Abstract
Plasma volume expansion is an important component of a successful pregnancy. The failure of maternal plasma volume expansion has been implicated in adverse obstetric outcomes such as pre-eclampsia, fetal growth restriction, and preterm birth. Altered iron homeostasis and elevated maternal hemoglobin concentrations have also been associated with adverse pregnancy outcomes; limited data have suggested that these effects may be mediated by inadequate plasma volume expansion. In addition, it has been noted that pregnant, obese women, compared with lean subjects, have decreased plasma volume expansion along with impaired iron homeostasis and increased inflammation. Current estimates of plasma volume expansion are outdated and do not necessarily reflect contemporary obstetric populations. Moreover, the validation of clinically applicable methods of plasma volume determination as well as enhanced methodologies should be a priority. Further study is needed to characterize diminished plasma volume expansion during pregnancy and to understand the potential role of impaired iron homeostasis and inflammation in adverse obstetric outcomes, especially in obese women.
Keywords: inflammation, iron, obesity, obstetric outcomes, plasma volume, pregnancy
INTRODUCTION
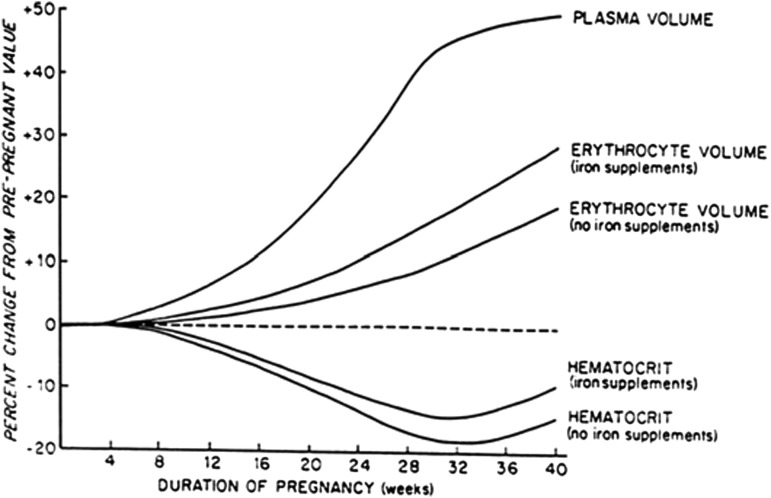
Plasma volume expansion is a well-documented aspect of pregnancy physiology that is essential to supporting successful pregnancy outcomes. The physiologic process of plasma volume expansion achieves a 30–50% increase from prepregnancy concentrations near full term (1). Plasma volume expansion, along with increases in red blood cell (RBC) volume, results in an expansion of the total blood volume in pregnancy. Plasma volume expansion is achieved by increased sodium and water reabsorption at the level of the kidney in addition to increased cardiac output and decreased systemic vascular resistance and systemic blood pressure (2). Plasma volume expansion begins as early as 6 wk of gestation, peaks around 32 wk of gestation, and plateaus until delivery (2, 3). Increased RBC volume is driven by progesterone-mediated increases in erythropoietin, although to a lesser extent than plasma volume (Figure 1) (1–3, 5, 6). This effect results in a dilutional decrease in hematocrit, which is known as the physiologic anemia of pregnancy with normal concentrations defined as ≥11.0 g/dL in the first and third trimesters and ≥10.5 g/dL in the second trimester (2, 6, 7).
FIGURE 1.
Relation between plasma volume, erythrocyte volume, and hematocrit in pregnancy. Values are expressed as means. Adapted from Pitkin (4) with permission.
Plasma volume expansion supports normal growth and the development of the fetus and protects against hemorrhage at the time of delivery. Normal fetal growth rates and birth weights are directly correlated with maternal volume status (1, 8–10). Conversely, diseases such as advanced diabetes, lupus nephritis, and pre-eclampsia can lead to a failure to adequately expand the plasma volume and, ultimately, to poor obstetric outcomes such as fetal growth restriction and fetal demise (2, 11, 12).
The relation between maternal plasma volume expansion and iron homeostasis during pregnancy may be associated with adverse obstetric outcomes (13). Moreover, there is a need to better understand the role of inflammation and obesity in both plasma volume expansion and iron homeostasis. Although available data are limited and emerging at best, the topic is worthy of consideration. A key aspect of the related research is a better understanding and estimation of plasma volume expansion during pregnancy. This review, which was prepared to support discussions that took place during an NIH workshop related to iron, describes the role of plasma volume expansion in pregnancy in normal and pathologic states. The paper focuses on the need for updated plasma volume estimates in pregnancy, the technical aspects of plasma and RBC volume estimation, and a novel method, to my knowledge, for clinical plasma volume estimation.
IRON HOMEOSTASIS, PLASMA VOLUME EXPANSION, AND OBSTETRIC OUTCOMES
The relation between maternal plasma volume expansion and iron homeostasis during pregnancy may play a role in the development of adverse obstetric outcomes such as pre-eclampsia, preterm birth, and low-birth-weight infants (13). Conditions that have been shown to affect plasma volume expansion in human and animal studies are pre-eclampsia, chronic hypertension, idiopathic fetal growth restriction, iron deficiency (ID), food restriction, and low protein intake (14). Hemoglobin and hematocrit decrease during pregnancy because the 50% increase in plasma volume exceeds the 25% increase in RBC mass (14). The maternal expansion in RBC mass and plasma volume do not occur at equal rates, thereby resulting in decreasing hemoglobin and hematocrit until a nadir at the end of the second trimester to the beginning of the third trimester that corresponds to maximal hemodilution, which is followed by gradual normalization in the third trimester. Therefore, the differentiation of the normal physiologic anemia of pregnancy from true maternal anemia during this period can be challenging. Conversely, identifying cases of inadequate plasma volume expansion may help to anticipate the development of adverse obstetric outcomes.
There is a well-documented U-shaped relation between maternal hemoglobin concentrations and adverse pregnancy outcomes. Optimal outcomes have been observed with maternal hemoglobin concentrations from 9.5 to 11 g/dL (14). At both increased and decreased hemoglobin concentrations, a greater incidence of adverse obstetric outcomes has been observed. Early pregnancy anemia and ID anemia have been associated with both preterm delivery as well as low-birth-weight infants (13, 15). Elevated maternal hemoglobin, hepcidin, serum iron, and serum ferritin (SF) concentrations have been noted in cases of pre-eclampsia, preterm delivery, and fetal growth restriction (16–18). What remains unclear is whether the underlying mechanism for adverse outcomes that are seen with elevated maternal hemoglobin concentrations is the result of a primary elevation in total body iron or a failure of plasma volume expansion that results in hemoconcentration.
There is limited evidence that both the failure of maternal plasma volume expansion and a primary elevation in total body iron may contribute to adverse outcomes such as pre-eclampsia and fetal growth restriction. Several studies have explored a primary elevation in total body iron as a potential cause of adverse outcomes in women with elevated hemoglobin concentrations via increased hypoxia and oxidative stress (15). One randomized controlled trial showed an increased incidence of pre-eclampsia in women who were undergoing iron supplementation (18). Another prospective study showed associations between elevated third-trimester SF concentrations and preterm delivery and markers of systemic infection (16). However, because these studies did not account for maternal plasma volume status, the role of maternal iron status in the development of pre-eclampsia, preterm birth, and fetal growth restriction remains uncertain.
Elevated rates of adverse pregnancy outcomes in women with chronic renal impairment from diseases such as lupus nephritis and advanced diabetes suggest that a failure of plasma volume expansion that is mediated by abnormal baseline renal function plays a causative role in the poor obstetric outcomes in these women. Rates of pre-eclampsia, fetal growth restriction, fetal demise, and preterm birth are correlated with the severity of baseline maternal renal impairment (19, 20). Reduced plasma volume expansion has been shown in normotensive women with idiopathic fetal growth restriction, thereby supporting a causative role for the failure of maternal plasma volume expansion (21). Further research is needed to understand the relation between maternal iron indexes, plasma volume expansion, and adverse obstetric outcomes.
Because the negative consequences of ID anemia in pregnancy have been well described, the practice of recommending iron supplementation to all pregnant women, regardless of iron status, is widespread in the United States (22). However, the potential consequences of iron supplementation in iron-replete women remain poorly understood and may include increased risk of hemoconcentration-mediated adverse obstetric outcomes. Further studies are needed in this area to determine the appropriateness of iron supplementation in iron-replete women.
Both inflammation and obesity can affect iron homeostasis, thus complicating attempts to articulate iron homeostasis and to relate changes in iron homeostasis to plasma volume expansion. Maternal systemic inflammation has increasingly been implicated in various adverse obstetric events such as the preterm premature rupture of membranes, preterm delivery, fetal malformations, and recurrent pregnancy loss (23). Normal pregnancy, itself, is an inflammatory state that is characterized by elevations in inflammatory markers such as C-reactive protein (CRP), granulocyte macrophage colony stimulating factor, leptin, and IL-4, -6, and -10 (14, 23, 24). Although early pregnancy and parturition are times of increased inflammation, midpregnancy appears to be an anti-inflammatory state under normal conditions (24). Therefore, although pregnancy itself has both proinflammatory and anti-inflammatory phases, the derangements in the mediation of normal inflammatory processes of pregnancy appear to predispose individuals to adverse outcomes.
Inflammation affects iron homeostasis in pregnancy as well. The concentration of SF (an iron-storage protein), which is typically decreased in states of ID, is also known to be physiologically elevated in normal pregnancy. SF, which is an acute phase reactant, is also known to be elevated in inflammatory and chronic disease states. This fact, in addition to the physiologic anemia of pregnancy, makes the characterization of derangements of iron homeostasis more challenging in pregnant women. A falsely elevated concentration of SF can mask ID in a pregnant woman with heightened inflammatory status (14). Increased SF concentrations have been associated with preterm birth, but this relation is thought to be mediated by inflammation for which an increased SF concentration, as an acute-phase reactant, is an indicator (16).
Hepcidin, which is the regulator of systemic iron bioavailability, is also affected by maternal inflammatory states. Hepcidin production is altered in response to total body iron, anemia, hypoxemia, inflammation, or infection (25). When systemic iron is decreased or in times of hypoxia or anemia, hepcidin production decreases, which in turn facilitates dietary iron absorption and mobilization from body stores. Conversely, when total body iron is increased or in times of inflammation or infection, hepcidin production increases, which leads to reduced dietary iron absorption and mobilization from body stores (25).
Typically, hepcidin concentrations are lower during pregnancy than in the nonpregnant state to maintain greater bioavailability of iron to the mother and fetus (17, 26). Hepcidin concentrations decrease progressively to their lowest amounts in the third trimester, which corresponds to the time of the highest fetal demand for iron (25). However, hepcidin concentrations have been shown to increase in various inflammatory states in pregnancy such as pre-eclampsia and obesity. Furthermore, states of systemic iron overload appear to impair the systemic response to inflammation and infection (27). A study of obese women in the second trimester showed elevated hepcidin concentrations compared with those of lean control subjects and a positive correlation with maternal CRP (26). Pre-eclamptic women were shown to have elevated hepcidin, IL-6, and SF concentrations with decreased mean corpuscular hemoglobin concentrations compared with those of healthy pregnant women (17). Further studies are needed to examine the relation between hepcidin concentrations, iron status, and pregnancy complications.
The relation between maternal obesity, inflammation, and iron homeostasis is incompletely understood. Obesity is a state of increased systemic inflammation (28). Formerly thought to be a relatively inert storage organ, adipose tissue is now recognized to be a metabolically active endocrine center (28). In studies of nonpregnant patients, inflammatory processes are thought to drive the end organ damage in obesity-related diseases such as cardiovascular disease and diabetes. Compared with lean pregnant women, obese gravidas have greater incidence of maternal and fetal morbidity and mortality including fetal issues, such as growth derangements, malformations, and demise, and maternal conditions, such as fatty liver disease, cardiac failure, diabetes, and pre-eclampsia (29). Although the majority of evidence linking the negative sequelae of obesity to inflammation is in nonpregnant individuals, inflammation is suspected to play a role in the adverse outcomes that are experienced by obese women in pregnancy as well (28).
In studies of pregnant women, inflammatory pathways have been linked to increased rates of glucose intolerance, insulin resistance, and gestational diabetes. Studies of obese pregnant women have shown increased concentrations of inflammatory mediators such as CRP, IL-6, TNF, and leptin compared with in lean controls (28). Inflammation has also been postulated to lead to the increased rates of pre-eclampsia in obese women in pregnancy, which are possibly mediated by chronic endothelial dysfunction and the decreased endothelium-dependent dilation of myometrial arteries in obese gravidas (30). Increased levels of reactive oxygen species and chronic hypoxemia have been implicated in the increased rates of certain fetal malformations that have been observed in fetuses of obese women (31). Although the mechanisms for adverse pregnancy outcomes in obese women in pregnancy are poorly understood, both systemic inflammation and local inflammation are thought to play a causative role. Further studies are needed to better characterize this relation.
ESTIMATION OF PLASMA VOLUME EXPANSION
Updated plasma volume estimates are needed
In recent decades, the US obstetric population has changed greatly in terms of increasing maternal age, obesity rates, and accompanying medical comorbidities. The mean age of pregnant women is now 26 y compared with 21 y in 1970 (32). Women >35 y of age now comprise >10% of first-time mothers, and women >40 y of age comprise almost 3% of first-time mothers (32). Obesity has increased in American women of reproductive age, with 34% of them now defined as obese [BMI (in kg/m2) ≥30], and 7.9% of them defined as severely obese (BMI ≥40) compared with a total obesity rate of only 8% in women in 1980 (29, 33). Concomitant with the trends in obesity and increasing age at pregnancy, the frequency of medical comorbidities such as diabetes and hypertension in pregnant women is also increasing (33, 34). These trends have all been linked to a greater incidence of adverse obstetric outcomes (35). The result is an obstetric population that is older, heavier, and plagued with more of the associated medical comorbidities that accompany those conditions.
Previous estimates of plasma volume may have little relevance to current obstetric populations. When the sentinel studies of plasma volume estimation were performed, obesity rates and maternal age were substantially lower than current estimates. Updated studies of plasma volume expansion in obese women and those with underlying medical comorbidities are needed to update our definition of normal plasma volume expansion in pregnancy and also to define the impact of increasingly frequent pathologic states on this process.
Our understanding of the impacts of pregnancy and obesity on plasma volume expansion is incomplete. Plasma volume in normal-weight nonpregnant women ranges from 65 to 85 mL/kg (36–38). In pregnancy, the plasma volume is known to expand to 100 mL/kg (range: 90–200 mL/kg) near term (Table 1) (1, 37, 38). As body mass increases, so does plasma volume. However, because fat mass is relatively underperfused compared with lean mass, the relation is curvilinear rather than linear, which results in estimates of 29–45 mL/kg in obese nonpregnant women (Table 1) (36, 38, 40). Plasma volume expansion in obese pregnant women remains understudied, although our recent publication suggests that it follows a similar trend to that in obese nonpregnant women (39). Investigation of abnormalities in plasma volume expansion in various disease states of pregnancy has also been limited, although some studies that were performed in pre-eclamptic patients have provided the basis for our understanding of this condition as one of volume contraction with decreased plasma volume estimates in pre-eclamptic women compared with in healthy and hypertensive controls (41). A more complete understanding of plasma volume expansion in the contemporary obstetric population as well as in various disease states is needed.
TABLE 1.
Total blood volume estimates obtained with the HES method and previously published estimates1
| Women | Previous studies, mL/kg | HES method, mL/kg |
| Lean nonpregnant | 652 | — |
| Obese nonpregnant | 45 | — |
| Lean pregnant | 100 (90–200)3 | 95 (35, 155)4 |
| Obese pregnant | — | 73 (29, 177) |
Adapted from Vricella et al. (39) with permission. HES, hydroxyethyl starch.
Mean (all such values).
Mean; range in parentheses.
Median; IQR in parentheses (all such values).
Technical aspects of plasma volume estimation
Whole blood is composed of both cellular and noncellular components. The cellular component of whole blood is composed primarily of RBCs, whereas plasma comprises the noncellular portion. Historically, blood volume estimates have been based on the dilution of a known quantity of an indicator substance that marks either the plasma or RBC volume. The most accurate methods for a whole blood volume estimation involve the direct measurement of both the RBC volume and the plasma volume with a subsequent summation to arrive at a whole blood volume measurement (42). Because of the technical challenge of the simultaneous measurement of both plasma and RBC components, many investigations have relied on the direct measurement of the plasma component followed by an estimation of whole blood volume through calculations involving the direct measurement, hematocrit, and a correction factor (whole body:peripheral hematocrit ratio) that accounts for the idea that the hematocrit varies with the location on the body where the blood is sampled (41, 42).
The following discussion of the technical aspects of blood volume estimation will focus on techniques for either direct plasma or direct RBC volume estimation. In cases where whole blood volume is described, these methods involve the direct measurement of the plasma component that is applied to an established equation including a measured hematocrit to arrive at an estimate of whole blood volume as the sum of the plasma and RBC components. The estimation of whole blood volume by the simultaneous direct measurement of both the plasma and RBC components is uncommon (42). A summary of the techniques described is presented in Table 2.
TABLE 2.
Techniques for plasma and RBC volume estimation1
| Method | Component | Marker | Method | Consideration |
| Radioisotope labeling | RBCs | 51Cr-RBCs | Nuclear medicine study | Radiation exposure |
| 53Cr-RBCs | Gas chromatography–mass spectrometry | Stable isotope | ||
| Plasma | 125I-HSA | Nuclear medicine study | Radiation exposure | |
| 131I-HSA | Blood volume analyzer kit2 | Greater ease of use and cost | ||
| Dye dilution | ||||
| Evans blue | Plasma | Azo dye | Spectrophotometry, dye-bound plasma proteins | Possible carcinogen |
| Expertise needed | ||||
| Indocyanine green | Plasma | Cyanine dye | Pulse spectrophotometry | Short half-life |
| HES method | Plasma | Colloid volume expander | Acid hydrolysis to glucose | Contemporary validation needed |
| CO rebreathing | RBCs | CO-bound hemoglobin | CO analyzer | Requires complete blood mixing |
| Concern for fetal exposure |
Cr-RBC, chromium-tagged red blood cell; HES, hydroxyethyl starch; I-HSA, iodine-tagged human serum albumin; RBC, red blood cell.
Plasma volume was determined with the use of the same technique as for 125I-HSA in a Food and Drug Administration–approved prepackaged kit (BVA-100; DAXOR Corp.).
Historically, techniques for blood volume estimation have fallen into 2 general categories: those that use isotopes and those that use dyes. Although isotope-based techniques have been used to estimate both plasma volume and RBC volume, dye techniques have been used primarily for plasma volume estimation. The carbon-monoxide rebreathing technique has been described as well, although its application to the contemporary obstetric population is limited because of concerns for fetal exposure (43).
Radioisotope labeling has been used to estimate both the RBC and plasma components and has formed the basis for many early studies of whole blood volume (1, 3, 36–38, 40). The gold standard for RBC volume determination is RBCs labeled with the chromium isotope (51Cr-RBC), which involves collecting a whole blood sample from the patient, tagging the patient’s own RBCs, reinjecting the patient with the sample, drawing a second sample after systemic mixing has occurred, and calculating the reactivity of the specimen to determine the degree of dilution that has occurred (42). Iodine isotopes (125I or 131I) that are tagged to human serum albumin have been used to estimate plasma volume through similar techniques (42). These techniques were also used in the obstetric population until the use of radioisotopes in human pregnancy was abandoned. More-contemporary studies have described the is use of the nonradioactive isotope chromium 53 as a safer alternative for estimating RBC volume in the obstetric population (43).
Plasma volume has also been estimated with the use of various dyes via a dye-dilution principle (42). Evans blue is an azo dye that strongly binds to plasma proteins. With experienced operators, the dye can give an accurate measure of plasma volume without concerns of exposure to radioactive substances. However, animal studies have indicated that Evans blue is a possible carcinogen, and the technique requires a high level of expertise to obtain reproducible results (42). Despite these concerns, Evans blue has been used frequently in studies of plasma volume expansion in pregnancy because it was considered to be more acceptable than the isotope-based methods.
Indocyanine green (ICG) dye is nonradioactive and nontoxic and has also been used to estimate plasma volume via an indicator-dilution method. The ICG technique is limited by its very short half-life that is due to rapid elimination by the liver. Therefore, multiple postinjection samples are needed to allow back extrapolation to calculate plasma volume at the time of injection; this aspect can decrease the accuracy of the technique. Alternatively, the rapid elimination of ICG makes it appealing for studies in which multiple sequential estimates are needed (42).
NOVEL AND CLINICALLY APPLICABLE METHOD FOR ASSESSING PLASMA VOLUME EXPANSION IN PREGNANCY
The previously used techniques for the estimation of RBC and plasma volumes have limited acceptability in the contemporary obstetric population as well as beyond the research setting. A more medically acceptable method would help to update our understanding of variations in maternal volume expansion with body habitus as well as various disease states. A more clinically applicable method would facilitate bedside estimates of maternal volume status, which could aid in the clinical management of disease states and maternal resuscitation. Our recently published study describes a promising technique that is both safe and clinically applicable for the contemporary study of obstetric patients (39).
We applied a dilutional method for whole-volume estimation using hydroxyethyl starch (HES), which is a plasma volume expander that is widely available and acceptable for use in pregnant women (39). The HES technique was originally described by Tschaikowsky et al. (44) in anesthetized neurosurgical patients and validated against the carbon-monoxide rebreathing method. Analytically, HES is hydrolyzed to constant proportions of glucose and hydroxyethyl glucose with boiling. With the use of HES as a dilution marker, plasma volume was calculated by hydrolyzing plasma specimens and measuring the resultant glucose concentrations before (G0) and after (G1) injection of HES. The change in glucose concentration (ΔG) was calculated as the difference in the glucose concentration in plasma before and after injection of HES whereby
 |
ΔG was used to estimate whole blood volume via the derived formula
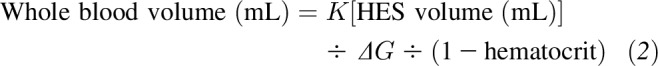 |
K is the constant 3082 mg%, which is the slope of the regression line obtained by plotting ΔG against the HES volume:plasma volume ratio for varying dilutions of HES in whole blood (44).
We used the change in glucose before and after HES injection, the patient’s hematocrit, and a previously derived equation to achieve an estimate of maternal whole blood volume. Our goal was to evaluate differences in whole blood volume on the basis of body habitus in pregnant women. We performed estimates of whole blood volume in 30 normal-weight and 30 obese pregnant women near term (39). We showed that obese pregnant women near term have a unit whole blood volume of 73 mL/kg, which is increased compared with that of 45 mL/kg in obese nonpregnant women but remains significantly lower than the estimated 100 mL/kg that was determined for lean pregnant women near term (Table 1). These findings, which require further study, have potential implications for the nutritional management, volume resuscitation, and anesthetic management of obese women in pregnancy. Such findings highlight the importance of updating studies of plasma and RBC volume expansion in the obstetric population.
Our current areas of study are an in vitro validation of this method with plans to perform in vivo methods in the future. If successful, we hope these methods would expand the ability to study plasma volume expansion and related disease states in contemporary obstetric populations.
SUMMARY AND FUTURE AREAS OF STUDY
The relation between plasma volume expansion, iron status, and pregnancy outcomes is incompletely understood. The increasing rates of maternal obesity and associated systemic inflammation are suspected to contribute to the adverse obstetric outcomes that are seen in these women. Our contemporary estimation of plasma volume expansion in obese gravidas shows that obese pregnant women fail to volume expand to the same extent as lean women do (39). This finding suggests that, without the same degree of hemodilution as lean women have, obese women should have higher hemoglobin concentrations. However, obese women are also known to have elevated hepcidin concentrations (presumably because of chronic inflammation) and, therefore, would be anticipated to have reduced iron absorption at the level of the gut and consequently a reduced hemoglobin concentration. Prospective simultaneous assessments of maternal plasma volume expansion, systemic iron status, and inflammatory markers are needed to better understand the relation between the failure of volume expansion, the hemoconcentration, and adverse obstetric outcomes. An improved understanding of the relation between chronic inflammation, plasma volume expansion, and iron homeostasis in pregnancy may contribute to our understanding of pathologic processes such as pre-eclampsia, fetal growth restriction, and fetal demise.
Acknowledgments
The sole author had responsibility for all parts of the manuscript. The author reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: CRP, C-reactive protein; HES, hydroxyethyl starch; ICG, indocyanine green; ID, iron deficiency; RBC, red blood cell; SF, serum ferritin.
REFERENCES
- 1.Pritchard JA. Changes in blood volume during pregnancy and delivery. Anesthesiology 1965;26:393–9. [DOI] [PubMed] [Google Scholar]
- 2.Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin 2012;30:317–29. [DOI] [PubMed] [Google Scholar]
- 3.Lund CJ, Donovan JC. Blood volume during pregnancy. Significance of plasma and red cell volumes. Am J Obstet Gynecol 1967;98:394–403. [PubMed] [Google Scholar]
- 4.Pitkin RM. Nutritional support in obstetrics and gynecology. Clin Obstet Gynecol 1976;19:489–513. [DOI] [PubMed] [Google Scholar]
- 5.Jepson JH. Endocrine control of maternal and fetal erythropoiesis. Can Med Assoc J 1968;98:844–7. [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 95: anemia in pregnancy. Obstet Gynecol 2008;112:201–7. [DOI] [PubMed] [Google Scholar]
- 7.Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep 1998;47:1–29. [PubMed] [Google Scholar]
- 8.Hytten FE, Paintin DB. Increase in plasma volume during normal pregnancy. J Obstet Gynaecol Br Emp 1963;70:402–7. [DOI] [PubMed] [Google Scholar]
- 9.Ueland K. Maternal cardiovascular dynamics. VII. Intrapartum blood volume changes. Am J Obstet Gynecol 1976;126:671–7. [PubMed] [Google Scholar]
- 10.Goodlin RC, Dobry CA, Anderson JC, Woods RE, Quaife M. Clinical signs of normal plasma volume expansion during pregnancy. Am J Obstet Gynecol 1983;145:1001–9. [DOI] [PubMed] [Google Scholar]
- 11.Brown MA, Gallery ED. Volume homeostasis in normal pregnancy and pre-eclampsia: physiology and clinical implications. Baillieres Clin Obstet Gynaecol 1994;8:287–310. [DOI] [PubMed] [Google Scholar]
- 12.Brown MA, Zammit VC, Mitar DM. Extracellular fluid volumes in pregnancy-induced hypertension. J Hypertens 1992;10:61–8. [DOI] [PubMed] [Google Scholar]
- 13.Scholl TO. Maternal iron status: relation to fetal growth, length of gestation, and iron endowment of the neonate. Nutr Rev 2011;69 Suppl 1:S23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao C, O’Brien KO. Pregnancy and iron homeostasis: an update. Nutr Rev 2013;71:35–51. [DOI] [PubMed] [Google Scholar]
- 15.Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr 2000;71:1280S–4S. [DOI] [PubMed] [Google Scholar]
- 16.Scholl TO. High third-trimester ferritin concentration: associations with very preterm delivery, infection, and maternal nutritional status. Obstet Gynecol 1998;92:161–6. [DOI] [PubMed] [Google Scholar]
- 17.Toldi G, Stenczer B, Molvarec A, Takats Z, Beko G, Rigo J Jr, Vasarhelyi B. Hepcidin concentrations and iron homeostasis in preeclampsia. Clin Chem Lab Med 2010;48:1423–6. [DOI] [PubMed] [Google Scholar]
- 18.Ziaei S, Norrozi M, Faghihzadeh S, Jafarbegloo E. A randomised placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin > or = 13.2 g/dl. BJOG 2007;114:684–8. Erratum in: BJOG 2007;114:1311. [DOI] [PubMed] [Google Scholar]
- 19.Fischer MJ, Lehnerz SD, Hebert JR, Parikh CR. Kidney disease is an independent risk factor for adverse fetal and maternal outcomes in pregnancy. Am J Kidney Dis 2004;43:415–23. [DOI] [PubMed] [Google Scholar]
- 20.Murakami S, Saitoh M, Kubo T, Koyama T, Kobayashi M. Renal disease in women with severe preeclampsia or gestational proteinuria. Obstet Gynecol 2000;96:945–9. [DOI] [PubMed] [Google Scholar]
- 21.Salas SP, Rosso P, Espinoza R, Robert JA, Valdes G, Donoso E. Maternal plasma volume expansion and hormonal changes in women with idiopathic fetal growth retardation. Obstet Gynecol 1993;81:1029–33. [PubMed] [Google Scholar]
- 22.Taylor CL, Brannon PM. Introduction to workshop on iron screening and supplementation in iron-replete pregnant women and young children. Am J Clin Nutr 2017;106(Suppl):1547S–54S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF III, Petraglia F. Inflammation and pregnancy. Reprod Sci 2009;16:206–15. [DOI] [PubMed] [Google Scholar]
- 24.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 2011;1221:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014;6:3062–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is Hepcidin the link? J Perinatol 2013;33:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr 2010;30:105–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denison FC, Roberts KA, Barr SM, Norman JE. Obesity, pregnancy, inflammation, and vascular function. Reproduction 2010;140:373–85. [DOI] [PubMed] [Google Scholar]
- 29.ACOG Practice Bulletin No 156: obesity in pregnancy. Obstet Gynecol 2015;126:e112–26. Erratum in: Obstet Gynecol 2016;128:1450. [DOI] [PubMed] [Google Scholar]
- 30.Bodnar LM, Ness RB, Harger GF, Roberts JM. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am J Epidemiol 2005;162:1198–206. [DOI] [PubMed] [Google Scholar]
- 31.Blomberg MI, Kallen B. Maternal obesity and morbid obesity: the risk for birth defects in the offspring. Birth Defects Res A Clin Mol Teratol 2010;88:35–40. [DOI] [PubMed] [Google Scholar]
- 32.Matthews TJ, Hamilton M. Mean age of mothers is on the rise: 2010-2014. NCHS Data Brief 2016;232:1–7. [PubMed] [Google Scholar]
- 33.World Health Organization. Global health observatory data: obesity [Internet]. Washington (DC): WHO; 2016. [cited 2016 Nov 17]. Available from: http://apps.who.int/gho/data/node.main.A896?lang=en.
- 34.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol 2013;25:124–32. [DOI] [PubMed] [Google Scholar]
- 35.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, Saade G, Eddleman K, Carter SM, Craigo SD, et al. Obesity, obstetric complications and cesarean delivery rate–a population-based screening study. Am J Obstet Gynecol 2004;190:1091–7. [DOI] [PubMed] [Google Scholar]
- 36.Huff RL, Feller DD. Relation of circulating red cell volume to body density and obesity. J Clin Invest 1956;35:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibson JG, Evans WA. Clinical studies of the blood volume. II. The relation of plasma and total blood volume to venous pressure, blood velocity rate, physical measurements, age and sex in ninety normal humans. J Clin Invest 1937;16:317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation 1977;56:605–12. [DOI] [PubMed] [Google Scholar]
- 39.Vricella LK, Louis JM, Chien E, Mercer BM. Blood volume determination in obese and normal-weight gravidas: the hydroxyethyl starch method. Am J Obstet Gynecol 2015;213:408.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander JK, Dennis EW, Smith WG, Amad KH, Duncan WC, Austin RC. Blood volume, cardiac output, and distribution of systemic blood flow in extreme obesity. Cardiovasc Res Cent Bull 1962–1963;1:39–44. [PubMed] [Google Scholar]
- 41.Silver HM, Seebeck M, Carlson R. Comparison of total blood volume in normal, preeclamptic, and nonproteinuric gestational hypertensive pregnancy by simultaneous measurement of red blood cell and plasma volumes. Am J Obstet Gynecol 1998;179:87–93. [DOI] [PubMed] [Google Scholar]
- 42.Ertl AC, Diedrich A, Raj SR. Techniques used for the determination of blood volume. Am J Med Sci 2007;334:32–6. [DOI] [PubMed] [Google Scholar]
- 43.Silver HM, Seebeck MA, Cowett RM, Patterson KY, Veillon C. Red cell volume determination using a stable isotope of chromium. J Soc Gynecol Investig 1997;4:254–8. [PubMed] [Google Scholar]
- 44.Tschaikowsky K, Meisner M, Durst R, Rugheimer E. Blood volume determination using hydroxyethyl starch: a rapid and simple intravenous injection method. Crit Care Med 1997;25:599–606. [DOI] [PubMed] [Google Scholar]