detected (i.e., not clinically detected) IDA in pregnant women. Therefore, the USPSTF was not able to recommend for or against screening for IDA in asymptomatic pregnant women.
SCREENING FOR IDA IN YOUNG CHILDREN
In 2015, the USPSTF chose not to update its recommendation regarding routine iron supplementation for young children. The widespread use of iron-fortified infant formula and foods has lowered the priority of routine clinician-prescribed supplementation for young children. In its most recent update, the USPSTF focused on screening for IDA in children aged 6–24 mo.
For screening tests, the USPSTF showed convincing older evidence that a hemoglobin measurement is sensitive but not specific for detecting IDA. However, no studies were shown that reported on the benefit or harm of treating IDA that was identified through screening on growth or neurodevelopment. In addition, no studies were shown that reported on the effect of changes in hematologic indexes on these same child health outcomes. Overall, the USPSTF showed that the evidence base was insufficient to determine the overall net benefit of screening for IDA in asymptomatic children aged 6–24 mo. These recommendations are not necessarily applicable to infants with additional known risk factors (e.g., prematurity) or with symptoms of anemia.
SUMMARY
The USPSTF identified a critical evidence gap regarding whether changing measures of hematologic laboratory indexes of pregnant women or infants lead to meaningful improvements in health outcomes of patients who are otherwise asymptomatic. Specifically, for pregnant women, studies are needed that evaluate the effect of iron supplementation during pregnancy on maternal and infant health outcomes such as (but not limited to) maternal quality-of-life factors such as fatigue, postpartum hemorrhage, neonatal mortality, premature birth, and low birth weight. Studies that evaluate the effect of improving hematologic indexes in pregnant women (such as hemoglobin or SF concentrations) on these health outcomes could also help address this critical evidence gap. Studies that directly evaluate the effect of screening for IDA in pregnant women on maternal, fetal, and infant health outcomes are similarly needed.
For young children, studies that evaluate the effect of improving hematologic indexes (such as SF or hemoglobin concentrations) in children aged 6–24 mo on health outcomes such as neurodevelopmental delays and cognitive or behavioral impairments are needed. Studies that evaluate the effect of screening for IDA in children aged 6–24 mo on these health outcomes are also needed. Evidence to help fill the gap and inform the USPSTF should include populations with similar risk of IDA as in the United States where nutritional and supplemental iron intakes are higher than in many developing countries. Other risk factors for anemia (e.g., parasites and chronic infection) in such studies should also be similar to those of the United States. These studies should be well designed, report prognostic baseline characteristics, and be adequately powered to detect differences in the clinical outcomes of interest.
Acknowledgments
The authors’ responsibilities were as follows—ARK: prepared the initial draft of the manuscript; TF, DCG, and MGP: critically reviewed and revised the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: IDA, iron deficiency anemia; SF, serum ferritin; USPSTF, US Preventive Services Task Force.
REFERENCES
- 1.Siu AL; US Preventive Services Task Force. Screening for iron deficiency anemia and iron supplementation in pregnant women to improve maternal health and birth outcomes: U.S. preventive services task force recommendation statement. Ann Intern Med 2015;163:529–36. [DOI] [PubMed] [Google Scholar]
- 2.Siu AL, US Preventive Services Task Force. Screening for iron deficiency anemia in young children: USPSTF recommendation statement. Pediatrics 2015;136:746–52. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 95: anemia in pregnancy. Obstet Gynecol 2008;112:201–7. [DOI] [PubMed] [Google Scholar]
- 4.Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics 2010;126:1040–50. [DOI] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force (USPSTF). US Preventive Services Task Force homepage [Internet]. [cited 2017 Sep 18]. Available from: https://www.uspreventiveservicestaskforce.org/.
- 6.Gupta PM, Hamner HC, Suchdev PS, Flores-Ayala R, Mei Z. Iron status of toddlers, nonpregnant females, and pregnant females in the United States. Am J Clin Nutr 2017;106(Suppl):1640S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantor AG, Bougatsos C, McDonagh M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy. Ann Intern Med 2015;163:400. [DOI] [PubMed] [Google Scholar]
- 8.McDonagh MS, Blazina I, Dana T, Cantor A, Bougatsos C. Screening and routine supplementation for iron deficiency anemia: a systematic review. Pediatrics 2015;135:723–33. [DOI] [PubMed] [Google Scholar]
- 9.US Preventive Services Task Force. Screening for iron deficiency anemia, including iron supplementation for children and pregnant women: recommendation statement. Am Fam Physician 2006;74:461–4. [Google Scholar]