Abstract
Biochemical assessment of iron status relies on serum-based indicators, such as serum ferritin (SF), transferrin saturation, and soluble transferrin receptor (sTfR), as well as erythrocyte protoporphyrin. These indicators present challenges for clinical practice and national nutrition surveys, and often iron status interpretation is based on the combination of several indicators. The diagnosis of iron deficiency (ID) through SF concentration, the most commonly used indicator, is complicated by concomitant inflammation. sTfR concentration is an indicator of functional ID that is not an acute-phase reactant, but challenges in its interpretation arise because of the lack of assay standardization, common reference ranges, and common cutoffs. It is unclear which indicators are best suited to assess excess iron status. The value of hepcidin, non–transferrin-bound iron, and reticulocyte indexes is being explored in research settings. Serum-based indicators are generally measured on fully automated clinical analyzers available in most hospitals. Although international reference materials have been available for years, the standardization of immunoassays is complicated by the heterogeneity of antibodies used and the absence of physicochemical reference methods to establish “true” concentrations. From 1988 to 2006, the assessment of iron status in NHANES was based on the multi-indicator ferritin model. However, the model did not indicate the severity of ID and produced categorical estimates. More recently, iron status assessment in NHANES has used the total body iron stores (TBI) model, in which the log ratio of sTfR to SF is assessed. Together, sTfR and SF concentrations cover the full range of iron status. The TBI model better predicts the absence of bone marrow iron than SF concentration alone, and TBI can be analyzed as a continuous variable. Additional consideration of methodologies, interpretation of indicators, and analytic standardization is important for further improvements in iron status assessment.
Keywords: iron analysis, ferritin, soluble transferrin receptor, total body iron, NHANES, iron methodologies
INTRODUCTION
Biochemical assessment of iron status relies mainly on serum-based indicators. The focus of indicators has largely been deficiency states, whereas questions of measuring iron repletion and iron overload have received less attention. Importantly, even though a panel of iron status indicators is routinely used in clinical practice and as part of national nutrition surveys, numerous challenges remain with the laboratory measurement and interpretation of these data.
This article provides an overview of commonly available indicators of iron status and their analytic challenges as well as discussions related to a ratio of 2 measures to determine status and newer indicators of iron status. Iron status assessment in NHANES is also reviewed. Currently, the Biomarkers of Nutrition for Development project aims to provide evidence-based advice to researchers with an interest in the role of nutrition in health (1). Iron is 1 of 6 nutrients in the Biomarkers of Nutrition for Development project, and the iron expert panel has assembled comprehensive information on the current state of the art with regard to specific iron biology and available indicators for assessing iron status at the individual and population level. On completion, this work will be available in the peer-reviewed literature.
COMMONLY USED IRON STATUS INDICATORS AND THEIR ANALYSIS
Nature of commonly used indicators
Iron balance is a tightly controlled process that can be reflected in a number of iron status indicators. Its hallmark is the rigorous regulation of absorption. There are 3 main body iron compartments that describe iron status inadequacy: iron stores, transport iron (iron to meet cellular requirements), and functional iron (iron available to tissues) (Table 1). Depletion of each compartment leads to a different iron deficiency stage (2). Short-term variations in physiologic iron needs are met by the release of iron stores, the majority of which are available as intracellular ferritin, predominantly in hepatocytes and specialized macrophages. The “gold standard” indicator that provides estimates of the size of the iron store is stainable bone marrow iron, but for obvious reasons it is not a practical measurement. Serum ferritin (SF) represents a small fraction of the body’s ferritin pool (Table 1), but the concentration of SF is reflective of the amount of iron stores (3). Once iron stores are depleted, the first stage of iron deficiency (ID) is reached, namely iron depletion, but there are no erythropoietic consequences yet.
TABLE 1.
Body iron compartments, their respective indicators, and the type of deficiency resulting from the depletion of each compartment
| Iron stores | Transport iron | Functional iron | |
| Commonly used indicators | Stainable bone marrow iron | Transferrin saturation1 (serum iron and either total iron-binding capacity, unsaturated iron-binding capacity, or transferrin) | Hemoglobin (whole blood) |
| Ferritin (serum) | Protoporphyrin2 (erythrocytes) | Hematocrit (whole blood) | |
| Soluble transferrin receptor (serum) | |||
| Deficiency | Iron depletion | Iron deficiency erythropoiesis | Iron deficient anemia |
Transferrin saturation is calculated as the ratio of iron to transferrin or iron to total iron-binding capacity; if unsaturated iron-binding capacity is measured, the total iron-binding capacity is calculated as the sum of iron and unsaturated iron-binding capacity.
Erythrocyte protoporphyrin can be measured directly as zinc protoporphyrin, the form present in erythrocytes, or as free erythrocyte protoporphyrin after extraction.
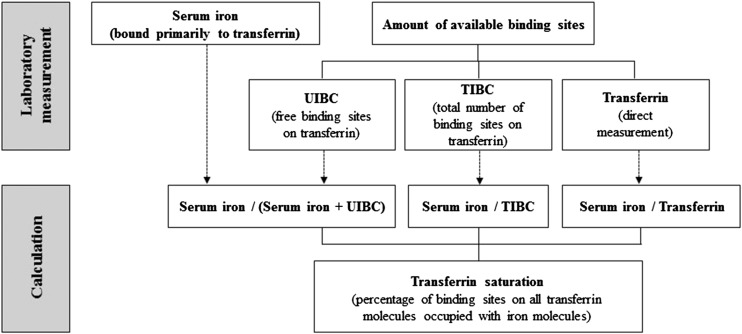
The iron supply provided by the transport iron compartment is mainly for red blood cell (RBC) production because the demand for iron for erythropoiesis is much larger than that for other tissues. If the supply can no longer be met, the second stage of ID, namely iron-deficient erythropoiesis, is reached without showing a notable decrease in hemoglobin concentration. Indicators that provide information about the adequacy of iron supply are transferrin saturation (TSAT) and the concentrations of erythrocyte protoporphyrin (EP) and soluble transferrin receptors (sTfRs) (4). TSAT represents the percentage of binding sites on all transferrin molecules occupied with iron molecules (Figure 1) and is calculated as the ratio of serum iron to transferrin or serum iron to total iron-binding capacity (TIBC). If unsaturated iron-binding capacity (UIBC) is measured, TIBC is calculated as the sum of serum iron and UIBC. The measured plasma or serum pool of iron is the fraction of iron that circulates bound primarily to transferrin. The so called non–transferrin-bound iron (NTBI), iron bound to low-molecular-weight proteins or other compounds, usually comprises <1% of the plasma total iron pool and is usually not detected in most routine assays (4). EP is a generic term for either the directly measured concentration of zinc protoporphyrin (ZPP), the form present in erythrocytes, or the free EP concentration measured after extraction. Although ZPP is often referred to as an indicator of iron status, for the purposes of this manuscript EP is used mainly because NHANES did not measure ZPP but instead measured free EP. sTfR is a truncated fragment of the transferrin receptor 1, and it circulates in plasma bound to transferrin.
FIGURE 1.
Laboratory measurement of iron indicators needed to calculate transferrin saturation. TIBC, total iron-binding capacity; UIBC, unsaturated iron-binding capacity.
Hemoglobin concentration is the key indicator for a functionally important iron deficit, specifically iron deficiency anemia (IDA) (Table 1) (5). The hematocrit or packed cell volume provides no additional information beyond hemoglobin. IDA is one of several nutritional anemias; depleted folate or vitamin B-12 stores can also cause anemia and low hemoglobin concentrations. Non–iron-related anemia can also be caused by blood loss, decreased or faulty RBC production, or destruction of RBCs.
Consideration of the common indicators requires that the biological confounding caused by inflammation be taken into account. Inflammation, a highly complex biological process (6), confounds the interpretation of iron status indicators, especially SF concentration because it increases in response to inflammation as well as to increased iron stores (Table 2). Discussions are ongoing regarding how to account for the amount of inflammation when interpreting iron status in a population (6).
TABLE 2.
Important confounders of iron status indicators1
| Confounder | Indicator and direction of change | Comment |
| Inflammation | SF ↑ | Ferritin is a positive acute-phase protein |
| Transferrin ↓ | Transferrin is a negative acute-phase protein | |
| Iron ↓ | The release of cytokines leads to increased uptake and retention of iron in reticuloendothelial system cells, e.g., iron becomes sequestered and is not available for transport to the bone marrow for erythropoiesis | |
| EP ↑ | ||
| Hemoglobin ↓ | ||
| Increased erythropoietic activity | EP, sTfR ↑ | In thalassemia, sickle cell anemia, and hemoglobinopathies |
| Lead poisoning | EP ↑ | Lead blocks the formation of heme and zinc protoporphyrin forms instead |
| Pregnancy | Hemoglobin ↓ | Plasma volume expansion results in hemodilution |
| Dehydration | Hemoglobin ↑ | The volume of fluid in blood drops and hemoglobin artificially rises |
| Smoking | Hemoglobin ↑ | Compensation for decreased oxygen intake in heavy smokers |
| Altitude | Hemoglobin ↑ | Compensation for decreased oxygen intake due at high altitude |
EP, erythrocyte protoporphyrin; SF, serum ferritin; sTfR, soluble transferrin receptor; ↑, increase in concentration; ↓, decrease in concentration.
In uncomplicated IDA (no inflammatory response), iron stores, transport iron, and functional iron (e.g., iron available to tissues) are all reduced (Table 3). As soon as the iron supply to erythropoiesis becomes insufficient, transferrin production is upregulated to increase iron transport, transferrin receptor production is upregulated to facilitate iron delivery to cells resulting in an increase in serum sTfR, and ZPP is produced instead of heme resulting in an increase in EP. SF and hemoglobin concentrations are important indicators in uncomplicated IDA.
TABLE 3.
Response of iron status indicators to a depletion of body iron compartments with and without concomitant inflammation and to an overload of body iron compartments1
| Compartment | Indicator | IDA | ACD | IDA + ACD | Overload |
| Stored iron | SF | Reduced | Normal to increased | Reduced to normal | Increased |
| Transport iron | Iron | Reduced | Reduced | Reduced | Increased |
| Transferrin | Increased | Reduced | Reduced | Reduced | |
| TSAT | Reduced | Reduced | Reduced | Increased | |
| EP | Increased | Increased | Increased | Reduced | |
| sTfR | Increased | Normal | Normal to increased | Normal | |
| Functional iron | Hemoglobin | Reduced | Reduced | Reduced | Normal |
| Inflammatory response | NA | Normal | Increased | Increased | NA |
ACD, anemia of chronic disease; EP, erythrocyte protoporphyrin; IDA, iron deficiency anemia; IDA + ACD, combined iron deficiency anemia and anemia of chronic disease; NA, not applicable; SF, serum ferritin; sTfR, soluble transferrin receptor; TSAT, transferrin saturation.
In anemia of chronic disease (ACD), the inflammatory response is increased, which causes iron stores to be sequestered (7). SF, being an acute-phase protein, appears normal to increased even though functional iron is reduced. Transport iron shows a mixed response with reduced serum iron and transferrin, increased EP, and normal sTfR concentration. When a combination of IDA and ACD exists, the inflammatory response is increased, iron stores are reduced to normal, and functional iron is reduced. Transport iron indicators show reduced serum iron and transferrin, a normal-to-increased sTfR concentration, and increased EP. When inflammation complicates the interpretation of iron status, it is helpful to have data on several indicators.
The same iron status indicators mentioned above are used to determine iron adequacy or repletion, but concentrations of the indicators are reflective of neither ID nor iron overload. In iron overload, SF and iron stores are increased and functional iron is normal. Transport iron indicators show increased serum iron, reduced transferrin and EP, and normal sTfR. High SF concentration has a high sensitivity but low specificity to diagnose iron overload in hemochromatosis (8–10). The high within-person variability of TSAT limits its usefulness as an initial screening test for hemochromatosis (11); however, elevated TSAT identifies patients at risk of organ iron overload who are eligible for further testing. EP has been shown to be of utility in distinguishing hemochromatosis from other conditions that lead to elevated SF as a result of ACD (12).
Finally, several other confounding factors should be noted for these status indicators (Table 2). Although sTfR and EP are more specific indicators of iron deficiency erythropoiesis, both are also elevated in increased erythropoietic activity, and EP is also elevated in lead poisoning. Hemoglobin is decreased in pregnancy due to hemodilution and increased in heavy smokers, people who live at high altitudes, and in a state of dehydration. The within-person biologic variability for serum iron (∼30%) is much higher than for SF (∼10–25%) or even sTfR (∼10%) (13–16), necessitating the analysis of >1 sample for clinical evaluation. However, this is typically not done in population surveys. Also, because of the relatively high diurnal variability of serum iron (but without a consistent pattern) (14, 17), collection of a specimen from fasting persons was recommended in the clinical setting, but was shown not to have an advantage over random sampling (11).
Analytic considerations
Preanalytical conditions
For serum-based iron status indicators, the preanalytical conditions required to obtain a valid sample for laboratory analysis are fairly easy to achieve (Supplemental Table 1). Whole blood needs to be refrigerated and processed the same day (iron) or within a few days (SF and sTfR) after blood collection to obtain serum (18, 19), serum is stable for ≥1 wk at 4°C and ≥1 y at ≤−20°C (20–22), and the sample can be subjected to ≤3 freeze-thawing cycles without negatively affecting the biomarker concentrations (20). For optimal long-term storage, serum should be stored at ≤−40°C. Data from our laboratory show that serum iron, SF, and sTfR were stable for ≥10 y when stored at −70°C. Although the preference for a particular anticoagulant in plasma and the influence of hemolysis and other preanalytical factors (e.g., bilirubin and lipemia) on the test result may be test specific, serum is usually the preferred matrix over plasma, and iron measurement is generally more affected by hemolysis than SF and sTfR. For whole blood–based iron status indicators, fresh whole blood needs to be analyzed directly for hemoglobin or refrigerated for 1–2 d before analysis (23), and refrigerated whole blood is stable for a week if protected from UV light for analysis of EP (4).
Laboratory methods
The measurement of hematologic indicators in general and hemoglobin in particular is widely available in clinical and research laboratories by flow cytometry on fully automated cell counters. These instruments can count different blood cells with good precision and high sample throughput. Battery-operated, hand-held hemoglobinometers can provide on-the-spot results from a single drop of blood collected in the field. However, these instruments require freshly collected blood, and if capillary blood is used, the proper sampling technique is critical to obtaining a valid specimen.
The measurement of serum-based biochemical indicators is generally carried out on fully-automated clinical analyzers available in many laboratories. Protein-based indicators such as SF, transferrin, and sTfR are measured by immunoassays, whereas serum iron, TIBC, and UIBC are measured on chemistry analyzers by using a colorimetric reaction with ferrine or ferrozine as a chromogen to form a color complex with iron. Most clinical analyzers measure UIBC because it is more easily automated than TIBC. The measurement precision of UIBC is good at high concentrations as found in iron depletion but worse at low concentrations in the presence of iron overload (24). Outside the United States, testing for UIBC and TIBC has been mostly replaced by transferrin. The precision of other biochemical indicators is good, with between-run CVs generally ≤5%.
Using clinical analyzers offers advantages, such as high sample throughput and quick turnaround time with minimum operator involvement, the availability of commercial kits for various instrument platforms, relatively low reagent cost (with the exception of sTfR), and generally good precision. Possibly the biggest caution for using commercial kits on clinical analyzers is that the user has no control over reagent lot-to-lot variation and assay reformulations. This is especially critical when the assay is used in longitudinal studies, such as national nutrition surveys, because small assay shifts could be misinterpreted as changes in population status. Another disadvantage of clinical analyzers is that the required sample volume is typically ≥150 μL, which may be problematic for pediatric or capillary samples. Lastly, clinical analyzers are moderately expensive and require regular maintenance and periodic technical service. Other less common analytic techniques to measure iron status indicators are summarized in Supplemental Table 2.
Standardization of iron status indicators
International reference materials are available for most iron status indicators from the National Institute of Standards and Technology [for iron in the form of an iron wire (SRM 937) or an iron standard solution (SRM 3126A)], the WHO through the United Kingdom National Institute of Biological Standards and Control [for hemoglobin (IS 98/708), SF (RM 94/572), and sTfR (RR 07/202)], or the European Institute for Reference Materials and Measurements [for transferrin (ERM-DA470)]. The National Institute of Standards and Technology reference materials provide certified values assigned by the use of high-order reference methods. However, physicochemical reference methods are not available for the protein-based iron status indicators to establish “true” concentrations, thus the value assignment has been done through consensus after analysis by common clinical assays (SF, transferrin) or after determining the protein content in the reference material (sTfR). As a result, moderate (SF, transferrin) to large (sTfR) assay differences, as discussed above, can be observed in proficiency testing programs and have been documented in other studies (25, 26). The difficulties in standardizing immunoassays have been reviewed (27) and include the heterogeneity of the antibodies used resulting in different epitope specificities, technical difficulties in producing a reference material that is identical to the circulating serum form, and difficulties in measuring intact proteins by mass spectrometry, including the absence of stable-isotopically labeled proteins that can be used as internal standards.
RATIO OF sTfR TO SF AS INDICATOR OF IRON STATUS
The ratio of sTfR to SF deserves a separate discussion. This indicator is currently reported as part of NHANES but is less well known. There are several ways to express the ratio, and confusion exists as to how it is calculated, what it means, and what laboratory data can be used to calculate and interpret the ratio (Table 4). SF is a sensitive indicator until body iron stores (mainly in hepatocytes) are depleted, but concentrations <12 μg/L are not indicative of the severity of the ID. On the other hand, serum sTfR is a sensitive indicator after body iron stores are depleted and concentrations keep increasing with increasing ID. Together, these 2 indicators assess the full range of iron status from severe deficiency to overload.
TABLE 4.
Approaches to express and interpret the ratio of sTfR to SF1
| Ratio | Calculation | sTfR assay used to establish ratio | Cutoff value and definition |
| Total body iron,2 mg/kg | − [log(sTfR/SF) − 2.28229]/0.1207 | In-house ELISA that is equivalent to Ramco assay and has known relation to Roche assay | ≤0: iron deficit>0: iron surplus |
| Simple ratio2 | sTfR/SF | In-house ELISA that is equivalent to Ramco assay and has known relation to Roche assay | ≤500: ample iron stores>500: depleted iron stores |
| sTfR index, mg/L | sTfR/log SF | First ELISA from R&D Systems; then adopted on Access Beckman Coulter analyzer | <1: ACD>2: IDA or IDA + ACD |
ACD, anemia of chronic disease; ELISA, enzyme linked immnosorbent assay; IDA, iron deficiency anemia; IDA + ACD, combined iron deficiency anemia and anemia of chronic disease; SF, serum ferritin; sTfR, soluble transferrin receptor.
Units of both sTfR and SF in the ratio are μg/L.
The logarithm of the ratio of sTfR to SF concentrations is linearly related to total body iron stores (TBI) expressed as mg/kg body weight, as shown in a unique phlebotomy study conducted in 14 healthy adult Caucasians (28). The sTfR assay used in that study was an in-house ELISA assay (29), shown to perform equivalently to the Ramco sTfR assay (30). The Ramco assay was shown to measure ∼50% higher than the Roche sTfR assay (30).
TBI is one of several terms found in the literature for this indicator; body iron is the original term used by the investigators who developed this methodology (28), but some reports also used body iron stores or total-body iron. Regardless of the term used, it is important to understand that TBI is not a measure of the quantity of iron in the individual’s body. It merely provides a quantitative estimate of the size of the body iron store when iron is present in the store (values >0 mg/kg) or the size of the functional deficit that would need to be corrected before iron could again be accumulated in the store in an individual who is iron deficient (values ≤0 mg/kg). It is expressed as a continuous variable that is conceptually easy to interpret and indicates the severity of the iron deficit at the low end of the spectrum and the magnitude of the iron surplus at the high end of the spectrum. Some investigators have proposed that the term “body iron index” might be more appropriate. The formula for this relation was validated by using data from 3 published studies (31): a nonrepresentative subset of healthy adult Caucasians participating in NHANES III (1988–1994) (31, 32), pregnant Jamaican women participating in an iron supplementation trial (33), and anemic Vietnamese women participating in an intervention trial with iron-fortified fish sauce (34). TBI has been used in a study that used an in-house multiplex ELISA sTfR assay believed to be equivalent to the Ramco assay (35) and in several NHANES data analyses that used the Roche sTfR assay (36–38).
Alternatively, the utility of the simple ratio of sTfR to SF concentrations (both expressed in μg/L) was confirmed in an iron supplementation trial in pregnant women in Jamaica, where the ratio of the supplemented group was significantly different from that in the nonsupplemented group (mean values of 470 compared with 1200, respectively) (33). However, because of large differences in sTfR assays, the cutoff for the simple ratio (≤500 ample iron stores, >500 depleted iron stores) is assay dependent and can only be used with data generated by an assay that performs equivalently to the Ramco or Roche assay. The simple ratio has been used by several investigators who used the Ramco sTfR assay (39–41) or an assay believed to be equivalent to the Ramco assay (42).
Finally, the sTfR index, calculated as the ratio of sTfR to the logarithm of SF, was introduced as an indicator to identify persons with depleted iron stores (43) and cutoff values (expressed in mg/L) were published to distinguish between ACD (<1) and IDA (>2) or both conditions (>2) (7). The sTfR index was adopted on the Access Beckman Coulter analyzer for differential diagnosis of IDA and ACD (44). The interpretation of the sTfR index is also assay dependent. One study that used the Roche sTfR assay (45) calculated the sTfR index, which is not appropriate because the Roche assay does not produce results comparable to the Beckman sTfR assay.
EMERGING IRON STATUS INDICATORS
Hepcidin, NTBI, and reticulocyte indexes are currently viewed as experimental indicators used mainly in the research setting. Hepcidin, the central regulator of iron homeostasis, increases with increasing iron status (46, 47). Hepcidin may be clinically useful in the diagnosis of some types of anemia, in the differentiation between IDA and ACD, and in patients with iron overload syndromes (48, 49). Because hepcidin is an important determinant of dietary iron absorption, it may guide safe iron supplementation in countries with a high infection burden (46). Its role in determining whether iron status is replete is unclear. Hepcidin is an acute-phase reactant (50), is suppressed in the presence of increased erythropoietic activity unrelated to iron status (51), and has a large (49%) intraindividual variability (52). Hepcidin can be measured with good reproducibility in serum or urine by mass spectrometry assays or immunoassays, but there are considerable assay differences and no standardization yet (53). A recent interlaboratory comparison study identified a commutable secondary reference material, which if used as a common calibrator could harmonize assay results to an achievable equivalence of 7.7% from the current 28.6% (53).
NTBI has a postulated role in the pathogenesis of iron toxicity due to oxidative damage and the risk of increased susceptibility to infectious diseases such as malaria (54). NTBI appears in the circulation of patients with iron overload or for a short time after the administration of an iron dose and may thus be of interest to distinguish between iron repletion and iron excess. The fraction of plasma NTBI that is redox active and can be chelated is designated labile plasma iron (55). The measurement of serum NTBI is fraught with technical difficulties related to the determination of heterogeneous chemical forms of circulating NTBI, as demonstrated in an international round robin by considerable method differences (40-fold variation) and large analytic variation (4.4–193%) (56). A second international round robin of current leading analytic assays for NTBI and labile plasma iron indicated good assay reproducibility but still relatively poor correlation and agreement between assays (57). Recently, a new NTBI assay system utilizing a conventional automated analyzer was reported to have good linearity, reproducibility, and comparability with HPLC (58).
Reticulocyte indexes are sensitive indicators for iron-deficient erythropoiesis (59). For example, reticulocyte hemoglobin content is useful in assessing the functional iron available for erythropoiesis during the previous 3–4 d, whereas reticulocyte volume is a useful indicator when monitoring the therapeutic response of anemias. Automated flow-cytometric analysis provides acceptable precision and bias, yet method-specific reference intervals have to be used currently (59). Standardization of these measurements is encouraged. Preanalytical variation (related to specimen transportation and storage) represents the major source of inaccurate test results. The high sample-related uncertainty requires that reticulocytes be analyzed without delay. Reticulocyte indexes have been included in the American Academy of Pediatrics guidelines for the evaluation of childhood anemia (60) and have been used in patients with chronic kidney disease to manage iron status and predict the responsiveness to intravenous iron and erythropoiesis stimulating agents (61).
IRON STATUS ASSESSMENT IN NHANES
Monitoring the iron status of the US population has been an important component of the NHANES since its inception in 1971. All NHANES surveys have included a battery of hematologic and biochemical indicators to provide the best possible assessment (Supplemental Table 3) (62, 63). A matrix describing the laboratory methods by indicator and survey cycle is shown in Supplemental Table 4. Details for each method can be found on the NHANES website (64).
Three models that used multiple iron status indicators were developed by an expert committee to assess the iron status of the US population by using data from NHANES II (1976–1980) (65). One of these models, called the ferritin model, has been used with data from several subsequent NHANES surveys conducted between 1988 and 2006 (32, 66, 67). The other 2 models (known as the mean cell volume model and hemoglobin shift model) have been discussed elsewhere (65). The ferritin model uses SF, TSAT, and EP. To meet the definition of ID by using the ferritin model, an individual had to have abnormal values for ≥2 indicators. Because the model included SF, it was believed to be more likely to capture the early stages of ID, including iron depletion (65).
Cutoffs to define abnormal values for the indicators used in the ferritin model were validated in clinically diagnosed patients whenever possible. This type of data was not available for children and adolescents, so cutoffs for these age groups were selected by identifying a value that kept the percentage of falsely identified individuals similar to that of women ages 20–44 y. Several types of studies provided data to validate the ferritin model for defining ID: 1) comparison of anemia rates among individuals with abnormal values for <2 compared with ≥2 indicators used in the ferritin model (65, 68–70), 2) assessment of whether the prevalence of ID (as defined by the ferritin model) followed expected patterns by selected demographic characteristics (65), 3) examination of whether the prevalence of ID by the ferritin model changed in response to oral iron treatment (71), 4) comparison of the ferritin model prevalence with the prevalence of iron depletion by estimated stores or bone marrow staining (72, 73), and 5) examination of whether including additional indicators in a regression model increased the predictive ability of the model above that observed for SF concentrations alone (74). One issue that complicates the interpretation of the preceding bone marrow studies is the use of different endpoints that may address slightly different stages in the development of ID. In specific, the ferritin model may assess a slightly later stage of ID than the presence or absence of bone marrow iron stores (the model includes TSAT and EP and thus detects iron-deficient erythropoiesis in addition to iron store depletion) and may therefore be expected to produce a lower prevalence. This is supported by findings from Cook et al. (72) that noted a similar prevalence between the ferritin model and estimated body iron stores of −100 mg/kg.
In the early 2000s interest grew to consider a simpler approach to define iron status in NHANES. Assays for some of the indicators used in the ferritin model were labor intensive and no longer widely used, and it was becoming increasingly difficult to find laboratories that were willing to continue performing them. A simpler approach involving fewer iron status indicators may also reduce survey complexity and cost. Around that time, the validation of the TBI model was published (31), a WHO recommendation suggesting the use of SF and sTfR to assess the iron status of populations became available (75), and the fully automated Roche sTfR assay that provided higher sample throughput and better precision than the Ramco sTfR assay was released (30). In 2005, the CDC convened a workshop with experts in iron metabolism to discuss whether the ferritin model could be replaced with the TBI model starting with NHANES 2003–2004 and what the most appropriate approach to assess iron status in pregnant women would be. The advantages of using a simpler model had to be weighed against the possible effect on the ability to assess changes in iron status over time if the ferritin model was replaced. An important aspect of assessing iron status of populations is to ensure coverage of all high-risk groups. Before 2003, iron status indicators were measured in the entire population. From 2003 on, NHANES concentrated on assessing iron status in children ages 1–5 y and women of reproductive age (12–49 y). Pregnant women were oversampled during NHANES 1999–2006, which provided a sufficient sample size to calculate baseline estimates for an objective on ID in pregnant women for Healthy People 2010, the nation’s prevention agenda (76).
One of the strengths of the TBI model was the derivation from the direct calculation of body iron stores from serial phlebotomy (25), a “gold standard” method for assessment of TBI. Like the ferritin model, the TBI model has also been indirectly validated by 1) assessment of whether the prevalence of ID as estimated by the model followed expected patterns by selected demographic characteristics, such as age and sex (31, 72, 77), 2) examination of the changes in body iron stores and the prevalence of low body iron stores in response to oral iron treatment (31), and 3) comparison of SF, sTfR, or the ratio of the 2 with absent stainable bone marrow iron in anemic patients (43, 78). Although the third group of studies provided some information, it cannot be seen as a direct validation because all the patients were anemic and the indicators (SF and sTfR) were not combined in the same mathematical manner and may represent a different construct.
There was limited evidence that the TBI model may be valid for pregnant women: the pattern of body iron stores in pregnant Jamaican and Bolivian women followed expected patterns (33, 77); unpublished data for US women in the third trimester of pregnancy presented at the workshop also supported its utility for this group.
The expert panel proposed that the ferritin model be replaced with the TBI model for assessing iron status in the population, including pregnant women. To permit secular trend analyses for Healthy People 2010, a gradual switch to the new model, with an overlap period during which both models can be applied (2003–2006), was recommended. The panel recommended that trimester-specific estimates be provided for pregnant women, regardless of which method was used to define their iron status. Table 5 provides advantages and disadvantages for these 2 models as well as a list of selected NHANES publications that used these 2 models.
TABLE 5.
Characteristics and selected NHANES publications for the serum ferritin and total body iron model
| Ferritin model | Total body iron model |
| Model characteristics | |
| Does not indicate degree of severity of iron deficiency | Assesses entire range of iron status |
| May assess slightly later stage of iron deficiency than the presence or absence of bone marrow iron stores (i.e., lower prevalence than serum ferritin alone) | Derived from direct calculation of body iron from serial phlebotomy, a “gold standard” method to assess body iron |
| May detect iron deficient erythropoiesis in addition to iron store depletion | Better predicts the absence of bone marrow iron than serum ferritin alone |
| Produces categorical yes/no estimate only | May provide a better estimate of the impact of iron intervention (amount of iron absorbed) |
| Total body iron can be analyzed as continuous variable | |
| Selected NHANES publications | |
| NHANES III (1988–1994) prevalence of iron deficiency in persons ≥1 y of age (32) | NHANES 2003–2006 iron deficiency in children aged 12–24 mo and nonpregnant women aged 12–49 y of age (36) |
| NHANES 1999–2000 iron deficiency in persons ≥1 y of age (66) | NHANES 1999–2006 iron status in pregnant women 15–39 y of age (37) |
| NHANES III (1988–1994) vs. 1999–2002 anemia prevalence among children aged 12–59 mo and women aged 20–49 y (67) | NHANES 2007–2010 iron, anemia, and iron deficiency anemia among children 1–5 y of age (38) |
SUMMARY AND RESEARCH NEEDS FOR IRON STATUS INDICATORS
The laboratory assessment of iron status relies on a combination of biochemical indicators. Although it has been pointed out that there is a need to link these iron indicators better to meaningful clinical outcomes (79), such efforts must be underpinned by a sound understanding of the indicators available. Diagnosis of ID through SF concentration is complicated by concomitant inflammation, as is the case with ACD. Lack of assay standardization for sTfR, an indicator of functional ID less affected by inflammation, results in a lack of common reference ranges and cutoffs. It is unclear which indicators are best suited to assess adequate or excess iron status. Whereas laboratory methods for commonly used iron indicators are widely available and provide good precision, the comparability of results across assay platforms could be further improved for SF and require harmonization for sTfR to become a more widely used indicator. High-order reference methods and certified reference materials are needed for iron status indicators. At the same time, the availability of simple yet reliable assays that can multiplex the measurement of several indicators using minimal specimen volume would be beneficial for pediatric and capillary samples. The utility of newer iron status indicators, such as hepcidin and NTBI, is being explored mainly in the research setting, but laboratory methods for these indicators require further improvements in terms of comparability and for NTBI also in terms of the definition of the clinically most relevant forms of NTBI.
Acknowledgments
The authors’ responsibilities were as follows—CMP: developed the materials for the workshop presentation and wrote the initial draft manuscript with critical input from ACL regarding NHANES measurements of iron status, and had primary responsibility for all content; and both authors: read and approved the final manuscript. Neither of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ACD, anemia of chronic disease; EP, erythrocyte protoporphyrin; ID, iron deficiency; IDA, iron deficiency anemia; NTBI, non–transferrin-bound iron; RBC, red blood cell; SF, serum ferritin; sTfR, soluble transferrin receptor; TBI, total body iron stores; TIBC, total iron-binding capacity; TSAT, transferrin saturation; UIBC, unsaturated iron-binding capacity; ZPP, zinc protoporphyrin.
REFERENCES
- 1.Raiten DJ, Namaste S, Brain B, Combs G Jr., L’Abbe MR, Wasantwisut E, Darnton-Hill I. Executive summary–biomarkers of nutrition for development: building a consensus. Am J Clin Nutr 2011;94:633S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hastka J, Lasserre JJ, Schwarzbeck A, Reiter A, Hehlmann R. Laboratory tests of iron status: correlation or common sense? Clin Chem 1996;42:718–24. [PubMed] [Google Scholar]
- 3.Worwood M. Indicators of the iron status of populations: ferritin. In: WHO, editor. Assessing the iron status of populations: including literature reviews: report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, Geneva, Switzerland, 6–8 April 2004. 2nd ed. Geneva (Switzerland): WHO; 2007.
- 4.Beard J. Indicators of the iron status of populations: free erythrocyte protoporphyrin and zinc protoporphyrin; serum and plasma iron, total iron binding capacity and transferrin saturation; and serum transferrin receptor. In: WHO, editor. Assessing the iron status of populations: including literature reviews: report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, Geneva, Switzerland, 6–8 April 2004. 2nd ed. Geneva (Switzerland): WHO; 2007.
- 5.Lynch S. Indicators of the iron status of populations: red blood cell parameters. In: WHO, editor. Assessing the iron status of populations: including literature reviews: report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, Geneva, Switzerland, 6–8 April 2004. 2nd ed. Geneva (Switzerland): WHO; 2007.
- 6.Raiten DJ, Sakr Ashour FA, Ross AC, Meydani SN, Dawson HD, Stephensen CB, Brabin BJ, Suchdev PS, van Ommen B. Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). J Nutr 2015;145:1039S–108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005;352:1011–23. [DOI] [PubMed] [Google Scholar]
- 8.European Association for the Study of the Liver. EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol 2010;53:3–22. [DOI] [PubMed] [Google Scholar]
- 9.Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011;54:328–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams PC, Kertesz AE, McLaren CE, Barr R, Bamford A, Chakrabarti S. Population screening for hemochromatosis: a comparison of unbound iron-binding capacity, transferrin saturation, and C282Y genotyping in 5,211 voluntary blood donors. Hepatology 2000;31:1160–4. [DOI] [PubMed] [Google Scholar]
- 11.Adams PC, Reboussin DM, Press RD, Barton JC, Acton RT, Moses GC, Leiendecker-Foster C, McLaren GD, Dawkins FW, Gordeuk VR, et al. . Biological variability of transferrin saturation and unsaturated iron-binding capacity. Am J Med 2007;120:999.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metzgeroth G, Schultheis B, Dorn-Beineke A, Hehlmann R, Hastka J. Zinc protoporphyrin, a useful parameter to address hyperferritinemia. Ann Hematol 2007;86:363–8. [DOI] [PubMed] [Google Scholar]
- 13.Ahluwalia N, Lammi-Keefe CJ, Haley NR, Beard JL. Day-to-day variation in iron-status indexes in elderly women. Am J Clin Nutr 1993;57:414–9. [DOI] [PubMed] [Google Scholar]
- 14.Lacher DA, Hughes JP, Carroll MD. Biological variation of laboratory analytes based on the 1999-2002 National Health and Nutrition Examination Survey. Natl Health Stat Rep 2010:1–7. [PubMed] [Google Scholar]
- 15.Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, Minchinela J, Perich C, Simón M. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest 1999;59:491–500. [DOI] [PubMed] [Google Scholar]
- 16.Cooper MJ, Zlotkin SH. Day-to-day variation of transferrin receptor and ferritin in healthy men and women. Am J Clin Nutr 1996;64:738–42. [DOI] [PubMed] [Google Scholar]
- 17.Dale JC, Burritt MF, Zinsmeister AR. Diurnal variation of serum iron, iron-binding capacity, transferrin saturation, and ferritin levels. Am J Clin Pathol 2002;117:802–8. [DOI] [PubMed] [Google Scholar]
- 18.Pearce CJ, Bosomworth MP. Storage of heparinized blood. Ann Clin Biochem 1991;28:112. [DOI] [PubMed] [Google Scholar]
- 19.van Eijsden M, van der Wal MF, Hornstra G, Bonsel GJ. Can whole-blood samples be stored over 24 hours without compromising stability of C-reactive protein, retinol, ferritin, folic acid, and fatty acids in epidemiologic research? Clin Chem 2005;51:230–2. [DOI] [PubMed] [Google Scholar]
- 20.Drammeh BS, Schleicher RL, Pfeiffer CM, Jain RB, Zhang M, Nguyen PH. Effects of delayed sample processing and freezing on serum concentrations of selected nutritional indicators. Clin Chem 2008;54:1883–91. [DOI] [PubMed] [Google Scholar]
- 21.Wilding P, Zilva JF, Wilde CE. Transport of specimens for clinical chemistry analysis. Ann Clin Biochem 1977;14:301–6. [DOI] [PubMed] [Google Scholar]
- 22.Jansen EH, Beekhof PK, Schenk E. Long-term stability of biomarkers of the iron status in human serum and plasma. Biomarkers 2013;18:365–8. [DOI] [PubMed] [Google Scholar]
- 23.Whitehead RD Jr., Zhang M, Sternberg MR, Schleicher RL, Drammeh B, Mapango C, Pfeiffer CM. Effects of preanalytical factors on hemoglobin measurement: a comparison of two HemoCue point-of-care analyzers. Clin Biochem 2017;50:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanck HM, Pfeiffer CM, Caudill SP, Reyes M, Gunter EW, Imperatore G, van Assendelft OW, Strider S, Dearth T. Serum iron and iron-binding capacity: a round-robin interlaboratory comparison study. Clin Chem 2003;49:1672–5. [DOI] [PubMed] [Google Scholar]
- 25.Blackmore S, Hamilton M, Lee A, Worwood M, Brierley M, Heath A, Thorpe SJ. Automated immunoassay methods for ferritin: recovery studies to assess traceability to an international standard. Clin Chem Lab Med 2008;46:1450–7. [DOI] [PubMed] [Google Scholar]
- 26.Thorpe SJ, Heath A, Sharp G, Cook J, Ellis R, Worwood M. A WHO reference reagent for the Serum Transferrin Receptor (sTfR): international collaborative study to evaluate a recombinant soluble transferrin receptor preparation. Clin Chem Lab Med 2010;48:815–20. [DOI] [PubMed] [Google Scholar]
- 27.Thorpe SJ. The development and role of international biological reference materials in the diagnosis of anaemia. Biologicals 2010;38:449–58. [DOI] [PubMed] [Google Scholar]
- 28.Skikne BS, Flowers CH, Cook JD. Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood 1990;75:1870–6. [PubMed] [Google Scholar]
- 29.Flowers CH, Skikne BS, Covell AM, Cook JD. The clinical measurement of serum transferrin receptor. J Lab Clin Med 1989;114:368–77. [PubMed] [Google Scholar]
- 30.Pfeiffer CM, Cook JD, Mei Z, Cogswell ME, Looker AC, Lacher DA. Evaluation of an automated soluble transferrin receptor (sTfR) assay on the Roche Hitachi analyzer and its comparison to two ELISA assays. Clin Chim Acta 2007;382:112–6. [DOI] [PubMed] [Google Scholar]
- 31.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101:3359–64. [DOI] [PubMed] [Google Scholar]
- 32.Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA 1997;277:973–6. [DOI] [PubMed] [Google Scholar]
- 33.Simmons WK, Cook JD, Bingham KC, Thomas M, Jackson J, Jackson M, Ahluwalia N, Kahn SG, Patterson AW. Evaluation of a gastric delivery system for iron supplementation in pregnancy. Am J Clin Nutr 1993;58:622–6. [DOI] [PubMed] [Google Scholar]
- 34.Thuy PV, Berger J, Davidsson L, Khan NC, Lam NT, Cook JD, Hurrell RF, Khoi HH. Regular consumption of NaFeEDTA-fortified fish sauce improves iron status and reduces the prevalence of anemia in anemic Vietnamese women. Am J Clin Nutr 2003;78:284–90. [DOI] [PubMed] [Google Scholar]
- 35.Engle-Stone R, Nankap M, Ndjebayi AO, Erhardt JG, Brown KH. Plasma ferritin and soluble transferrin receptor concentrations and body iron stores identify similar risk factors for iron deficiency but result in different estimates of the national prevalence of iron deficiency and iron-deficiency anemia among women and children in Cameroon. J Nutr 2013;143:369–77. [DOI] [PubMed] [Google Scholar]
- 36.Cogswell ME, Looker AC, Pfeiffer CM, Cook JD, Lacher DA, Beard JL, Lynch SR, Grummer-Strawn LM. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003-2006. Am J Clin Nutr 2009;89:1334–42. [DOI] [PubMed] [Google Scholar]
- 37.Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, Grummer-Strawn LM. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am J Clin Nutr 2011;93:1312–20. [DOI] [PubMed] [Google Scholar]
- 38.Gupta PM, Perrine CG, Mei Z, Scanlon KS. Iron, anemia, and iron deficiency anemia among young children in the United States. Nutrients 2016:8:E330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ronnenberg AG, Wood RJ, Wang X, Xing H, Chen C, Chen D, Guang W, Huang A, Wang L, Xu X. Preconception hemoglobin and ferritin concentrations are associated with pregnancy outcome in a prospective cohort of Chinese women. J Nutr 2004;134:2586–91. [DOI] [PubMed] [Google Scholar]
- 40.Akesson A, Bjellerup P, Berglund M, Bremme K, Vahter M. Soluble transferrin receptor: longitudinal assessment from pregnancy to postlactation. Obstet Gynecol 2002;99:260–6. [DOI] [PubMed] [Google Scholar]
- 41.van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: which measurements are valid? Br J Haematol 1998;103:817–24. [DOI] [PubMed] [Google Scholar]
- 42.Grant FK, Martorell R, Flores-Ayala R, Cole CR, Ruth LJ, Ramakrishnan U, Suchdev PS. Comparison of indicators of iron deficiency in Kenyan children. Am J Clin Nutr 2012;95:1231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 1997;89:1052–7. [PubMed] [Google Scholar]
- 44.Skikne BS, Punnonen K, Caldron PH, Bennett MT, Rehu M, Gasior GH, Chamberlin JS, Sullivan LA, Bray KR, Southwick PC. Improved differential diagnosis of anemia of chronic disease and iron deficiency anemia: a prospective multicenter evaluation of soluble transferrin receptor and the sTfR/log ferritin index. Am J Hematol 2011;86:923–7. [DOI] [PubMed] [Google Scholar]
- 45.Berlin T, Meyer A, Rotman-Pikielny P, Natur A, Levy Y. Soluble transferrin receptor as a diagnostic laboratory test for detection of iron deficiency anemia in acute illness of hospitalized patients. Isr Med Assoc J 2011;13:96–8. [PubMed] [Google Scholar]
- 46.Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood 2016;127:2809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011;117:4425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donker AE, Raymakers RA, Vlasveld LT, van Barneveld T, Terink R, Dors N, Brons PP, Knoers NV, Swinkels DW. Practice guidelines for the diagnosis and management of microcytic anemias due to genetic disorders of iron metabolism or heme synthesis. Blood 2014;123:3873–86. [DOI] [PubMed] [Google Scholar]
- 49.Kroot JJ, Tjalsma H, Fleming RE, Swinkels DW. Hepcidin in human iron disorders: diagnostic implications. Clin Chem 2011;57:1650–69. [DOI] [PubMed] [Google Scholar]
- 50.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 2003;101:2461–3. [DOI] [PubMed] [Google Scholar]
- 51.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood 2006;108:3730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy AT, Witcher DR, Luan P, Wroblewski VJ. Quantitation of hepcidin from human and mouse serum using liquid chromatography tandem mass spectrometry. Blood 2007;110:1048–54. [DOI] [PubMed] [Google Scholar]
- 53.van der Vorm LN, Hendriks JC, Laarakkers CM, Klaver S, Armitage AE, Bamberg A, Geurts-Moespot AJ, Girelli D, Herkert M, Itkonen O, et al. . Toward worldwide hepcidin assay harmonization: identification of a commutable secondary reference material. Clin Chem 2016;62:993–1001. [DOI] [PubMed] [Google Scholar]
- 54.Brissot P, Ropert M, Le Lan C, Loréal O.. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta 2012;1820:403–10. [DOI] [PubMed] [Google Scholar]
- 55.Cabantchik ZI. Labile iron in cells and body fluids: physiology, pathology, and pharmacology. Front Pharmacol 2014;5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobs EM, Hendriks JC, van Tits BL, Evans PJ, Breuer W, Liu DY, Jansen EH, Jauhiainen K, Sturm B, Porter JB, et al. . Results of an international round robin for the quantification of serum non-transferrin-bound iron: need for defining standardization and a clinically relevant isoform. Anal Biochem 2005;341:241–50. [DOI] [PubMed] [Google Scholar]
- 57.de Swart L, Hendriks JC, van der Vorm LN, Cabantchik ZI, Evans PJ, Hod EA, Brittenham GM, Furman Y, Wojczyk B, Janssen MC, et al. . Second international round robin for the quantification of serum non-transferrin-bound iron and labile plasma iron in patients with iron-overload disorders. Haematologica 2016;101:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito S, Ikuta K, Kato D, Shibusa K, Niizeki N, Tanaka H, Addo L, Toki Y, Hatayama M, Inamura J, et al. . Non-transferrin-bound iron assay system utilizing a conventional automated analyzer. Clin Chim Acta 2014;437:129–35. [DOI] [PubMed] [Google Scholar]
- 59.Piva E, Brugnara C, Spolaore F, Plebani M. Clinical utility of reticulocyte parameters. Clin Lab Med 2015;35:133–63. [DOI] [PubMed] [Google Scholar]
- 60.Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics 2010;126:1040–50. [DOI] [PubMed] [Google Scholar]
- 61.Ratcliffe LE, Thomas W, Glen J, Padhi S, Pordes BA, Wonderling D, Connell R, Stephens S, Mikhail AI, Fogarty DG, et al. . Diagnosis and management of iron deficiency in CKD: a summary of the NICE guideline recommendations and their rationale. Am J Kidney Dis 2016;67:548–58. [DOI] [PubMed] [Google Scholar]
- 62.Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C. Hematological and iron-related analytes–reference data for persons aged 1 year and over: United States, 1988-94. Vital Health Stat 11 2005:1–156. [PubMed] [Google Scholar]
- 63.CDC, National Center for Environmental Health. Second national report on biochemical indicators of diet and nutrition in the US population [Internet]. c2012. [cited 2016 Jun 23]. Available from: http://www.cdc.gov/nutritionreport/.
- 64.CDC , National Center for Health Statistics. National Health and Nutrition Examination Survey. 2009-2010 Lab methods [Internet]. [cited 2016 Oct 5]. Available from: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2009.
- 65.Summary of a report on assessment of the iron nutritional status of the United States population. Expert Scientific Working Group. Am J Clin Nutr 1985;42:1318–30. [DOI] [PubMed] [Google Scholar]
- 66.CDC. Iron deficiency–United States, 1999-2000. MMWR Morb Mortal Wkly Rep 2002;51:897–9. [PubMed] [Google Scholar]
- 67.Cusick SE, Mei Z, Freedman DS, Looker AC, Ogden CL, Gunter E, Cogswell ME. Unexplained decline in the prevalence of anemia among US children and women between 1988-1994 and 1999-2002. Am J Clin Nutr 2008;88:1611–7. [DOI] [PubMed] [Google Scholar]
- 68.Finch CA, Smith NJ, Cook JD, Labbe RF, Lipschitz DA. Laboratory parameters in the diagnosis of iron deficiency. Haematologia (Budap) 1974;8:177–82. [PubMed] [Google Scholar]
- 69.Cook JD, Finch CA, Smith NJ. Evaluation of the iron status of a population. Blood 1976;48:449–55. [PubMed] [Google Scholar]
- 70.Derman DP, Lynch SR, Bothwell TH, Charlton RW, Torrance JD, Brink BA. Serum ferritin as an index of iron nutrition in rural and urban South African children. Br J Nutr 1978;39:383–9. [DOI] [PubMed] [Google Scholar]
- 71.Berger J, Dyck JL, Galan P, Aplogan A, Schneider D, Traissac P, Hercberg S. Effect of daily iron supplementation on iron status, cell-mediated immunity, and incidence of infections in 6-36 month old Togolese children. Eur J Clin Nutr 2000;54:29–35. [DOI] [PubMed] [Google Scholar]
- 72.Cook JD, Skikne BS, Lynch SR, Reusser ME. Estimates of iron sufficiency in the US population. Blood 1986;68:726–31. [PubMed] [Google Scholar]
- 73.Hallberg L, Bengtsson C, Lapidus L, Lindstedt G, Lundberg PA, Hulten L. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. Br J Haematol 1993;85:787–98. [DOI] [PubMed] [Google Scholar]
- 74.Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med 1992;7:145–53. [DOI] [PubMed] [Google Scholar]
- 75.WHO, CDC. Assessing the iron status of populations: including literature reviews. Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, Geneva, Switzerland, 6–8 April 2004. 2nd ed. Geneva (Switzerland): WHO/CDC; 2007. [Google Scholar]
- 76.US Department of Health and Human Services. Healthy People 2010: understanding and improving health. 2nd ed. Washington (DC): US Department of Health and Human Services; 2000. [Google Scholar]
- 77.Cook JD, Boy E, Flowers C, Daroca Mdel C. The influence of high-altitude living on body iron. Blood 2005;106:1441–6. [DOI] [PubMed] [Google Scholar]
- 78.Means RT Jr., Allen J, Sears DA, Schuster SJ. Serum soluble transferrin receptor and the prediction of marrow aspirate iron results in a heterogeneous group of patients. Clin Lab Haematol 1999;21:161–7. [DOI] [PubMed] [Google Scholar]
- 79.USPSTF. Iron deficiency anemia in pregnant women: screening and supplementation [Internet]. c2015 [cited 2016 Oct 4]. Available from: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/iron-deficiency-anemia-in-pregnant-women-screening-and-supplementation.