Abstract
Clec4a has been reported to be an immune suppressor of dendritic cells (DCs), but its potential role in cancer therapy remains to be elucidated. The present study investigated whether downregulating the expression of Clec4a via skin delivery of small hairpin RNA (shRNA) using a gene gun produced stronger host immunity and inhibited tumor progression in animal models. Administration of Clec4a2 shRNA delayed tumor growth in both mouse bladder and lung tumor-bearing mouse models. The result was further confirmed with a compensation experiment showing that the antitumor effects induced by Clec4a2 shRNA were restored by co-injection of a plasmid expressing exogenous Clec4a2. Increased numbers of infiltrating CD4+ and CD8+ T cells at tumor sites were observed in mice treated with Clec4a2 shRNA. Splenocytes from mice with Clec4a2 shRNA administration exhibited stronger cytotoxic activity compared with splenocytes from control mice. CD8-deletion in vivo abrogated the antitumor effects elicited by Clec4a2 shRNA. Additionally, shClec4a enhanced the antitumor effects of the Neu DNA vaccine in the MBT-2 tumor model. In summary, the findings provide evidence that silencing of Clec4a2 expression via skin delivery of shRNA produces an effective antitumor response and that Clec4a2 shRNA may have therapeutic potential as an adjuvant for cancer immunotherapy.
Keywords: Clec4a2, Dcir1, immunotherapy, shRNA, gene gun
Introduction
Dendritic cells (DCs) are mature antigen-presenting cells that play a crucial role in the adaptive immune response, initiating immunity by capturing antigens and presenting them to naive T cells.1 In contrast, immature (or nonactivated) DCs induce immune tolerance or immune suppression by attenuating T cell activation or promoting the differentiation of regulator T cells. DCs have been reported to act as immunomodulators and negatively regulate DC-mediated immunity.2 They have also been shown to be potent targets of host immunity against diseases.3 For example, studies showed that indoleamine 2,3-dioxygenase (IDO)-expressing DCs suppressed T cell immunity by activation of regulator T cells, mediated by the degradation of tryptophan and inhibition of T cell proliferation, in various cancers.4, 5 As a result, several clinical trials are evaluating the therapeutic potential of targeting IDO enzymatic activity with small-molecule inhibitors.6
Clec4a (C-type lectin domain family 4 member a), a DC immunoreceptor (DCIR), belongs to the lectin-2 family of C-type lectin-like receptors and is commonly expressed by various immune cells, including CD14+ monocytes, CD15+ granulocytes, CD19+ B cells, and all DC subsets.7, 8, 9, 10 Human Clec4a is differentially expressed by DCs, depending on their stage of maturation and activation. In contrast to mouse Clec4a2 (Dcir1), which is expressed by DCs, macrophages, and B cells, mouse Clec4a4 (Dcir2) is only observed in the CD8− DC subtype.11 Blood monocyte-derived DCs strongly induce Clec4a expression, whereas interleukin-4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF) specifically downregulate Clec4a expression, in addition to the CD40 ligand lipopolysaccharide (LPS) and tumor necrosis factor alpha (TNF-α), all of which induce the maturation of DCs.7
Clec4a is unique among known C-type lectin receptors (CLRs), with an immunoreceptor tyrosine-based inhibitory signaling motif (ITIM) in its cytoplasmic tail and inhibitory capacity.12 The ITIM inhibitory signaling capacity of mouse Clec4a2 and Clec4a4 and human Clec4a is mediated through the activation of the phosphatases SHP-1 and SHP-2.9, 13, 14, 15 SHP signaling suppresses IL-12 and TNF expression induced by Toll-like receptor 8 (TLR8) in myeloid DCs and interferon-α (IFN-α) and TNF expression induced by TLR9 in plasmacytoid DCs.8, 9 Development of autoimmune diseases in a Clec4a2-deficient mice model is probably mediated via an abnormal increase in activated DCs and activated CD4+ T cells.16 Moreover, the inflammatory response and T cell immunity were suppressed by Clec4a-expressing DCs during microbial infection.17 DCIR knockout DCs increased the production of IL-12 and promoted Th1 immunity in a tuberculosis-infected model.18 In addition, targeting DCIRs with antigen-conjugated antibody triggered the priming of human CD8+ T cells via cross-presentation by DCs.10
Modulating the negative properties of DCs is a potent strategy in immunotherapy for enhancing patient immunity against cancer.19 Studies have demonstrated that the administration of DNA vaccines using a gene gun is a powerful approach to express specific antigens in antigen-presenting cells in vivo.20, 21 In previous studies, we provided evidence that the delivery of small hairpin RNA (shRNA), which targets an immune negative regulator, IDO, into skin DCs via a gene gun successfully produced antitumor responses in subcutaneous mouse tumor models22 and orthotopic and metastatic mouse liver tumor models.23 Additionally, skin administration of shRNA targeted thrombospondin 1 (another immune suppressor), delayed tumor growth, and prolonged the survival of tumor-bearing mice.24 According to the negative regulatory role of Clec4a2 in DCs, we hypothesized that downregulating the expression of Clec4a2 in DCs might elicit stronger immunity against tumors. In this study, we demonstrated that skin delivery of Clec4a2 shRNA using a gene gun elicited antitumor effects via an increase in CD8+ immunity.
Results
Skin Administration of Clec4a shRNA Delayed Tumor Growth in a Subcutaneous Mouse Tumor Model
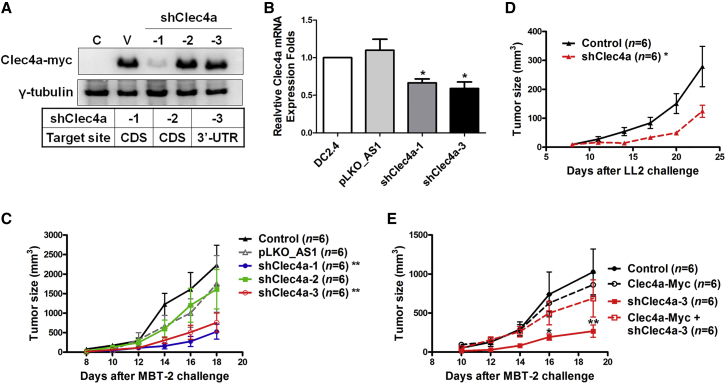
Skin delivery of shRNA through a gene gun was shown to have silencing effects in resident DCs in murine tumor models.22, 23, 24 To investigate whether downregulation of the expression of Clec4a2 in skin DCs induced an antitumor response, Clec4a2 shRNA was evaluated in a murine bladder tumor model. The knockdown efficiency of three Clec4a2 shRNAs with different targeting sequences (shClec4a-1 and 2, coding sequence [CDS] of Clec4a2 mRNA; shCle4a-3, 3′ UTR of Clec4a2 mRNA) were examined in 293T cells (Figure 1A). shClec4a-1 significantly downregulated the protein expression of co-transfected Clec4a2-myc (plasmid-encoded the CDS region) in 293T cells (Figure 1A). shClec4a-2 did not reduce the protein expression of Clec4a2-myc and may serve as another control shRNA (Figure 1A). To further evaluate the knockdown efficacy of shClec4a-3 (targeting the 3′ UTR), shClec4a-1 and shClec4a-3 were transfected into a murine dendritic cell line, DC2.4. Both shClec4a-3 and shClec4a-1 successfully decreased the endogenous mRNA expression of Clec4a2 in DC2.4 cells (Figure 1B). These results indicated that shClec4a-1 and shClec4a-3 are able to reduce Clec4a expression. In the MBT-2 tumor models, tumor growth was delayed in mice treated with skin administration of shClec4a-1 and shClec4a-3 compared with mice treated with a control plasmid. No effect was observed in mice treated with shClec4a-2, which has no effect on reducing Clec4a2 expression (Figure 1C). Another Clec4a2 shRNA, shClec4a2 (415), which targets Clce4a2 CDS and has a knockdown efficiency similar to that of shClec4a-1 (416) (Figure S1A), also elicited an antitumor response in the MBT-2 model (Figure S1B). To further evaluate the therapeutic effects induced by Celc4a2 shRNA, an LL2 (murine Lewis lung carcinoma) mouse lung tumor model and a B16F1 (murine melanoma) orthotopic mouse model were used. Skin delivery of shClec4a-1 via gene gun delayed tumor growth in LL2 tumor-bearing mice compared with mice without treatment (Figure 1D). Additionally, skin-administered shClec4a-1 induced an antitumor response in orthotopic B16F1 tumor mice compared with control mice (Figure S1C). To exclude the off-target effects of Clec4a2 shRNA, the expression of Clec4a2 was compensated by using a plasmid expressing Clec4a2-myc. Clec4a2-myc expression was not affected in the co-transfection of shClec4a-3 (targeting the 3′ UTR) and Clec4a2-myc plasmids (without the 3′ UTR) (Figure 1A). The therapeutic effects of shClec4a-3, which was delivered with or without Clec4a2-myc, were evaluated in tumor-bearing mice. There was no significant difference in tumor growth between mice receiving Clec4a2-myc and control mice (Figure 1E). Tumor size was decreased in mice treated with shClec4a-3 compared with those treated with Clec4a2-myc (Figure 1E). Moreover, co-delivery of a plasmid expressing Clec4a2 attenuated the therapeutic effects induced by shClec4a (Figure 1E). These results indicated that silencing Clec4a2 expression through skin administration of shRNA elicited an effective antitumor response.
Figure 1.
Skin Administration of shClec4a2 Induced Antitumor Responses in Mouse Tumor Models
(A) The knockdown efficacy of three Clec4a2 shRNAs (shClec4a-1 and 2 targeting the coding sequence [CDS] of Clec4a2 and shCle4a-3 targeting the 3′ UTR) were examined in 293T cells co-transfected with Clec4a2-myc (which plasmid-encoded the CDS of Clec4a2). Protein expression of Clec4a2-myc was measured using immunoblotting. C, parental control; V, pLKO_AS1+ Clec4a2-myc; shClec4a, Clec4a2 shRNA+ Clec4a2-myc. A pLKO_AS1 plasmid served as the vector control. β-Actin was used as an internal control. (B) The knockdown efficacy of shClec4a-1 and shCle4a-3 was examined in the DC2.4 cell line. mRNA expression of endogenous Clec4a2 in DC2.4 cells transfected with shClec4a-1, shCle4a-3, or pLKO_AS1 were detected using real-time PCR analysis. *p < 0.05, pLKO_AS1 versus shClec4a. ns, no statistical difference. A pLKO_AS1 plasmid served as the vector control. Bars represent the mean ± SEM of three independent experiments. HPRT was used as an internal control. (C) Mice bearing MBT-2 tumor cells were treated with Clec4a2 shRNA via gene gun. The tumor size was examined in C3H/HeN mice on the indicated days (*p < 0.05, pLKO_AS1 versus shClec4a). (D) Mice bearing LL2 tumor cells were treated with shClec4a-1 via gene gun. Tumor sizes were examined in C57BL/6 mice on the indicated days (*p < 0.05, control versus shClec4a). (E) MBT-2 tumor-bearing mice received Clec4a2-myc, shClec4a-3, or shClec4a-3 plus Clec4a2-myc, and the tumor sizes were examined (shClec4a-3 and Clec4a2-myc versus shClec4a-3, *p < 0.05 on day 16 and **p < 0.01 on day 19, respectively). shClec4a, Clec4a2 shRNA.
Immunological Mechanism of the shClec4a-Induced Antitumor Response
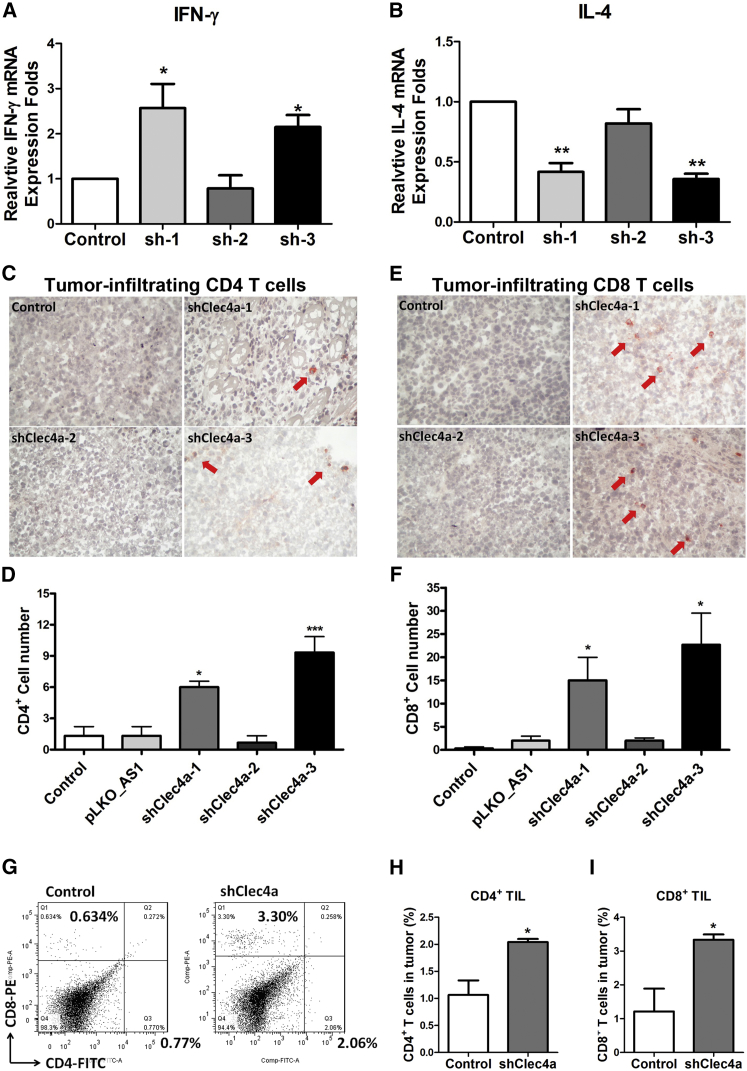
To investigate the immunological mechanisms underlying the antitumor responses induced by shClec4a, splenocytes expressing IFN-γ and IL-4, which are Th1- and Th2-related cytokines, were examined using the real-time PCR method. mRNA levels of IFN-γ were dramatically upregulated in the spleens of mice treated with shClec4a-1 and shClec4a-3 compared with the control group (Figure 2A). In contrast, the expression level of IL-4 was decreased in the spleens of mice treated with shClec4a-1 and shClec4a-3 (Figure 2B). A subsequent analysis of tumor-infiltrating lymphocytes by immunohistochemistry revealed that tumor-infiltrating CD4+ T cells were 4-fold and 6-fold higher in tumor-bearing mice that received shClec4a-1 and shClec4a-3 than in mice that received a vector control (Figures 2C and 2D). An 8- to 9-fold increase in tumor-infiltrating CD8+ T cells was observed in tumor-bearing mice that received shClec4a-1 and shClec4a-3 compared with mice that received the vector control (Figures 2E and 2F). The number of tumors infiltrating CD4+ and CD8+ T cells was significantly increased by administration of shClec4a-1 compared with the control (Figures 2G–2I) by using fluorescence-activated cell sorting (FACS) analysis. Taken together, these data suggested that downregulation of Clec4a induced an antitumor response and cellular immunity.
Figure 2.
The Immunological Mechanism of the Antitumor Response Induced by shClec4a2
(A and B) IFN-γ (A) and IL-4 (B) expression levels in splenocytes were measured with the real-time PCR method. The relative expression fold was compared with that of the control group (*p < 0.05, n = 3 mice per group, mean ± SEM). (C and E) Immunohistochemical staining of tumor-infiltrating CD4+ T cells (C) and CD8+ T cells (E) in the MBT-2 tumor-bearing mouse model (red arrows). (D and F) The cell count was performed at ×150 magnification. Three randomly chosen fields/samples from three mice were evaluated. (G) FACS analysis of tumor-infiltrating CD4+ T cells and CD8+ T cells from MBT-2 tumor-bearing mice. The bar chart represent the percentage of (H) CD4+ T cells and (I) CD8+ T cells in the tumors (n = 3 mice per group). Columns and bars represent mean values ± SEM. *p < 0.05 and ***p < 0.001 versus the vector control group.
Cellular Immunity Was Essential for shClec4a-Elicited Therapeutic Efficacy
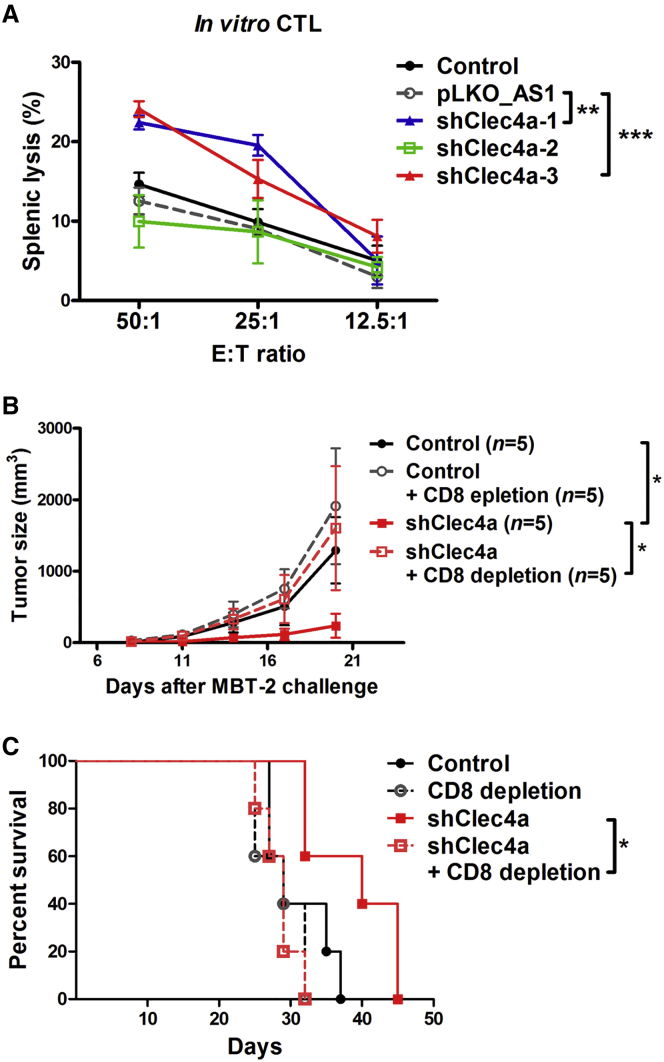
To explore the role of cellular immunity in silencing the Clec4a-induced antitumor response, the cytotoxic ability of splenic cells was evaluated using an in vitro cytotoxic T lymphocyte (CTL) assay. Splenocytes from mice that received shClec4a-1 and shClec4a-3 induced a stronger CTL response than those from mice that received a vector plasmid or no treatment (Figure 3A). Next, the role of CD8+ T cells in therapeutic effects induced by shClec4a was investigated by CD8-depletion in vivo. Depletion of CD8+ T cells abolished the therapeutic efficacy of shClec4a (Figure 3B). In addition, the survival rate of CD8 depletion tumor-bearing mice that received shClec4a was worse than that of tumor-bearing mice with CD8+ T cells that received shClec4a (Figure 3C). Together, these data indicated that cellular immunity was essential for the therapeutic effects and potent antitumor activity induced by Clec4a downregulation in DCs.
Figure 3.
CD8+ T Cell Immunity Was Essential for the shClec4a2-Induced Antitumor Response
(A) Splenocytes from mice treated with Clec4a2 shRNA were cytotoxic against tumor cells. Effector cells were isolated from mice that received the indicated treatments. MBT-2-luciferase cells were used as target cells. Cytotoxicity was measured by detecting the release of luciferase. **p < 0.01, vector control versus shClec4a-1; ***p < 0.001, vector control versus shClec4a-3. Bars represent the mean ± SEM of three independent experiments. (B) Tumor volume of MBT-2 cells in C3H/HeN mice with CD8 depletion. (C) Survival curve of MBT-2 tumor-bearing mice with CD8-depletion. *p < 0.05, shClec4a versus shClec4a and CD8 depletion. pLKO_AS1 served as the vector control. shClec4a, Clec4a2 shRNA-1.
Combination of the Neu DNA Vaccine and shClec4a Produced Stronger Antitumor Effects
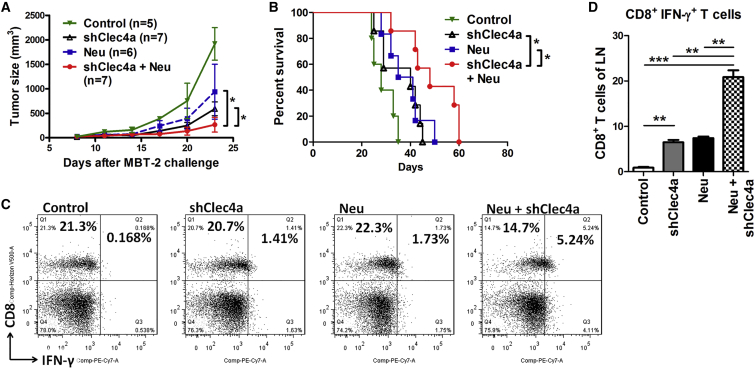
We have previously demonstrated the therapeutic effects of the Neu DNA vaccine in the MBT-2 mouse tumor model; therefore, we evaluated whether shCleca4 could be used as an anti-cancer adjuvant. Three different combinations—pRCMV (vector control of the Neu plasmid) plus shClec4a, Neu DNA vaccine plus pLKO (vector control of the shClec4a plasmid), or Neu DNA vaccine plus shClec4a—were skin-delivered into MBT-2 tumor-bearing mice. Mice vaccinated with the Neu DNA vaccine and shClec4a together exhibited smaller tumor sizes compared with mice vaccinated with the Neu DNA vaccine or shClec4a alone (Figure 4A). Mice vaccinated with both shClec4a and Neu DNA plasmids had longer survival than the other groups of mice (Figure 4B). To study the underlying mechanisms, the changes in activated CD8+ T cells and CD11c+ DCs were examined in tumor-draining lymph nodes (LNs) (Figure 4C). CD8+ IFN-γ+ T cells were significantly increased (2-folder higher) in LNs from mice that received both the Neu DNA vaccine and shClec4a compared with LNs from mice that received the Neu DNA vaccine or shClec4a alone (Figure 4D). Additionally, Th1 CD4 effector T cells, natural killer cells, and natural killer T cells produce large amounts of IFN-γ.25 The expression levels of IFN-γ+ on CD8− cells were enhanced in the Neu plus shClec4a group compared with the control, Neu-only, and shClec4a-only groups (7.6-fold, 2.52-fold, and 2.45-fold, respectively). These results suggested that silencing the expression of Clec4a augments the antitumor effects of the Neu DNA vaccine. shClec4a functions as an immunotherapeutic adjuvant in the animal tumor model.
Figure 4.
shClec4a2 Enhanced the Therapeutic Effects of the Neu DNA Vaccine
(A) MBT-2 tumor-bearing mice were administered shClec4a-1 and a plasmid encoding the intracellular domain of Neu (cyto-Neu). MBT-2 tumor volumes were measured at the indicated times (*p < 0.05, Neu+ shClec4a versus Neu or shClec4a on day 23). pRCMV was used as the vector control for human cyto-Neu. pLKO was used as the vector control for shClec4a. (B) Survival curve of MBT-2 tumor-bearing mice with the indicated treatments. (C) Lymphocytes from mice that received cyto-Neu, shClec4a-1, or cyto-Neu+ shClec4a-1 were isolated, and we examined the changes of cytotoxicity to CD8+ T cells. (D) Bar chart representing the percentage of CD8+ IFN-γ+ T cells in total CD8+ T cells (n = 3 mice per group, mean ± SEM). LN, tumor-draining lymph nodes. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Recent studies have demonstrated the effectiveness of various targeted therapeutic strategies in the activation of host immunity against tumor cells.26, 27 Modulating the negative properties of DCs by using RNAi approaches was shown to induce antitumor immunity and additive therapeutic efficiency in many preclinical cancer studies.22, 24, 28, 29 The present study indicated that skin administration of Clec4a2 shRNA elicited antitumor effects in a mouse tumor model. CD8-depletion in vivo abrogated the antitumor effects induced by skin delivery of Clec4a2 shRNA, suggesting that adaptive immunity was essential for the anticancer response. Additionally, the low silencing effect of shClec4a-2 was correlated with reduced antitumor effects. The observed therapeutic effects were significantly dependent on the silencing efficacy of shRNA targeting negative regulators of DCs.22, 24
Previous studies demonstrated that the use of monoclonal antibodies targeting immune cells was a promising approach for activating therapeutic antitumor immunity.30, 31 For example, antibodies against immune checkpoint signaling, cytotoxic T lymphocyte-associated antigen 4, programmed cell death protein 1 (PD-1), and the PD-1 ligand enhance antitumor immunity and produce strong clinical responses.32 CLRs are a large family of pattern recognition receptors that are commonly expressed on macrophages, neutrophils, and DCs. CLRs have one or more C-type lectin-like domain for recognition and internalization of glycosylated antigens. Ligand engagement of most CLRs, except Clec4a and Clec12a (myeloid inhibitory C-type lectin-like receptors), both of which contain an ITIM motif, results in the activation of an inflammatory response or cellular immunity.33 Myeloid inhibitory C-type lectin-like receptors were highly expressed in immature DCs and required SHP-1 and SHP-2 for ITIM-dependent inhibitory effects.34 Because of the internalization abilities of Clec4a and Clec12a, targeting these signal-inhibitory receptors with antigen-conjugated antibodies may be a potent approach to produce antigen-specific immunity against diseases. Antigen uptake and cross-presentation by targeting Clec4a and Clec12a of human DCs efficiently boosted CD8 T cell responses.10, 35 Importantly, in the present study, the delivery of Clec4a shRNA via a gene gun seemed to delay tumor growth in a tumor-bearing mouse model. Our findings provide evidence that downregulating Celc4a2 expression using a gene gun approach may produce similar therapeutic effects as those achieved using an antibody-targeted strategy. Our approach is not only achieved by activating a cellular response but also by attenuating intrinsic inhibitory signaling. The mechanisms underlying the different approaches should be evaluated to determine their potential usefulness in cancer immunotherapy in the future.
Clec4a2 is generally expressed in B cells, monocytes, macrophages, and DCs, but Celc4a4 is expressed in mouse CD8− DCs.11 On the other hand, skin epidermal areas contain abundant Langerhans cells (LCs), resident DCs, and some CD8+ T cells.36 The biolistic gene gun has been demonstrated to effectively transfect naked DNA plasmids into the skin epidermal area and promote the migration of plasmid-received LCs and resident DCs to drain LNs.21 Therefore, skin administration of Clec4a2 shRNA via gene gun may not interfere with the function of epithelial cells or other cells without Clec4a2 expression in skin epidermal areas, but it may interfere with local LCs or DCs.
Another CLR Clec4b1, DC immunoactivating receptor (DCAR), shares high identity (91% amino acid sequence) with the extracellular domain containing Clec4a. However, clec4b has a shorter intracellular domain and lacks the ITIM motif.37 Clec4b1/DCAR, highly expressed in DCs, mediates signal activation via the associated FcR-γ chain, which has an immunoreceptor tyrosine-based activation motif.37 DCARs have been identified in mice but not in humans, and the in vivo function of DCIRs and DCARs remain largely unknown.38 Antibodies targeting Clec4a2 should be used with caution because they could cross-react with DCAR in the animal experiment. Blocking Clec4a2 expression with shRNA may provide verification to evaluate the effects of antibody preparations when conducting in vivo animal experiments. Additionally, a member of the Clec4 family also has a similar nucleotide sequence that may cause the specificity of Clec4a2 shRNA. In the present study, shClec4a-1 and shClec4a-3 exhibited anti-tumor activity, but not shClec4a-2. The results of the BLAST analysis indicated that shClec4a-2 may target both Clec4a2 and Clec4b1 (DCAR) (Figure S2). shClec4a-2 had lower knockdown activity, and it may also exert off-target on the expression of Clec4b2, which may result in poor anti-tumor efficacy compared with shClec4a-1 and shClec4a-3.
Mature DCs have antigen capture and cross-presentation capacity in vivo and in vitro.39, 40 Migratory DCs could transfer exogenous antigen to LN-resident DCs to produce a CTL response.41 Communication between different DC subsets takes place in peripheral lymphoid organs. Consistent with previous findings, we demonstrated here that Clec4a shRNA injected into distal sites from the tumor lesion successfully elicited antitumor immunity. In our Clec4a shRNA study model, it is possible that the DCs captured antigen from circulating cancer cells and then migrated from the resident site to lymphoid organs, priming CD8 immunity. Another possibility is that DCs downregulated by Clec4a retained the ability to communicate with other DC populations that have captured tumor antigen in the draining LN after maturation.
In conclusion, noninvasive delivery of Clec4a2 shRNA induced antitumor effects in a mouse subcutaneous tumor model, and these therapeutic effects can be attenuated by using a compensation Clec4a2 plasmid. Cellular immunity was essential for the therapeutic effects induced by Clec4a2 shRNA, and the numbers of tumor-infiltrating CD8+ T cells and IFN-γ expression dramatically increased after injection of Clec4a2 shRNA. This study provides new evidence of the potent role of Clec4 in DC-based cancer therapy and suggests that Clec4a2 shRNA could be a promising anticancer adjuvant.
Materials and Methods
Cell Culture
The MBT-2 murine bladder carcinoma, LL2 murine Lewis lung carcinoma, B16F1 murine melanoma, and 293T cells lines used here have been described previously.42, 43 The murine DC2.4 cell line was a kind gift from Dr. Huan-Yao Lei.44 MBT-2, LL2, and 293T cells were grown in DMEM (Gibco, Carlsbad, CA, USA), and DC2.4 cells were grown in RPMI 1640 medium (Lonza, Walkersville, MD, USA). Both media were supplemented with 10% fetal bovine serum (Gibco), 100 U/mL of penicillin, and 100 mg/mL of streptomycin (HyClone, Logan, UT, USA). The cells were maintained at 37°C in a 5% CO2 incubator.
Plasmid Preparation
Clec4a2 shRNAs and pLKO_AS1 (vector control) were obtained from the National RNAi Core Facility (Academia Sinica, Taipei, Taiwan). TurboFect reagent (Thermo Fisher Scientific, Slangerup, Denmark) was used for co-transfection. The following target sequences were used: shClec4a-1 (416) (TRCN0000077416), 5′-ACT GCT TCT TAC ATC CCT GAT-3′; shClec4a-2 (TRCN0000077417), 5′-CCC AAA GGA TTG GAG GCT ATT-3′; shClec4a-3 (TRCN0000077413), 5′-GCA GCA TAT TAG ACA CAA GAT-3′; shClec4a2 (415) (TRCN0000077415), 5′-CAA TGA ATT GAA CTG CAC AAA-3′. A full-length murine Clec4a2 coding sequence was purchased from OriGene (OriGene, Rockville, MD, USA) and constructed into a pcDNA3.1 myc/His vector (Invitrogen, Carlsbad, CA, USA). All plasmid DNA was prepared using an EndoFree Plasmid Mega Kit (QIAGEN, Montreal, CA, USA).
Animal Tumor Model
Female 6- to 8-week-old C3H/HeN and C57BL/6 mice were obtained from the Laboratory Animal Center at National Cheng Kung University (Tainan, Taiwan). All protocols in this study involving mice were approved by the Animal Welfare Committee at National Cheng Kung University. To measure the therapeutic efficacy of shClec4a against an established tumor, MBT-2 cells (1 × 106 cells in 200 μL of PBS) were injected subcutaneously (s.c.) into C3H/HeN mice. To measure the therapeutic efficacy of shClec4a against an established tumor, LL2 cells (2 × 105 cells in 200 μL of PBS) were injected s.c. into C57BL/6 mice. To measure the therapeutic efficacy of shClec4a-1 against an orthotropic tumor, B16F1 cells (2 × 105 cells in 200 μL of PBS) were injected s.c. into C57BL/6 mice. On day 8, 10 μg of shRNA plasmid or 2 μg of Clec4a2-myc (for the compensation experiment) were administered to the abdominal skin of MBT-2 tumor-bearing mice. The plasmid was first dissolved in 20 μL of water. The injections were administered on days 8, 15, and 22 using a low pressure-accelerated gene gun (BioWare Technologies, Taipei, Taiwan) with 50 psi of helium gas pressure. The tumor volume was measured using calipers and was calculated using the following formula: volume = (A2 × B × 0.5236), where A and B represent the shortest and longest diameters, respectively. The mice were sacrificed when the tumor volume exceeded 2,500 mm3 or when they were expected to shortly become moribund.
Depletion of CD8+ T Cells In Vivo
For in vivo depletion of CD8+ T cells, 200 μg of anti-CD8 antibody (clone 53-6.7, BD Biosciences) or isotype control antibody were injected intraperitoneally (i.p.) into mice on days 7, 8, 15, and 22. Approximately 90% of the CD8+ T cells were depleted, as determined by flow cytometry analysis.
Western Blotting
The following antibodies were used in western blotting: anti-myc (OP10; Calbiochem, San Diego, CA, USA), which recognizes the myc tag of Clec4a2-myc, and anti-γ-tubulin rabbit polyclonal antibody (GTX113286; GeneTex, Hsinchu, Taiwan). To evaluate the knockdown efficiency of shClec4a, 293T cells were co-transfected with 0.4 μg of the Clec4a2-myc plasmid and 1.6 μg of pLKO_AS1 or Clec4a2 shRNAs (shClec4a-1, shClec4a-2, or shClec4a-3). After 24 hr, cell lysates were prepared by treating the cells with radioimmunoprecipitation assay (RIPA) lysis buffer (0.22 M NaCl, 0.38 M Tris-HCl [pH 7.5], 0.25% sodium deoxycholate, and 1% IGEPAL-630). The protein concentration was measured using a Micro BCA protein assay reagent kit (Pierce, Rockford, IL, USA). The polyvinylidene fluoride membranes were incubated overnight at 4°C with the primary antibody in TTBS containing 1% BSA. The secondary antibody was subsequently incubated with the membranes for 1 hr at room temperature, and the membranes were then washed extensively for 30 min with TTBS at room temperature. The blots were probed with an enhanced chemiluminescence (ECL) western blot detection system and visualized with the BioSpectrum AC imaging system (UVP, CA, USA) according to the manufacturer’s instructions.
Immunohistochemistry
Tumor-bearing mice were sacrificed 3 days after the third treatment. The tumors were removed from the mice, embedded in an optimal cutting temperature compound (Sakura Finetek USA, Torrance, CA), and then cryosectioned to a thickness of 5 μm. The immune cells were detected with anti-CD4 (clone GK 1.5; BD Biosciences, San Diego, CA, USA) and anti-CD8 (clone 53-6.7; BD Biosciences) antibodies. The tumor-infiltrating immune cells were counted at a magnification of 150×. Three randomly chosen fields/samples from three mice were evaluated.
Real-Time PCR
To measure the knockdown efficiency of Clec4a2 shRNA, DC2.4 cells were transfected with plasmids for 24 hr. After 24 hr of transfection, transfected cells were treated with puromycin (2 μg/mL) for 2 day. for enrichment. To measure the immune response induced by shClec4a, 3 days after the third treatment, spleens were collected from tumor-bearing mice that received the control treatment or the shClec4a-1 treatment. Total RNA was extracted from DC2.4 cells or splenocytes using TRIzol (Invitrogen). cDNA was synthesized using Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega, Madison, WI, USA). The following hypoxanthine-guanine phosphoribosyltransferase (HPRT), IFN-γ, and IL-4 sense and antisense primer sequences were used for real-time PCR: Clec4a2 forward, 5′-GCC CAA AGG ATT GGA GGC TA-3′; Clec4a2 reverse, 5′-CTG CTC TTC CTG GCT TTG GAʹ-3′; HPRT forward, 5′-GTT GGA TAC AGG CCA GAC TTT GTT Gʹ-3′; HPRT reverse, 5′-GAT TCA ACT TGC GCT CAT CTT AGG C-3′; IFN-γ forward, 5′-AAC GCT ACA CAC TGC ATC TTG G-3′; IFN-γ reverse, 5′-CAA GAC TTC AAA GAG TCT GAG G-3′; IL-4 forward, 5′-GAA TGT ACC AGG AGC CAT ATC-3′; and IL-4 reverse, 5′-CTC AGT ACT ACG AGT AAT CCAʹ-3′. HPRT served as an internal control. Real-time PCR was performed using an 7900HT real-time PCR instrument (Applied Biosystems, Foster City, CA, USA) and Fast SYBR Green Master Mix (Applied Biosystems). The cycling conditions were 10 min at 95°C and 45 cycles at 95°C for 15 s and 60°C for 60 s. The 2ΔΔCt method was used to calculate relative RNA expression, which was normalized with HPRT expression.
In Vitro CTL Activity Assay
The tumor-bearing mice were injected with the plasmid three times as described previously. Four days after the third DNA treatment, splenic cells were harvested and grown in RPMI 1640 medium with 25 mM HEPES and L-glutamate (Gibco, Rockville, MD, USA). The medium was supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% fetal bovine serum. The splenocytes were treated with target cell lysate for induction. After 3 days of incubation, nonadherent cells were harvested for use as effector cells. The effector cells were plated with MBT-2-luciferase cells, which were used as target cells. The target cells (5 × 103/well) were incubated for 8 hr in triplicate at 37°C with serial dilutions (50:1, 25:1, and 12.5:1) of effector cells. After 8 hr, the cells were pelleted by centrifugation, and the supernatant was collected. Specific lysis was calculated based on the amount of luciferase released into the supernatant, as measured by a conventional luciferase detection system (Promega, Madison, WI, USA). The test solution (100 μL) was mixed with 100 μL of the substrate (luciferin) and placed in a MiniLumat LB9506 luminometer (EG & G Berthold, Bad Wildbad, Germany). Light emission was recorded for 10 s.
Flow Cytometry Analysis
Lymphocytes were collected from the inguinal LNs of tumor-mearing mice 3 days after the second administration of Clec4a2 shRNA and/or Neu DNA vaccine. For incubation, 5 × 105 lymphocytes were treated with MBT-2 cell lysate (5 μg). Lymphocytes were filtered through a 0.7-μm cell strainer (BD Pharmingen, San Diego, CA) and stained with BV510 rat anti-mouse CD8a (563068, BD Pharmingen) and phycoerythrin (PE)-CyTM7 rat anti-mouse IFN-γ (561040, BD Pharmingen). The BD Pharmingen transcription factor buffer set was used for intracellular staining of IFN-γ. To analyze the tumor-infiltrating immune cells, tumor tissues were collected from the mice that received the shClec4a-1 injection three times. Tumor tissues were dilacerated and enzyme-digested for signal cell suspension (1 mg/mL collagenase A [Roche Diagnostics] and 100 IU/mL type I DNase [Sigma-Aldrich] for 2 hr at 37°C and 5% CO2). Suspension cells were filtered through a 0.7-μm cell strainer and stained with PE rat anti-CD8 (553032, BD Pharmingen) and fluorescein isothiocyanate (FITC) rat anti-CD4 (561828, BD Pharmingen) antibodies. A BD LSRFortessa (BD Pharmingen) instrument was used to determine protein expression. The lymphocytes were gated based on the side and forward scatter characteristics of T cells.
Statistical Analyses
Statistical analyses of the tumor curve were done using a two-way ANOVA test. Statistical analyses of mRNA expression in the real-time PCR experiments were performed using a one-way ANOVA. Kaplan-Meier analysis of the survival rates of the mice was performed. p < 0.05 was considered significant. All statistical analyses were done using GraphPad Prism 5 software.
Author Contributions
T.-Y.W. performed most of the experiments, prepared Figures 1, 4, S1, and S2, and wrote the paper. C.-J.L. performed the animal experiments and prepared Figures 1, 2, and 3. C.-Y.L. performed the animal experiments and prepared Figure S1. Y.-H.H. performed the flow cytometry analysis. M.-C.Y. and Y.-W.C. assisted with the animal experiments and cell culture. Y.-L.C. and H.-P.H. contributed to the experimental design and statistical analysis. The experiments were supported by Y.-H.C., J.-Y.C., and M.-D.L., who contributed to the experimental design and edited the manuscript. All authors reviewed the manuscript.
Conflicts of Interest
The authors declare that they have no competing interests.
Acknowledgments
This study was supported by Taiwan Ministry of Science and Technology grants MOST 105-2325-B-006-003 and MOST 105-2320-B-006-052.
Footnotes
Supplemental Information includes two figures and can be found with this article online at https://doi.org/10.1016/j.omtn.2017.10.015.
Supplemental Information
References
- 1.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Thompson A.G., Thomas R. Induction of immune tolerance by dendritic cells: implications for preventative and therapeutic immunotherapy of autoimmune disease. Immunol. Cell Biol. 2002;80:509–519. doi: 10.1046/j.1440-1711.2002.01114.x. [DOI] [PubMed] [Google Scholar]
- 3.Palucka K., Banchereau J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellor A.L., Munn D.H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 5.Munn D.H., Mellor A.L. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vacchelli E., Aranda F., Eggermont A., Sautès-Fridman C., Tartour E., Kennedy E.P., Platten M., Zitvogel L., Kroemer G., Galluzzi L. Trial watch: IDO inhibitors in cancer therapy. OncoImmunology. 2014;3:e957994. doi: 10.4161/21624011.2014.957994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates E.E., Fournier N., Garcia E., Valladeau J., Durand I., Pin J.J., Zurawski S.M., Patel S., Abrams J.S., Lebecque S. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J. Immunol. 1999;163:1973–1983. [PubMed] [Google Scholar]
- 8.Meyer-Wentrup F., Benitez-Ribas D., Tacken P.J., Punt C.J., Figdor C.G., de Vries I.J., Adema G.J. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Wentrup F., Cambi A., Joosten B., Looman M.W., de Vries I.J., Figdor C.G., Adema G.J. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J. Leukoc. Biol. 2009;85:518–525. doi: 10.1189/jlb.0608352. [DOI] [PubMed] [Google Scholar]
- 10.Klechevsky E., Flamar A.L., Cao Y., Blanck J.P., Liu M., O’Bar A., Agouna-Deciat O., Klucar P., Thompson-Snipes L., Zurawski S. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sancho D., Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Vliet S.J., García-Vallejo J.J., van Kooyk Y. Dendritic cells and C-type lectin receptors: coupling innate to adaptive immune responses. Immunol. Cell Biol. 2008;86:580–587. doi: 10.1038/icb.2008.55. [DOI] [PubMed] [Google Scholar]
- 13.Kanazawa N., Okazaki T., Nishimura H., Tashiro K., Inaba K., Miyachi Y. DCIR acts as an inhibitory receptor depending on its immunoreceptor tyrosine-based inhibitory motif. J. Invest. Dermatol. 2002;118:261–266. doi: 10.1046/j.0022-202x.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- 14.Richard M., Thibault N., Veilleux P., Gareau-Pagé G., Beaulieu A.D. Granulocyte macrophage-colony stimulating factor reduces the affinity of SHP-2 for the ITIM of CLECSF6 in neutrophils: a new mechanism of action for SHP-2. Mol. Immunol. 2006;43:1716–1721. doi: 10.1016/j.molimm.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Lambert A.A., Barabé F., Gilbert C., Tremblay M.J. DCIR-mediated enhancement of HIV-1 infection requires the ITIM-associated signal transduction pathway. Blood. 2011;117:6589–6599. doi: 10.1182/blood-2011-01-331363. [DOI] [PubMed] [Google Scholar]
- 16.Fujikado N., Saijo S., Yonezawa T., Shimamori K., Ishii A., Sugai S., Kotaki H., Sudo K., Nose M., Iwakura Y. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat. Med. 2008;14:176–180. doi: 10.1038/nm1697. [DOI] [PubMed] [Google Scholar]
- 17.Uto T., Fukaya T., Takagi H., Arimura K., Nakamura T., Kojima N., Malissen B., Sato K. Clec4A4 is a regulatory receptor for dendritic cells that impairs inflammation and T-cell immunity. Nat. Commun. 2016;7:11273. doi: 10.1038/ncomms11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troegeler A., Mercier I., Cougoule C., Pietretti D., Colom A., Duval C., Vu Manh T.P., Capilla F., Poincloux R., Pingris K. C-type lectin receptor DCIR modulates immunity to tuberculosis by sustaining type I interferon signaling in dendritic cells. Proc. Natl. Acad. Sci. USA. 2017;114:E540–E549. doi: 10.1073/pnas.1613254114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melief C.J. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Condon C., Watkins S.C., Celluzzi C.M., Thompson K., Falo L.D., Jr. DNA-based immunization by in vivo transfection of dendritic cells. Nat. Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 21.Lin C.C., Yen M.C., Lin C.M., Huang S.S., Yang H.J., Chow N.H., Lai M.D. Delivery of noncarrier naked DNA vaccine into the skin by supersonic flow induces a polarized T helper type 1 immune response to cancer. J. Gene Med. 2008;10:679–689. doi: 10.1002/jgm.1183. [DOI] [PubMed] [Google Scholar]
- 22.Yen M.C., Lin C.C., Chen Y.L., Huang S.S., Yang H.J., Chang C.P., Lei H.Y., Lai M.D. A novel cancer therapy by skin delivery of indoleamine 2,3-dioxygenase siRNA. Clin. Cancer Res. 2009;15:641–649. doi: 10.1158/1078-0432.CCR-08-1988. [DOI] [PubMed] [Google Scholar]
- 23.Huang T.T., Yen M.C., Lin C.C., Weng T.Y., Chen Y.L., Lin C.M., Lai M.D. Skin delivery of short hairpin RNA of indoleamine 2,3 dioxygenase induces antitumor immunity against orthotopic and metastatic liver cancer. Cancer Sci. 2011;102:2214–2220. doi: 10.1111/j.1349-7006.2011.02094.x. [DOI] [PubMed] [Google Scholar]
- 24.Weng T.Y., Huang S.S., Yen M.C., Lin C.C., Chen Y.L., Lin C.M., Chen W.C., Wang C.Y., Chang J.Y., Lai M.D. A novel cancer therapeutic using thrombospondin 1 in dendritic cells. Mol. Ther. 2014;22:292–302. doi: 10.1038/mt.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoenborn J.R., Wilson C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 26.Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantoff P.W., Higano C.S., Small E.J., Whitmore J.B., Frohlich M.W., Schellhammer P.F. Re: interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J. Natl. Cancer Inst. 2012;104:1107–1109. doi: 10.1093/jnci/djs279. author reply 1109–1112. [DOI] [PubMed] [Google Scholar]
- 28.Alshamsan A., Haddadi A., Hamdy S., Samuel J., El-Kadi A.O., Uludağ H., Lavasanifar A. STAT3 silencing in dendritic cells by siRNA polyplexes encapsulated in PLGA nanoparticles for the modulation of anticancer immune response. Mol. Pharm. 2010;7:1643–1654. doi: 10.1021/mp100067u. [DOI] [PubMed] [Google Scholar]
- 29.Hobo W., Maas F., Adisty N., de Witte T., Schaap N., van der Voort R., Dolstra H. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen-specific CD8+ T cells. Blood. 2010;116:4501–4511. doi: 10.1182/blood-2010-04-278739. [DOI] [PubMed] [Google Scholar]
- 30.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiner L.M., Surana R., Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan H., Kamiya T., Suabjakyong P., Tsuji N.M. Targeting C-Type Lectin Receptors for Cancer Immunity. Front. Immunol. 2015;6:408. doi: 10.3389/fimmu.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall A.S., Willment J.A., Lin H.H., Williams D.L., Gordon S., Brown G.D. Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J. Biol. Chem. 2004;279:14792–14802. doi: 10.1074/jbc.M313127200. [DOI] [PubMed] [Google Scholar]
- 35.Hutten T.J., Thordardottir S., Fredrix H., Janssen L., Woestenenk R., Tel J., Joosten B., Cambi A., Heemskerk M.H., Franssen G.M. CLEC12A-Mediated Antigen Uptake and Cross-Presentation by Human Dendritic Cell Subsets Efficiently Boost Tumor-Reactive T Cell Responses. J. Immunol. 2016;197:2715–2725. doi: 10.4049/jimmunol.1600011. [DOI] [PubMed] [Google Scholar]
- 36.Heath W.R., Carbone F.R. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013;14:978–985. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 37.Kanazawa N., Tashiro K., Inaba K., Miyachi Y. Dendritic cell immunoactivating receptor, a novel C-type lectin immunoreceptor, acts as an activating receptor through association with Fc receptor gamma chain. J. Biol. Chem. 2003;278:32645–32652. doi: 10.1074/jbc.M304226200. [DOI] [PubMed] [Google Scholar]
- 38.Kanazawa N. Dendritic cell immunoreceptors: C-type lectin receptors for pattern-recognition and signaling on antigen-presenting cells. J. Dermatol. Sci. 2007;45:77–86. doi: 10.1016/j.jdermsci.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Drutman S.B., Trombetta E.S. Dendritic cells continue to capture and present antigens after maturation in vivo. J. Immunol. 2010;185:2140–2146. doi: 10.4049/jimmunol.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delamarre L., Holcombe H., Mellman I. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J. Exp. Med. 2003;198:111–122. doi: 10.1084/jem.20021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allan R.S., Waithman J., Bedoui S., Jones C.M., Villadangos J.A., Zhan Y., Lew A.M., Shortman K., Heath W.R., Carbone F.R. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Lin C.C., Chou C.W., Shiau A.L., Tu C.F., Ko T.M., Chen Y.L., Yang B.C., Tao M.H., Lai M.D. Therapeutic HER2/Neu DNA vaccine inhibits mouse tumor naturally overexpressing endogenous neu. Mol. Ther. 2004;10:290–301. doi: 10.1016/j.ymthe.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Weng T.Y., Yen M.C., Huang C.T., Hung J.J., Chen Y.L., Chen W.C., Wang C.Y., Chang J.Y., Lai M.D. DNA vaccine elicits an efficient antitumor response by targeting the mutant Kras in a transgenic mouse lung cancer model. Gene Ther. 2014;21:888–896. doi: 10.1038/gt.2014.67. [DOI] [PubMed] [Google Scholar]
- 44.Huang K.J., Yang Y.C., Lin Y.S., Huang J.H., Liu H.S., Yeh T.M., Chen S.H., Liu C.C., Lei H.Y. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J. Immunol. 2006;176:2825–2832. doi: 10.4049/jimmunol.176.5.2825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.