Abstract
Both iron deficiency (ID) and excess can lead to impaired health status. There is substantial evidence of a U-shaped curve between the risk of adverse birth outcomes and maternal hemoglobin concentrations during pregnancy; however, it is unclear whether those relations are attributable to conditions of low and high iron status or to other mechanisms. We summarized current evidence from human studies regarding the association between birth outcomes and maternal hemoglobin concentrations or iron status. We also reviewed effects of iron supplementation on birth outcomes among women at low risk of ID and the potential mechanisms for adverse effects of high iron status during pregnancy. Overall, we confirmed a U-shaped curve for the risk of adverse birth outcomes with maternal hemoglobin concentrations, but the relations differ by trimester. For low hemoglobin concentrations, the link with adverse outcomes is more evident when hemoglobin concentrations are measured in early pregnancy. These relations generally became weaker or nonexistent when hemoglobin concentrations are measured in the second or third trimesters. Associations between high hemoglobin concentration and adverse birth outcomes are evident in all 3 trimesters but evidence is mixed. There is less evidence for the associations between maternal iron status and adverse birth outcomes. Most studies used serum ferritin (SF) concentrations as the indicator of iron status, which makes the interpretation of results challenging because SF concentrations increase in response to inflammation or infection. The effect of iron supplementation during pregnancy may depend on initial iron status. There are several mechanisms through which high iron status during pregnancy may have adverse effects on birth outcomes, including oxidative stress, increased blood viscosity, and impaired systemic response to inflammation and infection. Research is needed to understand the biological processes that underlie the U-shaped curves seen in observational studies. Reevaluation of cutoffs for hemoglobin concentrations and indicators of iron status during pregnancy is also needed.
Keywords: anemia, iron deficiency, pregnancy, iron supplementation, ferritin, soluble transferrin receptor, low birth weight, preterm birth, small-for-gestational age, stillbirth
INTRODUCTION
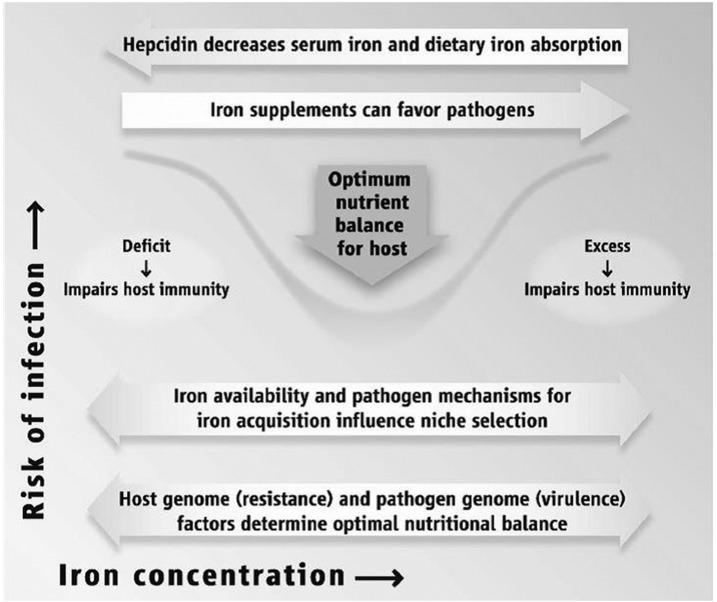
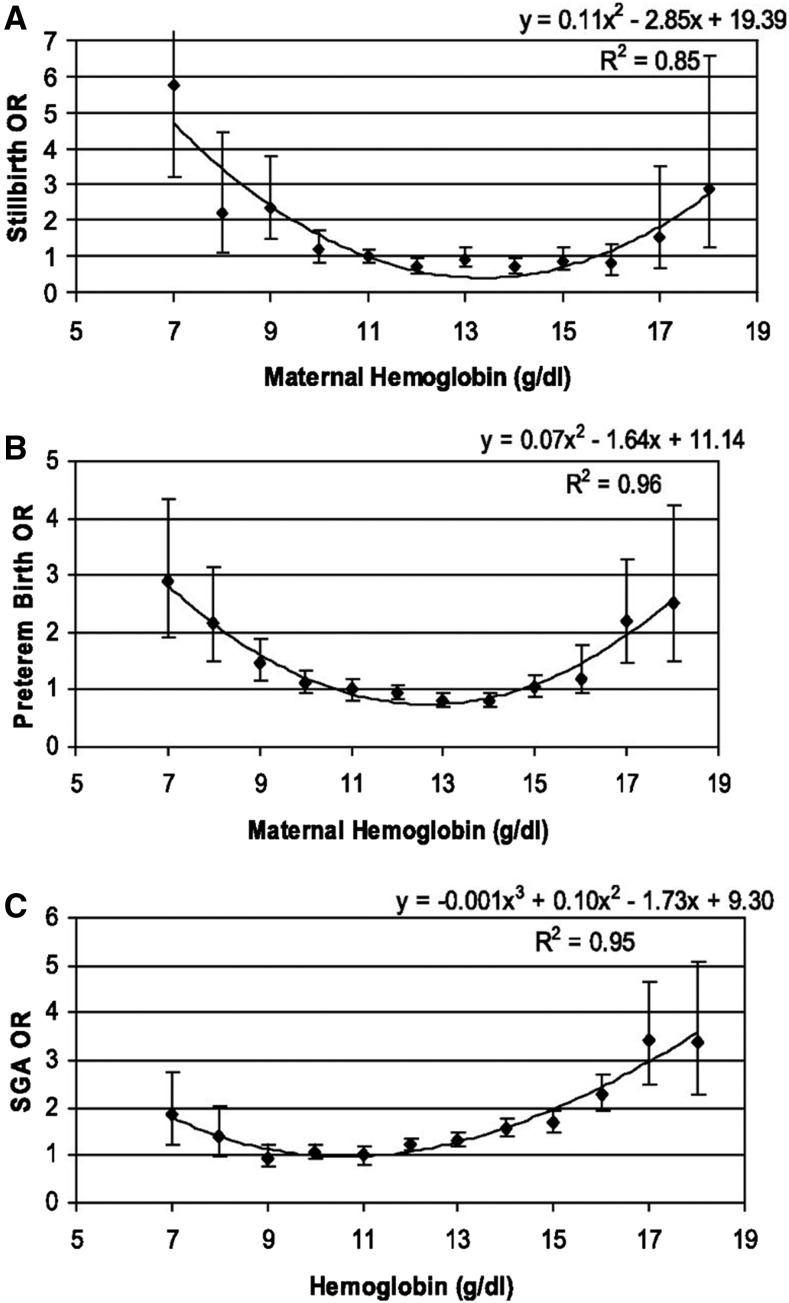
Both iron deficiency (ID) and iron excess can lead to impaired health status. Achieving optimal health outcomes requires a delicate balancing act so that iron intakes are neither too low nor too high as illustrated in Figure 1 for immune function (1). It has also been known for decades that there tends to be a U-shaped curve between the risk of adverse birth outcomes and maternal hemoglobin concentrations during pregnancy, with a higher risk of low birth weight (LBW) and preterm birth (PTB) observed among women with both low and high hemoglobin concentrations (2–5) as illustrated in Figure 2. Less clear is whether those relations are attributable to conditions of low and high iron status or to other mechanisms.
FIGURE 1.
A delicate balancing act. Reproduced from reference 1 with permission.
FIGURE 2.
ORs for stillbirth (A), preterm birth (B), and SGA (C), by maternal hemoglobin concentration. SGA, small-for-gestational-age. Reproduced from reference 4 with permission.
This review, conducted to support discussions at an NIH workshop, summarizes current evidence from human studies regarding the association between birth outcomes and maternal hemoglobin concentrations or indicators of iron status. We also briefly consider recent evidence regarding the effects of iron supplementation on birth outcomes among women at low risk of ID in early pregnancy. Lastly, we comment on potential mechanisms for adverse effects of high iron status during pregnancy and highlight some of the knowledge gaps and research needs.
ASSOCIATIONS BETWEEN MATERNAL HEMOGLOBIN OR IRON STATUS AND ADVERSE BIRTH OUTCOMES
Our review included observational studies in which selected birth outcomes [LBW, PTB, small-for-gestational age (SGA), and stillbirth] were examined relative to hemoglobin and iron status as defined by the researchers. We used 2 electronic databases (PubMed and Web of Science) to search for articles in English with no restriction on year of publication. Our specific search terms were: (“hemoglobin” or “anemia” or “iron status” or “iron deficiency”) and (“preterm birth” or “birth weight” or “stillbirth” or “small for gestational age” or “birth outcomes”). We also used the “snowball” technique of checking references cited in review articles or other research articles. We excluded studies that did not control for potentially confounding variables. In addition, we focused on studies that provided clear information on the timing of assessment of maternal hemoglobin or iron status during pregnancy, so that we could examine the nature of the associations with birth outcomes separately by the trimester when hemoglobin or iron status was measured. Twenty-seven observational studies were reviewed in total. Additionally, we reviewed data from recent trials we conducted in Ghana, Malawi, and Bangladesh (6–8), because these data included an indicator of iron status, soluble transferrin receptor (sTfR), not previously reported by any study examining the association between high iron status and birth outcomes and measured in only one study examining the association between low iron status and birth outcomes (9).
Tables 1–3 summarize the associations with maternal hemoglobin concentrations from 10 studies in the United States and Western Europe, 6 in Asia, one in Africa, one in South America, and one in the Middle East. In the first trimester (Table 1), a low hemoglobin concentration was significantly associated with LBW in all 3 of the studies that examined that relation and with PTB in 3 of the 5 studies that included that outcome. By contrast, in the second trimester (Table 2), low hemoglobin concentration was not associated with LBW in any of the 6 studies that examined that relation, and the associations with PTB, SGA, and stillbirth were inconsistent, with most studies showing no relation. A high hemoglobin concentration in the first trimester was associated with adverse birth outcomes in a few studies. In the second trimester, a high hemoglobin concentration was significantly associated with LBW in 2 of the 3 studies that examined that outcome, and there were mixed results for associations with PTB, SGA, and stillbirth.
TABLE 1.
Associations of a low or high hemoglobin concentration in the first trimester with birth outcomes1
| Low hemoglobin2 |
High hemoglobin2 |
||||||||||
| Study (ref), year | Hemoglobin cutoffs | n | Country | LBW | PTB | SGA | Stillbirth | LBW | PTB | SGA | Stillbirth |
| Delpisheh et al. (10), 2008 | <110 g/L | 270 | England | ↑ | |||||||
| Hämäläinen et al. (11), 2003 | <100 g/L | 597 cases, 22,202 controls | Finland | ↑ | o | o | o | ||||
| Maghsoudlou et al. (12), 2016 | <110, ≥140 g/L | 495 cases, 2888 controls | Iran | o | o | ||||||
| Ren et al. (13), 2007 | <110, ≥120 g/L | 88,149 | China | ↑ | ↑ | ↑ | o | o | o | ||
| Scanlon et al. (14), 2000 | <−1, >1 SD from the reference group | 173,031 | United States | ↑ | o | o | ↑ | ||||
| Stephansson et al. (15), 2000 | ≤115, ≥146 g/L | 702 cases, 702 controls | Sweden | (↑) | (↑) | ||||||
| Tomashek et al. (16), 2006 | <110, ≥146 g/L | 1375 cases, 4199 controls | United States | o | (↑) | ||||||
| Zhang et al. (17), 2009 | <90, ≥130 g/L | 160,700 | China | (↑) | ↓ | ||||||
| Zhang et al. (18), 2009 | <90, ≥120 g/L | 1354 cases, 163,313 controls | China | (↑) | o | ||||||
| Zhou et al. (19), 1998 | <110, ≥120 g/L | 829 | China | ↑ | ↑ | o | (↑) | (↑) | o | ||
↑ indicates a positive association (higher OR or RR of birth outcome), P < 0.05; (↑) indicates a marginally significant positive association, 0.05 ≥ P < 0.10; ↓ indicates a negative association (lower OR or RR for birth outcome), P < 0.05; (↓) indicates a marginally significant negative association, 0.05 ≥ P < 0.10; o indicates no association; a blank cell indicates the relation was not examined. LBW, low birth weight; PTB, preterm birth; ref, reference; SGA, small-for-gestational-age.
Low and high hemoglobin concentrations as defined by each study. Cutoffs used for each definition are provided in the second column.
TABLE 3.
Associations of a low or high hemoglobin concentration in the third trimester with birth outcomes1
| Low hemoglobin2 |
High hemoglobin2 |
||||||||||
| Study (ref), year | Hemoglobin cutoffs | n | Country or study type | LBW | PTB | SGA | Stillbirth | LBW | PTB | SGA | Stillbirth |
| Alwan et al. (21), 2015 | <105 g/L | 362 | England | (↓) | o | ||||||
| Chang et al. (5), 2003 | ≤95, >120 g/L | 918 | United States | o | ↑ | o | ↑ | ↑ | o | ||
| Hämäläinen et al. (11), 2003 | <100 g/L | 597 cases, 22,202 controls | Finland | o | o | o | o | ||||
| Hwang et al. (26), 2010 | <100 g/L | 377 cases, 3183 controls | Korean | ↑ | ↑ | ||||||
| Maghsoudlou et al. (12), 2016 | <110, ≥140 g/L | 495 cases, 2888 controls | Iran | ↓ | ↑ | ||||||
| Mohamed et al. (27), 2012 | <110, ≥120 g/L | 17,338 | United States | ↑ | ↑ | ↓ | ↓ | ||||
| Scanlon et al. (14), 2000 | <−1, >1 SD from the reference group | 173,031 | United States | o | ↓ | o | o | ||||
| Scholl and Hediger (28), 1994 | <110 g/L | 755 | United States | o | o | ||||||
| Verhoeff et al. (24), 2001 | <80 g/L | 1423 | Malawi | o | o | ||||||
| Xiong et al. (25), 2000 | <100–110 g/L | NA | Meta-analysis | (↓) | (↓) | o | |||||
| Zhang et al. (17), 2009 | <100, ≥120 g/L | 160,700 | China | ↓ | o | ||||||
| Zhang et al. (18), 2009 | <100, ≥120 g/L | 1354 cases, 163,313 controls | China | ↓ | ↑ | ||||||
| Zhou et al. (19), 1998 | <100, ≥120 g/L | 829 | China | o | o | o | o | o | o | ||
↑ indicates a positive association (higher OR or RR of birth outcome), P < 0.05; (↑) indicates a marginally significant positive association, 0.05 ≥ P < 0.10; ↓ indicates a negative association (lower OR or RR for birth outcome), P < 0.05; (↓) indicates a marginally significant negative association, 0.05 ≥ P < 0.10; o indicates no association; a blank cell indicates the relation was not examined. LBW, low birth weight; NA, not applicable; PTB, preterm birth; ref, reference; SGA, small-for-gestational-age.
Low and high hemoglobin concentrations as defined by each study. Cutoffs used for each definition are provided in the second column.
TABLE 2.
Associations of a low or high hemoglobin concentration in the second trimester with birth outcomes1
| Low hemoglobin2 |
High hemoglobin2 |
||||||||||
| Study (ref), year | Hemoglobin cutoffs | n | Country or study type | LBW | PTB | SGA | Stillbirth | LBW | PTB | SGA | Stillbirth |
| Abeysena et al. (20), 2010 | 110, >139 g/L | 817 | Sri Lanka | o | o | o | ↑ | o | o | ||
| Alwan et al. (21), 2015 | <110 g/L | 362 | England | o | (↑) | ||||||
| Chang et al. (5), 2003 | <105, >120 g/L | 918 | United States | o | o | o | ↑ | ↑ | o | ||
| Gonzales et al. (4), 2009 | <110, >129 g/L | 35,449 | Peru | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| Hämäläinen et al. (11), 2003 | <100 g/L | 597 cases, 22,202 controls | Finland | o | o | o | o | ||||
| Little et al. (22), 2005 | No definition | 222,614 | England | o | o | ||||||
| Scanlon et al. (14), 2000 | <−1, >1 SD from the reference group | 173,031 | United States | ↑ | o | o | ↑ | ||||
| Scholl et al. (23), 1992 | <105 g/L | 826 | United States | o | o | o | |||||
| Tomashek et al. (16), 2006 | <105, ≥146 g/L | 1375 cases, 4199 controls | United States | (↑) | (↑) | ||||||
| Verhoeff et al. (24), 2001 | <80 g/L | 1423 | Malawi | o | ↑ | ||||||
| Xiong et al. (25), 2000 | <100–110 g/L | NA | Meta-analysis | o | ↑ | o | |||||
| Zhang et al. (17), 2009 | <100, ≥120 g/L | 160,700 | China | o | o | ||||||
| Zhang et al. (18), 2009 | <100, ≥120 g/L | 1354 cases, 163,313 controls | China | o | o | ||||||
| Zhou et al. (19), 1998 | <100, ≥120 g/L | 829 | China | o | o | o | o | o | o | ||
↑ indicates a positive association (higher OR or RR of birth outcome), P < 0.05; (↑) indicates a marginally significant positive association, 0.05 ≥ P < 0.10; ↓ indicates a negative association (lower OR or RR for birth outcome), P < 0.05; (↓) indicates a marginally significant negative association, 0.05 ≥ P < 0.10; o indicates no association; a blank cell indicates the relation was not examined. LBW, low birth weight; NA, not applicable; PTB, preterm birth; ref, reference; SGA, small-for-gestational-age.
Low and high hemoglobin concentrations as defined by each study. Cutoffs used for each definition are provided in the second column.
In the third trimester (Table 3), very few studies demonstrated a link between a low hemoglobin concentration and a higher risk of LBW (1 of 6 studies), PTB (3 of 11 studies), SGA (1 of 8 studies), or stillbirth (0 of 3 studies); and in some cases, a low hemoglobin concentration was linked to a lower risk of adverse birth outcomes. A high hemoglobin concentration in the third trimester was significantly associated with stillbirth in 2 studies but inconsistently associated with LBW and PTB. For SGA, no relation was found in 3 studies.
Overall, there is substantial evidence for a U-shaped curve for the risk of adverse birth outcomes with maternal hemoglobin concentration, but the relations differ by trimester. For a low hemoglobin concentration, the link with adverse outcomes is more evident when the hemoglobin concentration was measured in early pregnancy, with these relations generally being much weaker or nonexistent when the hemoglobin concentration was measured in the second or third trimesters. Linkages between a high hemoglobin concentration and adverse birth outcomes are evident in all 3 trimesters, but the evidence is mixed.
The cutoffs used to define low or high hemoglobin concentrations in these studies differed considerably, which may have affected the likelihood of detecting relations with birth outcomes. In studies that performed analyses using multiple cutoffs, often only the more extreme cutoffs were significantly associated with adverse birth outcomes (5, 12, 17, 18). This is supported by a 2012 meta-analysis of 12 studies, which indicated that moderate to severe, but not mild, maternal anemia was associated with an increased risk of SGA (29). In a very large study conducted in the United Kingdom that demonstrated a U-shaped curve for the relation between the maternal hemoglobin concentration and LBW across many different racial-ethnic groups, the lowest incidence of LBW was among women with hemoglobin concentrations of 95–105 g/L (2). It is noteworthy that the WHO cutoff for anemia is <110 g/L, and these women all had hemoglobin concentrations below that cutoff.
The concept that the relation between the maternal hemoglobin concentration and birth outcomes differs depending on the time during pregnancy when hemoglobin concentration is assessed is supported by 2 very large studies that examined each trimester separately. Scanlon et al. (14) evaluated the risk of SGA and PTB among 173,371 pregnant women in the United States according to maternal hemoglobin concentration categorized into 7 levels: very low, low, low-normal, normal (reference group), high-normal, high, and very high. Women with a high or very high hemoglobin concentration in the first or second trimester had an elevated risk of SGA; this was less evident for the hemoglobin concentration in the third trimester, but those with a hemoglobin concentration below the reference range in late pregnancy had a lower risk of SGA. For PTB, those with a hemoglobin concentration lower than the reference range in the first or second trimester had an elevated risk of PTB, whereas those with a high hemoglobin concentration (but not those with a very high hemoglobin concentration) in the third trimester were at highest risk. Different associations by trimester were also found in a study of 164,667 women in China (17, 18); for both PTB and stillbirth, elevated risks were seen among women with a low hemoglobin concentration in the first trimester, but there was little association with the hemoglobin concentration in the second trimester, and the relations were reversed in the third trimester (i.e., elevated risks were associated with higher hemoglobin concentrations). Some of these shifts (as gestation progresses) in the slope of the relation between birth outcomes and the maternal hemoglobin concentration may be related to plasma volume expansion. The maternal plasma volume normally expands during pregnancy [mainly in the second and third trimesters (30, 31)] and is associated with lower concentrations of hemoglobin and other biomarkers. Thus, a high hemoglobin concentration in the second and third trimesters could be a marker of inadequate plasma volume expansion, which is associated with adverse birth outcomes.
Compared with hemoglobin, there is less evidence of the associations between maternal iron status and adverse birth outcomes, with 5 studies in North America and Western Europe, 1 in Australia, 1 in Africa, and 1 in Asia (Table 4). Generally, the serum ferritin (SF) concentration was used as an indicator of iron status, but some studies included other indicators, such as zinc protoporphyrin. In the first trimester, a low SF concentration was related to an increased risk of SGA in 1 of 2 studies, and a high SF was associated with PTB in another study, but no other significant associations were observed. In the second trimester, a low SF concentration was related to LBW and PTB (but not SGA) in one study, but no associations were observed in the other 2 studies examining these outcomes; a high SF concentration was related to an increased risk of LBW in 1 of 2 studies and PTB in 2 of 3 studies. In the third trimester, none of the 3 studies reported a significant association between low iron status and the risk of LBW or PTB, whereas a high SF concentration was associated with an increased risk of these outcomes in the 3 studies that examined these relations.
TABLE 4.
Associations of low or high iron status, by trimester, with birth outcomes1
| Low iron status2 |
High iron status2 |
||||||||||
| Study (ref), year | Iron status cutoffs | n | Country or study type | LBW | PTB | SGA | Stillbirth | LBW | PTB | SGA | Stillbirth |
| First trimester | |||||||||||
| Alwan et al. (21), 2015 | SF <15 μg/L | 362 | England | o | ↑ | ||||||
| Khambalia et al. (9), 2015 | SF <12 μg/L, sTfR ≥21.0 nmol/L, SF ≥75th percentile | 2254 | Australia | — | ↑ | ||||||
| Khambalia et al. (32), 2016 | SF <12 μg/L, sTfR ≥21.0 nmol/L, TBI <0 mg/kg | 4420 | Australia | o | o | ||||||
| Second trimester | |||||||||||
| Goldenberg et al. (33), 1996 | SF by quartiles | 580 | United States | o | o | ↑ | ↑ | ||||
| Scholl et al. (23), 1992 | SF <12 μg/L | 826 | United States | ↑ | ↑ | o | |||||
| Scholl (34), 1998 | SF ≥90th percentile | 1162 | United States | o | o | ||||||
| Tamura et al. (35), 1996 | SF median cutoff | 31 cases, 63 controls | United States | ↑ | |||||||
| Verhoeff et al. (24), 2001 | EP >2.7 μg ZPP/g hemoglobin and MCHC <32 g/dL | 1423 | Malawi | o | o | ||||||
| Third trimester | |||||||||||
| Goldenberg et al. (33), 1996 | SF by quartiles | 580 | United States | o | o | ↑ | |||||
| Lao et al. (36), 2000 | SF by quartiles | 488 | China | o | ↑ | ||||||
| Scholl (34), 1998 | SF ≥90th percentile | 1162 | United States | ↑ | ↑ | ||||||
| Verhoeff et al. (24), 2001 | EP >2.7 μg ZPP/g hemoglobin and MCHC <32 g/dL | 1423 | Malawi | o | o | ||||||
↑ indicates a positive association (higher OR or RR of birth outcome), P < 0.05; (↑) indicates a marginally significant positive association, 0.05 ≥ P < 0.10; ↓ indicates a negative association (lower OR or RR for birth outcome), P < 0.05; (↓) indicates a marginally significant negative association, 0.05 ≥ P < 0.10; o indicates no association; a blank cell indicates the relation was not examined. EP, erythrocyte protoporphyrin; LBW, low birth weight; MCHC, mean corpuscular hemoglobin concentration; PTB, preterm birth; ref, reference; SF, serum ferritin; SGA, small-for-gestational-age; sTfR, soluble transferrin receptor; TBI, total body iron stores; ZPP, zinc protoporphyrin.
Low and high iron status as defined by each study. Cutoffs used for each definition are provided in the second column.
Most of the above studies used the SF concentration as the indicator of iron status, and all of the reported associations between high iron status and adverse birth outcomes were based on a high SF concentration. This complicates the interpretation of the results because SF is an acute-phase protein that increases in response to inflammation or infection (37, 38), and thus the relations with adverse birth outcomes may reflect these conditions (or inadequate plasma volume expansion) rather than high iron status.
We analyzed the associations between maternal hemoglobin or iron status and birth outcomes in 3 recent trials in Ghana, Malawi, and Bangladesh (Table 5). The primary indicator of iron status in early (≤20 wk of gestation) and late (34–36 wk of gestation) pregnancy was sTfR, which increases with ID, but additional indicators included zinc protoporphyrin (Ghana and Malawi) and SF (Bangladesh). Analyses were conducted by using linear regression models to evaluate whether the hemoglobin concentration or iron status (as continuous variables) was significantly related to birth outcomes. In early pregnancy, a higher hemoglobin concentration was associated with a longer duration of gestation in all 3 cohorts and with higher birth weight and length-for-age z score (LAZ) in Malawi. Higher iron status in early pregnancy was associated with a longer duration of gestation in Malawi and Bangladesh and a higher birth weight, LAZ, and head-circumference-for-age z score in Malawi. In late pregnancy, there was a nonlinear relation of the hemoglobin concentration with the duration of gestation in Ghana (but not Malawi or Bangladesh) and no association with any other birth outcome in any of the sites. Higher iron status in late pregnancy was associated with a shorter duration of gestation in Bangladesh and a longer duration in Malawi and with lower birth weight (Ghana and Bangladesh), LAZ (Ghana and Bangladesh), and head-circumference-for-age z score (Bangladesh). Overall, a higher hemoglobin concentration and iron status measured in early pregnancy were generally associated with better birth outcomes, whereas when these indexes were measured in late pregnancy (Table 5), the hemoglobin concentration was not associated with birth outcomes and the higher iron status was associated with smaller birth size. The relations with iron status in late pregnancy were based not only on higher SF but also lower sTfR concentrations, and the latter cannot be explained by inadequate plasma volume expansion. Thus, these results support and strengthen the evidence from published studies indicating that the relations between birth outcomes and the maternal hemoglobin concentration or iron status differ depending on the trimester of pregnancy when these indexes are assessed.
TABLE 5.
Associations of maternal hemoglobin concentration and iron status with pregnancy outcomes in Ghana, Malawi, and Bangladesh [unpublished data (6–8)]1
| Duration of gestation |
Birth weight |
LAZ |
HCZ |
|||||||||
| Ghana | Malawi | Bangladesh | Ghana | Malawi | Bangladesh | Ghana | Malawi | Bangladesh | Ghana | Malawi | Bangladesh | |
| Early pregnancy | ||||||||||||
| Hemoglobin | + | + | + | o | + | o | o | + | o | o | (+) | o |
| Higher iron status (lower ZPP) | o | + | NA | o | o | NA | o | (+) | NA | o | o | NA |
| Higher iron status (lower sTfR) | o | + | o | o | + | o | (−) | + | o | o | + | o |
| Higher iron status (higher SF) | NA | NA | + | NA | NA | (−) | NA | NA | − | NA | NA | o |
| Late pregnancy | ||||||||||||
| Hemoglobin | Not linear | o | o | o | o | o | o | o | o | o | o | o |
| Higher iron status (lower ZPP) | o | + | NA | (−) | o | NA | (−) | o | NA | o | o | NA |
| Higher iron status (lower sTfR) | o | (+) | o | − | o | − | − | o | − | (−) | o | − |
| Higher iron status (higher SF) | NA | NA | − | NA | NA | − | NA | NA | − | NA | NA | − |
+ indicates a positive association, P < 0.05; (+) indicates a marginally significant positive association, 0.05 ≥ P < 0.10; − indicates a negative association, P < 0.05; (−) indicates a marginally significant negative association, 0.05 ≥ P < 0.10; o indicates no association. HCZ, head-circumference-for-age z score; LAZ, length-for-age z score; NA, not available (the relation was not examined); SF, serum ferritin; sTfR, soluble transferrin receptor; ZPP, zinc protoporphyrin.
POTENTIAL EFFECTS OF IRON SUPPLEMENTATION ON BIRTH OUTCOMES AMONG WOMEN AT LOW RISK OF ID IN EARLY PREGNANCY
Observational studies such as those described in the section above have inherent limitations that make it difficult to draw causal inferences regarding the potential effects of high iron status during pregnancy. Randomized controlled trials of iron supplementation thus provide critical information for this question.
Currently, WHO recommends daily supplementation with 30–60 mg elemental Fe/d (plus 400 μg folic acid) throughout pregnancy, and in settings where anemia in pregnant women is a severe public health problem (prevalence of 40% or higher), the daily dose of 60 mg is recommended instead of a lower dose (39). These recommendations were based on a Cochrane Review published in 2012, which reported that women assigned to daily iron supplementation during pregnancy had a reduced risk of giving birth to infants with LBW (RR: 0.81; 95% CI: 0.68, 0.97; 11 studies), maternal anemia at term (RR: 0.30; 95% CI: 0.19, 0.46; 14 studies), and maternal ID at term (RR: 0.43; 95% CI: 0.27, 0.66; 7 studies) (40). This review also reported an increased risk of side effects (RR: 2.36; 95% CI: 0.96, 5.82; 11 studies) and a high hemoglobin concentration (>130 mg/L) during the second and third trimesters of pregnancy (RR: 2.26; 95% CI: 1.40, 3.66; 10 studies). When this review was updated in 2015, the reduced risk of LBW became borderline significant (RR: 0.84; 95% CI: 0.69, 1.03; 11 studies) (41). There was no significant effect on any other birth outcomes. The authors of the 2015 review concluded that iron supplementation reduces the risk of maternal anemia and ID in pregnancy, but the positive effect on other maternal and infant outcomes is less clear. They stated that “implementation of iron supplementation recommendations may produce heterogeneous results depending on the populations’ background risk for LBW and anemia, as well as the level of adherence to the intervention.” The effect of iron supplementation may depend on a woman’s initial iron status. If her iron status is low, additional iron is probably beneficial, but if she is iron replete, it may be harmful. In meta-analyses in which all studies (and all individuals) are pooled together, there may be no overall main effects, but this may mask considerable heterogeneity in response.
Several studies suggest that iron supplementation among pregnant women at low risk of ID may adversely affect birth outcomes. Ziaei et al. (42) conducted a randomized controlled trial to evaluate effects of iron supplementation on pregnancy outcomes of Iranian women (n = 750) with a hemoglobin concentration ≥132 g/L early in the second trimester randomly assigned to receive 50 mg Fe or placebo daily for the rest of pregnancy. The group who received iron had significantly higher mean hemoglobin concentrations in the third trimester (137.5 compared with 125.6 mg/L, P < 0.001) but also were more likely to deliver an SGA infant (15.7% compared with 10.3%, P = 0.035) and to have hypertension disorder (2.7% compared with 0.8%, P = 0.05). There were no significant effects on the duration of pregnancy, preterm labor, or birth weight.
Shastri et al. (43) examined the association of oral iron supplementation with birth outcomes in nonanemic South Indian pregnant women. This was an observational study of 1196 nonanemic pregnant women (with a hemoglobin concentration ≥110 g/L in first trimester), all of whom had been prescribed 45 mg Fe/d plus 0.5 mg folic acid/d at the beginning of the second trimester. The investigators examined birth outcomes by tertile of actual supplemental iron intake (means: 33.6, 37.8, and 41.5 mg/d), adjusting for potential confounders, and found that infants of mothers in the highest tertile had a lower birth weight (−72 g), shorter duration of gestation (−0.6 wk), and higher risk of term LBW [16.8% compared with 8.5%; adjusted OR: 1.89; 95% CI: 1.26, 2.83] compared with infants of mothers in the lowest tertile of supplemental iron intake.
In the International Lipid-Based Nutrient Supplements-DYAD trial in Ghana, we also observed a discordance between increasing maternal hemoglobin concentration or iron status indicators and birth outcomes. In this study population, the prevalence of ID anemia at enrollment was relatively low [∼6%, with the use of a cutoff for anemia of hemoglobin concentration at <100 g/L as is recommended for African populations (44, 45)]. Women who received 60 mg Fe [iron-folic acid capsule (IFA)] from ≤20 wk of gestation until delivery had a lower prevalence of ID and anemia at 36 wk of gestation than women who received 20 mg Fe in either a lipid-based nutrient supplement (LNS) or a multiple-micronutrient capsule (46), but birth size was significantly greater in the LNS group compared with the IFA group especially among primiparous women (6). It is not possible to disentangle the effects of a lower compared with a higher dose of iron from those of the additional micronutrients provided via the LNS or multiple-micronutrient capsule, but the results illustrate that reducing maternal anemia and ID in the IFA group did not translate into greater birth size or a longer duration of gestation.
POTENTIAL MECHANISMS FOR ADVERSE EFFECTS OF EXCESS IRON DURING PREGNANCY
There are several mechanisms through which excess iron intake or high iron status during pregnancy may have adverse effects on birth outcomes. Although iron absorption is normally carefully regulated based on iron status, during pregnancy a substantial amount of iron may be absorbed even if a woman is iron replete because maternal hepcidin, which helps regulate iron absorption, is at least partially suppressed during pregnancy (47). As a result, the hemoglobin concentration may increase with prenatal iron supplementation even in iron-replete women (42). The resulting high hemoglobin concentration may increase blood viscosity and compromise placental blood flow (2). Second, excess iron intake may contribute to oxidative stress via postprandial increases in circulating non–transferrin bound iron, which can lead to lipid peroxidation and DNA damage of placental cells (48, 49). Third, iron overload may impair the systemic response to inflammation and infection (1), which could be associated with adverse birth outcomes (50, 51). Finally, there is also the potential for excess iron to alter the maternal gut microbiome (52) as well as increase the risk of copper and zinc deficiency (53), which may have implications for birth outcomes (54, 55).
CONCLUSIONS, KNOWLEDGE GAPS, AND RESEARCH NEEDS
In summary, our review confirms strong evidence of a U-shaped curve for the risk of adverse birth outcomes with maternal hemoglobin concentration, but the relations differ by trimester. For a low hemoglobin concentration, the link with adverse outcomes is more evident when the hemoglobin concentration is measured in early pregnancy. These relations generally became weaker or nonexistent when the hemoglobin concentration is measured in the second or third trimester. Associations between a high hemoglobin concentration and adverse birth outcomes are evident in all 3 trimesters, but the evidence is mixed. There is less evidence for associations between maternal iron status and adverse birth outcomes. Most studies used the SF concentration as the indicator of iron status, which makes the interpretation of the results challenging because SF increases in response to inflammation, infection, or inadequate plasma volume expansion. However, recent evidence from 2 different countries suggests that high iron status as assessed by low sTfR (a less problematic indicator in this situation) in late pregnancy is related to smaller birth size, which is concerning. The effect of iron supplementation during pregnancy may depend on initial iron status, and there are several potential mechanisms through which excess iron intake or high iron status during pregnancy may have adverse effects on birth outcomes, including oxidative stress, increased blood viscosity, impaired systemic response to inflammation and infection, and alterations in the maternal microbiome.
A limitation of this review is that, because of time limitations, the literature search did not follow all of the steps recommended for a formal systematic review. In particular, only one person read all of the articles and extracted the data presented. However, we note that additional work is moving forward with the WHO (56) that will build off this initial review to further explore maternal hemoglobin cutoffs.
Several knowledge gaps need to be addressed to clarify the relations between maternal hemoglobin concentration, iron status, and birth outcomes. Reevaluation of the most appropriate cutoffs for hemoglobin concentration and indicators of iron status during pregnancy is urgently needed, taking into account the range of values that is associated with optimal birth outcomes. Research is needed to understand the biological processes that underlie the U-shaped curves seen in observational studies: 1) is the higher risk of adverse outcomes at higher levels of hemoglobin concentration or certain iron indicators (e.g., SF) mainly the result of inadequate plasma volume expansion? 2) to what extent do the causes of failure to expand plasma volume (e.g., poor nutrition, diabetes, preeclampsia) contribute to adverse pregnancy outcomes? and 3) is the higher risk of adverse outcomes associated with higher concentrations of SF caused by inflammation or infection, rather than high iron status? Further investigation is also warranted with regard to potential mechanisms for adverse effects of supplemental iron and/or a high hemoglobin concentration during pregnancy, as described in the section above. Lastly, strategies to reduce the potentially adverse effects of prenatal iron supplementation should be evaluated, including consideration of the optimal dose and timing of iron supplementation, the effects of providing other nutrients simultaneously, the safety and efficacy of novel iron compounds, and the effects of iron-fortified foods compared with supplementation.
Acknowledgments
We thank Josh Jorgensen for his analysis of data from the International Lipid-Based Nutrient Supplements-DYAD-Malawi study and Susana Matias for her analysis of data from the Rang-Din Nutrition Study in Bangladesh and their permission to use those results in this review.
The authors’ responsibilities were as follows—BMO: performed the literature review; and both authors: wrote, reviewed, and approved the final manuscript. Neither of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ID, iron deficiency; IFA, iron–folic acid capsule; LAZ, length-for-age z score; LBW, low birth weight; LNS, lipid-based nutrient supplement; PTB, preterm birth; SF, serum ferritin; SGA, small-for-gestational-age; sTfR, soluble transferrin receptor.
REFERENCES
- 1.Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science 2012;338:768–72. [DOI] [PubMed] [Google Scholar]
- 2.Steer P, Alam MA, Wadsworth J, Welch A. Relation between maternal hemoglobin concentration and birth weight in different ethnic groups. BMJ 1995;310:489–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garn SM, Keating MT, Falkner F. Hematological status and pregnancy outcomes. Am J Clin Nutr 1981;34:115–7. [DOI] [PubMed] [Google Scholar]
- 4.Gonzales GF, Steenland K, Tapia V. Maternal hemoglobin level and fetal outcome at low and high altitudes. Am J Physiol Regul Integr Comp Physiol 2009;297:R1477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang SC, O’Brien KO, Nathanson MS, Mancini J, Witter FR. Hemoglobin concentrations influence birth outcomes in pregnant African-American adolescents. J Nutr 2003;133:2348–55. [DOI] [PubMed] [Google Scholar]
- 6.Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Zeilani M, Peerson JM, Arimond M, Vosti S, Dewey KG. Lipid-based nutrient supplement increases the birth size of infants of primiparous women in Ghana. Am J Clin Nutr 2015;101:835–46. [DOI] [PubMed] [Google Scholar]
- 7.Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Harjunmaa U, Lartey A, Nkhoma M, Phiri N, Phuka J, et al. The impact of lipid-based nutrient supplement provision to pregnant women on newborn size in rural Malawi: a randomized controlled trial. Am J Clin Nutr 2015;101:387–97. [DOI] [PubMed] [Google Scholar]
- 8.Mridha MK, Matias SL, Chaparro CM, Paul RR, Hussain S, Vosti SA, Harding KL, Cummins JR, Day LT, Saha SL, et al. Lipid-based nutrient supplements for pregnant women reduce newborn stunting in a cluster-randomized controlled effectiveness trial in Bangladesh. Am J Clin Nutr 2016;103:236–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khambalia AZ, Collins CE, Roberts CL, Morris JM, Powell KL, Tasevski V, Nassar N. High maternal serum ferritin in early pregnancy and risk of spontaneous preterm birth. Br J Nutr 2015;114:455–61. [DOI] [PubMed] [Google Scholar]
- 10.Delpisheh A, Brabin L, Drummond S, Brabin BJ. Prenatal smoking exposure and asymmetric fetal growth restriction. Ann Hum Biol 2008;35:573–83. [DOI] [PubMed] [Google Scholar]
- 11.Hämäläinen H, Hakkarainen K, Heinonen S. Anaemia in the first but not in the second or third trimester is a risk factor for low birth weight. Clin Nutr 2003;22:271–5. [DOI] [PubMed] [Google Scholar]
- 12.Maghsoudlou S, Cnattingius S, Stephansson O, Aarabi M, Semnani S, Montgomery SM, Bahmanyar S. Maternal haemoglobin concentrations before and during pregnancy and stillbirth risk: a population-based case-control study. BMC Pregnancy Childbirth 2016;16:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren A, Wang J, Ye RW, Li S, Liu JM, Li Z. Low first-trimester hemoglobin and low birth weight, preterm birth and small for gestational age newborns. Int J Gynaecol Obstet 2007;98:124–8. [DOI] [PubMed] [Google Scholar]
- 14.Scanlon KS, Yip R, Schieve LA, Cogswell ME. High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstet Gynecol 2000;96:741–8. [DOI] [PubMed] [Google Scholar]
- 15.Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. JAMA 2000;284:2611–7. [DOI] [PubMed] [Google Scholar]
- 16.Tomashek KM, Ananth CV, Cogswell ME. Risk of stillbirth in relation to maternal haemoglobin concentration during pregnancy. Matern Child Nutr 2006;2:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, Ananth CV, Rhoads GG, Li Z. The impact of maternal anemia on perinatal mortality: a population-based, prospective cohort study in China. Ann Epidemiol 2009;19:793–9. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Ananth CV, Li Z, Smulian JC. Maternal anaemia and preterm birth: a prospective cohort study. Int J Epidemiol 2009;38:1380–9. [DOI] [PubMed] [Google Scholar]
- 19.Zhou LM, Yang WW, Hua JZ, Deng CQ, Tao X, Stoltzfus RJ. Relation of hemoglobin measured at different times in pregnancy to preterm birth and low birth weight in Shanghai, China. Am J Epidemiol 1998;148:998–1006. [DOI] [PubMed] [Google Scholar]
- 20.Abeysena C, Jayawardana P, de A Seneviratne R. Maternal haemoglobin level at booking visit and its effect on adverse pregnancy outcome. Aust N Z J Obstet Gynaecol 2010;50:423–7. [DOI] [PubMed] [Google Scholar]
- 21.Alwan NA, Cade JE, McArdle HJ, Greenwood DC, Hayes HE, Simpson NA. Maternal iron status in early pregnancy and birth outcomes: insights from the Baby’s Vascular health and Iron in Pregnancy study. Br J Nutr 2015;113:1985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little MP, Brocard P, Elliott P, Steer PJ. Hemoglobin concentration in pregnancy and perinatal mortality: a London-based cohort study. Am J Obstet Gynecol 2005;193:220–6. [DOI] [PubMed] [Google Scholar]
- 23.Scholl TO, Hediger ML, Fischer RL, Shearer JW. Anemia vs iron deficiency: increased risk of preterm delivery in a prospective study. Am J Clin Nutr 1992;55:985–8. [DOI] [PubMed] [Google Scholar]
- 24.Verhoeff FH, Brabin BJ, van Buuren S, Chimsuku L, Kazembe P, Wit JM, Broadhead RL. An analysis of intra-uterine growth retardation in rural Malawi. Eur J Clin Nutr 2001;55:682–9. [DOI] [PubMed] [Google Scholar]
- 25.Xiong X, Buekens P, Alexander S, Demianczuk N, Wollast E. Anemia during pregnancy and birth outcome: a meta-analysis. Am J Perinatol 2000;17:137–46. [DOI] [PubMed] [Google Scholar]
- 26.Hwang HS, Kim YH, Kwon JY, Park YW. Uterine and umbilical artery Doppler velocimetry as a predictor for adverse pregnancy outcomes in pregnant women with anemia. J Perinat Med 2010;38:467–71. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed MA, Ahmad T, Macri C, Aly H. Racial disparities in maternal hemoglobin concentrations and pregnancy outcomes. J Perinat Med 2012;40:141–9. [DOI] [PubMed] [Google Scholar]
- 28.Scholl TO, Hediger ML. Anemia and iron-deficiency anemia: compilation of data on pregnancy outcome. Am J Clin Nutr 1994;59:492S–500S; discussion 500S–1S. [DOI] [PubMed] [Google Scholar]
- 29.Kozuki N, Lee AC, Katz J. Moderate to severe, but not mild, maternal anemia is associated with increased risk of small-for-gestational-age outcomes. J Nutr 2012;142:358–62. [DOI] [PubMed] [Google Scholar]
- 30.Institute of Medicine. Subcommittee on nutritional status and weight gain during pregnancy. Nutrition during pregnancy. Washington (DC): National Academy Press; 1990. [Google Scholar]
- 31.Klebanoff MA, Shiono PH, Berendes HW, Rhoads GG. Facts and artifacts about anemia and preterm delivery. JAMA 1989;262:511–5. [PubMed] [Google Scholar]
- 32.Khambalia AZ, Collins CE, Roberts CL, Morris JM, Powell KL, Tasevski V, Nassar N. Iron deficiency in early pregnancy using serum ferritin and soluble transferrin receptor concentrations are associated with pregnancy and birth outcomes. Eur J Clin Nutr 2016;70:358–63. [DOI] [PubMed] [Google Scholar]
- 33.Goldenberg RL, Tamura T, DuBard M, Johnston KE, Copper RL, Neggers Y. Plasma ferritin and pregnancy outcome. Am J Obstet Gynecol 1996;175:1356–9. [DOI] [PubMed] [Google Scholar]
- 34.Scholl TO. High third-trimester ferritin concentration: associations with very preterm delivery, infection, and maternal nutritional status. Obstet Gynecol 1998;92:161–6. [DOI] [PubMed] [Google Scholar]
- 35.Tamura T, Goldenberg RL, Johnston KE, Cliver SP, Hickey CA. Serum ferritin: a predictor of early spontaneous preterm delivery. Obstet Gynecol 1996;87:360–5. [DOI] [PubMed] [Google Scholar]
- 36.Lao TT, Tam KF, Chan LY. Third trimester iron status and pregnancy outcome in non-anaemic women; pregnancy unfavourably affected by maternal iron excess. Hum Reprod 2000;15:1843–8. [DOI] [PubMed] [Google Scholar]
- 37.Finch CA, Bellotti V, Stray S, Lipschitz DA, Cook JD, Pippard MJ, Huebers HA. Plasma ferritin determination as a diagnostic tool. West J Med 1986;145:657–63. [PMC free article] [PubMed] [Google Scholar]
- 38.Baynes R, Bezwoda W, Bothwell T, Khan Q, Mansoor N. The non-immune inflammatory response: serial changes in plasma iron, iron-binding capacity, lactoferrin, ferritin and C-reactive protein. Scand J Clin Lab Invest 1986;46:695–704. [DOI] [PubMed] [Google Scholar]
- 39.WHO. Guideline: daily iron and folic acid supplementation in pregnant women. Geneva (Switzerland): WHO; 2012. [PubMed] [Google Scholar]
- 40.Peña-Rosas JP, De-Regil LM, Dowswell T, Viteri FE. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2012;12:CD004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2015:CD004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziaei S, Norrozi M, Faghihzadeh S, Jafarbegloo E. A randomised placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin > or = 13.2 g/dl. BJOG 2007;114:684–8. Erratum in: BJOG 2007;114(10):1311. [DOI] [PubMed] [Google Scholar]
- 43.Shastri L, Mishra PE, Dwarkanath P, Thomas T, Duggan C, Bosch R, McDonald CM, Thomas A, Kurpad AV. Association of oral iron supplementation with birth outcomes in non-anaemic South Indian pregnant women. Eur J Clin Nutr 2015;69:609–13. [DOI] [PubMed] [Google Scholar]
- 44.INACG Steering Committee. Adjusting hemoglobin values in program surveys [Internet]. c2002 [cited 2014 Jan 10]. Available from: http://pdf.usaid.gov/pdf_docs/PNACQ927.pdf.
- 45.WHO/UNICEF/UNU. Iron deficiency anaemia - assessment, prevention and control. A guide for programme managers. Geneva (Switzerland): WHO; 2001. p. 1–132. [Google Scholar]
- 46.Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Zeilani M, Baldiviez LM, Oaks BM, Vosti S, Dewey KG. Impact of small-quantity lipid-based nutrient supplement on hemoglobin, iron status and biomarkers of inflammation in pregnant Ghanaian women. Matern Child Nutr 2017;13:e12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014;6:3062–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000;149:43–50. [DOI] [PubMed] [Google Scholar]
- 49.Casanueva E, Viteri FE. Iron and oxidative stress in pregnancy. J Nutr 2003;133:1700S–8S. [DOI] [PubMed] [Google Scholar]
- 50.Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol 2010;116:393–401. [DOI] [PubMed] [Google Scholar]
- 51.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015;64:731–42. [DOI] [PubMed] [Google Scholar]
- 53.Ziaei S, Janghorban R, Shariatdoust S, Faghihzadeh S. The effects of iron supplementation on serum copper and zinc levels in pregnant women with high-normal hemoglobin. Int J Gynaecol Obstet 2008;100:133–5. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Hu YF, Hao JH, Chen YH, Su PY, Wang Y, Yu Z, Fu L, Xu YY, Zhang C, et al. Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: a population-based birth cohort study. Sci Rep 2015;5:11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pathak P, Kapil U. Role of trace elements zinc, copper and magnesium during pregnancy and its outcome. Indian J Pediatr 2004;71:1003–5. [DOI] [PubMed] [Google Scholar]
- 56.WHO. Use and interpretation of haemoglobin concentrations for assessing anaemia status in individuals and populations [Internet]. 2016 [cited 2017 May 9]. Available from: http://www.who.int/nutrition/callforauthors_anaemia_status/en/.