Abstract
Pregnant women are particularly vulnerable to iron deficiency due to the high iron demands of pregnancy. To avoid the adverse birth outcomes that are associated with maternal iron deficiency anemia, both Canada and the United States recommend universal iron supplementation for pregnant women. Although the benefits of iron supplementation in anemic women are well recognized, insufficient data are currently available on the maternal and neonatal benefits and harms of universal iron supplementation in developed countries as evidenced by the recent conclusions of the US Preventive Services Task Force on the need for further data that address existing gaps. As part of an effort to evaluate the impact of the current North American prenatal iron supplementation policy, this review highlights the lack of national data on longitudinal changes in iron status in pregnant North American women, emphasizes possible limitations with the original longitudinal hemoglobin data used to inform the current CDC reference hemoglobin values, and presents additional normative data from recent longitudinal research studies of iron status in North American pregnant women. Further longitudinal data in North American pregnant women are needed to help identify those who may benefit most from supplementation as well as to help determine whether there are adverse effects of iron supplementation in iron-replete women.
Keywords: pregnancy, anemia, hemoglobin, ferritin, inflammation, erythropoietin, serum transferrin receptor, hepcidin
INTRODUCTION
Gestational anemia remains an important public health problem in both developed and developing countries. Globally, the prevalence of anemia during pregnancy has been reported to be highly variable, ranging from 17% to 31% in Europe and North America, 44–53% in South East Asia, and 53–61% in Africa (1). According to global data compiled by the WHO, the prevalence of gestational anemia in North America is mild but highly variable (5–19.9%) (2), with minority women, those with higher parity, and those in lower socioeconomic groups having increased risks of iron deficiency (ID) and anemia (3, 4). Although maternal iron supplementation has been shown to decrease the risk of maternal anemia and ID (5, 6), national screening data on anemia and iron status in North American pregnant women remain limited.
To identify anemia in North American pregnant women, universal anemia screening is recommended by the CDC (7), the American Academy of Family Physicians (8), the American Congress of Obstetricians and Gynecologists (9), and the US Department of Veterans Affairs and Department of Defense (10). However, the US Preventive Services Task Force (USPSTF) report concluded that the existing body of evidence is inconclusive with respect to the ability of prenatal iron supplementation to improve maternal or infant clinical health outcomes and highlighted the need for further data to target existing gaps in the current literature (6). Normative population-based data are needed to understand the need for, and benefits of, universal prenatal iron supplementation. At present, national iron status screening programs and the availability of population-based longitudinal hematologic data are limited in both the United States and Canada.
NATIONAL DATA ON IRON STATUS AND ANEMIA IN THE UNITED STATES AND CANADA
In the United States, no national programs collect longitudinal data on maternal iron status across gestation. Cross-sectional data on the national prevalence of anemia are regularly monitored by NHANES, which has assessed hemoglobin and serum ferritin (SF) concentrations as part of their survey panel. Initially, this survey only included a limited number of pregnant women. When Healthy People 2010 highlighted the need to obtain data on ID in pregnant women, subsequent NHANESs (1999–2006) oversampled pregnant women (11). To compile data on an additional indicator of iron status, soluble transferrin receptor (sTfR) concentration was included in NHANES studies starting from NHANES 2003. With the inclusion of sTfR concentration, the SF and sTfR data could be used to estimate total body iron (TBI) stores in pregnant women. Additional iron indicator data in pregnant women were obtained by using surplus sera available from NHANES 1999–2002 for the evaluation of sTfR and SF (12).
Although national US data on iron status are limited, a number of longitudinal studies have evaluated the impact of maternal iron supplementation on iron status. The USPSTF identified a total of 12 longitudinal iron supplementation trials that evaluated routine iron supplementation; of these, only 3 were undertaken in North America and all 3 of these studies were from the United States (13–15). These US studies tended to focus on higher-risk populations (higher parity, predominantly minority, adolescent pregnancies, and lower socioeconomic status women), limiting the amount of normative iron supplementation data on North American women who may enter pregnancy with replete or excess total iron stores. A detailed summary of all data evaluated by the USPSTF has recently been published (16), along with a summary of main findings (6).
The first national Canadian study to obtain any population-based data on hemoglobin and anemia was the 1970–1972 Nutrition Canada Survey (NCS) (17, 18). This study evaluated 6727 adults (>20 y of age) and measured hemoglobin and mean corpuscular hemoglobin concentrations. Some pregnant women were sampled in this survey, but concerns have been raised that this group may not have been a representative population sample (19), and, to our knowledge, no data were published on the pregnant women sampled. Concerns with the lack of pregnancy data in the NCS were subsequently highlighted by The Canadian Pediatric Society (19).
In 2003, planning and development were initiated for the Canadian Health Measures Survey (CHMS) 2007–2008. This survey obtains national, cross-sectional health data on ∼5500 persons in each 2-y nationally representative sampling strategy. To date, 5 cycles have been undertaken: cycle 1 (2007–2009), cycle 2 (2009–2011), cycle 3 (2012–2014), cycle 4 (2014–2015), and cycle 5 (in the field now). Cycle 1 included those aged 6–79 y, but all subsequent studies sampled individuals between the ages of 3–79 y. Details of the CHMS have been published (20). Among the CHMSs completed to date, there has only been one evaluation of iron status that used the CHMS cycle 2 data (21). In this analysis, cross-sectional national data on hemoglobin concentrations were published for males and females across the full age range sampled, but no data were presented for the pregnancy cohort (estimated to be comprised of only 38 pregnant women) (22). Several smaller cross-sectional studies in pregnant Canadian women and pregnant adolescents (23–29) have been undertaken, and a summary of these data has been compiled (21). In this review, the prevalence of ID (SF <12 μg/L) was stated to range from 3–66%; 59% of pregnant women in the NCS were said to have depleted their iron stores, and 1% had iron deficiency anemia (IDA). However, definitions used to denote depleted iron stores and IDA and a description of the data used to obtain these figures were not provided (21).
There are currently a number of ongoing longitudinal Canadian pregnancy and birth cohort studies that are investigating various maternal and neonatal health outcomes. One such study is the Alberta Pregnancy Outcomes and Nutrition (APrON) Study (30). As of 2012, this longitudinal cohort study had enrolled ∼2000 pregnant Canadian women (aged ≥16 y and at <27 wk of gestation at enrollment) from the central and southern regions of Alberta, Canada (in or near Calgary and Edmonton). The APrON study is unique in that this study is collecting longitudinal data on iron status across gestation, including hematocrit and hemoglobin and SF concentrations, and infant blood samples will be obtained at 3 mo of age. Children will be followed to 3 y of age, providing additional opportunities to explore possible associations between developmental outcomes in relation to maternal iron status.
REFERENCE VALUES USED TO CLASSIFY GESTATIONAL ANEMIA IN NORTH AMERICA
Given the lack of national longitudinal hemoglobin data in North American pregnant women, it is of interest to examine the source of the reference data that are currently used to classify gestational anemia in North American pregnant women. In 1987, the CDC published mean hemoglobin concentrations across the range of 12–40 wk of gestation along with the fifth percentile for reported hemoglobin concentrations (Table 1) (31). Anemia during pregnancy is currently defined as a blood hemoglobin concentration or hematocrit below the fifth percentile by using these trimester-specific normative values of <11 g/dL in the first and third trimester and <10.5 g/dL in the second trimester. Anemia cutoffs vary by trimester to account for the known decrease in hemoglobin that occurs as a result of the 45–50% increase in plasma volume (36, 37) that peaks at ∼24 wk of gestation (36) and results in an increase in the total red blood cell hemoglobin mass of ∼30% (38). Additional CDC criteria are available to adjust cutoffs for the confounding effects of maternal cigarette smoking, residence at high altitude, and African American race (7, 31, 39, 40).
TABLE 1.
CDC trimester-specific mean hemoglobin concentrations and anemia cutoffs1
| Trimester |
||||||||
| 1 | 2 |
3 |
||||||
| Weeks of gestation | 12 | 16 | 20 | 24 | 28 | 32 | 36 | 40 |
| Mean hemoglobin, g/dL | 12.2 | 11.8 | 11.6 | 11.6 | 11.8 | 12.1 | 12.5 | 12.9 |
| Anemia, g/dL | <11.0 | <10.5 | <11.0 | |||||
As detailed in the original publication (31), data used to establish the CDC guidelines were based on pooled longitudinal data from 4 European surveys in healthy pregnant women studied 3–4 decades ago in Finland, Sweden, and the United Kingdom (32–35). In these 4 studies women received daily iron supplements containing between 65 and 200 mg elemental Fe (32–35) and 3 of the studies included a placebo group of women who did not receive supplemental iron during pregnancy (32–34). Maternal hematologic status was longitudinally evaluated ≤8 times between 12 and 40 wk of gestation. In some studies, bone marrow aspirates were obtained during pregnancy to evaluate iron stores in relation to other hematologic measures (32, 34). One study also included longitudinal measures of iron absorption by using radiolabeled 59-iron (34).
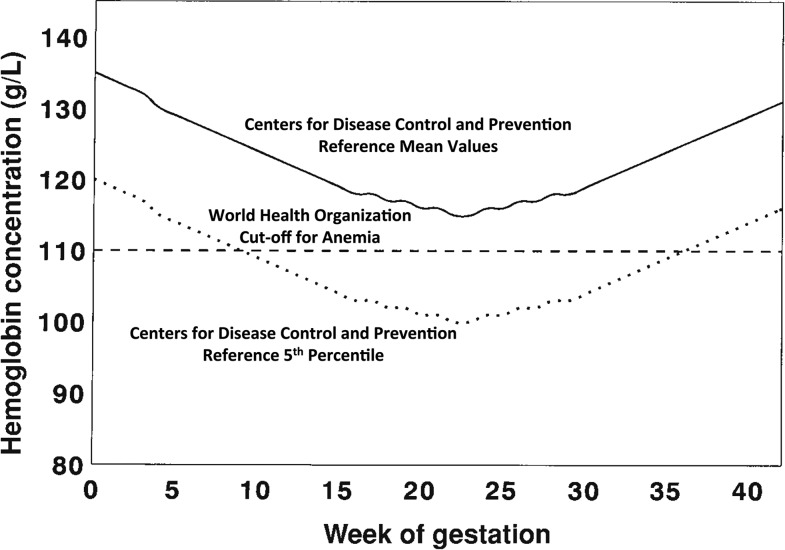
Several aspects of these reference data could potentially influence their current applicability. First, data were obtained from relatively small study populations, ranging from a low of 32 women (32) to 267 women in the largest study (35), with a combined sample size of 427 participants in total. Second, the racial composition of the reference populations was not specified but is assumed to be predominantly white. In the United States, black or African-American individuals currently represent 13.3% of the US population, and minorities, defined as those who are not white alone, represent 38.4% of the US population (41). At present, 19.9% of the Canadian population belongs to a minority group; and the percentage of Canadian immigrants from Africa has grown from 1.5% to 13.0% between 1961 and 2012 (42). Third, prepregnancy BMI [(ppBMI) in kg/m2] of the CDC reference populations was provided for 2 of the 4 studies and averaged ∼21 (32, 33). Given that 54% of US women enter pregnancy while overweight or obese (43), 46% of Canadian women are currently overweight or obese (44), and that obesity may cause alterations in maternal iron status (45), the impact of maternal ppBMI on response to iron supplementation may not have been adequately captured. Finally, the populations used to generate these reference data received between 65 and 200 mg supplemental Fe/d, which, at the high end, is >7 times higher than the current Recommended Dietary Allowance for US and Canadian pregnant women (27 mg/d) (46). Despite possible differences in the current characteristics of North American pregnant women, the 1989 CDC data continue to provide the reference data with which other US and Canadian data are evaluated. For example, Beaton (47) published these data in graphical form in 2000 when presenting a review of iron requirements across pregnancy (Figure 1).
FIGURE 1.
Longitudinal changes in hemoglobin concentrations across pregnancy are presented by using the CDC reference mean values (solid black line). The dotted line represents the fifth percentile of the CDC reference data. The WHO definition of anemia is 110 g/L across pregnancy (shown by the horizontal dashed line). Reproduced from reference 47 with permission.
ADDITIONAL FACTORS THAT AFFECT HEMOGLOBIN CONCENTRATIONS ACROSS GESTATION
The most current published American College of Obstetrics and Gynecology report on anemia was published in 2008 and highlighted additional risk factors for gestational anemia, including minority race, adolescent pregnancy, poor dietary quality, heavy menses, and short interpregnancy intervals (9). Several other risk factors for low hemoglobin were also identified in otherwise healthy obstetric populations including low-income women, food-insecure women, pregnant adolescents, and women with multiple gestations (4, 48–53). These higher-risk, but otherwise healthy, obstetric populations have frequently not been adequately represented in the national surveys to date. In 2011 in the United States, 1100 adolescents gave birth each day and 1 in 10 new mothers was an adolescent on the basis of data from the CDC (54). Similarly, by using 2011 data from the CDC, multiple births comprised ∼3.5% of all births in the United States (55).
LONGITUDINAL CHANGES IN IRON INDICATORS ACROSS GESTATION IN NORTH AMERICAN WOMEN
Multiple indicators are available to help diagnose ID and IDA during pregnancy. These indicators have been reviewed, and common cutoffs for ID and IDA have been published (56). Three regulatory hormones to date have been found to be integral to the maintenance of iron homeostasis: erythropoietin [(EPO) regulated in response to hypoxia], erythroferrone (couples iron homeostasis to erythropoiesis), and hepcidin (regulated by body iron stores, hypoxia, and inflammation) (57). Only a limited number of relatively small US studies examined longitudinal changes in a full complement of iron status indicators in relation to iron regulatory hormones across pregnancy in North American women, as detailed below. As expected, research data on iron status often come from obstetric populations at higher risk of maternal ID and IDA.
Two recent published longitudinal studies evaluated correlations between hemoglobin and a full panel of iron status indicators (SF, sTfR, and serum iron concentrations and TBI), anemia-related nutrients (folate and vitamin B-12), inflammatory markers (IL-6 and C-reactive protein concentrations), and 2 iron regulatory hormones (EPO and hepcidin concentrations) across gestation (50, 58). These studies were undertaken in obstetric populations known to be at higher risk of ID and IDA [pregnant adolescents (n = 253) and women carrying multiple fetuses (n = 83)]. Although the size of these studies was limited and each focused on a higher-risk population, these data provide opportunities to 1) evaluate longitudinal changes in hemoglobin concentration in relation to iron status indicators, 2) assess relative associations between iron status indicators during pregnancy and anemia at term, 3) evaluate associations between inflammation and iron status indicators across gestation, and 4) identify relations between iron status indicators that may be shared between 2 obstetric populations expected to be at greater risk of anemia and ID.
In the adolescent cohort, iron status was evaluated in 253 pregnant teens (≤18 y of age at entry). Maternal blood samples were taken during pregnancy and at delivery, and cord blood was obtained from neonates at birth. In these healthy adolescents, self-reported data on ppBMI indicated that 21% of teens were overweight and 18% were obese at entry into prenatal care. In the entire cohort of 253 adolescents, the prevalence of anemia increased significantly from 3% to 5% (in those at <28 wk of gestation) to 25% by late pregnancy. The prevalence of anemia in late gestation was 5 times that reported in NHANES III data. At delivery, ID based on sTfR concentration (>8.5 mg/L) or TBI (<0 mg/kg) was 1.5 times or 2.4 times higher, respectively (50), than that reported using NHANES III data (12).
At both midgestation and delivery, relative associations among hemoglobin concentration, iron status indicators, and regulatory hormones were evaluated. Of note, the highest correlations between hemoglobin concentration and iron indicators at midgestation were found between EPO and hemoglobin concentrations, with EPO concentration explaining the largest portion of variance in hemoglobin concentration at both midgestation (13%, n = 113; P = 0.0001) and at delivery (19%, n = 192; P < 0.0001). Teens with EPO concentrations above the 75th percentile at delivery had a 2.6 times higher risk of anemia at delivery. EPO concentration was also significantly correlated with all other iron indicators evaluated at both midgestation and at delivery. Moreover, EPO concentration was not associated with IL-6 concentration at either time point sampled (50).
In these adolescents, significant increases in IL-6, SF, and hepcidin concentrations were evident at delivery when compared with values measured during late gestation. A 1.6-fold increase in IL-6 was associated with a 31% increase in serum hepcidin concentration from midgestation (26.0 ± 3.3 wk) to delivery (39.3 ± 2.6 wk) (50). Two publications reported equations to adjust SF concentration for concurrent inflammation (59, 60); at this time, there are no equations available to adjust SF or hepcidin concentrations for the presence of inflammation during pregnancy, which limits the utility and the interpretation of SF and hepcidin concentrations, particularly in late gestation and at term.
The interpretation of iron status indicators that also function as acute-phase proteins (SF and hepcidin concentrations) may also be affected by ppBMI, obesity, and excess gestational weight gain. Several studies examined the impact of maternal obesity on iron status across gestation with mixed results (61–64). In one of the studies, nearly 50% of the adolescent population was overweight or obese, and the majority (63%) had excessive gestational weight gains; however, with the exception of serum iron, no correlations were found among maternal ppBMI or gestational weight gain and hemoglobin concentration or iron status indicators (SF or sTfR concentration or TBI) (61). Maternal IL-6 and hepcidin concentrations were only significantly associated with one another at delivery, not at midgestation. In these pregnant teens, in contrast to expectations, neonates born to obese and overweight mothers had significantly higher hemoglobin concentrations at birth (61). These results suggest that adiposity-associated inflammation does not override the influence of low iron status on hepcidin signaling pathways in this group who was at higher risk of gestational anemia.
With the use of the same methodology, relations between hemoglobin concentration, iron indicators, and iron regulatory hormones were explored in a cohort of 83 healthy women carrying twins (n = 64), triplets (n = 18), or quadruplets (n = 1) (58). Anemia was present in 45% of women studied during the third trimester, which is 4-fold higher than the national prevalence observed among women carrying singletons (12). Women with anemia during the second trimester had a nearly 2-fold increased risk of anemia at delivery, even though, as expected among women carrying multiple fetuses, 65% of these women delivered prematurely (51). Women with depleted iron stores during pregnancy (SF <12 μg/L) had a 2-fold higher risk of anemia at delivery. Similar to findings in the adolescents, women with EPO concentrations above the 75th percentile during pregnancy had a 3-fold greater risk of anemia at delivery, and EPO concentration was also the only indicator during pregnancy that was significantly associated with hemoglobin concentration at delivery (58). Comparable to the pregnant adolescent cohort, IL-6 concentrations at delivery in women carrying multiple fetuses were significantly positively associated with both SF and hepcidin concentrations. A segmented regression model was used to identify the cutoff of IL-6 concentration at delivery that was associated with an increase in hepcidin concentrations. Women with IL-6 concentrations >18.4 pg/mL at delivery were found to have significantly higher hepcidin concentrations, reinforcing the need to account for inflammation when evaluating SF and hepcidin concentrations at term (58). Pooled data from these 2 cohorts are shown in Table 2, highlighting the correlations observed between hemoglobin and the iron status indicators measured.
TABLE 2.
Correlations between hemoglobin concentrations and iron status indicators in a cohort of pregnant adolescents and women carrying multiple fetuses1
| SF | sTfR | TBI | EPO | |
| Pregnancy (26.0 ± 3.4 wk of gestation) | ||||
| Hemoglobin | ||||
| Nonanemic | ||||
| r | 0.07 | 0.16 | −0.02 | −0.23 |
| P | 0.43 | 0.052 | 0.80 | 0.008 |
| n | 144 | 144 | 144 | 133 |
| Anemic | ||||
| r | 0.25 | −0.46 | 0.41 | −0.57 |
| P | 0.04 | 0.0001 | 0.0009 | <0.0001 |
| n | 64 | 64 | 64 | 58 |
| SF | ||||
| Nonanemic | ||||
| r | — | −0.19 | ND | −0.11 |
| P | — | 0.01 | ND | 0.16 |
| n | — | 173 | ND | 160 |
| Anemic | ||||
| r | — | −0.39 | ND | −0.48 |
| P | — | 0.0003 | ND | <0.0001 |
| n | — | 79 | ND | 72 |
| sTfR | ||||
| Nonanemic | ||||
| r | — | — | ND | 0.25 |
| P | — | — | ND | 0.002 |
| n | — | — | ND | 160 |
| Anemic | ||||
| r | — | — | ND | 0.53 |
| P | — | — | ND | <0.0001 |
| n | — | — | ND | 72 |
| TBI | ||||
| Nonanemic | ||||
| r | — | — | — | −0.21 |
| P | — | — | — | 0.008 |
| n | — | — | — | 160 |
| Anemic | ||||
| r | — | — | — | −0.60 |
| P | — | — | — | <0.0001 |
| n | — | — | — | 72 |
| Delivery (39.2 ± 2.7 wk of gestation) | ||||
| Hemoglobin | ||||
| Nonanemic | ||||
| r | 0.24 | −0.15 | 0.26 | −0.27 |
| P | 0.0006 | 0.04 | 0.0002 | 0.0002 |
| n | 200 | 202 | 200 | 186 |
| Anemic | ||||
| r | 0.21 | −0.26 | 0.27 | −0.43 |
| P | 0.07 | 0.02 | 0.02 | 0.0002 |
| n | 75 | 76 | 75 | 68 |
| SF | ||||
| Nonanemic | ||||
| r | — | −0.16 | ND | −0.25 |
| P | — | 0.02 | ND | 0.0004 |
| n | — | 210 | ND | 194 |
| Anemic | ||||
| r | — | −0.46 | ND | −0.42 |
| P | — | <0.0001 | ND | 0.0002 |
| n | — | 85 | ND | 77 |
| sTfR | ||||
| Nonanemic | ||||
| r | — | — | ND | 0.17 |
| P | — | — | ND | 0.02 |
| n | — | — | ND | 195 |
| Anemic | ||||
| r | — | — | ND | 0.52 |
| P | — | — | ND | <0.0001 |
| n | — | — | ND | 77 |
| TBI | ||||
| Nonanemic | ||||
| r | — | — | — | −0.28 |
| P | — | — | — | <0.0001 |
| n | — | — | — | 194 |
| Anemic | ||||
| r | — | — | — | −0.54 |
| P | — | — | — | <0.0001 |
| n | — | — | — | 77 |
Correlations between iron status indicators are shown during pregnancy and at delivery for those who remained nonanemic or for those who developed anemia across gestation. EPO, erythropoietin; ND, not determined (because TBI is calculated by using SF and transferrin receptor); SF, serum ferritin; sTfR, soluble transferrin receptor; TBI, total body iron.
A summary of comparisons between anemia and depleted iron stores across pregnancy from published NHANES data (12), the 2 higher-risk populations mentioned previously (50, 58), and preliminary unpublished data from the APrON cohort (R Bell and C Field, University of Alberta, Department of Agricultural, Food and Nutritional Science, personal communication, 2016) is presented in Table 3. By late gestation, the fraction of the Canadian APrON population with anemia was 5%, only half of that noted on the basis of US NHANES data. This may be a consequence of the fact that the Canadian women studied tended to be of higher socioeconomic status, 70% had an undergraduate degree or higher, 87% were white, and >80% of women indicated that they took daily prenatal supplements each trimester. Of note, despite their lower risk of anemia, similar numbers of Canadian women had depleted SF concentrations by late gestation while receiving prenatal iron supplementation.
TABLE 3.
Anemia and ID in 4 North American pregnant populations1
| Trimester, % (n) |
|||||
| Study, year (reference) | Iron status variable | 1 | 2 | 3 | Delivery, % (n) |
| NHANES (ages 12–49 y) | ID; SF <12 μg/L | 7.3 (189) | 23.7 (416) | 39.2 (384) | — |
| Mei et al., 2011 (12) | Anemia | 2.7 (189) | 2.2 (416) | 10.8 (384) | — |
| APrON2 (ages 16–40 y) | ID; SF <12 μg/L | 3 (286) | 10 (1020) | 40 (847) | — |
| Unpublished | Anemia | 2 (540) | 2 (1894) | 5 (1673) | — |
| Teens (ages 13–18 y) | ID; SF <12 μg/L | 6.5 (137) | 22.2 (99) | 34.8 (46) | 21.4 (206) |
| Lee et al., 2014 (50) | Anemia | — | 32.5 (123) | 42.3 (85) | 35 (228) |
| Multiples (ages 20–46 y) | ID; SF <12 μg/L | — | 37 (73) | — | 26.2 (61) |
| Ru et al., 2016 (58) | Anemia | — | 31.5 (73) | — | 42.6 (61) |
APrON, Alberta Pregnancy Outcomes and Nutrition Study; ID, iron deficiency; SF, serum ferritin.
Unpublished data from the APrON cohort were provided by R Bell and C Field (University of Alberta, Department of Agricultural, Food and Nutritional Science, personal communication, 2016).
Longitudinal comparisons of maternal iron status across gestation from these 4 studies highlight another finding of note. Whereas considerably more women were anemic in the adolescent and multiple-gestation cohorts, depleted iron stores at delivery were less common in these 2 groups as a consequence of inflammation that increased across pregnancy (50, 58). Approaches to control for the presence of pregnancy-associated changes in inflammation are needed to fully interpret SF and hepcidin concentrations across gestation, particularly at delivery due to labor-associated changes in systemic inflammation.
GAPS IN KNOWLEDGE AND DIRECTIONS FOR FUTURE RESEARCH
How does baseline maternal iron status influence response to prenatal iron supplementation?
Given the known significant associations between maternal iron status and absorption of nonheme iron during pregnancy (65, 66), one would predict that maternal responses to inorganic iron supplementation would be heavily influenced by baseline iron status. A large iron supplementation study in Burkina Faso (n = 1268) evaluated the response to prenatal iron supplementation as a function of baseline hemoglobin status and found significant increases in hemoglobin concentrations only in women who were anemic at baseline (67). Although the study population in Burkina Faso was at greater risk of maternal anemia and nutritional deficiencies across gestation when compared with North American women, this study draws attention to the mixed results that may be found when the response to supplementation is not evaluated in relation to baseline iron status.
Does variability in dietary heme intake affect response to prenatal iron supplementation?
Few studies have evaluated the response to prenatal iron supplementation in relation to dietary heme and nonheme iron intake. With the use of stable iron isotopes, heme iron absorption was 3-fold higher than the absorption of nonheme iron in nonpregnant women (50.5% compared with 15.2% respectively; n = 11; P < 0.001), but in pregnant women (67% of whom had undetectable hepcidin), the difference observed between heme and nonheme iron absorption was substantially reduced (47.7% compared with 40.4%; n = 18; P = 0.04) (66). Unlike nonheme iron, the absorption of heme iron was not associated with iron status or hepcidin in the nonpregnant or pregnant women (66). Moreover, dietary iron of heme origin was also found to be preferentially transported to the fetus (68). Lack of consideration of maternal dietary heme and total dietary iron content likely adds additional variability to the interpretation of responses to prenatal iron supplementation.
How does maternal iron status affect fetal development and neonatal iron stores at birth?
Optimal maternal iron requirements across gestation must supply the iron required for maternal homeostasis but also must be sufficient to fully endow the neonate with the iron needed during the first 6 mo of life. Unfortunately, few human studies have evaluated maternal iron status and the impact of prenatal iron supplementation on neonatal iron stores at birth, and there are many confounding issues (e.g., time to cord clamping) (69) that may further complicate the evaluation of these associations. In both adolescent and multiple-births cohorts (58, 70), highly significant correlations were found between maternal iron status at term and neonatal iron status at birth. Moreover, 21% of neonates born to the adolescents were anemic (cord hemoglobin <13 g/dL) (71). In the neonates born to the women carrying multiple fetuses, ∼19% were anemic at birth (72). Greater attention to both maternal and neonatal iron status is needed when evaluating dietary iron requirements across pregnancy, and more data are needed to determine the degree to which maternal iron status and prenatal iron supplementation affect fetal iron acquisition. This also requires a greater understanding of the role of the human placenta in mediating iron exchange in response to maternal and neonatal iron status and systemic iron regulatory signals.
What factors other than ID contribute to maternal anemia?
IDA often explains a relatively small percentage of gestational anemia, drawing attention to the need to evaluate other causes of maternal anemia and hematologic status across pregnancy. In pregnant adolescents, anemia and IDA were found in 25% and 6%, respectively, of the teens at delivery (50). Similarly, anemia was found in 45% and IDA was present in 18% of the women carrying multiple fetuses at delivery (58). Folate and vitamin B-12 status of these 2 populations was adequate. Deficiencies of vitamin A (73), selenium (74), and vitamin D (75, 76) have been linked to anemia. In the adolescent cohort described above, 31% and 18% of teens had 25-hydroxyvitamin D [25(OH)D] concentrations <16 or <12 ng/mL, respectively (77). This may contribute to the anemia observed, because the odds of anemia at delivery were 8 times greater in teens with 25(OH)D <20 ng/mL, and this association was found to be mediated in part by interactions between 25(OH)D status and EPO (75). More data are needed to fully explore nutrient-nutrient interactions that affect hemoglobin and the risk of anemia during pregnancy.
What controls iron homeostasis across gestation?
To date, only ∼30% of the variability in iron absorption during pregnancy can be explained by iron status indicators and regulatory hormones (66). For iron, 5 genes are known to cause iron overload disorders in humans (human hemochromatosis protein, ferroportin 1, transferrin receptor 2, hemojuvelin, and hepcidin) (78). The role of genetic variability in proteins that affect iron homeostasis during pregnancy has yet to be explored but has relevance to targeting those at increased risk of gestational anemia.
CONCLUSIONS
More data are clearly needed to evaluate the impact of universal iron supplementation and its relative benefits and harms to maternal and neonatal iron status. At present, longitudinal studies that characterize determinants of hemoglobin concentrations across gestation are limited, and more data are needed to establish cutoffs of iron indicators in relation to the trimester of pregnancy and to evaluate factors that may increase or decrease the risk of gestational anemia. At this time, less than half of the variability in iron absorption during pregnancy can be explained by iron status indicators and hepcidin, and additional research is needed to identify key determinants of iron homeostasis across gestation. The CDC has provided factors to adjust the anemia cutoffs during pregnancy in relation to maternal race, but the degree to which this altered hemoglobin distribution may also affect the cutoffs used for other iron status indicators has not been adequately explored. Longitudinal assessment of maternal iron status across gestation should also consider the impact of the timing of maternal iron supplementation to determine whether there are key gestational windows in which maternal iron availability may be particularly beneficial for optimal fetal development. Current data on responses to maternal iron supplementation may be difficult to interpret without accounting for variability in the baseline iron status and racial composition of the populations studied, the variable timing of the pregnancy measures obtained, and the need to adjust for inflammation if relying on iron status indicators that also function as acute-phase proteins. Of concern, prenatal iron requirements needed to prevent maternal anemia may not be the same as those needed to support fetal health, brain development, and optimal fetal iron endowment at birth. As research in this field moves forward, answers to these questions will inform subsequent recommendations designed to promote maternal and fetal health during this key life stage.
Acknowledgments
We thank Rhonda Bell and Catherine Field for sharing their scientific insights from unpublished APrON data on longitudinal changes in iron status among pregnant Canadian women, and Homeira Hamayelimehrabani and Ye Shen for their statistical evaluation of the existing APrON data. We also thank Kevin Cockell and Didier Garriguet for providing information on Canadian data that evaluated gestational anemia and iron status in pregnant Canadian women.
The authors’ responsibilities were as follows—KOO: had primary responsibility for the final content; and both authors: wrote the manuscript, and read and approved the final manuscript. Neither of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: APrON, Alberta Pregnancy Outcomes and Nutrition Study; CHMS, Canadian Health Measures Survey; EPO, erythropoietin; ID, iron deficiency; IDA, iron deficiency anemia; NCS, Nutrition Canada Survey; ppBMI, prepregnancy BMI; SF, serum ferritin; sTfR, soluble transferrin receptor; TBI, total body iron (stores); USPSTF, US Preventive Services Task Force; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Breymann C. Iron deficiency anemia in pregnancy. Semin Hematol 2015;52:339–47. [DOI] [PubMed] [Google Scholar]
- 2.WHO; de Benoist B, McLean E, Egli I, Cogswell M, editors. World-wide prevalence of anaemia 1993-2005: WHO Global Database on Anaemia. Geneva (Switzerland): WHO; 2005. [Google Scholar]
- 3.Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA 1997;277:973–6. [DOI] [PubMed] [Google Scholar]
- 4.Scholl TO. Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr 2005;81(Suppl):1218S–22S. [DOI] [PubMed] [Google Scholar]
- 5.Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2015;22:CD004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantor AG, Bougatsos C, McDonagh M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy. Ann Intern Med 2015;163:400. [DOI] [PubMed] [Google Scholar]
- 7.CDC. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep 1998;47:1–29. [PubMed] [Google Scholar]
- 8.American Academy of Family Physicians. Clinical preventive service recommendation: iron deficiency anemia [Internet]. Leawood (KS): American Academy of Family Physicians; 2006. [cited 2017 Sep 19]. Available from: http://www.aafp.org/patient-care/clinical-recommendations/all/iron-deficiency-anemia.html.
- 9.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 95: anemia in pregnancy. Obstet Gynecol 2008;112:201–7. [DOI] [PubMed] [Google Scholar]
- 10.Department of Veteran Affairs; Department of Defense. VA/DoD clinical practice guideline for management of pregnancy [Internet]. Washington (DC): Department of Veteran Affairs, Department of Defense; 2009. [cited 2017 Sep 19]. Available from: http://www.healthquality.va.gov/guidelines/WH/up/.
- 11.CDC. Analytic and reporting guidelines. The National Health and Nutrition Examination Survey (NHANES). Atlanta (GA): CDC; 2006. [Google Scholar]
- 12.Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, Grummer-Strawn LM. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am J Clin Nutr 2011;93:1312–20. [DOI] [PubMed] [Google Scholar]
- 13.Siega-Riz AM, Hartzema AG, Turnbull C, Thorp J, McDonald T, Cogswell ME. The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: a randomized controlled trial. Am J Obstet Gynecol 2006;194:512–9. [DOI] [PubMed] [Google Scholar]
- 14.Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. Am J Clin Nutr 2003;78:773–81. [DOI] [PubMed] [Google Scholar]
- 15.Meier PR, Nickerson HJ, Olson KA, Berg RL, Meyer JA. Prevention of iron deficiency anemia in adolescent and adult pregnancies. Clin Med Res 2003;1:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonagh M, Cantor A, Bougatsos C, Dana T, Blazina I. Routine iron supplementation and screening for iron deficiency anemia in pregnant women: a systematic review to update the U.S. Preventive Services Task Force recommendation. Rockville (MD): Agency for Healthcare Research and Quality; 2015. [PubMed] [Google Scholar]
- 17.Health and Welfare Canada. Nutrition Canada national survey. Ottawa (Canada): Information Canada; 1973. [Google Scholar]
- 18.Sabry ZI, Campbell E, Campbell JA, Forbes AL. Nutrition Canada—a national nutrition survey. Nutr Rev 1974;32:105–11. [DOI] [PubMed] [Google Scholar]
- 19.Nutrition Committee of the Canadian Paediatric Society. The Nutrition Canada Survey: a review. Statement by the Nutrition Committee of the Canadian Paediatric Society. Can Med Assoc J 1976;115:775–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Tremblay MS, Connor Gorber S. Canadian health measures survey: brief overview. Can J Public Health 2007;98:453–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper MJ, Cockell KA, L’Abbé MR. The iron status of Canadian adolescents and adults: current knowledge and practical implications. Can J Diet Pract Res 2006;67:130–8. [DOI] [PubMed] [Google Scholar]
- 22.Cooper M, Greene-Finestone L, Lowell H, Levesque J, Robinson D.. Iron sufficiency of Canadians. Health Rep 2012;23:41–8. [PubMed] [Google Scholar]
- 23.Godel JC, Pabst HF, Hodges PE, Johnson KE. Iron status and pregnancy in a northern Canadian population: relationship to diet and iron supplementation. Can J Public Health 1992;83:339–43. [PubMed] [Google Scholar]
- 24.Turgeon O’Brien H, Larocque I, Desmeules C. Depletion des reserves en fer chez un group d’adolecentes et de femmes enceintes de la region de Quebec. [Depletion of iron reserves in a group of teenagers and pregnant women in the Quebec region.] J Praticien 1994;140:529 (in French). [Google Scholar]
- 25.Turgeon O’Brien H, Santure M, Maziade J. The association of low and high ferritin levels and anemia with pregnancy outcome. Can J Diet Pract Res 2000;61:121–7. [PubMed] [Google Scholar]
- 26.Rioux MF, Michaud J. Maternal anemia in the southeast and northeast regions of New Brunswick and the impact on hematological parameters and the growth of the newborn. Can J Diet Pract Res 2001;62:70–5. [PubMed] [Google Scholar]
- 27.Gadowsky SL, Gale K, Wolfe SA, Jory J, Gibson R, O’Connor DL. Biochemical folate, B12, and iron status of a group of pregnant adolescents accessed through the public health system in southern Ontario. J Adolesc Health 1995;16:465–74. [DOI] [PubMed] [Google Scholar]
- 28.Valberg LS, Sorbie J, Ludwig J, Pelletier O. Serum ferritin and the iron status of Canadians. Can Med Assoc J 1976;114:417–21. [PMC free article] [PubMed] [Google Scholar]
- 29.Hodgins S, Dewailly E, Chatwood S, Bruneau S, Bernier F. Iron-deficiency anemia in Nunavik: pregnancy and infancy. Int J Circumpolar Health 1998;57(Suppl 1):135–40. [PubMed] [Google Scholar]
- 30.Kaplan BJ, Giesbrecht GF, Leung BM, Field CJ, Dewey D, Bell RC, Manca DP, O’Beirne M, Johnston DW, Pop VJ, et al. . The Alberta Pregnancy Outcomes and Nutrition (APrON) cohort study: rationale and methods. Matern Child Nutr 2014;10:44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CDC. CDC criteria for anemia in children and childbearing-aged women. MMWR Morb Mortal Wkly Rep 1989;38:400–4. [PubMed] [Google Scholar]
- 32.Puolakka J. Serum ferritin as a measure of iron stores during pregnancy. Acta Obstet Gynecol Scand Suppl 1980;95:1–31. [DOI] [PubMed] [Google Scholar]
- 33.Taylor DJ, Mallen C, McDougall N, Lind T. Effect of iron supplementation on serum ferritin levels during and after pregnancy. Br J Obstet Gynaecol 1982;89:1011–7. [DOI] [PubMed] [Google Scholar]
- 34.Svanberg B, Arvidsson B, Norrby A, Rybo G, Solvell L. Absorption of supplemental iron during pregnancy—a longitudinal study with repeated bone-marrow studies and absorption measurements. Acta Obstet Gynecol Scand Suppl 1975;48:87–108. [DOI] [PubMed] [Google Scholar]
- 35.Sjostedt J, Manner P, Nummi S, Ekenved G. Oral iron prophylaxis during pregnancy—a comparative study on different dosage regimens. Acta Obstet Gynecol Scand Suppl 1977;60:3–9. [DOI] [PubMed] [Google Scholar]
- 36.Lund CJ, Donovan JC. Blood volume during pregnancy: significance of plasma and red cell volumes. Am J Obstet Gynecol 1967;98:394–403. [PubMed] [Google Scholar]
- 37.Bothwell TH, Charlton RW, Cook JD, Finch CA. Iron metabolism in man. Oxford (United Kingdom): Blackwell Scientific Publications; 1979. [Google Scholar]
- 38.De Leeuw NK, Lowenstein L, Hsieh YS. Iron deficiency and hydremia in normal pregnancy. Medicine (Baltimore) 1966;45:291–315. [DOI] [PubMed] [Google Scholar]
- 39.WHO/CDC. Assessing the iron status of populations. Geneva (Switzerland): WHO Press; 2004. [Google Scholar]
- 40.WHO/UNICEF/United Nations University. Iron deficiency anemia; assessment, prevention and control: a guide for programme managers. Geneva (Switzerland): WHO Press; 2001. WHO/NHD/01.3. [Google Scholar]
- 41.US Census Bureau. QuickFacts, United States [Internet]. [cited 2016 Oct 10]. Available from: https://www.census.gov/quickfacts/table/PST045215/00#headnote-js-a.
- 42.Statistics Canada. Canadian demographics at a glance: second edition [Internet]. [cited 2016 Oct 10]. Available from: http://www.statcan.gc.ca/pub/91-003-x/2014001/section02/24-eng.htm.
- 43.Gilmore LA, Klempel-Donchenko M, Redman LM. Pregnancy as a window to future health: excessive gestational weight gain and obesity. Semin Perinatol 2015;39:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Statistics Canada. Body mass index, overweight or obese, self-reported, adult, by sex (percent) [Internet]. 2016. [cited 2016 Oct 10]. Available from: http://www.statcan.gc.ca/tables-tableaux/sum-som/101/cst01/health81b-eng.htm.
- 45.Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014;6:3062–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Institute of Medicine, Food Nutrition Board. Dietary Reference Intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 47.Beaton GH. Iron needs during pregnancy: do we need to rethink our targets? Am J Clin Nutr 2000;72:265S–71S. [DOI] [PubMed] [Google Scholar]
- 48.Scholl TO, Hediger ML. Anemia and iron-deficiency anemia: compilation of data on pregnancy outcome. Am J Clin Nutr 1994;59(Suppl):492S–500S; discussion 500S–1S. [DOI] [PubMed] [Google Scholar]
- 49.Olausson H, Goldberg GR, Laskey MA, Schoenmakers I, Jarjou LM, Prentice A. Calcium economy in human pregnancy and lactation. Nutr Res Rev 2012;25:40–67. [DOI] [PubMed] [Google Scholar]
- 50.Lee S, Guillet R, Cooper EM, Westerman M, Orlando M, Pressman E, O’Brien KO. Maternal inflammation at delivery affects assessment of maternal iron status. J Nutr 2014;144:1524–32. [DOI] [PubMed] [Google Scholar]
- 51.Jarjou LM, Prentice A, Sawo Y, Laskey MA, Bennett J, Goldberg GR, Cole TJ. Randomized, placebo-controlled, calcium supplementation study in pregnant Gambian women: effects on breast-milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. Am J Clin Nutr 2006;83:657–66. [DOI] [PubMed] [Google Scholar]
- 52.Park CY, Eicher-Miller HA. Iron deficiency is associated with food insecurity in pregnant females in the United States: National Health and Nutrition Examination Survey 1999-2010. J Acad Nutr Diet 2014;114:1967–73. [DOI] [PubMed] [Google Scholar]
- 53.Kawakita T, Wilson K, Grantz KL, Landy HJ, Huang CC, Gomez-Lobo V. Adverse maternal and neonatal outcomes in adolescent pregnancy. J Pediatr Adolesc Gynecol 2016;29:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.CDC. Vital signs: teen pregnancy—United States, 1991-2009. MMWR Morb Mortal Wkly Rep 2011;60:414–20. [PubMed] [Google Scholar]
- 55.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep 2015;64:1–65. [PubMed] [Google Scholar]
- 56.Cao C, O’Brien KO. Pregnancy and iron homeostasis: an update. Nutr Rev 2013;71:35–51. [DOI] [PubMed] [Google Scholar]
- 57.Kim A, Nemeth E. New insights into iron regulation and erythropoiesis. Curr Opin Hematol 2015;22:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ru Y, Pressman E, Cooper E, Guillet R, Katzman P, Kent T, Bacak S, O’Brien KO. Iron deficiency and anemia are prevalent in women carrying multiples. Am J Clin Nutr 2016;104:1052–60. [DOI] [PubMed] [Google Scholar]
- 59.Knowles J, Thurnham DI, Phengdy B, Houamboun K, Philavong K, Keomoungkhone I, Keovilay K. Impact of inflammation on the biomarkers of iron status in a cross-sectional survey of Lao women and children. Br J Nutr 2013;110:2285–97. [DOI] [PubMed] [Google Scholar]
- 60.Thurnham DI, Northrop-Clewes CA, Knowles J. The use of adjustment factors to address the impact of inflammation on vitamin A and iron status in humans. J Nutr 2015;145(Suppl):1137S–43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao C, Pressman EK, Cooper EM, Guillet R, Westerman M, O’Brien KO. Prepregnancy body mass index and gestational weight gain have no negative impact on maternal or neonatal iron status. Reprod Sci 2016;23:613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garcia-Valdes L, Campoy C, Hayes H, Florido J, Rusanova I, Miranda MT, McArdle HJ. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int J Obes (Lond) 2015;39:571–8. [DOI] [PubMed] [Google Scholar]
- 63.Jones AD, Zhao G, Jiang YP, Zhou M, Xu G, Kaciroti N, Zhang Z, Lozoff B. Maternal obesity during pregnancy is negatively associated with maternal and neonatal iron status. Eur J Clin Nutr 2016;70:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is hepcidin the link? J Perinatol 2013;33:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Brien KO, Zavaleta N, Caulfield LE, Yang DX, Abrams SA. Influence of prenatal iron and zinc supplements on supplemental iron absorption, red blood cell iron incorporation, and iron status in pregnant Peruvian women. Am J Clin Nutr 1999;69:509–15. [DOI] [PubMed] [Google Scholar]
- 66.Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O’Brien KO. Utilization of iron from an animal-based iron source is greater than that of ferrous sulfate in pregnant and nonpregnant women. J Nutr 2010;140:2162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberfroid D, Huybregts L, Habicht JP, Lanou H, Henry MC, Meda N, d’Alessandro U, Kolsteren P. Randomized controlled trial of 2 prenatal iron supplements: is there a dose-response relation with maternal hemoglobin? Am J Clin Nutr 2011;93:1012–8. [DOI] [PubMed] [Google Scholar]
- 68.Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O’Brien KO. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr 2012;142:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonald SJ, Middleton P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev 2008 Apr 16;2:CD004074. [DOI] [PubMed] [Google Scholar]
- 70.Wagner CL, McNeil RB, Johnson DD, Hulsey TC, Ebeling M, Robinson C, Hamilton SA, Hollis BW. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: a combined analysis. J Steroid Biochem Mol Biol 2013;136:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee S, Guillet R, Cooper EM, Westerman M, Orlando M, Kent T, Pressman E, O’Brien KO. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res 2016;79:42–8. [DOI] [PubMed] [Google Scholar]
- 72.Ru Y, Pressman E, Guillet R, Cooper B, Katzman P, Caveglia S, O’Brien KO. Variable iron status among twins and triplets at birth. FASEB J 2014;28:636.6. [Google Scholar]
- 73.Semba RD, Bloem MW. The anemia of vitamin A deficiency: epidemiology and pathogenesis. Eur J Clin Nutr 2002;56:271–81. [DOI] [PubMed] [Google Scholar]
- 74.Semba RD, Ricks MO, Ferrucci L, Xue Q, Guralnik JM, Fried LP. Low serum selenium is associated with anemia among older adults in the United States. Eur J Clin Nutr 2009;63:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas CE, Guillet R, Queenan RA, Cooper EM, Kent TR, Pressman EK, Vermeylen FM, Roberson MS, O’Brien KO. Vitamin D status is inversely associated with anemia and serum erythropoietin during pregnancy. Am J Clin Nutr 2015;102:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith EM, Tangpricha V. Vitamin D and anemia: insights into an emerging association. Curr Opin Endocrinol Diabetes Obes 2015;22:432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young BE, McNanley TJ, Cooper EM, McIntyre AW, Witter F, Harris ZL, O’Brien KO. Vitamin D insufficiency is prevalent and vitamin D is inversely associated with parathyroid hormone and calcitriol in pregnant adolescents. J Bone Miner Res 2012;27:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol 2007;69:69–85. [DOI] [PubMed] [Google Scholar]