Abstract
Inflammation has a major impact on iron homeostasis. This review focuses on acute and chronic inflammation as it affects iron trafficking and, as a result, the availability of this essential micronutrient to the host. In situations of microbial infection, not only the host is affected but also the offending microorganisms, which, in general, not only require iron for their own growth but have evolved mechanisms to obtain it from the infected host. Key players in mammalian iron trafficking include several types of cells important to iron acquisition, homeostasis, and hematopoiesis (enterocytes, hepatocytes, macrophages, hematopoietic cells, and in the case of pregnancy, placental syncytiotrophoblast cells) and several forms of chaperone proteins, including, for nonheme iron, the transport protein transferrin and the intracellular iron-storage protein ferritin, and for heme iron, the chaperone proteins haptoglobin and hemopexin. Additional key players are the cell membrane–associated iron transporters, particularly ferroportin (FPN), the only protein known to modulate iron export from cells, and finally, the iron-regulatory hormone hepcidin, which, in addition to having antibacterial activity, regulates the functions of FPN. Interestingly, the impact of infection on iron homeostasis differs among pathogens whose mode of infection is mainly intracellular or extracellular. Understanding how inflammation affects each of these processes may be crucial for understanding how inflammation affects iron status, indicators of iron sufficiency, and iron supplementation during inflammation and how it may potentially result in a beneficial or detrimental impact on the host.
Keywords: acute phase response, C-reactive protein, ferroportin, hepatocyte, hepcidin, infancy, intra- and extracellular pathogens, macrophage, pregnancy
INTRODUCTION
Inflammation is now recognized as a significant contributor, even a causal agent, in chronic metabolic diseases. The inflammatory response results in significant modifications to nutrient transport, tissue distribution, and cellular metabolism (1, 2). There is no doubt that inflammation has a significant impact on iron homeostasis; however, the mechanisms are far less clear. Current evidence has provided important clues, namely concerning the importance of the hepcidin-ferroportin axis (3), and the competition that exists between the mammalian host and infectious microbes for iron (4, 5). There are still important challenges, and opportunities, in understanding how inflammation per se and the plasma biomarkers of inflammation used clinically are related to iron homeostasis and its indicators in both iron-sufficient and iron-depleted populations. This review was developed to support discussions that took place during the NIH workshop.
CELLS INVOLVED IN IRON HOMEOSTASIS
Because there is no excretory route, iron homeostasis in organisms is regulated at the level of iron uptake (6). Most iron is absorbed by enterocytes in the upper small intestine (duodenum). Iron is taken up at the apical surface mainly through the mediation of divalent metal-ion transport proteins; this process is considered to be relatively unregulated. Within the intestinal absorptive cell, iron can be trafficked to different subcellular compartments (e.g., to ferritin), but the net absorptive process depends on the export of iron by ferroportin (FPN) (7), a channel protein of the solute carrier family (SLC40A1) on the basolateral membrane through which ferrous iron exits, to be oxidized extracellularly to ferric iron by a ferroxidase, and thus prepared for binding to the high-affinity binding sites of its plasma transport protein, transferrin. The importance of FPN is exemplified by the embryonic lethality of the FPNnull/null knockout mouse (8). More will be said later about the regulation of the process of iron export by hepcidin and the impact of inflammation on the export of intracellular iron. In general, iron supplementation is expected, in a mass action manner, to increase the uptake of iron at the apical surface, and thus the intracellular ferritin-bound iron content; however, just how much iron leaves the enterocyte will depend on the quantity of FPN protein available for iron export. Iron that is not exported from the enterocytes can be expected to be eliminated in sloughed cells (7).
Other cells involved in iron homeostasis include tissue-resident macrophages (9) in the splenic red pulp and liver, which together comprise most of the reticuloendothelial system. Approximately 80% of liver-resident macrophages (Kupffer cells) line the hepatic venous sinusoids (10). Macrophages are crucial to the recycling of iron obtained from catabolism of spent red blood cells (RBCs), and recycling is crucial to maintain a normal rate of RBC formation, and thus for the prevention of anemia (9). Macrophages also store excess iron—for example, in situations of unrestrained iron absorption from the diet, as in hereditary hemochromatosis (11). Moreover, tissue macrophages are, as will be noted later, also reservoirs for several intracellular pathogens and, furthermore, intimately involved in the innate immune response through the production of inflammatory factors. Hematopoietic cells in splenic and bone marrow use iron but may be inadequately supplied in states of inflammation, leading to the anemia of inflammation or anemia of chronic disease (12, 13). Finally, in pregnancy, the syncytiotrophoblast cells of the placenta, which interface between the maternal and fetal circulations, function in iron homeostasis and fetal development by transferring iron derived from maternal transferrin vectorially across the cell, which is released on the fetal side by a similar mechanism as occurs in enterocytes involving FPN; however, in this situation, the exported iron becomes bound after oxidation to ferric iron to fetal transferrin. Maternal-to-fetal iron transfer is greatest in the third trimester of pregnancy, concomitant with the greatest rate of fetal growth (14).
PROTEINS INVOLVED IN IRON HOMEOSTASIS
Free iron is toxic due to its participation in un- or poorly regulated cellular and extracellular redox reactions (15), including the well-known Fenton (Haber-Weiss) reaction in which hydrogen peroxide and ferrous salts generate reactive species (free radicals) capable of oxidizing a wide variety of organic substrates (16). Thus, biological mechanisms to bind and sequester iron are essential for controlling oxidant production and, hence, natural and induced oxidative damage. Nonheme iron is sequestered and transported in plasma by transferrin, which possesses 2 high-affinity binding sites for 1 atom each of ferric iron. Heme iron, in addition to being contained within RBCs in hemoglobin, is bound extracellularly by the plasma protein haptoglobin, whereas “free” heme is bound intracellularly to hemopexin. These proteins function to limit the concentration of free inorganic iron and heme-bound iron within the extracellular space, within cells, or both. Heme has been described as “a double-edged sword” (17). In moderate quantities and bound to protein, it is essential; in large amounts and free, it can become toxic by mediating oxidative stress and inflammation. Heme toxicity underlies much of the pathology of sepsis and several hemolytic disorders (18).
ACUTE AND CHRONIC INFLAMMATION AND ITS IMPACT ON IRON TRAFFICKING
Acute inflammation is part of the body’s natural response to infection or injury and can rightly be considered an adaptive response, as long as it remains within healthy limits. The response generally begins locally and represents a highly evolutionarily conserved program of reactions related to innate immunity, which are relatively “hard wired” (i.e., modifiable but not preventable). The acute response to infection and inflammation is closely related to immune defense, wound healing, and tissue repair. Key features are the recruitment of white blood cells to the site of injury, through chemotactic and other mechanisms, and release of proinflammatory cytokines and chemokines, among which the TNF, IL-1, IL-6, and interferon (IFN) families of proteins are predominant or most studied. These proteins function as signals that initiate changes in metabolism (19), which include the hepatic acute phase response (APR). The APR, as its name implies, is a very rapid response initiated by the insult and, optimally, sufficiently strong to deal with the injury or infection, and then to be resolved back to “baseline” homeostatic conditions. Although chronic inflammation may begin locally, it is characterized by persistence over time, dissemination, and failure to become completely resolved or quelled, and is thus described as chronic, which implies a survivable, long-term state that is sometimes referred to as “low grade” or mild inflammation.
The classical medical description of inflammation includes the cardinal signs: pain, heat, redness, and swelling (i.e., dolor, caldor, rubor, turgor). The term “inflammation” as it is often meant or inferred in relation to chronic diseases is harder to define, because the origins of the condition itself are less clear. In fact, side-by-side comparisons between models of acute and chronic inflammation are scarce, and the use of the same term, inflammation, for both of them may mask differences yet to be appreciated. Thus, although it seems safe to say that their general features are similar, more research is needed to compare and elucidate them.
Chronic inflammation may result, instead of from acute injury, from metabolic disturbances, such as long-term tissue damage such as caused by hypoxia, cell death, cellular necrosis, or autophagy, arthritis, and other autoimmune disorders, or from other nonacute injuries that also result in the recruitment of phagocytic and immune cells and in the production of proinflammatory cytokines. Conditions that have now become common in the general population, such as obesity, are linked to increased inflammation (20), which may be chronic and “low grade” compared with the inflammation of acute infection; nonetheless, the body’s pool of metabolically active adipose tissue is large, and the inflammation-related cytokines and adipokines produced therein are in intimate contact with other tissues through endocrine and paracrine interactions (21).
IMPORTANCE OF THE LIVER IN THE APR
The APR, described >8 decades ago by Tillet and Francis (see reference 10), is a highly conserved process found in all mammals (19, 22–25). When infectious agents or their invoked cytokines enter the systemic circulation (sepsis or sterile inflammation with an elevation of proinflammatory cytokines), the liver becomes a central organ of the inflammatory APR, which can be attributed to the following several features of the liver:
1) Its anatomical location between the gut and other viscera.
2) Its dual venous blood supply, including the portal vein through which the liver obtains intestinally derived materials, including microbes that have breached the intestinal epithelial barrier, immune cells educated in the environment of the intestine (including and differing in the lamina propria, intraepithelial lymphocyte compartment, and specialized gut-associated lymphoid tissue) (26), and a host of nutritional and other factors including water-soluble nutrients, food-borne and absorbed toxins, and cytokines produced in the intestine. Of note, the intestine is the body’s largest reservoir of immune cells (27), including tolerogenic T lymphocytes, and others with inflammatory potential (26, 28). Interestingly, the composition of these cells differs in neonatal and adult life, with few epithelial T cells in neonates (29). Nevertheless, at all ages, the portal vein connects a major component of the body’s innate and adaptive immune system (intestine, spleen, and pancreas) with the liver (30).
3) The proximity of the apical surface of hepatocytes to the venous sinusoids, separated only by loose, fenestrated endothelial cells, such that these cells readily filter and take up blood-borne materials.
4) The intrinsic functions of the hepatocytes in central energy metabolism, including glucose utilization and fatty acid transport. Thus, the utilization of all 3 major fuel sources becomes altered during the APR, which can be considered a means to redistribute building blocks for tissue repair at sites of injury, at the (temporary) expense of normal hepatic metabolism, in ways that provide an advantage to host survival.
5) Hepatic synthesis of most of the plasma proteins.
It is this latter function, specifically protein synthesis, that most research on the APR and acute phase (AP) proteins has addressed. Human AP proteins, which number >180, include proteins of the complement system, coagulation factors, antiproteases, transport proteins, and inflammatory response proteins (19). Most of the AP proteins are induced during inflammation and many of them exert crucial effector functions—for example, in the regulation of blood clotting and as opsonins (24). The best-known biomarker of inflammation, C-reactive protein (CRP), named for its role in reacting to the C-polysaccharide component of Streptococcus pneumoniae, is an opsonic protein. Several major AP proteins, considered to be markers of inflammation, are noted in Figure 1. In humans, CRP and serum amyloid protein (SAA) are the most prominent responding proteins, with increases of ≤100-fold during inflammation. In the rat, α2-macroglobulin and αl-acid glycoprotein (AGP) are most prominent (31). Although each AP protein exhibits changes in its concentration in plasma after the induction of the APR, the magnitude and duration of response differ among them; CRP and SAA proteins are induced very rapidly and to very high concentrations after exposure to an inflammatory stimulus, whereas haptoglobin and fibrogen, for example, increase less rapidly and dramatically (23). Albumin, a major regulator of oncotic pressure, and transferrin as well as several other nutrient transport proteins are negative AP proteins (22, 23, 31) that are reduced in concentration.
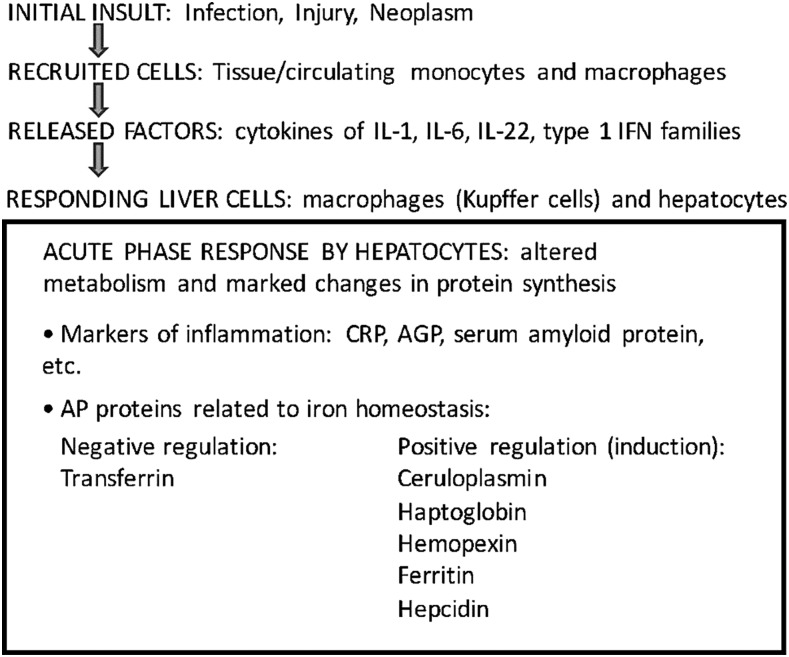
FIGURE 1.
Schematic of pathway from initial insult to the induction of the AP response. See Kilicarslan et al. (24) and Trautwein et al. (31) for reviews of AP proteins. AGP, α1-acid glycoprotein; AP, acute phase; CRP, C-reactive protein; IFN, interferon.
At least 5 AP proteins are directly involved in iron trafficking (Figure 1): transferrin, the transport/redox protein ceruloplasmin, the chaperone proteins haptoglobin and hemopexin, and the intracellular iron chelator ferritin, which is also present in small amounts in plasma, all of which are induced in the APR (24). Although few reviews of AP proteins have, until recently, listed a sixth factor, hepcidin, it should now be considered an important AP protein with regard to iron homeostasis (10). As discussed further below, the APR affects the distribution of iron to cells throughout the body and has significant implications for the availability of iron to the host and to microbes in the case of infectious diseases.
REGULATION OF THE SYNTHESIS OF HEPATIC AP PROTEINS
It has become traditional to categorize AP proteins as either class I AP genes, exemplified by CRP, haptoglobin, SAA, complement C3, hemopexin, haptoglobin, and AGP (10, 31), which are mainly regulated by IL-1 or by combinations of IL-1 plus IL-6 or IL-1, IL-6, and glucocorticoids, and class II genes, exemplified by α2-macroglobulin, α1-antichymotrypsin, α1-antitrypsin, and fibrinogen, for which IL-6 and glucocorticoids are the major inducers (31). However, current studies have shown that the situation is likely more complex, involving differential regulation by multiple factors. IL-1 and IL-6 still function as lead regulators, but the APR is further shaped by hormones and other regulatory factors in a manner determined by different inflammatory stimuli (10). The IL-1 family proteins (IL-1β being the most studied) may be the most complex, with both inductive and inhibitory activity (10). IL-1 generally signals via pathways that lead to the induction of the nuclear transcription factor κB, a major regulator of proinflammatory signaling (31). IL-6, originally identified as a B cell differentiation factor, is a multifunctional cytokine whose deregulation is implicated in several disease processes, including autoimmune diseases and chronic inflammatory proliferative diseases (32). IL-6 signals through its cell surface IL-6–binding protein, IL-6R, coupled to the accessory signaling protein glycoprotein 130; these signals are transduced from the cell surface intracellularly through several additional protein factors including the protein signal transducer and activator of transcription (STAT) 3 (33).
As reviewed by Bode et al. (10), signals from infection, injury, inflammation, or neoplasms induce a response by macrophages and monocytes and other inflammatory cells that results in the release of mediators. These factors then, in liver, influence Kupffer cells and sinusoidal endothelial cells to produce additional cytokines (IL-1, IL-6, TNF) that are received by receptors on the adjacent hepatocytes and regulate the APR. Examples based on model studies in isolated murine hepatocytes show that IL-1β can either suppress the induction of protein synthesis by IL-6, with little to no induction by IL-1β alone as in the case of γ-fibrinogen mRNA expression; or as shown for hepcidin mRNA, IL-1β can strongly synergize with IL-6, whereas neither cytokine by itself is a strong inducer [Figure 1B, C in (10)]. These studies represent the potential and complexity of cytokine regulation of the APR. However, further studies in primary human hepatocytes, especially those that are representative of different metabolic and physiologic states, including trimesters of pregnancy and age (infancy to adult), would be very desirable for a better understanding of the impact of inflammatory mediators, in addition to IL-1β and IL-6, on the hepatic APR and AP protein production under a range of conditions.
With regard to the differences in response to IL-1β and IL-6 reported by Bode et al. (10), several potential mechanisms were suggested, including cross-regulation of IL-1β and IL-6 soon after cell surface signaling, intracellular induction or suppression involving specific protein factors, competition at the level of transcription factor binding to DNA elements, and sequestration of transcription factors on cryptic or unproductive DNA sites, any or all of which could affect the transcriptional regulation (the main form of regulation) of the production of AP proteins (10). To move from bench to bedside, or public health, these mechanisms, too, will be important to elucidate in cells that represent various physiologic conditions.
HEPCIDIN AS AN AP PROTEIN IN THE RESPONSE TO INFLAMMATION
Liver is the major site of hepcidin synthesis, as indicated by higher concentrations of hepcidin antimicrobrial peptide (HAMP) mRNA in hepatocytes, compared with other organs and cells; however, it is interesting that HAMP mRNA is also detectable, albeit at lower concentrations, in other tissues and cell types and may be synthesized in cells that also express FPN [reviewed in (34)]. As noted above, HAMP is regulated mainly transcriptionally. The hepcidin protein is first translated as an 84-amino acid preprohormone, cleaved co-translationally to a 60-amino acid prohormone, and secreted as a 25-amino acid hormone. A shortened form, hepcidin-20 (Hep-20), which lacks the first 5 N-terminal amino acids, appears to have antimicrobial activity but lacks iron regulatory activity (34, 35). Hepcidin was initially called liver-expressed antimicrobial protein (LEAP) 1 on the basis of its antimicrobial function (36).
A variety of factors may contribute to the regulation of HAMP expression, mostly shown in vitro, including a suppressive effect of hepatocyte nuclear factor (HNF) 4 (37), induction by factors related to endoplasmic reticulum stress (38), and factors related to oxygen and oxidant and antioxidant signaling (39), as well as genetic factors (34, 40). As listed in Table 1, numerous factors and physiologic states result in the increased or decreased expression of HAMP and concentrations of hepcidin in plasma. Therefore, hepcidin concentrations are very sensitively regulated, and it can be anticipated that iron efflux from cells is regulated in parallel. As the “master regulator” of iron metabolism, the hepcidin-ferroportin axis serves to control iron absorption (enterocytes), iron in the extracellular space (via sequestration in macrophages), and transplacental iron transport, via regulation of FPN on syncytiotrophoblasts (4, 5, 10, 14).
TABLE 1.
Hepcidin characteristics and regulation1
| Characteristics | Site of synthesis or action | Functions and regulation |
| Peptide hormone (25-amino acid); short form, Hep-20 | Multiple (4); presumably where bacteria reside (35) | “Innate nutritional response” factor; antimicrobial activity (greater for Hep-20?) |
| “Master regulator” of iron metabolism | Binds and assists further degradation of FPN present on the basolateral membrane of enterocytes (7, 8); liver and splenic macrophages; placental syncytiotrophoblast cells (14) | Acts in conjunction with FPN protein to limit the efflux/export of iron from FPN-expressing cells |
| Synthesis is mainly regulated at the level of transcription | Liver parenchymal cells; others? (mRNA detected extrahepatic tissues (34) | Induced by hyperferremia, cytokines (IL-1, IL-6, and IL-22; type I interferons), LPS/toll-like receptor 4 signaling, intracellular innate inflammatory responses, and repressed by hypoferremia/iron deficiency, anemia, tissue hypoxia (increased erythropoietic drive) (13, 34)2 |
FPN, ferroportin; Hep-20, hepcidin-20; mRNA, messenger RNA.
Underlined text indicates factors related to inflammation.
THE APR AS A MEANS TO DEPRIVE MICROBES OF NUTRIENTS
Excellent reviews (4, 5) have addressed the fundamental competition for iron between the host (human or animal) and infectious agents, many if not most of which require iron for growth, and therefore iron availability constitutes a virulence factor. In the host-microbe competition for this essential nutrient, microbes have evolved sophisticated strategies to outcompete the host, including the production of siderophores with high affinity for iron. Soares and Wiess (4) comment on host mechanisms that are cytotoxic to the offending microbes, but also, “However, there are also resistance mechanisms….that prevent pathogens from accessing metabolites and/or nutrients that are essential for their survival and/or proliferation,” a defense strategy they refer to as “nutritional immunity,” which comprises ways that the host attempts to limit iron availability to the infectious bacteria. These investigators present the general principle that “Adaptive responses supplying Fe to microbes increase, in most cases, their pathogenicity, while those withholding Fe from microbes limit their virulence” (4).
What host responses help in compensation? This question is interesting because the appropriate response depends on whether the pathogen resides intracellularly [examples such as Candida, Chlamydia, Legionella, Salmonella, and Mycobacterium species (4)] or extracellularly (examples such as blood-phase malaria). The issue is important because the location of the pathogen may affect the outcome to host supplementation with iron. With regard to extracellular iron and extracellular pathogens, clinical and epidemiologic studies have shown that host iron overload is associated with poor clinical outcomes in diseases such as AIDS, malaria, and tuberculosis (4) and that dietary iron supplementation (assumed to increase extracellular iron initially) can exacerbate overall mortality rate in areas of endemic infectious diseases (5). Unbound iron in the host is a source of iron to extracellular pathogens; moreover, extra- and intracellular unbound iron can generate toxic free radicals and potentially result in tissue damage. Therefore, counteracting mechanisms to limit the concentration of unbound iron are extremely important. These include the chaperoning of iron by transferrin in plasma [which also targets iron via the interaction of transferrin with the plasma membrane-associated transferrin receptor (TfR), CD71]; lactoferrin, which is present in milk and other secretions (41); ferritin as an intracellular chaperone and high-capacity sequestrant of iron; hemoglobin, as the functionally important carrier of most iron; haptoglobin, as the chaperone of unbound hemoglobin [such as that released from RBCs at sites of injury and hemolysis (9, 18)]; and hemopexin, an intracellular chaperone for free heme, which interacts with the enzyme heme oxygenase 1 (HO-1), to catabolize free heme (17). It is also relevant that transferrin is normally only partially saturated with iron, providing a reserve of “iron binding capacity” to take up free iron when it is in excess.
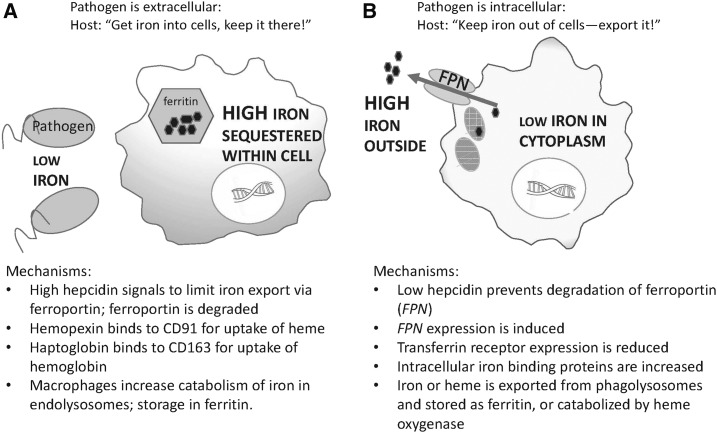
The ability of macrophages to take up and store iron is well known (9), and Kupffer cells have an especially high capacity for the storage of excess iron (42). Schematically (Figure 2), macrophage iron metabolism should be regulated differentially in response to the presence of extracellular pathogens (Figure 2A) and intracellular pathogens (Figure 2B), the common “theme” being to reduce the co-compartmentalization of the pathogen or pathogens and available iron. Several potential mechanisms are listed in Figure 2.
FIGURE 2.
Schematic of differential adaptive responses to segregate iron from microbes in the situation with extracellular infectious agents in which sequestration of iron intracellularly generally favors the host’s response (A) and with intracellular infectious agents in which removal of iron from the cell generally favors the host’s response to infection (B). (See references 4 and 5 for additional information). FPN, ferroportin.
The microvasculature is also sensitive to iron status and the products of RBC damage [reviewed in (4)]. Free heme is a danger signal; oxidized heme released from infected RBCs has multiple effects on tissue microvasculature and tissue damage, including damaged RBCs, can induce phagocytosis and the release of heme, which may scavenge nitrous oxide and cause local vasoconstriction. Released oxidized heme, as a ligand for G-protein-coupled receptors on polymorphonuclear leukocytes, may stimulate the release of reactive oxygen species and cause more damage; and released oxidized heme may serve as a ligand for Toll-like receptors on endothelial cells, causing endothelial inflammation. Scavenging of heme iron and oxidized heme are important for reducing their presence in the extracellular fluid, and HO-1 is an important intracellular control mechanism for the degradation of free heme (43). Moreover, one of its reaction products, carbon monoxide, is cytoprotective (43, 44).
PREGNANCY AND INFANCY
Information on the mechanisms of the regulation of iron transport and metabolism, as affected by inflammation, in pregnancy and in newborns is extremely limited. With respect to CRP concentrations as a general indicator of inflammation, systemic CRP concentrations in pregnancy have been found to be within the range that is normal for healthy, nonpregnant individuals (45), or categorized as “normal/insignificant” (<1 mg/dL) (24). Yet, whether more local tissue differences exist is not well known. Studies of implantation have shown that a controlled, local immune response, characterized by the presence of IL-6, IL-8, TNF, and T-helper 1 cells, is essential for implantation, which, if blocked, results in implantation failure (46). Similarly, parturition requires a local uterine or systemic inflammatory response (47). Thus, understanding the impact of “inflammation” on iron status in pregnancy will require studies that specifically consider local and systemic inflammation and that integrate knowledge of iron-regulatory pathways with acceptable biomarkers of inflammation. Hepcidin concentrations decrease and become very low in the course of pregnancy but are higher in pregnancy with inflammation (14). Concentrations normally increase around delivery (48). Key questions (14) are to what extent the variation in hepcidin concentrations is actually regulatory for iron status and how do we interpret the biological significance of these differences?
Finally, syncytiotrophoblast cells take up iron from maternal holo-transferrin via apical TfR, have some capacity to store iron, and release ferrous iron to the fetal circulation through the mediation of FPN on the fetal side for oxidation and removal by fetal transferrin. The placenta also expresses HO-1. Both placental HO-1 expression and exhaled carbon monoxide concentrations were reported to be lower in women with severe pre-eclampsia, which is consistent with the idea of a pathogenic role for low HO-1, whereas an in vitro experiment suggested that the induction of HO-1 and carbon monoxide production reduced the expression of antiangiogenic factors (49).
With regard to infants, recent results by Hedengran et al. (48) suggest a spike, with considerable variability, in neonatal blood hepcidin concentrations 1–2 d after birth. Another study in healthy full-term infants born in Sweden and therefore presumably from well-nourished iron-replete mothers showed a sharp increase in the concentration of plasma inflammatory markers 1 d after birth, with the elevation in IL-6 slightly preceding CRP and SAA (50). This could represent a normal physiologic response to change from the intrauterine to the extrauterine environment and concomitant physiologic changes in lung function (51). Whether these differences are significant as signals for changes in iron homeostasis still must be tested. In infants in an Indonesian population with widespread iron insufficiency, significant correlations were found between ferritin concentrations, as an iron-related AP protein, and both CRP and AGP concentrations, although infants with elevations in either CRP or AGP did not differ from infants in whom both of these proteins were elevated (52).
CONCLUSIONS
Mechanisms by which inflammatory processes regulate iron homeostasis in iron-replete and iron-deficient pregnant women and infants deserve additional study. Pregnancy per se may be too broad a category, and effects at different stages, including the time of implantation, pregnancy maintenance, and parturition, may be necessary to parse out differences due to differences intrinsic to these physiologic states. Studies in children from birth and after the time when prenatal iron stores are exhausted are also needed. Because hepcidin is now well established as a major regulator, additional studies on the meaning of HAMP expression in extrahepatic tissues would also be timely.
A key question is to what extent small differences in inflammatory status (and how these should be assessed) affect hepcidin concentrations and to what extent these differences actually result in tangible changes in iron transport, intracellular iron storage and metabolism, and functional outcomes. Overlaying all of this is the effect of infection, the differences in response to extracellular and intracellular microbes, and their impact on health and survival.
Acknowledgments
The author was solely responsible for the manuscript. The author had no conflict of interest related to the study.
Footnotes
Abbreviations used: AGP, α1-acid glycoprotein; AP, acute phase; APR, acute phase response; CRP, C-reactive protein; FPN, ferroportin; HAMP, hepcidin antimicrobrial peptide; HO-1, heme oxygenase 1; RBC, red blood cell; SAA, serum amyloid protein.
REFERENCES
- 1.Thurnham DI. Interactions between nutrition and immune function: using inflammation biomarkers to interpret micronutrient status. Proc Nutr Soc 2014;73:1–8. [DOI] [PubMed] [Google Scholar]
- 2.Raiten DJ, Sakr Ashour FA, Ross AC, Meydani SN, Dawson HD, Stephensen CB, Brabin BJ, Suchdev PS, van Ommen B. Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). J Nutr 2015;145(Suppl):1039S–108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson GJ, Wang F. Essential but toxic: controlling the flux of iron in the body. Clin Exp Pharmacol Physiol 2012;39:719–24. [DOI] [PubMed] [Google Scholar]
- 4.Soares MP, Wiess G. The Iron age of host–microbe interactions. EMBO Rep 2015;16:1482–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science 2012;338:768–72. [DOI] [PubMed] [Google Scholar]
- 6.Gasche C, Lomer MCE, Cavill I, Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut 2004;53:1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganz T. Cellular iron: ferroportin is the only way out. Cell Metab 2005;1:155–7. [DOI] [PubMed] [Google Scholar]
- 8.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 2005;1:191–200. [DOI] [PubMed] [Google Scholar]
- 9.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol 2013;14:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bode JG, Albrecht U, Håussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins—regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kB-dependent signaling. Eur J Cell Biol 2012;91:496–505. [DOI] [PubMed] [Google Scholar]
- 11.Pietrangelo A, Trautwein C. Mechanisms of disease: the role of hepcidin in iron homeostasis–implications for hemochromatosis and other disorders. Nat Clin Pract Gastroenterol Hepatol 2004;1:39–45. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal N, Prchal JT. Anemia of chronic disease (anemia of inflammation). Acta Haematol 2009;122:103–8. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am 2014;28:671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014;6:3062–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gozzelino R. The pathophysiology of heme in the brain. Curr Alzheimer Res 2016;13:174–84. [DOI] [PubMed] [Google Scholar]
- 16.Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett 1995;82–83:969–74. [DOI] [PubMed] [Google Scholar]
- 17.Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev 2003;55:551–71. [DOI] [PubMed] [Google Scholar]
- 18.Smith A, McCulloh RJ. Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders. Front Physiol 2015;6:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabay C, Kushner I. Acute phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–54. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006;83(Suppl):461S–5S. [DOI] [PubMed] [Google Scholar]
- 21.Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells 2014;37:365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldred AR, Schreiber G. The negative acute phase proteins. In: Mackiewicz A, Kushner I, Bauman H, editors. Acute phase proteins molecular biology, biochemistry, and clinical applications. Boca Raton (FL): CRC Press; 1993. p. 21–37. [Google Scholar]
- 23.Gitlin JD, Colten HR. Molecular biology of the acute phase proteins. In: Pick E, Landy M, editors. Lymphokines. San Diego (CA): Academic Press; 1987. p. 123–53. [Google Scholar]
- 24.Kilicarslan A, Uysal A, Roach EC. Acute phase reactants. Acta Medica (Cordoba) 2013;2:2–7. [Google Scholar]
- 25.Schreiber G, Tsykin A, Aldred AR, Thomas T, Fung W-P, Dickson PW, Cole T, Birch H, De Jong FA, Milland J. The acute phase response in the rodent. Ann N Y Acad Sci 1989;557:61–85. [DOI] [PubMed] [Google Scholar]
- 26.van Wijk F, Cheroutre H. Mucosal T cells in gut homeostasis and inflammation. Expert Rev Clin Immunol 2010;6:559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampson HA. Food allergies. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s gastrointestinal and liver disease. Philadelphia: Elsevier, Saunders; 2015. [Google Scholar]
- 28.Harrison OJ, Powrie FM. Regulatory T cells and immune tolerance in the intestine. Cold Spring Harb Perspect Biol 2013;5:a018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozing J, de Geus B. Changes in the intestinal lymphoid compartment throughout life: implications for the local generation of intestinal T cells. Int Rev Immunol 1995;12:13–25. [DOI] [PubMed] [Google Scholar]
- 30.Burroughs AK. The hepatic artery, portal venous system and portal hypertension: the hepatic veins and liver in circulatory failure. In: Dooley JS, Lok ASF, Burroughs AK, Heathcote EJ, editors. Sherlock’s diseases of the liver and biliary system. London: Wiley Blackwell; 2011. p. 152–209. [Google Scholar]
- 31.Trautwein C, Böker K, Manns MP. Hepatocyte and immune system: acute phase reaction as a contribution to early defence mechanisms. Gut 1994;35:1163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev 2002;13:357–68. [DOI] [PubMed] [Google Scholar]
- 33.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000;19:2548–56. [DOI] [PubMed] [Google Scholar]
- 34.Piperno A, Mariani R, Trombini P, Girelli D. Hepcidin modulation in human diseases: from research to clinic. World J Gastroenterol 2009;15:538–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maisetta G, Petruzzelli R, Brancatisano FL, Esin S, Vitali R, Campa M, Batoni G. Antimicrobial activity of human hepcidin 20 and 25 against clinically relevant bacterial strains: effect of copper and acidic pH. Peptides 2010;31:1995–2002. [DOI] [PubMed] [Google Scholar]
- 36.Krause A, Neitz S, Mägert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett 2000;480:147–50. [DOI] [PubMed] [Google Scholar]
- 37.Shi W, Wang H, Zheng X, Jiang X, Xu Z, Shen H, Li M. HNF-4alpha negatively regulates hepcidin expression through BMPR1A in HepG2 cells. Biol Trace Elem Res 2017;176:294–304. [DOI] [PubMed] [Google Scholar]
- 38.Harrison-Findik DD, Lu S. The effect of alcohol and hydrogen peroxide on liver hepcidin gene expression in mice lacking antioxidant enzymes, glutathione peroxidase-1 or catalase. Biomolecules 2015;5:793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayele HK, Balesaria S, Srai SK. Phytoestrogens modulate hepcidin expression by Nrf2: implications for dietary control of iron absorption. Free Radic Biol Med 2015;89:1192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bayele HK, Srai SKS. Genetic variation in hepcidin expression and its implications for phenotypic differences in iron metabolism. Haematologica 2009;94:1185–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legrand D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem Cell Biol 2012;90:252–68. [DOI] [PubMed] [Google Scholar]
- 42.Papanastasiou DA, Vayenas DV, Vassilopoulos A, Repanti M. Concentration of iron and distribution of iron and transferrin after experimental iron overload in rat tissues in vivo: study of the liver, the spleen, the central nervous system and other organs. Pathol Res Pract 2000;196:47–54. [DOI] [PubMed] [Google Scholar]
- 43.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 2010;50:323–54. [DOI] [PubMed] [Google Scholar]
- 44.Origassa CS, Câmara NO. Cytoprotective role of heme oxygenase-1 and heme degradation derived end products in liver injury. World J Hepatol 2013;5:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haram K, Augensen K, Elsayed S. Serum protein pattern in normal pregnancy with reference to acute-phase reactants. Br J Obstet Gynaecol 1983;90:139–45. [DOI] [PubMed] [Google Scholar]
- 46.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 2011;1221:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 2006;11:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hedengran KK, Nelson D, Andersen MR, Stender S, Szecsi PB. Hepcidin levels are low during pregnancy and increase around delivery in women without iron deficiency—a prospective cohort study. J Matern Fetal Neonatal Med 2016;29:1506–8. [DOI] [PubMed] [Google Scholar]
- 49.Levytska K, Kingdom J, Baczyk D, Drewlo S. Heme oxygenase-1 in placental development and pathology. Placenta 2013;34:291–8. [DOI] [PubMed] [Google Scholar]
- 50.Marchini G, Berggren V, Djilali-Merzoug R, Hansson LO. The birth process initiates an acute phase reaction in the fetus–newborn infant. Acta Paediatr 2000;89:1082–6. [DOI] [PubMed] [Google Scholar]
- 51.Hooper SB, Te Pas AB, Kitchen MJ. Respiratory transition in the newborn: a three-phase process. Arch Dis Child Fetal Neonatal Ed 2016;101:F266–71. [DOI] [PubMed] [Google Scholar]
- 52.Wieringa FT, Dijkhuizen MA, Clive E, West CE, Northrop-Clewes CA, Muhilal. Estimation of the effect of the acute phase response on indicators of micronutrient status in Indonesian infants. J Nutr 2002;132:3061–6. [DOI] [PubMed] [Google Scholar]