Abstract
The aims of this investigation were to determine the distribution in the gastrointestinal (GI) tract of Eudragit S-100 encapsulated colon-specific sodium alginate microspheres containing 5-fluorouracil (5-FU) in rats, and to perform pharmacokinetic and pharmacodynamic studies. Comparisons were with a control immediate-release (IR) formulation of 5-FU. 5-FU was distributed predominantly in the upper GI tract from the IR formulation but was distributed primarily to the lower part of the GI tract from the microsphere formulation. No drug was released in the stomach and intestinal regions from the colon-specific microspheres. Significantly, a high concentration of the active drug was achieved in colonic tissues from the colon-specific microspheres (P < 0.001), which was higher than the IC50 required to halt the growth of and/or kill colon cancer cells. Colon cancer was induced in rats by subcutaneous injection of 1,2-dimethylhydrazine (40 mg kg−1) for 10 weeks. The tumours induced were non-invasive adenocarcinomas and were in Duke’s stage A. The 5-FU formulations were administered for 4 weeks after tumour induction. Non-significant reductions in tumour volume and multiplicity were observed in animals given the colon-specific microspheres. Enhanced levels of liver enzymes (SGOT, SGPT and alkaline phosphatase) were found in animals given the IR formulation of 5-FU, and values differed significantly (P < 0.001) from those in animals treated with the colon-specific microspheres. Elevated levels of serum albumin and creatinine, and leucocytopenia and thrombocytopenia were observed in the animals given the IR formulation. In summary, Eudragit S-100 coated alginate microspheres delivered 5-FU to colonic tissues, with reduced systemic side-effects. A long-term dosing study is required to ascertain the therapeutic benefits.
Introduction
The incidence of colorectal cancer (CRC) is highest in developed countries and lowest in developing countries. CRC is the third most common form of malignancy in the USA. The American Cancer Society estimated that about 1444920 cases of colorectal cancer will be diagnosed in 2007, and about 559650 people will die from the disease (Jemal et al 2007). Surgery is generally the first-line treatment option for patients with early-stage CRC. This approach affords a relatively positive prognosis, although it is less effective in patients with more advanced disease (patients with lymph node involvement have a 50% risk of relapse following resection). For this reason, adjuvant chemotherapy is often prescribed for patients with Duke’s stage C disease, in an effort to reduce the risk of recurrence. For patients with advanced disease, chemotherapy or the best supportive care is usually the mainstay of treatment (Ishikawa et al 1998; Meropol 1998). Currently, 5-fluorouracil (5-FU) remains the single most effective chemotherapeutic agent for the treatment CRC (Labianca etal 2001). The cytotoxic effects of 5-FU have been well characterized, and include: inhibition of the target enzyme thymidylate synthase by the 5-FU metabolite 5-fluoro-2′-deoxyuridine-5′-mono-phosphate; incorporation of 5-FU into RNA, with subsequent alterations in RNA processing and function; and incorporation of 5-FU into DNA, with resultant DNA damage (Wilkinson & Crumley 1976; Glazer & Peale 1979; Ardalan et al 1980; Kufe et al 1981; Major et al 1982).
The oral bioavailability of 5-FU is erratic, so the drug is usually administered by the intravenous route (Hahn et al 1975): however, it produces systemic side-effects, which reduce patient compliance, and a high failure rate due to interactions with sites other than those associated with tumours (Djordjeuic et al 1993; Herrmann 1993). Patients prefer oral rather than intravenous treatment, but are unwilling to sacrifice tumour response. In terms of drug delivery, site-specific delivery of 5-FU to receptor sites has the potential to reduce the systemic side-effects and to increase the pharmacological response. Various approaches have been used to target the colon via the oral route, including prodrugs, pH-dependent systems, time-dependent systems, microflora-activated systems, intestinal-pressure-controlled colon delivery capsules, and CODES technology (Yang et al 2002).
The colonic region is inhabited by more than 400 species of bacteria, which mostly ferment non-starch polysaccharides such as alginate, chitosan, dextran and pectin (Yang et al 2002). This concept has been exploited to trigger drug release and hence delivery to the colon (Tuğcu-Demiröz et al 2006; Wu et al 2007). The hostile environment of the stomach forces polysaccharide-based delivery systems to dissolve/disintegrate and release the drug in the upper part of the gastrointestinal (GI) tract, leading to failure of the delivery system. This problem can be obviated by coating the polysac-charide matrix with enteric polymers such as Eudragit or ethylcellulose (Yang et al 2002; Nykänen et al 2004; Zambito et al 2005; Mundargi et al 2007). Most of these are single-unit systems. Because of the variation in transit time through the colon, drug release can be incomplete when these colon-specific single units are not disintegrated, and thus treatment will remain ineffective. Diarrhoea is the one of the major side-effects and toxicity-limiting factors of 5-FU therapy (Cortesi et al 1990) and can result in ineffective treatment. This problem can be circumvented by reducing the carrier size, and the development of a multiparticulate colon-specific drug-delivery system, since size-dependent GI retention has been reported in earlier studies (Watts et al 1992). Multiparticulate delivery systems of 5-FU based on dual concepts, namely a pH-dependent and microflora-activated system, have been shown to be useful in in-vitro (Jain et al 2007) and pharmacokinetic (Wei et al 2008) studies. These researchers did not attempt to test the pharmacodynamics of a 5-FU colon-specific formulation for the treatment of CRC, however.
Rahman and co-workers have previously prepared core microspheres of alginate and 5-FU by cross-linking with calcium chloride, and coating with Eudragit S-100 using a solvent evaporation technique, in order to prevent drug release in the upper part of the GI tract. Using in-vitro methods, these colon-specific Eudragit S-100 coated alginate microspheres improved the availability of 5-FU in the colonic region for the treatment of CRC (Rahman et al 2006).
The current study focuses on the GI distribution, pharmacokinetics and pharmacodynamics of the same formulation of Eudragit S-100 encapsulated sodium alginate colon-specific microspheres (average particle size 123.47 ± 5.60 μm, drug content 98.50%, drug release > 95% at pH 7.4 and sustained delivery up to 16 h) given to male Wister rats, in comparison with an immediate-release (IR) preparation of 5-FU as a control.
Materials and Methods
Materials
5-FU was a gift from the Dabur Research Foundation (Sahibabad, India). Eudragit S-100 was provided by Ranbaxy Laboratories (Gurgaon, India). 1,2-Dimethylhydrazine (DMH) and 5-bromouracil (internal standard (IS)) were purchased from Sigma-Aldrich (Munich, Germany). Kits for measurement of serum glutamate oxaloacetate transaminase (SGOT), glutamate pyruvate transaminase (SGPT), albumin, creatinine and alkaline phosphatase were purchased from Bayer India Ltd (Mumbai, India). Sodium alginate, sodium carboxymethyl-cellulose (SCMC), microcrystalline cellulose PH 102, colloidal silicon dioxide, magnesium stearate and formalin were purchased from S.D. Fine Chemicals Ltd (Mumbai, India). All other solvents and reagents used were of HPLC or analytical grade.
Animals
Male Wister rats, 3 months of age, weighing 200–250 g, were obtained from the Central Animal House Facility, Hamdard University, New Delhi, India. They were housed in propylene cages (six rats per cage) with wood-chip bedding in an animal room under constant conditions (22± 2°C and 44± 5% relative humidity) with a 12 h light–dark cycle. Rats had free access to drinking water and standard rodent feed. All animals used in the experiments received care in compliance with the Principle of Laboratory Animal Care and Guidance for the Care and Use of Laboratory Animals. The experimental protocol was approved by the Institutional Ethical Committee, Hamdard University, New Delhi, India (approval no. CPCSEA/165/2004).
Pharmacokinetic and gastrointestinal distribution studies
The efficacy of colon-specific 5-FU microspheres in targeting drug locally to the colon was assessed in pharmacokinetic and GI tract distribution studies, and was evaluated in a rat model in comparison with a control treatment (an IR formulation of 5-FU composed of: 5-FU powder (50% w/w), microcrystalline cellulose PH 102 (49% w/w), colloidal silicon dioxide (0.5% w/w) and magnesium stearate (0.5% w/w). Formulation suspensions were prepared in 0.5% w/v SCMC.
Animals were fasted for 24 h before the studies, and during the course of the studies; water was available ad libitum.
Rats were divided into two groups of 45 animals. Group I received the microsphere formulation; group II received the control IR formulation. The dose of 5-FU to be administered was calculated according to the surface area of the rat’s colon (0.0023× 500× 7= 8.05mg) (Preezel & Webling 1971). Animals were given the appropriate formulation containing the equivalent of 8.05 mg of 5-FU by oral gavage. Three animals from each group were killed by deep ether anaesthesia at 0.5, 1, 1.5, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22 and 24 h after drug administration.
Immediately after death, carcasses were placed on ice packs and opened by bilateral thoracotomy. Blood (5 mL) was obtained by intracardiac puncture and was collected into centrifuge tubes. Samples were centrifuged at 2000 g for 10min and the serum was stored at −20°C for later measurement of 5-FU concentrations by HPLC.
The entire GI tract was removed, and mesenteric and fatty tissues were separated. The GI tract was segmented into stomach, small intestine, caecum and colon. The contents of the lumen were removed by gentle pressure with wet scissors, and organs were cut longitudinally and washed with normal saline (0.9% w/v sodium chloride) to remove the remaining luminal contents. Caecal and colon contents were taken from the caecum and colon at each time point. Organs were weighed and cut into small pieces and homogenized at 4°C with an Ultra-turrex (type T 25) mixer (IKA-Werke, Staufen, Germany). The homogenate was then centrifuged at 2000 g for 10 min at 4°C. The fatty layer was discarded, and the amount of drug in the supernatant was determined by HPLC.
Pharmacokinetic data were analysed by fitting to a non-compartmental model using WinNonlin software, version 5.2 (Pharsight Corporation, Mountain View, CA, USA).
HPLC analysis of 5-fluorouracil
The HPLC system (Shimadzu HPLC Class VP series, Kyoto, Japan) consisted of two LC 10AT VP pumps, a variable-wavelength programmable UV-Vis detector (SPD-10A VP), a system controller (SCL-10A VP) and a reverse-phase C18 column (150 × 4.6mm i.d., particle size 5μm; Merck, Mumbai, India) and a manual injection valve was equipped with a 20 μL sample loop, and was equipped with Class-VP series software (version 5.0, Shimadzu, Kyoto, Japan).
The concentration of 5-FU in the serum or tissue homogenates was determined using the method reported by Jung etal (1997), with some modifications. The mobile phase was methanol, acetic acid and water (4:0.05:95.95, by volume), delivered at a flow rate of 1.0mLmin−1. 5-FU and the IS (5-bromouracil) were detected at 254nm. An aliquot (0.5mL) of serum or tissue content was measured into the centrifuge tube, followed by the addition of 0.5mL 10% v/v perchloric acid and 100μL IS (200μg mL−1), vortexed for 2min and centrifuged at 2000g for 10min. The supernatant was filtered through a 0.2μm membrane and 20μL was injected onto the column. Retention times of 5-FU and the IS were 6.4 and 3.1min, respectively. Calibration curves were constructed by spiking varying amounts of 5-FU (1–50 μg mL−1) and fixed amounts of IS in blank serum and blank tissues. The ratio of the peak areas of 5-FU:IS was plotted against the theoretical 5-FU concentration, and used to determine the 5-FU concentration in samples. A linear relationship was observed between 5-FU concentration and peak area in the range 1–50 μg mL−1. Recovery ranged from 87.2% to 101.1%, and the lower limit of quantification was 0.1μgmL−1. The coefficient of variation (CV) ranged from 7.2 to 11.5% for serum and various tissues. The intra-day and inter-day precision was less than 9.5% (CV) and 14.1% (CV), respectively.
Pharmacodynamic studies
After 2 weeks’ acclimatization, rats were randomly divided into two groups. Group II rats were given 40 mg kg−1 subcutaneous injections of DMH (10% w/v solution in sterile normal saline) in the groin region weekly for 10 weeks (Li & Li 2003). Rats in group I were given normal saline at the same time for the same period of experiment. After the last DMH dose, 10 animals from group II and six from group I were killed by deep ether anesthesia. After cancer induction, the remaining animals in group II were divided into five groups. The treatment protocols for the subgroups are outlined in Table 1. Formulations were suspended in 0.5% w/v SCMC. Group IIc and IIe were given colon-specific micro-spheres or the IR formulation equivalent to 8.05 mg 5-FU for 4 weeks by gavage, respectively. Group IIa, IIb and IId were given SCMC solution, placebo IR and placebo microspheres formulations suspended in SCMC, respectively. After 1, 2, 3 and 4 weeks’ treatment with the 5-FU formulations, six animals from groups IIc and IIe were killed by deep ether anaesthesia; the controls were killed at the end of the study. The duration of the experiment was 14 weeks.
Table 1.
Treatment protocols
| Group | No. rats | Treatment |
|---|---|---|
| II | 12 | Saline |
| II | 82 | 1,2-Dimethylhydrazine |
| IIa | 06 | Sodium carboxymethylcellulose |
| IIb | 06 | Control of group IIc |
| IIc | 24 | Microsphere formulation |
| IId | 06 | Control of group IIe |
| IIe | 24 | Immediate-release formulation |
Tissue processing
The carcass was opened by bilateral thoracotomy immediately after death and blood (10 mL) withdrawn by cardiac puncture. Sodium-EDTA was added to 2 mL blood for determination of white blood cell (WBC) and platelet counts. The remaining blood was centrifuged at 2000 g for 10 min to separate the serum for biochemical estimations.
Buffered formalin-phosphate (10%) was immediately injected into the colon by intubation into the anus, so that the colon and small intestine were distended and fixed. The colons were removed and placed on plastic sheets, flushed with normal saline and then cut longitudinally. The number and location of tumours in each animal were determined. Tumour width (W) and length (L) were measured with callipers. Tumour volume (TV) was determined using the formula TV = (L × W2)/2 (Yoon et al 1999). Colon samples were fixed between sheets of Whatman filter paper and stored in 10% buffered formalin-phosphate for histological examinations. Afterwards, colon tissues were embedded in paraffin and sectioned and stained with haematoxylin and eosin. The stained sections were examined histologically for tumour type and Duke’s stage.
Biochemical estimations
WBC and platelets were determined by a cell counter (Sysmex-K100, Transasia Biomedicals Ltd, Mumbai, India). SGOT and SGPT, alkaline phosphatase, creatinine and albumin concentrations were estimated by a UV kinetic method (Begemeyer et al 1978), p-nitrophenyl phosphate (PNPP) method (Orhanovic & Pavela-Vrancic 2000), alkaline picric acid method (Michael & Greggory 2000) and BCG (bromocresol green) method (Guijarro etal 1996), respectively. Levels of SGOT, SGPT, albumin, creatinine and alkaline phosphatase in the serum were determined using a clinical chemistry analyser (ERBA Chem-5 Plus Transasia Biomedicals Ltd, Mumbai, India).
Statistical analysis
GraphPad Instat 3 software was used for statistical analysis (GraphPad Software Inc., San Diego, USA). Data are expressed as mean± s.d. Data were analysed by one-way analysis of variance followed by the Student–Newman–Keul multiple comparison test. The unpaired Student’s t-test was used where applicable. Differences were considered significant at P<0.05.
Results
Pharmacokinetics
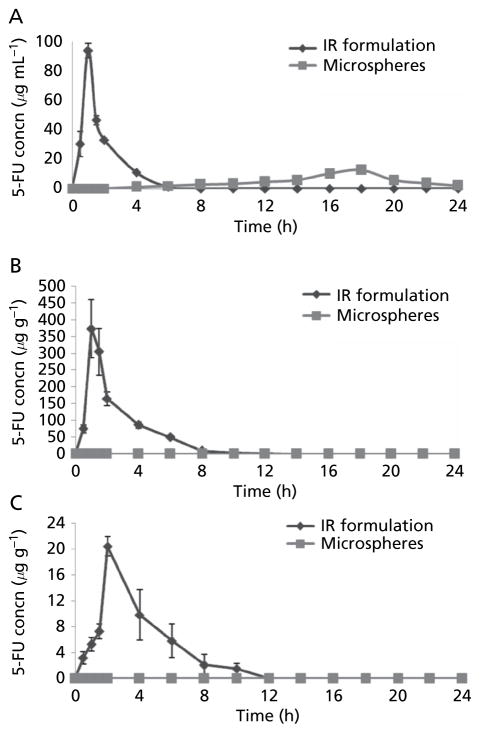
Serum 5-FU concentration vs time profiles are shown in Figure 1A. Pharmacokinetic parameters are shown in Table 2. The two formulations produced markedly different serum drug concentration profiles. The lag time of 5-FU was 4 h in the case of colon-specific microspheres. The mean peak plasma concentration (Cmax) was markedly different for the two formulations: 94.12 ± 5.12 μg mL−1 vs 12.51 ± 2.81μg mL−1 for the IR formulation and colon-specific microspheres, respectively. Time to Cmax (Tmax) was 0.83 ± 0.28 h for the IR formulation, which was significantly shorter than for the microspheres (17.30 ± 1.154 h).
Figure 1.
Concentrations of 5-FU in (A) serum (B) stomach tissue homogenate and (C) intestinal tissue homogenate after administration of colon-specific microspheres and immediate-release (IR) formulations of 5-FU. Data are mean ± s.d. (n = 3).
Table 2.
Pharmacokinetic parameters of 5-FU after administration of colon-specific microspheres and immediate-release formulation in rats. Values are mean ± s.d. (n = 3).
| Parameter | Microsphere formulation | Immediate-release formulation |
|---|---|---|
| Cmax (μg mL−1) | 12.51 ± 2.81 | 94.12 ± 5.21 |
| Tmax (h) | 17.30 ± 1.154 | 0.83 ± 0.280 |
| AUC (μg h mL−1) | 104.14 ± 8.42 | 150.10 ± 11.58 |
| Mean residence time (h) | 15.50 ± 2.73 | 1.78 ± 0.31 |
| Relative bioavailability (%) | 69.37 ± 7.49 | – |
Cmax, maximum plasma concentration; Tmax, time to Cmax; AUC, area under the plasma concentration–time curve (0–24 h).
The mean residence time (MRT) was 15.50 ± 2.73 h for the colon-specific microspheres, which was 8.7 times higher than for the IR formulation (1.78 ± 0.31 h). The area under the plasma concentration–time curve (AUC) was 150.11μg h ml−1 for the IR formulation, which was significantly higher than that for the colon-specific microspheres (104.14 μg h mL−1). All of the differences in pharmacokinetic parameters (Cmax, Tmax, AUC, MRT) for the two formulations were significant (P < 0.001).
Distribution
The distribution of 5-FU to the stomach and small intestine after administration of colon-specific microspheres and the IR formulation are shown in Figures 1B and 1C. Results indicate the total amount in the upper part of the GI tract. The mean peak concentrations of 5-FU in the stomach and small intestine tissues from the IR formulation were 374.37± 87.65 μg g−1 at 1 h and 20.41± 1.52μg g−1 at 2 h. No 5-FU was released in stomach and small intestine from the colon-specific microspheres, indicating that the Eudragit S-100 coating remained intact during transit of the micro-spheres through the stomach and small intestine. A sharp decrease in the concentration of 5-FU was found with the IR formulation, which may be attributed to absorption, systemic distribution and further movement to the small intestinal region.
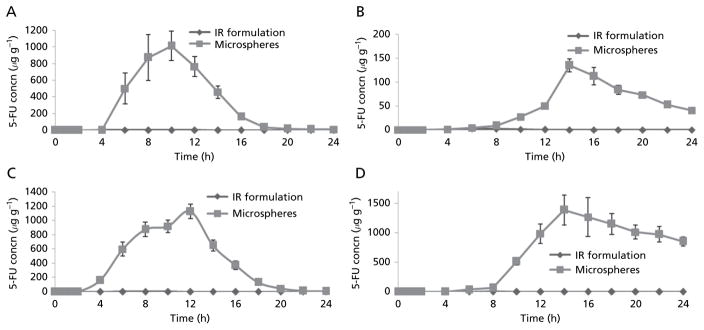
Distribution of 5-FU to the caecal contents, caecum tissues, colon contents and colon tissues after administration of the IR formulation and colon-specific microspheres is shown in Figure 2. Significant differences in 5-FU concentrations in colon tissues were observed after administration of the two formulations (P <0.001). The mean peak 5-FU concentrations in caecal contents, caecum tissues, colon contents and colon tissues were 1015.73 ± 176.41 μgg−1, 135.09 ± 13.82 μgg−1, 1128.33 ± 101.56 μgg−1 and 1387.35 ± 256.43 μgg−1, respectively, for the colon-specific microspheres vs 1.52± 0.08μgg−1, 2.53 ± 1.02 μgg−1, 0.51 ± 0.08 μgg−1 and 0.79 ± 0.28 μgg−1, respectively, for the IR formulation. A higher 5-FU concentration was achieved with the colon-specific microspheres at all time points. 5-FU was still detectable in the caecum and colon after 24 h in the case of colon-specific microspheres: 5-FU concentrations in caecal contents, caecum tissues, colon contents and colon tissues 24h after administration of the micro-spheres were 8.42 ± 0.98 μgg−1, 40.96 ± 5.86 μgg−1, 6.51 ± 1.29 μgg−1 and 854.25 ± 76.19 μgg−1, respectively, whereas 5-FU was not detectable after 24 h in the case of the IR formulation. This high concentration in the colon could be ascribed to protection of the core alginate microspheres from the environment of the stomach and small intestine by the Eudragit S-100 coating, thereby preventing drug release in the upper part of the GI tract.
Figure 2.
Concentrations of 5-FU in homogenates of caecum contents (A) caecum tissues (B) colon contents (C) and colon tissues (D) af administration of colon-specific microspheres and immediate-release (IR) formulations of 5-FU. Data are mean ± s.d. (n = 3).
Pharmacodynamic studies
Body weight
All of the rats survived after dosing of the carcinogen in group II. By the end of the tenth week, there was a significant difference in average body weight between animals treated with DMH and those in the control group (results not shown, P < 0.0001). Similarly, there were significant differences in average weights (Table 3) of animals after 14 weeks between group IIc and group IIe (P < 0.001).
Table 3.
Macroscopic findings and histopathology in rats after treatment with DMH (to induce colon cancer) and 5-FU formulations. Values are mean ± s.d. The treatment of the groups is given in Table 1
| Parameter | Group IIa | Group IIb | Group IIab | Group IIbb | Group IIcb | Group IIdb | Group IIeb |
|---|---|---|---|---|---|---|---|
| Weight (g) | 320 ± 25 | 379 ± 27 | 362 ± 19 | 349 ± 23 | 298 ± 16 | 388 ± 23 | 191 ± 24 |
| Tumour incidence, number (%) | 10 (100) | 6 (100) | 6 (100) | 6 (100) | 23 (95.8) | 6 (100) | 24 (100) |
| Survival (%) | 100 | 100 | 100 | 100 | 100 | 100 | 66.66 |
| Total number of tumours | 13 | 17 | 18 | 19 | 10 | 18 | 11 |
| Number of adenomas (%) | 2 (15.4) | 3 (17.6) | 4 (22.2) | 3 (15.8) | 1 (10) | 3 (16.7) | 1 (14.3) |
| Number of adenocarcinomas (%) | 11 (84.6) | 14 (82.4) | 14 (77.8) | 16 (84.2) | 9 (90) | 15 (83.3) | 10 (85.7) |
| Tumour multiplicity | 2.16 ± 0.75 | 2.83 ± 0.98 | 3.00 ± 1.09 | 3.16 ± 1.17 | 1.66 ± 0.81 | 3.00 ± 0.89 | 2.75 ± 0.95 |
| Tumour volume (mm3) | 1.57 ± 1.34 | 1.89 ± 1.37 | 1.79 ± 1.09 | 1.81 ± 1.59 | 1.25 ± 1.23 | 1.77 ± 1.11 | 1.51 ± 1.45 |
Values after 10 weeks;
values after 14 weeks.
Tumour incidence, distribution, volume and multiplicity
Macroscopic findings are summarized in Table 3. The tumour incidence was 100% in the group treated with the IR formulation of 5-FU. There was a non-significant decrease in the number of tumours in groups treated with the colon-specific micro-spheres. Tumours were distributed predominantly in the proximal and middle colon. Tumour multiplicity was decreased in group IIc compared with group IIe but this was not significant. A non-significant reduction in tumour volume was achieved after treatment with the 5-FU colon-specific microspheres (group IIc) compared with group IIe (treated with the IR 5-FU formulation).
Tumour histopathology
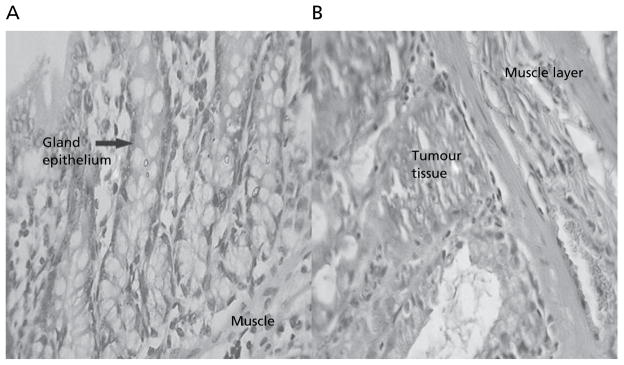
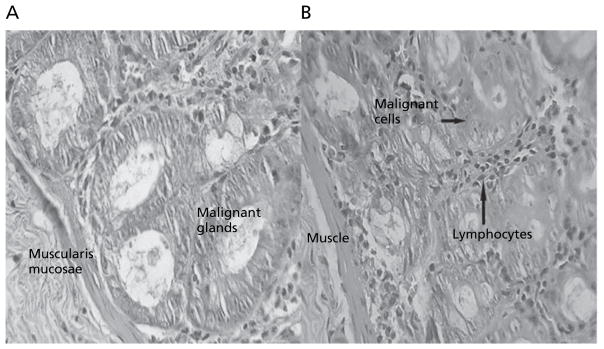
Animals in group II treated with DMH showed development of well-differentiated non-invasive adenocarcinoma restricted to the mucosa (Figure 3). The tumour tissues comprised of glands lined by cells with large, hyperchromatic nuclei. Some glands showed obstruction of the lumen. Lymphocytic infiltration was minimal and limited to occasional clusters only. Rats of group IIe, treated with the IR formulation of 5-FU, showed minimal lymphocytic infiltration of the tumour (Figure 4A). After 4 weeks’ treatment, animals in group IIc, treated with the colon-specific microspheres, showed well differentiated adenocarcinoma that was restricted to the submucosa (Figure 4B). There was, however, a significant degree of lymphocytic infiltration within and around the malignant glands in the mucosa.
Figure 3.
Normal colon tissues (A) and colon tissues after treatment with DMH for 10 weeks (B) (magnification × 40).
Figure 4.
Colon tissues after induction of tumours with DMH for 10 weeks, followed by 4 weeks’ treatment with 5-FU in the immediate-release formulation (A) or colon-specific microspheres (B). Magnification (×40).
Biochemical parameters
Biochemical parameters are shown in Table 4. After 4 weeks’ treatment with the 5-FU formulations in carcinogen-treated animals, significantly higher serum levels of SGOT, SGPT and alkaline phosphatase were observed in group IIe compared with group IIc (P < 0.001). Similarly, significant differences in WBC count, platelet count, serum albumin and serum creatinine concentrations were observed between the two groups of animals (P < 0.001).
Table 4.
Biochemical parameters in rats treated with immediate-release 5-FU and colon-specific microspheres. Data are mean ± s.d. (n = 6). Treatment of groups is shown in Table 1
| Parameter | Group Ia | Group IIa | Group IIaa | Group IIba | Group IIca | Group IIda | Group IIea,b |
|---|---|---|---|---|---|---|---|
| WBC (mm−3) | 6800 ± 234 | 7633 ± 665 | 8124 ± 561 | 7299 ± 360 | 5200 ± 655 | 7546 ± 790 | 2400 ± 264 |
| Platelets (Lacs mm−3) | 3.45 ± 0.34 | 5.46 ± 1.22 | 6.16 ± 1.01 | 4.55 ± 0.87 | 3.11 ± 0.59 | 4.58 ± 0.87 | 2.17 ± 0.56 |
| Serum albumin (g dL−1) | 1.13 ± 0.23 | 1.82 ± 0.32 | 1.67 ± 0.27 | 1.73 ± 0.23 | 2.59 ± 0.41 | 1.77 ± 0.23 | 3.89 ± 0.43 |
| Serum creatinine (mg dL−1) | 0.356 ± 0.04 | 0.656 ± 0.08 | 0.603 ± 0.05 | 0.659 ± 0.07 | 0.749 ± 0.04 | 0.652 ± 0.04 | 0.948 ± 0.06 |
| Alkaline phosphatase (IU L−1) | 21.5 ± 4.6 | 81.2 ± 20.5 | 96.2 ± 17.7 | 70.2 ± 15.3 | 147.2 ± 18.0 | 65.7 ± 11.3 | 571.5 ± 45.3 |
| SGOT (IU L−1) | 15.5 ± 2.6 | 81.8 ± 12.5 | 75.9 ± 15.1 | 62.3 ± 17.6 | 147.5 ± 14.6 | 55.1 ± 9.0 | 228.0 ± 27.4 |
| SGPT (IU L−1) | 22.3 ± 5.5 | 48.7 ± 7.2 | 52.4 ± 6.3 | 37.5 ± 8.3 | 116.5 ± 18.6 | 38.12 ± 8.6 | 265.5 ± 28.3 |
SGOT, serum glutamate oxloacetate transaminase; SGPT, serum glutamate pyruvate transaminase.
Values after 14 weeks;
n = 4.
Discussion
The recommended dose of 5-FU for the average-risk patient with CRC in good nutritional status with adequate haematopoietic function is 12 mgkg−1 intravenously once daily for 4 consecutive days. If no toxicity is observed, then the dose is decreased to 6 mgkg−1 on days 6, 8, 10 and 12, after which treatment is discontinued. Maintenance therapy is continued by intravenous administration (10–15 mgkg−1 per week as a single dose) by either repeating the first course every 30 days after the last day of the previous course of therapy, or when toxic signs resulting from the initial course of therapy have subsided. This may require 9–45 courses of therapy over 12–60 months (Chabner et al 1996).
The daily intravenous dose of 5-FU is fixed at 500 mg m−2 body surface area (Chabner et al 1996). According to this dosage regimen, an adult dose of 5-FU to be administered intravenously is 600 mg (mean body surface area = 1.2 m2). Thus, the dose of 5-FU to be administered specifically to the human colon would be 100 mg (mean colon surface area = 0.20 m2) (Sandle 1998). Hence, targeting delivery of 5-FU to the colon reduces the dose, and therefore the side-effects of 5-FU therapy.
Alginate was chosen for preparation of the core micro-spheres, as this material is digested by the microflora present in the colon. These microspheres were coated with an acrylate polymer, Eudragit S-100, which only dissolves at pH values above 7.0, thus preventing 5-FU release in the physiological environment of the stomach and small intestine.
Absorption was delayed in the case of the microspheres formulation, shown by a long Tmax, indicating that the Eudragit S-100 coating effectively prevented drug release in the upper part of the GI tract. Enzymatic degradation of polysaccharides by enzymes of the colonic microflora is a slow process and usually requires 12 h for complete degradation (Yang et al 2000); release is thus further modulated by slow degradation of alginate. Cmax was higher with the IR formulation because most of the dose was absorbed in the upper part of the GI tract, which has a large surface area available for absorption. Similarly, AUC was higher with the IR formulation. Values for Tmax, Cmax and AUC were all lower in the case of the microspheres, which would account for the low systemic toxicity – less drug is available systemically for interaction with non-tumour cells, increasing the safety and efficacy index of the microsphere formulation. The MRT of the microsphere formulation was higher than with the control IR formulation, indicating that the drug was slowly and steadily absorbed by colonic tissue, evoking its effect on cancerous tissue of the colon. These results were in agreement with previous researchers (Wei et al 2008).
The IC50 for 5-FU varies from 15 to 7770 μM (median 390 μM) for various cancers (Fischel et al 1995) and from 5.5 to 160 μM in resistant colon cell lines (Copur et al 1995). The concentration of drug in the tissue/organ is the marker of its specificity, efficacy and pharmacological response. The distribution of 5-FU in the GI tract showed that a high concentration of 5-FU was achieved in the colonic tissues with the colon-specific microspheres compared with the IR formulation. The concentration achieved 6, 10, 14 and 24 h after administration of the colon-specific microspheres was 268.91 ± 41.21 μM (34.98 ± 5.36 μg g−1), 3977.18 ± 517.76 μM (517.34 ± 67.31 μg g−1), 10665.60 ± 1971.37 μM (1387.35 ± 256.43μg g−1) and 6567.26± 585.72μM (854.25± 76.19μg g−1), respectively. The maintained concentration was well above the IC50 of 5-FU for resistant colon cell lines, indicating that no or little drug release occurred in the non-target site and the concentration of 5-FU achieved would be able to halt the tumour growth.
The carcinogen DMH was used to induce colon tumours in male Wister rats, a model known to closely parallel the human disease in terms of disease presentation and gross and microscopic pathology (LaMont & O’Gorman 1978). DMH is a procarcinogen which requires metabolic activation to its active electrophilic carcinogenic forms through a series of oxidative steps in the liver. Metabolism of DMH in the liver produces azoxymethane (a known colon carcinogen), ultimately leading to the generation of a methyldiazonium ion and carbonium ion, an active carcinogen electrophile which then manifests its activity in the colon (Fiala 1977; Fiala et al 1987).
Changes in tumour size and the survival of the rats are determinants of the safety and the efficacy of the anticancer drug-delivery system. Four 4 weeks’ treatment of animals with the colon-specific microspheres resulted in reductions in tumour volume and multiplicity, although these differences were not significant. Duke’s staging can provide accurate prognostic information. In the present study, tumours in all of the groups were Duke’s stage A and remained at this stage even after 4 weeks’ treatment with the 5-FU formulations. However, marked lymphocyte infiltration was seen within and around the tumour cells in animals treated with the colon-specific microspheres, indicating that the body was responding to 5-FU therapy. Moreover, survival rate was lower in rats treated with the IR formulation compared with the colon-specific formulation. High survival rates of animals treated with the colon-specific formulation indicated that the drug was delivered to the target site, with minimal systemic exposure to the drug.
5-FU therapy provokes severe haematological, mucosal and digestive side-effects, and 5-FU undergoes anabolism and catabolism in the liver. The biochemical changes produced by the colon-specific microspheres were less significant than with the IR control. SGOT, SGPT and alkaline phosphatase are indicators of liver function. These enzymes were less affected in animals treated with the colon-specific microspheres. Four weeks’ treatment with the IR formulation of 5-FU produced significant decreases in body weight and in food intake; diarrhoea was also observed in these animals, which could be attributed to mucositis of the GI tract due to systemic exposure of the body to the drug. Leucocytopenia and thrombocytopenia were significant in animals treated with the IR formulation compared with the colon-specific microspheres (P < 0.0005). Serum albumin and creatinine were markedly elevated in animals given the IR formulation. The elevation of these enzymes and biochemical parameters in animals treated with the IR formulation was due to systemic exposure of the body to the drug. Changes were less marked in animals treated with the colon-specific microspheres, a reflection of its site-specific drug release and activity, with low systemic absorption. This targeted delivery of 5-FU produced significant changes in the biochemical parameters, but these were less pronounced than with the IR formulation, which suggests that the colon-specific formulation was successful in delivering the drug to the colon region, with low systemic toxicity. Moreover, morphological and histopathological changes produced were also insignificant, indicating that chronic dosing studies are needed to explore the potential use of this formulation for the oral treatment of colon cancer.
Conclusions
Colon cancer is highly prevalent in Western nations. The treatment of choice is 5-FU, but it causes severe systemic side-effects. The efficacy of colon cancer therapy is likely to be improved by localized delivery of 5-FU to the colon. Eudragit S-100 encapsulated alginate microspheres were prepared and evaluated in male Wistar rats, in an attempt to increase the local concentration of 5-FU in the colonic tissues. The pharmacokinetic and GI tract distribution studies demonstrate that the concentration achieved in the colon tissue was higher than the IC50 of 5-FU for colon tumour cells, with low systemic exposure to the drug. Biochemical parameters were not changed significantly with the colon-specific microspheres formulation. Thus, the tested delivery system for 5-FU is promising for the treatment of colon cancer. Clinical studies in humans are required to explore the safety and efficacy of this novel but practical concept.
Acknowledgments
The authors are grateful to the Dabur Research Foundation, India, and Ranbaxy Research Laboratories, India, for providing the gift samples of 5-FU and Eudragit S-100 microspheres. Dr Ziyaur Rahman wishes to acknowledge the Hamdard National Foundation, New Delhi, India, for awarding the HNF fellowship. This work was partially supported by grant P20RR021929 from the National Center For Research Resources.
Contributor Information
Ziyaur Rahman, Department of Pharmaceutics, Faculty of Pharmacy, Hamdard University, New Delhi-110062, India. Department of Pharmaceutics, School of Pharmacy, University of Mississippi, University, MS 38677, USA.
Kanchan Kohli, Department of Pharmaceutics, Faculty of Pharmacy, Hamdard University, New Delhi-110062, India.
Shuang-Qing Zhang, Department of Pharmaceutics, School of Pharmacy, University of Mississippi, University, MS 38677, USA.
Roop K. Khar, Department of Pharmaceutics, Faculty of Pharmacy, Hamdard University, New Delhi-110062, India
Mushir Ali, Department of Pharmaceutics, Faculty of Pharmacy, Hamdard University, New Delhi-110062, India.
Naseem A. Charoo, Department of Pharmaceutics, Faculty of Pharmacy, Hamdard University, New Delhi-110062, India
Mohammad Tauseef, Department of Physiology, VP Chest Institute, University of Delhi, New Delhi-110007, India.
Areeg A. A. Shamsher, Department of Pharmacology, Faculty of Pharmacy, Hamdard University, New Delhi-110062, India
Noorullah N. Mohammed, Department of Pharmaceutics, School of Pharmacy, University of Mississippi, University, MS 38677, USA
Michael A. Repka, Department of Pharmaceutics, School of Pharmacy, University of Mississippi, University, MS 38677, USA
References
- Ardalan B, Cooney DA, Jayaram HN, Carrico CK, Glazar RI, Macdonald J, Schein PS. Mechanisms of sensitivity and resistance of murine tumors to 5-fluorouracil. Cancer Res. 1980;40:1431–1437. [PubMed] [Google Scholar]
- Begemeyer HU, Scheibe P, Wahlefeld AW. Optimization of methods for aspartate aminotransferase and alanine aminotransferase. Clin Chem. 1978;24:58–73. [PubMed] [Google Scholar]
- Chabner BA, Allegra CJ, Curt GA, Calabresi P. Anti-neoplastic agents. In: Hardman JG, Limbird LE, Berry PM, Raymond WR, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 9. Mcgraw-Hill; New Delhi: 1996. pp. 1233–1287. [Google Scholar]
- Copur S, Aiba K, Drake JC, Allegra CJ, Chu E. Thymidylate synthase gene amplification in human colon cancer cell lines resistant to 5-fluorouracil. Biochem Pharmacol. 1995;49:1419–1426. doi: 10.1016/0006-2952(95)00067-a. [DOI] [PubMed] [Google Scholar]
- Cortesi E, Ashelter AM, Goacchini N, Pellegrini A, Frati L, Ficorella C, Mazzei N, Marchetti P. Efficacy and toxicity of 5-fluorouracil and folates in advanced colon cancer. J Chemother. 1990;2:47–50. doi: 10.1080/1120009x.1990.11739005. [DOI] [PubMed] [Google Scholar]
- Djordjeuic B, Lange CS, Allison RR, Rotman M. Response of primary colon cancer cells in hybrid spheroids to fluorouracil. Cancer Invest. 1993;11:291–293. doi: 10.3109/07357909309024854. [DOI] [PubMed] [Google Scholar]
- Fiala ES. Investigation into metabolism and mode of action of the colon carcinogen 1,2-dimethylhydrazine and azoxymethane. Cancer. 1977;40:2436–2445. doi: 10.1002/1097-0142(197711)40:5+<2436::aid-cncr2820400908>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Fiala ES, Sohn OS, Hamilton SR. Effects of chronic dietary ethanol on the in vivo and in vitro metabolism of methyl-azoxymethanol and on methylazoxymethanol induced DNA methylation in the rat colon and liver. Cancer Res. 1987;47:5939–5943. [PubMed] [Google Scholar]
- Fischel JL, Etienne MC, Spector T, Formento P, Renee N, Milano G. Dihydropyrimidine dehydrogenase: a tumoral target for fluorouracil modulation. Clin Cancer Res. 1995;1:991–996. [PubMed] [Google Scholar]
- Glazer RI, Peale AL. The effect of 5-fluorouracil on the synthesis of nuclear RNA in L1210 cells in vitro. Mol Pharmacol. 1979;16:270–277. [PubMed] [Google Scholar]
- Guijarro C, Massay ZA, Wiederkehr MR, Ma JZ, Kasiske BL. Serum albumin and mortality after renal transplantation. Am J Kidney Dis. 1996;27:117–123. doi: 10.1016/s0272-6386(96)90038-4. [DOI] [PubMed] [Google Scholar]
- Hahn RG, Moertel CG, Schutt AJ. A double blind comparison of intensive course 5-fluorouracil by oral versus intravenous route in the treatment of colorectal carcinoma. Cancer. 1975;35:1031–1035. doi: 10.1002/1097-0142(197504)35:4<1031::aid-cncr2820350403>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Herrmann R. Systemic treatment of colorectal cancer. Eur J Cancer. 1993;29A:583–586. doi: 10.1016/s0959-8049(05)80156-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Utoh M, Sawada N, Nishida M, Fukase Y, Sekigochi F, Ishitsuka H. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol. 1998;55:1091–1097. doi: 10.1016/s0006-2952(97)00682-5. [DOI] [PubMed] [Google Scholar]
- Jain A, Gupta Y, Jain SK. Potential of calcium pectinate beads for target specific drug release to colon. J Drug Target. 2007;15:285–294. doi: 10.1080/10611860601146134. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Jung M, Berger G, Pohlen U, Pauser S, Reszka R, Buhr HJ. Simultaneous determination of 5-fluorouracil and its metabolites in serum and tissue by high performance liquid chromatography. J Chromatogr B. 1997;702:193–202. doi: 10.1016/s0378-4347(97)00368-x. [DOI] [PubMed] [Google Scholar]
- Kufe DW, Major PP, Egan EM, Loh E. 5-fluoro-2′-deoxyuridine incorporation in L1210 DNA. J Biol Chem. 1981;256:8885–8888. [PubMed] [Google Scholar]
- Labianca RF, Beretta GD, Pessi MA. Disease management considerations: disease management considerations. Drugs. 2001;61:1751–1764. doi: 10.2165/00003495-200161120-00006. [DOI] [PubMed] [Google Scholar]
- LaMont JT, O’Gorman TA. Experimental colon cancer. Gastroenterology. 1978;75:1157–1169. [PubMed] [Google Scholar]
- Li W, Li C-B. lack of inhibitory effects of lactic acid bacteria on 1,2-dimethylhydrazine-induced colon tumors in rats. World J Gastroenterol. 2003;9:2469–2473. doi: 10.3748/wjg.v9.i11.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major PP, Egan E, Derrick D, Kufe DW. 5-fluorouracil incorporation in DNA of human breast carcinoma cells. Cancer Res. 1982;42:3005–3009. [PubMed] [Google Scholar]
- Meropol NJ. Clinical oncology update, oral fluoropyrimi-dines in the treatment of colorectal cancer. Eur J Cancer. 1998;34:1509–1513. doi: 10.1016/s0959-8049(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Michael L, Greggory C. Effect of calcium and magnesium on urinary oxalate excretion after oxalate loads. J Urol. 2000;163:1565–1569. [PubMed] [Google Scholar]
- Mundargi RC, Patil SA, Agnihotri SA, Aminabhavi TM. Development of polysaccharide-based colon targeted drug delivery systems for the treatment of amoebiasis. Drug Dev Ind Pharm. 2007;3:255–264. doi: 10.1080/03639040600897127. [DOI] [PubMed] [Google Scholar]
- Nykänen P, Sten T, Jürjenson H, Veski P, Marvola M. Citric acid as a pH-regulating additive in granules and the tablet matrix in enteric-coated formulations for colon-specific drug delivery. Pharmazie. 2004;59:268–273. [PubMed] [Google Scholar]
- Orhanovic S, Pavela-Vrancic M. Alkaline phosphatase activity in sea waters, influence of reactive conditions on the kinetic parameters of ALP. Croatica Chem Acta. 2000;73:819–830. [Google Scholar]
- Preezel NC, Webling DDA. The length and mucosal surface area of the small and large gut in young rat. J Anat. 1971;108:295–296. [PMC free article] [PubMed] [Google Scholar]
- Rahman Z, Kohli K, Khar RK, Ali M, Charoo NA, Shamsher AAA. Characterization of 5-fluorouracil microspheres for colon-specific delivery. AAPS Pharm Sci Tech. 2006;7(2):E1–E9. doi: 10.1208/pt070362. article 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandle GI. Salt and water absorption in the human colon: a modern appraisal. Gut. 1998;43:294–299. doi: 10.1136/gut.43.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuğcu-Demiröz F, Acartürk F, Takka S. Investigation of colon-specific dosage forms of ondansetron prepared with natural polymers. Pharmazie. 2006;61:916–919. [PubMed] [Google Scholar]
- Watts PJ, Barrow L, Steed KP, Wilson CG, Spiller RC, Mellia CD, Davies MC. The transit rate of different sized model dosage forms through the human colon and he effects of a lactulose-induced catharsis. Int J Pharm. 1992;87:215–221. [Google Scholar]
- Wei H, Qing D, De-Ying C, Bai X, Li-Fang F. Study on colon-specific pectin/ethylcellulose film-coated 5-fluorouracil pellets in rats. Int J Pharm. 2008;348:35–45. doi: 10.1016/j.ijpharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Wilkinson DS, Crumley J. The mechanism of 5-fluorouridine toxicity in Novikoff hepatoma cells. Cancer Res. 1976;36:4032–4038. [PubMed] [Google Scholar]
- Wu B, Deng D, Lu Y, Wu W. Biphasic release of indomethacin from HPMC/pectin/calcium matrix tablet: II. Influencing variables, stability and pharmacokinetics in dogs. Eur J Pharm Biopharm. 2007 Oct 5; doi: 10.1016/j.ejpb.2007.10.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Yang L, Chu JS, Fix JA. Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int J Pharm. 2002;235:1–15. doi: 10.1016/s0378-5173(02)00004-2. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Eto H, Lin CM, Nakamura H, Pawlik TM, Song SU, Tanabe KK. Mouse endostatin inhibits the formation of lung and liver metastases. Cancer Res. 1999;59:6251–6256. [PubMed] [Google Scholar]
- Zambito Y, Baggiani A, Carelli V, Serafini MF, Di G. Matrices for site-specific controlled-delivery of 5-fluorouracil to descending colon. J Control Release. 2005;102:669–677. doi: 10.1016/j.jconrel.2004.11.001. [DOI] [PubMed] [Google Scholar]