Abstract
During pregnancy, iron needs to increase substantially to support fetoplacental development and maternal adaptation to pregnancy. To meet these iron requirements, both dietary iron absorption and the mobilization of iron from stores increase, a mechanism that is in large part dependent on the iron-regulatory hormone hepcidin. In healthy human pregnancies, maternal hepcidin concentrations are suppressed in the second and third trimesters, thereby facilitating an increased supply of iron into the circulation. The mechanism of maternal hepcidin suppression in pregnancy is unknown, but hepcidin regulation by the known stimuli (i.e., iron, erythropoietic activity, and inflammation) appears to be preserved during pregnancy. Inappropriately increased maternal hepcidin during pregnancy can compromise the iron availability for placental transfer and impair the efficacy of iron supplementation. The role of fetal hepcidin in the regulation of placental iron transfer still remains to be characterized. This review summarizes the current understanding and addresses the gaps in knowledge about gestational changes in hematologic and iron variables and regulatory aspects of maternal, fetal, and placental iron homeostasis.
Keywords: anemia, hepcidin, iron regulation, placenta, pregnancy
IRON REQUIREMENTS DURING PREGNANCY
During pregnancy, physiologic iron demands increase substantially to support fetoplacental development and maternal adaptation to pregnancy. Table 1 summarizes iron economy during pregnancy [the estimates are based on a 120-lb (54-kg) woman]. Baseline maternal body iron losses during 9 mo have been estimated at ∼230 mg (5) and would be higher were it not for the cessation of menstruation. The development of the placenta and fetus requires ∼360 mg Fe. An additional 450 mg Fe is needed to expand maternal red blood cell (RBC) mass during pregnancy. Thus, ∼1 g of iron must be acquired during pregnancy to preserve the maternal iron balance and support fetoplacental development. Some of that iron is recycled after pregnancy when the erythrocyte mass contracts to prepregnancy concentrations with the exception of the iron that is lost through bleeding at delivery (∼150 mg). Therefore, the average net pregnancy-related loss of iron to the mother has been estimated to be 740 mg. However, iron requirements are not uniform throughout the 3 trimesters of pregnancy. In the first trimester, the requirements (estimated at ∼0.8 mg/d) are lower than before pregnancy because menstruation stops. As pregnancy advances, maternal RBC mass increases and placental and fetal growth accelerates, which result in the rise in physiologic iron requirements to 3.0–7.5 mg/d in the third trimester (1).
TABLE 1.
Iron balance in pregnancy1
| Iron fate | Amount, mg |
| Fetal iron | 270 |
| Placental iron | 90 |
| Baseline maternal body iron loss | 230 |
| Expansion of maternal RBC mass | 450 |
| Total iron needs during pregnancy | 1040 |
| RBC-mass contraction after delivery (450 mg) minus the blood lost at delivery (150 mg) | −300 |
| Net pregnancy iron loss to the mother | 740 |
To meet the accelerating physiologic iron requirements, both dietary iron absorption and the mobilization of iron from stores need to increase. Many women enter pregnancy with insufficient iron stores to meet the needs of the pregnancy. In the United States, the prevalence of iron deficiency (ID) in women of child-bearing age has been reported to be 12% with a higher rate in Black and Hispanic women (19% and 22%, respectively) (6). Because ID and ID anemia (IDA) during pregnancy have been associated with adverse outcomes for the mother and the child, including increased risk of maternal mortality, premature birth, low birth weight, and neurodevelopmental impairment in infants (7, 8), iron supplementation has been nearly universally recommended during pregnancy. Nevertheless, in the developed world, more women are iron replete than iron deficient when they become pregnant, thus prompting considerations of potential risks of indiscriminate iron supplementation.
REGULATION OF IRON AVAILABILITY DURING PREGNANCY
As assessed by the uptake of stable or radioactive iron isotopes, nonheme iron absorption during pregnancy increases as gestation progresses (2, 9). It is likely that heme absorption increases in a similar manner (10). Moreover, iron stores are efficiently mobilized during pregnancy, as reflected by the decreased liver and spleen iron contents in animal models compared with nonpregnancy concentrations (11–13). Both of these processes increase iron availability for transfer across the placenta and for maternal hematologic adaptation.
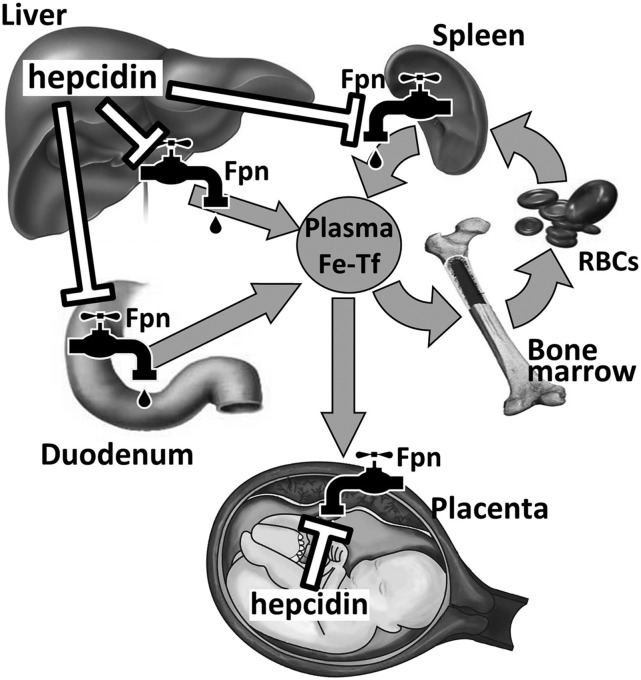
The regulation of iron availability during pregnancy is at least in part dependent on maternal hepcidin concentrations. Hepcidin, which is an iron-regulatory hormone, is produced by the liver and controls plasma iron concentrations and tissue iron distribution (14). Hepcidin acts by inhibiting the following major iron flows into plasma: intestinal iron absorption, release from macrophages that recycle iron from old RBCs, and mobilization of stored iron from the liver (Figure 1). Hepcidin exerts its effects through its receptor the iron exporter ferroportin. Ferroportin is expressed in all the tissues that actively export iron into plasma (15). Hepcidin binds to ferroportin and triggers its degradation, resulting in iron sequestration in target cells and decreased iron flow into plasma. Thus, iron delivery to consuming tissues (e.g., bone marrow and placenta with fetus) is inversely correlated with hepcidin concentrations.
FIGURE 1.
Hepcidin-ferroportin interaction controls systemic iron homeostasis. By causing degradation of Fpn, hepcidin decreases iron supply into plasma. Thus, lowering of maternal hepcidin during pregnancy increases iron bioavailability for placental transfer. Fetal hepcidin may control placental Fpn and the transfer of iron into fetal circulation. Fpn, ferroportin; RBC, red blood cell; Tf, transferrin.
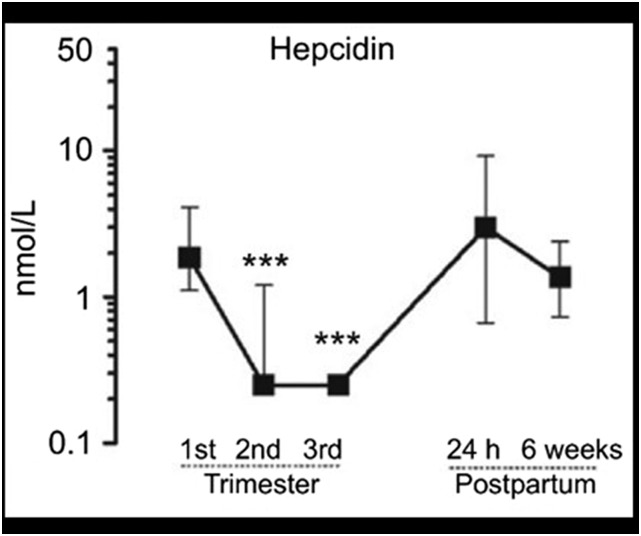
Relatively few studies have examined hepcidin during pregnancy, but initial reports have indicated that, in healthy pregnancies, maternal hepcidin concentrations are decreased in the second and third trimesters in humans (Figure 2) (16, 17) or during the third week in rats (18). The lowering of maternal hepcidin would allow an increased supply of iron into the circulation both from the enhanced absorption of dietary iron and the enhanced release of iron from stores. One study in 19 pregnant women who ingested stable iron isotopes in their third trimesters confirmed that the net dietary nonheme and heme iron that was transferred to the fetus was inversely correlated with maternal serum hepcidin (measured at delivery) (10).
FIGURE 2.
Median (IQR) serum hepcidin concentrations in 31 women during pregnancy and postpartum. ***Compared with first-trimester values, P < 0.0001. Reproduced from reference 16 with permission.
To our knowledge, the mechanism of maternal hepcidin suppression during pregnancy is completely unknown. Plasma dilution may partially contribute, but the magnitude of hepcidin decrease cannot be explained by only a 30–50% increase in plasma volume. Moreover, plasma dilution would not explain the profound suppression of hepatic hepcidin messenger RNA that has been observed in animal studies (18). The gradual development of ID may also be a signal to suppress hepcidin. Hepcidin is lowest in pregnant women with iron-restricted erythropoiesis; however, even mothers with replete iron stores have low hepcidin concentrations at delivery (19), thus suggesting that maternal hepcidin may be actively suppressed during pregnancy. The identification of the pregnancy-related hepcidin suppressors is an important goal for the understanding of iron regulation during pregnancy.
Major stimuli that are known to regulate hepcidin production include iron (both circulating and stored iron increase hepcidin), erythropoietic activity (suppresses hepcidin), and inflammation (increases hepcidin) (14). The regulation of hepcidin by all of these pathways appears to be preserved during pregnancy (20) but at a lower set point as pregnancy advances. In human studies, throughout pregnancy and even at delivery, maternal hepcidin concentrations are positively correlated with serum ferritin (SF) and transferrin saturation (TSAT) and inversely correlated with soluble transferrin receptor (sTfR) and hemoglobin, indicating the stimulation of hepcidin production by iron and the suppression of hepcidin production by ID and erythropoietic activity (10, 16, 17, 19, 21). However, immediately after delivery, serum hepcidin concentrations increase (Figure 2), presumably because of dramatic physiologic changes that are associated with labor and delivery and are not correlated with SF or serum iron concentrations.
To our knowledge, how iron supplementation affects maternal hepcidin during pregnancy is not known. The ingestion of iron supplements in nonpregnant adults increases hepcidin rapidly (22–24) and, consequently, decreases iron absorption. If iron supplementation in pregnancy has the same effect on maternal hepcidin, daily iron supplementation may not be optimal to achieve the most efficient iron absorption. Indeed, a 2015 Cochrane review of randomized trials from 15 countries showed that maternal and infant outcomes at birth were not better with daily iron supplementation compared with intermittent iron supplementation, but intermittent supplementation was associated with fewer side effects (25).
Hepcidin concentrations measured in either serum or urine were not correlated with inflammatory markers in healthy pregnancies (16, 21), including those with multiple gestations (26), thereby suggesting that the mild inflammation that occurs in healthy pregnancies is not sufficient to increase hepcidin. However, it is possible that hepcidin may be inappropriately increased in complicated pregnancies that are associated with more intense inflammation, and this increase could compromise iron availability during pregnancy. Elevated hepcidin would also be expected to impair iron absorption from supplements that are commonly prescribed to pregnant women and could even impair the efficacy of intravenous iron therapy by trapping iron in macrophages. Mildly elevated serum hepcidin concentrations have been reported in obese compared with lean pregnant women (27, 28) and in pre-eclamptic compared with healthy pregnancies (29). These concentrations did not have an obvious negative impact on hematologic or iron variables in the mother or neonate in these studies, suggesting that hepcidin concentrations were still sufficiently low to allow effective iron utilization during pregnancy. Systematic studies of hepcidin in complicated pregnancies are needed to determine the extent of hepcidin elevation in different conditions and the impact of elevated hepcidin on pregnancy outcomes.
Apart from the hepcidin-dependent mechanism that regulates iron absorption and recycling, additional hepcidin-independent mechanisms may exist in pregnancies, but this possibility remains to be characterized. Concentrations of the apical iron transporter in duodenal enterocytes divalent metal transporter 1 (DMT1) and the associated ferrireductase duodenal cytochrome B were also increased in an animal model of pregnancy (18), but the regulatory mechanisms are unknown. One such mechanism could be related to the stabilization of the transcription factor hypoxia-inducible factor (HIF)-2α in the duodenum. In mouse models, ID and anemia promote the accumulation of HIF-2α, which mediates the increased expression of ferroportin, DMT1, and duodenal cytochrome B (30). Whether these HIF-2α–dependent duodenal mechanisms regulate iron absorption in pregnant women remains to be determined.
ROLE OF FETAL HEPCIDIN IN REGULATING PLACENTAL IRON TRANSFER
During pregnancy, not only maternal but also fetal hepcidin could determine the rate of placental iron transfer (Figure 1). In this scenario, maternal hepcidin would regulate the amount of iron that is presented to the placenta for uptake, whereas fetal hepcidin would regulate the export of iron from the placenta into the fetal circulation. Ferroportin is expressed on the basolateral side of the placental syncytiotrophoblast, facing fetal circulation, and would be expected to be accessible only by fetal hepcidin. The transgenic overexpression of fetal hepcidin in mice confirmed that fetal hepcidin can regulate placental ferroportin (31). Overexpressing fetuses developed severe ID and had decreased viability. However, whether endogenous fetal hepcidin in a normal or complicated pregnancy contributes to the regulation of placental transfer remains to be evaluated. Thus far, animal studies have shown very low concentrations of fetal hepcidin during normal gestation (31, 32). This finding suggests that fetal hepcidin would not exert much effect on the placental ferroportin in healthy pregnancies. In humans, only hepcidin from cord blood has been evaluated. Cord blood hepcidin concentrations were higher than maternal concentrations and showed no correlation with maternal hepcidin at delivery (20), but an interpretation of these measurements is confounded by the physiologic effects of delivery. Indeed, another study showed a positive association between cord hepcidin (at delivery) and maternal hepcidin at midgestation (33).
CHANGES IN HEMATOLOGIC VARIABLES
Like many organ systems during pregnancy, the maternal hematologic system undergoes profound physiologic changes to accommodate the development of the fetus and placenta (a summary is shown in Table 2). The total blood volume (plasma volume plus RBC volume) increases ∼1.5 L to facilitate the blood flow in the uterus and placenta for nutrient and oxygen delivery to the fetus and to blunt the effects of blood loss at delivery (35).
TABLE 2.
Hematologic changes in normal pregnancy1
| Change | |
| Plasma volume | Increases 30–50% |
| RBC mass | Increases 20–30% |
| Hemoglobin concentration | Decreases |
| RBC life span | Decreases slightly |
| Erythropoietin | Increases |
Adapted from reference 34 with permission. RBC, red blood cell.
The plasma volume starts increasing during the first trimester and expands until 30–34 wk, reaching a 30–50% greater volume than in nonpregnant women (36, 37). A lesser increase in plasma volume is associated with pathologies such as intrauterine growth restriction and preeclampsia (38).
The RBC mass starts to increase at 8–10 wk of gestation and continues to increase until delivery. Compared with prepregnancy concentrations, the RBC mass increases by 15–20% in women who are not taking iron supplements and by 20–30% in women who are taking iron supplements (34). The RBC life span has been reported to be slightly decreased during normal pregnancies (∼9% decrease in rats and assumed to be similar in women) (39, 40).
Erythropoietin production increases during pregnancy and drives the increase in RBC mass. Erythropoietin concentrations approximately double by the end of the third trimester (41). As in nonpregnant adults, the kidney is the main source of maternal erythropoietin during pregnancy (42, 43). However, the cause of the baseline erythropoietin increase during pregnancy is still uncertain. Nevertheless, erythropoietin production can be modulated by iron and anemia during pregnancy. In human studies, the erythropoietin increase was greater with ID (41), and conversely, iron supplementation was associated with lower erythropoietin concentrations in the third trimester (44).
Fetal RBC production is independent of its maternal counterpart. Maternal erythropoietin does not cross the placenta (45, 46). The fetus produces its own erythropoietin but mostly in the liver, which is also its main erythropoietic organ. After 30 wk of gestation, the fetus also starts producing erythropoietin in the kidney (47). Fetal erythropoietin production (as measured by the cord blood concentration) is higher when the mother is anemic or has other hypoxic complications (e.g., smoking, fetal growth restriction, and intrauterine fetal hypoxia) (48–50).
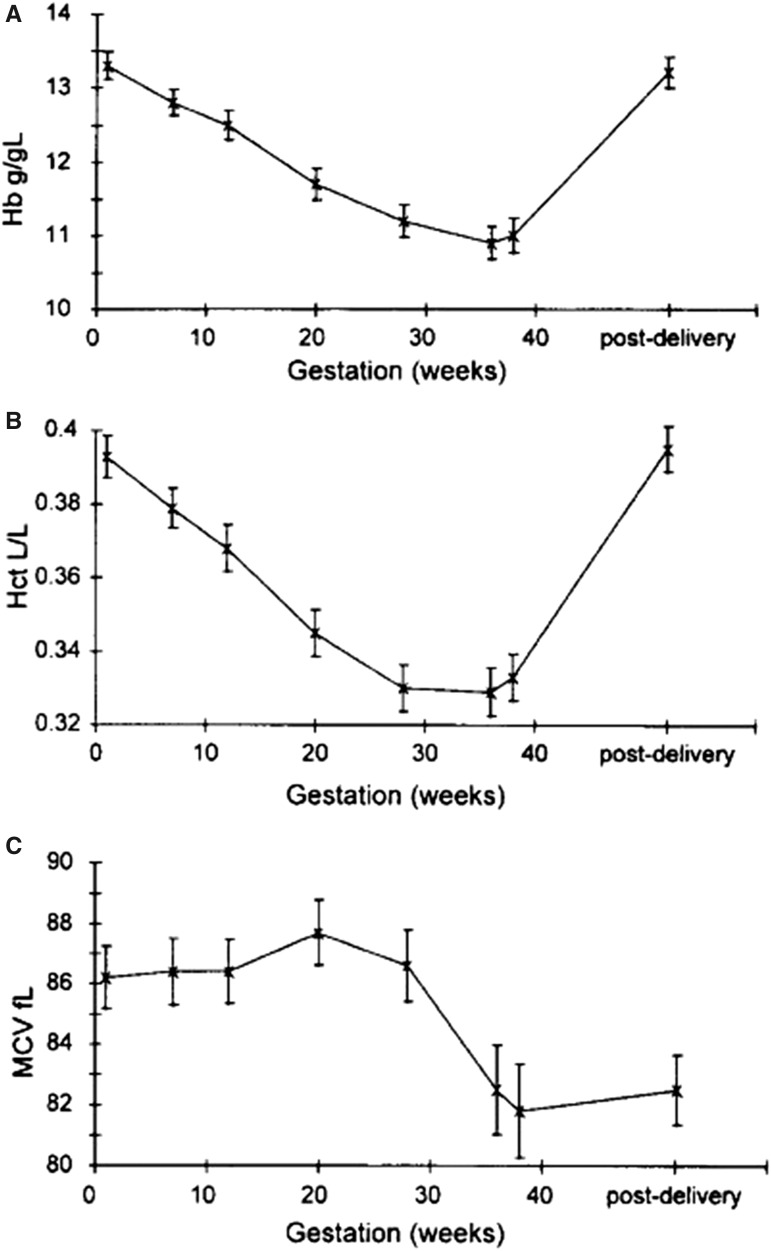
Physiologic anemia of pregnancy occurs during a healthy pregnancy as a consequence of a greater increase in the plasma volume relative to the increase in RBC mass. In women who are not taking iron supplements, the hemoglobin concentration and hematocrit decrease steadily to reach a nadir at ∼28–36 wk (on average, ∼2 g/dL lower than prepregnancy hemoglobin concentrations) (Figure 3) (51). The mean corpuscular volume mildly decreases between 26 and 38 wk, which is likely because the placental iron transfer is most intense during this period, thereby decreasing the iron availability for maternal erythropoiesis. Iron supplementation has been reported to result in ∼1-g/dL higher hemoglobin concentration at term compared with those in unsupplemented women (52, 53).
FIGURE 3.
Mean (95% CI) Hb (A), Hct (B), and MCV (C) values during normal, unsupplemented pregnancy in 69 women. Reproduced from reference 51 with permission. Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume.
Both low and high hemoglobin concentrations during pregnancy are associated with adverse outcomes (54). IDA in pregnant women is associated with reduced physical and mental performance, maternal cardiovascular strain, increased risk of peripartum blood transfusions, and other complications. In severe and very severe anemia, maternal mortality is increased as well. Maternal hemoglobin concentrations <9 g/dL are associated with increased risk of premature birth, intrauterine growth retardation, and fetal death (7). Despite the anemia-defining cutoffs of 10.5–11 g/dL (39), an analysis of nearly 150,000 pregnancies in the United Kingdom showed that the lowest rate of perinatal mortality was shown when maternal hemoglobin concentrations during pregnancy were between 9 and 11 g/dL (54). The highest birth weight was also recorded in mothers whose hemoglobin concentrations fell to 9–11 g/dL during pregnancy (55). These favorable outcomes may be related to an optimal plasma volume expansion (incidentally resulting in slightly lower hemoglobin concentrations) rather than to any beneficial effect of IDA. These reports raise questions of the appropriateness of anemia cutoffs and which other variables should be considered in evaluating anemia during pregnancy.
The absence of a decrease in the hemoglobin concentration during pregnancy is also associated with poor outcomes including preeclampsia, intrauterine growth retardation, preterm birth, and stillbirth (56–58). When the lowest recorded maternal hemoglobin concentrations were >11 g/dL, perinatal mortality increased (54). A higher hemoglobin concentration is thought to be related to the failure to increase the plasma volume, and adverse consequences may be caused by increased blood viscosity and decreased placental perfusion (54).
CHANGES IN IRON VARIABLES DURING PREGNANCY
The SF concentration is the most frequently used marker of iron stores. Ferritin is secreted mostly by macrophages and, to a lesser extent, by hepatocytes, in proportion to their intracellular iron contents; thus, SF is proportional to body iron stores. However, because ferritin production is also regulated by inflammatory cytokines, SF may not accurately reflect iron stores in the presence of inflammation.
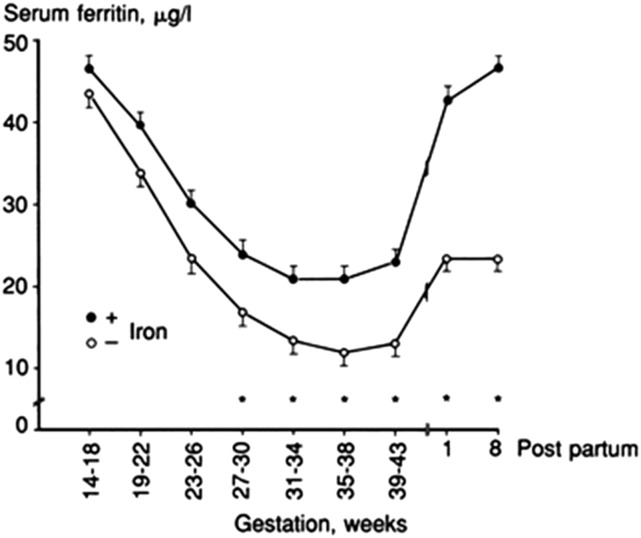
In pregnancy, SF concentrations gradually decrease to reach the lowest concentrations in the third trimester (52, 59, 60) (Figure 4). In addition to hemodilution, this decrease likely reflects efficient iron mobilization from stores in agreement with the progressive hepcidin decrease during pregnancy. Iron supplementation results in a lesser SF decrease in the third trimester (Figure 4). Higher SF concentrations in the second or third trimester (61–64) are associated with less favorable pregnancy outcomes including increased risk of preterm delivery. However, apart from reflecting higher iron stores in the mother, higher SF could also reflect the presence of inflammation in complicated pregnancies or the failure of the plasma volume to expand. Whether maternal iron excess, itself, contributes to adverse outcomes is less clear (64) and is an important research question.
FIGURE 4.
Geometric mean ± SEM serum ferritin concentrations during pregnancy in 63 women with iron supplementation and 57 women without iron supplementation. Reproduced from reference 52 with permission. SF, serum ferritin.
Similar to other iron variables, serum iron and TSAT both decrease during pregnancy but less so in iron-supplemented pregnancies (52, 53). The plasma iron compartment is very small compared with iron stores (several milligrams compared with several hundred milligrams), is subject to diurnal variation, and can change rapidly, e.g., after iron ingestion. Because of these effects, serum iron and TSAT are inferior to SF in diagnosing ID (53, 65).
sTfR is generated by cleavage and by vesicular shedding of transferrin receptor 1 (TfR1) from the plasma membrane during erythroid maturation. The amount of sTfR reflects both the number of young erythrocytes and the degree of their ID because cellular TfR1 concentrations are regulated by intracellular iron via the iron-responsive element-binding proteins (IRPs) IRP1 and IRP2. In pregnancy, sTfR concentrations do not seem to change compared with nonpregnant concentrations unless maternal erythropoiesis is iron deficient (66). Thus, sTfR concentration mays only mildly increase by the third trimester in the iron-replete population but increase substantially in women with IDA. Furthermore, because sTfR is not regulated by inflammation, sTfR is a better indicator of iron-deficient erythropoiesis than SF is in the presence of inflammation.
PLACENTAL IRON TRANSPORT
During pregnancy, the placenta retains ∼90 mg Fe for its own function, and transports, on average, 270 mg Fe to the fetus. Most of the iron transfer to the fetus occurs during the third trimester (67), and this transfer coincides with the lowest maternal hepcidin expression, which allows for a maximal rate of iron supply into the maternal circulation. Maternal transferrin production steadily increases during pregnancy (34), which may function to increase iron delivery to the placenta.
The transport of nonheme iron across the placenta to the fetus is unidirectional; iron is not transferred from the fetus to the mother (67). Despite its importance in fetal development, the mechanism of placental iron transport is incompletely understood. The uptake of iron transferrin from the maternal circulation is mediated by TfR1 on the placental syncytiotrophoblast (Figure 5) (68). TfR1 is located on the apical membrane of the syncytiotrophoblast (69, 70), and the TfR1-transferrin complex is internalized via clathrin-coated vesicles, similar to iron-transferrin endocytosis that occurs in other epithelia (71). In the acidic environment of the vesicle, iron dissociates from transferrin, and ferric iron is reduced to ferrous iron by ferrireductases, possibly 6-transmembrane epithelial antigen of prostate 3 and 4 (STEAP 3 and 4) (72). After iron is released from transferrin, the TfR1–apotransferrin complex recycles back to the membrane, apotransferrin is released, and the cycle repeats. Maternal ID has been associated with increased placental TfR1 expression in humans and in animal models (73, 74). The likely mechanism is the development of placental ID when the mother is iron deficient whereby a low intracellular iron concentration in trophoblast cells may increase TfR1 expression via the IRP1 and IRP2 regulators.
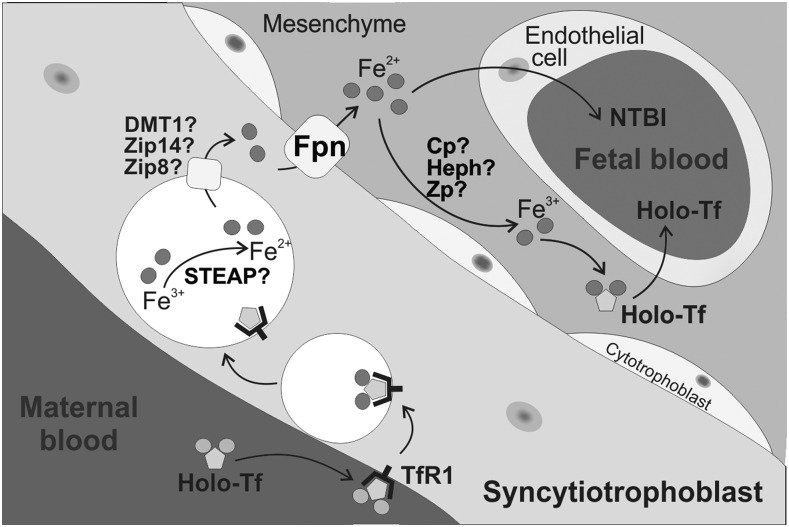
FIGURE 5.
Placental iron transport. On the apical side of the syncytiotrophoblast, maternal iron Tf binds to TfR1. After internalization, iron dissociates from Tf, is reduced by a ferrireductase, and is exported from the endosome into the cytoplasm, possibly via DMT1 or another transporter. Iron is exported from the syncytiotrophoblast by the iron exporter Fpn and eventually oxidized by a ferroxidase to be loaded onto fetal Tf or possibly transferred into fetal circulation as NTBI. How the iron is transported across the fetal endothelium is unclear. Cp, ceruloplasmin; DMT1, divalent metal transporter 1; Fpn, ferroportin; Heph, hephaestin; NTBI, non–transferrin-bound iron; STEAP, 6-transmembrane epithelial antigen of prostate; Tf, transferrin; TfR1, transferrin receptor 1; Zip, Zrt/Irt-like protein; Zp, zyklopen.
How iron is transported from the vesicle into cytoplasm is not fully understood, but iron transporters DMT1, Zrt/Irt-like protein (ZIP) 8, and ZIP14 have been identified as potential candidates. DMT1, which plays a critical role for endosomal iron release in erythroid cells (75), strongly localizes to the human placental syncytium (70, 76). However, the discovery that neonatal DMT1-null mice have normal iron contents (76) suggests that DMT1 is not the sole endosomal iron transporter in the placenta. ZIP8 is also abundantly expressed in the placenta (77). ZIP8 hypomorphic embryos are severely anemic in utero and do not survive >48 h after birth (78); however, whether this outcome is related to ZIP8 function in placental iron transport or also in fetal RBCs needs to be clarified. ZIP14 is also highly expressed in the mouse placenta. ZIP14 mutant mice have no abnormal birth phenotype other than low birth weight (79), which suggests that ZIP14 plays a nonessential or redundant role in placenta.
Iron is transported out of the syncytiotrophoblast by ferroportin (69, 80) (Figure 5). The complete knockout of ferroportin is embryonic lethal, whereas the conditional knockout of ferroportin that preserves its expression in the placenta results in normal embryonic development and birth (15), thus confirming the essential role of ferroportin in placental iron export. Ferroportin likely exports iron into the fetal stroma. Once there, iron still needs to cross the endothelium to reach the fetal circulation. With consideration that non–transferrin-bound iron is present in the fetal circulation (81), it is possible that some form of non–transferrin-bound iron is transported across fetal endothelial cells. Alternatively, after being exported from the syncytiotrophoblast by ferroportin, iron may be oxidized to the Fe+3 form before loading onto fetal transferrin. There are 3 known mammalian multicopper ferroxidases: ceruloplasmin, hephaestin, and zyklopen. Although all of them have been detected in the placenta (82–84), knockout mouse models have indicated that none of them are essential or that they have redundant roles (85–87). Once iron is loaded onto fetal transferrin, it may be transported to the fetal circulation through endothelial cells although this mechanism is unclear (68). Fundamental questions remain regarding the physiology of iron transport from the mother to the fetus.
Whether heme is transported across the placenta and what role heme transporters play in the placenta are much less understood. Feline leukemia virus subgroup C receptor–related protein (FLVCR1) is a heme exporter that is highly expressed in the placenta (88). It has the following 2 isoforms: FLVCR1a is expressed on the cell surface, and FLVCR1b is expressed on mitochondria, but the role of each isoform and their localization and regulation remain to be determined. Maternal anemia is associated with lower placental FLVCR1 expression (89), but the biological implication of this observation is not yet understood. More research is needed to determine the specific roles of placental iron transporters and regulators, their interactions, and the control of the placental iron transport by maternal iron status and fetal iron status.
CONCLUSION AND FUTURE DIRECTIONS
Although the importance of iron for maternal health and fetal development during pregnancy is well appreciated, major gaps exist in our understanding of iron regulation during pregnancy. Future directions include defining the role and regulation of maternal and fetal hepcidin, elucidating the mechanism and regulation of placental iron transport, and understanding how iron supplementation interacts with these processes in healthy and complicated pregnancies. Correlating descriptive studies in human pregnancies with detailed mechanistic and molecular studies in animal models will be necessary to make progress on these important questions.
Acknowledgments
The authors’ responsibilities were as follows—both authors: wrote the manuscript and read and approved the final manuscript. EN is a consultant and stockholder of Intrinsic LifeSciences (a company developing hepcidin diagnostics) and Silarus Therapeutics (a company developing erythroferrone-targeted therapeutics). ALF reported no conflict of interest related to the study.
Footnotes
Abbreviations used: DMT1, divalent metal transporter 1; FLVCR, feline leukemia virus subgroup C receptor-related protein; HIF, hypoxia-inducible factor; ID, iron deficiency; IDA, iron deficiency anemia; IRP, iron-responsive element-binding protein; RBC, red blood cell; SF, serum ferritin; sTfR, soluble transferrin receptor; TfR1, transferrin receptor 1; TSAT, transferrin saturation; ZIP, Zrt/Irt-like protein.
REFERENCES
- 1.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr 2000;72:257S–64S. [DOI] [PubMed] [Google Scholar]
- 2.Barrett JF, Whittaker PG, Williams JG, Lind T. Absorption of non-haem iron from food during normal pregnancy. BMJ 1994;309:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodnough LT, Nemeth E. Iron deficiency and related disorders In: Greer JP, Arber DA, Glader B, List AF, Means RT, Paraskevas F, Rodgers GM, editors. Wintrobe’s clinical hematology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 4.World Health Organization, Food and Agricultural Organization of the United Nations. Vitamin and mineral requirements in human nutrition. Geneva (Switzerland): World Health Organization; 2004. [Google Scholar]
- 5.Hallberg L, Rossander-Hulten L. Iron requirements in menstruating women. Am J Clin Nutr 1991;54:1047–58. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). Iron deficiency–United States, 1999-2000. MMWR Morb Mortal Wkly Rep 2002;51:897–9. [PubMed] [Google Scholar]
- 7.Breymann C. Iron deficiency anemia in pregnancy. Semin Hematol 2015;52:339–47. [DOI] [PubMed] [Google Scholar]
- 8.Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med 2007;12:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milman N. Iron and pregnancy–a delicate balance. Ann Hematol 2006;85:559–65. [DOI] [PubMed] [Google Scholar]
- 10.Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O’Brien KO. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr 2012;142:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubbard AC, Bandyopadhyay S, Wojczyk BS, Spitalnik SL, Hod EA, Prestia KA. Effect of dietary iron on fetal growth in pregnant mice. Comp Med 2013;63:127–35. [PMC free article] [PubMed] [Google Scholar]
- 12.Lin W, Kirksey A. Effects of different levels of dietary iron on pregnancy superimposed upon growth in the rat. J Nutr 1976;106:543–54. [DOI] [PubMed] [Google Scholar]
- 13.Gao G, Liu SY, Wang HJ, Zhang TW, Yu P, Duan XL, Zhao SE, Chang YZ. Effects of pregnancy and lactation on iron metabolism in rats. Biomed Res Int 2015;2015:105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta 2012;1823:1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 2005;1:191–200. [DOI] [PubMed] [Google Scholar]
- 16.van Santen S, Kroot JJ, Zijderveld G, Wiegerinck ET, Spaanderman ME, Swinkels DW. The iron regulatory hormone hepcidin is decreased in pregnancy: a prospective longitudinal study. Clin Chem Lab Med 2013;51:1395–401. [DOI] [PubMed] [Google Scholar]
- 17.Bah A, Pasricha SR, Jallow MW, Sise EA, Wegmuller R, Armitage AE, Drakesmith H, Moore SE, Prentice AM. Serum hepcidin concentrations decline during pregnancy and may identify iron deficiency: analysis of a longitudinal pregnancy cohort in the Gambia. J Nutr 2017;147:1131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millard KN, Frazer DM, Wilkins SJ, Anderson GJ. Changes in the expression of intestinal iron transport and hepatic regulatory molecules explain the enhanced iron absorption associated with pregnancy in the rat. Gut 2004;53:655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rehu M, Punnonen K, Ostland V, Heinonen S, Westerman M, Pulkki K, Sankilampi U. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol 2010;85:345–52. [DOI] [PubMed] [Google Scholar]
- 20.Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014;6:3062–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulze KJ, Christian P, Ruczinski I, Ray AL, Nath A, Wu LS, Semba RD. Hepcidin and iron status among pregnant women in Bangladesh. Asia Pac J Clin Nutr 2008;17:451–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Moretti D, Goede JS, Zeder C, Jiskra M, Chatzinakou V, Tjalsma H, Melse-Boonstra A, Brittenham G, Swinkels DW, Zimmermann MB. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015;126:1981–9. [DOI] [PubMed] [Google Scholar]
- 23.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 2004;113:1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood 2008;112:4292–7. [DOI] [PubMed] [Google Scholar]
- 25.Peña-Rosas JP, De-Regil LM, Gomez MH, Flores-Urrutia MC, Dowswell T. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2015;10:CD009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ru Y, Pressman E, Cooper E, Guillet R, Katzman P, Kent T, Bacak S, O’Brien K. Iron deficiency and anemia are prevalent in women with multiple gestations. Am J Clin Nutr 2016;104:1052–60. [DOI] [PubMed] [Google Scholar]
- 27.Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is Hepcidin the link? J Perinatol 2013;33:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao C, Pressman EK, Cooper EM, Guillet R, Westerman M, O’Brien KO. Prepregnancy body mass index and gestational weight gain have no negative impact on maternal or neonatal iron status. Reprod Sci 2016;23:613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toldi G, Stenczer B, Molvarec A, Takats Z, Beko G, Rigo J Jr, Vasarhelyi B. Hepcidin concentrations and iron homeostasis in preeclampsia. Clin Chem Lab Med 2010;48:1423–6. [DOI] [PubMed] [Google Scholar]
- 30.Shah YM, Xie L. Hypoxia-inducible factors link iron homeostasis and erythropoiesis. Gastroenterology 2014;146:630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA 2002;99:4596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willemetz A, Lenoir A, Deschemin JC, Lopez-Otin C, Ramsay AJ, Vaulont S, Nicolas G. Matriptase-2 is essential for hepcidin repression during fetal life and postnatal development in mice to maintain iron homeostasis. Blood 2014;124:441–4. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Guillet R, Cooper EM, Westerman M, Orlando M, Kent T, Pressman E, O’Brien KO. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res 2016;79:42–8. [DOI] [PubMed] [Google Scholar]
- 34.Bauer KA. Hematologic changes in pregnancy. In: Post TW, editor. UpToDate. Waltham (MA): UpToDate; 2016. [Google Scholar]
- 35.Ramsey M. Normal hematological changes during pregnancy and the puerperium. In: Pavord S, Hunt B, editors. The obstetric hematology manual. Cambridge (United Kingdom): Cambridge University Press; 2010. p. 1–11. [Google Scholar]
- 36.Pritchard JA. Changes in blood volume during pregnancy and delivery. Anesthesiology 1965;26:393–9. [DOI] [PubMed] [Google Scholar]
- 37.Peck TM, Arias F. Hematologic changes associated with pregnancy. Clin Obstet Gynecol 1979;22:785–98. [DOI] [PubMed] [Google Scholar]
- 38.Faupel-Badger JM, Hsieh CC, Troisi R, Lagiou P, Potischman N. Plasma volume expansion in pregnancy: implications for biomarkers in population studies. Cancer Epidemiol Biomarkers Prev 2007;16:1720–3. [DOI] [PubMed] [Google Scholar]
- 39.Lurie S, Danon D. Life span of erythrocytes in late pregnancy. Obstet Gynecol 1992;80:123–6. [PubMed] [Google Scholar]
- 40.Lurie S, Mamet Y. Red blood cell survival and kinetics during pregnancy. Eur J Obstet Gynecol Reprod Biol 2000;93:185–92. [DOI] [PubMed] [Google Scholar]
- 41.McMullin MF, White R, Lappin T, Reeves J, MacKenzie G. Haemoglobin during pregnancy: relationship to erythropoietin and haematinic status. Eur J Haematol 2003;71:44–50. [DOI] [PubMed] [Google Scholar]
- 42.Zanjani ED, Peterson EN, Gordon AS, Wasserman LR. Erythropoietin production in the fetus: role of the kidney and maternal anemia. J Lab Clin Med 1974;83:281–7. [PubMed] [Google Scholar]
- 43.Clapp JF III, Little KD, Widness JA. Effect of maternal exercise and fetoplacental growth rate on serum erythropoietin concentrations. Am J Obstet Gynecol 2003;188:1021–5. [DOI] [PubMed] [Google Scholar]
- 44.Barton DP, Joy MT, Lappin TR, Afrasiabi M, Morel JG, O’Riordan J, Murphy JF, O’Herlihy C. Maternal erythropoietin in singleton pregnancies: a randomized trial on the effect of oral hematinic supplementation. Am J Obstet Gynecol 1994;170:896–901. [DOI] [PubMed] [Google Scholar]
- 45.Malek A, Sager R, Eckardt KU, Bauer C, Schneider H. Lack of transport of erythropoietin across the human placenta as studied by an in vitro perfusion system. Pflugers Arch 1994;427:157–61. [DOI] [PubMed] [Google Scholar]
- 46.Widness JA, Schmidt RL, Sawyer ST. Erythropoietin transplacental passage–review of animal studies. J Perinat Med 1995;23:61–70. [DOI] [PubMed] [Google Scholar]
- 47.Dame C, Fahnenstich H, Freitag P, Hofmann D, Abdul-Nour T, Bartmann P, Fandrey J. Erythropoietin mRNA expression in human fetal and neonatal tissue. Blood 1998;92:3218–25. [PubMed] [Google Scholar]
- 48.Erdem A, Erdem M, Arslan M, Yazici G, Eskandari R, Himmetoglu O. The effect of maternal anemia and iron deficiency on fetal erythropoiesis: comparison between serum erythropoietin, hemoglobin and ferritin levels in mothers and newborns. J Matern Fetal Neonatal Med 2002;11:329–32. [DOI] [PubMed] [Google Scholar]
- 49.Jazayeri A, Tsibris JC, Spellacy WN. Fetal erythropoietin levels in growth-restricted and appropriately grown neonates with and without abnormal fetal heart rate tracings: a comparison with cord blood gases and Apgar scores. J Perinatol 1999;19:255–9. [DOI] [PubMed] [Google Scholar]
- 50.Teramo KA, Widness JA. Increased fetal plasma and amniotic fluid erythropoietin concentrations: markers of intrauterine hypoxia. Neonatology 2009;95:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whittaker PG, Macphail S, Lind T. Serial hematologic changes and pregnancy outcome. Obstet Gynecol 1996;88:33–9. [DOI] [PubMed] [Google Scholar]
- 52.Milman N, Agger AO, Nielsen OJ. Iron supplementation during pregnancy. Effect on iron status markers, serum erythropoietin and human placental lactogen. A placebo controlled study in 207 Danish women. Dan Med Bull 1991;38:471–6. [PubMed] [Google Scholar]
- 53.Romslo I, Haram K, Sagen N, Augensen K. Iron requirement in normal pregnancy as assessed by serum ferritin, serum transferrin saturation and erythrocyte protoporphyrin determinations. Br J Obstet Gynaecol 1983;90:101–7. [DOI] [PubMed] [Google Scholar]
- 54.Little MP, Brocard P, Elliott P, Steer PJ. Hemoglobin concentration in pregnancy and perinatal mortality: a London-based cohort study. Am J Obstet Gynecol 2005;193:220–6. [DOI] [PubMed] [Google Scholar]
- 55.Steer PJ. Maternal hemoglobin concentration and birth weight. Am J Clin Nutr 2000;71:1285S–7S. [DOI] [PubMed] [Google Scholar]
- 56.Huisman A, Aarnoudse JG. Increased 2nd trimester hemoglobin concentration in pregnancies later complicated by hypertension and growth retardation. Early evidence of a reduced plasma volume. Acta Obstet Gynecol Scand 1986;65:605–8. [DOI] [PubMed] [Google Scholar]
- 57.Murphy JF, O’Riordan J, Newcombe RG, Coles EC, Pearson JF. Relation of haemoglobin levels in first and second trimesters to outcome of pregnancy. Lancet 1986;1:992–5. [DOI] [PubMed] [Google Scholar]
- 58.Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. JAMA 2000;284:2611–7. [DOI] [PubMed] [Google Scholar]
- 59.Larsson A, Palm M, Hansson LO, Axelsson O. Reference values for clinical chemistry tests during normal pregnancy. BJOG 2008;115:874–81. [DOI] [PubMed] [Google Scholar]
- 60.Milman N, Bergholt T, Byg KE, Eriksen L, Hvas AM. Reference intervals for haematological variables during normal pregnancy and postpartum in 434 healthy Danish women. Eur J Haematol 2007;79:39–46. [DOI] [PubMed] [Google Scholar]
- 61.Tamura T, Goldenberg RL, Johnston KE, Cliver SP, Hickey CA. Serum ferritin: a predictor of early spontaneous preterm delivery. Obstet Gynecol 1996;87:360–5. [DOI] [PubMed] [Google Scholar]
- 62.Scholl TO. High third-trimester ferritin concentration: associations with very preterm delivery, infection, and maternal nutritional status. Obstet Gynecol 1998;92:161–6. [DOI] [PubMed] [Google Scholar]
- 63.Goldenberg RL, Tamura T, DuBard M, Johnston KE, Copper RL, Neggers Y. Plasma ferritin and pregnancy outcome. Am J Obstet Gynecol 1996;175:1356–9. [DOI] [PubMed] [Google Scholar]
- 64.Lao TT, Tam KF, Chan LY. Third trimester iron status and pregnancy outcome in non-anaemic women; pregnancy unfavourably affected by maternal iron excess. Hum Reprod 2000;15:1843–8. [DOI] [PubMed] [Google Scholar]
- 65.van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: which measurements are valid? Br J Haematol 1998;103:817–24. [DOI] [PubMed] [Google Scholar]
- 66.Carriaga MT, Skikne BS, Finley B, Cutler B, Cook JD. Serum transferrin receptor for the detection of iron deficiency in pregnancy. Am J Clin Nutr 1991;54:1077–81. [DOI] [PubMed] [Google Scholar]
- 67.Cao C, O’Brien KO. Pregnancy and iron homeostasis: an update. Nutr Rev 2013;71:35–51. [DOI] [PubMed] [Google Scholar]
- 68.Cao C, Fleming MD. The placenta: the forgotten essential organ of iron transport. Nutr Rev 2016;74:421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bastin J, Drakesmith H, Rees M, Sargent I, Townsend A. Localisation of proteins of iron metabolism in the human placenta and liver. Br J Haematol 2006;134:532–43. [DOI] [PubMed] [Google Scholar]
- 70.Georgieff MK, Wobken JK, Welle J, Burdo JR, Connor JR. Identification and localization of divalent metal transporter-1 (DMT-1) in term human placenta. Placenta 2000;21:799–804. [DOI] [PubMed] [Google Scholar]
- 71.Srai SK, Bomford A, McArdle HJ. Iron transport across cell membranes: molecular understanding of duodenal and placental iron uptake. Best Pract Res Clin Haematol 2002;15:243–59. [DOI] [PubMed] [Google Scholar]
- 72.Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood 2006;108:1388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Best CM, Pressman EK, Cao C, Cooper E, Guillet R, Yost OL, Galati J, Kent TR, O’Brien KO. Maternal iron status during pregnancy compared with neonatal iron status better predicts placental iron transporter expression in humans. FASEB J 2016;30:3541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gambling L, Czopek A, Andersen HS, Holtrop G, Srai SK, Krejpcio Z, McArdle HJ. Fetal iron status regulates maternal iron metabolism during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol 2009;296:R1063–70. [DOI] [PubMed] [Google Scholar]
- 75.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA 1998;95:1148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest 2005;115:1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang CY, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J Biol Chem 2012;287:34032–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gálvez-Peralta M, He L, Jorge-Nebert LF, Wang B, Miller ML, Eppert BL, Afton S, Nebert DW. ZIP8 zinc transporter: indispensable role for both multiple-organ organogenesis and hematopoiesis in utero. PLoS One 2012;7:e36055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hojyo S, Fukada T, Shimoda S, Ohashi W, Bin BH, Koseki H, Hirano T. The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS One 2011;6:e18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, et al. . Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 2000;403:776–81. [DOI] [PubMed] [Google Scholar]
- 81.Evans P, Cindrova-Davies T, Muttukrishna S, Burton GJ, Porter J, Jauniaux E. Hepcidin and iron species distribution inside the first-trimester human gestational sac. Mol Hum Reprod 2011;17:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guller S, Buhimschi CS, Ma YY, Huang ST, Yang L, Kuczynski E, Zambrano E, Lockwood CJ, Buhimschi IA. Placental expression of ceruloplasmin in pregnancies complicated by severe preeclampsia. Lab Invest 2008;88:1057–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li YQ, Bai B, Cao XX, Yan H, Zhuang GH. Ferroportin 1 and hephaestin expression in BeWo cell line with different iron treatment. Cell Biochem Funct 2012;30:249–55. [DOI] [PubMed] [Google Scholar]
- 84.Chen H, Attieh ZK, Syed BA, Kuo YM, Stevens V, Fuqua BK, Andersen HS, Naylor CE, Evans RW, Gambling L, et al. . Identification of zyklopen, a new member of the vertebrate multicopper ferroxidase family, and characterization in rodents and human cells. J Nutr 2010;140:1728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA 1999;96:10812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 1999;21:195–9. [DOI] [PubMed] [Google Scholar]
- 87.Fuqua B, Lu Y, Darshan D, Frazer D, Wilkins S, Page K, Vulpe C, Anderson G. The role of multicopper ferroxidases in mammalian iron homeostasis (995.2). FASEB J 2014;28. [Google Scholar]
- 88.Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, Vaughn MB, Kaplan J, Palis J, et al. . A heme export protein is required for red blood cell differentiation and iron homeostasis. Science 2008;319:825–8. [DOI] [PubMed] [Google Scholar]
- 89.Jaacks LM, Young MF, Essley BV, McNanley TJ, Cooper EM, Pressman EK, McIntyre AW, Orlando MS, Abkowitz JL, Guillet R, et al. . Placental expression of the heme transporter, feline leukemia virus subgroup C receptor, is related to maternal iron status in pregnant adolescents. J Nutr 2011;141:1267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]