Abstract
In donor hearts from mini pigs, overtime cold preservation and ischemia-reperfusion injury cause poor graft quality and impaired heart function. Blockage of complement, apoptosis, and inflammation is considered a strategy for attenuating ischemia-reperfusion injury and protecting cardiac function. Minipig donor hearts were perfused and preserved in Celsior solution or transfection reagent containing Celsior solution with scramble siRNA or siRNAs targeting complement 3, caspase-8, caspase-3, and nuclear factor κB-p65 genes at 4°C and subsequently hemo-reperfused ex vivo (38°C) or transplanted into recipients. The protective effect of the siRNA solution was evaluated by measuring cell apoptosis, structural alteration, protein markers for tissue damage and oxidative stress, and cardiac function. We found a reduction in cell apoptosis, myocardial damage, and tissue inflammation by reduced biochemistry and markers and protein expression of proinflammatory cytokines and improvement in cardiac function, as shown by the improved hemodynamic indices in 12-hr-preserved siRNA-treated hearts of both ex vivo and orthotopic transplantation models. These findings demonstrate that blockade of inflammation and apoptosis pathways using siRNA can prolong cold preservation time and better protect donor heart function in cardiac transplantation of large animals, which may be beneficial for human heart preservation.
Keywords: ischemia, reperfusion, small interfering RNA, heart transplantation, porcine model, working heart model
Introduction
For patients with end-stage heart failure, cardiac transplantation is the only effective treatment. However, several hurdles need to be overcome for successful transplantation, such as ischemia-reperfusion injury (IRI) and limited cold preservation time. IRI causes tissue damage because of the loss of blood supply and resultant ischemia as well as inflammation and re-oxygenation during reperfusion. IRI is associated with delayed donor graft function and organ failure as well as chronic organ dysfunction and mortality.1, 2, 3, 4 In the clinic, utilizing cold preservation solution to perfuse and store donor hearts ex vivo is popular because of its simplicity and low cost. However, the metabolism cannot be completely shut off, resulting in an accumulation of harmful cellular metabolites and, consequently, inflammation and organ injury during reperfusion.5, 6 The current safe myocardial preservation time is 4–6 hr,7, 8 which restricts the geographic distance between donor and recipient to an approximately 500-mile radius. The limited storage time window intensifies the conflict between a shortage of donor hearts and the increasing need for cardiac transplantation. Therefore, new solutions that can attenuate IRI and extend organ preservation time are critically needed.
IRI induces activation of multiple signaling pathways and regulates the expression of various genes, subsequently causing cell apoptosis and necrosis, which promote organ damage.9, 10, 11, 12 Apoptosis, complement activation, and inflammation pathways are the best-defined and most studied causative factors associated with IRI. Recent studies suggest that adding synthetic small interfering RNA (siRNA) to solutions has the potential to effectively inhibit IRI-caused damage in organ preservation.13, 14, 15 Indeed, many studies have been performed to limit cardiac IRI by siRNA, but only a few studies are involved in cardiac transplantation. siRNAs targeting tumor necrosis factor alpha (TNF-α), complement 3 (C3), and Fas genes in the cold preservation solution have been shown to improve the structure, function, and survival of transplanted hearts in mice; however, most studies are conducted in murine models,16, 17 and small animals are often unable to model human disease. Swine provide an excellent model for the human condition, given their similarities in anatomy, physiology, and immunology.15 The large heart/body weights and high degree of similarity of the cardiovascular system also make the swine a valuable and highly relevant model for human cardiovascular research.18 In this study, an ex vivo hemoperfused system was used to perfuse the coronary arteries directly and fill the atria and ventricles with whole blood, which was considered to represent in situ physiological cardiac function ex vivo, and orthotopic transplantation was undertaken subsequently. These 2 models are necessary to mimic the human situation as closely as possible.
siRNA delivery is a current hurdle for clinical application. In previous studies, a large quantity of naked siRNA is added to the preservation solution, which appears to work in small animals such as rats. However, a high dose of siRNA may not be feasible for clinic use or porcine cardiac grafts. Transfection reagents (TRs) have been extensively used either in vitro19 or in vivo20 for siRNA delivery; the reagents also allow for minimization of the siRNA dosage, which is more suitable for clinical application. In the present study, we used chemically modified siRNAs targeting C3, nuclear factor κB-p65 (p65), caspase-8, and caspase-3, the key molecules in complement activation, inflammation, and apoptosis, respectively, to develop a new solution containing siRNA TR mixture, which was used to perfuse and preserve porcine donor hearts. The new solution was also used to re-perfuse the hearts in the isolated working heart model or orthotopic transplantation model.
Results
siRNA with TR Efficiently Knocked Down the Expression of C3, Caspase-8, p65, and Caspase-3 and Enhanced Cardiac Tissue Survival
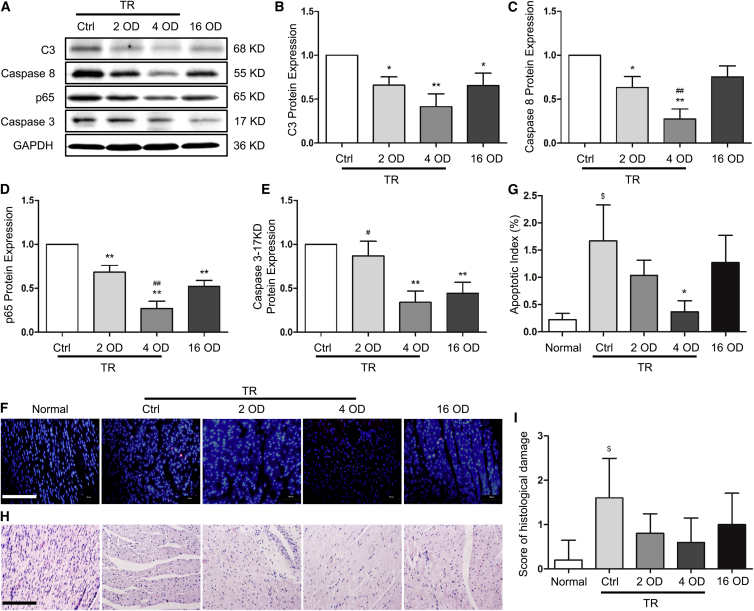
The knockdown efficiency of the 12 siRNAs targeting C3, caspase-8, p65, and caspase-3 (3 siRNAs for each gene) was tested in porcine kidney 15 (PK15) and swine testicular (ST) cells, and Figure S1 displays an average result of the 2 cell lines. The most effective siRNAs for each individual gene were selected for subsequent experiments, and their sequences are described in Materials and Methods. Because preservation of donor hearts using siRNA solution in large animal models has not been reported, and TR is widely used in different cells and rodents to assist with siRNA delivery, we tested the siRNA efficiency with or without TR in porcine hearts preserved in Celsior solution. 12 donor hearts were perfused and preserved in 500 mL Celsior solution containing 2 optical density (OD) siRNAs (1 OD = 33 μg) with 10 μL RNAiMAX TR (2 OD group), 4 OD siRNAs or scramble siRNA (control [Ctrl] group) with 20 μL RNAiMAX TR (4 OD group; the ratio is 5 μL TR/OD RNA), and 16 OD siRNAs without TR (16 OD group), respectively, at 4°C for 24 hr and 3 hearts for each group. Protein expression in the heart tissue samples was detected by western blotting. As shown in Figures 1A–1E, siRNAs with TR inhibited the expression of C3, caspase-8, p65, and caspase-3 (17 knockdown [KD], active form of caspase-3) in a dose-dependent manner, and 4 OD of siRNA with TR showed the strongest knockdown efficiency (p < 0.01 versus Ctrl). The 16 OD siRNA without TR inhibited the expression of the target genes, but the knockdown efficiency was much lower compared with the 4 OD siRNA with TR, suggesting that TR makes a significant difference in delivering siRNA into heart tissue.
Figure 1.
siRNA Efficiency, Cardiac Tissue Survival, and Structure in Grafts Preserved with or without Transfection Reagent
Donor hearts were isolated, perfused and preserved (4°C) using transfection reagent (TR)-containing Celsior solution with 4 OD scramble siRNA (control group, Ctrl) or with 2 OD and 4 OD siRNA, or TR-free Celsior solution with 16 OD siRNA. 24 hr after preservation, tissue samples were collected. Fresh myocardium was used as a control (Normal). (A–E) siRNA efficacy was detected by western blot (A), and the expression of complement 3 (C3) (B), caspase-8 (C), p65 (D), and cleaved caspase-3 (17 KD) (E) was quantified by normalizing to GAPDH and relative to the Ctrl (set as 1). (F) Cardiac tissue survival was detected by TUNEL staining. (G) The apoptotic index was calculated by counting the apoptotic (red) and total cells in 10 fields at 400× magnification and is shown as the percentage of apoptotic cells (red) in total cells in each group. (H) Myocardial structural alteration was examined by H&E staining. (I) The semiquantitative score of histopathological damage was assessed in H&E sections in 5 fields at 400× magnification. 1 OD = 33 μg. Scale bars, 200 μm. Data shown are mean ± SEM. *p < 0.05, **p < 0.01 versus Ctrl; #p < 0.05, ##p < 0.01 versus the 16 OD group; $p < 0.05 versus Normal (n = 3).
Cardiac cell apoptosis is one of the consequences of cold preservation or IRI. Therefore, we examined whether the siRNAs protected cardiac cell survival. As shown in Figures 1F and 1G, more apoptotic cells were observed in myocardium preserved in Ctrl or siRNA without TR compared with heart tissues preserved in solution with siRNA and TR, suggesting that siRNAs with TR enhance cardiac cell survival during cold preservation.
Moreover, we examined myocardium structural damage in H&E-stained tissue sections by using normal myocardium as a control. As shown in Figure 1H, because of the lack of hematic re-perfusion, no cellular infiltration and hemorrhage were observed in any sections. However, more severe interstitial edema and ischemic damage were observed in the control and 16 OD siRNA without TR preserved groups, consistent with terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) staining, whereas the histological structure in the 2 OD/TR and 4 OD/TR groups showed no significant difference compared with the normal heart (Figure 1I).
Preservation Solution with siRNA Improved Cardiac Tissue Survival and Reduced Structural Damage in Isolated Working Hearts
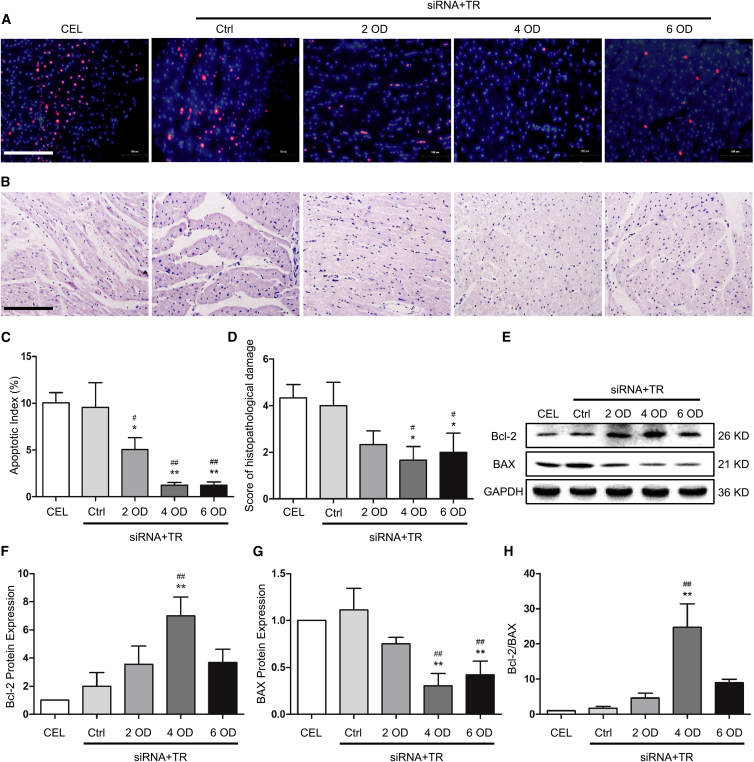
Cold preservation and subsequent reperfusion often cause cardiac cell apoptosis. Although TR-containing siRNA solution appeared to reduce apoptosis in cold-preserved hearts, it is unclear whether the solution has the same effect on heart tissue after reperfusion. To test this, we incubated porcine hearts in 500 mL Celsior solution (regular preservation, CEL) or 500 mL TR-containing Celsior solution with 4 OD scramble siRNA (Ctrl) or 2, 4, or 6 OD siRNAs (siRNA+TR groups, ratio of siRNA:TR = 1 OD:5 μL). After 12 hr of cold preservation, the heart grafts were re-perfused and allowed to beat in working heart mode for 3 hr. As shown in Figures 2A and 2C, siRNAs with TR dramatically reduced apoptosis compared with the 2 control groups, CEL and Ctrl. Importantly, 4 and 6 OD siRNA-containing solution appeared to produce the most efficient protection, as shown by fewer apoptotic cells and less structural damage compared with other groups (Figures 2A–2D), but there was no significant difference between these 2 groups, consistent with the siRNA knockdown efficiency (Figures S2A–S2E). To confirm this observation, we detected the Bcl-2/BAX ratio; Bcl-2 and BAX have been shown to be downregulated and upregulated after cardiac reperfusion, respectively.21 As shown in Figures 2E–2H, compared with the CEL and Ctrl groups, hearts treated with siRNA-TR displayed increased Bcl-2 but decreased BAX protein expression and subsequently increased Bcl-2/BAX ratios. Among the siRNA groups, treatment of 4 OD siRNA with TR led to the highest level of Bcl-2/BAX ratios.
Figure 2.
The Effect of siRNA Solution on Survival and Structure of Isolated Working Heart Tissues
(A) Cell apoptosis of the isolated hearts was analyzed by TUNEL staining. (B) Myocardial structure was analyzed by H&E staining. (C) The apoptotic index was calculated by counting the apoptotic (red) and total cells in 10 fields at 400× magnification and is shown as the percentage of apoptotic cells (red) in total cells in each group. (D) The semiquantitative score of histopathological damage was assessed in H&E sections in 5 fields at 400× magnification. Scale bar, 200 μm. (E–G) Expression of the apoptosis-related proteins Bcl-2 and BAX was measured by western blot (E), and the protein levels were quantified by normalizing to GAPDH and relative to the CEL group (set as 1) for Bcl-2 (F) and BAX (G), respectively. (H) Bcl-2/BAX ratio. CEL, Celsior solution without siRNA group; Ctrl, Celsior solution with scramble siRNA and TR group. Data shown are mean ± SEM. *p < 0.05, **p < 0.01 versus CEL; #p < 0.05, ##p < 0.01 versus Ctrl (n = 3).
The New Preservation Solution with siRNA Reduced Heart Tissue Damage
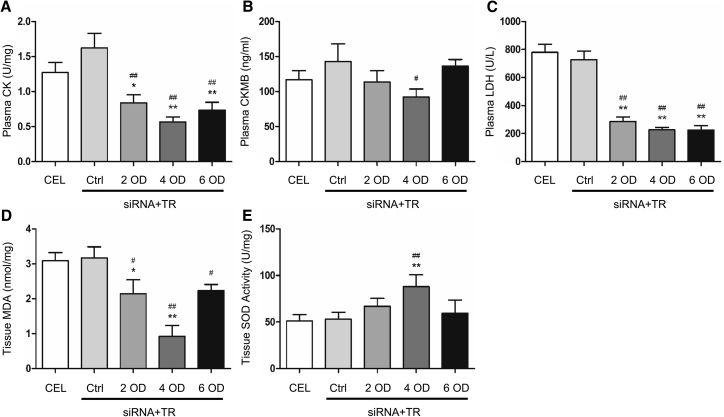
Preservation of heart tissue with siRNAs reduced the levels of creatine kinase (CK), creatine kinase MB (CKMB), lactate dehydrogenase (LDH), and malonaldehyde (MDA) and increased superoxide dismutase (SOD) activity (Figure 3). The solution containing 4 OD siRNA-TR compound appeared to have the most dramatic effect on reducing myocardial damage (Figure 3). These results suggest that the new solution provides better protection for the porcine heart.
Figure 3.
Biochemistry Alterations in Isolated Hearts
(A–E) Plasma and tissue samples were collected from isolated hearts for measuring the levels of creatine kinase (CK, A), creatine kinase MB (CKMB, B), lactate dehydrogenase (LDH, C), malonaldehyde (MDA, D), and superoxide dismutase (SOD) activity (E) as described in Materials and Methods. Data shown are mean ± SEM. *p < 0.05, **p < 0.01 versus CEL; #p < 0.05, ##p < 0.01 versus Ctrl (n = 3).
The New Preservation Solution with siRNA Improved the Function of Isolated Working Hearts
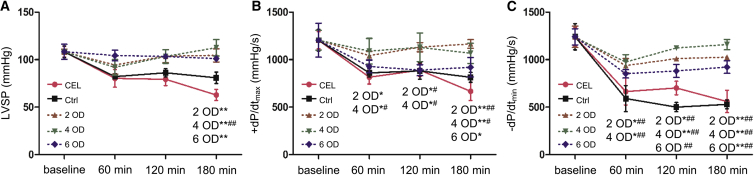
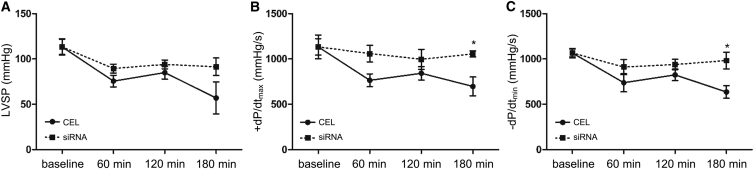
Cardiac function was evaluated by left ventricular hemodynamic parameters. As shown in Figure 4, left ventricular systolic pressure (LVSP) and left ventricular maximal rates of increase and decrease of developed pressure (+dP/dtmax and −dP/dtmin, respectively) values were higher for the hearts of siRNAs-TR groups compared with the CEL and Ctrl groups. The solution with 4 OD siRNA produced the best outcome compared with other treatments, and this amount of siRNA produced a better outcome after 120 min of reperfusion in LVSP and −dP/dtmin compared with 2 or 6 OD siRNA solution. These data indicate that myocardial function preservation does not necessarily increase with an increased amount siRNA in the preservation solution. 4 OD siRNA appeared to be appropriate for cardiac function recovery.
Figure 4.
Hemodynamic Parameters of Isolated Working Hearts
During reperfusion of isolated hearts, left ventricular systolic pressure (LVSP), left ventricular maximal rates of increase and decrease of developed pressure (+dP/dtmax and −dP/dtmin) were recorded 0 min (baseline), 60 min, 120 min, and 180 min after reperfusion. The value of each parameter was compared with the corresponding baseline mean value, respectively. (A) LVSP. (B) +dP/dtmax. (C) −dP/dtmin. Data shown are the mean ± SEM. For each corresponding time point, *p < 0.05, **p < 0.01 versus CEL; #p < 0.05, ##p < 0.01 versus Ctrl (n = 3).
siRNA-Containing Solution Improved the Cardiac Function of Transplanted Hearts
Based on the results of the isolated working hearts, we hypothesized that 4 OD siRNA with TR would also provide better protection for porcine hearts undergoing cardiac orthotopic transplantation than the conventional method. To prove this hypothesis, 6 pairs of adult male mini pigs were used as donors and recipients. 6 grafts were divided into 2 groups and preserved in 1,000 mL Celsior solution with or without 8 OD siRNA and 40 μL TR compound (equal to 20 μL TR and 4 OD siRNA in 500 mL Celsior solution). The hearts without siRNA treatment were preserved for the standard 6 hr (CEL), and the hearts with siRNA treatment were prolonged to 12 hr preservation (siRNA). All 6 hearts resumed beating after 1 or 2 defibrillations and were allowed to beat for 3 hr. The hemodynamic parameters were recorded during the beating. The siRNAs effectively knocked down the expression of C3, caspase-8, p65, and caspase-3 in the transplanted hearts (Figures S2F and S2G). siRNA-treated hearts exhibited a higher LVSP, +dP/dtmax, and –dP/dtmin than the clinical regularly preserved hearts (CEL) after reperfusion (Figure 5). The significant differences were evident 180 min after initiation of reperfusion, suggesting that the siRNA solution enhanced cardiac function of the transplanted hearts and prolonged the preservation time.
Figure 5.
Hemodynamic Parameters of Transplanted Hearts
During the reperfusion of the transplanted hearts, LVSP, +dP/dtmax, and −dP/dtmin were recorded 0 min (baseline), 60 min, 120 min, and 180 min after the heartbeat started. The value of each parameter was compared with the corresponding baseline mean value, respectively. (A) LVSP. (B) +dP/dtmax. (C) −dP/dtmin. Data shown are the mean ± SEM. *p < 0.05 versus the CEL group at the corresponding time point (n = 3).
The New siRNA Preservation Solution Enhanced Cardiac Tissue Survival and Reduced Tissue Damage in Transplanted Hearts
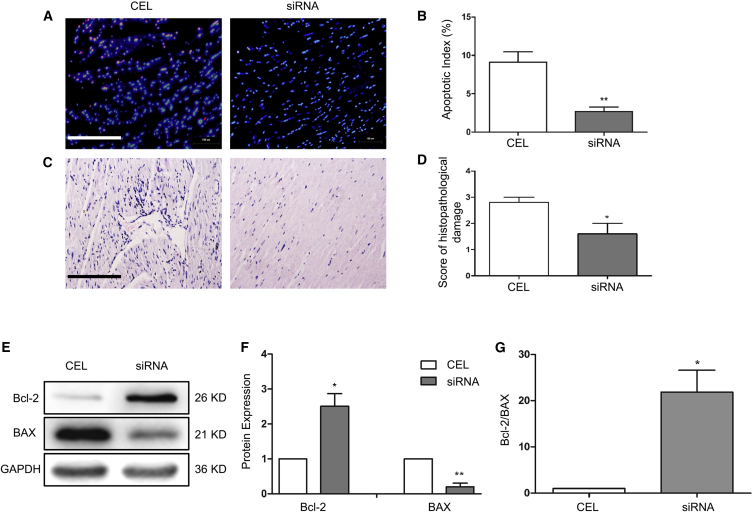
To determine how heart function was improved, all transplanted hearts were sampled for analysis of cell apoptosis and potential myocardial damage. As shown in Figures 6A–6D, although the donor grafts preserved in solution with 4 OD siRNA underwent a longer ischemia period, they had fewer apoptotic cells (Figures 6A and 6B) and a lower degree of structural damage (Figures 6C and 6D) compared with the standard Celsior-preserved hearts. In addition, transplanted heart tissues with siRNA treatment also exhibited a higher Bcl-2/BAX ratio compared with CEL hearts (Figures 6E–6G), indicating that the siRNA solution protected the prolonged cold storage hearts from apoptosis and structural damage.
Figure 6.
Cardiac Tissue Survival and Structure after Transplantation
(A) Cell apoptosis of the transplanted heart tissues was detected by TUNEL assay. (B) The apoptotic index was calculated by counting the apoptotic (red) and total cells in 10 fields at 400× magnification and is shown as the percentage of apoptotic cells (red) in total cells in each group. (C) Myocardial structural alteration was analyzed by H&E staining. (D) The semiquantitative score of histopathological damage was assessed in H&E sections in 5 fields at 400× magnification. Scale bar, 200 μm. (E and F) Expression of the apoptosis-related proteins Bcl-2 and BAX was measured by western blot (E), and the protein levels were quantified by normalizing to GAPDH and relative to the CEL group (set as 1) (F). (G) Bcl-2/BAX ratio. Data shown are mean ± SEM. *p < 0.05, **p < 0.01 versus CEL (n = 3).
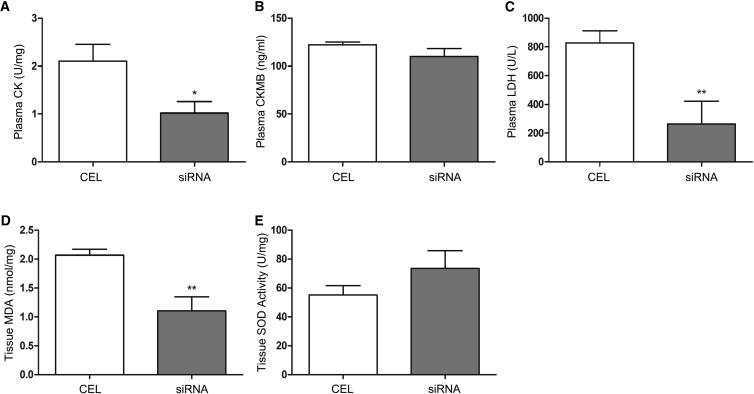
Moreover, biochemistry analyses of the transplanted hearts found that siRNA treatment caused less damage to the hearts, as shown by the lower levels of CK, CKMB, LDH, and MDA and a higher level of SOD activity compared with conventional preservation (Figure 7), further demonstrating that the siRNA solution was effective in attenuating myocardial tissue damage in transplanted hearts that underwent 12 hr cold preservation.
Figure 7.
Biochemistry Alterations in Transplanted Hearts
(A–E) Plasma and tissue samples were collected from the transplanted hearts for measuring the levels of CK (A), CKMB (B), LDH (C), MDA (D), and SOD activity (E) as described in Materials and Methods. Data shown are mean ± SEM. *p < 0.05, **p < 0.01 versus CEL; n = 3.
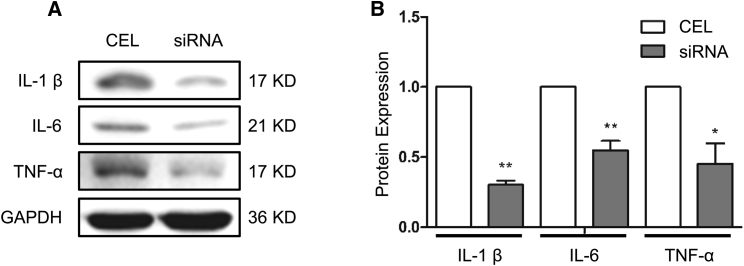
siRNA Solution Reduced Cardiac Tissue Inflammation
IRI upregulates cytokines, leading to inflammation, which contributes to graft dysfunction in the heart.19, 22, 23, 24 Therefore, we examined the inflammatory status of the hearts after reperfusion by detecting the expression of interleukin-1β (IL-1β), IL-6, and TNF-α, the typical proinflammatory cytokines, in the transplanted heart tissue. As shown in Figure 8, siRNA solution-treated hearts exhibited much less expression of IL-1β, IL-6, and TNF-α compared with CEL hearts. These data suggest that siRNA solution protects transplanted hearts from I/R injury (IRI)-induced inflammation, which may have contributed to the overall improved function in siRNA solution-preserved hearts.
Figure 8.
The Inflammation Status of the Transplanted Hearts
(A) Interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α) protein expression was detected by western blotting. (B) The protein levels were quantified by normalizing to GAPDH and compared with the CEL group (set as 1). Data shown are the mean ± SEM. *p < 0.05, **p < 0.01 versus CEL; n = 3.
Discussion
This study has developed, for the first time, a new solution containing liposome TR and a siRNA mix targeting C3, p65, caspase-8, caspase-3 that can preserve porcine donor hearts for a longer time than the conventional preservation method and improve heart function, as demonstrated in both the isolated working heart model and orthotopic transplantation model.
Because of the growing conflict between the shortage and demand of qualified donor hearts, a new solution that can prolong storage time while maintaining organ function is urgently needed in clinical cardiac transplantation.
That IRI is the major cause contributing to the loss of function, long-term allograft changes, or low survival rate of the recipient, inevitably occurred in cardiac transplantation dose need appropriate referencing to cardiac-related experimental and clinical evidence. Therefore, preventing IRI is critical for successful transplantation. IRI leads to organ injury by activating multiple signaling pathways. Complement activation, cell apoptosis, and inflammatory pathways have been well confirmed as factors directly correlating with IRI.14 Indeed, targeting the central regulators of these pathways—e.g., C3, caspase-8, caspase-3, and p65—has significantly attenuated IRI-caused damage to transplanted porcine hearts, as shown by the reduction in both apoptosis rate and myocardial damage markers, including LDH, CK, CKMB, and MDA and the increased SOD activity in these hearts.
Chemical compounds,25, 26 antibody,20 or viral vectors27 have been successfully used to reduce IRI-caused damage. However, various defects hinder their application in organ preservation. In contrast, RNAi using siRNA appears to be a better approach because of its high inhibitory activity, limited duration of action, minimal toxicity, and precise specificity.13 Because siRNA delivery and serum stability are obstacles for siRNA-based therapies, chemically modified, 2′-O-methylpurines (2′OMe) siRNA has been chosen to enhance serum stability because of its resistance to RNase activity and enhanced siRNA potency.28 On the other hand, for murine donor organ perfusion, a small volume of solution with a high concentration of siRNA may be feasible because of the very small size of murine organs. In this context, mouse hearts and kidneys have been perfused and preserved with siRNA cocktail solution containing 100 μg/mL and 1.5 mg/mL of siRNAs, respectively.14, 29 However, the methods used in small animals are not suitable for clinical use because a large volume of solution administrated to large animals or humans requires a large amount of siRNAs, which is costly. Instead of choosing highly concentrated siRNA solution, Yang et al.15 used a much lower dosage of serum-stabilized siRNA (cold preservation, 7.5 μg/mL, 9 OD; i.v. after transplantation, 27.3 OD) to protect pig donor kidneys and also achieved a promising effect, which provided a better siRNA administration strategy for donor organ preservation of large animals. Indeed, porcine hearts preserved with solution containing 16 OD siRNAs exhibit a higher apoptosis index compared with the lower TR-containing siRNA solution. Liposome TR efficiently helps intracellular siRNA delivery in vitro and in vivo.30 TR allows us to use a lower amount of siRNAs while achieving a higher potency, and, so far, 20 μL TR did not appear to cause significant toxicity, such as cell contraction and tendency to induce inflammatory,31, 32 because there are no significant differences between the CEL and Ctrl groups in apoptosis, tissue structural damage, and cardiac function of the ex vivo re-perfused hearts. In cold-preserved hearts, TR-containing siRNA solution decreases cell apoptosis and structural damage compared with the non-TR solution.
Gene silencing experiments in mammalian cells showed that the specificity of siRNA is concentration-dependent. siRNA at high concentrations (≈100 nM, 1 nM = 13.2 μg/μL) may nonspecifically alter the expression of a significant number of genes, many of which are known to be involved in apoptosis and the stress response. Reduction of the siRNA concentration (20 nM) can eliminate this nonspecific effect without lowering the knockdown efficiency.33, 34, 35 Indeed, effective siRNA duplexes typically produce potent silencing at 1 to 10 nM concentrations.36 These studies underscore the importance of determining the concentration for siRNA to optimize silencing efficiency a with minimal amount of siRNA to avoid nonspecific off-target effects. We found that the 4 OD siRNA mix with 20 μL TR appears to be an ideal combination for an equal knockdown efficiency and better heart function preservation compared with the 6 OD siRNAs with 40 μL TR compounds. A higher concentration than 4 OD of siRNA might also trigger unwanted nonspecific effects. In clinical applications, such as prevention of IRI, the off-target effects manifest as graft dysfunction.13 Therefore, we predict, for regular preservation in the clinic with a 2 L volume, a 16 OD siRNA and 80 μL TR mixture is likely to achieve optimal potency.
We have established a blood-perfused system for isolated porcine hearts. The system can be run in coronary perfusion mode (Langendorff) or working heart mode. During the left atrium-perfused working heart mode, left ventricular hemodynamics can be recorded for cardiac function analysis. The system also allows us to perfuse multiple-sized hearts, including small-sized hearts from juvenile pigs, which are difficult for orthotopic transplantation. In addition, use of a mixture of autologous blood and colloid solution with near-normal hematocrit and hemoglobin allows us to minimize the need for donor blood or supporting animals.37 Moreover, reperfusion of isolated hearts can be punctually operated after a precise ischemia period because hearts have virtually no anastomoses between adjacent coronary perfusion beds. Most importantly, the protection and function of siRNA+TR solution-preserved porcine hearts tested by using the isolated working heart model are consistent with the transplanted heart model. Our results suggest that this model may be a very useful tool for preclinical cardiac research.
Although the siRNA solution appears to be able to prolong cold preservation time while attenuating tissue injury, certain limitations need to be addressed prior to clinical translation. Off-target effects may occur during administration of foreign RNA with specific sequences,38 especially delivered by liposomal vehicles,39 which need further investigation. Additionally, optimal concentration of siRNA and vehicle, delivery timing, and systemic compensative responses need to be fully assessed for optimized outcomes. Because of technical equipment issues, we observed the donor grafts for only 3 hr, which cannot predict heart function at a later time. Therefore, long-term cardiac function and survival rate would be our priority in a future study. Despite these limitations, we have demonstrated that the multiple-target siRNA solution can extend the preservation time for donor grafts, attenuate IRI, and protect cardiac function in porcine models of heart transplantation, which provides a principal of concept for potential bench-to-bed implication.
Materials and Methods
Animals
The hearts of 27 juvenile male mini pigs (10∼15 kg) were used in cold preservation and the isolated working heart model. 12 juvenile orthologous male mini pigs (50∼60 kg) were paired as donors and recipients, and their hearts were used for cardiac transplantation. All animals were provided by the laboratory animal center of Shanghai Jiao Tong University and received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals. All experimental procedures were performed with approval from the Laboratory Animal Ethical Committee of Shanghai Jiao Tong University.
siRNA Preparation
A total of 12 pairs of double-stranded 2′OMe-modified siRNAs targeting C3, p65, caspase-8, and caspase-3 mRNA were designed and synthesized by GenePharma (Shanghai, China), 3 pairs of siRNA for each gene. The sequence of the 4 most effective pairs (each for one gene) were as follows: C3, 5′-GCAUCAACACACCCGACAATT-3′ (sense) and 5′-UUGUCGGGUGUGUUGAUGCTT-3′ (antisense); caspase-8, 5′-GCAAGACCUUUAGUGAUCUTT-3′ (sense) and 5′-AGAUCACUAAAGGUCUUGCTT-3′ (antisense); p65, 5′-GCACCGGAUUGAGGAGAAATT-3′ (sense) and 5′-UUUCUCCUCAAUCCGGUGCTT-3′ (antisense); caspase 3, 5′-GCUUGAGCUUAUGCACAUUTT-3′ (sense) and 5′-AAUGUGCAUAAGCUCAAGCTT-3′ (antisense). They were used in the preservation solution.
Gene Transfection In Vitro
The PK15 and ST cell lines were cultured in DMEM (Thermo Fisher Scientific, Waltham, MA) with 10% fetal bovine serum (FBS, Thermo Fisher Scientific) at 37°C under a humidified atmosphere of 95% air/5% CO2. Cells were seeded in 24-well plates (5 × 104 cells per well) and allowed to grow overnight to reach 60%∼70% confluence. C3, caspase-8, p65, caspase-3, or scramble siRNA (nonsense negative control) was mixed with Lipofectamine RNAiMAX reagent (Thermo Fisher Scientific) and Opti-MEM medium (Thermo Fisher Scientific) and incubated at room temperature for 30 min. siRNA-reagent complex was added to cells and cultured for 48 hr. Individual experiments were repeated 3 times on PK15 and ST cells.
qPCR
Total RNA was extracted from cultured cells using the RNA Simple Total RNA kit (Tiangen, Beijing, China). cDNA was reverse-transcribed using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) by following the manufacturer’s protocol. Primers used in qPCR included the following: C3, 5′-GCATTGACATCAAACGGGACT-3′ (forward) and 5′-TTCCACTGCCCCATGTTGACC-3′ (reverse); caspase 8, 5′-TGCCACAACCTACTTTCACC-3′ (forward) and 5′-CACTACCCCTTCAATCTAGCC-3′ (reverse); p65, 5′-ACCTGGCATCCGTCGACAAC-3′ (forward) and 5′-ACCCTGTCACCAAGCGAGT-3′ (reverse); caspase 3, 5′-GCACCTGGTTACTATTCTG-3′ (forward) and 5′-GTAAGAATGTGCATAAGCTC-3′ (reverse); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-TCTTCCAGGAGCGAGATCCC-3′ (forward) and 5′-CCACAACATACGTAGCACCA-3′ (reverse). The qPCR reaction was performed on a StepOnePlus real-time PCR system (Thermo Fisher Scientific) using Hieff qPCR SYBR Green Master Mix (Yeasen, Shanghai, China). After a hot start (10 min at 95°C), amplification was performed for 35 cycles (15 s at 95°C, 60 s at 60°C). The expression of C3, caspase-8, p65, and caspase-3 mRNA was normalized with the GAPDH level and was relative to the negative control using the 2−ΔΔCt method. Each sample was tested in triplicate, and samples were obtained from 3 independent experiments to calculate the mean and SEM.
Anesthetic Protocol
Anesthesia was induced intramuscularly (IM) with a mixture of atropine (0.05 mg/kg), Zoletil (3 mg/kg), and xylazine (1.5 mg/kg). A 22G i.v. catheter was placed in the marginal ear vein, lactated Ringer’s solution was administrated to maintain volume, and propofol (2 mg/kg, i.v.) was given when needed. After intubation using an endotracheal (ET) tube (Medtronic, Minneapolis, MN), pressure-controlled ventilation with air and supplemental oxygen was established. Anesthesia was maintained by 2%∼3% isoflurane. Before the start of surgery, vecuronium bromide (0.1 mg/kg, i.v.) and tolfenamic acid (2 mg/kg, IM) were administrated.
Donor Heart Retrieval and Preservation
A median sternotomy was performed, and then heparin (500 IU/kg) was applied. A homemade catheter was placed into the left ventricle through the apex and secured by purse string sutures for cardiac hemodynamic measurement, and the in situ hemodynamic data were recorded as baseline. Before the hearts were isolated, as much autologous blood as possible was collected. After the superior and inferior vena cava were ligated, the ascending aorta was cross-clamped, and 500 mL cold (4°C) Celsior solution (Genzyme, Boston, MA) containing 100 mg lidocaine was infused into the coronary artery, with an incision in the pulmonary veins and inferior vena cava for intracardiac decompression. Thereafter, the heart was excised.
Scramble siRNA (Ctrl) or the mixture of siRNAs (a mixture of 4 equal doses of target siRNAs, siRNA group, siRNA) were blended with Lipofectamine RNAiMAX TR, and then the siRNA-TR mixtures were added into 33 mL Celsior solution, followed by incubation the room temperature for 30 min. A non-siRNA-TR group was also included to represent the clinical procedure; only pure Celsior solution was given to the heart (CEL). When the heart was isolated, it was transferred into an organ bag surrounded by cold saline and ice. Then 467 mL cold (4°C) Celsior solution was infused into the coronary artery (the aorta root pressure was 20 cm H2O hydrostatic pressure), 33 mL siRNA-TR-Celsior solution was given to the heart, and the donor graft was immersed in the effusive solution. For the non-siRNA-TR group, CEL, only 500 mL Celsior solution was given. Then the bag was sealed and stored at 4°C. A total of 500 mL Celsior solution was sufficient for heart grafts from 10- to 15-kg piglets that were used in cold preservation and the ex vivo working heart model. When the transplantation was carried out, the Celsior volume and siRNA-TR quantity were doubled as the size of the transplanted donor hearts is 2 times as large as the hearts for ex vivo reperfusion.
Ex Vivo Working Heart Model
The working heart model was implemented using a newly designed and assembled perfusion system containing a medical extracorporeal membrane oxygenator (ECMO, Medtronic), an Ultrafilter, preload and afterload chambers, circulation tubes and cannulas of different sizes (Medtronic), an air trap with filter (Medtronic), a heart chamber, a thermostatic chamber around the heart chamber to avoid heat loss, 4 rotary pumps, a physiological recorder (Chengdu TME Technology, Chengdu, China), and a computer for cardiac hemodynamic data recording. This system was used to perfuse the heart in either coronary perfusion Langendorff mode or working heart mode. A schematic overview and description of this system is shown in Figure S3. The perfusate consisted of 800 mL blood (mainly autologous blood; stored blood from blood donors was added after cross-matching test when needed), 200 mL hydroxyethyl starch solution, 1 g fructosediphosphates, 20 IU insulin, 40 mg methylprednisolone, 5 g glucose, 0.5 g magnesium sulfate heptahydrate, 0.5 mg calcium chloride, and 1 g sodium bicarbonate. Before perfusion, the perfusate was warmed to 38°C, circulated, and gassed with a mix of 95% oxygen (3 L/min) and 5% carbon dioxide (0.15 L/min) for maximal oxygenation. Arterial blood gas and electrolytes were adjusted to be in accordance with the normal condition, and the hematocrit was maintained at no less than 22%.
After cold preservation, a cannula was put on the aorta, and the pulmonary artery was shortened to 3 cm. A purse string suture was placed on the left atrium to secure the preload tube during working heart reperfusion, and the rest of the openings of the heart were sutured. Following cannulation, the aorta was connected to the perfusion system, and perfusion of the coronary arteries was started at an initial pressure of 40 mmHg and blood flow of 20 mL/min and gradually increased to 70 mmHg and 70∼80 mL/min. Langendorff perfusion was continued until the cardiac beat resumed and stabilized. After 10∼15 min spontaneous cardiac rhythm, the system was switched to working heart mode. The left atrium was then connected to the preload chamber, the preload volume was increased gradually, the left atrium pressure was maintained at a physiology level of 10∼15 mmHg, and the blood flow was set at 300 mL/min. Under this condition, the heart began to eject (Figures S4A–S4E). Dopamine, epinephrine, and lidocaine were added to maintain cardiac function during ex vivo perfusion. In working heart mode, hearts were allowed to beat for 180 min. All hearts were administrated the same dosage of cardiotonic drugs.
Cardiac Transplantation
A total of 6 pairs of male mini pigs weighing 50∼60 kg were used as donors and recipients. Anesthesia was performed as described above. The recipient was kept on the operation table with a heating pad. A catheter was placed into the right carotid artery to monitor the arterial blood pressure. With a median sternotomy, the heart of the recipient was exposed, and 500 IU/kg heparin was given i.v. When the activated clotting time was longer than 480 s, a standard cardiopulmonary bypass (CBP) was established, and the recipient heart was removed after the aorta was cross-clamped. The donor heart was transferred into cold saline, and the left atrium and the great vessels were trimmed. The donor heart was then sutured onto the recipient in compliance with standard orthotopic heart transplantation procedures using the bicaval method (Figures S4F and S4G). Before de-clamping the aorta, methylprednisolone (500 mg) and lidocaine (100 mg) were administrated. After the aorta was de-clamped, reperfusion started, and cardiotonic and defibrillation were given when needed. The CBP system was kept running at full flow (3.5 L/min) for the first 90 min; after that, the flow was decreased gradually. 120 min later, CBP ceased with injection of 50 mg protamine, and the heart was allowed to work for one more hour. All hearts were administrated the same dosage of cardiotonic drugs during reperfusion. At 180 min, plasma samples were collected before cardioplegia, and then the donor hearts were arrested.
Left Ventricle Hemodynamic Measurement
During the reperfusion period, left ventricle (LV) hemodynamic parameters, including LVSP, +dP/dtmax, and −dP/dtmin, were recorded and analyzed using the BL-420F data acquisition and analysis system (Chengdu TME Technology). These 3 parameters were assessed at 0 min (baseline), 60 min, 120 min, and 180 min during ex vivo working heart or in vivo transplantation, and the value of each parameter was normalized by the corresponding baseline mean value, respectively.
Sample Collection
Blood samples were collected from the coronary venous sinus using a syringe with a 24G needle at 180 min (before cardioplegia, 38°C) of working heart mode or transplantation. After centrifugation, plasma samples were collected and stored at −80°C to detect the myocardial injury (MI) markers. Heart tissue samples were collected from the left ventricular anterior wall (after cardioplegia, 4°C). Half of them were stored in liquid nitrogen for protein and RNA extraction or MI measurement, and the rest were fixed with 10% formaldehyde solution for TUNEL analysis and pathohistological assessment.
Western Blot Analysis
Heart tissues from the left ventricle were homogenized in radioimmunoprecipitation analysis (RIPA) lysis buffer (Beyotime, Beijing, China), and 20 μg of protein was separated by 10% or 15% SDS-PAGE gels and transferred onto polyvinylidene fluoride (PVDF) membranes (0.22 μm, Merck Millipore, Darmstadt, Germany). The membranes were blocked with 5% (w/v) non-fat milk. Antibodies against C3 (1:1,000), caspase-8 (1:50), p65 (1:1,000), caspase-3 (1:250), IL-1β (1:1,000), IL-6 (1:500), TNF-α (1:1,000), Bcl-2 (1:1,000), BAX (1:1,000), and GAPDH (1:10,000) (Abcam, Cambridge, MA) were used for immunoblotting at 4°C overnight, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000, Beyotime). Immunoreactive bands were visualized using enhanced chemiluminesence substrate (Merck Millipore) and a Bio-Image analysis system (Tanon, Shanghai, China). The semiquantitative results were expressed as OD × mm2 and normalized to the GAPDH level (Image Pro Plus, Rockville, MD).
Myocardial Apoptosis Measurement
Cell apoptosis was detected by TUNEL assay using a TUNEL detection kit (Yeasen). Briefly, formalin tissue was embedded in paraffin, and 5-μm tissue sections were de-paraffinized and gradient-washed with ethanol. Proteinase K solution was added, and the samples were incubated at room temperature for 30 min, followed by incubation with 100 μL of equilibration buffer containing terminal deoxynucleotidyl transferase (TdT) at room temperature for another 10–30 min. Then 5 μL of TdT solution and 45 μL of fluorescein-labeled dUTP were added to each sample and mixed thoroughly. For the sham group, only fluorescein labeled dUTP was added without TdT. The samples were then sealed and covered with reaction solution and incubated at 37°C for 60 min. DAPI was used for nuclear staining. Labeled apoptotic cells were analyzed under a red fluorescence excitation wavelength of 651 nm. Apoptosis cells were observed at 400× magnification in 10 fields using an inverted fluorescence microscope (IX71, Olympus, Japan).
Histopathological Assessment
5-μm paraffin tissue sections were stained with H&E.40 These sections were examined in a blinded fashion by a pathologist. The percentage of histological changes in grafts was scored with a semiquantitative scale designed to evaluate the degree of 4 criteria: interstitial edema, cellular infiltration, hemorrhage, and ischemic injury. Each criterion was graded from mild to severe by the percentage of injury: 0 (< 1%); 1 (1%∼25%); 2 (26%∼50%); 3 (51%∼75%); and 4 (>75%).
Measurement of MI Markers
The levels of CK, CKMB, LDH, MDA, and SOD activity are protein markers well characterized for myocardial tissue damage.41, 42 The CK, CKMB, LDH content in plasma samples and MDA and SOD activity content in cardiac tissue homogenate were measured using commercially available kits (Creatine Kinase Assay Kit, Creatine Kinase MB Isoenzyme Assay Kit, Lactate Dehydrogenase Assay Kit, Malonaldehyde Assay Kit TBA Method, and Total Superoxide Dismutase Assay Kit Hydroxylamine Method; Nanjing Jiancheng Biochemical, Nanjing, China) according to the manufacturer’s protocols.
Statistical Analysis
Results are expressed as mean ± SEM. Statistical analyses were performed using GraphPad Prism 5 statistical software (San Diego, CA). Differences between two groups were analyzed by unpaired t test. Differences among multiple groups were analyzed by one-way ANOVA with a Bonferroni post-test (a homogeneity of variance test was conducted before analysis). A two-sided p < 0.05 was considered statistically significant.
Author Contributions
J.W., Y.W., and S.X. designed and performed the laboratory experiments. J.W., Y.W., S.X., Q.Z., and S.L. performed the surgical experiments. J.W. and S.C. analyzed the experiments and wrote the manuscript. Y.W., X.H., and L.C. conceived the project and supervised the entire study.
Acknowledgments
We thank Xianda Li and Duodan Li (CCI Facility, Covidien Management Consulting Co. Ltd., Shanghai, China) for help with the heart transplantation surgeries. This work was supported by Medicine Cross Fund of Shanghai Jiaotong University Grant YG2013MS35, Natural Science Foundation of China grants 31572525 and 31270186, and the Shanghai Jiao Tong University Agri-X Foundation (2015).
Footnotes
Supplemental Information includes four figures and can be found with this article online at https://doi.org/10.1016/j.omtn.2017.10.020.
Contributor Information
Xiuguo Hua, Email: hxg@sjtu.edu.cn.
Yongyi Wang, Email: m13918763677@163.com.
Supplemental Information
References
- 1.Coulson M.T., Jablonski P., Howden B.O., Thomson N.M., Stein A.N. Beyond operational tolerance: effect of ischemic injury on development of chronic damage in renal grafts. Transplantation. 2005;80:353–361. doi: 10.1097/01.tp.0000168214.84417.7d. [DOI] [PubMed] [Google Scholar]
- 2.Kouwenhoven E.A., de Bruin R.W., Bajema I.M., Marquet R.L., Ijzermans J.N. Cold ischemia augments allogeneic-mediated injury in rat kidney allografts. Kidney Int. 2001;59:1142–1148. doi: 10.1046/j.1523-1755.2001.0590031142.x. [DOI] [PubMed] [Google Scholar]
- 3.Knight R.J., Dikman S., Liu H., Martinelli G.P. Cold ischemic injury accelerates the progression to chronic rejection in a rat cardiac allograft model. Transplantation. 1997;64:1102–1107. doi: 10.1097/00007890-199710270-00003. [DOI] [PubMed] [Google Scholar]
- 4.Schmid C., Heemann U., Tilney N.L. Factors contributing to the development of chronic rejection in heterotopic rat heart transplantation. Transplantation. 1997;64:222–228. doi: 10.1097/00007890-199707270-00007. [DOI] [PubMed] [Google Scholar]
- 5.Carden D.L., Granger D.N. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Jassem W., Heaton N.D. The role of mitochondria in ischemia/reperfusion injury in organ transplantation. Kidney Int. 2004;66:514–517. doi: 10.1111/j.1523-1755.2004.761_9.x. [DOI] [PubMed] [Google Scholar]
- 7.Jahania M.S., Sanchez J.A., Narayan P., Lasley R.D., Mentzer R.M., Jr. Heart preservation for transplantation: principles and strategies. Ann. Thorac. Surg. 1999;68:1983–1987. doi: 10.1016/s0003-4975(99)01028-0. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum D.H., Peltz M., DiMaio J.M., Meyer D.M., Wait M.A., Merritt M.E., Ring W.S., Jessen M.E. Perfusion preservation versus static preservation for cardiac transplantation: effects on myocardial function and metabolism. J. Heart Lung Transplant. 2008;27:93–99. doi: 10.1016/j.healun.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Menke J., Sollinger D., Schamberger B., Heemann U., Lutz J. The effect of ischemia/reperfusion on the kidney graft. Curr. Opin. Organ Transplant. 2014;19:395–400. doi: 10.1097/MOT.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 10.Boros P., Bromberg J.S. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am. J. Transplant. 2006;6:652–658. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 11.Gluba A., Banach M., Hannam S., Mikhailidis D.P., Sakowicz A., Rysz J. The role of Toll-like receptors in renal diseases. Nat. Rev. Nephrol. 2010;6:224–235. doi: 10.1038/nrneph.2010.16. [DOI] [PubMed] [Google Scholar]
- 12.Bouma H.R., Ploeg R.J., Schuurs T.A. Signal transduction pathways involved in brain death-induced renal injury. Am. J. Transplant. 2009;9:989–997. doi: 10.1111/j.1600-6143.2009.02587.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z.X., Min W.P., Jevnikar A.M. Use of RNA interference to minimize ischemia reperfusion injury. Transplant. Rev. (Orlando) 2012;26:140–155. doi: 10.1016/j.trre.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Zheng X., Lian D., Wong A., Bygrave M., Ichim T.E., Khoshniat M., Zhang X., Sun H., De Zordo T., Lacefield J.C. Novel small interfering RNA-containing solution protecting donor organs in heart transplantation. Circulation. 2009;120:1099–1107. doi: 10.1161/CIRCULATIONAHA.108.787390. 1 p following 1107. [DOI] [PubMed] [Google Scholar]
- 15.Yang C., Zhao T., Zhao Z., Jia Y., Li L., Zhang Y., Song M., Rong R., Xu M., Nicholson M.L. Serum-stabilized naked caspase-3 siRNA protects autotransplant kidneys in a porcine model. Mol. Ther. 2014;22:1817–1828. doi: 10.1038/mt.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X., Zheng X., Sun H., Feng B., Chen G., Vladau C., Li M., Chen D., Suzuki M., Min L. Prevention of renal ischemic injury by silencing the expression of renal caspase 3 and caspase 8. Transplantation. 2006;82:1728–1732. doi: 10.1097/01.tp.0000250764.17636.ba. [DOI] [PubMed] [Google Scholar]
- 17.Zheng X., Zhang X., Sun H., Feng B., Li M., Chen G., Vladau C., Chen D., Suzuki M., Min L. Protection of renal ischemia injury using combination gene silencing of complement 3 and caspase 3 genes. Transplantation. 2006;82:1781–1786. doi: 10.1097/01.tp.0000250769.86623.a3. [DOI] [PubMed] [Google Scholar]
- 18.Milani-Nejad N., Janssen P.M. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol. Ther. 2014;141:235–249. doi: 10.1016/j.pharmthera.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang Y., Christopher K., Finn P.W., Colson Y.L., Perkins D.L. Graft produced interleukin-6 functions as a danger signal and promotes rejection after transplantation. Transplantation. 2007;84:771–777. doi: 10.1097/01.tp.0000281384.24333.0b. [DOI] [PubMed] [Google Scholar]
- 20.Haug C.E., Colvin R.B., Delmonico F.L., Auchincloss H., Jr., Tolkoff-Rubin N., Preffer F.I., Rothlein R., Norris S., Scharschmidt L., Cosimi A.B. A phase I trial of immunosuppression with anti-ICAM-1 (CD54) mAb in renal allograft recipients. Transplantation. 1993;55:766–772. doi: 10.1097/00007890-199304000-00016. discussion 772–773. [DOI] [PubMed] [Google Scholar]
- 21.Czabotar P.E., Lessene G., Strasser A., Adams J.M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 22.Lattmann T., Hein M., Horber S., Ortmann J., Teixeira M.M., Souza D.G., Haas E., Tornillo L., Münter K., Vetter W., Barton M. Activation of pro-inflammatory and anti-inflammatory cytokines in host organs during chronic allograft rejection: role of endothelin receptor signaling. Am. J. Transplant. 2005;5:1042–1049. doi: 10.1111/j.1600-6143.2005.00807.x. [DOI] [PubMed] [Google Scholar]
- 23.Ishii D., Schenk A.D., Baba S., Fairchild R.L. Role of TNFalpha in early chemokine production and leukocyte infiltration into heart allografts. Am. J. Transplant. 2010;10:59–68. doi: 10.1111/j.1600-6143.2009.02921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seto T., Kamijo S., Wada Y., Yamaura K., Takahashi K., Komatsu K., Otsu Y., Terasaki T., Fukui D., Amano J. Upregulation of the apoptosis-related inflammasome in cardiac allograft rejection. J. Heart Lung Transplant. 2010;29:352–359. doi: 10.1016/j.healun.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 25.McLaren A.J., Friend P.J. Trends in organ preservation. Transpl. Int. 2003;16:701–708. doi: 10.1007/s00147-003-0659-2. [DOI] [PubMed] [Google Scholar]
- 26.Jani A., Ljubanovic D., Faubel S., Kim J., Mischak R., Edelstein C.L. Caspase inhibition prevents the increase in caspase-3, -2, -8 and -9 activity and apoptosis in the cold ischemic mouse kidney. Am. J. Transplant. 2004;4:1246–1254. doi: 10.1111/j.1600-6143.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 27.Swenson K.M., Ke B., Wang T., Markowitz J.S., Maggard M.A., Spear G.S., Imagawa D.K., Goss J.A., Busuttil R.W., Seu P. Fas ligand gene transfer to renal allografts in rats: effects on allograft survival. Transplantation. 1998;65:155–160. doi: 10.1097/00007890-199801270-00002. [DOI] [PubMed] [Google Scholar]
- 28.Chernolovskaya E.L., Zenkova M.A. Chemical modification of siRNA. Curr. Opin. Mol. Ther. 2010;12:158–167. [PubMed] [Google Scholar]
- 29.Zheng X., Zang G., Jiang J., He W., Johnston N.J., Ling H., Chen R., Zhang X., Liu Y., Haig A. Attenuating Ischemia-Reperfusion Injury in Kidney Transplantation by Perfusing Donor Organs With siRNA Cocktail Solution. Transplantation. 2016;100:743–752. doi: 10.1097/TP.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 30.Foged C., Nielsen H.M., Frokjaer S. Phospholipase A2 sensitive liposomes for delivery of small interfering RNA (siRNA) J. Liposome Res. 2007;17:191–196. doi: 10.1080/08982100701530373. [DOI] [PubMed] [Google Scholar]
- 31.Stewart M.J., Plautz G.E., Del Buono L., Yang Z.Y., Xu L., Gao X., Huang L., Nabel E.G., Nabel G.J. Gene transfer in vivo with DNA-liposome complexes: safety and acute toxicity in mice. Hum. Gene Ther. 1992;3:267–275. doi: 10.1089/hum.1992.3.3-267. [DOI] [PubMed] [Google Scholar]
- 32.Filion M.C., Phillips N.C. Major limitations in the use of cationic liposomes for DNA delivery. Int. J. Pharm. 1998;162:159–170. [Google Scholar]
- 33.Semizarov D., Frost L., Sarthy A., Kroeger P., Halbert D.N., Fesik S.W. Specificity of short interfering RNA determined through gene expression signatures. Proc. Natl. Acad. Sci. USA. 2003;100:6347–6352. doi: 10.1073/pnas.1131959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Persengiev S.P., Zhu X., Green M.R. Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs) RNA. 2004;10:12–18. doi: 10.1261/rna5160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson A.L., Bartz S.R., Schelter J., Kobayashi S.V., Burchard J., Mao M., Li B., Cavet G., Linsley P.S. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz D.S., Hutvágner G., Haley B., Zamore P.D. Evidence that siRNAs function as guides, not primers, in the Drosophila and human RNAi pathways. Mol. Cell. 2002;10:537–548. doi: 10.1016/s1097-2765(02)00651-2. [DOI] [PubMed] [Google Scholar]
- 37.Schuster A., Grünwald I., Chiribiri A., Southworth R., Ishida M., Hay G., Neumann N., Morton G., Perera D., Schaeffter T., Nagel E. An isolated perfused pig heart model for the development, validation and translation of novel cardiovascular magnetic resonance techniques. J. Cardiovasc. Magn. Reson. 2010;12:53. doi: 10.1186/1532-429X-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Judge A.D., Sood V., Shaw J.R., Fang D., McClintock K., MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 39.Sioud M. Innate sensing of self and non-self RNAs by Toll-like receptors. Trends Mol. Med. 2006;12:167–176. doi: 10.1016/j.molmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Hashmi S., Al-Salam S. Acute myocardial infarction and myocardial ischemia-reperfusion injury: a comparison. Int. J. Clin. Exp. Pathol. 2015;8:8786–8796. [PMC free article] [PubMed] [Google Scholar]
- 41.Bodor G.S. Biochemical Markers of Myocardial Damage. EJIFCC. 2016;27:95–111. [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigo R., Libuy M., Feliú F., Hasson D. Oxidative stress-related biomarkers in essential hypertension and ischemia-reperfusion myocardial damage. Dis. Markers. 2013;35:773–790. doi: 10.1155/2013/974358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.