Abstract
Understanding the iron status in pregnant women in Europe provides a foundation for considering the role of iron screening and supplementation. However, available reports and studies have used different approaches that challenge the devising of overall summaries. Moreover, data on pregnant women are limited, and thus, data on women of reproductive age provide useful background information including baseline iron stores in pregnant women. This review considered data that are available from >15 European countries including national surveys and relevant clinical studies. In European women of reproductive age, median or geometric mean serum ferritin (SF) concentrations were estimated at 26–38 μg/L. Approximately 40–55% of this population had small or depleted iron stores (i.e., SF concentration ≤30 μg/L), and 45–60% of this population had apparently replete iron stores. The prevalence of iron deficiency (ID) and iron deficiency anemia (IDA) was 10–32% and 2–5%, respectively, depending on the cutoffs used. Approximately 20–35% of European women of reproductive age had sufficient iron stores (SF concentration >70 μg/L) to complete a pregnancy without supplementary iron. During pregnancy, European women in controlled supplementation trials who were not receiving iron supplements displayed increasing prevalences of ID and IDA during pregnancy, which peaked in the middle to late third trimester. Available evidence has suggested that, in gestational weeks 32–39, the median or geometric mean SF concentrations were 6–21 μg/L, and prevalences of ID and IDA were 28–85% and 21–35%, respectively. Women who were taking iron supplements had higher iron status and lower prevalences of ID and IDA, which were dependent on the dose of iron and compliance. The data suggest that, in Europe, the iron status of reproductive-aged women varies by region and worsens in pregnancy without iron supplementation.
Keywords: European women, iron deficiency, iron deficiency anemia, iron overload, iron replete, iron status, pregnancy, premenopausal women
INTRODUCTION
Describing the iron status of pregnant women is often challenged by the limited nature of available data. Such descriptions can be enhanced by examining the iron status of women of reproductive age whereby successful pregnancy is in part dependent on the condition of the woman before becoming pregnant. Prepregnancy body iron stores are important because during pregnancy there is a marked physiologic increase in the demands for absorbed iron to expand the woman’s red blood cell mass and to secure an adequate iron supply for the function of the placenta and the growing fetus. The total amount of iron that is needed for a normal pregnancy is ∼1000–1200 mg (1). To complete a normal pregnancy without taking iron supplements and without developing iron deficiency (ID) or iron deficiency anemia (IDA), the woman should have body iron stores at conception of ≥500 mg (2), which correspond to serum ferritin (SF) concentrations of 70–80 μg/L (1). In short, the iron status of the reproductive-aged woman is important in terms of the iron stores that are needed for pregnancy, and this consideration enhances the understanding of the iron status of pregnant women.
This review, which encompasses both pregnant women and women of reproductive age, was intended to support discussions during an NIH workshop that was focused on the screening and supplementation of pregnant women and young children. The review reflects data that were gleaned from a literature search that used PubMed (www.ncbi.nlm.nih.gov/pubmed) and other sources. Although intake of iron is not the focus of this review, the general nature of iron intake in European women is worth noting. Recommended intake of iron for reproductive-aged women varies from 14.8 to 20 mg/d depending on the country (3, 4). This intake can be compared with that in the United States and Canada where the recommended intake is 18 mg/d (5). For pregnancy, recommendations in Europe vary from supplementation increases of 27 mg/d (5) to 40 mg/d (3), whereas the European Food Safety Authority (4) recommends no increase in iron intake over that recommended for the nonpregnant woman on the basis of the assumption of increased iron absorption. Such enhanced absorption has been reported to have been seen in pregnant women with ID or IDA (6) but not in those with replete stores (7). There are several European studies (1, 8–10) and national monitoring surveys (11–19) that were conducted in the past 15 y on iron intake in reproductive-aged and pregnant women that have suggested that the majority of women did not consume recommended iron intakes. Of course, iron status and stores are influenced by a number of factors beyond dietary iron intake including the bioavailability of the iron consumed as well as the magnitude of blood loss from menstruation and parity.
ASSESSING IRON STATUS: CUTOFFS FOR SF AND HEMOGLOBIN
In the typical assessment of iron status, SF concentrations are considered to be reflective of mobilizable body iron stores, whereas hemoglobin concentrations are used, often in conjunction with SF concentrations, as indicators of functional iron. SF concentrations are affected by infection and inflammation, which limit their interpretation as has been highlighted by other authors in these proceedings (20, 21), but the measure remains commonly used.
For the majority of studies that are presented in this review, ID is defined as an SF concentration <15 μg/L or, in some studies, as an SF concentration <12 μg/L and, in a few studies, as an SF concentration <10 μg/L (22). The distributions of SF concentrations in various populations are skewed to the right, but a normal distribution can be obtained by the log10 transformation of SF concentrations. Therefore, the medians or geometric means (i.e., antilog10 mean values) should be used in the statistical analyses and in the description of the distributions of SF concentrations. The often-used and -quoted arithmetic mean yields a falsely high mean value and is not representative of the true normal distribution.
Before the era of SF analysis that began in the 1970s, a histochemical assessment of bone marrow hemosiderin iron was used to determine whether mobilizable body iron stores were present. Several studies have shown that individuals with no stainable bone marrow hemosiderin iron had SF concentrations ≤30 μg/L (22, 23). Therefore, the presence of small or depleted iron stores is defined as an SF concentration ≤30 μg/L. Individuals with an SF concentration >30 μg/L are considered to have stainable bone marrow hemosiderin iron and, therefore, to be iron replete. These individuals have mobilizable body iron stores of ∼230 mg, which correspond to the iron content in one blood donation of 500 mL. In the majority of studies, an iron overload in women of reproductive age is defined as an SF concentration >200 μg/L and, in one study, as an SF concentration ≥110 μg/L (24). To our knowledge, definitions of iron replete and excess iron stores are not available for pregnant women.
Hemoglobin concentrations are used to characterize anemia, and cutoffs for anemia and IDA have been provided by the WHO (25). For nonpregnant women, the WHO defines anemia as hemoglobin concentrations <120 g/L and defines IDA as hemoglobin concentrations <120 g/L and SF concentrations <15 or <12 μg/L. In pregnant women globally, including Europe, the WHO defines anemia as hemoglobin concentrations <110 g/L and defines IDA as hemoglobin concentrations <110 g/L and SF concentrations <15 or <12 μg/L.
IRON STATUS IN APPARENTLY HEALTHY WOMEN OF REPRODUCTIVE AGE
The WHO report on the global prevalence of anemia (25) states that 19.9% of nonpregnant women aged 15–49 y living in the UN European Region have anemia, which is predominantly due to ID. On the basis of these data, anemia is more prevalent in Eastern Europe than in Northern, Western, and Southern Europe.
A number of European epidemiologic studies have examined iron status in women of reproductive age. In the beginning of the SF era, a phlebotomy study in English premenopausal women (n = 10) showed mean mobilizable body iron stores of 210 mg (26). An overview of European iron-status studies in menstruating women has previously been presented by Hallberg (27). The prevalence of ID was 4–33%, with the lowest in Iceland and the highest in Sweden. In turn, 67–96% of the women displayed sufficient iron stores to avoid IDA. However, a direct comparison of the different studies is not possible because the studies used different first-generation, nonstandardized SF assays; also, the SF concentration cutoffs for ID varied from <20 to <10 μg/L. A later review of iron-status studies in menstruating women in Europe was conducted by Hercberg et al. (28). Depending on the SF assays and cutoffs, a similar prevalence of ID (11–13%) was reported compared with that in the earlier study, with the lowest in Finland and the highest in Sweden. In turn, this finding suggested that 67–89% of the women displayed sufficient iron stores.
An overview of European studies on iron status in women of reproductive age is shown in Table 1. In most countries, the median or geometric mean SF concentrations were quite similar and ranged from 29 μg/L in a large Norwegian study (24) to 38 μg/L in the comprehensive Danish studies (31, 37). Four studies of Danish women at the various ages of 30–40 y (38), 40 y (31), 16–31 y (37), and 18–23 y, showed comparable SF concentrations with geometric means of 35–38 μg/L across the 1994–1999 study period. A comparable geometric mean SF concentration (34 μg/L) was reported in French women aged 35 y (33), but slightly lower median SF concentrations were reported in Norwegian women aged 20–49 y (29 μg/L) (24) and in Belgian women aged 18–39 y (26 μg/L) (29). In contrast, a number of studies reported arithmetic means for SF concentrations in reproductive-aged women in Finland (30, 32), Italy (32), Netherlands (32, 34), Poland (32), and Spain (36) that were consistently, but falsely, higher at 30–47 μg/L (due to the use of arithmetic mean). On the basis of the geometric mean for 30–40-y-old Danish women (38), the calculated mean iron store was 263 mg.
TABLE 1.
Iron status in healthy women of reproductive age in Europe1
| Study | Country | Sample, n | Age, y | SF, μg/L | ID,2 % | IDA,3 % | Iron overload,4 % |
| Pynaert et al. (29) | Belgium | 599 | 18–39 | 26 (16–48)5 | 23.0 | 3.0 | NA |
| Borch-Iohnsen et al. (24) | Norway | 1484 | 20–39 | — | — | — | — |
| 20–29 | 32 (18–51)5 | 19.6 | 3.76 | 3.37 | |||
| 30–39 | 27 (15–47)5 | 24.2 | 5.36 | 2.77 | |||
| Lahti-Koski et al. (30) | Finland | 118 | 20–49 | 32 ± 308 | 32.1 | 39 | NA |
| Milman et al. (31) | Denmark | 380 | 40 | 39 (10, 170)10 | 12.4 | 1.8411, | 2.9 |
| 2.4511,12 | |||||||
| van de Vijver et al. (32) | Finland | 82 | 20–23 | 3013 | 11.314 | NA | NA |
| Netherlands | 96 | 20–23 | 4713 | 10.814 | NA | NA | |
| Poland | 96 | 20–23 | 4213 | 3.114 | NA | NA | |
| Italy | 99 | 20–23 | 3313 | 8.414 | NA | NA | |
| Galan et al. (33) | France | 2432 | 35–44 | — | 22.715 | 4.415 | NA |
| 35–39 | 33.7 (5, 116) | — | — | — | |||
| 40–44 | 32.4 (5, 116) | — | — | — | |||
| Brussaard et al. (34) | Netherlands | 75 | 20–49 | 39 ± 36 | 16.016 | 4.0 | NA |
| Milman et al. (35) | Denmark | 213 | 18–23 | — | — | — | — |
| 18–19 | 30 (10, 89) | 13.9 | 4.217 | 0 | |||
| 20–21 | 32 (8, 86) | 13.9 | 017 | 0 | |||
| 22–23 | 40 (12, 119) | 7.9 | 1.317 | 0 | |||
| Quintas et al. (36) | Spain | 130 | 19–35 | 37 (3–141)18 | 23.119 | 3.9 | NA |
| Milman et al. (37) | Denmark | 284 | 16–31 | 45 (13, 102)10 | 9.5 | 4.9 | 0.4 |
| Milman and Kirchhoff (38) | Denmark | 704 | 30 and 40 | 35 (6, 135)10 | 17.2 | 2.3 | 1.3 |
ID, iron deficiency; IDA, iron deficiency anemia; NA, not applicable; SF, serum ferritin.
SF concentration <15 or <16 μg/L.
Hemoglobin concentration <120 g/L and an SF concentration compatible with ID.
SF concentration >200 μg/L.
Median; IQR in parentheses (all such values).
SF concentration <12 μg/L and hemoglobin concentration <120 g/L.
SF concentration >109 μg/L.
Arithmetic mean ± SD (all such values).
Approximate value.
Geometric mean; 5th, 95th percentiles in parentheses (all such values).
SF concentration <13 μg/L and hemoglobin concentration <5th percentile in the group of women.
Premenopausal group.
Arithmetic mean.
SF concentration <12 μg/L. Extrapolated from figure.
Menstruating women only.
SF concentration <10 μg/L.
SF concentration <13 μg/L and hemoglobin concentration <121 g/L.
Arithmetic mean; observed range in parentheses.
Defined by level one of the Multiple Biochemical Indexes model.
The reported prevalence of ID ranged widely with age and geographic location, but overall, the prevalence of IDA was lower than that of ID. For Danish women, ID (SF concentration <15 μg/L) was low with prevalences ranging from 11.7% in women aged 18–23 y (35) to 17.2% in women aged 30–40 y (38). The ID prevalence was comparable in Dutch (30, 34) and Finnish (32) women aged 20–49 y, ranging from 11.3% to 16%, was lower in Polish and Italian women aged 20–23 y, ranging from 3.1% to 8.4%, and was somewhat higher in Norwegian (24), Belgian (29), French (33), and Spanish (36) women aged 18–49 y, ranging from 22% to 23.4%. The prevalence of ID in Finnish women as reported by van de Vijver et al. (32) was 11.3% in women aged 20–23 y but differed from that of Lahti-Koski et al. (30) who reported a prevalence of 32.1% in Finnish women aged 20–49 y. However, age ranges differed in these 2 studies. The prevalence of IDA (hemoglobin concentration <120 g/L and SF concentration <12 μg/L) was lower than that of ID and was comparable in Danish (2.3–4.9%), Norwegian (4.7%), Belgian (3%), Dutch (4.0%), Finnish (5.9%), French (4.4%), Spanish (3.9%), and Swedish (6.9%) women of reproductive age.
The prevalence of small or depleted iron stores (SF concentration <30 μg/L) in women of reproductive age was higher than that of ID, whereas the prevalence of replete iron stores varied more. In Danish women aged 30–40 y (38), 39.9% had small or depleted iron stores; ∼60% had replete iron stores, and only 28.4% had concentrations >60 μg/L (i.e., sufficient iron stores to undergo pregnancy without iron supplementation). In Norwegian women (24), ∼50% had small or depleted iron stores (SF concentration ≤30 μg/L), whereas ∼50% had replete iron stores.
Iron-overload data were only available for Danish and Norwegian women. Across the 4 Danish studies, the prevalence of iron overload (SF concentration >200 μg/L) was low, ranging from none in the youngest Danish women (35, 37) to 1.3% in those aged 20–40 y (38). Iron overload (SF concentration ≥110 μg/L) was slightly higher at 3.8% of the Norwegian women of reproductive age (24).
IRON STATUS IN PREGNANT WOMEN
According to the WHO report on anemia (25), in the UN European Region, 24.5% of pregnant women aged 15–49 y have anemia that is predominantly caused by ID. However, there has been a lack of European epidemiologic studies that have assessed iron status from early pregnancy to delivery in women who were not taking iron supplements. Nowadays, such studies cannot be performed because of ethical concerns. Consequently, the best estimates of iron status are based on individuals who are not taking an iron supplement as have been reported from nationwide epidemiologic studies, from placebo controlled studies, and from cross-sectional studies in pregnant women who, for various reasons, were not taking iron supplements, although these latter types of studies pose interpretational challenges because of self-selection bias.
To our knowledge, 1 cohort study and 2 nationally representative studies of iron status in pregnant women have been conducted in Belgium (n = 1311) (39), Switzerland (n = 381) (40), and Germany (n = 378) (41). The prevalence of ID in Belgium was 6% and 23% (SF concentration <15 μg/L) in the first and third trimesters, respectively. The prevalence of ID was 19% (SF concentration <12 μg/L) in Switzerland and Germany. The prevalence of IDA (hemoglobin concentration <110 g/L and SF concentration <15 μg/L) was 16% in Belgium and 3% in Switzerland although 65–66% of the Belgian and Swiss women took iron supplements. In Germany, 12% of the women had IDA on the basis of the slightly different criteria of a hemoglobin concentration <110 g/L and soluble transferrin receptor concentration >3.3 mg/L. Swiss pregnant women who were taking iron supplements in the third trimester had substantially higher SF concentrations than did those who were not taking supplements. German pregnant women who were not taking an iron supplement had greater risks of ID and IDA. Most pregnant women in Belgium, Switzerland, and Germany have adequate iron status in pregnancy, and the authors of the study in Switzerland concluded that iron supplements have a positive impact on iron status. Finally, most pregnant women in Belgium, Switzerland, and Germany appear to have adequate iron status in pregnancy.
Iron status without iron supplements
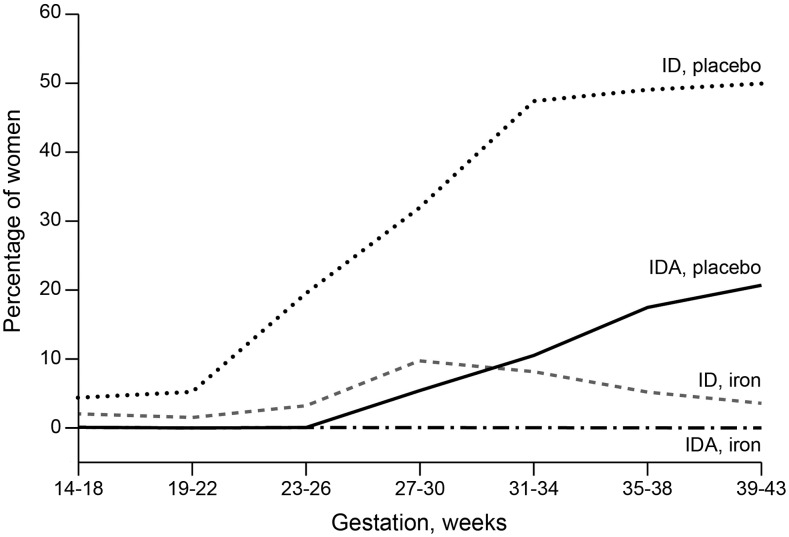
In controlled studies, groups who were not taking iron supplements (42–49) had geometric mean SF concentrations ranging from 7.7 to 12 μg/L in British (47), Danish (44), French (49), and Spanish (42) pregnant women; the arithmetic mean SF concentrations ranged from 6 to 21 μg/L, which can be falsely high. The prevalence of ID ranged from a low of 28% in Danish pregnant women (44) to a high of 85% in Norwegian pregnant women (43). The prevalence of IDA ranged from a low of 4% in Norwegian pregnant women (43) to a high of 35% in Spanish pregnant women (42). The prevalences of ID and IDA increased gradually during pregnancy and peaked in the middle or late third trimester as shown in Figure 1.
FIGURE 1.
ID and IDA during pregnancy in healthy Danish women taking a placebo or 66 mg Fe2+/d from 14 to 18 wk gestation to delivery. The graph was created from data published in references 44 and 50. ID was defined as a serum ferritin concentration <12 μg/L; IDA was defined as a serum ferritin concentration <12 μg/L and hemoglobin below the discriminatory values for anemia in iron-replete women on the basis of data from reference 50. ID, iron deficiency; IDA, iron deficiency anemia.
Iron status with iron supplements
An overview of European placebo-controlled and other experimental studies of iron supplementation in pregnant women is shown in Table 2. Results are presented for the iron-status indicator for both control and supplemented treatment groups to facilitate the interpretation of the intervention. In addition, iron status in the late third trimester (gestational weeks 32–39) is presented to facilitate a comparison of the studies.
TABLE 2.
Experimental studies of iron supplementation in healthy pregnant women in Europe1
| 32–39-wk test range |
||||||||||
| SF, μg/L |
ID, % (95% CI) |
IDA, % (95% CI) |
||||||||
| Study | Country | Sample, n | Study period, wk | Treatment, Fe2+ mg/d | C | Treatment | C | Treatment | C | Treatment |
| Milman et al. (51)2 | Denmark | IG1: 40; | 15 | IG1: 253 B; | NA | IG1: 21 (9, 58)4; | — | IG1: 14.85; | — | IG1: 06; |
| IG2: 40 | IG2: 507 D | IG2: 30 (8, 154)4 | IG2: 13.85 | IG2: 3.46 | ||||||
| Ribot et al. (42) | Spain | IG1: 169; | 10 | IG1: <608; | 7.7 (7.1, 8.3)4 | IG1: 9.3 (8.6, 10.0)4; | 81.5 (71.8, 91.2)4,9 | IG1: 70.3 (63.4, 77.2)4,9; | 35.2 (23.2, 47.2)4,7 | IG1: 22.2 (15.9, 28.5)4,6; |
| IG2: 38; | IG2: 60–1008; | IG2: 13.2 (12.6, 13.8)4; | IG2: 47.2 (31.3, 63.1)4,9; | IG2: 11.4 (1.3, 21.5)4,6; | ||||||
| IG3: 90; | IG3: >1008 | IG3: 14.9 (14.2, 15.6)4 | IG3: 37.6 (27.6, 47.6)4,9 | IG3: 14.6 (7.3, 21.9)4,6 | ||||||
| C: 61 | ||||||||||
| Milman et al. (52)10 | Denmark | IG1: 61; | 18 | IG1: 207; | NA | IG1: 15.5 ± 2.011; | — | IG1: 28.812; | — | IG1: 1013; |
| IG2: 66; | IG2: 407; | IG2: 22.4 ± 1.711; | IG2: 11.112; | IG2: 4.513; | ||||||
| IG3: 73; | IG3: 607; | IG3: 22.9 ± 1.711; | IG3: 10.012; | IG3: 013; | ||||||
| IG4: 69; | IG4: 807 | IG4: 26.9 ± 1.711 | IG4: 9.012 | IG4: 1.513 | ||||||
| Eskeland et al. (43) | Norway | HIG:24; NHIG: 25; C:20 | 20 | HIG: 3.6 mg Fe2+ heme and 24 mg Fe2+ 7; | — | — | 8514 | HIG: 2914; NHIG: 5214 | 415 | 015 |
| NHIG: 277 | ||||||||||
| Thomsen et al. (53)10 | Denmark | IG1: 22; | 16 | IG1: 1007; | — | IG1: 28.0 (19.0, 36.7)16; | IG2: 7217 | IG1: 517 | — | — |
| IG2: 21 | IG2: 1818 | IG2: 10.0 (8.5, 15.5)16 | ||||||||
| Milman et al. (44) | Denmark | IG: 100; | 12 | 6619 | 1220 | 2120 | 289 | 39 | 213 | 03 |
| C: 107 | ||||||||||
| Galan et al. (49) (in French) | France | IG: 81; | 12 | 4521 | 1022 | 1922 | 659 | 309 | — | 4 |
| C: 84 | ||||||||||
| Romslo et al. (45) | Norway | IG: 22; | 12 | 2007 | 6.0 ± 523 | 24.0 ±1323 | 8324 | 524 | — | — |
| C: 23 | ||||||||||
| Foulkes and Goldie (48) | United Kingdom | IG: 251; | 12 | 1007 | — | — | — | — | — | — |
| C: 250 | ||||||||||
| Taylor et al. (47) | United Kingdom | IG: 21; | 12 | 657 | 6 (2.5, 12.5)16 | 15.8 (7.0, 41.8)16 | — | — | — | — |
| C: 24 | ||||||||||
| Puolakka et al. (46) | Finland | IG: 16; | 16 | 2007 | 21 (12, 35)16 | 63 (43, 92)16 | — | — | — | — |
| C: 16 | ||||||||||
C, control group; HIG, heme iron group; ID, iron deficiency; IDA, iron deficiency anemia; IG, intervention group; NA, not applicable; NHIG, non–heme iron group; SF, serum ferritin.
Noninferiority study.
Ferrous-bisglycinate.
Geometric mean; 2.5th, 97.5th percentiles in parentheses.
SF concentration <15 μg/L.
Hemoglobin concentration <110 g/L and SF concentration <12 μg/L.
Ferrous sulfate.
Different iron compounds.
SF concentration <12 μg/L.
Dose-response study.
Geometric mean ± SD.
SF concentration <13 μg/L.
SF concentration <13 μg/L and hemoglobin concentration <6.6 mmol/L (106 g/L), 6.5 mmol/L (105 g/L), or 7.2 mmol/L (115 g/L).
SF concentration <11.5 μg/L.
Hemoglobin concentration <100 g/L and SF concentration <15 μg/L as determined through reported treatment failures.
Arithmetic mean; 2.5th, 97.5th percentiles in parentheses.
SF concentration <15 μg/L.
Ferrous fumarate in a multivitamin and multimineral tablet.
Ferrous fumarate.
Geometric mean derived from figure.
Ferrous betainate hydrochloride.
Geometric mean.
Arithmetic mean ± SD.
SF concentration <10 μg/L.
Iron status improved in ferrous iron–supplemented pregnant women compared with in women who were taking a placebo. In an overview of European iron-status studies in women (28), ID was reported in 25–77% of pregnant women, and IDA was reported in 6–30% of pregnant women with a lower prevalence in women who were taking iron supplements. Placebo-controlled and experimental studies that are shown in Table 2 provided a range of ferrous iron supplements from 18 to 200 mg/d, which began to be taken between 12 and 18 wk gestation and continued through term. In the controlled studies, geometric mean SF concentrations in the iron-supplemented groups varied with the supplemental dose late in term (32–39 wk gestation). Although it is difficult to generalize across the studies because of overlapping response ranges, a higher supplemental dosage seemed to result in slightly higher geometric mean SF concentrations, but within studies and across studies, no readily apparent relation could be characterized. Women who were taking doses of iron from 18 to 27 mg/d attained SF concentrations ranging from a geometric mean of 15.5 μg/L (52) to 21 μg/L (51) or an arithmetic mean of 10 μg/L (53). Women who were taking doses from 40 to 60 mg/d attained SF concentrations ranging from a geometric mean of 9.3 μg/L (42) to 30 μg/L (51). Women who were taking higher doses of 60–100 mg/d attained geometric mean SF concentrations of 13.9 μg/L (42) to 22.9 μg/L (52) or an arithmetic mean of 28 μg/L (53). Finally, women who were taking the highest doses of >100–200 mg/d attained a geometric mean SF concentration of 14.9 μg/L (42) or an arithmetic mean of 24 μg/L (45) or 27 μg/L (46).
Increasing the supplemental iron dosage, when considered across and within studies, appeared to result in decreased prevalences of ID and IDA although, again, the overlapping response ranges made it hard to discern a consistent dose-response pattern. The prevalence of ID in the iron-supplemented groups ranged from higher rates of 14.8% (54) to 72% (53) or from 13.8% (30) to 70% (42) with the lowest and intermediate doses of 18–27 and 40–60 mg/d, respectively, to lower rates from 3% (44) to 37% (42) with the highest doses >60–200 mg/d. The prevalence of IDA was lower than that of ID and ranged from none (43) to 10% (51) with the lowest dose of 18–27 mg/d and from none (44) to 1.5% (51) with the highest dose of 60–100 mg/d. As shown in Figure 1, the prevalence of ID and IDA was lower in iron-supplemented women than in placebo-treated women.
CONCLUSIONS
There exist only scarce data that have been published in English on iron status in women in Eastern European countries. Further surveys are needed in these areas. In studies dated from 1992 (38), iron status in predominantly Western European women of reproductive age appeared to be similar to that in Western countries with respect to the low prevalence of IDA and was somewhat more variable with respect to the prevalence of ID. The overall prevalence of IDA ranges from only 2–5%, and the prevalence of ID in various countries ranges from 10% to 33%. These prevalences indicated that >70% of the women had adequate iron status, but body iron stores may display greater variation between countries. Median SF concentrations ranged from 26 to 38 μg/L, suggesting that, in most countries, ∼40–50% of women of reproductive age may have small or depleted iron stores (SF concentrations ≤30 μg/L), whereas 50–60% of women of reproductive age have replete iron stores. When women become pregnant, demands for absorbed iron increase, which some women cannot meet exclusively by dietary iron intake. However, women with preconception SF concentrations >60–70 μg/L have sufficient body iron stores of 400–500 mg to complete pregnancy without taking iron supplements. Approximately 28% of Danish premenopausal women were reported to have such sufficient iron stores, but to our knowledge, the prevalence of sufficient iron stores for women in other countries is not known. As an estimate, depending on the individual country, ∼20–35% of the female population of reproductive age may have sufficient iron stores and, consequently, may not need supplementary iron during pregnancy.
In women of reproductive age, iron overload is relatively rare, ranging from 0.4% to 3.0%. The prevalence of iron overload increases slightly with age even in menstruating women. However, it should be stressed that, in populations of Northern European descent, the high iron (HFE)-gene variants C282Y and H63D, which are responsible for the development of HFE hemochromatosis, are quite common (55). Approximately 0.3–0.7% of women (i.e., 3–7/1000 women) are C283Y homozygous and at risk of developing iron overload with organ damage; 10–15% of women are C282Y heterozygous and generally have a slightly higher iron status than do women without this variant because of increased iron absorption (56). Furthermore, in Southern Europe, non-HFE hemochromatosis is being recognized as a growing problem as our knowledge in this field is expanding (57). It is important to identify women with a genetic predisposition of being at risk of iron overload before supplementation or treatment with iron because any kind of iron administration may aggravate their disorder or disease.
Only a few European studies in pregnant women, comprising a small number of participants, have investigated iron status in women who were not taking iron supplements. However, all of the studies have shown that iron status decreases markedly during pregnancy. In the third trimester, many women display ID, and ≤26% of women may have IDA in accordance with the established criteria (Figure 1, Table 2). In contrast, studies have shown that pregnant women who were taking iron supplements consistently had a higher iron status and lower frequencies of ID and IDA, which were dependent on the dose of iron and on their compliance in taking the prescribed supplements (Figure 1, Table 1). The 2012 Cochrane Review (58) on daily iron supplementation in pregnancy noted the positive relation of iron supplements with low birth weight and reduced risk of maternal anemia.
During pregnancy, SF concentrations display a U-shaped curve with decreasing concentrations in the first and second trimesters, a nadir in gestational weeks 35–38, and an increase in the postpartum period (44). This variation is observed both in iron-supplemented and nonsupplemented women. To our knowledge, the cause of this U-shaped distribution is not known, but it may be due to the hemodilution of pregnancy or other factors such as inflammation as discussed by Vricella (59) in these proceedings. The SF concentration is increased by infection or inflammation, and thus, its interpretation should always consider the presence of inflammation. However, the U-shaped distribution merits reconsideration of the current common cutoffs for ID in pregnancy. Future research should examine cutoffs to define ID, IDA, iron repletion, and iron overload, especially in the second and third trimesters of pregnancy relative to maternal and fetal health outcomes. Future research also needs to determine SF cutoffs in pregnant women relative to both the proinflammatory and anti-inflammatory periods that occur in gestation. Such studies should analyze SF and inflammatory markers, which has not been done in the referred studies either in reproductive-aged or pregnant women.
Iron prophylaxis in pregnancy is still a controversial issue, and few European countries have established national guidelines with respect to iron supplementation. Iron supplements improve iron status and its hematologic indicators in pregnant women (58), whereas the overall health benefit or risk relative to the woman and the fetus or newborn has been less well documented. Future research is needed to assess iron status longitudinally from conception and early pregnancy to clarify how many women will be definite or possible candidates for iron supplementation, how many women can complete pregnancy without supplementation, and how many women have iron overload or high iron stores and, thus, might experience adverse effects from iron supplementation.
Future research also needs to assess the effect of individualized iron-supplementation programs in pregnant women on the basis of their iron status at conception and early pregnancy and given individualized doses of iron supplements that are tailored according to iron status. Research needs to determine, according to different baseline SF concentration, which supplemental doses of iron are the minimally effective doses to prevent ID and IDA in ∼95% of the women and to ensure beneficial outcomes for the mother and fetus without adverse effects. In addition, research needs to assess the most effective supplementation in terms of daily or alternate-day regimens.
Acknowledgments
The authors’ responsibilities were as follows—NM, CLT, and PMB: developed the text; NM and JM: compiled the data tables; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: HFE, high iron (hemochromatosis); ID, iron deficiency; IDA, iron deficiency anemia; SF, serum ferritin.
REFERENCES
- 1.Milman N. Iron and pregnancy–a delicate balance. Ann Hematol 2006;85:559–65. [DOI] [PubMed] [Google Scholar]
- 2.Milman N. Iron prophylaxis in pregnancy–general or individual and in which dose? Ann Hematol 2006;85:821–8. [DOI] [PubMed] [Google Scholar]
- 3.Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: integrating nutrition and physical activity. Copenhagen (Denmark): Nordisk Ministerråd; 2014. [Google Scholar]
- 4.EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for iron. EFSA J 2015;13:4186–249. [Google Scholar]
- 5.Institute of Medicine Food Nutrition Board. Dietary Reference Intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 6.Barrett JF, Whittaker PG, Williams JG, Lind T. Absorption of non-haem iron from food during normal pregnancy. BMJ 1994;309:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien KO, Zavaleta N, Caulfield LE, Yang DX, Abrams SA. Influence of prenatal iron and zinc supplements on supplemental iron absorption, red blood cell iron incorporation, and iron status in pregnant Peruvian women. Am J Clin Nutr 1999;69:509–15. [DOI] [PubMed] [Google Scholar]
- 8.Leclercq C, Arcella D, Piccinelli R, Sette S, Le Donne C, Turrini A. The Italian National Food Consumption Survey INRAN-SCAI 2005-06: main results in terms of food consumption. Public Health Nutr 2009;12:2504–32. [DOI] [PubMed] [Google Scholar]
- 9.Alwan NA, Greenwood DC, Simpson NA, McArdle HJ, Godfrey KM, Cade JE. Dietary iron intake during early pregnancy and birth outcomes in a cohort of British women. Hum Reprod 2011;26:911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrakos G, Panagopoulos P, Koutras I, Kazis A, Panagiotakos D, Economou A, Kanellopoulos N, Salamalekis E, Zabelas A. A comparison of the dietary and total intake of micronutrients in a group of pregnant Greek women with the Dietary Reference Intakes. Eur J Obstet Gynecol Reprod Biol 2006;127:166–71. [DOI] [PubMed] [Google Scholar]
- 11.Thorgeirsdottir H, Valgeirsdottir H, Gunnarsdottir I. National Dietary Survey of the Icelandic Nutrition Council 2010–2011. Main findings: Directorate of Health , Icelandic Food and Veterinary Authority. Reykjavik (Iceland): University of Iceland Unit for Nutrition Research; 2011. [Google Scholar]
- 12.Amcoff E, Edberg A, Barbieri HE, Lindroos AK, Nalsen C, Pearson M, Lemming EW. Riksmaten vuxna 2010–11. Livsmedels- och näringsintag bland vuxna i Sverige. Resultat från matvaneundersökningen utförd 2010–11. [Food and nutrient intake in adults in Sweden 2010–11.] Uppsala (Sweden): Livsmedelsverket; 2012. (in Swedish; summary, figures, and tables in English). [Google Scholar]
- 13.Helldán A, Kosonen M, Tapanainen H. The National FINDIET 2012 Survey. Helsinki (Finland): National Institute For Health and Welfare; 2013. [Google Scholar]
- 14.Pedersen AN, Christensen T, Matthiessen J, Knudsen VK, Sørensen MR, Biltoft-Jensen AP, Hinsch H, Ygil KH, Kørup K, Saxholt E, et al. Danskernes kostvaner 2011-2013. [Dietary habits in Denmark 2011-2013. Main results.] Lyngby (Denmark): DTU Fødevareinstituttet, Danmarks Tekniske Universitet; 2015. (in Danish). [Google Scholar]
- 15.Food Standards Agency. National Diet and Nutrition Survey: adults aged 19 to 64 years. Vol. 3 London: Food Standards Agency; 2003. [Google Scholar]
- 16.Public Health England, UK Food Standards Agency. National Diet and Nutrition Survey results from years 1, 2, 3 and 4 (combined) of the rolling programme (2008/2009 – 2011/2012) Bates B, Lennox A, Prentice A, Bates C, Page P, Nicholson S, Swan G, editors. London: Public Health England; 2014. [Google Scholar]
- 17.Irish Universities Nutrition Alliance. Summary report on food and nutrient intakes, physical measurements, physical activity patterns and food choice motives Walton J, editor. National Adult Nutrition Survey. Cork (Ireland): Irish Universities Nutrition Alliance; 2011. [Google Scholar]
- 18.van Rossum CTM, Fransen HP, Verkaik-Kloosterman J, Buurma-Rethans EJM, Ocke MC. Dutch National Food Consumption Survey 2007-2010: diet of children and adults aged 7 to 69 years. Bilthoven (Netherlands): National Institute for Public Health and the Environment; 2011. [Google Scholar]
- 19.Agence Francaise de Securite. Sanitaire des Aliments. Etude individuelle nationale des consommations alimentaire 2 (INCA2) In: Lafay L, editor. Maisons-Alfort (France): Agence Francaise de Securite Sanitaire des Aliments. 2009. [Google Scholar]
- 20.Ross AC. Impact of chronic and acute inflammation on extra- and intracellular iron homeostasis. Am J Clin Nutr 2017;106(Suppl):1581S–7S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suchdev PS, Williams AM, Mei Z, Flores-Ayala R, Pasricha S-R, Rogers LM, Namaste SML. Assessment of iron status in settings of inflammation: challenges and potential approaches. Am J Clin Nutr 2017;106(Suppl):1626S–33S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milman N. Serum ferritin in Danes: studies of iron status from infancy to old age, during blood donation and pregnancy. Int J Hematol 1996;63:103–35. [DOI] [PubMed] [Google Scholar]
- 23.Milman N, Pedersen NS, Visfeldt J. Serum ferritin in healthy Danes: relation to marrow haemosiderin iron stores. Dan Med Bull 1983;30:115–20. [PubMed] [Google Scholar]
- 24.Borch-Iohnsen B, Sandstad B, Asberg A. Iron status among 3005 women aged 20-55 years in Central Norway: the Nord-Trondelag Health Study (the HUNT study). Scand J Clin Lab Invest 2005;65:45–54. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. The global prevalence of anaemia in 2011. Geneva (Switzerland): WHO; 2015. [Google Scholar]
- 26.Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol 1973;26:770–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallberg L. Results of surveys to assess iron status in Europe. Nutr Rev 1995;53:314–22. [DOI] [PubMed] [Google Scholar]
- 28.Hercberg S, Preziosi P, Galan P. Iron deficiency in Europe. Public Health Nutr 2001;4:537–45. [DOI] [PubMed] [Google Scholar]
- 29.Pynaert I, Delanghe J, Temmerman M, De Henauw S. Iron intake in relation to diet and iron status of young adult women. Ann Nutr Metab 2007;51:172–81. [DOI] [PubMed] [Google Scholar]
- 30.Lahti-Koski M, Valsta LM, Alfthan G, Tapanainen H, Aro A. Iron status of adults in the capital area of Finland. Eur J Nutr 2003;42:287–92. [DOI] [PubMed] [Google Scholar]
- 31.Milman N, Byg KE, Ovesen L. Iron status in Danes 1994. II: prevalence of iron deficiency and iron overload in 1319 Danish women aged 40-70 years. Influence of blood donation, alcohol intake and iron supplementation. Ann Hematol 2000;79:612–21. [DOI] [PubMed] [Google Scholar]
- 32.van de Vijver LP, Kardinaal AF, Charzewska J, Rotily M, Charles P, Maggiolini M, Ando S, Vaananen K, Wajszczyk B, Heikkinen J, et al. Calcium intake is weakly but consistently negatively associated with iron status in girls and women in six European countries. J Nutr 1999;129:963–8. [DOI] [PubMed] [Google Scholar]
- 33.Galan P, Yoon HC, Preziosi P, Viteri F, Valeix P, Fieux B, Briancon S, Malvy D, Roussel AM, Favier A, et al. Determining factors in the iron status of adult women in the SU.VI.MAX study. SUpplementation en VItamines et Mineraux AntioXydants. Eur J Clin Nutr 1998;52:383–8. [DOI] [PubMed] [Google Scholar]
- 34.Brussaard JH, Brants HA, Bouman M, Lowik MR. Iron intake and iron status among adults in the Netherlands. Eur J Clin Nutr 1997;51 Suppl 3:S51–8. [PubMed] [Google Scholar]
- 35.Milman N, Ulrik CS, Graudal N, Jordal R. Iron status in young Danes. Evaluation by serum ferritin and haemoglobin in a population survey of 634 individuals aged 14-23 yr. Eur J Haematol 1997;58:160–6. [DOI] [PubMed] [Google Scholar]
- 36.Quintas ME, Requejo AM, Ortega RM, Redondo MR, Lopez-Sobaler AM, Gaspar MJ. The female Spanish population: a group at risk of nutritional iron deficiency. Int J Food Sci Nutr 1997;48:271–9. [DOI] [PubMed] [Google Scholar]
- 37.Milman N, Clausen JO, Jordal R. Iron status in young Danish men and women: a population survey comprising 548 individuals. Ann Hematol 1995;70:215–21. [DOI] [PubMed] [Google Scholar]
- 38.Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol 1992;64:22–7. [DOI] [PubMed] [Google Scholar]
- 39.Vandevijvere S, Amsalkhir S, Van Oyen H, Egli I, Moreno-Reyes R. Iron status and its determinants in a nationally representative sample of pregnant women. J Acad Nutr Diet 2013;113:659–66. Erratum in: J Acad Nutr Diet 2013;113:1253. [DOI] [PubMed] [Google Scholar]
- 40.Hess SY, Zimmermann MB, Brogli S, Hurrell RF. A national survey of iron and folate status in pregnant women in Switzerland. Int J Vitam Nutr Res 2001;71:268–73. [DOI] [PubMed] [Google Scholar]
- 41.Bergmann RL, Gravens-Muller L, Hertwig K, Hinkel J, Andres B, Bergmann KE, Dudenhausen JW. Iron deficiency is prevalent in a sample of pregnant women at delivery in Germany. Eur J Obstet Gynecol Reprod Biol 2002;102:155–60. [DOI] [PubMed] [Google Scholar]
- 42.Ribot B, Aranda N, Giralt M, Romeu M, Balaguer A, Arija V. Effect of different doses of iron supplementation during pregnancy on maternal and infant health. Ann Hematol 2013;92:221–9. [DOI] [PubMed] [Google Scholar]
- 43.Eskeland B, Malterud K, Ulvik RJ, Hunskaar S. Iron supplementation in pregnancy: is less enough? A randomized, placebo controlled trial of low dose iron supplementation with and without heme iron. Acta Obstet Gynecol Scand 1997;76:822–8. [DOI] [PubMed] [Google Scholar]
- 44.Milman N, Agger AO, Nielsen OJ. Iron supplementation during pregnancy. Effect on iron status markers, serum erythropoietin and human placental lactogen. A placebo controlled study in 207 Danish women. Dan Med Bull 1991;38:471–6. [PubMed] [Google Scholar]
- 45.Romslo I, Haram K, Sagen N, Augensen K. Iron requirement in normal pregnancy as assessed by serum ferritin, serum transferrin saturation and erythrocyte protoporphyrin determinations. Br J Obstet Gynaecol 1983;90:101–7. [DOI] [PubMed] [Google Scholar]
- 46.Puolakka J, Janne O, Pakarinen A, Jarvinen PA, Vihko R. Serum ferritin as a measure of iron stores during and after normal pregnancy with and without iron supplements. Acta Obstet Gynecol Scand Suppl 1980;95:43–51. [DOI] [PubMed] [Google Scholar]
- 47.Taylor DJ, Mallen C, McDougall N, Lind T. Effect of iron supplementation on serum ferritin levels during and after pregnancy. Br J Obstet Gynaecol 1982;89:1011–7. [DOI] [PubMed] [Google Scholar]
- 48.Foulkes J, Goldie DJ. The use of ferritin to assess the need for iron supplements in pregnancy. J Obstet Gynaecol 1982;3:11–6. [Google Scholar]
- 49.Galan P, Wainer R, De Benaze C, Hercberg S. Prevention de l’anemie ferriprive au cours de la grossesse: effet de la supplementation precoce en fer [Prevention of iron deficiency anemia during pregnancy: effect of early iron supplementation.] In: Hercberg S, Galan P, Dupin H, editors. Recent knowledge on iron and folate deficiencies in the world. Paris: INSERM; 1990. p. 615–21 (in French). [Google Scholar]
- 50.Milman N, Byg KE, Agger AO. Hemoglobin and erythrocyte indices during normal pregnancy and postpartum in 206 women with and without iron supplementation. Acta Obstet Gynecol Scand 2000;79:89–98. [DOI] [PubMed] [Google Scholar]
- 51.Milman N, Jonsson L, Dyre P, Pedersen PL, Larsen LG. Ferrous bisglycinate 25 mg iron is as effective as ferrous sulfate 50 mg iron in the prophylaxis of iron deficiency and anemia during pregnancy in a randomized trial. J Perinat Med 2014;42:197–206. [DOI] [PubMed] [Google Scholar]
- 52.Milman N, Bergholt T, Eriksen L, Byg KE, Graudal N, Pedersen P, Hertz J. Iron prophylaxis during pregnancy–how much iron is needed? A randomized dose- response study of 20-80 mg ferrous iron daily in pregnant women. Acta Obstet Gynecol Scand 2005;84:238–47. [DOI] [PubMed] [Google Scholar]
- 53.Thomsen JK, Prien-Larsen JC, Devantier A, Fogh-Andersen N. Low dose iron supplementation does not cover the need for iron during pregnancy. Acta Obstet Gynecol Scand 1993;72:93–8. [DOI] [PubMed] [Google Scholar]
- 54.Milman N, Kirchhoff M, Jorgensen T. Iron status markers, serum ferritin and hemoglobin in 1359 Danish women in relation to menstruation, hormonal contraception, parity, and postmenopausal hormone treatment. Ann Hematol 1992;65:96–102. [DOI] [PubMed] [Google Scholar]
- 55.Milman N, Pedersen P. Evidence that the Cys282Tyr mutation of the HFE gene originated from a population in Southern Scandinavia and spread with the Vikings. Clin Genet 2003;64:36–47. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen P, Melsen GV, Milman N. Frequencies of the haemochromatosis gene (HFE) variants C282Y, H63D and S65C in 6,020 ethnic Danish men. Ann Hematol 2008;87:735–40. [DOI] [PubMed] [Google Scholar]
- 57.Pietrangelo A, Caleffi A, Corradini E. Non-HFE hepatic iron overload. Semin Liver Dis 2011;31:302–18. [DOI] [PubMed] [Google Scholar]
- 58.Peña-Rosas JP, De-Regil LM, Dowswell T, Viteri FE. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2012;12:CD004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vricella LK. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am J Clin Nutr 2017;106(Suppl):1620S–5S. [DOI] [PMC free article] [PubMed] [Google Scholar]