Abstract
This report addresses the evidence and the uncertainties, knowledge gaps, and research needs identified by participants at the NIH workshop related to iron screening and routine iron supplementation of largely iron-replete pregnant women and young children (6–24 mo) in developed countries. The workshop presentations and panel discussions focused on current understanding and knowledge gaps related to iron homeostasis, measurement of and evidence for iron status, and emerging concerns about supplementing iron-replete members of these vulnerable populations. Four integrating themes emerged across workshop presentations and discussion and centered on 1) physiologic or developmental adaptations of iron homeostasis to pregnancy and early infancy, respectively, and their implications, 2) improvement of the assessment of iron status across the full continuum from iron deficiency anemia to iron deficiency to iron replete to iron excess, 3) the linkage of iron status with health outcomes beyond hematologic outcomes, and 4) the balance of benefit and harm of iron supplementation of iron-replete pregnant women and young children. Research that addresses these themes in the context of the full continuum of iron status is needed to inform approaches to the balancing of benefits and harms of screening and routine supplementation.
Keywords: iron replete, iron excess, screening, supplementation, iron homeostasis, pregnancy, infancy, U-shaped risk curve
INTRODUCTION
The NIH Office of Dietary Supplements held a public workshop in September 2016 to consider the evidence, knowledge gaps, and research needs relevant to screening for iron status and supplementing iron-replete pregnant women and young children ages 6–24 mo, primarily in developed countries (1). The purpose of the workshop was not to develop clinical or policy recommendations. Although the importance of identifying and addressing iron deficiency (ID) was recognized, the workshop emphasized issues related to supplementation of iron-replete individuals given the limited attention to these concerns. Despite recognition of the importance of treating ID and ID anemia (IDA), the lower prevalence of ID and IDA in developed countries, particularly in the United States, Canada, and Europe, further focused the discussion on these largely iron-replete populations (2). Finally, the workshop concluded with discussions on integrating the scientific understandings surrounding screening and supplementation to inform identification of evidence gaps and research needs. This report addresses the integrating themes and their related facets; it does not serve as an inclusive summary of the entire workshop, which can be viewed online (1).
Four overarching and integrating themes emerged for pregnancy and young children related to iron screening and supplementation: 1) elucidating the adaptations in iron homeostasis to pregnancy and early infancy and implications for iron status and response to supplementation, 2) improving the assessment of iron status in pregnancy and young children, 3) linking iron status to maternal and infant health outcomes beyond hematology and IDA, and 4) supplementing iron-replete pregnant women and young children. These integrating themes were complementary; for each theme current knowledge is summarized followed by an exploration of the uncertainties, gaps in this knowledge, and research needs evident from the experts’ presentations and analyses as well as workshop discussion. Direct applicability to the concerns of the US Preventive Services Task Force (USPSTF) are highlighted where relevant, but the themes extended beyond the USPSTF focus. Addressing these research needs could inform the body of evidence that the USPSTF will consider when it next updates its guidelines and be generally useful to those interested in iron during pregnancy and young childhood.
An initial impetus for the workshop was the recent USPSTF conclusion that there was insufficient evidence to determine the benefits or harms of screening for anemia and iron supplementation of asymptomatic pregnant women (3) and screening for anemia in young children in the United States (4). This determination precluded the USPSTF from recommending for or against screening and universal supplementation.
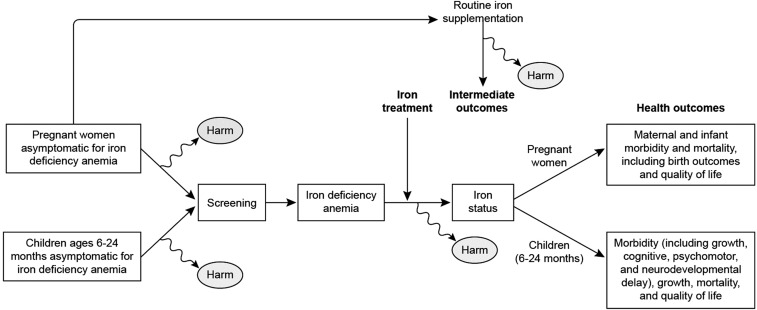
The health outcomes used in the USPSTF review are exemplified in the adapted analytic framework (Figure 1) from the systematic reviews that informed the USPSTF deliberations (5, 6). Importantly, these outcomes did not include hematologic measures, such as hemoglobin and serum ferritin (SF), which they considered as intermediate outcomes that have not been linked to relevant clinical outcomes. A number of other organizations, including the CDC (7), the American Academy of Pediatrics (8), and UpToDate (9), based current recommendations for screening and supplementation on hematologic measures and concluded that there are benefits for screening and supplementation. Yet the uncertainties in the evidence require scientific judgement, which results in different expert panels reaching different conclusions and recommendations from the same body of evidence. Thus, the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (10) and the Australian Department of Health (11) do not recommend supplementation of infants or pregnant women, respectively. For this reason, a discussion point during the workshop was the ability, as well as the need, to evaluate blood and serum measures for iron relative to relevant health outcomes.
FIGURE 1.
Integrated analytic framework developed to combine and illustrate the 3 analytic frameworks used by the US Preventive Services Task Force for benefits and harms of anemia screening and routine iron supplementation of pregnant women and young children 6–24 mo (5, 6).
ELUCIDATING ADAPTATIONS IN IRON HOMEOSTASIS TO PREGNANCY AND EARLY INFANCY: UNCERTAINTIES AND IMPLICATIONS
Iron homeostasis is tightly regulated after early infancy in response to 4 major factors—iron status, erythropoiesis, inflammation, and hypoxia—as discussed by Anderson and Frazer (12) and Lönnerdal (13) in this supplement issue. Briefly, hepcidin produced by the liver in response to these factors regulates both net intestinal absorption and mobilization of iron from its primary stores in hepatocytes. Thus, hepcidin ensures tissue iron needs are met but not exceeded. However, other findings suggest that physiologic adaptations occur in pregnancy that suppress hepcidin to a lower “setpoint” even while retaining responsiveness to these key factors (14). Circulating hepcidin concentrations decline in the second and third trimesters to nearly undetectable levels (15) even in iron-replete pregnant women (16). This “resetting” of iron homeostatic regulation increases intestinal iron absorption and availability from stores. In the suckling young infant and mammal, regulation of iron absorption by hepcidin may be refractory to some extent even though hepcidin and its target, ferroportin, are present (13). Limited evidence suggests that iron absorption is not regulated by iron supplementation or status before 6 mo in infants (17) or day 10 in rat pups (18, 19), but is regulated in older infants at 9 mo or rat pups at day 20. This developmental adaptation also results in increased iron absorption and availability. These adaptations in iron homeostasis occur when erythropoiesis and tissue iron needs are elevated in both pregnant women and young infants. The extent to which such adaptations might enhance susceptibility to high-level iron supplementation among iron-replete pregnant women and young infants is unknown. However, this possibility needs to be investigated because the primary protective mechanism preventing excess uptake and subsequent excess iron stores may not be fully operating under these adaptations in the hepcidin-dependent homeostatic regulation (12).
The mechanisms of iron homeostatic regulation “resetting” are not understood in either pregnancy or early infancy and likely differ between the two. In pregnancy, a regulatory factor not yet identified is proposed to reset the regulation of hepcidin to a lower level, even though it is still responsive to its usual regulation by iron status, erythropoiesis, inflammation, and hypoxia (14). Regulation of placental iron transport is also not well understood in response to either maternal status or fetal needs but requires elucidation, particularly with respect to the roles of maternal and fetal hepcidin (14, 20). Further research is needed to understand how maternal iron homeostatic regulation is reset to enhance dietary iron absorption and mobilization of iron from maternal stores to meet substantive maternal and fetal needs for iron, totaling ∼1000–1200 mg (14, 21).
Little is known about the mechanism that results in refractoriness of the iron homeostatic regulatory system in the young infant (<6 mo) (13). Typically, iron intakes of exclusively breastfed infants are low at 0.27 mg/d (13), and the high iron needs in the first 4–6 mo are met by the iron stores of 80 mg/kg (13). Conceivably, the evolution of infants and other mammals led to unrestrained absorption of limited dietary iron (2). However, with iron supplementation and iron-fortified formula, some young infants may be exposed to higher dietary intakes during this early period with less functional regulation to limit net iron absorption. Thus, the short- and long-term health outcomes of high iron intakes through either iron-fortified formula or direct supplementation of iron-replete infants need to be understood.
Other knowledge gaps exist relative to how effectively this adapted iron homeostatic regulation in pregnancy and early infancy responds to its usual regulators, such as iron status and inflammation, whether acutely with infection, chronically with obesity, or physiologically with mild inflammation in pregnancy. The effective response of the adapted iron homeostatic regulation is also not well understood relative to baseline prepregnancy iron status (replete, deficient, or excess) or longitudinal changes in iron status occurring throughout pregnancy. Limited evidence indicates the reset system does respond, but it is uncertain how effectively it does. Based on limited longitudinal studies, SF concentration, an indicator of iron stores, decreases to a nadir in the third trimester in healthy pregnancies (14). Also unclear is whether the initial reset is itself influenced by maternal or infant baseline status, whether low or high. Further, no information is available on the role of genetic factors and their differential distribution among ethnic subgroups, such as specific alleles of hemochromatosis protein and the iron transporter ferroportin (22). Also unknown is how factors posing a higher risk of adverse outcomes, such as preeclampsia (14), infections, adolescence (23), or excessive gestational weight gain, interact with this reset homeostatic regulation as well as what, if any, effect such an interaction has on health outcomes and iron status of the pregnant woman.
Iron is also differentially prioritized to developing tissues with competing high demands for iron (20). Briefly, erythropoiesis has priority for iron at the expense of the tissue needs of the brain and other organs during key developmental periods in utero and in infancy during a negative iron balance. Yet brain ID, which can occur without concomitant IDA, may have long-term adverse health outcomes related to cognitive and psychomotor development and function. The mechanisms, whereby differential tissue prioritization occurs, are unknown but need to be better understood to evaluate how adaptations in iron homeostatic regulation influence this prioritization and how this differential prioritization influences indicators and their interpretation relative to iron status.
In summary, iron homeostasis is altered in pregnancy and early infancy through physiologic and developmental adaptations in the hepcidin regulation of iron. These adaptations are likely critical to ensuring availability of iron to key erythropoietic, placental, and developing tissues with high iron demands during these developmental periods. However, the mechanisms whereby these adaptations occur are largely unknown, but they may increase vulnerability to excess iron absorption. Also unknown are many interrelated facets of how these adaptations affect homeostatic response to regulatory factors, including iron status and inflammation, response to supplementation, and either benefit or harms of routine supplementation across the full continuum of iron status from IDA to ID to iron repletion to iron excess.
IMPROVING ASSESSMENT OF IRON STATUS IN PREGNANCY AND YOUNG CHILDREN: CHALLENGES AND OPPORTUNITIES
Central to effective screening of asymptomatic pregnant women and young children is the full spectrum of iron status including IDA, ID, iron repletion, and iron excess. Such consideration becomes more important during pregnancy and young childhood because of the prioritization of iron to erythropoiesis in IDA even in the final and extreme stages of ID. Before the point when screening identifies IDA, the critical development of the brain, heart, and other tissues may already have been permanently impaired by ID in those tissues (12, 20). Further, the recent report by Petry et al. (24) suggests that only ∼25% of anemia is attributable to ID. Therefore, improvements in screening will depend in part on improving indicators that are particularly informative of tissue ID. Improvements will also require measures beyond hematologic indicators as well as indicators that have been evaluated relative to health outcomes, particularly critical for USPSTF priorities, and that can be reliably reproduced with minimal confounding by factors such as inflammation.
One challenge to measuring the full continuum of iron status is the lack of cutoffs established relative to health outcomes. Current cutoffs for pregnancy and young children reflect population-based cutoffs that are derived from the lowest percentile distribution, typically lower than the fifth percentile. As discussed by O’Brien and Ru (23), these cutoffs for anemia based on the hemoglobin concentration in pregnancy were established by the CDC based on 4 small longitudinal studies conducted >30 y ago. A strength of the CDC trimester-specific cutoffs is their consideration of hemodilution due to plasma volume expansion during pregnancy. However, as Vricella (25) notes, the gynecologic population was leaner and younger in the past with lower morbidity, such as gestational diabetes mellitus (GDM), which may have affected plasma volume expansion and, therefore, hemodilution. Currently available longitudinal studies of hemoglobin concentrations or other hematologic iron indicators, such as SF concentration, may be too limited to reconsider these population cutoffs. Thus, longitudinal studies in today’s gynecologic population might yield different cutoffs. For SF concentration, the same cutoffs, admittedly variable in range from <10 to <15 μg/L, are used for both nonpregnant women of reproductive age and pregnant women (25, 26) and, therefore, fail to consider hemodilution with plasma volume expansion. In addition, ranges of “normal” vary among clinical laboratories (26, 27). Total body iron stores (TBI), currently used in NHANES, is the log ratio of SF to soluble transferrin receptor (sTfR) concentrations and is likely independent of plasma volume expansion, although this has not been determined. Further, TBI has been evaluated only in a small number of adults, none of whom were pregnant (28). The measure is attractive, however, as it reflects the full range of iron stores from depleted (ID, IDA) to replete to excess even though agreed-on cutoffs for replete and excess have not been established (29).
The USPSTF noted a critical knowledge gap and research need related to whether changing hematologic indicators in pregnant women or young children reflects “meaningful improvements in health outcomes” (30). This gap reflects the lack of evaluation of the full spectrum of iron status with any currently used indicator relative to a nonhematologic health outcome (26). Yet, such health outcome–evaluated indicators would allow for meaningful cutoffs with clinical relevance for assessing the full continuum of iron status. In addition, the outcomes evaluated might differ in relation to low and high exposures. Although hematologic indicators are widely used in clinical, public health, and research settings, these have not been related to nonhematologic health outcomes even though their relation to anemia is well accepted. Hence, they can be considered intermediate outcomes. The value of relying on hematologic measures compared with the type of health outcomes specified in the USPSTF review may be debated, and the workshop could not resolve this specific point. The need to relate hematologic measures to health outcomes was recognized by the USPSTF and is important in terms of screening to ensure that ID before its extreme outcome of IDA can be identified and treated in pregnant women and young children to assure normal development of critical tissues.
Novel indicators of iron status are needed, especially for pregnant women and young children. Although of interest, hepcidin (14, 28) may be less suitable as an indicator in pregnant women and young infants because of the resetting of iron homeostasis discussed above. Emerging interest in erythropoietin in pregnant women (23) may not reflect how well iron needs of nonerythropoietic tissue are being met. Evaluation of TBI in pregnancy and young children might be helpful but may also primarily reflect erythropoietic iron needs. Key to the assessment of not only ID but also iron repletion and excess in pregnancy and young children must be consideration of indicators beyond those exclusive to hematologic outcomes. Innovative research with the use of system biology approaches, including proteomics, metabolomics, and genomics, may offer opportunities to explore novel indicators of the full continuum of iron status, differential tissue iron availability, and health outcomes.
Limitations in methods, including assay procedures and standardization as well as confounding (especially by inflammation) of common hematologic indicators for iron status, result in the potential for misclassification and serve to challenge interpretation. Collectively, these limit the precision, accuracy, and thereby the determination of the prevalence of not only IDA and ID in these vulnerable populations but conditions of iron repletion and iron excess as well. Although WHO international standard materials based on “consensus” values are available for some indicators (i.e., SF concentration) and in development for others (i.e., sTfR), there are no standard reference methods and, thus, no standard reference materials. Hoofnagle (27) noted that in the absence of standardization imprecise measures and inflated CIs can occur and in turn present interpretative challenges. Developing standard reference methods will likely prove challenging because of the relatively low abundance of the proteins to be measured (27, 28). What might be helpful to the field are quality assurance and harmonization programs. Without such programs, synthesizing the evidence across studies is difficult because different indicators and different cutoffs are used. These methodologic and resultant interpretational challenges in assessing iron status may result in misclassification of individuals as iron deplete when they may be iron replete or vice versa.
A key confounder of the current hematologic indicators of iron status is inflammation because many of these indicators are acute-phase proteins, including SF (2, 27, 28, 31, 32) and perhaps to a lesser extent sTfR (32). Even hepcidin concentration, the promising emergent indicator, is an acute-phase protein and will, therefore, be confounded by inflammation. However, the hepcidin-mediated anti-infective mechanism also serves to limit iron availability to tissues in need and can result in an anemia of chronic disease. The multinational project Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia (BRINDA) explores approaches to adjust indicators of iron status for inflammation by using linear regression models based on indicators of acute (C-reactive protein) and chronic (α1-acid glycoprotein) inflammation. These adjustments have been developed and applied to SF concentration and TBI to reassess the prevalence of ID and IDA (32). For US women of reproductive age, the adjustment of TBI from NHANES resulted in a small increase in ID prevalence of 7% points. These linear regression adjustments, however, were derived from multinational, cross-sectional datasets that did not include pregnant women but did include preschool children (age 6–59 mo). Thus, it remains unknown if such approaches based on linear regression and predictive adjustments are suitable for adjustment for inflammation during pregnancy. Enhancing concern about whether this approach would be feasible in pregnant women is the lack of correlation between hepcidin concentration and inflammation during pregnancy, although this correlation has not been evaluated in the presence of infection or severe inflammation (14). Furthermore, indicators of inflammation do not correlate with indicators of iron status in pregnant adolescents except at delivery (23). The relation of current iron indicators with inflammation during pregnancy remains to be determined as do potential approaches for adjusting for inflammation if such a relation is demonstrated. A related knowledge gap concerns the relation of inflammation in early infancy and iron homeostasis and status.
In summary, critical knowledge gaps and methodologic challenges limit the accurate assessment of iron status in individuals and populations throughout the life cycle. Furthermore, current indicators use population-based cutoffs that may not be appropriate for current older and more obese pregnant women. Lacking are indicators that are not confounded by inflammation or that can be appropriately adjusted for inflammation specifically in pregnancy and young infants and that are established relative to health outcomes relevant to pregnancy and young childhood. Finally, novel indicators of iron status beyond anemia and hematologic intermediate outcomes are needed to ensure that iron needs of less prioritized but critically important tissues, such as the heart and brain, are met to avoid permanent damage to function of these tissues.
LINKING IRON STATUS TO MATERNAL AND INFANT HEALTH OUTCOMES BEYOND HEMATOLOGIC INDICATORS AND IDA
A critical evidence gap identified by the USPSTF (30) based on its systematic review of the evidence (5, 6) was the lack of any study that investigated the impact of the changing iron status of pregnant women on maternal health outcomes. Some evidence was identified on the relation between maternal iron status and infant health outcomes, including birth weight. However, the evidence was limited because of inconsistent findings and small effect sizes within normal ranges for those reporting improved outcomes with maternal iron supplementation. Presentations and discussion at the workshop echoed this lack of evidence linking iron status to health outcomes either in terms of indicators of iron status (26) or studies examining changes in iron status (13, 14, 21, 23, 33, 34). Both the USPSTF (1) and the discussion at the workshop noted evidence that supplementation or fortification improved hematologic indicators. It is important to understand the distinction between health outcomes and hematologic indicators. The USPSTF defines health outcomes of interest as “patient-centered,” namely those outcomes that a patient can perceive. The Agency for Healthcare Research and Quality also defines health outcomes in terms of general health or quality-of-life outcomes, which aligns with the USPSTF definition of patient-centered health outcomes (35). For screening and routine supplementation, the USPSTF considered as possible health outcomes maternal and child mortality and morbidity; birth outcomes, such as preterm birth and low birth weight; growth; developmental (cognitive, neurodevelopmental delay, psychomotor) outcomes; and quality of life (Figure 1). Other maternal health outcomes, such as cognition and depression, have not been considered and were not discussed during the workshop but are also potential outcomes to assess. What is not included in either USPSTF or Agency for Healthcare Research and Quality definitions are indicators of status except when they are intermediate outcomes linked to a health outcome. For example, the USPSTF only considers changes in “biometric measures,” such as hematologic indicators of iron status, relevant when they are linked to patient-centered outcomes, which establishes them as intermediate outcomes (30). Thus, among currently used indicators for iron status, only anemia and IDA would be considered biometric measures linked to a patient-centered health outcome, i.e., fatigue. However, in most research, indicators and not patient-centered outcomes are reported. This evidence gap highlights the research need to identify relevant health outcomes perceivable by the patient or indicators validated to such health outcomes.
Added impetus for identifying and prioritizing health outcomes or establishing intermediate outcomes comes from the paucity of evidence demonstrating that improving the iron status of pregnant women with ID and their neonates affects health outcomes. Although pregnant women with IDA give birth to neonates at a higher risk of low iron stores, this is an intermediate, not a health, outcome. This evidence gap is not to say that one can conclude that leaving ID or IDA untreated is supported by the evidence (13, 26, 33). Rather, it says that the evidence does not demonstrate the benefit, in part because many studies examine only intermediate outcomes and hematologic indicators. Consideration of the short- and long-term impact on health outcomes requires consideration of differential tissue-specific prioritization of iron. Identification of tissue-specific outcomes is critical because the damage to lower-priority developing tissues, such as the brain, appear to be permanent and, thus, must be prevented to avoid adverse effects. The USPSTF sums up the critical research need simply as understanding whether improving the measurement of hematologic indicators of iron status results in “meaningful improvements in health outcomes” among asymptomatic pregnant women and young children (30).
SUPPLEMENTING IRON-REPLETE PREGNANT WOMEN AND YOUNG CHILDREN: ISSUES AND UNCERTAINTIES
Iron supplementation of pregnant women or young children who have diagnosed IDA or ID has proven beneficial in restoring hematologic indicators of iron (26, 30) as noted above, but the benefits and harms are uncertain for providing routine iron supplementation to largely iron-replete pregnant women and young infants in developed countries. Yet adaptations of iron homeostasis to pregnancy and early infancy raise the possibility that higher absorption rates might, with high intakes, result in excess iron exposure in those who are iron replete or have high stores, but further research is needed to examine this possibility. Excess iron accumulates preferentially in some target tissues, such as the brain, stem cells, and erythropoietic cells (36) as well as the pancreas (37). As a pro-oxidant, excess iron can result in increased reactive oxygen species, oxidative stress, and potentially oxidative damage (36). Given the regulation of transferrin-bound iron uptake by cells, the focus has been on the less-regulated uptake of non–transferrin bound iron (NTBI), which has been associated with oxidative damage and cytotoxicity (38). Although once believed to occur only at high exposure, recent evidence indicates that NTBI occurs in women of reproductive age transiently within 2 h after consuming a supplement with a meal and to an even greater degree without a meal (38). However, the magnitude of NTBI concentrations has not been determined after the consumption of supplemental iron or iron-fortified formula in pregnant women or young infants. Research is needed to determine the NTBI response in both pregnant women and young infants, given the adaptations of iron homeostasis that result in enhanced absorption and the emerging evidence of risk of adverse outcomes with high iron exposure among those who are iron replete. Such exposure might lead to elevated iron stores (high iron status), albeit not to a toxic overload. Furthermore, understanding the relations among baseline iron status, iron supplementation dosage, NTBI concentrations postsupplement, and these adverse outcomes is important in developing approaches to supplementing these populations.
Emerging evidence of adverse outcomes of supplementation or high iron status in iron-replete pregnant women and young children derives from studies in both developed and developing countries linking high iron status to a variety of health outcomes including GDM (observational case-control and prospective cohort studies and limited randomized controlled trials), preterm birth (observational studies and randomized controlled trials), and impaired fetal growth (observational studies) (2, 33, 37). The nature of this evidence is summarized in the evidence map presented in Table 1 in terms of the types of evidence (mechanistic, observational, or randomized controlled trials) with a summary of the nature of the totality of the evidence. Consistent evidence indicates congruent results across the studies, whereas inconsistent evidence indicates discordant results across studies. The limited availability of evidence and its inconsistency is readily apparent for most outcomes through this evidence map, as the inconsistent findings for GDM reflect. Further, high iron status has been measured by using various hematologic indicators, including hemoglobin and SF concentrations in the observational studies. Associations with high hemoglobin concentrations are potentially confounded by a failure in plasma volume expansion rather than high iron status. Associations with high SF concentrations are potentially confounded by inflammation. Further, some of these adverse outcomes, such as GDM and preterm birth, also associate with inflammation. Understanding the individual contributions and the interaction of high iron exposure and inflammation in these adverse outcomes is needed.
TABLE 1.
Evidence map for adverse health outcomes associated with high SF concentration, high hemoglobin concentration, high iron intake, or iron supplementation in pregnant women and young children1
| Study type |
||||||||
| Observational |
RCT |
|||||||
| Health outcome | Mechanistic: in vitro | Retrospective | Case control2 | Prospective | Primary | Secondary | Nature of evidence based on iron exposure indicator | |
| GDM/T2D-PP3 | —4 | √ | √ | √ | √ | Supplementation: inconsistent5 SF concentration: consistent;6 intake: inconsistent |
||
| Preterm birth7 | √ | √ | √ | Hemoglobin concentration: inconsistent across trimesters SF concentration; consistent |
||||
| Impaired fetal growth8 | √ | √ | √ | Supplementation: consistent; hemoglobin concentration: inconsistent across trimesters SF concentration: inconsistent |
||||
| Impaired infant/child growth9 | √ | Supplementation to iron replete: consistent | ||||||
| Long-term impaired cognitive development10 | √ | Supplementation to iron replete: limited | ||||||
| Diarrhea11 | √ | √ | Supplementation or fortification: inconsistent | |||||
| Intermediate outcome | ||||||||
| Microbiome change12 | √ | √ | √ | Supplementation: inconsistent | ||||
A check (√) indicates available evidence for each study type. GDM, gestational diabetes mellitus; RCT, randomized controlled trial; SF, serum ferritin; T2D-PP, type 2 diabetes postpartum.
Includes nested studies.
Only one animal study (rats) on high-fructose diet induced GDM; there was no additional effect on GDM with a moderate 83% increase in dietary iron (49).
Inconsistent indicates discordant results. In this case, discordant results for supplemental iron and GDM from observational studies and RCT.
Consistent indicates concordant results. In this case, concordant results reported associating SF with GDM risk from observational studies.
From reference 61.
From reference 62.
Other emerging evidence from supplementation trials reveals an increased risk of diarrhea and altered microbiome profiles (34, 67) and impaired linear growth in iron-replete infants (67). In addition, neurodegenerative diseases, such as Parkinson and Alzheimer diseases, have been associated with high brain iron concentrations and high dietary iron intakes in adult animal models but have not been studied relative to vulnerable periods when iron could be accumulated in utero and in early infancy (36). Yet concern exists regarding the effect of high iron exposure in utero and in early infancy on the development of chronic disease in the adult offspring, particularly for neurodegenerative diseases. Further, embryonic and adult stem cells may be vulnerable to high iron exposure, resulting in altered cell fates and differentiation, which have led to ineffective erythropoiesis and anemia in selected animal models. Such an adverse impact on stem cells could contribute to developmental origins of health and disease (DOHaD) (36).
Research is needed to evaluate the relation of high iron exposure in pregnancy and young children relative to these health and intermediate outcomes given the emerging and uncertain nature of this evidence. Factors to be considered in future research include the mechanisms whereby high iron exposure in these critical periods may affect short- and long-term adverse health outcomes. A possible mechanism considered may be oxidative stress and damage of pancreatic β cells resulting in diabetes (37), of brain cells resulting in neurodegenerative diseases (36), and of stem cells potentially contributing to DOHaD (36). Although not yet studied, the role of iron in altering the epigenome should be explored as a mechanism of its role in DOHaD. In addition, unabsorbed iron from supplements may promote differentially the pathogenic gastrointestinal microbiome to shift the microbiome profile, a proposed mechanism supported by in vitro model systems (34). The mechanisms explaining how iron supplementation of iron-replete infants may impair growth in some contexts need to be determined. Of particular interest are the interactions of excess iron with key nutrients important for growth, such as zinc and copper (67). The mechanisms underlying the risk of adverse outcomes with high exposure to dietary iron during pregnancy and young childhood need to be understood and might inform development of supplements and fortificants with minimal risk. Factors that need to be clarified include dosage, developmentally vulnerable periods, and the interaction of baseline iron status with supplementation in terms of dosage and the form of the dietary iron (heme or nonheme) or supplement (chemical form). Research is needed to determine what underlies the heterogeneity of response to supplementation (33). Some of the aforementioned factors could contribute to this heterogeneity of response, as could genetic factors. Research also needs to assess whether specific genetic alleles in hemochromatosis protein that increase the risk of excess iron while protecting against ID (22) interact with high iron exposure, resulting in an increased risk of adverse outcomes. Environmental context has also been posited as a factor in heterogeneity of microbiome and diarrheal response to iron supplementation (34), but these contexts have not been explored or identified. Overall, the emerging and uncertain evidence for a diverse array of adverse outcomes of iron supplementation or high iron status and its potential interaction with inflammation emphasizes the need to consider the balance of benefits and harms of supplementation of largely iron-replete pregnant women and young children. Little attention has been paid systematically to the right side of the U-shaped risk curve for iron with high intakes, but the emerging evidence suggests that such investigation is needed (2).
RESEARCH NEEDS AND OPPORTUNITIES
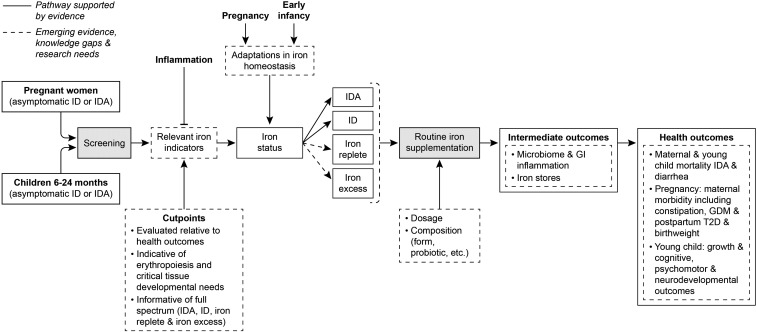
Knowledge gaps, major research needs, and sample-specific topics were identified for each of the 4 integrating themes across the workshop presentations and discussion (Table 2). These themes and research needs center on 1) elucidating the mechanisms, impact, and interaction with baseline status associated with the physiologic and developmental adaptations in iron homeostasis to pregnancy and early infancy, 2) improving the assessment of iron status across the full continuum from IDA to ID to iron replete to iron excess, 3) determining the relation of iron status to the health outcomes most relevant to pregnant women and young children, and 4) determining the balance of benefit and harm of iron supplementation of pregnant women and young children. An analytic framework in Figure 2 depicts the pathway from screening to supplementation to health outcomes, as well as the gaps in our knowledge that relate directly to these themes and research needs. Such research is needed to inform the balancing of benefits and harms of screening and routine supplementation of those at both low and high iron status in the face of increasing mild inflammation from obesity and higher exposure to iron through fortification of the food supply in developed countries. The findings will advance our understanding of the U-shaped risk of health outcomes with both ID and iron excess. The ultimate goal of these identified tasks is to inform better those who develop recommendations for clinical practice and who formulate public health policy. However, the workshop did not and could not reflect clinical guidance. Such guidance evolves as the evidence does. Thus, practitioners and policy makers will need to incorporate newer understandings about iron homeostasis relative to iron needs for these vulnerable groups along with the impact of improved methodologies for determining iron status as they are published. This will ensure adequacy of iron during the critical development periods in these populations.
TABLE 2.
Integrating themes and related research needs identified by 2016 NIH workshop on iron screening and supplementation of pregnant women and young children (6–24 mo)1
| Research needs |
||
| Integrating themes | Major focal area | Examples of specific topics |
| Elucidating adaptations of iron homeostasis to pregnancy and early infancy: uncertainties and implications | Mechanism(s) of “reset” of hepcidin to lower regulatory level | Molecular regulation in pregnancy and early infancy Relative roles of systemic and local regulatory factors Relative maternal and fetal regulation of placental iron transport Responsiveness to iron status of mother and fetus (baseline, supplementation, maternal dietary forms and bioavailability, non–transferrin bound iron, etc.) |
| Responsiveness to other drivers of hepcidin-regulated homeostasis | Role of inflammation (physiologic, obesity, infection, etc.), erythropoiesis, and hypoxia | |
| Interaction with genetic and ethnic factors | Role of alleles of HFE and ferroportin in this adaptation | |
| Differential prioritization of iron to maternal, fetal and infant tissues during pregnancy, development and growth | Mechanism of this differential prioritization and its relation to adapted iron homeostasis | |
| Improving assessment of iron status: challenges and opportunities in pregnancy and young children | Measurement | Standardization and accuracy |
| Appropriateness of indicators across the full spectrum of iron status (ID, IDA, iron repletion, and iron excess) during pregnancy and young childhood | Cutoffs and interpretation relative to health outcomes beyond ID and IDA | |
| Limited assessment of infants aged 0–12 mo in the United States | Development of noninvasive measures or blood spot methods for use in large surveys | |
| Inflammation | Validation for correction during pregnancy and in young infants Identification and validation of indicators not subject to confounding by inflammation |
|
| Innovative and nonhematological measures | Identification and validation of nonhematologic indicators that enable the assessment of ID of tissues with lower priority for iron before IDA Use of system biology approaches (proteomics, metabolomics, and genomics) to identify relevant indicators of early ID |
|
| Linking iron status to maternal and infant health outcomes: beyond hematology and IDA | Prioritization and relevance of health outcomes | Determine which outcomes are most informative of iron status across the full spectrum from IDA to ID to iron replete to iron excess |
| Indicators of differential tissue prioritization | Evaluate indicators relative to health outcomes relevant to specific tissues, such as neurodevelopmental delay, cognitive development, and psychomotor development | |
| Supplementing iron-replete pregnant women and young children: issues and uncertainties | Prioritization and relevance of possible short- and long-term health outcomes | Maternal GDM and postpartum T2D Fetal and young child growth Maternal and young child morbidity (diarrhea, constipation, etc.) Early iron excess and neurodegenerative disease, cognitive development, psychomotor development to understand the role of excess iron, and DOHaD Maternal and neonatal mortality |
| Determination of mechanisms of heterogeneity of response | Interaction of high dietary iron with alleles of HFE Environmental and personal factors of responders and nonresponders |
|
| Determination of short- and long-term intermediate outcomes | Microbiome profiles, GI inflammation, stem cell alterations, damage to β cells, and epigenome alterations | |
DOHaD, developmental origins of health and disease; GDM, gestational diabetes mellitus; GI, gastrointestinal; HFE, hemochromatosis protein; ID, iron deficiency; IDA, iron deficiency anemia; T2D, type 2 diabetes.
FIGURE 2.
Analytic framework reflecting emerging science, knowledge gaps, and research needs for the pathway from universal screening of asymptomatic pregnant women and young children 6–24 mo (including routine iron supplementation) to relevant health outcomes as conceptualized by using discussions that took place throughout the workshop on screening and supplementation of iron-replete pregnant women and young children (1). Solid lines reflect pathway components supported by current evidence; dashed lines reflect pathway components suggested by emerging evidence or indicative of gaps in our knowledge for which additional research is needed. GDM, gestational diabetes mellitus; GI, gastrointestinal; ID, iron deficiency; IDA, iron deficiency anemia; T2D, type 2 diabetes.
Acknowledgments
We thank Elizabeta Nemeth, Michael Georgieff, and Gary Brittenham for their helpful input in developing this manuscript.
The authors’ responsibilities were as follows—PMB: developed the manuscript; CLT and PJS: critiqued and provided input; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: DOHaD, developmental origins of health and disease; GDM, gestational diabetes mellitus; ID, iron deficiency; IDA, iron deficiency anemia; NTBI, non–transferrin bound iron; SF, serum ferritin; sTfR, soluble transferrin receptor; TBI, total body iron stores; USPSTF, US Preventive Services Task Force.
REFERENCES
- 1.NIH Office of Dietary Supplements. Iron screening and supplementation in iron-replete pregnant women and young children workshop, September 28–29 [Internet]. c2016 [cited 2017 Feb 13]. Available from: https://ods.od.nih.gov/pubs/NIH_Iron_Workshop_Agenda.pdf.
- 2.Taylor CL, Brannon PM. Introduction to workshop on iron screening and supplementation in iron-replete pregnant women and young children. Am J Clin Nutr 2017;106(Suppl):1547S–54S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siu AL; US Preventive Services Task Force. Screening for iron deficiency anemia and iron supplementation in pregnant women to improve maternal health and birth outcomes: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2015;163:529–36. [DOI] [PubMed] [Google Scholar]
- 4.Siu AL; US Preventive Services Task Force. Screening for iron deficiency anemia in young children: USPSTF recommendation statement. Pediatrics 2015;136:746–52. [DOI] [PubMed] [Google Scholar]
- 5.McDonagh M, Cantor A, Bougatsos C, Dana T, Blazina I. Routine iron supplementation and screening for iron deficiency anemia in pregnant women: a systematic review to update the U.S. Preventive Services Task Force recommendation. Rockville (MD): Agency for Healthcare Research and Quality; 2015. [PubMed] [Google Scholar]
- 6.McDonagh MS, Blazina I, Dana T, Cantor A, Bougatsos C. Screening and routine supplementation for iron deficiency anemia: a systematic review. Pediatrics 2015;135:723–33. [DOI] [PubMed] [Google Scholar]
- 7.CDC. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep 1998;47:1–29. [PubMed] [Google Scholar]
- 8.Baker RD, Greer FR; Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics 2010;126:1040–50. [DOI] [PubMed] [Google Scholar]
- 9.Garner CD. Nutrition in pregnancy In: Post TW, editor. UpToDate. Waltham (MA): UpToDate; 2017. [Google Scholar]
- 10.Domellöf M, Braegger C, Campoy C, Colomb V, Decsi T, Fewtrell M, Hojsak I, Mihatsch W, Molgaard C, Shamir R, et al. Iron requirements of infants and toddlers. J Pediatr Gastroenterol Nutr 2014;58:119–29. [DOI] [PubMed] [Google Scholar]
- 11.Australian Government Department of Health. Clinical practice guidelines antenatal care - module I 10.4 nutritional supplements [Internet]. c2013 [cited 2017 May 5]. Available from: http://www.health.gov.au/internet/publications/publishing.nsf/Content/clinical-practice-guidelines-ac-mod1~part-b~lifestyle-considerations~nutritional-supplements.
- 12.Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr 2017;106(Suppl):1559S–66S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lönnerdal B. Development of iron homeostasis in infants and young children. Am J Clin Nutr 2017;106(Suppl):1575S–80S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr 2017;106(Suppl):1567S–74S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Santen S, Kroot JJ, Zijderveld G, Wiegerinck ET, Spaanderman ME, Swinkels DW. The iron regulatory hormone hepcidin is decreased in pregnancy: a prospective longitudinal study. Clin Chem Lab Med 2013;51:1395–401. [DOI] [PubMed] [Google Scholar]
- 16.Rehu M, Punnonen K, Ostland V, Heinonen S, Westerman M, Pulkki K, Sankilampi U. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol 2010;85:345–52. [DOI] [PubMed] [Google Scholar]
- 17.Domellöf M, Lönnerdal B, Abrams SA, Hernell O. Iron absorption in breast-fed infants: effects of age, iron status, iron supplements, and complementary foods. Am J Clin Nutr 2002;76:198–204. [DOI] [PubMed] [Google Scholar]
- 18.Leong WI, Bowlus CL, Tallkvist J, Lönnerdal B. Iron supplementation during infancy–effects on expression of iron transporters, iron absorption, and iron utilization in rat pups. Am J Clin Nutr 2003;78:1203–11. [DOI] [PubMed] [Google Scholar]
- 19.Leong WI, Bowlus CL, Tallkvist J, Lönnerdal B. DMT1 and FPN1 expression during infancy: developmental regulation of iron absorption. Am J Physiol Gastrointest Liver Physiol 2003;285:G1153–61. [DOI] [PubMed] [Google Scholar]
- 20.Georgieff MK. Iron assessment to protect the developing brain. Am J Clin Nutr 2017;106(Suppl):1588S–93S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milman N, Taylor CL, Merkel J, Brannon PM. Iron status in pregnant women and women of reproductive age in Europe. Am J Clin Nutr 2017;106(Suppl):1655S–62S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordeuk VR, Brannon PM. Ethnic and genetic factors of iron status in women of reproductive age. Am J Clin Nutr 2017;106(Suppl):1594S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien KO, Ru Y. Iron status of North American pregnant women: an update on longitudinal data and gaps in knowledge from the United States and Canada. Am J Clin Nutr 2017;106(Suppl):1647S–54S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petry N, Olofin I, Hurrell RF, Boy E, Wirth JP, Moursi M, Donahue Angel M, Rohner F. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients 2016;8:E693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vricella LK. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am J Clin Nutr 2017;106(Suppl):1620S–5S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daru J, Colman K, Stanworth SJ, De La Salle B, Wood EM, Pasricha S-R. Serum ferritin as an indicator of iron status: what do we need to know? Am J Clin Nutr 2017;106(Suppl):1634S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoofnagle AN. Harmonization of blood-based indicators of iron status: making the hard work matter. Am J Clin Nutr 2017;106(Suppl):1615S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr 2017;106(Suppl):1606S–14S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta PM, Hamner HC, Suchdev PS, Flores-Ayala R, Mei Z. Iron status of toddlers, nonpregnant females, and pregnant females in the United States. Am J Clin Nutr 2017;106(Suppl):1640S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemper AR, Fan T, Grossman DC, Phipps MG. Gaps in evidence regarding iron deficiency anemia in pregnant women and young children: summary of US Preventive Services Task Force recommendations. Am J Clin Nutr 2017;106(Suppl):1555S–58S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross AC. Impact of chronic and acute inflammation on extra- and intracellular iron homeostasis. Am J Clin Nutr 2017;106(Suppl):1581S–7S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suchdev PS, Williams AM, Mei Z, Flores-Ayala R, Pasricha S-R, Rogers LM, Namaste SML. Assessment of iron status in settings of inflammation: challenges and potential approaches. Am J Clin Nutr 2017;106(Suppl):1626S–33S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dewey KG, Oaks BM. U-shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. Am J Clin Nutr 2017;106(Suppl):1694S–702S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paganini D, Zimmermann MB. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: a review. Am J Clin Nutr 2017;106(Suppl):1688S–93S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velentgas P, Dreyer NA, Wu AW. Outcome definition and measurement In: Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, editors. Developing a protocol for observational comparative effectiveness research: a user’s guide AHRQ Publication No 12(13)-EHC099. Rockville (MD): Agency for Healthcare Research and Quality; 2013. p. 71–92. [PubMed] [Google Scholar]
- 36.Wessling-Resnick M. Excess iron: considerations related to development and early growth. Am J Clin Nutr 2017;106(Suppl):1600S–5S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, Rawal S. Dietary iron intake, iron status, and gestational diabetes. Am J Clin Nutr 2017;106(Suppl):1672S–80S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brittenham GM, Andersson M, Egli I, Foman JT, Zeder C, Westerman ME, Hurrell RF. Circulating non-transferrin-bound iron after oral administration of supplemental and fortification doses of iron to healthy women: a randomized study. Am J Clin Nutr 2014;100:813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu S, Li F, Zhou J, Liu Z. The relationship between body iron status, iron intake and gestational diabetes: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darling AM, Mitchell AA, Werler MM. Preconceptional iron intake and gestational diabetes mellitus. Int J Environ Res Public Health 2016;13:E525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bao W, Chavarro JE, Tobias DK, Bowers K, Li S, Hu FB, Zhang C. Long-term risk of type 2 diabetes in relation to habitual iron intake in women with a history of gestational diabetes: a prospective cohort study. Am J Clin Nutr 2016;103:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bo S, Menato G, Villois P, Gambino R, Cassader M, Cotrino I, Cavallo-Perin P. Iron supplementation and gestational diabetes in midpregnancy. Am J Obstet Gynecol 2009;201:158.e1– 6. [DOI] [PubMed] [Google Scholar]
- 43.Helin A, Kinnunen TI, Raitanen J, Ahonen S, Virtanen SM, Luoto R. Iron intake, haemoglobin and risk of gestational diabetes: a prospective cohort study. BMJ Open 2012;2:e001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowers K, Yeung E, Williams MA, Qi L, Tobias DK, Hu FB, Zhang C. A prospective study of prepregnancy dietary iron intake and risk for gestational diabetes mellitus. Diabetes Care 2011;34:1557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowers KA, Olsen SF, Bao W, Halldorsson TI, Strm M, Zhang C. Plasma concentrations of ferritin in early pregnancy are associated with risk of gestational diabetes mellitus in women in the Danish National Birth Cohort. J Nutr 2016;146:1756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Scholl TO, Stein TP. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: the Camden study. Diabetes Care 2006;29:1077–82. [DOI] [PubMed] [Google Scholar]
- 47.Chan KK, Chan BC, Lam KF, Tam S, Lao TT. Iron supplement in pregnancy and development of gestational diabetes–a randomised placebo-controlled trial. BJOG 2009;116:789–97, discussion 97–8. [DOI] [PubMed] [Google Scholar]
- 48.Kinnunen TI, Luoto R, Helin A, Hemminki E. Supplemental iron intake and the risk of glucose intolerance in pregnancy: re-analysis of a randomised controlled trial in Finland. Matern Child Nutr 2016;12:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zein S, Sitti F, Osman M, Arnaud J, Batandier C, Gauchez AS, Rachidi S, Couturier K, Hininger-Favier I. Middle iron-enriched fructose diet on gestational diabetes risk and on oxidative stress in offspring rats. Biol Trace Elem Res 2017;175:405–13. [DOI] [PubMed] [Google Scholar]
- 50.Tamura T, Goldenberg RL, Johnston KE, Cliver SP, Hickey CA. Serum ferritin: a predictor of early spontaneous preterm delivery. Obstet Gynecol 1996;87:360–5. [DOI] [PubMed] [Google Scholar]
- 51.Goldenberg RL, Tamura T, DuBard M, Johnston KE, Copper RL, Neggers Y. Plasma ferritin and pregnancy outcome. Am J Obstet Gynecol 1996;175:1356–9. [DOI] [PubMed] [Google Scholar]
- 52.Scholl TO. High third-trimester ferritin concentration: associations with very preterm delivery, infection, and maternal nutritional status. Obstet Gynecol 1998;92:161–6. [DOI] [PubMed] [Google Scholar]
- 53.Lao TT, Tam KF, Chan LY. Third trimester iron status and pregnancy outcome in non-anaemic women; pregnancy unfavourably affected by maternal iron excess. Hum Reprod 2000;15:1843–8. [DOI] [PubMed] [Google Scholar]
- 54.Hwang JY, Lee JY, Kim KN, Kim H, Ha EH, Park H, Ha M, Kim Y, Hong YC, Chang N. Maternal iron intake at mid-pregnancy is associated with reduced fetal growth: results from Mothers and Children’s Environmental Health (MOCEH) study. Nutr J 2013;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dewey KG, Domellöf M, Cohen RJ, Landa Rivera L, Hernell O, Lönnerdal B. Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J Nutr 2002;132:3249–55. [DOI] [PubMed] [Google Scholar]
- 56.Domellöf M, Cohen RJ, Dewey KG, Hernell O, Rivera LL, Lönnerdal B. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J Pediatr 2001;138:679–87. [DOI] [PubMed] [Google Scholar]
- 57.Majumdar I, Paul P, Talib VH, Ranga S. The effect of iron therapy on the growth of iron-replete and iron-deplete children. J Trop Pediatr 2003;49:84–8. [DOI] [PubMed] [Google Scholar]
- 58.Lind T, Seswandhana R, Persson LÅ, Lönnerdal B. Iron supplementation of iron-replete Indonesian infants is associated with reduced weight-for-age. Acta Paediatr 2008;97:770–5. [DOI] [PubMed] [Google Scholar]
- 59.Idjradinata P, Watkins WE, Pollitt E. Adverse effect of iron supplementation on weight gain of iron-replete young children. Lancet 1994;343:1252–4. [DOI] [PubMed] [Google Scholar]
- 60.Pasricha SR, Hayes E, Kalumba K, Biggs BA. Effect of daily iron supplementation on health in children aged 4-23 months: a systematic review and meta-analysis of randomised controlled trials. Lancet Glob Health 2013;1:e77–86. [DOI] [PubMed] [Google Scholar]
- 61.Lozoff B, Castillo M, Clark KM, Smith JB. Iron-fortified vs low-iron infant formula: developmental outcome at 10 years. Arch Pediatr Adolesc Med 2012;166:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paganini D, Uyoga MA, Zimmermann MB. Iron fortification of foods for infants and children in low-income countries: effects on the gut microbiome, gut inflammation, and diarrhea. Nutrients 2016;8:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dostal A, Lacroix C, Bircher L, Pham VT, Follador R, Zimmermann MB, Chassard C. Iron modulates butyrate production by a child gut microbiota in vitro. MBio 2015;6:e01453–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015;64:731–42. [DOI] [PubMed] [Google Scholar]
- 65.Dostal A, Baumgartner J, Riesen N, Chassard C, Smuts CM, Zimmermann MB, Lacroix C. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: a randomised, placebo-controlled intervention trial in South African children. Br J Nutr 2014;112:547–56. [DOI] [PubMed] [Google Scholar]
- 66.Zimmermann MB, Chassard C, Rohner F, N’Goran EK, Nindjin C, Dostal A, Utzinger J, Ghattas H, Lacroix C, Hurrell RF. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d’Ivoire. Am J Clin Nutr 2010;92:1406–15. [DOI] [PubMed] [Google Scholar]
- 67.Lönnerdal B. Excess iron intake as a factor in growth, infections, and development of infants and young children. Am J Clin Nutr 2017;106(Suppl):1681S–7S. [DOI] [PMC free article] [PubMed] [Google Scholar]