Abstract
OBJECTIVES
The aim of this study was to describe associations of the presence of lipid-rich necrotic core (LRNC) in the proximal superficial femoral artery (SFA) with lower extremity peripheral artery disease (PAD) event rates and systemic cardiovascular event rates.
BACKGROUND
LRNC in the coronary and carotid arteries is associated with adverse outcomes but has not been studied previously in lower extremity arteries.
METHODS
Participants with ankle-brachial index (ABI) values <1.00 were identified from Chicago medical centers and followed annually. Magnetic resonance imaging was used to characterize SFA atherosclerotic plaque at baseline. Medical records for hospitalizations and procedures after baseline were adjudicated for lower extremity revascularization, amputation, and critical limb ischemia and also for new coronary events, ischemic stroke, and mortality.
RESULTS
Of 254 participants with PAD, 62 (24%) had LRNC and 149 (59%) had calcium in the SFA at baseline. Cox regression analyses were adjusted for age, sex, race, comorbidities, baseline ABI, and other confounders. SFA LRNC was associated with an increased incidence of the combined outcome of lower extremity amputation, critical limb ischemia, ABI decline >0.15, and revascularization at 47-month follow-up (hazard ratio: 2.18; 95% confidence interval: 1.27 to 3.75; p = 0.005). The association of SFA LRNC with PAD events was maintained even when this combined outcome excluded lower extremity revascularization (hazard ratio: 2.58; 95% confidence interval: 1.25 to 5.33; p = 0.01). LRNC in the SFA was not associated with all-cause mortality, acute coronary events, or stroke.
CONCLUSIONS
Among patients with PAD, LRNC in the SFA was associated with higher rates of clinical PAD events, and this association was independent of ABI. Further study is needed to determine whether interventions that reduce SFA LRNC prevent PAD events.
Keywords: femoral artery, lipid rich necrotic core, MRI, vascular medicine
Coronary artery atherosclerotic plaque with a lipid-rich necrotic core (LRNC) and thin fibrous cap is associated with plaque rupture, thrombus formation, and an acute coronary event or progression of coronary artery atherosclerosis (1–4). Similar associations have been reported for the presence of LRNC in the carotid arteries and subsequent cerebrovascular events (5–8). However, the significance of LRNC in the lower extremity arteries is unknown.
We studied whether the presence of magnetic resonance imaging (MRI)–measured LRNC in superficial femoral artery (SFA) plaque is associated with higher rates of lower extremity peripheral artery disease (PAD) events, including amputation, hospitalization for critical limb ischemia, significant ankle-brachial index (ABI) decline, and lower extremity revascularization. We hypothesized that compared with the absence of LRNC, the presence of LRNC in the SFA would be associated with a higher rate of PAD events. We also studied whether MRI-detected calcium in SFA plaque and whether greater SFA plaque volume and smaller SFA luminal area were associated with higher PAD event rates.
Preliminary evidence suggests that local plaque characteristics may also provide information about risk for cardiovascular events in distant artery beds (9–14). Therefore, we studied whether LRNC and calcium in the SFA were associated with higher rates of acute coronary events, ischemic stroke, and all-cause mortality compared with the absence of LRNC or calcium in the SFA. We also studied whether greater MRI-measured plaque volume in the SFA and smaller luminal area in the SFA were associated with higher rates of acute coronary events, ischemic stroke, and mortality.
METHODS
SUBJECTS
Participants were part of the WALCS (Walking and Leg Circulation Study) III cohort, a longitudinal observational study designed to examine the association of MRI-measured atherosclerotic plaque characteristic in the SFA with functional impairment and decline in men and women with PAD (15). Enrollment occurred between October 26, 2007, and April 2010 (15,16). Participants were identified from among all patients diagnosed with PAD in the noninvasive vascular laboratories or in vascular surgery, cardiology, and/or general medical practices at 4 Chicago-area hospitals according to our Institutional Review Board approved methods. We also invited patients in a general medicine practice age 70 years and older who did not have histories of PAD to be screened with the ABI. Those with ABI values <1.00 were invited to participate. The Institutional Review Boards at all participating sites approved the protocol. Participants gave written informed consent. MRI was performed between January 2008 and April 2010. Because MRI data collection initially required more time than anticipated, between January 2008 and May 2008, a randomly selected 50% subset of participants underwent plaque composition imaging. Once MRI data collection had become more efficient, all participants underwent MRI for plaque composition (between June 2008 and April 2010) (16).
INCLUSION AND EXCLUSION CRITERIA
The inclusion criterion for WALCS III was an ABI <1.00 (17–19).
WALCS III exclusion criteria have been described (15,16) and are summarized here. Potential participants with dementia and those with Mini-Mental Status Examination scores <23 were excluded because it was unclear whether they could answer questions accurately (20). Nursing home residents, wheelchair-bound patients, and patients with foot or leg amputations were excluded because of their impaired functioning. Non-English-speaking patients were excluded. Patients with recent major surgery or contraindications to MRI were excluded. Potential participants requiring oxygen, those who stopped a 6-min walk test because of dyspnea, and those with severe knee osteoarthritis were excluded (21).
ABI MEASUREMENT
The ABI was used to document the presence and severity of PAD. After participants rested supine for 5 min, a handheld Doppler probe (Nicolet Vascular Pocket-Dop II, Natus Medical, Golden, Colorado) was used to measure systolic pressure in the right brachial, dorsalis pedis, and posterior tibial arteries and left dorsalis pedis, posterior tibial, and brachial arteries. Pressure measurements were repeated in reverse order. The ABI was calculated in each leg by dividing average pressures in that leg by the average of the 4 brachial pressures (22). The ABI in the leg in which MRI was measured was used for analyses.
MRI
We imaged the SFA of the leg with the lowest ABI. MRI data were obtained with a 1.5-T platform (Espree, Siemens Medical Solutions, Malvern, Pennsylvania) using a 4-element phased-array surface coil (Nova Medical, Wilmington, Massachusetts). We imaged the proximal SFA because its superficial location was more amenable to high-quality images than the distal SFA. The bifurcation of the common femoral artery was the reference point. Images were collected with a standard, proton density–weighted turbo spin echo acquisition (repetition time [TR] 2,160 ms; echo time [TE] 8 ms; bandwidth 230 Hz/pixel; turbo factor 15). The field of view was 120 × 120 mm2, and images were acquired on a 192 × 192 matrix to yield an in-plane spatial resolution of 0.625 × 0.625 mm2. Three signal averages were acquired.
Twelve contiguous 2.5-mm cross-sectional images in the short-axis plane were obtained, beginning at the common femoral artery bifurcation into the SFA and moving distally using 2-dimensional bright-blood time-of-flight and proton density–weighted images. Bright-blood 2-dimensional time-of-flight images (TR 31.0 ms; TE 7.2 ms) were registered to the proton density–weighted images and acquired using an identical field of view, slice thickness, and imaging matrix. This method has excellent test-retest reliability (15,23). Additional turbo spin echo images were acquired with TR and TE adjusted to provide T1-weighted (TR 800 ms; TE 8 ms) and T2-weighted (TR 2,160 ms; TE 50 ms) images, respectively. These images were prescribed with identical thickness, location, number of slices, and signal saturation (fat saturation and regional blood saturation) as the proton density–weighted images. The additional contrast weighting was used for plaque characterization.
For analysis of plaque area, wall thickness, and luminal area, 2 physician readers with cardiovascular imaging training used CASCADE software (University of Washington, Seattle, Washington). Poor-quality images were excluded, using established criteria (24). Readers traced the outer boundary and the lumen of each cross-sectional image to quantify wall thickness, wall area, and luminal area. Images for each participant were assigned to 1 primary reviewer, and tracings of arterial boundaries were reviewed by the second reviewer to ensure accuracy. Disagreements were resolved via discussion and consensus. Plaque area measurements were normalized for artery size by dividing each measure by the median of the total vessel area (15,25). Luminal area was normalized per slice using total vessel area because of variation in vessel dimensions due to patient size. An assessment of test-retest reliability among a 6% subsample showed coefficient of variation percentage values of 5.8 and 8.9 for mean and maximum plaque area, respectively; the values were 7.9 for mean and 12.9 for minimum percentage luminal area, respectively (15).
The presence of LRNC and calcium was determined at each artery cross section using validated methods (26–28). Images were evaluated at the University of Washington Core Reading Center in the Vascular Imaging Laboratory by 2 readers. Tissue types were identified on the basis of signal intensities relative to the sartorius muscle. LRNC is hypointense on T2-weighted images, isointense or slightly hyperintense on T1-weighted images, and isointense on proton density–weighted and time-of-flight images. Calcium is hypointense on T1-weighted, T2-weighted, and time-of-flight images.
BASELINE COMORBIDITIES
Comorbidities assessed at baseline were diabetes mellitus, hypertension, myocardial infarction (MI), and angina. Comorbidities were identified and confirmed using algorithms developed for the Women’s Health and Aging Study (29). The algorithms combine data from patient report, medical record review, medications, laboratory values, and a primary care physician questionnaire. Hypertension was defined as patient report of physician-diagnosed hypertension or physician designation of hypertension on the primary care physician questionnaire.
OTHER BASELINE MEASURES
Height and weight were measured at baseline. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Low-density lipoprotein cholesterol was determined using a homogenous direct method from Roche Diagnostics (Indianapolis, Indiana). High-density lipoprotein cholesterol was measured using a direct enzymatic colorimetric assay. Cigarette smoking history was determined by self-report. Participants brought medication bottles or medication lists to their study visit. A study investigator (M.M.M.) identified which participants were taking statin and antiplatelet medications. The presence of exertional leg symptoms and classic symptoms of intermittent claudication were defined using the San Diego Claudication Questionnaire (30,31).
ADJUDICATION OF LOWER EXTREMITY AND CARDIOVASCULAR EVENTS
Participants returned to the medical center for follow-up 1, 2, and 4 years after baseline. At follow-up, participants were interviewed to identify new hospitalizations and underwent repeat ABI measurement. At 3-year follow-up, participants were interviewed by telephone to identify new hospitalizations. Medical records for all hospitalizations after baseline were retrieved and reviewed independently by 2 investigators (M.M.M. and J.W.) using an abstraction form. Differences between reviewers were resolved by discussion. Reviewers were blinded to MRI measures.
Lower extremity PAD events
Lower extremity events indicating PAD progression were defined as medical record–documented hospitalization for critical limb ischemia, amputation, or lower extremity revascularization. Decline in the ABI by >0.15 in the leg with the MRI measurement was defined as PAD progression (31).
Adjudication of acute coronary events
Acute coronary events were defined as MI, hospitalization for unstable angina, and cardiac death. Additional outcomes were ischemic stroke and all-cause mortality. Criteria for MI were derived from the ARIC (Atherosclerosis Risk In Communities) and MESA (Multi-Ethnic Study of Atherosclerosis) studies (32,33). An acute MI required 2 of the following criteria: 1) chest pain; 2) abnormal electrocardiographic findings consistent with an MI (ST-segment elevation, new left bundle branch block, new Q waves); and 3) abnormal cardiac enzymes (troponin >2 times the upper limit of normal). Criteria for unstable angina were derived from the MESA and LIFE (Lifestyle Interventions and Independence for Elders) studies (33,34). Unstable angina was defined as nonelective hospital admission with a discharge diagnosis of acute coronary ischemia that was not definite or probable MI. Clinical symptoms were required. In addition, 1 of the following was required: 1) treatment with nitrates, heparin, or beta-blockers; 2) coronary revascularization during the hospitalization; 3) >70% obstruction of a coronary artery by angiography during hospitalization; or 4) an electrocardiogram showing horizontal or down-sloping ST-segment depression or abnormal ST-segment elevation >1 mm, and these findings were present only during chest pain. Acute coronary death consisted of definite fatal MI, definite coronary heart disease death, and possible coronary heart disease death (33,34).
Ischemic stroke and mortality
The outcome of ischemic stroke was based on criteria from the LIFE study (34) and consisted of acute onset of neurological symptoms combined with an imaging study consistent with acute ischemic stroke. Deaths were ascertained from medical records and from proxies if medical records were not available.
STATISTICAL ANALYSES
Baseline characteristics were compared between PAD participants with versus without LRNC and with versus without calcium at baseline. Student t tests were used for continuous variables, and chi-square tests were used for categorical variables. Kaplan-Meier analyses compared cumulative rates of PAD events, acute coronary events, stroke, and all-cause mortality between participants with and those without LRNC and with and those without calcium, using the log-rank test for statistical significance. PAD events, acute coronary events, stroke, and all-cause mortality were compared across tertiles of mean luminal area and mean plaque area using Kaplan-Meier curves and log-rank tests.
Associations of plaque LRNC and plaque calcium with PAD events, cardiovascular outcomes, and mortality were evaluated using Cox proportional hazards models, adjusting for age, sex, race, diabetes, hypertension, history of MI, angina, cholesterol values, BMI, smoking, statin use, antiplatelet therapy, and baseline ABI. Similarly, associations of mean plaque area tertiles and mean luminal area tertiles with outcomes were evaluated using Cox proportional hazards models, adjusting for age, sex, race, diabetes, hypertension, history of MI, angina, cholesterol values, BMI, smoking, statins, antiplatelet therapy, and baseline ABI. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
In total, 473 participants with PAD underwent MRI of the SFA. Sixteen were excluded because of poor image quality. Of the remaining 457 participants, 305 underwent additional MRI data collection (T1- and T2-weighted imaging) for plaque composition. Of these, 13 (4.3%) did not return for any follow-up visits and were considered lost to follow-up, 37 (12.0%) were excluded because of missing data on cholesterol, and 1 (0.32%) was excluded because of missing data on smoking, leaving 254 for plaque composition analyses. Median follow-up duration was 47 months. For the analyses of plaque volume and luminal area, of the 457 participants with valid MRI data, 20 (4.0%) did not return for any follow-up visits, 57 (12.0%) were excluded because of missing data on cholesterol, and 1 (0.22%) was excluded because of missing data on smoking, leaving 379 for analyses of plaque and luminal area. Three participants with PAD were identified from ABI screening of general medicine patients age >70 years, and 2 of these participants underwent MRI of plaque composition.
Of 254 participants with plaque composition data, 62 (24.4%) had LRNC and 149 (58.7%) had calcium in the SFA. Seventy (27.6%) had classic symptoms of intermittent claudication, 41 (16.1%) were asymptomatic, and 143 (56.3%) had exertional leg symptoms that were not consistent with classic intermittent claudication. LRNC in the SFA was associated with a lower prevalence of former smokers and higher prevalence rates of current smoking and male sex compared with absence of LRNC (Table 1). LRNC was also associated with lower high-density lipoprotein cholesterol levels. Calcium was associated with older age, lower BMI, and higher high-density lipoprotein and was more prevalent in men, former smokers, and those using statins at baseline (Table 1).
TABLE 1.
Associations of Superficial Femoral Artery Plaque Composition With Baseline Characteristics in Participants With Peripheral Artery Disease
| Total (n = 254) |
Absence of LRNC (n = 192) |
Presence of LRNC (n = 62) |
p Value | Absence of Calcium (n = 105) |
Presence of Calcium (n = 149) |
p Value | |
|---|---|---|---|---|---|---|---|
| Age, yrs | 68.53 ± 9.96 | 68.72 ± 9.33 | 67.94 ± 11.76 | 0.591 | 66.15 ± 11.69 | 70.20 ± 8.17 | 0.001 |
|
| |||||||
| ABI | 0.67 ± 0.17 | 0.68 ± 0.18 | 0.66 ± 0.16 | 0.308 | 0.70 ± 0.18 | 0.66 ± 0.17 | 0.054 |
|
| |||||||
| Male | 181 (71.3) | 130 (67.7) | 51 (82.3) | 0.028 | 67 (63.8) | 114 (76.5) | 0.028 |
|
| |||||||
| African American | 83 (32.7) | 60 (31.3) | 23 (37.1) | 0.393 | 37 (35.2) | 46 (30.9) | 0.465 |
|
| |||||||
| Never smoker | 27 (10.6) | 18 (9.4) | 9 (14.5) | 0.253 | 19 (18.1) | 8 (5.4) | 0.001 |
|
| |||||||
| Former smoker | 163 (64.2) | 133 (69.3) | 30 (48.4) | 0.003 | 58 (55.2) | 105 (70.5) | 0.013 |
|
| |||||||
| Current smoker | 64 (25.2) | 41 (21.4) | 23 (37.1) | 0.013 | 28 (26.7) | 36 (24.2) | 0.651 |
|
| |||||||
| Diabetes | 108 (42.5) | 78 (40.6) | 30 (48.4) | 0.282 | 44 (41.9) | 64 (43.0) | 0.868 |
|
| |||||||
| BMI, kg/m2 | 29.89 ± 6.54 | 30.14 ± 6.72 | 29.10 ± 5.92 | 0.277 | 31.09 ± 7.29 | 29.04 ± 5.82 | 0.013 |
|
| |||||||
| HDL-C, mg/dl | 49.01 ± 17.40 | 50.37 ± 18.36 | 44.80 ± 13.27 | 0.028 | 46.43 ± 14.08 | 50.83 ± 19.24 | 0.047 |
|
| |||||||
| LDL-C, mg/dl | 92.08 ± 31.97 | 93.43 ± 33.16 | 87.90 ± 27.83 | 0.237 | 95.56 ± 32.54 | 89.63 ± 31.45 | 0.146 |
|
| |||||||
| Pulmonary disease | 95 (37.4) | 70 (36.5) | 25 (40.3) | 0.585 | 40 (38.1) | 55 (36.9) | 0.848 |
|
| |||||||
| Cancer | 40 (15.7) | 32 (16.7) | 8 (12.9) | 0.479 | 16 (15.2) | 24 (16.1) | 0.851 |
|
| |||||||
| Angina | 54 (21.3) | 41 (21.4) | 13 (21.0) | 0.948 | 17 (16.2) | 37 (24.8) | 0.097 |
|
| |||||||
| MI | 44 (17.3) | 30 (15.6) | 14 (22.6) | 0.208 | 15 (14.3) | 29 (19.5) | 0.283 |
|
| |||||||
| Stroke | 42 (16.5) | 31 (16.1) | 11 (17.7) | 0.769 | 18 (17.1) | 24 (16.1) | 0.827 |
|
| |||||||
| Heart failure | 37 (14.6) | 31 (16.1) | 6 (9.7) | 0.209 | 16 (15.2) | 21 (14.1) | 0.799 |
|
| |||||||
| Hypertension | 237 (93.3) | 182 (94.8) | 55 (88.7) | 0.139 | 98 (93.3) | 139 (93.3) | 0.989 |
|
| |||||||
| On statin medication at baseline | 196 (77.2) | 152 (79.2) | 44 (71.0) | 0.181 | 74 (70.5) | 122 (81.9) | 0.033 |
|
| |||||||
| Antiplatelet therapy | 205 (80.7) | 156 (81.3) | 49 (79.0) | 0.700 | 79 (75.2) | 126 (84.6) | 0.064 |
|
| |||||||
| Leg symptoms | |||||||
| Intermittent claudication | 70 (27.6) | 60 (31.3) | 10 (16.1) | 24 (22.9) | 46 (30.9) | ||
| Exertional leg symptoms other than intermittent claudication | 143 (56.3) | 104 (54.2) | 39 (62.9) | 0.058 | 59 (56.2) | 84 (56.4) | 0.135 |
| Asymptomatic | 41 (16.1) | 28 (14.6) | 13 (21.0) | 22 (21.0) | 19 (12.8) | ||
Values are mean ± SD for continuous variables and n (%) for categorical variables. ABI values are from the leg in which MRI measures were obtained.
ABI = ankle-brachial index; BMI = body mass index; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; LRNC = lipid-rich necrotic core; MI = myocardial infarction; MRI = magnetic resonance imaging.
Among participants with plaque composition data, 66 experienced 1 or more PAD events during followup. Of these, 38 (58%) had lower extremity revascularization, 8 (12%) had amputation or critical limb ischemia, and 31 (47%) experienced ABI declines of >0.15 compared with baseline. Seventy-five participants had acute coronary events, ischemic stroke, or death. Of these, 38 (51%) had acute coronary events, 15 (20%) had ischemic stroke, and 45 (60%) died of any cause during follow-up.
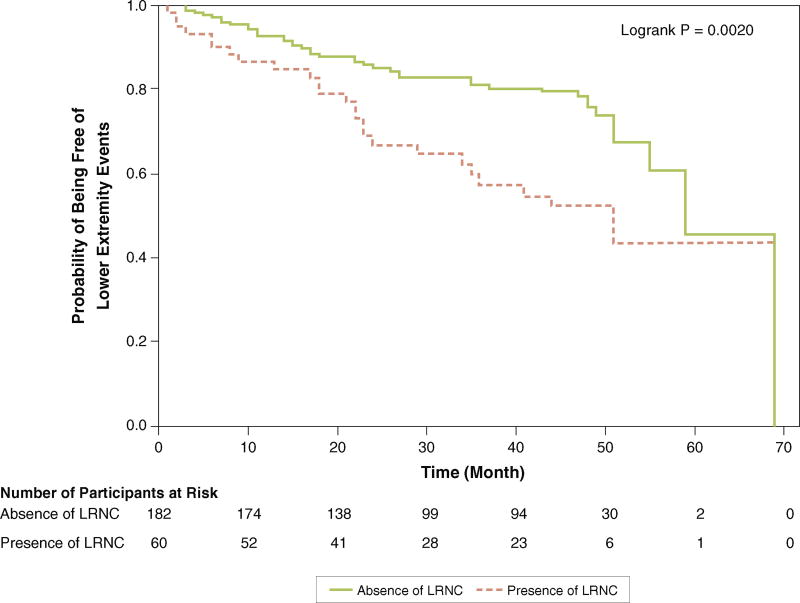
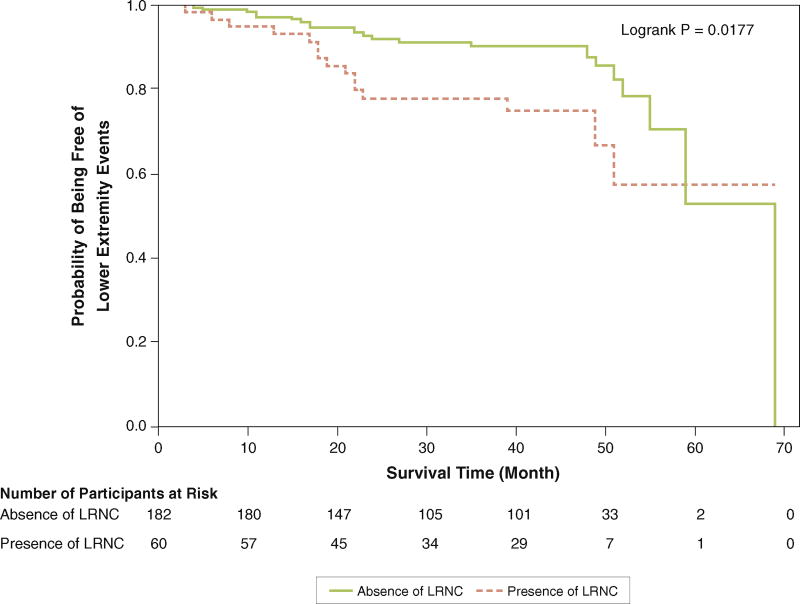
Participants with LRNC in the SFA had higher rates of PAD events, measured by the combined outcome of lower extremity revascularization, amputation, hospitalization for critical limb ischemia, or ABI decline >0.15, compared with those without LRNC in the SFA (Figure 1, Table 2). This association remained after excluding lower extremity revascularization from the composite endpoint (Figure 2, Table 2). There were no significant associations of LRNC with acute coronary events, ischemic stroke, or all-cause mortality (Table 2).
FIGURE 1. Association of Presence Versus Absence of Lipid-Rich Necrotic Core and Subsequent Rates of Lower Extremity Events.
Lower extremity events are defined as the first occurrence of 1 of the following: lower extremity amputation, critical limb ischemia, ankle-brachial index decline of >0.15, or lower extremity revascularization. LRNC = lipid-rich necrotic core.
TABLE 2.
Associations of Lipid-Rich Necrotic Core and Calcium in the Superficial Femoral Artery With Subsequent Lower Extremity Peripheral Artery Disease Events, Acute Coronary Events, Ischemic Stroke, and All-Cause Mortality
| LRNC Present in SFA (n = 62) |
LRNC Absent in SFA (n = 192) |
p Value | Calcium in SFA (n = 149) |
No Calcium in SFA (n = 105) |
p Value | |
|---|---|---|---|---|---|---|
| Lower extremity PAD events | ||||||
|
| ||||||
| ≥1 PAD event* | 25 (41.7) | 41 (22.5) | 0.004 | 33 (23.4) | 33 (32.7) | 0.110 |
| ≥1 PAD event other than revascularization† | 15 (25.0) | 23 (12.6) | 0.022 | 17 (12.1) | 21 (20.8) | 0.065 |
| Any lower extremity revascularization | 16 (25.8) | 22 (11.5) | 0.006 | 19 (12.8) | 19 (18.1) | 0.240 |
| Amputation or critical limb ischemia | 4 (6.5) | 4 (2.1) | 0.102 | 4 (2.7) | 4 (3.8) | 0.721 |
| ABI decline >0.15 | 11 (18.6) | 20 (11.1) | 0.131 | 13 (9.4) | 18 (17.8) | 0.053 |
|
| ||||||
| Acute coronary events, ischemic stroke, all-cause mortality | ||||||
|
| ||||||
| Coronary events, stroke, or all-cause mortality‡ | 18 (29.0) | 57 (29.7) | 0.922 | 44 (29.5) | 31 (29.5) | 0.999 |
| Acute coronary events | 10 (16.1) | 28 (14.6) | 0.767 | 16 (10.7) | 22 (21.0) | 0.025 |
| Ischemic stroke | 3 (4.8) | 12 (6.3) | 1.000 | 8 (5.4) | 7 (6.7) | 0.666 |
| All-cause mortality | 12 (19.4) | 33 (17.2) | 0.698 | 29 (19.5) | 16 (15.2) | 0.385 |
Values are n (%).
Patients who experienced 1 or more lower extremity PAD events, consisting of lower extremity amputation, hospitalization for critical limb ischemia, ABI decline of >0.15, or lower extremity revascularization.
Patients with 1 or more PAD events, consisting of lower extremity amputation, hospitalization for critical limb ischemia, or ABI decline of >0.15.
Participants who experienced 1 or more events. Participants may have had more than one type of event.
PAD = peripheral artery disease; SFA = superficial femoral artery; other abbreviations as in Table 1.
FIGURE 2. Association of Presence Versus Absence of Lipid-Rich Necrotic Core and Subsequent Rates of Lower Extremity Events Other Than Lower Extremity Revascularization.
Lower extremity events are defined as the first occurrence of 1 of the following: lower extremity amputation, critical limb ischemia, or ankle-brachial index decline of >0.15. LRNC = lipid-rich necrotic core.
Adjusting for age, sex, race, cholesterol values, smoking, BMI, history of MI, angina, hypertension, diabetes, statin use, antiplatelet therapy, and baseline ABI, LRNC in the SFA was associated with an increased hazard for a PAD event (p = 0.005) and for any PAD event other than revascularization (p = 0.010) (Table 3). LRNC in the SFA was also associated with an increased hazard for lower extremity revascularization (p = 0.013) (Table 3). There were no associations of SFA LRNC with other individual PAD events, acute coronary events, ischemic stroke, or all-cause mortality (Table 3).
TABLE 3.
Adjusted and Unadjusted Associations of Lipid-Rich Necrotic Core in the Superficial Femoral Artery With Subsequent Lower Extremity Peripheral Artery Disease Events, Acute Coronary Events, Ischemic Stroke, and All-Cause Mortality
| Model 1, Unadjusted | Model 2, Fully Adjusted | |||
|---|---|---|---|---|
|
|
|
|||
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Lower extremity PAD events | ||||
|
| ||||
| Any PAD event* | 2.15 (1.30–3.54) | 0.003 | 2.18 (1.27–3.75) | 0.005 |
| Any PAD event excluding revascularization† | 2.16 (1.12–4.16) | 0.021 | 2.58 (1.25–5.33) | 0.010 |
| Lower extremity revascularization | 2.47 (1.30–4.70) | 0.006 | 2.42 (1.20–4.86) | 0.013 |
| Amputation or hospitalization for critical limb ischemia. | 3.12 (0.78–12.49) | 0.107 | 2.75 (0.56–13.61) | 0.215 |
| ABI decline >0.15 | 1.68 (0.80–3.53) | 0.169 | 2.20 (0.97–5.01) | 0.06 |
|
| ||||
| Coronary and cerebrovascular events and all-cause mortality | ||||
|
| ||||
| Acute coronary event, ischemic stroke, or all-cause mortality | 0.97 (0.57–1.65) | 0.911 | 0.86 (0.49–1.51) | 0.608 |
| Acute coronary events | 1.11 (0.54–2.29) | 0.771 | 0.86 (0.38–1.94) | 0.711 |
| Ischemic stroke | 0.76 (0.21–2.69) | 0.671 | 0.80 (0.21–3.10) | 0.752 |
| All-cause mortality | 1.12 (0.58–2.18) | 0.728 | 0.93 (0.45–1.89) | 0.831 |
Model 2 adjusted for age, sex, race, diabetes, hypertension, history of MI, angina, LDL-C, HDL-C, BMI, cigarette smoking, statin use, antiplatelet therapy, and baseline ABI.
Patients who experienced 1 or more lower extremity PAD events, consisting of lower extremity amputation, hospitalization for critical limb ischemia, ABI decline of >0.15, or lower extremity revascularization.
Patients with 1 or more PAD events, consisting of lower extremity amputation, hospitalization for critical limb ischemia, or ABI decline of >0.15.
In unadjusted analyses, SFA calcium was not associated with PAD events (Table 2). However, calcium in the SFA was associated with a lower rate of acute coronary events (Table 2). Adjusting for age, sex, race, cholesterol values, smoking, BMI, MI, angina, hypertension, diabetes, statin use, antiplatelet therapy, and baseline ABI, there was no association of SFA calcium with subsequent PAD events or acute coronary events, ischemic stroke, or all-cause mortality (Table 4). There was no association of SFA plaque area or luminal area with any outcome (Table 5).
TABLE 4.
Adjusted and Unadjusted Associations of Calcium in the Superficial Femoral Artery With Subsequent Lower Extremity Peripheral Artery Disease Events, Acute Coronary Events, Ischemic Stroke, and All-Cause Mortality
| Model 1, Unadjusted | Model 2, Fully Adjusted | |||
|---|---|---|---|---|
|
|
|
|||
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Lower extremity PAD events | ||||
|
| ||||
| Any PAD event* | 0.68 (0.42–1.10) | 0.12 | 0.70 (0.40–1.21) | 0.203 |
| Any PAD event excluding revascularization† | 0.60 (0.31–1.13) | 0.115 | 0.73 (0.34–1.59) | 0.432 |
| Lower extremity revascularization | 0.70 (0.37–1.32) | 0.265 | 0.71 (0.35–1.48) | 0.365 |
| Amputation or hospitalization for critical limb ischemia | 0.73 (0.18–2.93) | 0.66 | 2.00 (0.35–11.32) | 0.433 |
| ABI decline >0.15 | 0.56 (0.28–1.16) | 0.118 | 0.53 (0.22–1.32) | 0.175 |
|
| ||||
| Coronary events, ischemic stroke, and all-cause mortality | ||||
|
| ||||
| Acute coronary event, ischemic stroke, or all-cause mortality | 1.05 (0.66–1.66) | 0.839 | 1.11 (0.66–1.87) | 0.69 |
| Acute coronary events | 0.54 (0.28–1.02) | 0.058 | 0.59 (0.28–1.25) | 0.169 |
| Ischemic stroke | 0.85 (0.31–2.33) | 0.747 | 0.72 (0.21–2.47) | 0.596 |
| All-cause mortality | 1.41 (0.77–2.60) | 0.267 | 1.66 (0.82–3.38) | 0.159 |
Model 2 adjusted for age, sex, race, diabetes, hypertension, history of MI, angina, LDL-C, HDL-C, BMI, cigarette smoking, statin use, antiplatelet therapy, and baseline ABI.
Patients who experienced 1 or more lower extremity PAD events, consisting of lower extremity amputation, hospitalization for critical limb ischemia, ABI decline of >0.15, or lower extremity revascularization.
Patients with 1 or more PAD events, consisting of lower extremity amputation, hospitalization for critical limb ischemia, or ABI decline of >0.15.
TABLE 5.
Unadjusted Associations of Plaque Quantity and Luminal Area With Cardiovascular Outcomes
| Plaque Area | Luminal Area | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Tertile 1 (Lowest Plaque Quantity) |
Tertile 2 | Tertile 3 (Highest Plaque Quantity) |
Trend p Value |
Tertile 1 (Largest Luminal Area) |
Tertile 2 | Tertile 3 (Smallest Luminal Area) |
p Value | |
| Lower extremity PAD events | ||||||||
|
| ||||||||
| ≥1 PAD event* | 37/119 (31.09) | 38/120 (31.67) | 33/120 (27.50) | 0.748 | 39/120 (32.50) | 37/120 (30.83) | 32/119 (26.89) | 0.624 |
| ≥1 PAD event other than revascularization† | 27/119 (22.69) | 24/120 (20.00) | 20/120 (16.67) | 0.504 | 28/120 (23.33) | 24/120 (20.00) | 19/119 (15.97) | 0.360 |
| Any lower extremity revascularization | 20/126 (15.87) | 18/127 (14.17) | 17/126 (13.49) | 0.858 | 20/126 (15.87) | 17/127 (13.39) | 18/126 (14.29) | 0.851 |
| Amputation or critical limb ischemia | 5/126 (3.97) | 3/127 (2.36) | 4/126 (3.17) | 0.718 | 5/126 (3.97) | 3/127 (2.36) | 4/126 (3.17) | 0.718 |
| ABI decline >0.15 | 23/118 (19.49) | 21/119 (17.65) | 16/118 (13.56) | 0.461 | 24/118 (20.34) | 20/119 (16.81) | 16/118 (13.56) | 0.381 |
|
| ||||||||
| Coronary events, ischemic stroke, and all-cause mortality | ||||||||
|
| ||||||||
| Coronary events, stroke, or all-cause mortality‡ | 29/126 (23.02) | 42/127 (33.07) | 39/126 (30.95) | 0.179 | 31/126 (24.60) | 39/127 (30.71) | 40/126 (31.75) | 0.402 |
| Acute coronary events | 17/126 (13.50) | 24/127 (18.90) | 12/126 (9.50) | 0.097 | 16/126 (12.70) | 23/127 (18.10) | 14/126 (11.10) | 0.242 |
| Ischemic stroke | 9/126 (7.14) | 7/127 (5.51) | 7/126 (5.56) | 0.826 | 9/126 (7.14) | 7/127 (5.51) | 7/126 (5.56) | 0.826 |
| All-cause mortality | 17/126 (13.49) | 26/127 (20.47) | 25/126 (19.84) | 0.279 | 21/126 (16.67) | 22/127 (17.32) | 25/126 (19.84) | 0.786 |
Values are n/N (%).
Patients who experienced 1 or more lower extremity PAD events, consisting of lower extremity amputation, hospitalization for critical limb ischemia, ABI decline of >0.15, or lower extremity revascularization.
Patients with 1 or more PAD events, consisting of lower extremity amputation, hospitalization for critical limb ischemia, or ABI decline of >0.15.
Participants who experienced 1 or more events. Participants may have had more than one type of event.
DISCUSSION
Our results indicate that the presence of LRNC in the SFA is associated with a higher rate of clinically important PAD events, measured by a composite outcome of lower extremity amputation, hospitalization for critical limb ischemia, significant ABI decline, and lower extremity revascularization. This association was observed even after excluding lower extremity revascularizations from the composite PAD outcome. Associations were independent of potential confounders, including PAD severity, measured by the ABI. After adjusting for confounders, none of the plaque measures (LRNC, calcium, or plaque burden) was associated with events that occurred remotely from the lower extremities such as acute coronary events or ischemic stroke.
Histopathologic studies from the coronary arteries demonstrate that coronary artery atherosclerotic plaque with an LRNC and a thin fibrous cap is associated with increased risk for plaque rupture and thrombus formation, resulting in an acute coronary event or progression of coronary artery atherosclerosis (1–3). Similar associations have been reported for the carotid arteries (4–8). Takaya et al. (7) reported that a larger LRNC, measured by MRI, was associated with a higher rate of ipsilateral cerebrovascular events over a mean follow-up of 38.2 months. However, to our knowledge, the clinical significance of LRNC in the SFA has not been reported previously.
Guzman et al. (35) reported that computed tomography–measured calcium in the tibial artery was associated with amputation risk in 229 patients with PAD. The finding reported here, that calcium in the SFA is not related to subsequent lower extremity events, is likely related to several factors. First, computed tomography is more sensitive for detecting calcium than MRI. Second, only a short proximal segment of the SFA was imaged in the present study, whereas the prior study shows that calcification is more prevalent in distal segments of lower extremity arteries. Our finding that plaque burden was not associated with lower extremity events is consistent with a previous study showing that plaque burden in the SFA is not associated with decline in 6-min walk (36).
Preliminary evidence supported the hypothesis that local plaque characteristics convey risk for atherosclerotic events in distant vascular beds. For example, plaque morphology and the presence of arterial LRNC is typically consistent throughout the vascular tree (11–13). In a case-control study, Underhill et al. (14) compared MRI-measured carotid artery characteristics between 97 participants with >50% coronary artery stenosis and 94 participants with no angiographic evidence of coronary artery disease. Participants with coronary artery disease had a higher prevalence of carotid artery LRNC than those without coronary artery disease. A separate study of community-dwelling men and women in MESA without clinically evident cardiovascular disease reported that MRI-measured carotid intima-media thickness and the presence of MRI-detected carotid artery LRNC or calcium were each associated with increased rates of cardiovascular events (37). However, a recent histopathologic study of 176 arterial segments from 60 amputated limbs in patients with PAD reported intimal thickening without atheromatous changes in 68% of lower extremity arteries. In some arteries, intimal thickening was so severe that it occluded the artery, but intimal thickening was not accompanied by atheromatous change (38). This study suggests that the arterial histopathology in the lower extremity arteries of PAD patients differs from arterial histopathology in the coronary or cerebrovascular arteries.
STUDY LIMITATIONS
First, although the sensitivity and specificity of MRI for plaque composition in the carotid artery, including calcium and LRNC, range from 84% to 100% (26), MRI is not optimally sensitive for detecting arterial calcium.
Second, we imaged a proximal segment of the SFA in the leg with the lowest ABI. Although a previous study showed that the presence of LRNC in 1 lower extremity artery is highly correlated with LRNC in the opposite leg (13), it is conceivable that the composition of plaque in the short proximal segment of the SFA that we imaged does not reflect plaque composition in the rest of the lower extremity artery bed.
Third, the outcome of lower extremity revascularization is determined in part by clinician determinations about patient disability from PAD and ability to safely undergo revascularization. However, LRNC predicted lower extremity events even after revascularizations were excluded.
Fourth, we did not collect data on whether calcium was eccentric or concentric within the artery.
Fifth, we did not collect data on Rutherford classification.
CONCLUSIONS
Among patients with PAD, LRNC in the SFA was associated with higher rates of lower extremity PAD events, independent of PAD severity, measured by the ABI. Further study is needed to determine whether interventions that reduce LRNC in the SFA can prevent lower extremity outcomes in PAD.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Among patients with lower extremity PAD who underwent MRI of the proximal segment of the SFA, 24% had LRNC in the SFA. The presence of LRNC in the SFA was associated with a higher incidence of the combined outcome of lower extremity amputation, critical limb ischemia, significant ABI decline, and lower extremity revascularization over 47-month follow-up.
TRANSLATIONAL OUTLOOK
Further study is needed to confirm that LRNC in the SFA is an important predictor of adverse lower extremity events, independent of the ABI. Further study is also needed to determine whether interventions that reduce SFA LRNC prevent lower extremity events in people with PAD.
Acknowledgments
This work was funded by the National Heart, Lung, and Blood Institute (grants R01-HL109244 and R01-HL083064). Dr. Yuan has received grant funding from Philips Healthcare and is a member of the Philips Radiology Medical Advisory Network. Drs. Kramer and McDermott have received research funding from Novartis. Mr. Hippe has received grant funding from Philips Healthcare and GE Healthcare.
ABBREVIATIONS AND ACRONYMS
- ABI
ankle-brachial index
- BMI
body mass index
- LRNC
lipid-rich necrotic core
- MI
myocardial infarction
- MRI
magnetic resonance imaging
- PAD
peripheral artery disease
- SFA
superficial femoral artery
- TE
echo time
- TR
repetition time
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 2.Schaar JA, Muller JE, Falk E, et al. Terminology for high-risk and vulnerable coronary artery plaques: report of a meeting on the vulnerable plaque, June 17 and 18 2003. Santorini, Greece. Eur Heart J. 2004;25:1077–82. doi: 10.1016/j.ehj.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–71. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Fayad ZA, Moreno PR, Poon M, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque. Part II: approaches by noninvasive computed tomographic/magnetic resonance imaging. J Am Coll Cardiol. 2005;46:1209–18. doi: 10.1016/j.jacc.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 5.Gronholdt ML. Ultrasound and lipoproteins as predictors of lipid-rich, rupture-prone plaques in the carotid artery. Arterioscler Thromb Vasc Biol. 1999;19:2–13. doi: 10.1161/01.atv.19.1.2. [DOI] [PubMed] [Google Scholar]
- 6.Gronholdt ML, Nordestgaard BG, Schroeder TV, Vorstrup S, Sillesen H. Ultrasonic echolucent carotid plaques predict future strokes. Circulation. 2001;104:68–73. doi: 10.1161/hc2601.091704. [DOI] [PubMed] [Google Scholar]
- 7.Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events. Stroke. 2006;37:818–23. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 8.Oikawa M, Ota H, Takaya N, Miller Z, Hatsukami TS, Yuan C. Carotid magnetic resonance imaging. A window to study atherosclerosis and identify high-risk plaques. Circ J. 2009;73:1765–73. doi: 10.1253/circj.cj-09-0617. [DOI] [PubMed] [Google Scholar]
- 9.De Kleijn DPV, Moll FL, Hellings WE, et al. Local atherosclerotic plaques are a source of prognostic biomarkers for adverse cardiovascular events. Arterioscler Thromb Vasc Biol. 2010;30:612–9. doi: 10.1161/ATVBAHA.109.194944. [DOI] [PubMed] [Google Scholar]
- 10.Hurks R, Peeters W, Derksen WJM, et al. Biobanks and the search for predictive biomarkers of local and systemic outcome in atherosclerotic disease. Thromb Haemost. 2009;101:48–54. [PubMed] [Google Scholar]
- 11.Mauriello A, Sangiorgi G, Fratoni S, et al. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree: a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol. 2005;45:1585–93. doi: 10.1016/j.jacc.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Rothwell PM, Villagra R, Gibson R, Donders RC, Warlow CP. Evidence of a chronic systemic cause of instability of atherosclerotic plaques. Lancet. 2000;355:19–24. doi: 10.1016/s0140-6736(99)04470-0. [DOI] [PubMed] [Google Scholar]
- 13.Vink A, Schoneveld AH, Richard W, et al. Plaque burden, arterial remodeling and plaque vulnerability: determined by systemic factors? J Am Coll Cardiol. 2001;38:718–23. doi: 10.1016/s0735-1097(01)01444-9. [DOI] [PubMed] [Google Scholar]
- 14.Underhill HR, Yuan C, Terry JG, et al. The composition and morphology of the carotid artery are associated with coronary artery disease: a case-control high resolution magnetic resonance imaging study. J Cardiovasc Magn Reson. 2008;10:31. doi: 10.1186/1532-429X-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott MM, Liu K, Carr J, et al. Superficial femoral artery plaque, the ankle-brachial index, and leg symptoms in peripheral arterial disease: the Walking and Leg Circulation Study (WALCS) III. Circ Cardiovasc Imaging. 2011;4:246–52. doi: 10.1161/CIRCIMAGING.110.962183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polonsky TS, Liu K, Tian L, et al. High-risk plaque in the superficial femoral artery of people with peripheral artery disease: prevalence and associated clinical characteristics. Atherosclerosis. 2014;237:169–76. doi: 10.1016/j.atherosclerosis.2014.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ankle Brachial Index Collaboration. Fowkes FG, Murray GD, et al. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott MM, Guralnik JM, Tian L, et al. Associations of borderline and low normal ankle-brachial index values with functional decline at 5-year follow-up: the WALCS (Walking and Leg Circulation Study) J Am Coll Cardiol. 2009;53:1056–62. doi: 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JC, Criqui MH, Denenberg JO, McDermott MM, Golomb BA, Fronek A. Exertional leg pain in patients with and without peripheral arterial disease. Circulation. 2005;112:3501–8. doi: 10.1161/CIRCULATIONAHA.105.548099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heun R, Papassotiropoulos A, Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int J Geriatr Psychiatry. 1998;13:368–80. doi: 10.1002/(sici)1099-1166(199806)13:6<368::aid-gps775>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 22.McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–71. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 23.Cai J, Hatsukami TS, Ferguson MS, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–44. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 24.Underhill HR, Yarnykh VL, Hatsukami TS, et al. Carotid plaque morphology and composition: initial comparison between 1.5- and 3.0-T magnetic field strengths. Radiology. 2008;248:550–60. doi: 10.1148/radiol.2482071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDermott MM, Liu K, Carroll TJ, et al. Superficial femoral artery plaque and functional performance in peripheral arterial disease: walking and leg circulation study (WALCS III) J Am Coll Cardiol Img. 2011;4:730–9. doi: 10.1016/j.jcmg.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051–6. doi: 10.1161/hc4201.097839. [DOI] [PubMed] [Google Scholar]
- 27.Parmar JP, Rogers WJ, Mugler JP, III, et al. Magnetic resonance imaging of carotid atherosclerotic plaque in clinically suspected acute transient ischemic attack and acute ischemic stroke. Circulation. 2010;122:2031–8. doi: 10.1161/CIRCULATIONAHA.109.866053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saam T, Ferguson MS, Yarnykh VL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25:234–9. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 29.Guralnik JMFL, Simonsick EM, Kasper JD, Lafferty ME. The Women’s Health and Aging Study: health and social characteristics of older women with disability. Bethesda, MD: National Institutes of Health; NIH Publication 1995; No. 95–4009: Appendix E. [Google Scholar]
- 30.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 31.McLafferty RB, Moneta GL, Taylor LM, Porter JM. Ability of ankle-brachial index to detect lower extremity atherosclerotic disease progression. Arch Surg. 1997;132:836–40. doi: 10.1001/archsurg.1997.01430320038005. [DOI] [PubMed] [Google Scholar]
- 32.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–33. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 33.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 34.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–96. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guzman RJ, Brinkley DM, Schumacher PM, Donahue RM, Beavers H, Qin X. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;51:1967–74. doi: 10.1016/j.jacc.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott MM, Carr J, Liu K, et al. Collateral vessel number, plaque burden, and functional decline in peripheral artery disease. Vasc Med. 2014;19:281–8. doi: 10.1177/1358863X14540362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zavodni AE, Wasserman BA, McClelland RL, et al. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the Multi-Ethnic Study of Atherosclerosis (MESA) Radiology. 2014;271:381–9. doi: 10.1148/radiol.14131020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Neill WC, Han KH, Schenider TM, Hennigar RA. Prevalence of nonatheromatous lesions in peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2015;35:439–47. doi: 10.1161/ATVBAHA.114.304764. [DOI] [PubMed] [Google Scholar]