Abstract
Introduction
Vocal fold paresis and paralysis are common conditions. Treatment options include augmentation laryngoplasty and voice therapy. The optimal management for this condition is unclear. The objective of this study was to assess possible neuromuscular compensation mechanisms that could potentially be used in the treatment of vocal fold paresis and paralysis.
Methods
In an in vivo canine model we examined three conditions: (1) unilateral right recurrent laryngeal nerve (RLN) paresis and paralysis, (2) unilateral superior laryngeal nerve paralysis (SLN), and (3) unilateral vagal nerve paresis and paralysis. Phonatory acoustics and aerodynamics were measured in each of these conditions. Effective compensation was defined as improved acoustic and aerodynamic profile.
Results
The most effective compensation for all conditions was increasing RLN activation and decreasing glottal gap. Increasing RLN activation increased the percentage of possible phonatory conditions that achieved phonation onset. SLN activation generally led to decreased number of total phonation onset conditions within each category. Differential effects of SLN (CT muscle) activation were seen: Ipsilateral SLN activation could compensate for RLN paralysis, normal CT compensated well in unilateral SLN paralysis, and in vagal paresis/paralysis contralateral SLN and RLN display antagonistic relationships.
Conclusions
Methods to improve glottal closure should be the primary treatment for large glottal gaps. Neuromuscular compensation is possible for paresis. This study provides insights into possible compensatory mechanisms in vocal fold paresis and paralysis.
Keywords: Vocal fold paresis, vocal fold paralysis, recurrent laryngeal nerve, superior laryngeal nerve, voice therapy, canine
INTRODUCTION
Vocal fold paresis and paralysis is common and present in one of six new patients evaluated for dysphonia1. The terminology vocal fold paresis and paralysis implies that vocal fold hypomobility or immobility is present and attributable to neurologic injury to the vagus nerve or the recurrent laryngeal nerve2. The most common complaint of patients with vocal fold insufficiency is alteration of voice quality and/or loudness3. This may be due to several factors: (1) If there is a large glottal gap, airflow requirements are too high for adequate subglottal pressure to be reached for phonation4, (2) If glottal closure is adequate but vocal fold tension and stiffness asymmetries are beyond the normal range, aperiodic and chaotic vibration can affect the acoustic output5, and (3) The decreased tension of the vocal fold may be unable to resist the lateralizing flow pressure and simply get “blown apart” and unable to sustain glottal vibration, especially as subglottal pressure increases with increasing vocal intensity1,6. In practical terms, in vocal fold insufficiency greater airflow is required to reach phonation onset pressure due to decreased laryngeal resistance7. In vagal paralysis, the flow/pressure relationship is further skewed and significantly more airflow is required for phonation onset.5
Treatment options for vocal fold insufficiency include augmentation laryngoplasty, medialization thyroplasty, or voice therapy8. Additional interventions such as laryngeal reinnervation and arytenoid adduction are applicable for paralysis conditions9. Voice therapy is the most commonly prescribed therapy for vocal fold paresis and isolated SLN paresis/paralysis. Therapy is targeted to encourage compensation by the intact laryngeal muscles8,7. The treatment methodologies used by speech pathologists to treat vocal fold insufficiency (e.g.: glissando maneuver to activate the cricothyroid muscles, hard glottal attacks to strengthen adductory muscles, resonant voice therapy, etc.) assume that physiological laryngeal compensatory mechanisms from normal muscles are available to compensate for the weakened laryngeal muscles. Currently no studies adequately evaluate the effects of voice therapy on SLN paralysis. One suggested treatment algorithm indicates that treatment should begin with speech therapy focusing on building cricothyroid muscle strength using techniques such a glissando maneuvers10. Surgical intervention by injection augmentation or medialization thyroplasty is generally recommended for profound glottal incompetence or if results of voice therapy are inadequate2, 8, 7.
The incidence of vocal fold paresis and paralysis continues to be considerably high in clinical practice. Previous studies have reported a prevalence of mild vocal fold hypomobility in 46% among patients with voice complaints, 71% among singing teachers with complaints of technical difficulties, and 23% among singing teachers with no vocal complaints11,12. Siu et al. performed a systematic review and compared a battery of treatments for vocal fold paralysis including medialization thyroplasty, injection thyroplasty, arytenoid adduction, and laryngeal reinnervation. They found no definitive evidence suggesting superiority of any of these techniques based on acoustic and aerodynamic parameters, perceptual voice outcomes and laryngoscopic findings3. Currently there is no preferred method of treatment for vocal fold hypomobility. The optimal management continues to be debated, especially in regards to nonsurgical techniques. The objective of this study is to assess what neuromuscular compensation mechanisms are possible that may be used in the treatment of vocal fold paresis and paralysis. This is an effort to answer the clinical question – in a larynx with unilateral paresis and paralysis, are the remaining muscles able to adequately compensate to maintain optimal phonation?
MATERIAL AND METHODS
In vivo canine model
This in vivo canine study protocol was approved by the Institutional Animal Research Committee of the University of California, Los Angeles. One male mongrel dog, 20 months old, was used in this study. Surgical exposure of the larynx and the recurrent laryngeal nerves (RLN) and superior laryngeal nerves (SLN) was as described previously13,14. The nerve branches to the posterior cricoarytenoid (PCA) muscle, Galen’s anastomosis, and the internal superior laryngeal nerve branches were divided bilaterally to eliminate their effects during nerve stimulation. Appropriately sized tripolar cuff electrodes (Ardiem Medical, Indiana, PA) were applied to the RLN and SLN to stimulate the adductor muscles and cricothyroid muscles respectively. Neuromuscular stimulation for each activation level was performed as previously described, 0.1 millisecond cathodic pulses at 100Hz for 1,500 milliseconds.11, 12
The larynx was stimulated in various combinations of RLN and SLN activation levels (see below) while a subglottal tube attached to the upper trachea provided rostral airflow for phonation. The airflow was computer controlled (MCS Series Mass Flow Controller, Alicat Scientific, Tucson, AZ) to increase the airflow rate linearly from 300 to 1600 ml/s during the 1,500 ms nerve stimulation duration. The airflow was increased in such a manner to continuously increase subglottic pressure (Psub) to reach phonation onset pressure (Pth) and beyond until maximal airflow level was reached. A heated humidifier (HumiCare 200; Gruendler Medical, Freudenstadt, Germany) warmed and humidified the airflow at the glottic level to 37.5 degrees Celsius and 100% relative humidity.
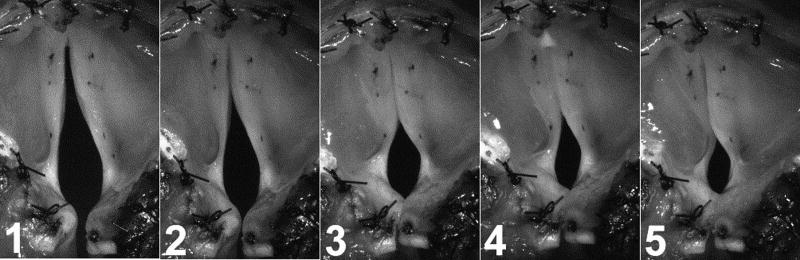
A high-speed digital video camera (Phantom v210, Vision Research Inc., Wayne, NJ) imaged laryngeal vibration at 3000 frames/s during nerve stimulation. The distance from the camera to the larynx remained constant for all conditions. To assess posture changes still photos were taken when the final pre-phonatory shape was achieved at a given stimulation level (Figure 1).
Figure 1.
Still images from high speed video selected after onset of final pre-phonatory posture demonstrating progressively increasing glottal closure with increasing left RLN activation (levels 1–5) in the setting of right RLN paralysis. At the highest level of left RLN stimulation (level 5), the left vocal fold is hyperadduction and a height mismatch between the vocal folds is present.
Neuromuscular conditions tested
The aim of this research was to induce paralysis and paresis typically seen in clinical practice, and to investigate if the remaining muscles could compensate for the deficit. Compensation was defined in terms of presence of phonation onset at each of the possible activation conditions, as well as the fundamental frequency (F0) profile and aerodynamic requirements (phonation onset pressure and airflow). With this in mind the following three conditions were tested:
Unilateral RLN paralysis: The right RLN was not stimulated to mimic unilateral RLN paralysis. The left RLN was stimulated in evenly spaced stimulation grades from levels 1 through 5 to simulate severe paresis (level 1) to just supramaximal RLN activation (level 5). At each of these constant left RLN levels, the left and right SLN were stimulated over 6 grades (threshold to maximal activation) in all possible left/right combinations. Thus, including zero stimulation condition a total of 49 total left/right SLN combinations (activation states) were possible per left RLN activation level. Muscle activation plots (MAPs) were then produced in color coded form to display results (see results section).
Unilateral SLN paralysis: The left SLN was not stimulated to mimic unilateral CT muscle paralysis. The left RLN was stimulated over five graded levels to simulate severe left RLN paresis (level 1) to normal activation (level 5). At each of the constant left RLN level, the right RLN and SLN were stimulated over 6 grades (threshold to maximal activation) in all combinations (thus including zero stimulation condition a total of 49 total activation condition possible per left RLN level). In this manner the compensation by left RLN as well as right RLN and SLN at various levels could be assessed in unilateral left CT paralysis.
Unilateral vagal paresis: In this condition the left SLN and RLN were activated concurrently at 5 constant graded levels to simulate severe vagal paresis (level 1) to normal function (level 5). At each of the constant left vagal activation level, the right RLN and SLN were stimulated over 7 grades (threshold to maximal activation) in all combinations (thus total 64 activation conditions, including zero stimulation). In this manner the compensation by right RLN and SLN at various levels could be assessed in unilateral left vagal paresis.
Measurement of Experimental Parameters (F0, Pth, Airflow)
Acoustic and aerodynamic data were recorded using a probe tube microphone (Model 4128; Bruel &Kjaer North America, Norcross, GA) and a pressure transducer (MKS Baratron 220D; MKS Instruments, Andover, MA) mounted flush with the inner wall of the subglottic inflow tube about 5cm below the inferior border of the glottis. The subglottal acoustic pressure signal was used to determine fundamental frequency (F0) at phonation onset. The acoustic signal, spectrogram, and digital video kymograms (DVK) of glottal vibration were simultaneously displayed on a computer screen to determine onset of phonation. The first four acoustic cycles after phonation onset was used to determine the F0. The corresponding mean subglottic pressure (Psub) at phonation onset represented the phonation onset pressure (Pth). The corresponding airflow (Q) at phonation onset was also recorded.
RESULTS
Unilateral RLN Paralysis
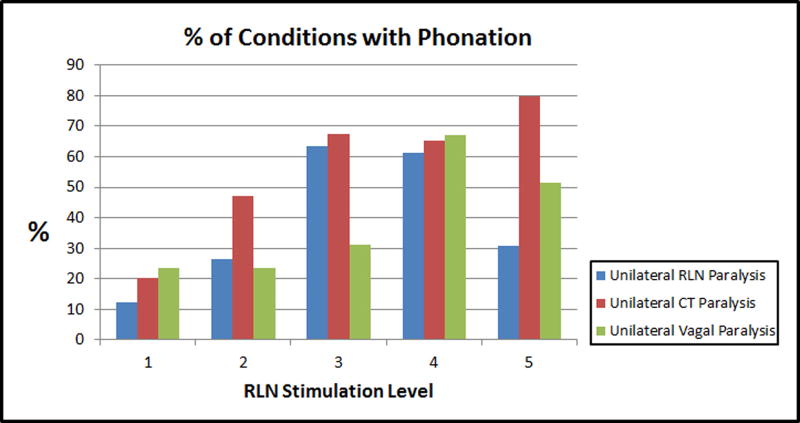
A right RLN paralysis was induced, and the effects of differential left/right activation of CT muscles were tested at five constant levels of left RLN activation. Pre-phonatory glottal closure progressively increased as left RLN activation increased (Figure 1). Accordingly, phonation onset occurred in 12%, 27%, 63%, 61%, and 31% of possible activation conditions tested for left RLN activation levels 1–5, respectively (Figure 2). At lower levels of left RLN activation the glottal gap was larger and the subglottal pressure required for phonation onset could not be achieved even with the maximal airflow levels in many possible activation conditions. At the highest left RLN stimulation (level 5) the left vocal fold was hyperadducted, there was a height mismatch between the true vocal folds, and number of conditions with phonation onset decreased.
Figure 2.
Effects of increasing RLN stimulation level on the percentage of conditions achieving phonation onset in three experimental conditions (see text).
While increasing RLN activation increased the number of phonation onset conditions, increasing SLN activation decreased it. Higher levels of SLN stimulation led to cessation of phonation onset, which could be countered by increased left RLN activation. Left/right SLN effects were similar except at left RLN level 3, where a differential effect of CT activation for phonation onset was seen (Figure 3). Here, phonation onset occurred at many conditions with highest right CT activation levels but not with any of the highest left CT activation levels. Additionally, higher F0 range was possible at lower phonation onset pressure (higher airflow rate) with right CT activation than with left CT activation (Figure 3). Interestingly, at supramaximal left RLN activation level, fewer phonatory conditions were seen, and more phonatory conditions were possible at higher left CT activation than right (data not shown). These examples illustrate the antagonistic roles CT and adductor play in phonation onset parameters.
Figure 3.
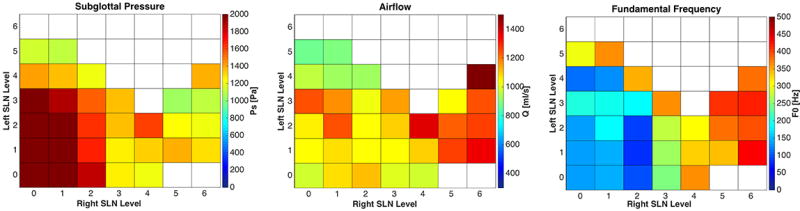
Effects of combinations of left/right SLN graded activation on subglottal pressure, airflow, and fundamental frequency in right RLN paralysis and left RLN stimulation level 3. Compensation by right CT muscle is demonstrated by the presence of phonation onset occurring with the highest levels of right CT activation but not with the highest level of left CT activation. Increased F0 range is also seen with right CT activation conditions. White squares represent conditions not achieving phonation onset.
Unilateral CT Paralysis
A left CT paralysis was modeled at 5 constant levels of left RLN activation (severe paresis to normal) and all combinations of right SLN right RLN stimulation over 6 levels of activation. As left RLN activation increased from levels 1–5, glottal gap decreased, and phonation occurred in 20%, 47%, 67%, 65% and 79% of possible activation conditions, respectively (Figure 2). For any constant low level activation of left or right RLN, increasing right CT activation led to cessation of phonation onset. However, this deleterious effect of right CT activation on phonation was skewed towards low right RLN activation levels, i.e. this effect was more prominent at low right RLN activation while increasing right RLN activation eliminated this effect, while increasing left RLN activation was less effective in countering this effect. An expected progression of increasing F0 range was seen with the right SLN activation, but bilateral RLN activation was needed to reach the high F0 (Figure 4).
Figure 4.
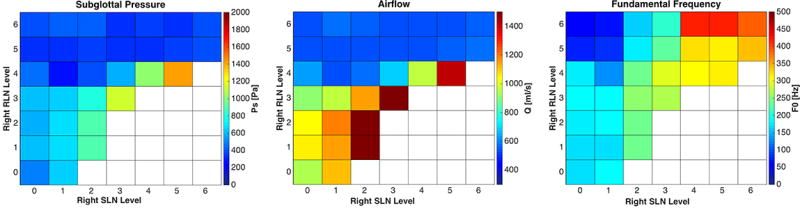
Effects of combinations of right RLN/SLN graded activation on subglottal pressure, airflow, and fundamental frequency in left SLN paralysis at left RLN stimulation level 4. Increasing right SLN activation leads to decreased phonation onset conditions while increasing right RLN activation compensates and leads to increase in phonation onset conditions with improved F0 range. White squares represent conditions not achieving phonation onset.
Unilateral Vagal Paresis
Left vagal paresis was simulated over five levels of paresis (level 1 severe paresis to level 5 normal). Increasing left vagal stimulation improved glottal closure and phonation onset occurred in 23%, 23%, 31%, 67%, and 51% of possible activation conditions for level 1–5, respectively (Figure 2). Similar to left CT paralysis condition, less conditions reached phonation onset as the right CT activation was increased. This was countered by increasing right RLN activation and thus increasing glottal closure (Figure 5). The subglottal pressure requirements at these junctions were low and very similar across conditions, indicating minimal stiffness changes over adjacent activation conditions but increasing airflow requirements due to glottal gap.
Figure 5.
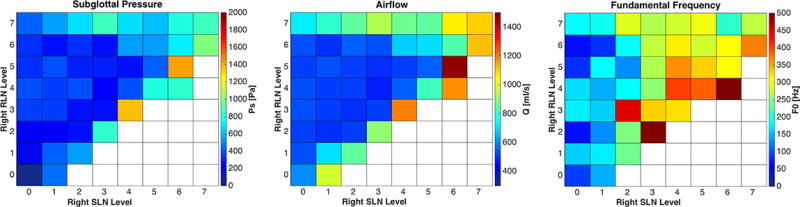
Effects of combinations of right RLN and right SLN graded activation on subglottal pressure, airflow, and fundamental frequency in left vagal paresis at activation level 4. Increasing right SLN activation leads to decreased phonation onset conditions while increasing right RLN activation compensates and leads to increase in phonation onset conditions with improved F0 range. White squares represent conditions not achieving phonation onset.
DISCUSSION
Currently the most common treatment methods are voice therapy and vocal fold medialization procedures. In voice therapy the goal is to teach the patient to compensate with the remaining muscles. However, the physiological capacity to compensate using the remaining functional muscles is not clear. In this study, we evaluated these compensatory mechanisms in three common clinical scenarios: unilateral RLN paralysis, unilateral SLN paralysis, and vagal paresis. The most consistent positive effect on phonation was seen with glottal adduction. In all three conditions, as the glottal gap decreased with increasing RLN activation, the number of compensatory conditions for phonation onset increased (Figure 2).
SLN activation was still required to increase the F0, but the effects of SLN activation were variable across the conditions tested. In RLN paralysis, SLN activation led to cessation of phonation onset. This is likely due to larger glottal gap from SLN activation as well as some other phonatory shape change from SLN activation. This effect is not necessarily due to increased tension from SLN activation because as SLN activation increased, the phonation onset pressure actually decreased. But airflow requirement increased, which may reflect decreased laryngeal resistance, perhaps due to vocal fold abductory effects from SLN activation when the glottis is in an adducted position that has been previously described15. To some degree, more conditions phonated in the right SLN activation quadrant than the left SLN activation quadrant; thus ipsilateral SLN activation appears to compensate in RLN paralysis to some degree. Activation of the CT elongates the vocal fold and increases vocal fold tension. The increased tension has been shown to increase the restraining forces of the vocal fold and prevent the vocal folds from being “blown apart” by aerodynamic forces16. This may explain the findings, as well as the “paralytic falsetto” often seen in patients with unilateral vocal fold paralysis. RLN activation antagonizes this CT effect and vice versa. This can be appreciated more as the left RLN activation increased. While increasing glottal adduction is the best method to reach phonation onset, hyper-compensation by the intact Left RLN was also deleterious to sound generation, as seen with decreased number of phonatory conditions present with supramaximal contralateral RLN activation. Hypercompensation by the intact vocal fold can lead to height mismatch, thus leading to poor phonatory posture. This explains why there were fewer phonatory conditions at the highest compensatory state. Overcompensation by the intact RLN is also a poor compensatory maneuver.
In unilateral SLN paralysis, neuromuscular compensation was straightforward. If both RLN were normal, phonation onset occurred at low subglottal pressures and flow requirement. Activation of the contralateral SLN was able to raise the pitch. If concurrent RLN paralysis is present, SLN activation led to aphonia due to increased flow requirement beyond the maximal flow levels used. Thus, in unilateral SLN paralysis, the normal CT muscle can adequately compensate to control the pitch range. This is likely the reason many unilateral SLN injuries remain asymptomatic unless the patient has high vocal requirements.
In vagal paresis and paralysis, just as in the other conditions, as the SLN is stimulated and the CT activation increased, phonation onset ceases at higher SLN levels. Again, as glottal closure is achieved with increasing right RLN activation number of conditions of phonation onset increases and the requirement for subglottal pressure decreases.
Nasseri and Maragos performed both type I and type IV thyroplasty in nine patients with weakness of a unilateral SLN. The type IV thyroplasty is a cricothyroid approximation and specifically addresses cricothyroid muscle weakness17. Good results were reported. There were postoperative improvements in subjective dysphonia, objective visual and acoustic measures. The study by Yin and Zhang also supports this concept. The medialization enhances the compensatory activity of the thyroarytenoid muscle, leading to improvement in vocal quality and range18.
In terms of flow, decreasing flow is required with increasing RLN activation. This is logical, as increased glottal closure is achieved with increasing RLN activation. Therefore, as the glottal gap is decreased less airflow is required to produce voice. As the cricothyroid muscle is activated at increasing levels, and increases vocal fold tension and slightly abducts it from an adducted position, more airflow is required to achieve phonation onset.
This study has several limitations. Compensation by the PCA muscle was not investigated in this study. The PCA muscle is mostly involved in the production of voiceless fricatives and in devoicing19. It may also support the arytenoid complex during phonation. The role of the PCA muscle on neuromuscular compensation in vocal fold insufficiency should be evaluated in future studies. In addition, this is a study of acute injury, without time for compensation related to reinnervation that may be present in a chronically paralyzed or paretic larynx. Finally, this study was performed in a canine larynx. However, the canine larynx matches the human larynx in size and neuromuscular anatomy. The fundamental frequency range and subglottal pressures required for phonation in the canine larynx are also nearly identical20. The invasive experiments described in this study requiring precise control of muscle activation cannot ethically be performed in humans. Thus the in vivo canine larynx is an excellent biomechanical model for the human larynx and much of our current understanding of how the human larynx functions has been investigated in the canine larynx.
CONCLUSIONS
The data presented here demonstrate that improving glottal closure is the best compensatory maneuver in vocal fold paresis and paralysis. In each of the conditions, the most consistent effect on increasing the number of phonation onset conditions and F0 range was increasing glottal closure. Thus medialization or augmentation procedures should remain the mainstay of therapy. Compensation by SLN is possible and is needed for F0 control in all conditions. SLN activation most consistently compensates for deficits in unilateral RLN paralysis and unilateral SLN paralysis cases.
Acknowledgments
Financial Disclosures:
This study was supported by Grant R01DC011300 from the National Institutes of Health.
Footnotes
Conflict of Interest:
None
Presented as an oral presentation at the annual meeting of the American Laryngological Association, Chicago IL, May 18 2016.
References
- 1.Stager SV. Vocal fold paresis: etiology, clinical diagnosis and clinical management. Curr Opin Otolaryngol Head Neck Surg. 2014;22(6):444–449. doi: 10.1097/MOO.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 2.Reiter R, Hoffmann TK, Pickhard A, Brosch S. Hoarseness-causes and treatments. Dtsch Arztebl Int. 2015;112(19):329–337. doi: 10.3238/arztebl.2015.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siu J, Tam S, Fung K. A comparison of outcomes in interventions for unilateral vocal fold paralysis: A systematic review. Laryngoscope. 2015 doi: 10.1002/lary.25739. [DOI] [PubMed] [Google Scholar]
- 4.Chhetri DK, Neubauer J. Differential roles for the thyroarytenoid and lateral cricoarytenoid muscles in phonation. Laryngoscope. 2015;125(12):2772–2777. doi: 10.1002/lary.25480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herzel H, Berry D, Titze I, Steinecke I. Nonlinear dynamics of the voice: Signal analysis and biomechanical modeling. Chaos. 1995;5(1):30–34. doi: 10.1063/1.166078. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z. Restraining mechanisms in regulating glottal closure during phonation. J Acoust Soc Am. 2011;130(6):4010–4019. doi: 10.1121/1.3658477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orestes MI, Chhetri DK. Superior laryngeal nerve injury: effects, clinical findings, prognosis, and management options. Curr Opin Otolaryngol Head Neck Surg. 2014;22(6):439–443. doi: 10.1097/MOO.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller S. Voice therapy for vocal fold paralysis. Otolaryngol Clin North Am. 2004;37(1):105–119. doi: 10.1016/S0030-6665(03)00163-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Wan P, Yu Y, Li M, Xu Y, Huang P, Huang Z. Types and timing of therapy for vocal fold paresis/paralysis after thyroidectomy: a systematic review and meta-analysis. J Voice. 2014;28(6):799–808. doi: 10.1016/j.jvoice.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Dursun G, Boynukalin S, Ozgursoy OB, Coruh I. Long-term results of different treatment modalities for glottic insufficiency. Am J Otolaryngol. 2008;29(1):7–12. doi: 10.1016/j.amjoto.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Heman-Ackah YD, Barr A. Mild vocal fold paresis: understanding clinical presentation and electromyographic findings. J Voice. 2006;20(2):269–281. doi: 10.1016/j.jvoice.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Heman-Ackah YD, Batory M. Determining the etiology of mild vocal fold hypomobility. J Voice. 2003;17(4):579–588. doi: 10.1067/s0892-1997(03)00085-7. [DOI] [PubMed] [Google Scholar]
- 13.Chhetri DK, Neubauer J, Sofer E, Berry DA. Influence and interactions of laryngeal adductors and cricothyroid muscles on fundamental frequency and glottal posture control. J Acoust Soc Am. 2014;135(4):2052–2064. doi: 10.1121/1.4865918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chhetri DK, Neubauer J, Berry DA. Neuromuscular control of fundamental frequency and glottal posture at phonation onset. J Acoust Soc Am. 2012;131(2):1401–1412. doi: 10.1121/1.3672686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka S, Hirano M, Umeno H. Laryngeal behavior in unilateral superior laryngeal nerve paralysis. Ann Otol Rhinol Laryngol. 1994;103(2):93–97. doi: 10.1177/000348949410300202. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Luu TH. Asymmetric vibration in a two-layer vocal fold model with left-right stiffness asymmetry: experiment and simulation. J Acoust Soc Am. 2012;132(3):1626–1635. doi: 10.1121/1.4739437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasseri SS, Maragos NE. Combination thyroplasty and the "twisted larynx:" combined type IV and type I thyroplasty for superior laryngeal nerve weakness. J Voice. 2000;14(1):104–111. doi: 10.1016/s0892-1997(00)80100-9. [DOI] [PubMed] [Google Scholar]
- 18.Yin J, Zhang Z. Interaction between the thyroarytenoid and lateral cricoarytenoid muscles in the control of vocal fold adduction and eigenfrequencies. J Biomech Eng. 2014;136(11) doi: 10.1115/1.4028428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirose H. Posterior cricoarytenoid as a speech muscle. Ann Otol Rhinol Laryngol. 1976;85(3 pt 1):335–342. [PubMed] [Google Scholar]
- 20.Chhetri DK, Rafizadeh S. Young's modulus of canine vocal fold cover layers. J Voice. 2014;28(4):406–410. doi: 10.1016/j.jvoice.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]