Abstract
Importance
CJD is a fatal neurodegenerative disorder associated with the accumulation of infectious abnormal PrP through a mechanism of templated misfolding. A recent report has described the detection of abnormal PrP in vCJD patient urine using protein misfolding by cylic amplification, which was apparently absent in the more common sporadic form of CJD (sCJD). A non-invasive diagnostic test could improve early diagnosis of sCJD, and by screening donations, mitigate the potential risks of prion transmission through human urine-derived pharmaceuticals. Here we describe the adaptation of the direct detection assay, developed originally as a blood test for vCJD, for the detection of disease-associated PrP in urine samples from sCJD patients.
Objectives
To determine the feasibility of sCJD diagnosis by adaptation of an established vCJD diagnostic blood test to urine.
Design
Retrospective, cross sectional study.
Setting
Urine samples obtained during the MRC PRION-1 trial, the National Prion Monitoring Cohort study, and/or referred to the National Prion Clinic or Dementia Research Centre at the National Hospital for Neurology and Neurosurgery in the United Kingdom.
Participants
Anonymised urine samples from healthy non-neurological control subjects (n=91), patients with non-prion neurodegenerative diseases (n=34) and patients with prion disease (n=37) of which 20 were sCJD.
Main Outcome Measure
Presence of sCJD infection determined by an assay that captures, enriches, and detects disease-associated prion protein isoforms.
Results
The assay’s specificity for prion disease was 100% [CI: 97% to 100%] with no false positive reactions from 125 controls, including 34 from a range of neurodegenerative diseases. In contrast to a previous study which used a different methodology, sensitivity to vCJD infection was low with only 1 out of 13 patients testing positive whilst sensitivity to sCJD was unexpectedly high at 40% [CI:19-64%].
Conclusions
We determined 40% of sCJD urine samples as positive. This is the first demonstration of an assay that can detect sCJD infection in urine or any target analyte outside of the central nervous system. Urine detection could allow the development of rapid, molecular diagnostics for sporadic CJD and has implications for other neurodegenerative diseases where disease-related assemblies of misfolded proteins might also be present in urine.
Introduction
Prion diseases are lethal, transmissible neurodegenerative conditions of humans and animals caused by misfolding and aggregation of the prion protein (PrP)1;2. It is the transmissibility of disease that is a defining feature of the group, and has resulted in several high profile epidemics of acquired prion disease, including for example, kuru in humans, bovine spongiform encephalopathy (BSE) in cattle and its human counterpart variant Creutzfeldt-Jakob disease (vCJD)3. Prion disease may also occur as sporadic CJD (sCJD) without any obvious exposure to an infectious source, the clinical syndrome being rapidly progressive with a median survival of around four months4. Central to the pathogenesis of prion disease is the autocatalytic, templated remodelling of the host’s normal cellular prion protein (PrPC) leading to accumulation of abnormal PrP isoforms typified by insoluble aggregates often termed PrPSc 5–8. It is increasingly recognised that more common neurodegenerative diseases such as Alzheimer’s disease also involve similar seeding processes and worldwide efforts are now being made to establish the precise role of prion-like mechanisms in a range of human diseases9–12.
As a consequence of widespread exposure to BSE prions, it is thought that as many as 1 in 2000 of the UK population may be prion carriers13. Significant public health concerns are associated with the transmissibility of prion diseases and the potential prevalence of vCJD infection has required widespread precautionary measures to limit iatrogenic transmission. The risks of iatrogenic transmission from individuals with sCJD have long been established, with the primary sources being contaminated neurosurgical instruments and devices14 and cadaveric donor-derived materials such as dura mater and pituitary growth hormone15. The incidence of sCJD is around 2 persons per million per year, which typically occurs with increasing incidence from late middle age onwards and has a uniform gender and geographical distribution. The lifetime risk of sCJD is around 1 in 5000 in the UK. Unfortunately, approximately half of patients are only diagnosed at a clinically advanced state16.
The presence of PrPSc and other abnormal isoforms of PrP is a specific and potentially sensitive diagnostic indicator for prion disease and methods for their detection have been developed that can be applied to a range of tissues and fluids17–21. The impetus for the development of diagnostic methods has been the emergence of vCJD which is characterised by a widespread peripheral pathology22 and the infection of blood23;24 and urine25. Despite the development of an assay for the diagnosis of vCJD using blood21;26 and the reporting of vCJD infection being detectable in urine25, the application of such techniques to sCJD has been confounded by the restriction of significant pathology to the central nervous system (CNS). Coincident with the reporting of detection in the urine of patients with vCJD was the failure to detect any signs of infection in sCJD patient samples using the same technique25. However, the method used has never been convincingly demonstrated to detect sCJD and notably no positive controls were included in this study. Whilst the application of amyloid seeding assays to cerebrospinal fluid (CSF) have proved effective for the diagnosis of sCJD, the invasive nature of this procedure has limited their application to patients with a high index of suspicion of prion disease27.
A non-invasive pre-mortem biochemical test for sCJD diagnosis could significantly reduce the extended time currently taken to establish a firm diagnosis, which can comprise the majority of a patient’s remaining life16, and reduce the threshold for testing in patients with relatively early, non-specific clinical features. Here we report the application to urine testing of an adapted blood assay, originally developed for vCJD21. Strikingly, the assay was able to detect sCJD infection in almost half of the patient samples, the first demonstration of an assay capable of diagnosing sCJD from urine or any peripheral analyte.
Methods
Source of urine samples
Patient and control urine samples were obtained with informed consent from patients enrolled in the MRC PRION-1 trial28, National Prion Monitoring Cohort study, and/or referred to the National Prion Clinic or Dementia Research Centre at the National Hospital for Neurology and Neurosurgery. CJD diagnoses were made according to established criteria27;29 All samples were collected using standard urinalysis preservative (boric acid, sodium formate and sodium borate) and stored frozen at -70°C in multiple aliquots of 2ml and thawed before use. Samples were analysed blind to the operators and only decoded after analysis and determination of positive or negative status.
Testing procedure
Samples were tested using a modification of a method previously described21. Briefly, 400μL of each sample was diluted 1:1 into 400μL buffer [200mM Tris, 4%w/v BSA, 4%w/v CHAPS, 2 tablets complete protease inhibitors (Roche), 80 units Benzonase (Grade II, Merck)] containing 23mg of Capture Matrix (CM) [sub-45μm stainless steel particles, Goodfellow, Cambridge, UK] and incubated overnight. The CM was isolated using a magnetic rack, supernatant discarded, and washed repeatedly with 1mL PBS + 0.05%v/v Tween-20 (PBST). After the final wash, all liquid was removed and the CM heated at 110°C. To each tube 50µL of biotinylated primary antibody (ICSM18, D-Gen, Ltd) prepared at 1µg/mL in PBS + 1%v/v Tween-20 (PBST*) was added and incubated at 37°C for 1 hour. Samples were washed repeatedly with 1mL PBST isolating CM each time. Each sample was then incubated with High Sensitivity NeutrAvidin-HRP (Pierce) prepared at a 1:100 000 dilution in PBST* at 37°C for 45 minutes. Samples were again washed repeatedly with 1mL PBST isolating CM each time. To each sample 60µL of SuperSignal ELISA Femto chemiluminescent substrate (Pierce) was added and CM was evenly distributed into three replicate wells of a black 96-well plate (Greiner). Each plate included set of six quality control samples (5 negative control normal urine samples and one positive control). An additional 80µL of chemiluminescent substrate was added to each well immediately prior to the plate scan. The total luminescent output over a fixed integration time was measured for each well using a M1000 plate reader (Tecan).
Positivity criteria and data analysis
Samples were scored reactive if the mean chemiluminescence from three replicate wells exceeded an on-plate cut-off threshold of the mean plus five standard deviations of five negative control normal urine samples. Thus, samples with a “ratio relative to cut-off” greater than one were scored reactive. All samples were tested twice with those that were repeat-reactive, ie reactive in both anlayses considered positive for prion disease. Non-reactive and single-reactive samples were considered negative.
Conductivity measurements
In order to control for the possibility of dehydration in prion disease patients producing higher urine direct detection assay (DDA) signals, an approximation to the concentration levels of urine samples was determined by conductivity measurement. Conductivity is known to correlate with urine osmolality and specific gravity30;31 and was determined using a low volume conductivity meter (Horiba Laqua Twin B-771). Calibration of the conductivity meter was performed using a 1.41mS/cm standard solution and the conductivity of urine samples determined by taking the mean of three independent measurements from three separate aliquots of 100μl of urine sample, washing the electrode with ddH2O between readings.
Results
DDA analysis of urine from patients with prion disease
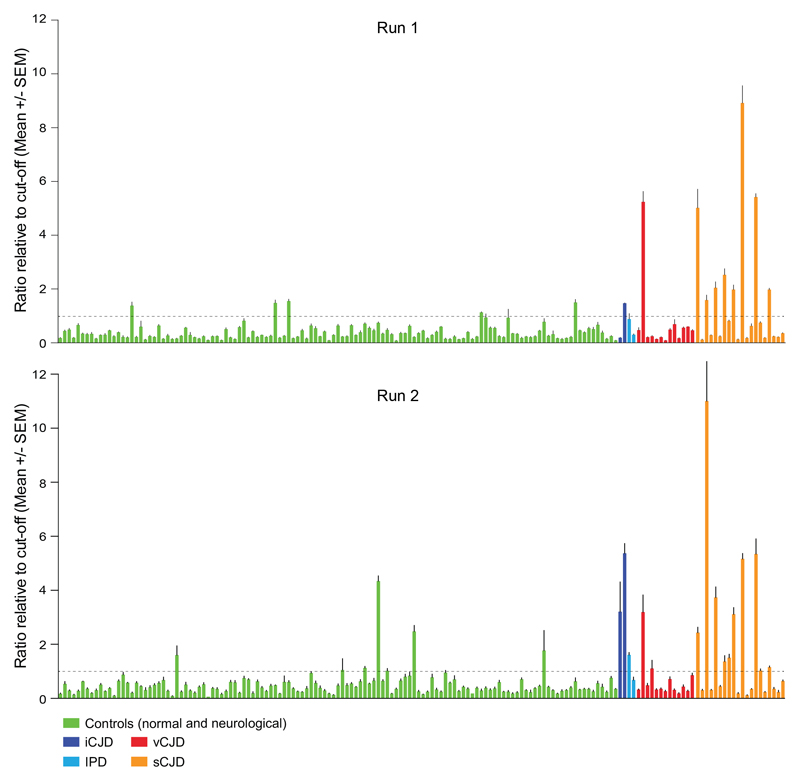
A panel of urine samples obtained from a total of 37 patients with confirmed prion disease and 125 controls from both normal individuals and patients with neurological diseases other than prion disease were analysed using an adaptation of a previously reported DDA blood test for vCJD infection. The samples were tested in groups of 12 with a quality control panel on each plate, consisting of five negative normal controls and one positive control. Each sample was tested in triplicate wells across two independent runs. An arbitrary ‘cut off’ was determined from the mean plus 5 S.D.’s of the panel of five normal samples in the quality control panel. A ratio for the mean chemiluminescence of each sample to the plate ‘cut off’ was calculated for each sample and if greater than 1 a sample was classified as reactive. Only samples repeat reactive in both test runs were scored as positive. Of the 162 blinded samples tested, 15 and 22 samples were scored as reactive in the first and second test runs respectively (Figure 1), with 10 samples scored repeat reactive across the two test runs. All of the 10 positive samples were from patients with prion disease, one from a case of vCJD, one from a growth hormone related case of iCJD and eight from patients with sCJD. A summary of the major clinical findings in all 37 cases of prion disease is provided in eTable 1.
Figure 1. Assaying a blind panel of 162 urine samples.
Testing was performed in two independent assays on a blind panel of 162 urine samples from patients with diagnoses of; sCJD (orange bars, n=20), vCJD (red bars, n=13), inherited prion disease (IPD, pale blue bars, n=2), growth hormone related iatrogenic CJD (iCJD, dark blue bars, n=2), healthy normal and neurological disease controls (green bars, n=91 and n=34 respectively). Neurological disease controls comprised; Alzheimer’s disease (AD, n=21), Huntington’s disease (HD, n=3), fronto-temporal dementia (FTD, n=3), familial Alzheimer’s disease (FAD, n=2), early onset dementia (EOD, n=1) and referrals to the Nation Prion Clinic determined as non-prion disease (n=4). Runs 1 and 2 represent data from the individual assay runs of blind samples. Data are shown as the chemiluminescent signal ratio relative to a cut-off determined from the mean + 5 x SD of normal controls (see Methods).
There were no clear associations with any aspects of the clinical history, disease progression, or investigations. The National Prion Clinic have developed a functionally-orientated rating scale to measure CJD disease progression termed the MRC Prion Disease Rating Scale 4. The mean MRC Scale was 4.0/20 for positive cases, and 5.0 for negative cases (P=0.57, t-test). The patient with least disease progression of sCJD had an MRC Scale of 10/20, however at present most sCJD in the UK is diagnosed at advanced clinical stages for a variety of reasons; we had no samples from any patient with sCJD at earlier disease stages. All genotypes at polymorphic codon 129 of the prion protein gene, an important susceptibility factor and disease modifier32, were represented in both DDA positive and negative cases. Typical investigation findings, including periodic sharp wave complexes on EEG, abnormal signal on diffusion weighted MRI brain in the cortex and basal ganglia, and abnormal 14-3-3 protein levels in CSF were found in an expected proportion of DDA positive and negative cases (eTable 1).
Based on the criteria of a sample being repeat reactive in two independent runs, 10/37 (27%) of the prion disease urine samples were identified in the absence of any false positive reactions. However, considering sCJD in isolation, sensitivity of detection was 8/20 samples or 40% (CI:19-64%) with 100% (CI:97-100%) specificity.
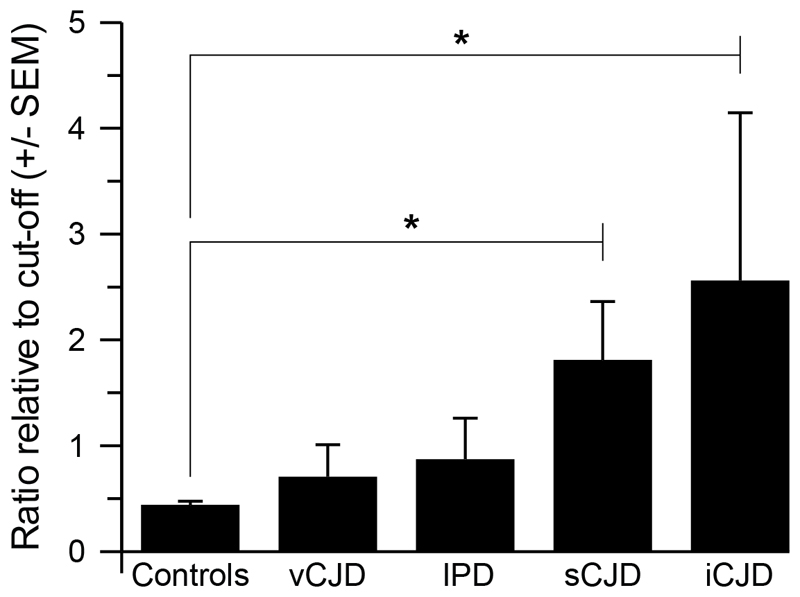
Comparison of the mean DDA results expressed as RRC for the four types of prion disease analysed (Figure 2) confirms significantly elevated abnormal PrP levels in both sCJD and iCJD with respect to controls (p<0.0001), but with levels in both vCJD and IPD failing to achieve statistical significance, possibly due to the low numbers of samples analysed.
Figure 2. Discrimination of various forms of prion disease from normal and neurological controls.
Data shown are the group mean ratios relative to cut-offs (RRC) for both assay runs combined for the groups of samples indicated; 91 normal controls, 34 neurological disease controls (for details see Figure 1 Legend), 13 vCJD, 20 sCJD, 2 IPD and 2 iCJD. Error bars represent the standard error of the mean (SEM). The two tailed p values for comparison of control samples to prion disease cohorts support a significant difference for both sCJD and iCJD (* p <0.0001).
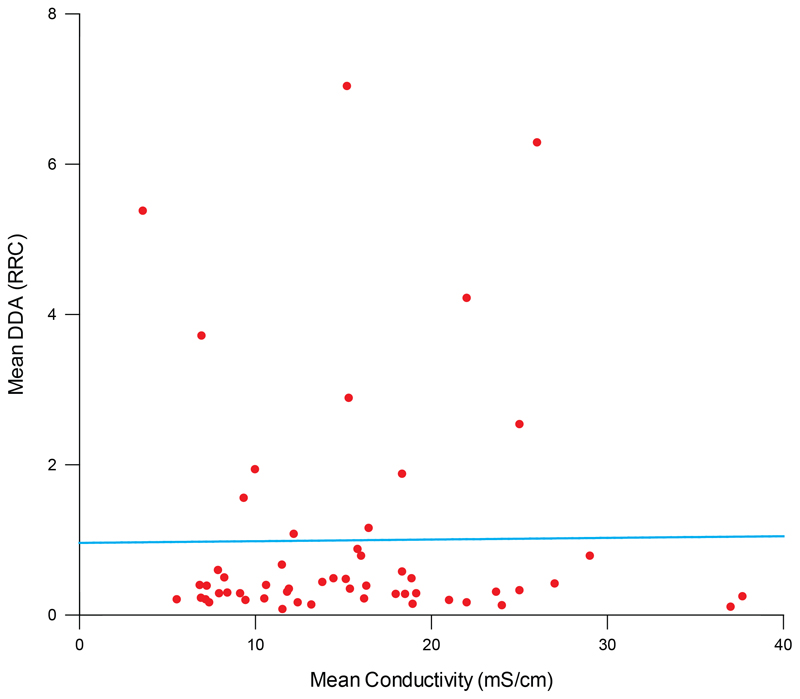
Conductivity of urine samples as a measure of concentration
Conductivity, a simplified measure of osmolality which correlates with urine concentration30;31, was measured in approximately a third of the samples (n=55), where sufficient volume was available. No correlation was observed between urine concentration and DDA signals (Figure 3) confirming patient hydration was not the cause of elevated abnormal PrP signals in urine.
Figure 3. Correlation of urine sample DDA values and conductivity.
The conductivities of 55 urine samples were quantified as milliSiemens cm-1 and plotted versus the mean of the previously determined RRC values on the y-axis. A Pearson correlation co-efficient was calculated as 0.058 for the relationship between the two values which was not significantly different from 0 (p=0.68, two tailed t test). The line superimposed on the data is the best fit of a linear regression, highlighting the lack of correlation with a gradient of 0.002.
Discussion
Blood is a commonly used analyte for range of diagnostic and prognostic tests, being readily accessible with minimal risk in almost all patients. We have previously reported the development and validation of a blood test for vCJD21;26 which despite excellent performance characteristics against vCJD was unable to detect infection in the blood of patients with sCJD. An accurate diagnosis of sCJD in patients with advanced symptomatic disease patients can usually be achieved16 without recourse to molecular diagnostics but the ability to diagnose patients much earlier would have obvious benefits, particular with respect to early entry into therapeutic trials.
The ability of disease-associated abnormal PrP to catalyse the conversion of recombinant PrP into amyloid conformations has been exploited in the development of amyloid-seeding-assays capable of detecting the presence of prion disease infection in a variety of tissues and fluids18;19;33. However, the detection of sCJD prion infection has been limited to the tissues of the central nervous system and cerebro-spinal fluid (CSF). In one study, analysis of CSF obtained from patients with sporadic CJD using amyloid seeding has indicated the detection limit is sufficient for excellent discrimination of affected individuals from controls with a sensitivity of around 90% 34. However, the requirement for CSF limits the application of the assay as the collection of such samples is an invasive procedure that is generally performed only when significant neurological symptoms and signs are evident.
An alternative to blood as a peripheral analyte, and one that can be readily obtained for routine diagnostic use, is urine. Our study has shown for the first time that it is feasible to identify sCJD using a urine sample by capture of disease-associated PrP on a stainless steel matrix and detection using anti-PrP monoclonal antibodies. Whilst the sensitivity of diagnosis is relatively low, at 40%, the high mean signals obtained in the assay suggest this could be improved considerably by pre-treatment or processing of large volumes of urine prior to assay. It is likely the current diagnostic sensitivity could be significantly improved by improvements in the analytic sensitivity of the assay but it is unclear if this could approach 100% as there is likely to be variability in the presence and concentration of abnormal PrP in the urine of individual patients. The high mean signals observed in the two samples from cases of iatrogenic growth-hormone related CJD suggest such an assay may also have significant sensitivity to cases of secondary CJD infection, although this will require the analysis of larger numbers of samples. The detection of abnormal misfolded prion protein in the urine of CJD patients is somewhat unexpected and intriguingly suggests other neurodegenerative diseases associated with protein misfolding may have diagnostic urine signatures. An unparalleled advantage of urine as an analyte is the easy availability of large sample volumes for research which will facilitate the development of protocols for the isolation and characterisation of the specific form of abnormal PrP present, leading to improved diagnostic sensitivity.
The origin of disease-associated PrP in the urine of sCJD patients is unknown but it is clear that urine contains PrPC, largely in a truncated form that carries only a partial glycosylphosphatidylinositol (GPI) anchor lacking the associated lipid moiety35;36, and therefore there is the potential for prion replication in situ as well as accumulation from the glomerular filtration of blood. Although urine is normally considered to lack detectable protein in the absence of kidney disease, in fact, normal kidney function results in trace protein concentrations of between 50-200 µg ml-1, sufficient to result in the accumulation of abnormal PrP derived from blood. It is notable that although abnormal PrP is present in urine it is not readily detectable in blood. A plausible explanation is the enrichment of abnormal PrP by the action of filtration in the kidney to concentrations that are detectable, but this could also be explained by active prion replication in the kidney or urinary tract. Certainly all the requirements for prion amplification are present; normal PrPC, disease-associated abnormal PrP and a slightly denaturing environment provided by urea. However it is important to note that transmission studies have indicated the potential prion titres in native urine are low (<0.38 infectious units ml-1)37 or absent, and there are no reported cases of CJD resulting from sexual transmission with the evidence from experimental models indicating that this is unlikely to occur38;39. The complex milieu of blood contributes to greater difficulties in specifically detecting disease-related signals whereas urine is relative ‘clean’ analyte with low background levels of protein which may be sufficient to account for the apparent discrepancy alone.
Prion protein has previously been identified in commercially available injectable urine-derived gonadotropin products that are used to treat infertility in women40 and undescended testes in boys41 and are available for illicit use in body building and as performance enhancing drugs42. This fact, coupled with the detection of abnormal PrP in the urine of vCJD patients25, has raised questions about the safety of such products should the purification methods result in the co-purification of prions as is known to have been the case for human cadaveric pituitary-derived hormones15. Typically urine is sourced from post-menopausal women with a median age of over 50 years, an age cohort where sCJD can be expected, although exclusion criteria exist for donations, analogous to those in place for donating blood. Despite transmission studies indicating the potential prion titres in native urine are low37, it is conceivable that commercial purification of hormone products could concomitantly enrich for prion infectivity. Our finding, that the urine of sCJD patients contains abnormal PrP is consistent with concerns that urine-derived fertility products could contain infectious prions as sCJD remains the most common form of prion disease and has a uniform world-wide incidence.
Our findings are in marked contrast to those recently reported using the technique of protein misfolding by cyclic amplification (PMCA) where a high sensitivity for the detection of vCJD infection in urine was observed and yet none of 68 patient samples from sCJD tested positive25. A critical limitation to the interpretation of this finding is that the assay methodology (PMCA) has never been convincingly demonstrated to detect sCJD and indeed no positive controls were included in this study. Parallel CJD strain-dependent differences are observed with other assay methods, some of which are attributable to differences in peripheral pathogenesis21;22;43;44 but some of which remain unexplained45.
Implications for practice and conclusions
The detection of disease-related prion proteins in the urine of patients with sCJD is the first demonstration that sCJD can be diagnosed from urine of any peripheral analyte by biochemical means. This offers the possibility of improved early diagnosis of sCJD and also the potential precautionary screening of human urine-derived pharmaceuticals.
Supplementary Material
Acknowledgments
We thank the patients and families affected by neurodegenerative diseases for their support of this work, Dr Gill Rumsby for providing anonymised urine samples, Mr Richard Newton for his help preparing the figures and Dr Jonathan Wadsworth for helpful discussion. Graham Jackson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was funded by the UK Medical Research Council. The Department of Health (England) and the University College London Hospital NIHR Biomedical Research Centre funded the National Prion Monitoring Cohort study. The sponsors provided financial support for the research but were not involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; the decision to submit the manuscript for publication.
Footnotes
Competing interests: The authors declare that they have a conflict of interest relevant to this manuscript. JC is a Director and JC and GSJ are shareholders of D-Gen Limited (London), an academic spin-out company working in the field of prion disease diagnosis, decontamination, and therapeutics. D-Gen supplied the ICSM18 antibody used in this study.
Ethical approval: These studies were approved by the local research ethics committees of the UCL Institute of Neurology and the National Hospital for Neurology and Neurosurgery. Patient urine samples were obtained with informed consent from patients.
References
- (1).Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- (2).Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hill AF, Desbruslais M, Joiner S, et al. The same prion strain causes vCJD and BSE. Nature. 1997;389:448–50. 526. doi: 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- (4).Thompson AG, Lowe J, Fox Z, et al. The Medical Research Council Prion Disease Rating Scale: a new outcome measure for prion disease therapeutic trials developed and validated using systematic observational studies. Brain. 2013;136:1116–1127. doi: 10.1093/brain/awt048. [DOI] [PubMed] [Google Scholar]
- (5).Griffith JS. Self Replication and scrapie. Nature. 1967;215:1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- (6).Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- (7).Oesch B, Westaway D, Walchli M, et al. A Cellular Gene Encodes Scrapie Prp 27-30 Protein. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- (8).Bueler H, Aguzzi A, Sailer A, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- (9).Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Prusiner SB. Biology and genetics of prions causing neurodegeneration. Annu Rev Genet. 2013;47:601–623. doi: 10.1146/annurev-genet-110711-155524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Goedert M. NEURODEGENERATION. Alzheimer's and Parkinson's diseases: The prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science. 2015;349:1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- (12).Jaunmuktane Z, Mead S, Ellis M, et al. Evidence for human transmission of amyloid-beta pathology and cerebral amyloid angiopathy. Nature. 2015;525:247–250. doi: 10.1038/nature15369. [DOI] [PubMed] [Google Scholar]
- (13).Gill ON, Spencer Y, Richard-Loendt A, et al. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey. BMJ. 2013;347:f5675. doi: 10.1136/bmj.f5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Bernoulli C, Siegfried J, Baumgartner G, et al. Danger of accidental person-to-person transmission of Creutzfeldt-Jakob disease by surgery. Lancet. 1977;1:478–479. doi: 10.1016/s0140-6736(77)91958-4. [DOI] [PubMed] [Google Scholar]
- (15).Powell-Jackson J, Weller RO, Kennedy P, Preece MA, Whitcombe EM, Newsom-Davis J. Creutzfeldt-Jakob disease after administration of human growth hormone. Lancet. 1985 Aug 3;:244–245. doi: 10.1016/s0140-6736(85)90292-2. [DOI] [PubMed] [Google Scholar]
- (16).Paterson RW, Torres-Chae CC, Kuo AL, et al. Differential Diagnosis of Jakob-Creutzfeldt Disease. Arch Neurol. 2012:1–5. doi: 10.1001/2013.jamaneurol.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- (18).Colby DW, Zhang Q, Wang S, et al. Prion detection by an amyloid seeding assay. Proc Natl Acad Sci U S A. 2007;104:20914–20919. doi: 10.1073/pnas.0710152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Atarashi R, Moore RA, Sim VL, et al. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods. 2007;4:645–650. doi: 10.1038/nmeth1066. [DOI] [PubMed] [Google Scholar]
- (20).Edgeworth JA, Jackson GS, Clarke AR, Weissmann C, Collinge J. Highly sensitive, quantitative cell-based assay for prions adsorbed to solid surfaces. Proc Natl Acad Sci USA. 2009;106:3479–3483. doi: 10.1073/pnas.0813342106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Edgeworth JA, Farmer M, Sicilia A, et al. Detection of prion infection in variant Creutzfeldt-Jakob disease: a blood-based assay. Lancet. 2011;377:487–493. doi: 10.1016/S0140-6736(10)62308-2. [DOI] [PubMed] [Google Scholar]
- (22).Wadsworth JD, Joiner S, Hill AF, et al. Tissue distribution of protease resistant prion protein in variant CJD using a highly sensitive immuno-blotting assay. Lancet. 2001;358:171–180. doi: 10.1016/s0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- (23).Llewelyn CA, Hewitt PE, Knight RS, et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- (24).Wroe SJ, Pal S, Siddique D, et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet. 2006;368:2061–2067. doi: 10.1016/S0140-6736(06)69835-8. [DOI] [PubMed] [Google Scholar]
- (25).Moda F, Gambetti P, Notari S, et al. Prions in the urine of patients with variant Creutzfeldt-Jakob disease. N Engl J Med. 2014;371:530–539. doi: 10.1056/NEJMoa1404401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Jackson GS, Burk-Rafel J, Edgeworth JA, et al. Population Screening for Variant Creutzfeldt-Jakob Disease Using a Novel Blood Test: Diagnostic Accuracy and Feasibility Study. JAMA Neurol. 2014;71:340. doi: 10.1001/jamaneurol.2013.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132:2659–2668. doi: 10.1093/brain/awp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Collinge J, Gorham M, Hudson F, et al. Safety and efficacy of quinacrine in human prion disease (PRION-1 study): a patient-preference trial. Lancet Neurology. 2009;2009:334–344. doi: 10.1016/S1474-4422(09)70049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Poser S, Mollenhauer B, Krauss A, et al. How to improve the clinical diagnosis of Creutzfeldt-Jakob disease. Brain. 1999;122:2345–2351. doi: 10.1093/brain/122.12.2345. [DOI] [PubMed] [Google Scholar]
- (30).Wang JM, Wen CY, Lin CY, Li JY, Lee CH, Wu MF. Evaluating the performance of urine conductivity as screening for early stage chronic kidney disease. Clin Lab. 2014;60:635–643. doi: 10.7754/clin.lab.2013.130628. [DOI] [PubMed] [Google Scholar]
- (31).Genain C, Tellier P, Syrota A, Pocidalo JJ, Hans M. Infinite dilution conductimetry of plasma and urine: correlation with osmolality. Clin Chim Acta. 1978;88:177–182. doi: 10.1016/0009-8981(78)90167-5. [DOI] [PubMed] [Google Scholar]
- (32).Palmer MS, Dryden AJ, Hughes JT, Collinge J. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature. 1991;352:340–342. doi: 10.1038/352340a0. [DOI] [PubMed] [Google Scholar]
- (33).Atarashi R, Wilham JM, Christensen L, et al. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat Methods. 2008;5:211–212. doi: 10.1038/nmeth0308-211. [DOI] [PubMed] [Google Scholar]
- (34).McGuire LI, Peden AH, Orru CD, et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol. 2012;72:278–285. doi: 10.1002/ana.23589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Narang HK, Dagdanova A, Xie Z, Yang Q, Chen SG. Sensitive detection of prion protein in human urine. Exp Biol Med (Maywood) 2005;230:343–349. doi: 10.1177/153537020523000508. [DOI] [PubMed] [Google Scholar]
- (36).Dagdanova A, Ilchenko S, Notari S, et al. Characterization of the prion protein in human urine. J Biol chem. 2010;285:30489–30495. doi: 10.1074/jbc.M110.161794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Notari S, Qing LT, Pocchiari M, et al. Assessing Prion Infectivity of Human Urine in Sporadic Creutzfeldt-Jakob Disease. Emerging Infectious Diseases. 2012;18:21–28. doi: 10.3201/eid1801.110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Morales R, Pritzkow S, Hu PP, Duran-Aniotz C, Soto C. Lack of prion transmission by sexual or parental routes in experimentally infected hamsters. Prion. 2013:7. doi: 10.4161/pri.26747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Sarradin P, Melo S, Barc C, et al. Semen from scrapie-infected rams does not transmit prion infection to transgenic mice. Reproduction. 2008;135:415–418. doi: 10.1530/REP-07-0388. [DOI] [PubMed] [Google Scholar]
- (40).Van Dorsselaer A, Carapito C, Delalande F, et al. Detection of prion protein in urine-derived injectable fertility products by a targeted proteomic approach. PLoS ONE. 2011;6:e17815. doi: 10.1371/journal.pone.0017815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Pyorala S, Huttunen NP, Uhari M. A Review and Metaanalysis of Hormonal Treatment of Cryptorchidism. Journal of Clinical Endocrinology & Metabolism. 1995;80:2795–2799. doi: 10.1210/jcem.80.9.7673426. [DOI] [PubMed] [Google Scholar]
- (42).Handelsman DJ. Clinical review: The rationale for banning human chorionic gonadotropin and estrogen blockers in sport. J Clin Endocrinol Metab. 2006;91:1646–1653. doi: 10.1210/jc.2005-2569. [DOI] [PubMed] [Google Scholar]
- (43).Glatzel M, Abela E, Maissen M, Aguzzi A. Extraneural pathologic prion protein in sporadic Creutzfeldt-Jakob disease. N Engl J Med. 2003;349:1812–1820. doi: 10.1056/NEJMoa030351. [DOI] [PubMed] [Google Scholar]
- (44).Douet JY, Zafar S, Perret-Liaudet A, et al. Detection of infectivity in blood of persons with variant and sporadic Creutzfeldt-Jakob disease. Emerg Infect Dis. 2014;20:114–117. doi: 10.3201/eid2001.130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Orru CD, Groveman BR, Raymond LD, et al. Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains. PLoS Pathog. 2015;11:e1004983. doi: 10.1371/journal.ppat.1004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.