Abstract
The provision of iron via supplementation or the fortification of foods has been shown to be effective in preventing and treating iron deficiency and iron deficiency anemia in infants and young children. However, iron is a pro-oxidative element and can have negative effects on biological systems even at moderate amounts. An increasing number of studies have reported adverse effects of iron that was given to infants and young-children populations who initially were iron replete. These effects include decreased growth (both linear growth and weight), increased illness (usually diarrhea), interactions with other trace elements such as copper and zinc, altered gut microbiota to more pathogenic bacteria, increased inflammatory markers, and impaired cognitive and motor development. If these results can be confirmed by larger and well-controlled studies, it may have considerable programmatic implications (e.g., the necessity to screen for iron status before interventions to exclude iron-replete individuals). A lack of understanding of the mechanisms underlying these adverse outcomes limits our ability to modify present supplementation and fortification strategies. This review summarizes studies on the adverse effects of iron on various outcomes; suggests possible mechanisms that may explain these observations, which are usually made in clinical studies and intervention trials; and gives examples from animal models and in vitro studies. With a better understanding of these mechanisms, it may be possible to find novel ways of providing iron in a form that causes fewer or no adverse effects even when subjects are iron replete. However, it is apparent that our understanding is limited, and research in this area is urgently needed.
Keywords: children, development, excess iron, growth, infants, infections, iron deficiency
INTRODUCTION
Iron deficiency (ID) is the most common single-nutrient deficiency worldwide, and infants and young children are among those individuals who are most affected because of their rapid growth. Although the consequences of ID may not be pronounced, it is well known to have adverse effects on cognitive and motor development (1, 2). During early life, ID has caused altered metabolism and neurotransmission, myelination, and gene and protein profiles in experimental animals, and 6- to 24-mo-old infants with ID anemia (IDA) are at risk of poorer cognitive, motor, social-emotional, and neurophysiologic development in short- and long-term outcomes (3). These effects have been shown to be irreversible in several studies (1, 4). Because the provision of iron by the supplementation or fortification of foods has been shown to be effective in preventing and treating ID and IDA, there are many such programs in both resource-rich and -constrained settings worldwide (5). The benefits of such programs are indisputable; however, reports are accumulating on various adverse effects of iron that is given to iron-replete infants and young children (6, 7). These effects include decreased growth, increased morbidity, interactions with other trace elements (copper and zinc), impaired development, and changed gut microflora. Some of these effects may be due to immature iron homeostasis in infants (8), but other effects may be caused by more indirect mechanisms. This review describes these reports and potential underlying mechanisms.
EFFECTS OF IRON EXCESS ON GROWTH OF IRON-REPLETE INFANTS AND CHILDREN
In a study on the appropriate age to start iron supplementation of breastfed infants, we gave exclusively breastfed infants iron drops (1 mg FeSO4 · kg−1 · d−1) or a placebo from 4 or 6 to 9 mo of age (9). This issue was of interest because, at the time, the WHO was considering altering their recommendation to start iron supplementation from 6 to 4 mo of age. This study, which was funded by the USDA, was conducted in Honduras, which represented a lower socioeconomic population with known poor iron status, and in Sweden, which represented a higher socioeconomic group with adequate iron status. We showed that iron status increased in the Honduran infants but not in the Swedish infants, and iron status was similar at 9 mo of age regardless of whether iron supplementation was started at 4 or 6 mo of age. After these results and other studies, the WHO retained their recommendation to start iron supplementation at 6 mo of age.
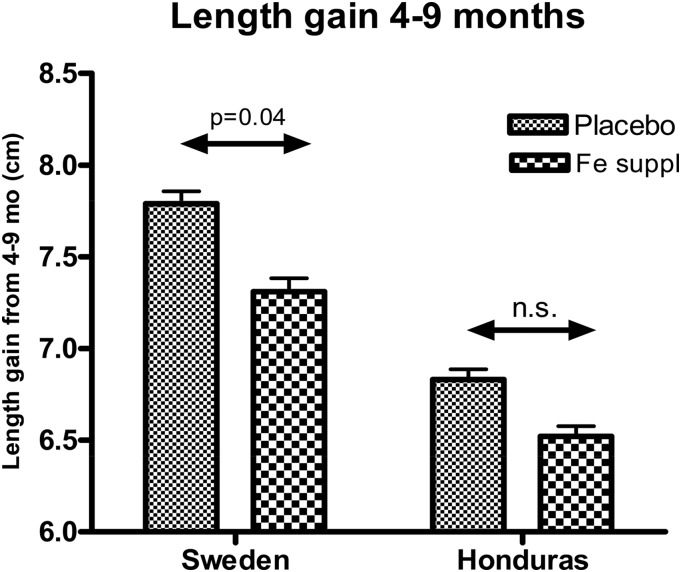
To us, a surprising finding in our study was that linear growth was significantly (P = 0.04) reduced (moderate effect size) in the Swedish infants who were given iron compared with in infants given the placebo (Figure 1) (10). Although this was not first seen in the Honduran cohort, we subsequently showed that iron-replete infants (hemoglobin concentration ≥110 g/L) who were given iron had lower length gain than that of infants with low iron status who were given iron.
FIGURE 1.
Length gain of infants in Sweden and Honduras who were given iron drops or a placebo from 4 to 6 mo of age. Adapted from Dewey et al. (10) with permission. Fe suppl, iron supplementation.
Several other investigators have shown an adverse effect of iron on growth. Idjradinata et al. (11) reported that iron-replete, 12- to 18-mo-old children in Indonesia who were given 3 mg Fe · kg−1 · d−1 for 4 mo had significantly slower weight gain than infants did who were given a placebo. Further, Majumdar et al. (12) showed that 6- to 24-mo-old iron-replete children in India who were given 2 mg Fe · kg−1 · d−1 for 4 mo had significantly slower weight gain and linear growth than in children who were given a placebo. We conducted a trial in Indonesia in which infants were given daily, from 6 to 12 mo of age, either iron (10 mg FeSO4) or zinc (10 mg ZnCl2), both (10 mg each), or a placebo orally (13). Iron supplementation increased iron status and cognitive development, whereas zinc supplementation increased zinc status (plasma zinc) and growth. When analyzing the data by dividing the cohort into infants who were iron replete at the start of the intervention and those who were not, iron supplementation resulted in significantly decreased weight-for-age and weight-for-height z scores in iron-replete infants but not in those who were not iron replete (Table 1). Capozzi et al. (14) followed infants from 6 to 24 mo of age and showed that breastfed infants who were fed unfortified foods had lower hematologic values than infants did who fed iron-fortified formulas and foods, and the formula-fed infants had lower hematologic values than infants did who were given oral iron supplementation (1 mg · kg−1 · d−1). However, infants who were given oral iron supplements had significantly lower length than the other groups did at 12 and 18 mo of age. In addition, Ziegler et al. (15) showed that breastfed infants who were given iron drops (7 mg FeSO4/d) daily from 4 to 9 mo of age had significantly reduced weight gain and a trend toward reduced length gain compared with in unsupplemented infants. However, another study by Friel et al. (16) did not show an adverse effect of iron supplementation (7.5 mg FeSO4/d) of breastfed infants in Canada on growth from 1 to of 6 mo of age. It is possible that the proportion of iron-replete infants was higher in Iowa than in the Canadian cohort as was indicated by other studies on iron status from the same sites and investigators.
TABLE 1.
Anthropometric, hematologic, and biochemical outcomes of iron-supplemented and unsupplemented iron-replete and non–iron-replete infants at the 12-mo follow-up1
| Iron replete (n = 154)2 |
Non–iron replete (n = 452) |
|||||
| Fe (n = 80) | Nonsupplemented (n = 74) | P3 | Fe+ (n = 220) | Fe− (n = 232) | P4 | |
| WAZ5 | −1.99 ± 1.02 | −1.58 ± 1.13 | <0.001 | −1.60 ± 1.03 | −1.63 ± 1.05 | 0.45 |
| ΔWAZ5 | −1.45 ± 0.46 | −1.03 ± 0.63 | <0.001 | −1.23 ± 0.56 | −1.27 ± 0.54 | 0.44 |
| WLZ5 | −1.37 ± 1.17 | −0.85 ± 1.27 | 0.001 | −0.87 ± 1.10 | −0.89 ± 1.06 | 0.39 |
| LAZ5 | −0.79 ± 1.05 | −0.83 ± 0.93 | 0.83 | −0.79 ± 0.84 | −0.82 ± 0.92 | 0.68 |
| Hemoglobin,5 g/L | 119.2 ± 15.3 | 117.6 ± 15.4 | 0.43 | 116.7 ± 14.9 | 113.6 ± 15.8 | 0.03 |
| SF,6 μg/L | 47.5 ± 2.0 | 20.7 ± 2.2 | 0.04 | 36.8 ± 2.6 | 11.7 ± 3.7 | <0.001 |
| Serum zinc,5 μmol/L | 9.7 ± 2.5 | 10.5 ± 3.1 | 0.04 | 10.3 ± 3.3 | 10.9 ± 4.1 | 0.12 |
Adapted from Lind et al. (13) with permission. Fe−, no Fe provided; Fe+, Fe drops; LAZ, length-for-age z score; SF, serum ferritin; WAZ, weight-for-age z score; WLZ, weight-for-length z score; ΔWAZ, weight-for-age change from baseline to follow-up.
Hemoglobin concentration ≥113 g/L and SF concentration ≥33 μg/L at 6 mo of age.
ANOVA for difference between Fe+ and Fe− iron-replete infants.
ANOVA for difference between Fe+ and Fe− non–iron-replete infants.
All values are means ± SDs.
All values are antilogs of mean ln values ± SDs.
Note that not all studies have shown an adverse effect of iron on growth of iron-replete infants. For example, Gahagan et al. (17) examined whether short- or long-term growth was impaired by iron supplementation in a post hoc analysis of a previous study of healthy, breastfed, low- to middle-income Chilean infants who were randomly assigned to iron supplementation or usual nutrition at 6 or 12 mo. A subgroup of infants (n = 273) were identified as iron sufficient at the time of assignment. Growth trajectories did not differ during or after supplementation, thereby indicating no adverse effect of iron. Because the underlying mechanisms behind the decreased growth observed are not known, it is difficult to discern what factors are associated with the different outcomes shown in these studies. However, concerns over the effects of iron supplementation on growth remain, and a systematic review and meta-analysis by Pasricha et al. (18) that included 35 studies and a total number of 42,306 infants and children from 4 to 23 mo of age showed that daily iron supplementation resulted in significantly reduced length gain and weight gain compared with in controls. In this meta-analysis, initial iron status was not considered, thus showing that there may be an overall negative effect of iron on growth even when a significant proportion of the infants initially had low iron status because the majority of the studies were from less developed countries with a strong rationale for iron interventions.
EFFECTS OF IRON EXCESS ON TRACE-ELEMENT STATUS OF IRON-REPLETE INFANTS AND CHILDREN
It is well known that trace elements compete for uptake mechanisms in the small intestine and that the excess of one trace element can impair the absorption of another trace element (19, 20). For example, excess iron has been shown to decrease the absorption of zinc and copper and, consequently, zinc and copper status. In a carefully conducted metabolic balance study, Haschke et al. (21) showed that feeding infants formula that contained 10.2 mg Fe/L resulted in significantly lower copper absorption (13.4% compared with 27.5%; P < 0.01) than in infants fed formula with 2.5 mg Fe/L. The authors concluded that iron in amounts that are present in iron-fortified formulas has a measurable effect on copper utilization although they doubted that this effect would be clinically relevant. In most cases, these interactions have been shown for trace elements that are given orally apart from meals. For example, using radioisotopes, we have shown that oral iron supplements decreased zinc absorption in human adults when ingested separately from a meal, whereas no decrease was shown when the supplements were consumed in a meal (22). Because the addition of histidine, which is a dietary ligand for zinc, to the oral dose of iron that was given with zinc apart from a meal also eliminated the interaction between iron and zinc, we hypothesized that dietary ligands that are present in a meal facilitate the absorption of zinc and reduce the possibility of an interaction between the 2 elements. Since that time, we have shown that interactions between iron, zinc, and copper do occur, and this occurrence can be seen even when they are given in a meal.
In a study of Swedish infants (23) who were fed formula with varying iron concentrations from 6 wk to 6 mo of age, we showed that copper status was reduced when infants were fed formula with a higher concentration of iron (7 mg/L) than with a lower concentration (4 mg Fe/L). At 6 mo of age, infants who were fed the formula with a higher iron content had significantly lower concentrations of serum copper and ceruloplasmin, which is an indicator of copper status, than did infants who were fed formula with lower iron and the breastfed reference group. These lower indexes of copper status were still within the range of normal values and were not associated with any clinical signs of copper deficiency, but because the difference in the 2 amounts of iron was very modest, it raises the possibility that a high iron-to-copper ratio in the diet may impair copper status.
In a study of low-birth-weight infants who were given iron supplements from 4 to 20 wk of age, infants who received the higher dose of iron (13.8 mg/d) had significantly lower erythrocyte copper and zinc superoxide dismutase (SOD) activity, which is another indicator of copper status, than did infants who received a lower dose of iron (7 mg/d) or no iron (24). In our study in Honduras and Sweden described previously (9), we also showed that iron drops that were given to term breastfed infants reduced concentrations of erythrocyte copper and zinc SOD (25). The concentrations of copper and zinc SOD were still in the normal range, but because this enzyme is involved in protection against free-radical–mediated pro-oxidative events, it may be prudent to monitor the copper status of infants who are given iron supplements. However, this observation may be difficult to do in clinical practice, particularly in preterm infants because the volume of blood that is available for analysis is very limited. Another study by Friel et al. (16), with a limited number of subjects, did so but showed no adverse effect of iron supplements that were given to breastfed infants in Canada on copper and zinc SOD. The dose of iron supplementation, duration of the treatment, age of the infants, and their underlying nutritional status may explain the differences shown between studies.
In a study in Indonesia, we supplemented infants with iron (10 mg/d), zinc (10 mg/d), iron and zinc (10 mg each/d), or a placebo from 6 to 12 mo of age (26). Iron supplementation increased iron status (hemoglobin and serum ferritin), and zinc supplementation increased zinc status (serum zinc); however, adding iron to the zinc supplement resulted in some improvement of serum zinc but not as much as in the zinc-only group. In addition, the group given only iron had a significantly higher proportion of low serum zinc values than did the placebo group. These findings are in agreement with several reports in human adults that have shown that high concentrations of iron can inhibit zinc absorption when given in a water solution (22, 27, 28).
EFFECTS OF IRON EXCESS ON MORBIDITY OF IRON-REPLETE INFANTS AND CHILDREN
In the study that we conducted in breastfed infants in Sweden and Honduras (10), we showed that iron-replete infants (defined as having a hemoglobin concentration ≥105 g/L) who were given iron drops had significantly more diarrheal illness than infants did who were given a placebo. In contrast, anemic infants (hemoglobin concentration <105 g/L) who were given iron had significantly less diarrhea than infants did who were given a placebo. These results are in agreement with a large (n = 900/group), cluster-randomized trial in Pakistan by Soofi et al. (29), which showed that infants aged 6–18 mo who received a micronutrient powder containing iron with or without zinc had a significant reduction in IDA. Thus, there was improved iron status in both groups, but there was also a significant increase in the proportion of days with diarrhea and an increased incidence of diarrhea in both groups. A large supplementation trial in children <3 y of age in an area with a high prevalence of malaria had to be stopped because of an increased incidence of severe infections in the iron-supplemented groups (30). In that trial, increased risk of a poor outcome was observed in iron-supplemented children who had adequate initial iron status. A systematic review and meta-analysis (18) of the effect of daily iron supplementation on health in children aged 4–23 mo showed that iron supplementation substantially reduced risks of ID and IDA but increased risks of vomiting and fever. There are several possible mechanisms behind these observations, and they may well work together. The reduction in diarrhea in anemic infants may be manifested by an effect of iron on restoring immune functions that are iron dependent and, thus, compromised by underlying ID (31). Instead, the negative effect of iron in iron-replete infants may be due to a stimulatory effect of iron on pathogens that require iron for growth and proliferation, thus altering the microflora (also see below under “Effects of Iron Excess on the Gut Microbiota of Iron-Replete Infants and Children”) and increasing risk of diarrheal disease. It is also possible that iron inhibits the expression of inducible nitric oxide synthase, which then downregulates the synthesis of nitric oxide in macrophages (6). This effect may be particularly relevant for the macrophage defense against Plasmodium falciparum and may explain the previous observations on iron and malaria.
EFFECTS OF IRON EXCESS ON COGNITIVE DEVELOPMENT OF IRON-REPLETE INFANTS AND CHILDREN
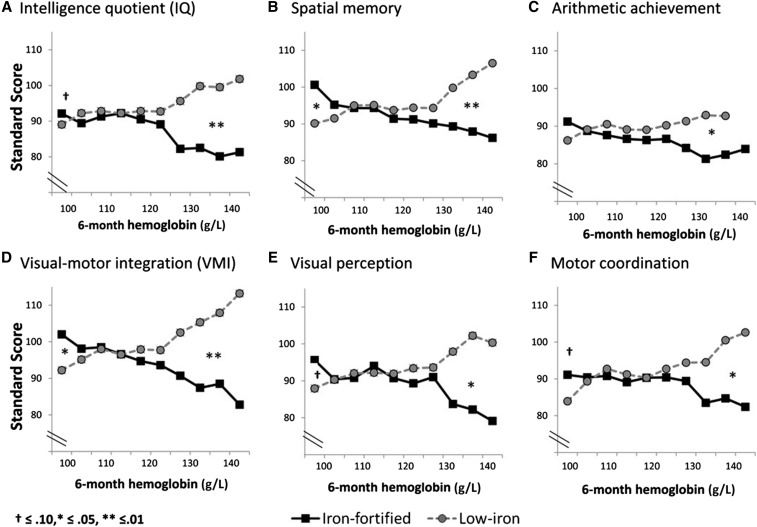
Our studies on an adverse effect on linear growth of iron drops given to iron-replete infants (10) and observations by others caused Lozoff et al. (32) to re-examine data from a previous large intervention study that they had conducted in Chilean infants who were given infant formula with 2 amounts of iron fortification (33). In this study, healthy, term infants (n = 835) were fed a low-iron formula (2.3 mg/L) or iron-fortified formula (12.7 mg/L) from 6 to 12 mo of age. At 10 y of age, 473 children were assessed; children who had been given an iron-fortified formula as infants scored lower on every developmental outcome, which was significant for spatial memory and visual-motor integration and suggestive for IQ, arithmetic achievement, visual perception, and motor coordination (Figure 2). Infants with high hemoglobin concentrations (>128 g/L) at 6 mo of age showed a poorer outcome on these measures if they received the iron-fortified formula (large effect sizes), whereas those with low hemoglobin concentrations (<105 g/L) showed a better outcome (small effect sizes). The authors concluded that long-term development may be adversely affected in infants who receive iron-fortified formula (at 12.7 mg/L). Unfortunately, there have been very few studies that have evaluated long-term developmental outcomes of infants who are given iron according to their initial iron status.
FIGURE 2.
Developmental outcomes of infants fed iron-fortified infant formula compared with low-iron infant formula at 10 y of age. Adapted from Lozoff et al. (32) with permission.
Studies in experimental animals have also supported an adverse effect of iron that is given during the neonatal period on cognitive development and the brain. Fredriksson et al. (34) treated mice with 0, 3.7, or 37 mg ferrous iron orally on postnatal days (PDs) 10–12. Mice that were given the higher iron dose showed a lack of habituation of spontaneous activity in test chambers at 3 mo of age. This dose is considerably higher than what infants may be exposed to, but it is of concern that mice that were fed the lower dose also showed these effects although they were less pronounced. This study showed that a modest amount of iron that is provided during a short period during the neonatal period can have long-lasting effects on neurobehavior. Kaur et al. (35) administered iron by oral gavage at doses that were equivalent to those present in iron-fortified formula to mouse pups from days 10–17 postnatally and showed loss of striatal dopamine, progressive midbrain neurodegeneration, and enhanced vulnerability to toxic injury. Although brain maturation in mice is quite different from that in human infants, the authors proposed that elevated neonatal iron intake may be a potential risk factor for later development of Parkinson disease. That early life iron exposure can have long-term consequences for brain iron homeostasis was shown by Dornelles et al. (36) who analyzed the gene expression of transferrin receptor, H-ferritin, and iron regulatory protein 2 in the cortex, hippocampus, and striatum of rats who had been given 10 mg ferrous iron/kg body weight during PDs 12–14 when they were 15-d, 90-d, and 2-y old. All of these proteins that are involved in iron homeostasis regulation were dysregulated by early iron exposure, and the authors suggested that this involvement may explain the misregulation shown in neurologic disorders. Miwa et al. (37) also exposed rats to iron (10 mg ferrous iron/kg body weight) during PDs 12–14 and showed that the rats, as adults, had significantly elevated concentrations of prostate apoptosis response-4 (Par-4) and caspase-3 in the hippocampus, adjacent cortex, and striatum, which were indicative of increased apoptosis. However, in aged, iron-treated animals, these markers were lower than in control rats. The authors suggested that this result may be interpreted as an acceleration of aging progressive damages that are caused by iron excess.
In a recent study (38), we gave normal and growth-restricted rat pups iron (30 or 150 μg/d) at amounts that were similar to those that were ingested by human infants who were fed medium and high amounts of formula fortification from birth to PD 20 and followed the rats to PD 56. At PD 20, hematology, tissue iron, and the hepatic metabolome were measured. The plasma metabolome and colonic microbial ecology were assessed at PDs 20 and 56. Passive avoidance (PD-40) tests were used to evaluate cognitive development. Iron supplementation increased iron status in a dose-dependent manner. Passive avoidance was significantly lower in rats that were given high iron than in controls. In the plasma and liver, excess iron increased 3-hydroxybutyrate and decreased several amino acids, urea, and myoinositol. Inositol is involved in brain function and is considered a conditionally essential nutrient in preterm infants (39). Iron supplementation did not have a pronounced effect on the abundance of major individual bacterial species, which may be explained by the sanitized conditions of the animal room, but a significant difference in the abundance of strict anaerobes was observed. In control rats, there was an increasing proportion of strict anaerobes from PDs 20 to 56 (19.4–28.3%); however, this effect was no longer significant in rats that were given medium or high iron. One mechanism to explain these changes could be that higher amounts of free reactive iron [Fe(II)] may induce free radical damage to the gastrointestinal tract and subsequently release oxygen by the Haber-Weiss reaction, thereby causing oxidative stress that reduces the amount of strict anaerobes to slow maturation to an adult-like gut microbial profile. We concluded that excess iron adversely affects cognitive development, which may be a consequence of altered metabolism or shifts in the gut microbiota.
EFFECTS OF IRON EXCESS ON THE GUT MICROBIOTA OF IRON-REPLETE INFANTS AND CHILDREN
Iron has long been recognized as an important factor affecting microbial growth. Many pathogens require iron for their growth and have acquired strong mechanisms for acquiring iron from the environment, e.g., by secreting iron chelators (e.g., siderophores) that facilitate the bacterial uptake of iron. Mammals, in turn, have developed mechanisms to withhold iron from iron-requiring pathogens to protect against infection. Lactoferrin in breast milk is one such example; 15–20% of protein in breast milk is lactoferrin, and it is largely unsaturated with iron (40). Lactoferrin has an unusually high affinity for iron (Kass ∼1023) and can successfully compete with bacterial siderophores for iron in the gut. Because of this action, lactoferrin can create an iron-poor environment in the gut of breastfed infants. Early studies have shown that human milk and human milk lactoferrin have a strong bacteriostatic effect on pathogenic Escherichia coli in vitro and that this growth-inhibitory effect is abolished with the addition of iron (41). Many other studies have shown these effects both in vitro and in vivo for both lactoferrin and other iron-binding proteins. Oral iron may also have more systemic effects on bacterial growth. Cross et al. (42) recently showed that ex vivo growth of sentinel bacteria in adult serum samples that were collected before and 4 h after oral supplementation with 2 mg FeSO4/kg showed markedly elevated bacterial growth in serum that was collected after iron supplementation. The growth of several gram-negative pathogens was very strongly correlated with transferrin saturation, suggesting bacteriostatic control by this iron-binding protein. The growth of Staphylococcus aureus, which preferentially acquires heme iron, was unaffected. Their results suggest that modest oral iron supplements with highly soluble iron, as are usually used in low-income settings, may promote bacteremia by increasing early bacterial growth before the induction of immune responses.
Thus, there is a conceptual connection between iron and the gut microflora in infants and children, and the topic is discussed in detail elsewhere in these proceedings (43). A study that was conducted by Zimmermann et al. (44) showed that anemic children in Côte d’Ivoire had an unfavorable ratio of fecal Enterobacteria to Bifidobacteria and Lactobacilli at baseline and that children who were fed iron-fortified biscuits showed an increase in the number of Enterobacteria and a decrease in Lactobacilli compared with in controls. The anemic children also had an increase in fecal calprotectin, which is a marker of gut inflammation. The outcomes of a subsequent study in South African schoolchildren (45) were in contrast to the Ivorian study in that iron supplements did not increase gut inflammation; moreover, at baseline, there was no evidence of greater fecal Enterobacteria. The Zimmermann group (45) suggested that the local context is a consideration relative to the effect of iron supplementation on the gut microbiome, and supplementation is likely to be more problematic where hygiene is low. They noted reports of adverse impacts of iron supplementation on the gut microbiota in developed countries with a low enteropathogenic burden (46) but suggested that infants are in the process of developing bacterial ecosystems and may be more vulnerable overall to alterations than are older children. The recent study of the Zimmermann group (47) in Kenyan children supports the suggestion that the administration of iron-containing products to African infants in areas of poor hygiene causes adverse shifts in the gut microbiome.
The form of iron in complementary foods can also affect the gut microbiota in infants. Krebs et al. (48) studied 5-mo-old breastfed infants who were randomly assigned to receive pureed meats, iron-fortified cereals, or iron- and zinc-fortified cereals as the first and primary complementary foods to 9–10 mo of age. Although infants in the cereal groups had iron intakes that were 2–3 times higher than those of the meat group, and no differences in abundance or diversity of potentially pathogenic bacteria were observed, the abundances of several bacterial groups differed between these 2 groups such as a significantly higher abundance of the butyrate-producing Clostridium group XIVa in the meat group. Because short-chain fatty acids are considered beneficial for host health and for providing additional energy to host cells (49), it is possible that higher intake of iron had a negative effect on the gut microflora. Overall, the study of Krebs et al. (48) emphasizes that different forms of iron in weaning foods can affect the gut microflora, which may have further consequences. Dostal et al. (50) have shown, in an in vitro colonic fermentation model that mimicked a child’s microbiota, that different iron conditions affect butyrate production as well as genes that are involved in butyrate synthesis. Both low iron conditions and excess iron showed adverse effects, suggesting that there may an optimal range for iron in the gut to control butyrate production. That oxidative events that are triggered by iron may be involved in alterations in the gut microbiota was shown in a recent study by Tang et al. (51). Iron-deficient infants and toddlers in Colorado were given therapeutic iron (6 mg · kg−1 · d−1) with or without vitamin E for 8 wk. The therapy was effective in treating ID, and the authors showed no changes over time in serum cytokines IL-4, TNF-α, or fecal calprotectin, but the relative abundance of Roseburia (phylum Firmicutes), which is a butyrate producer, increased in the iron plus vitamin E group. Thus, antioxidants that are given with iron may have a beneficial effect on the gut microflora and butyrate production. It is evident that safer formulations are needed to ascertain a reduction in IDA without changing the gastrointestinal microflora and increasing morbidity (52).
MECHANISMS BEHIND ADVERSE EFFECTS OF GIVING IRON TO IRON-REPLETE INFANTS AND YOUNG CHILDREN
It should be re-emphasized that providing iron to infants who are ID or have IDA overall has many beneficial effects and that giving iron to a population with high prevalences of ID and IDA most likely has a positive outcome. However, if iron can cause harm in these age groups when given to individuals who are iron replete, we should try to determine the mechanisms behind these effects, particularly on growth and cognitive development, and try to reduce or eliminate them. This is not an easy task; the adverse outcomes are diverse, and it is unlikely that they are the result of a common mechanism. Rather, iron may affect several pathways that also can have interactive effects. It is also important to delineate the sequence of events (e.g., do proinflammatory events that are caused by iron affect the microbiota or do iron-mediated alterations in the microbiota cause a local inflammatory environment?). The involvement of pro-oxidative effects of iron, the competition and interaction with other elements such as copper and zinc for absorptive pathways, disturbances in the microflora and its effect on immune function, metabolism, and health as well as communication along the gut-brain axis that affects cognition and development all need to be considered.
CONCLUSIONS
Long-term studies on the effects of providing iron via supplementation or fortification on the gut microflora, immune function and inflammation, illness, growth, and cognitive development are needed in populations with defined iron status as are studies on the mechanisms underlying these outcomes. However, conducting such studies is ethically complicated because adverse effects have been observed in several studies.
Acknowledgments
The author’s responsibilities were as follows—The sole author conducted the review, wrote the manuscript, and read and approved the final manuscript. BL is emeritus in the Department of Nutrition at University of California, Davis. The author reported no conflict of interest related to the study.
Footnotes
Abbreviations used: ID, iron deficiency; IDA, iron deficiency anemia; PD, postnatal day; SOD, superoxide dismutase.
REFERENCES
- 1.Lozoff B. Iron deficiency and child development. Food Nutr Bull 2007;28:S560–71. [DOI] [PubMed] [Google Scholar]
- 2.Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev 2011;69 Suppl 1:S43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hare DJ, Arora M, Jenkins NL, Finkelstein DI, Doble PA, Bush AI. Is early-life iron exposure critical in neurodegeneration? Nat Rev Neurol 2015;11:536–44. [DOI] [PubMed] [Google Scholar]
- 4.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 2006;64:S34– 43; discussion S72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernell O, Fewtrell MS, Georgieff MK, Krebs NF, Lönnerdal B. Summary of current recommendations on iron provision and monitoring of iron status for breastfed and formula-fed infants in resource-rich and resource-constrained countries. J Pediatr 2015;167:S40–7. [DOI] [PubMed] [Google Scholar]
- 6.Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr 2006;84:1261–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lönnerdal B, Georgieff MK, Hernell O. Developmental physiology of iron absorption, homeostasis, and metabolism in the healthy term infant. J Pediatr 2015;167:S8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lönnerdal B. Development of iron homeostasis in infants and young children. Am J Clin Nutr 2017;106(Suppl):1575S–80S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domellöf M, Cohen RJ, Dewey KG, Hernell O, Rivera LL, Lönnerdal B. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J Pediatr 2001;138:679–87. [DOI] [PubMed] [Google Scholar]
- 10.Dewey KG, Domellöf M, Cohen RJ, Landa Rivera L, Hernell O, Lönnerdal B. Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J Nutr 2002;132:3249–55. [DOI] [PubMed] [Google Scholar]
- 11.Idjradinata P, Watkins WE, Pollitt E. Adverse effect of iron supplementation on weight gain of iron-replete young children. Lancet 1994;343:1252–4. [DOI] [PubMed] [Google Scholar]
- 12.Majumdar I, Paul P, Talib VH, Ranga S. The effect of iron therapy on the growth of iron-replete and iron-deplete children. J Trop Pediatr 2003;49:84–8. [DOI] [PubMed] [Google Scholar]
- 13.Lind T, Seswandhana R, Persson LÅ, Lönnerdal B. Iron supplementation of iron-replete Indonesian infants is associated with reduced weight-for-age. Acta Paediatr 2008;97:770–5. [DOI] [PubMed] [Google Scholar]
- 14.Capozzi L, Russo R, Bertocco F, Ferrara D, Ferrara M. Effect on haematological and anthropometric parameters of iron supplementation in the first 2 years of life. Risks and benefits. Hematology 2011;16:261–4. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler EE, Nelson SE, Jeter JM. Iron status of breastfed infants is improved equally by medicinal iron and iron-fortified cereal. Am J Clin Nutr 2009;90:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friel JK, Aziz K, Andrews WL, Harding SV, Courage ML, Adams RJ. A double-masked, randomized control trial of iron supplementation in early infancy in healthy term breast-fed infants. J Pediatr 2003;143:582–6. [DOI] [PubMed] [Google Scholar]
- 17.Gahagan S, Yu S, Kaciroti N, Castillo M, Lozoff B. Linear and ponderal growth trajectories in well-nourished, iron-sufficient infants are unimpaired by iron supplementation. J Nutr 2009;139:2106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasricha SR, Hayes E, Kalumba K, Biggs BA. Effect of daily iron supplementation on health in children aged 4-23 months: a systematic review and meta-analysis of randomised controlled trials. Lancet Glob Health 2013;1:e77–86. [DOI] [PubMed] [Google Scholar]
- 19.Lönnerdal B. Iron-zinc-copper interactions. In: Micronutrient interactions: impact on child health and nutrition. Washington: (DC): USAID and FAO, ILSI Press; 1998. p. 3–10. [Google Scholar]
- 20.Fischer Walker C, Kordas K, Stoltzfus RJ, Black RE. Interactive effects of iron and zinc on biochemical and functional outcomes in supplementation trials. Am J Clin Nutr 2005;82:5–12. [DOI] [PubMed] [Google Scholar]
- 21.Haschke F, Ziegler EE, Edwards BB, Fomon SJ. Effect of iron fortification of infant formula on trace mineral absorption. J Pediatr Gastroenterol Nutr 1986;5:768–73. [DOI] [PubMed] [Google Scholar]
- 22.Sandström B, Davidsson L, Cederblad Å, Lönnerdal B. Oral iron, dietary ligands and zinc absorption. J Nutr 1985;115:411–4. [DOI] [PubMed] [Google Scholar]
- 23.Lönnerdal B, Hernell O. Iron, zinc, copper and selenium status of breast-fed infants and infants fed trace element fortified milk-based infant formula. Acta Paediatr 1994;83:367–73. [DOI] [PubMed] [Google Scholar]
- 24.Barclay SM, Aggett PJ, Lloyd DJ, Duffty P. Reduced erythrocyte superoxide dismutase activity in low birth weight infants given iron supplements. Pediatr Res 1991;29:297–301. [DOI] [PubMed] [Google Scholar]
- 25.Domellöf M, Dewey KG, Cohen RJ, Lönnerdal B, Hernell O. Iron supplements reduce erythrocyte copper-zinc superoxide dismutase activity in term, breastfed infants. Acta Paediatr 2005;94:1578–82. [DOI] [PubMed] [Google Scholar]
- 26.Lind T, Lönnerdal B, Stenlund H, Ismail D, Seswandhana R, Ekström EC, Persson LÅ. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: interactions between iron and zinc. Am J Clin Nutr 2003;77:883–90. [DOI] [PubMed] [Google Scholar]
- 27.Valberg LS, Flanagan PR, Chamberlain MJ. Effects of iron, tin, and copper on zinc absorption in humans. Am J Clin Nutr 1984;40:536–41. [DOI] [PubMed] [Google Scholar]
- 28.Solomons NW, Jacob RA. Studies on the bioavailability of zinc in humans: effects of heme and nonheme iron on the absorption of zinc. Am J Clin Nutr 1981;34:475–82. [DOI] [PubMed] [Google Scholar]
- 29.Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, Zaidi AK, Bhutta ZA. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet 2013;382:29–40. [DOI] [PubMed] [Google Scholar]
- 30.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 2006;367:133–43. [DOI] [PubMed] [Google Scholar]
- 31.Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr 2001;131:568S–79S; discussion 80S. [DOI] [PubMed] [Google Scholar]
- 32.Lozoff B, Castillo M, Clark KM, Smith JB. Iron-fortified vs low-iron infant formula: developmental outcome at 10 years. Arch Pediatr Adolesc Med 2012;166:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter T, Pino P, Pizarro F, Lozoff B. Prevention of iron-deficiency anemia: comparison of high- and low-iron formulas in term healthy infants after six months of life. J Pediatr 1998;132:635–40. [DOI] [PubMed] [Google Scholar]
- 34.Fredriksson A, Schroder N, Eriksson P, Izquierdo I, Archer T. Neonatal iron exposure induces neurobehavioural dysfunctions in adult mice. Toxicol Appl Pharmacol 1999;159:25–30. [DOI] [PubMed] [Google Scholar]
- 35.Kaur D, Peng J, Chinta SJ, Rajagopalan S, Di Monte DA, Cherny RA, Andersen JK. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol Aging 2007;28:907–13. [DOI] [PubMed] [Google Scholar]
- 36.Dornelles AS, Garcia VA, de Lima MN, Vedana G, Alcalde LA, Bogo MR, Schroder N. mRNA expression of proteins involved in iron homeostasis in brain regions is altered by age and by iron overloading in the neonatal period. Neurochem Res 2010;35:564–71. [DOI] [PubMed] [Google Scholar]
- 37.Miwa CP, de Lima MN, Scalco F, Vedana G, Mattos R, Fernandez LL, Hilbig A, Schroder N, Vianna MR. Neonatal iron treatment increases apoptotic markers in hippocampal and cortical areas of adult rats. Neurotox Res 2011;19:527–35. [DOI] [PubMed] [Google Scholar]
- 38.Alexeev EE, He X, Slupsky CM, Lönnerdal B. Effects of iron supplementation on growth, gut microbiota, metabolomics and cognitive development of rat pups. PLoS One 2017;12:e0179713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carver JD, Stromquist CI, Benford VJ, Minervini G, Benford SA, Barness LA. Postnatal inositol levels in preterm infants. J Perinatol 1997;17:389–92. [PubMed] [Google Scholar]
- 40.Fransson GB, Lönnerdal B. Iron in human milk. J Pediatr 1980;96:380–4. [DOI] [PubMed] [Google Scholar]
- 41.Bullen JJ, Rogers HJ, Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. BMJ 1972;1:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cross JH, Bradbury RS, Fulford AJ, Jallow AT, Wegmuller R, Prentice AM, Cerami C. Oral iron acutely elevates bacterial growth in human serum. Sci Rep 2015;5:16670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paganini D, Zimmermann MB. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: a review. Am J Clin Nutr 2017;106(Suppl):1688S–93S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmermann MB, Chassard C, Rohner F, N’Goran EK, Nindjin C, Dostal A, Utzinger J, Ghattas H, Lacroix C, Hurrell RF. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d’Ivoire. Am J Clin Nutr 2010;92:1406–15. [DOI] [PubMed] [Google Scholar]
- 45.Dostal A, Baumgartner J, Riesen N, Chassard C, Smuts CM, Zimmermann MB, Lacroix C. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: a randomised, placebo-controlled intervention trial in South African children. Br J Nutr 2014;112:547–56. [DOI] [PubMed] [Google Scholar]
- 46.Mevissen-Verhage EA, Marcelis JH, Harmsen-Van Amerongen WC, de Vos NM, Verhoef J. Effect of iron on neonatal gut flora during the first three months of life. Eur J Clin Microbiol 1985;4:273–8. [DOI] [PubMed] [Google Scholar]
- 47.Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015;64:731–42. [DOI] [PubMed] [Google Scholar]
- 48.Krebs NF, Sherlock LG, Westcott J, Culbertson D, Hambidge KM, Feazel LM, Robertson CE, Frank DN. Effects of different complementary feeding regimens on iron status and enteric microbiota in breastfed infants. J Pediatr 2013;163:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 2012;9:577–89. [DOI] [PubMed] [Google Scholar]
- 50.Dostal A, Lacroix C, Bircher L, Pham VT, Follador R, Zimmermann MB, Chassard C. Iron modulates butyrate production by a child gut microbiota in vitro. MBio 2015;6:e01453-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang M, Frank DN, Sherlock L, Ir D, Robertson CE, Krebs NF. Effect of vitamin E with therapeutic iron supplementation on iron repletion and gut microbiome in US iron deficient infants and toddlers. J Pediatr Gastroenterol Nutr 2016;63:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paganini D, Uyoga MA, Zimmermann MB. Iron fortification of foods for infants and children in low-income countries: effects on the gut microbiome, gut inflammation, and diarrhea. Nutrients 2016;8:E494. [DOI] [PMC free article] [PubMed] [Google Scholar]