Abstract
Background: Total-body iron stores (TBI), which are calculated from serum ferritin and soluble transferrin receptor concentrations, can be used to assess the iron status of populations in the United States.
Objective: This analysis, developed to support workshop discussions, describes the distribution of TBI and the prevalence of iron deficiency (ID) and ID anemia (IDA) among toddlers, nonpregnant females, and pregnant females.
Design: We analyzed data from NHANES; toddlers aged 12–23 mo (NHANES 2003–2010), nonpregnant females aged 15–49 y (NHANES 2007–2010), and pregnant females aged 12–49 y (NHANES 1999–2010). We used SAS survey procedures to plot distributions of TBI and produce prevalence estimates of ID and IDA for each target population. All analyses were weighted to account for the complex survey design.
Results: According to these data, ID prevalences (± SEs) were 15.1% ± 1.7%, 10.4% ± 0.5%, and 16.3% ± 1.3% in toddlers, nonpregnant females, and pregnant females, respectively. ID prevalence in pregnant females increased significantly with each trimester (5.3% ± 1.5%, 12.7% ± 2.3%, and 27.5% ± 3.5% in the first, second, and third trimesters, respectively). Racial disparities in the prevalence of ID among both nonpregnant and pregnant females exist, with Mexican American and non-Hispanic black females at greater risk of ID than non-Hispanic white females. IDA prevalence was 5.0% ± 0.4% and 2.6% ± 0.7% in nonpregnant and pregnant females, respectively.
Conclusions: Available nationally representative data suggest that ID and IDA remain a concern in the United States. Estimates of iron-replete status cannot be made at this time in the absence of established cutoffs for iron repletion based on TBI. The study was registered at clinicaltrials.gov as NCT03274726.
Keywords: ferritin, soluble transferrin receptor, total-body iron, iron deficiency, toddlers, women of reproductive age, hemoglobin
INTRODUCTION
Monitoring the iron status of US toddlers and women of reproductive age (WRA), including both nonpregnant and pregnant women, is an important element of NHANES and includes different measures to assess iron status, each with their own strengths and limitations (1–6). Starting in the mid-1980s, iron deficiency (ID) in the United States was evaluated using a multivariable approach called the ferritin model (5, 7); however, in 2003, a new model was proposed for estimating total-body iron stores (TBI) on the basis of the ratio of soluble transferrin receptor (sTfR) to serum ferritin (SF). Published data on the advantages of TBI to assess the iron status of populations are described elsewhere (8, 9). Briefly, TBI (expressed as μg/kg), as a measurement of iron status, has the following advantages: independence from hemoglobin, which shifts the focus from screening and prevention of anemia to ID; fewer laboratory measurements (than the ferritin model); and estimation of the entire distribution of iron stores based on body weight. Additionally, the assay methods for the calculation of TBI are readily automated, and previous publications show adequate agreement between the estimated prevalence of ID by TBI and the ferritin model in high-risk groups (6, 10, 11). Positive values of TBI represent iron stores, and negative values represent tissue ID. The suggested cutoff for defining ID is <0 mg/kg (8, 9). No cutoff has been suggested for iron replete and excess.
Prevalence estimates of ID among toddlers, nonpregnant females, and pregnant females using data from NHANES 2007–2010, 2003–2006, and 1999–2006, respectively, have been published previously (10–12); however, data on TBI exist in NHANES through 2010. This analysis, developed to support workshop discussions, describes the distribution of TBI and the prevalence of ID and ID anemia (IDA) among toddlers (12–23 mo), nonpregnant females (15–49 y), and pregnant females (12–49 y) using data from NHANES 1999–2010.
METHODS
Data source
NHANES uses a stratified multistage probability sample and represents the total, civilian, noninstitutionalized population in the United States. Surveys are conducted by the CDC’s National Center for Health Statistics (NCHS) via household interview followed by a standardized physical examination in a mobile examination center (MEC). The NHANES protocol was approved by the NCHS Research Ethics Review Board. NHANES data are released in 2-y cycles. Procedures for NHANES data collection and analysis are published elsewhere (13).
Serum sTfR and SF assays for the 2003–2010 specimens and the surplus specimens from 1999 to 2002 were analyzed at the CDC's National Center of Environmental Health. Methodological details were described previously (10, 11). In brief, SF was measured using 2 different methods during NHANES 1999–2010 (14–19). Because of methodological differences between the 2 assays, SF concentrations were statistically adjusted to be comparable across NHANES cycles. This was accomplished before data release by applying 3 piecewise linear regression equations described in detail elsewhere (18, 20). Hemoglobin was measured as part of a complete blood count in the MEC (14–19).
TBI was calculated based on sTfR (expressed as μg/L) and SF (expressed as μg/L) concentrations by using a formula from Cook et al. (8) and Skikne et al. (9) (Equation 1):
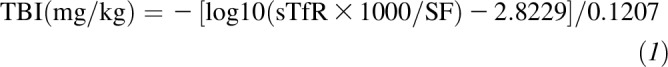 |
For this calculation, sTfR concentrations were converted to those equivalent to the Flowers assay, which was used in the development of the body iron model (8, 9) (Equation 2):
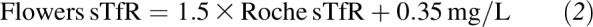 |
Sample selection
For the purposes of this analysis, we pooled data from different time periods to obtain adequate sample sizes to produce stable estimates of ID and IDA. For toddlers 12–23 mo of age (age range requested for workshop purposes), we pooled data from 2003 to 2010 as data on SF and sTfR concentrations were not available for toddlers ≥1 y in NHANES until 2003. For nonpregnant females 15–49 y of age, 2007–2010 data provided an adequate sample size for stable prevalence estimates, and for pregnant females, 1999–2010 data were pooled (including surplus specimens, NHANES 1999–2002). Historically, pregnant women were oversampled in NHANES, but this practice ended in 2006; therefore, additional survey years were pooled to produce stable estimates of ID and IDA for pregnant women. In addition, starting in 2007, pregnancy status of teenagers was not released into the publicly available dataset because of concerns regarding confidentiality (21, 22).
We restricted our sample to those who attended the MEC to undergo blood collection for biochemical analyses (toddlers, n = 1285; nonpregnant females, n = 3681; pregnant females, n = 1349). We excluded participants who were missing hemoglobin, sTfR, or SF measurements (toddlers, n = 670; nonpregnant females, n = 263; pregnant females, n = 66); these participants did not significantly differ with regard to survey year, sex, age, race, or family income from those who were included in the study sample. A complete blood count, which contains hemoglobin measurements, was given first priority in the MEC. SF and sTfR were performed after the complete blood count profile was completed. An exploratory analysis revealed that 427 toddlers did not have their blood drawn (complete blood count profile was not performed) in NHANES 2003–2010. Of these, 312 (73.1%) refused (their proxies) to participate in phlebotomy, 106 (24.8%) had physical limitations including unsuitable veins or vein collapse, and the remaining 9 (2.1%) could not attend the MEC or stay the full time allotted for phlebotomy procedures (R Storandt, NCHS, personal communication, 2016). Our final sample included 615 toddlers, 3418 nonpregnant females, and 1283 pregnant females.
Outcome
ID was defined as TBI <0 mg/kg. IDA was defined as the presence of both ID and anemia, based on the CDC’s hemoglobin concentration thresholds (5): hemoglobin <110 g/L for toddlers 12–23 mo; hemoglobin <120 g/L for nonpregnant females 15–49 y of age; and hemoglobin <110, <105, and <110 g/L for pregnant females in the first, second, and third trimesters, respectively (5). For any woman who did not know or was not asked about the length of the pregnancy (n = 212), the trimester was categorized as unknown, and a hemoglobin value of <110 g/L was used for defining anemia.
Covariates
Data on sex (of the child), age, race/ethnicity, family income, pregnancy status, trimester, and parity were collected during the household questionnaire. Race/ethnicity was based on self-reported data and was categorized into non-Hispanic white, non-Hispanic black, Mexican American, and other. Pregnancy status was based on a positive urine pregnancy test or self-reported pregnancy. Urine pregnancy tests were administered to all females aged 12–49 y in NHANES 1999–2006 and all women 20–44 y from NHANES 2007 onward. As a result, pregnancy status for females 12–19 y old was not included in the publically available dataset; for the purposes of this analysis, females 12–19 y of age, from NHANES 2007–2010, were assumed to be nonpregnant.
Trimester was defined as the number of months of gestation reported by the mother. First trimester was defined as ≤3 mo, second trimester as 4–6 mo, and third trimester as ≥7 mo. The trimester was recorded as unknown for women who did not know, were not asked, or did not report how long they had been pregnant. Parity was based on the self-reported number of pregnancies resulting in a live birth among females aged ≥12 y and was categorized into 0, 1, or ≥2 births. The ratio of family income to poverty was based on the US Department of Health and Human Services’ poverty guidelines (13).
Statistical analysis
We used SAS survey procedures (Version 9.3; Research Triangle Institute) to plot distributions of TBI and produce weighted prevalence estimates of ID and IDA for each target population. We created 8-y (NHANES 2003–2010), 4-y (NHANES 2007–2010), and 12-y (NHANES 1999–2010) weight variables for toddlers, nonpregnant females, and pregnant females, respectively, based on the 2-y MEC weight to account for the complex NHANES survey design.
For toddlers, nonpregnant females, and pregnant females, we present prevalence estimates of ID and IDA by survey cycle, age, race/ethnicity, and family income. Prevalence estimates for nonpregnant and pregnant females were further stratified by parity, and estimates for pregnant females were also stratified by trimester. Some sample sizes varied due to missing data on covariates. NCHS advises against publication of unstable estimates. Unstable estimates were based on NCHS criteria: those with relative SEs [(SE of prevalence/prevalence) × 100] ≥30% (23). Logistic regression pairwise comparison was used to examine differences in prevalence of ID and IDA within each sociodemographic stratification. We did not account for multiple comparisons.
Lastly, we conducted a secondary analysis to explore the effect of inflammation on our results. We acknowledge that inflammation affects iron indicators (24–26). C-reactive protein (CRP), the only biomarker of inflammation available in NHANES, is measured in those aged ≥3 y. Therefore, only prevalence estimates for nonpregnant and pregnant females were adjusted for inflammation. To account for inflammation in our samples of nonpregnant and pregnant females, we excluded those with elevated CRP (>5 mg/L). Excluding those with elevated CRP reduced our sample size to 2533 nonpregnant females and 624 pregnant females.
RESULTS
The following population characteristics were reflected in our sample. Among toddlers 12–23 mo there were similar proportions of males and females (51.6% and 48.4%, respectively) (Table 1).The proportion of toddlers who were non-Hispanic white, non-Hispanic black, and Mexican American were 51.4%, 15.9%, and 19.6%, respectively. For 42.4% of toddlers, the family income was <130% of the poverty:income ratio. Among nonpregnant females, there were similar proportions by survey year (Table 2). Approximately half were ages 35–49 y, and the proportions who were nulliparous, primiparous, and multiparous (≥2 births) were 27.0%, 18.7%, and 54.3%, respectively. More than 1 in 4 nonpregnant females had a family income <130% of the poverty:income ratio. Among pregnant females, more than half were 20–34 y of age and in either their second or third trimester of pregnancy (Table 3).
TABLE 1.
Demographics and prevalence estimates of ID, based on TBI, among US toddlers aged 12–23 mo from NHANES 2003–20101
| Demographic characteristics |
|||
| n | % (95% CI) | Prevalence of ID based on TBI,2 % (95% CI) | |
| Total | 615 | — | 15.1 (11.7, 18.5) |
| Survey years | |||
| 2003–2004 | 162 | 26.9 (19.5, 34.3) | 16.1 (8.2, 24.0)a,b |
| 2005–2006 | 133 | 20.6 (14.3, 26.9) | 18.4 (10.3, 26.5)a |
| 2007–2008 | 137 | 22.5 (17.1, 27.9) | 19.1 (11.6, 26.5)a |
| 2009–2010 | 183 | 30.0 (24.1, 35.9) | 8.8 (5.5, 12.1)b |
| Sex | |||
| Female | 307 | 51.6 (45.5, 57.6) | 12.1 (7.8, 16.5)a |
| Male | 308 | 48.4 (42.4, 54.5) | 18.2 (12.9, 23.5)a |
| Age, mo | |||
| 12–17 | 293 | 47.3 (42.6, 52.1) | 13.4 (9.4, 17.3)a |
| 18–23 | 322 | 52.7 (47.9, 57.4) | 16.6 (11.4, 21.9)a |
| Race/ethnicity | |||
| NH white | 176 | 51.4 (43.9, 58.8) | 12.5 (6.7, 18.3)a |
| NH black | 154 | 15.9 (12.8, 18.9) | 13.7 (7.7, 19.6)a |
| Mexican American | 199 | 19.6 (14.9, 24.3) | 19.1 (13.2, 25.0)a |
| Other | 86 | 13.1 (9.4, 16.9) | 20.9 (11.4, 30.4)a |
| Family income | |||
| <130% of poverty:income ratio | 338 | 42.4 (37.0, 47.7) | 18.4 (13.7, 23.0)a |
| ≥130% of poverty:income ratio | 242 | 57.6 (52.3, 63.0) | 12.1 (7.2, 16.9)a |
ID was defined as TBI <0 mg/kg. ID, iron deficiency; NH, non-Hispanic; TBI, total-body iron stores.
All analyses were weighted and took into account the complex survey design. Within a group, values with different superscript letters had significantly different prevalence estimates (logistic regression, P < 0.05).
TABLE 2.
Demographics and prevalence estimates of ID, based on TBI, and the prevalence of IDA, based on TBI and hemoglobin, among US nonpregnant females aged 15–49 y from NHANES 2007–20101
| Demographic characteristics |
||||
| n | % (95% CI) | Prevalence of ID based on TBI,2 % (95% CI) | Prevalence of IDA based on TBI and low hemoglobin concentrations,2 % (95% CI) | |
| Total | 3418 | — | 10.4 (9.3, 11.5) | 5.0 (4.2, 5.8) |
| Survey years | ||||
| 2007–2008 | 1566 | 49.6 (45.2, 54.1) | 11.3 (9.8, 12.9)a | 5.2 (3.9, 6.6)a |
| 2009–2010 | 1852 | 50.4 (45.9, 54.8) | 9.4 (7.9, 10.9)b | 4.8 (4.0, 5.7)a |
| Age, y | ||||
| 15–19 | 636 | 13.5 (11.9, 15.1) | 10.8 (7.8, 13.8)a | 5.2 (3.0, 7.4)a,b |
| 20–34 | 1299 | 39.7 (37.1, 42.2) | 8.8 (7.1, 10.5)a | 3.7 (2.9, 4.6)a |
| 35–49 | 1483 | 46.8 (44.7, 49.0) | 11.6 (9.7, 13.4)a | 6.1 (4.7, 7.5)b |
| Parity | ||||
| 0 | 522 | 27.0 (24.9, 29.1) | 8.7 (6.3, 11.1)a | 4.8 (3.0, 6.6)a,b |
| 1 | 423 | 18.7 (16.6, 20.8) | 7.4 (4.3, 10.5)a | 3.0 (1.5, 4.5)a |
| ≥2 | 1364 | 54.3 (51.8, 56.8) | 12.3 (10.5, 14.1)b | 6.1 (4.9, 7.3)b |
| Race/ethnicity | ||||
| NH white | 1425 | 63.7 (58.0, 69.4) | 8.3 (6.8, 9.8)a | 3.0 (2.3, 3.8)a |
| NH black | 658 | 13.0 (10.4, 15.5) | 15.7 (12.8, 18.5)b | 11.8 (9.6, 14.0)b |
| Mexican American | 705 | 9.9 (6.9, 12.9) | 14.4 (12.3, 16.5)b | 8.5 (6.8, 10.3)c |
| Other | 630 | 13.5 (10.4, 16.6) | 12.1 (7.8, 16.4)a,b | 5.4 (2.2, 8.6)a,c |
| Family income | ||||
| <130% of poverty:income ratio | 1265 | 27.9 (24.8, 31.0) | 11.8 (9.4, 14.3)a | 6.1 (4.4, 7.9)a |
| ≥130% of poverty:income ratio | 1884 | 72.1 (69.0, 75.2) | 9.8 (8.4, 11.1)a | 4.6 (3.7, 5.4)a |
ID was defined as TBI <0 mg/kg, and IDA was defined as TBI <0 mg/kg and hemoglobin <120 g/L. ID, iron deficiency; IDA, iron deficiency anemia; NH, non-Hispanic; TBI, total-body iron stores.
All analyses were weighted and took into account the complex survey design. Within a group, values with different superscript letters had significantly different prevalence estimates (logistic regression, P < 0.05).
TABLE 3.
Demographics and prevalence estimates of ID, based on TBI, among US pregnant females aged 12–49 y from NHANES 1999–20101
| Demographic characteristics |
|||
| n | % (95% CI) | Prevalence of ID based on TBI,2 % (95% CI) | |
| Total | 1283 | — | 16.3 (13.6, 18.9) |
| Survey years | |||
| 1999–2000 | 258 | 22.5 (17.8, 27.2) | 24.5 (19.7, 29.3)a |
| 2001–2002 | 319 | 20.8 (18.1, 23.5) | 14.4 (8.8, 20.0)b |
| 2003–2004 | 241 | 14.6 (11.4, 17.8) | 12.4 (7.4, 17.3)b |
| 2005–2006 | 352 | 21.9 (18.1, 25.7) | 18.5 (12.7, 24.3)a,b |
| 2007–2008 | 49 | 8.8 (6.3, 11.4) | —3 |
| 2009–2010 | 64 | 11.3 (8.4, 14.3) | —3 |
| Age, y | |||
| 12–19 | 161 | 6.3 (4.5, 8.1) | 18.4 (9.7, 27.1)a |
| 20–29 | 722 | 55.4 (51.1, 59.7) | 19.2 (15.3, 23.0)a |
| 30–49 | 397 | 38.3 (34.1, 42.5) | 11.9 (7.0, 16.8)a |
| Trimester | |||
| First | 210 | 18.8 (15.4, 22.2) | 5.3 (2.3, 8.3)a |
| Second | 447 | 30.9 (26.5, 35.3) | 12.7 (8.1, 17.4)b |
| Third | 414 | 29.4 (24.8, 34.0) | 27.5 (20.6, 34.4)c |
| Unknown | 212 | 20.9 (17.1, 24.7) | 15.6 (7.1, 24.1)b,c |
| Parity | |||
| 0 | 386 | 32.7 (27.2, 38.1) | 12.4 (6.1, 18.8)a |
| 1 | 404 | 35.6 (30.5, 40.6) | 14.4 (9.0, 19.7)a |
| ≥2 | 379 | 31.8 (27.6, 35.9) | 26.2 (19.4, 33.1)b |
| Race/ethnicity | |||
| NH white | 547 | 53.5 (47.9, 59.2) | 12.1 (8.5, 15.7)a |
| NH black | 203 | 14.8 (11.2, 18.4) | 27.8 (19.3, 36.4)b |
| Mexican American | 385 | 16.5 (13.4, 19.6) | 20.7 (16.4, 25.1)b |
| Other | 148 | 15.2 (10.6, 19.7) | —3 |
| Family income | |||
| <130% of poverty:income ratio | 438 | 27.3 (23.4, 31.2) | 18.1 (12.9, 23.3)a |
| ≥130% of poverty:income ratio | 758 | 72.7 (68.8, 76.6) | 15.9 (12.6, 19.1)a |
ID was defined as TBI <0 mg/kg. ID, iron deficiency; NH, non-Hispanic; TBI, total-body iron stores.
All analyses were weighted and took into account the complex survey design. Within a group, values with different superscript letters had significantly different prevalence estimates (logistic regression, P < 0.05).
These estimates were suppressed due to concerns regarding the stability of the estimates.
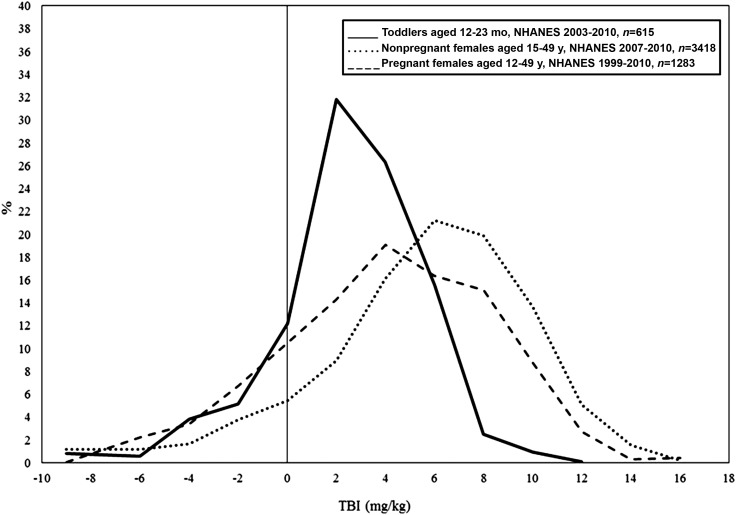
The weighted distributions of TBI are presented in Figure 1. For each population, the distributions are symmetrical and approximately normally distributed. Mean TBI was 2.6 mg/kg (95% CI: 2.3, 2.9 mg/kg), 5.6 mg/kg (95% CI: 5.4, 5.8 mg/kg), and 4.1 mg/kg (95% CI: 3.6, 4.5 mg/kg) for toddlers, nonpregnant females, and pregnant females, respectively. There is currently no recommended cutoff for iron repletion and excess. Thus, we are unable to make conclusions regarding the proportion of iron-replete toddlers and WRA. Further research is needed to establish a cutoff for iron repletion and excess in the United States.
FIGURE 1.
Distributions of TBI (calculated from serum ferritin and soluble transferrin receptor concentrations) in the United States by target population from NHANES. TBI, total-body iron stores.
Toddlers aged 12–23 mo
The prevalence of ID among toddlers was 15.1% (95% CI: 11.7%, 18.5%), and this varied significantly by survey year (Table 1). We observed variation in the prevalence of ID among toddlers when stratifying by sex, age, race/ethnicity, and family income. However, differences were not significant, and patterns will need to be confirmed with additional data. The overall and stratified prevalence estimates of IDA were suppressed due to concerns regarding stability of the estimates.
Nonpregnant females aged 15–49 y
Approximately 1 in 10 nonpregnant females (10.4%; 95% CI: 9.3%, 11.5%) had ID, including 5.0% with IDA (95% CI: 4.2%, 5.8%) (Table 2). Significant differences in the prevalence of ID existed by survey year, parity, and race/ethnicity. Multiparous women had a significantly higher prevalence of ID than nulliparous and primiparous women. The prevalence of ID among non-Hispanic blacks was significantly higher than that of non-Hispanic whites, but not higher than that of Mexican Americans. Significant differences in the prevalence of IDA existed by age, parity, race/ethnicity (Table 2). For example, nonpregnant women 35–49 y of age had a significantly higher prevalence of IDA than nonpregnant women 20–34 y of age. Multiparous women had a significantly higher prevalence of IDA than did primiparous women, but did not have a higher prevalence than nulliparous women. Racial and ethnic differences in the prevalence of IDA were evident as well. Non-Hispanic blacks had a higher prevalence of IDA than non-Hispanic whites, Mexican Americans, and other race/ethnicities.
Pregnant females aged 12–49 y
Among the full sample of pregnant females 16.3% (95% CI: 13.6%, 18.9%) had ID, including 2.6% (95% CI: 1.3%, 4.0%) with IDA. The prevalence of ID increased with trimester, e.g., the prevalence of ID was >2 times higher in the second trimester than in the first trimester, and >2 times higher in the third trimester than in the second trimester. Multiparous pregnant females had the highest prevalence of ID compared with that among nulliparous and primiparous females. The prevalence of ID among both non-Hispanic blacks and Mexican Americans was ∼2 times higher than that among non-Hispanic whites. Prevalence estimates did not differ by family income. Stratified prevalence estimates of IDA were suppressed due to concerns regarding stability of the estimates.
Secondary analysis
After excluding females with elevated CRP, prevalence of ID, based on TBI, was 11.4% (95% CI: 10.2%, 12.7%) and 15.2% (95% CI: 10.9%, 19.5%) among nonpregnant and pregnant females, respectively. The CIs for these prevalence estimates overlap with those for nonpregnant (Table 2) and pregnant females (Table 3) in our primary analysis. Therefore, it is unlikely that these estimates will differ significantly from those produced in our primary analysis. Additional stratifications using CRP exclusions are not shown.
After excluding females with elevated CRP, the prevalence of IDA for nonpregnant females was 3.4% (95% CI: 2.7%, 4.1%), which differs significantly from the prevalence of IDA for nonpregnant females in our primary analysis. However, both prevalence estimates are low. The prevalence of IDA among pregnant females was suppressed due to concerns regarding the stability of the estimate.
DISCUSSION
In summary, according to the most recent nationally representative data in the United States, the overall prevalence of ID for toddlers 12–23 mo and for nonpregnant and pregnant females was 15.1%, 10.4%, and 16.3%, respectively. The prevalence of ID among toddlers did not differ significantly when stratifying by race, sex, or family income. Racial disparities in ID exist among WRA; non-Hispanic blacks and Mexican Americans had the highest prevalence of ID, regardless of pregnancy status. Our analysis showed that among all females, ID is most prevalent among those who are multiparous. Additionally, pregnant females in their third trimester of pregnancy had the highest prevalence of ID. Accounting for inflammation by using the exclusion approach based on CRP values alone did not alter our prevalence estimates of ID for nonpregnant and pregnant females; however, prevalence estimates of IDA among nonpregnant females may differ significantly.
The racial and socioeconomic variation in ID underscores the need for programs, such as the Special Supplemental Nutrition Program for Women, Infants, and Toddlers, that target lower-income women and provide education and support for mothers, infants, and toddlers to meet their nutrient needs. However, our results also suggest that ID is not limited to specific populations. Approximately 1 in 10 toddlers, pregnant females, and nonpregnant females who are non-Hispanic white or have a poverty:income ratio ≥130% are also at risk of having ID. This suggests that all women and toddlers could benefit from programs and policies that support adequate nutrition.
A major strength of this study is the use of data from NHANES. NHANES is a nationally representative data source; therefore, we were able to produce reliable estimates for various subsets of the US population. Additionally, we used TBI, which is the standard indicator for assessment of iron status in the United States.
Monitoring the iron status of the US population can present several challenges. We were able to present the distribution of TBI and examine the prevalence of ID among toddlers and nonpregnant and pregnant females. However, without established cutoffs for iron repletion or iron excess based on TBI, we cannot determine these prevalence estimates; this suggests a need for additional research. In addition, we were not able to correct for inflammation and infection as has been done in previous studies. In the presence of inflammation, SF and sTfR concentrations increase, such that estimates of ID by using each indicator are inversely related (27). Although it has been hypothesized that the inflammation effects on SF and sTfR might cancel each other out when measuring TBI, a recent study showed that inflammation had a significant effect on TBI, suggesting the need for adjustment for inflammation among toddlers 6–59 mo and WRA (27). Additionally, similar studies showed that the prevalence of IDA is altered in the presence of inflammation and infection (28, 29). It is important to note that these studies accounted for inflammation by using 2 measurements, CRP and α-1 acid glycoprotein (AGP), of which only CRP is available in NHANES. We performed a secondary analysis in which we excluded women with elevated CRP concentrations. This exclusion approach is subject to limitations (30). To summarize, the exclusion approach resulted in a loss of precision due to reductions in sample size and may therefore introduce bias. Furthermore, the exclusion approach relies on established cutoffs for inflammation and thus may not capture the full spectrum of inflammation in a population, especially in low-infection settings such as the United States. The recommended analytic approach from the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) group, regarding adjusting for inflammation, is to use regression correction to adjust sTfR by using AGP (27, 31) and to correct SF by using both AGP and CRP (32). Unfortunately, AGP is not collected in NHANES, limiting our ability to adjust the ID and IDA estimates for inflammation. It is possible that the estimates produced in our primary and secondary analyses underestimate the prevalence of ID and IDA; for example, pooled analyses from the BRINDA project showed that inflammation correction increased estimated ID prevalence by using TBI by 14% in preschool-aged children and by 3% in WRA (27). Including biomarkers of infection and inflammation could help improve the accuracy of assessments of the iron status in the United States.
These data are subject to several limitations. Although TBI is the standard measure of iron status in the United States, its use worldwide is limited. SF concentration is more commonly used globally to assess iron status. Direct comparisons between iron status using the 2 different methods (TBI compared with SF only) should not be made. In an effort to provide information for comparison with other studies that assess the iron status of populations using SF, we calculated the unadjusted prevalence (± SE) of ID in the United States based on SF concentration (<12 μg/L for toddlers and <15 μg/L for nonpregnant and pregnant females) and found 15.3% ± 1.7%, 15.8% ± 0.8%, and 31.6% ± 1.9% among toddlers, nonpregnant females, and pregnant females, respectively. A recent BRINDA publication by Namaste et al. (30) comparing various methods for adjusting SF for infection and inflammation found that those with inflammation (CRP and/or AGP) and/or malaria infection had higher SF concentrations than did those without inflammation or infection. To our knowledge, the BRINDA project has focused on the effects of inflammation on iron indicators in young children and nonpregnant women. Further research is needed to determine the appropriate method for adjusting iron indicators for inflammation and infection among pregnant women. Therefore, we produced adjusted prevalence estimates of ID, based on SF, and found that among nonpregnant females, 22.5% (95% CI: 20.9%, 24.1%) were iron deficient. Adjusting for inflammation resulted in a statistically significant increase in the prevalence of ID, based on SF concentration. Second, the data used to calculate prevalence estimates for this study are older because data on iron indicators in NHANES do not exist beyond 2010. Third, the sample size of eligible toddlers was reduced because of missing nutritional biochemistry data. The demographic characteristics for toddlers included in our eligible sample compared with those not included were not statistically different, and therefore we do not have evidence of bias based on demographic differences. Fourth, starting in 2007, pregnant women were no longer oversampled, and urine pregnancy results were made publically available for women 20–44 y only, thus explaining the reduction in the sample size of pregnant females aged 12–19 y (21, 22). This may have led to potential misclassification bias, although given the low pregnancy rates among teenagers in the United States, we estimate that the impact on our prevalence estimates would be minimal (33).
Better assessment of the complete spectrum of iron status in the United States requires the development of cutoffs for iron replete and excess, as well as the consideration of biomarkers of infection and inflammation. Available nationally representative data suggest that ID and IDA remain a concern in the United States.
Acknowledgments
The authors’ responsibilities were as follows—PMG and ZM: had full access to all of the data in the study and were responsible for the integrity of the data and accuracy of the data analysis; HCH, PSS, and RF-A: critically revised the manuscript; ZM and PMG: performed the statistical analysis and drafted the manuscript; and all authors: participated in the study design and interpretation of the data, and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AGP, α-1-acid glycoprotein; BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; CRP, C-reactive protein; ID, iron deficiency; IDA, iron deficiency anemia; MEC, mobile examination center; NCHS, National Center for Health Statistics; SF, serum ferritin; sTfR, soluble transferrin receptor; TBI, total-body iron stores; WRA, women of reproductive age (pregnant and nonpregnant).
REFERENCES
- 1.US Department of Health and Human Services. Tracking healthy people 2010. Washington (DC): US Department of Health and Human Services; 2000. [Google Scholar]
- 2.Lynch S. Case studies: iron. Am J Clin Nutr 2011;94:673S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke RM, Leon JS, Suchdev PS. Identification, prevention and treatment of iron deficiency during the first 1000 days. Nutrients 2014;6:4093–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coad J, Pedley K. Iron deficiency and iron deficiency anemia in women. Scand J Clin Lab Invest Suppl 2014;244:82–9, discussion 9. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep 1998;47:1–29. [PubMed] [Google Scholar]
- 6.Pfeiffer CM, Looker AC. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr 2017;106(Suppl):1606S–14S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA 1997;277:973–6. [DOI] [PubMed] [Google Scholar]
- 8.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101:3359–64. [DOI] [PubMed] [Google Scholar]
- 9.Skikne BS, Flowers CH, Cook JD. Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood 1990;75:1870–6. [PubMed] [Google Scholar]
- 10.Cogswell ME, Looker AC, Pfeiffer CM, Cook JD, Lacher DA, Beard JL, Lynch SR, Grummer-Strawn LM. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003-2006. Am J Clin Nutr 2009;89:1334–42. [DOI] [PubMed] [Google Scholar]
- 11.Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, Grummer-Strawn LM. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am J Clin Nutr 2011;93:1312–20. [DOI] [PubMed] [Google Scholar]
- 12.Gupta PM, Perrine CG, Mei Z, Scanlon KS. Iron, anemia, and iron deficiency anemia among young children in the United States. Nutrients 2016;8:E330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR. National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. Vital Health Stat 2 2013:1–24. [PubMed] [Google Scholar]
- 14.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey lab methods 2009-2010 [Internet]. 2011. [cited 2016 Sep 12]. Available from: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2009.
- 15.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey lab methods 2007-2008 [Internet]. 2009. [cited 2016 Sep 12]. Available from: https://www.cdc.gov/nchs/nhanes/nhanes2007-2008/lab_methods_07_08.htm.
- 16.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey lab methods 1999-2000 [Internet]. 2000. [cited 2016 Sep 12]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/ContinuousNhanes/Manuals.aspx?BeginYear=1999.
- 17.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey lab methods 2001-2002 [Internet]. 2002. [cited 2016 Sep 12]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&CycleBeginYear=2001.
- 18.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey lab methods 2003-2004 [Internet]. 2004. [cited 2016 Sep 12]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&CycleBeginYear=2003.
- 19.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey lab methods 2005-2006 [Internet]. 2006. [cited 2016 Sep 12]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&CycleBeginYear=2005.
- 20.CDC, National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES) analytic guidelines June 2004 version 2003-2004 [Internet]. 2004. [cited 2016 Sep 12]. Available from: https://wwwn.cdc.gov/Nchs/Data/Nhanes/2003-2004/nhanes_general_guidelines_june_04.pdf.
- 21.Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, Hirsch R, Burt VL, Johnson CL. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital Health Stat 2 2013;2:1–23. [PubMed] [Google Scholar]
- 22.Johnson CL, Dohrmann SM, Burt VL, Mohadjer L. National Health and Nutrition Examination Survey: sample design, 2011–2014. Vital Health Stat 2 2014;2:1–33. [PubMed] [Google Scholar]
- 23.CDC, National Center for Health Statistics. NHANES 1999-2000 Addendum to the NHANES III analytic guidelines. Atlanta (GA): National Center for Health Statistics; 2002. [Google Scholar]
- 24.Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJ, et al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106(Suppl):359S–71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohner F, Namaste SM, Larson LM, Addo OY, Mei Z, Suchdev PS, Williams AM, Sakr Ashour FA, Rawat R, Raiten DJ, et al. Adjusting soluble transferrin receptor concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106(Suppl):372S–82S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrill RD, Burke RM, Northrop-Clewes CA, Rayco-Solon P, Flores-Ayala R, Namaste SM, Serdula MK, Suchdev PS. Factors associated with inflammation among preschool children and women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106(Suppl):348S–58S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mei Z, Namaste SM, Serdula M, Suchdev PS, Rohner F, Flores-Ayala R, Addo OY, Raiten DJ. Adjusting total body iron for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106(Suppl):383S–89S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engle-Stone R, Aaron GJ, Huang J, Wirth JP, Namaste SM, Williams AM, Peerson JM, Rohner F, Varadhan R, Addo OY, et al. Predictors of anemia among preschool children: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106(Suppl):402S–15S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wirth JP, Woodruff BA, Engle-Stone R, Namaste SM, Temple VJ, Petry N, Macdonald B, Suchdev PS, Rohner F, Aaron GJ. Predictors of anemia among women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106(Suppl):416S–27S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Namaste SM, Aaron GJ, Varadhan R, Peerson JM, Suchdev PS, BRINDA Working Group. Methodological approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106(Suppl):333S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO/CDC. Assessing the iron status of populations: a report of a joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level. 2nd ed. Geneva (Switzerland): WHO/CDC; 2007. [Google Scholar]
- 32.Suchdev PS, Namaste S, Aaron G, Raiten D, Brown KH, Flores-Ayala R. Overview of the Biomarkers Reflecting Inflammation and Determinants of Anemia project. Adv Nutr 2016;7:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtin SC, Abma JC, Ventura SJ, Henshaw SK. Pregnancy rates for U.S. women continue to drop. NCHS Data Brief No. 136. Hyattsville (MD): National Center for Health Statistics; 2013. [PubMed] [Google Scholar]