Abstract
Germ cells develop as a cyst of interconnected sibling cells in a broad range of organisms in both sexes. A well-established function of intercellular connectivity is to transport cytoplasmic materials from ‘nurse’ cells to oocytes, a critical process for developing functional oocytes in ovaries of many species. However, there are situations where connectivity exists without a nursing mechanism, and the biological meaning of such connectivity remains obscure. In this review, we summarize the current knowledge on the formation of intercellular connectivity, and discuss its meaning by visiting multiple examples of germ cell connectivity observed in evolutionarily distant species.
Keywords: germ cell connectivity, Drosophila, fusome, DNA damage, genome integrity
Why do germ cells develop as interconnected cysts?
As early as the 19th century, cysts of interconnected germ cells were described by biologists of the time including Sertoli (1877) and von Ebner (1878) [1]. Since then, it became evident that stable intercellular bridges connecting the cytoplasm of germ cells is a common feature of developing germ cells in both female and male metazoans [2–4]. In addition, some somatic cells have also been shown to be connected through intercellular bridges [5].
The mechanisms that underlie the formation of interconnected germ cell cysts have been intensively investigated [4]. However, why interconnected cysts are formed in the germline of many organisms has remained unclear, except for in meroistic ovaries where only a subset of germ cells are specified as oocytes while the others differentiate into nurse cells (Figure 1). In this case, intercellular bridges allow nurse cells to transport their cytoplasmic contents into oocytes, facilitating the development of oocytes [6]. Oocytes acquire much of their cytoplasmic materials such as organelles and fate determining mRNAs from nurse cells, whereas their own nuclei remain mostly quiescent.
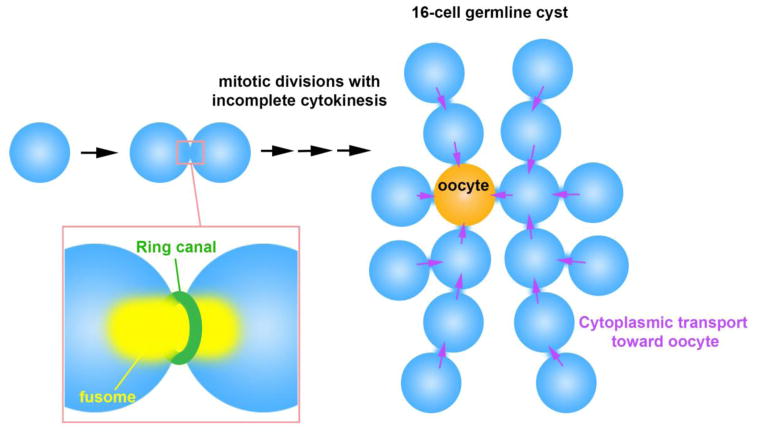
Figure 1. Nursing mechanism through germ cell connectivity in meroistic ovaries.
In meroistic ovaries, where only a subset of germ cells within the cyst are chosen to become an oocyte, sister germ cells (nurse cells) donate their cytoplasm to the oocyte through ring canals. Shown is the example of the Drosophila female germline cyst, where 16 interconnected cells develop into one oocyte and 15 nurse cells. The fusome is a germline specific membranous organelle that runs through ring canal and disassembles before cytoplasmic transport begins.
The Drosophila ovary, where 15 nurse cells support the development of an oocyte, has been the premier model of meroistic ovaries to study how germ cell connectivity is established and how it contributes to oocyte development. Accordingly, our knowledge has been framed around the idea of a ‘nursing mechanism’ carried out by the nurse cells to nurture oocytes. However, broad evolutionary conservation of germ cell connectivity such as in panoistic ovaries (where all germ cells become oocytes) and in the male germline (Figure 2) urges us to think that the nursing mechanism is unlikely the only reason for how and why germ cell connectivity might have arisen during the evolution of multicellular organisms.
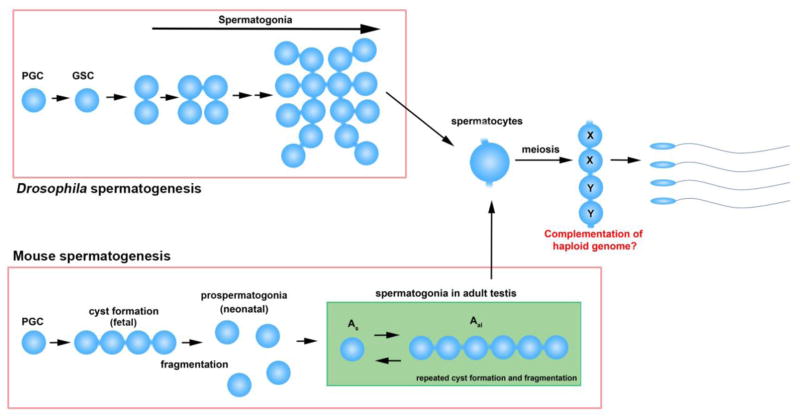
Figure 2. Germ cell cyst formation during spermatogenesis.
Despite the lack of a nursing mechanism, male germ cells also develop germline cysts. Germ cell connectivity in post-meiotic spermatids has been proposed to help complement haploid genomes (X chromosome products shared by Y-containing spermatids, and vice versa). Note that spermatocytes are still interconnected with sibling spermatocytes while undergoing meiosis and spermiogenesis (although depicted as a single cell). Mitotically-dividing spermatogonia are also interconnected with sibling cells both in Drosophila and mice.
In this review, we attempt to discuss the functionality of germ cell connectivity across multiple systems beyond the meroistic ovaries. First, we will briefly review the molecular mechanisms by which intercellular connectivity is established in the ovary of Drosophila. Then we will describe germ cell connectivity in evolutionarily distant species. Finally, we discuss recent findings on germ cell connectivity in the Drosophila male germline and propose that germ cell connectivity might help protect the genomic integrity of germ cells.
Mechanisms to establish germ cell connectivity: ring canal and fusome
The structure of germ cell intercellular bridges has been best studied in the Drosophila female germline. Asymmetric division of germline stem cells (GSCs) produces one self-renewing GSC and one cystoblast, the latter of which proceeds to differentiate. The cystoblast undergoes 4 mitotic divisions with incomplete cytokinesis, resulting in formation of a cyst with 16 interconnected germ cells (Figure 1). During these mitotic divisions, the contractile ring is stabilized without completely pinching off sister cells. These stabilized contractile rings result in intercellular bridges referred to as ring canals. Ring canals are outlined by the actin cytoskeleton, which grow considerably in size from 0.5 μm to ~10 μm in diameter [7]. The first step in ring canal formation is the appearance of phospho-tyrosine residues along the contractile ring during mitosis [8, 9]. The ring canal is initially composed of contractile ring components such as Anillin, kinesin motor MKLP (mitotic kinesin-like protein, Pavarotti in Drosophila), and Myosin II. As ring canals grow, these initial components disappear, whereas the core components of mature ring canals, such as Hts-RC and Kelch, are loaded onto the ring canals [10–12]. The expansion of ring canals is critical for oocyte development by allowing for the intercellular transport of cytoplasmic materials from nurse cells to the oocyte.
In the Drosophila male germline, four mitotic divisions with incomplete cytokinesis yield a cyst of 16 spermatogonia, all of which subsequently undergo the meiotic program as spermatocytes (Figure 2). Ring canals do not grow in size, likely reflecting the lack of need for the large scale cytoplasmic transport that is observed during oocyte development. Ring canals in the male germline do not have Hts-RC or Kelch, critical ring canal components in the female germline. Instead, male ring canals remain associated with contractile ring components such as Anillin, Septins (Peanut, Sep1, Sep2), and Pavarotti [13, 14].
In mammals, germ cells also develop intercellular bridges during gametogenesis in both males and females [1]. Intercellular bridges are observed in germ cells in fetal ovaries and testes, forming germ cell cysts [1, 15]. Cysts subsequently fragment, resulting in individual primary oocytes or prospermatogonia in neonatal gonads [16]. In adult testes, germ cells again undergo incomplete cytokinesis through spermatogonial and meiotic divisions [17]. Spermatogonial populations undergo repeated cycles of cyst fragmentation and branching (Figure 2) [17]. The molecular composition of the intercellular bridges in adult testes was revealed by proteomic analysis. Several proteins that are involved in somatic cytokinesis (such as MKLP1, RacGap, SEPT2, SEPT7, SEPT9, and Anillin) were shown to be localized to intercellular bridges in adult mouse testes [18]. Mice with germ cell-specific knockout of RacGAP or non-muscle Myosin IIB showed a male infertility phenotype [19, 20]. Germline-specific components of intercellular bridges, such as Tex14 and RBM44, were also discovered [21, 22]. Tex14 was identified as a key protein that blocks the cytokinesis machinery containing CEP55, TSG101 and ALIX, thus leading to stable intercellular bridges in germ cells in adult testes [23]. Male mice with a Tex14 null mutation are infertile [21]. Although Tex14 also localizes to the intercellular bridges in female germ cells, Tex14 mutant female mice are fertile [24].
In Drosophila, ring canals are filled with a membranous organelle called the fusome (Figure 1). The fusome, found both in males and females, is a germline-specific membranous organelle that is considered to be a derivative of the endoplasmic reticulum [25]. The fusome adopts a branched morphology that runs through all germ cells within a cyst (Figure 1). The core fusome components are Hts-Fus/Adducin-like (a product of the hu-li tai shao gene, which produces a peptide that is cleaved into Hts-Fus and Hts-RC, a component of ring canals as described above), α- and β-Spectrin, and Ankyrin [26–28]. The fusome likely functions to facilitate communication among germ cells within a cyst. It is known to be associated with cell cycle regulators (Cyclin A, B, E, Cdk1, and Cyclin degradation factors)[29–32], and loss of the fusome results in disruption of cell cycle synchronization within the cyst. These results show that the fusome plays a critical role in promoting the sharing of information among the germ cells within a cyst, leading to cell cycle synchronization. Moreover, fusome-associated Aurora B and Survivin may contribute to the formation of cyst by preventing complete abscission via inhibition of Cyclin B, which promotes abscission [30, 33].
Fusome-like structures have been observed in germ cells of multiple vertebrate systems, including the Xenopus ovary and the dogfish testis [34, 35], suggesting that ring canals and fusomes are ubiquitous mechanisms for achieving germ cell connectivity. However, EM studies on mouse ovaries suggest that there is no fusome-equivalent structure [36]. Mouse male germ cells also appear to lack fusome-like structure [1]. Despite the apparent lack of fusome, mouse ovarian germ cell cysts are synchronized in meiotic progression, suggesting that there are yet-to-be identified mechanism(s) that allow synchronization among germ cells with the cysts [16].
In summary, connectivity/cyst formation is a ubiquitous phenomenon observed in germ cells of both sexes in a broad range of organisms.
Function of germ cell connectivity: meroistic ovaries
The function of germ cell connectivity is best understood in meroistic ovaries, in which a subset of germ cells is chosen to become the oocyte while the remaining germ cells differentiate as nurse cells. In the Drosophila ovary, after 4 mitotic divisions, only one germ cell develops as the oocyte, whereas the remaining 15 germ cells become nurse cells. During and soon after the mitotic divisions, a polarity within connected germ cells is set up such that the oocyte can collect materials (e.g. mRNAs and organelles such as mitochondria) from nurse cells [37–39]. During this process, polarization of microtubules within the cyst plays a fundamental role: all the centrosomes move into the oocyte, thus the minus ends of microtubules are concentrated in the oocyte [40]. This allows dynein-mediated directional transport of cargos (such as fate-determining mRNAs), leading to oocyte determination. Failure to set up this polarity or in early transport of oocyte fate determinant(s) results in defective oocyte specification, leading to 16 nurse cells and no oocyte [41]. Even if oocyte fate is correctly specified, later defects in transporting nurse cell contents into oocyte results in defective oocyte growth, leading to underdeveloped oocytes and thus sterility [41].
Recent studies demonstrated that mouse ovaries are also meroistic, where some germ cells function as nurse cells to donate their cytoplasm to their sister cells that will develop into oocytes [42]. The function of intercellular bridges in allowing cytoplasmic transport during mammalian oocyte differentiation was initially suggested by studies using EM. Organelles such as mitochondria, ER and free ribosomes were found within the bridge in rabbit and mouse fetal ovaries [36, 43, 44]. Recent study by lineage-labeling further revealed that organelles (centrosomes, Golgi complexes and mitochondria) redistribute extensively within germline cysts during mouse oocyte differentiation, where the future oocytes collect organelles whereas the remaining cells donate them [42]. Subsequently, in the neonatal mouse ovary, cysts fragment into individual germ cells and those that have collected organelles from sibling cells to form a Balbiani body (aggregation of centrosomes, Golgi and mitochondria) become the primary oocytes. The germ cells that lack a Balbiani body, presumably nurse cells, undergo apoptosis. When cytoplasmic transport is blocked by inhibitors of microtubule polymerization or dynein, primary oocytes contain less cytoplasm and are defective in their ability to develop into later stage oocytes, suggesting that cytoplasmic augmentation via intracyst transport is critical for oocyte development [42].
These studies establish the importance of germ cell connectivity in oocyte development in meroistic ovaries.
Evolution of germ cell connectivity: nursing mechanism does not explain everything
Whereas meroistic ovaries present a clear case for the function of intercellular bridges, the nursing mechanisms is unlikely the only reason for forming intercellular bridges. For example, male germ cells in a broad range of species (insects, fish [45], mammals [46, 47]) develop as a cyst of interconnected cells, yet these male germ cells obviously do not utilize a nursing mechanism (Figure 2). It has been suggested that intercellular connectivity might help complement genomic contents of haploid cells: after meiosis, half of the spermatids lack the X chromosome and the other half lack the Y chromosome. As the X chromosome is essential for cells’ viability and the Y chromosome is essential for male fertility, haploid gametes may need access to the other chromosomes’ products, which may be supported by germ cell connectivity [48–51]. Also, it has been postulated that germ cell connectivity might prevent meiotic drive, although no evidence has been found thus far [52]. However, the need for connectivity in post-meiotic haploid cells does not explain why connectivity in pre-meiotic diploid spermatogonia exists.
In another example, primitive insects such as grasshoppers, cockroaches, and stoneflies have panoistic ovaries, where all germ cells develop as oocytes without ever developing nurse cells. This implies that oocyte development does not always require intercellular bridges to aid cytoplasmic transport from nurse cells. Yet curiously, in several species with panoistic ovaries, mitotic germ cells are interconnected with cytoplasmic bridges, followed by separation of individual germ cells, each of which become an oocyte [53–56].
These observations clearly point to the possibility that cytoplasmic connectivity might have more functions beyond ‘oocyte nursing’. Although no comprehensive effort has been made to determine when germ cell connectivity arose during evolution, there are several examples where researchers describe germ cell connectivity in diverse species, including early-diverging animals such as Cnidarians, Ctenophores, and Poriferans (i.e. non-Bilaterian phyla). In hydra (belonging to Cnidaria, a phylum that diverged roughly 720 million years ago [57]), germ cells are connected both in spermatogenesis and oogenesis, and the utilization of a nurse cell mechanism during oocyte development already makes an appearance [58, 59].
In Poriferans (e.g. sponges, calcarea), germ cells are often generated from transdifferentiation of somatic cells. When they undergo gametogenesis, it appears that only post-meiotic spermatids are connected by cytoplasmic bridges without evidence for earlier spermatogonial connectivity [60, 61], an observation consistent with the hypothesis that male germ cells need to complement their haploid genome by connectivity [48].
Interestingly, evidence of intercellular connectivity goes back possibly before the emergence of metazoans. Choanoflagellates are believed to be the closest relative of animals. During their life cycle, they alternate between the unicellular phase as single-flagellar swimming cells and the colony-forming phase. During the colony-forming phase of the choanoflagellate S. rosetta, a single cell undergoes multiple rounds of cell divisions with incomplete cytokinesis, leading to the formation of a colony of up to ~50 cells [62, 63]. It was shown that intercellular bridges connect the cells within the colony, although it remains entirely unknown what purposes (if any) these bridges may serve. However, this suggests that stable intercellular bridges connecting mitotic cells emerged fairly early during evolution, likely predating the emergence of metazoans and before the evolution of an oocyte nursing mechanism. Future studies in choanoflagellates might provide insights into the potential roles of germ cell connectivity outside of the nursing mechanism.
Increasing sensitivity to DNA damage by germ cell connectivity
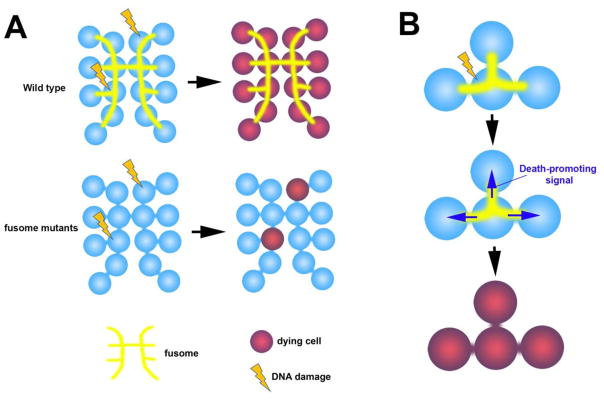
Recent work in the Drosophila testis has shed light on an additional purpose for germ cell connectivity outside of the nursing mechanism. It was shown that in response to DNA damage by irradiation, all spermatogonia within a cyst die in synchrony even when only a subset of them exhibit detectable DNA damage (Figure 3A) [64]. Such synchronized cell death was disrupted in mutants of the fusome, suggesting that intracyst communication mediated by the fusome plays a critical role in synchronized spermatogonial death (Figure 3) [64]. It awaits future investigation to understand how the death-promoting signal may be shared among germ cells within the cyst. The signal may be transmitted through fusome-associated cell death regulators, as is the case for fusome-associated cell cycle regulators in synchronized cell cycle, as described above. Alternatively, death-promoting agents (signals or organelles) might be transported along the microtubules organized by the fusome.
Figure 3. Fusome-mediated sharing of death-promoting signal may enhance sensitivity of spermatogonia to DNA damage.
A. In the Drosophila testis, all spermatogonia within a cyst die in synchrony even when only a subset of spermatogonia have DNA damage. This is mediated by the fusome, as spermatogonia die individually in fusome mutants.
B. The fusome might facilitate the sharing of death-promoting signal among spermatogonia, leading to all-or-none cell death.
Irrespective of the mechanisms, fusome-mediated sharing of death-promoting signal(s) leads to the death of spermatogonia that are not sufficiently damaged to die on their own. Sharing of such signals would effectively increase the sensitivity of the germline to the DNA damage by lowering the threshold of damage per cyst needed to commit to cell death. It might be that by increasing connectivity, germ cells might be able to ‘ cast a bigger net’ to detect DNA damages (and possibly other cellular stresses) for an increased surveillance mechanism for the genomic integrity.
Sensitivity of the germline to DNA damaging agents compared to somatic cells is a long-held observation [65], and has been speculated to be a means by which organisms protect gametes’ genomic integrity. During the evolution of metazoans as germ cells segregated from somatic cells, it might have been necessary to introduce a ‘double standard’ in the surveillance mechanisms of genomic integrity. Whereas germ cells could enjoy the highest level of DNA damage surveillance (where any subpar genome can be culled), such stringent quality control could compromise the development of somatic tissues: cells in the developing embryo undergo a series of concerted fate determination processes (e.g. induction of cell fate in one cell type by another), requiring the presence of all participating cells to be in the right place and time thus limiting the ability to afford discarding too many cells. Therefore, somatic cells may have to tip the balance toward keeping cells alive rather than maintaining the highest level of genomic quality.
Thus, germ cell connectivity that allows intracyst communication to share death-promoting signals might serve as a mechanism that confer a high sensitivity to DNA damage, possibly discriminating germ cells from somatic cells.
Conclusion and future direction
As described here, germ cell connectivity is a broadly conserved phenomenon with its evolutionary root possibly found in pre-metazoan species (e.g. choanoflagellates), although for what purpose intercellular connectivity serves in such pre-metazoan species remains unclear. It is possible that, once intercellular connectivity evolved in pre-metazoan species, it started serving many other purposes such as oocyte nursing and increased sensitivity to DNA damage. It awaits future investigation to understand for what purpose intercellular connectivity may serve in other examples of intercellular connectivity (see Outstanding Questions). It is of particular interest to understand the evolutionary origin of the purpose of intercellular connectivity, which will provide fundamental insights into universality and particularities of intercellular connectivity.
Outstanding question.
What is the evolutional origin for intercellular connectivity? It will be of particular interest to understand the function of intercellular connectivity in pre-metazoan species such as choanoflagellates.
Are there any unknown functions for germ cell connectivity, and if so, what are they?
What are the signals that mediate germ cell death, and how fusome mediates the propagation of such death signals?
Trends Box.
Germ cell connectivity is observed in broad range of organisms, including early diverging metazoans.
In meroistic ovaries, germ cell connectivity functions to transport cytoplasmic material from nurse cells to oocyte.
In Drosophila male germline, germ cell connectivity may serve as a mechanism to increase the sensitivity to DNA damage. When only a subset of germ cells within a cyst are damaged, all germ cells within the cyst die, increasing the net sensitivity to DNA damage.
Acknowledgments
We thank the Yamashita lab members for discussion. We thank Dr. Nicole King for answering questions regarding S. rosetta, Drs. Dan Barbash, Amamda Larracuente, Harmit Malik, Robert Unckless, and Colin Meiklejohn for commenting on meiotic drive. The research in the Yamashita lab is supported by Howard Hughes Medical Institute and National Institute of General Medical Sciences (R01 GM118308-01). Kevin Lu is supported by National Institute of Aging (F30 AG050398-01A1). The research in the Lei lab is supported by the Jones Foundation for Reproductive Medicine and the University of Michigan start-up fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenbaum MP, et al. Germ cell intercellular bridges. Cold Spring Harb Perspect Biol. 2011;3(8):a005850. doi: 10.1101/cshperspect.a005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fawcett DW, et al. The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. J Biophys Biochem Cytol. 1959;5(3):453–60. doi: 10.1083/jcb.5.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch EA, King RC. The origin and early differentiation of the egg chamber of Drosophila melanogaster. J Morphol. 1966;119(3):283–303. doi: 10.1002/jmor.1051190303. [DOI] [PubMed] [Google Scholar]
- 4.Haglund K, et al. Structure and functions of stable intercellular bridges formed by incomplete cytokinesis during development. Commun Integr Biol. 2011;4(1):1–9. doi: 10.4161/cib.4.1.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLean PF, Cooley L. Protein equilibration through somatic ring canals in Drosophila. Science. 2013;340(6139):1445–7. doi: 10.1126/science.1234887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spradling A. Developmental Genetics of Oogenesis. New York: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- 7.Cooley L. Drosophila ring canal growth requires Src and Tec kinases. Cell. 1998;93(6):913–5. doi: 10.1016/s0092-8674(00)81196-4. [DOI] [PubMed] [Google Scholar]
- 8.Robinson DN, Cooley L. Stable intercellular bridges in development: the cytoskeleton lining the tunnel. Trends Cell Biol. 1996;6(12):474–9. doi: 10.1016/0962-8924(96)84945-2. [DOI] [PubMed] [Google Scholar]
- 9.Robinson DN, Cooley L. Drosophila kelch is an oligomeric ring canal actin organizer. J Cell Biol. 1997;138(4):799–810. doi: 10.1083/jcb.138.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelso RJ, et al. Drosophila Kelch regulates actin organization via Src64-dependent tyrosine phosphorylation. J Cell Biol. 2002;156(4):703–13. doi: 10.1083/jcb.200110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudson AM, Cooley L. Drosophila Kelch functions with Cullin-3 to organize the ring canal actin cytoskeleton. J Cell Biol. 2010;188(1):29–37. doi: 10.1083/jcb.200909017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson AM, et al. Actin Cytoskeletal Organization in Drosophila Germline Ring Canals Depends on Kelch Function in a Cullin-RING E3 Ligase. Genetics. 2015;201(3):1117–31. doi: 10.1534/genetics.115.181289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hime GR, et al. Assembly of ring canals in the male germ line from structural components of the contractile ring. J Cell Sci. 1996;109(Pt 12):2779–88. doi: 10.1242/jcs.109.12.2779. [DOI] [PubMed] [Google Scholar]
- 14.Eikenes AH, et al. Spatiotemporal control of Cindr at ring canals during incomplete cytokinesis in the Drosophila male germline. Dev Biol. 2013;377(1):9–20. doi: 10.1016/j.ydbio.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 15.McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262(1):1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 16.Lei L, Spradling AC. Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development. 2013;140(10):2075–81. doi: 10.1242/dev.093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara K, et al. Mouse Spermatogenic Stem Cells Continually Interconvert between Equipotent Singly Isolated and Syncytial States. Cell Stem Cell. 2014;14(5):658–72. doi: 10.1016/j.stem.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenbaum MP, et al. Conversion of midbodies into germ cell intercellular bridges. Dev Biol. 2007;305(2):389–96. doi: 10.1016/j.ydbio.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lores P, et al. Deletion of MgcRacGAP in the male germ cells impairs spermatogenesis and causes male sterility in the mouse. Dev Biol. 2014;386(2):419–27. doi: 10.1016/j.ydbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Yang F, et al. Non-muscle myosin IIB is essential for cytokinesis during male meiotic cell divisions. Dev Biol. 2012;369(2):356–61. doi: 10.1016/j.ydbio.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenbaum MP, et al. TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci U S A. 2006;103(13):4982–7. doi: 10.1073/pnas.0505123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwamori T, et al. Identification and characterization of RBM44 as a novel intercellular bridge protein. PLoS One. 2011;6(2):e17066. doi: 10.1371/journal.pone.0017066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwamori T, et al. TEX14 interacts with CEP55 to block cell abscission. Mol Cell Biol. 2010;30(9):2280–92. doi: 10.1128/MCB.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenbaum MP, et al. Mouse TEX14 is required for embryonic germ cell intercellular bridges but not female fertility. Biol Reprod. 2009;80(3):449–57. doi: 10.1095/biolreprod.108.070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snapp EL, et al. The fusome mediates intercellular endoplasmic reticulum connectivity in Drosophila ovarian cysts. Mol Biol Cell. 2004;15(10):4512–21. doi: 10.1091/mbc.E04-06-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin H, et al. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120(4):947–56. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- 27.de Cuevas M, et al. alpha-spectrin is required for germline cell division and differentiation in the Drosophila ovary. Development. 1996;122(12):3959–68. doi: 10.1242/dev.122.12.3959. [DOI] [PubMed] [Google Scholar]
- 28.Yue L, Spradling AC. hu-li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes Dev. 1992;6(12B):2443–54. doi: 10.1101/gad.6.12b.2443. [DOI] [PubMed] [Google Scholar]
- 29.Lilly MA, et al. Cyclin A associates with the fusome during germline cyst formation in the Drosophila ovary. Dev Biol. 2000;218(1):53–63. doi: 10.1006/dbio.1999.9570. [DOI] [PubMed] [Google Scholar]
- 30.Mathieu J, et al. Aurora B and cyclin B have opposite effects on the timing of cytokinesis abscission in Drosophila germ cells and in vertebrate somatic cells. Dev Cell. 2013;26(3):250–65. doi: 10.1016/j.devcel.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohlmeyer JT, Schupbach T. Encore facilitates SCF-Ubiquitin-proteasome-dependent proteolysis during Drosophila oogenesis. Development. 2003;130(25):6339–49. doi: 10.1242/dev.00855. [DOI] [PubMed] [Google Scholar]
- 32.Varadarajan R, et al. Myt1 inhibition of Cyclin A/Cdk1 is essential for fusome integrity and premeiotic centriole engagement in Drosophila spermatocytes. Mol Biol Cell. 2016;27(13):2051–63. doi: 10.1091/mbc.E16-02-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenhart KF, DiNardo S. Somatic Cell Encystment Promotes Abscission in Germline Stem Cells following a Regulated Block in Cytokinesis. Dev Cell. 2015 doi: 10.1016/j.devcel.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kloc M, et al. Formation, architecture and polarity of female germline cyst in Xenopus. Dev Biol. 2004;266(1):43–61. doi: 10.1016/j.ydbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Loppion G, et al. Study of the potential spermatogonial stem cell compartment in dogfish testis, Scyliorhinus canicula L. Cell Tissue Res. 2008;332(3):533–42. doi: 10.1007/s00441-008-0590-z. [DOI] [PubMed] [Google Scholar]
- 36.Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125(17):3323–8. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann R. Germ Plasm Biogenesis--An Oskar-Centric Perspective. Curr Top Dev Biol. 2016;116:679–707. doi: 10.1016/bs.ctdb.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bastock R, St Johnston D. Drosophila oogenesis. Curr Biol. 2008;18(23):R1082–7. doi: 10.1016/j.cub.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Cox RT, Spradling AC. A Balbiani body and the fusome mediate mitochondrial inheritance during Drosophila oogenesis. Development. 2003;130(8):1579–90. doi: 10.1242/dev.00365. [DOI] [PubMed] [Google Scholar]
- 40.Theurkauf WE, et al. Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development. 1992;115(4):923–36. doi: 10.1242/dev.115.4.923. [DOI] [PubMed] [Google Scholar]
- 41.Huynh JR, St Johnston D. The origin of asymmetry: early polarisation of the Drosophila germline cyst and oocyte. Current biology : CB. 2004;14(11):R438–49. doi: 10.1016/j.cub.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 42.Lei L, Spradling AC. Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science. 2016 doi: 10.1126/science.aad2156. [DOI] [PMC free article] [PubMed]
- 43.Zamboni L, Gondos B. Intercellular bridges and synchronization of germ cell differentiation during oogenesis in the rabbit. J Cell Biol. 1968;36(1):276–82. [PubMed] [Google Scholar]
- 44.Ruby JR, et al. The occurrence of intercellular bridges during oogenesis in the mouse. J Morphol. 1969;127(3):307–39. doi: 10.1002/jmor.1051270304. [DOI] [PubMed] [Google Scholar]
- 45.Grier HJ. Sperm development in the teleost Oryzias latipes. Cell Tissue Res. 1976;168(4):419–31. doi: 10.1007/BF00215993. [DOI] [PubMed] [Google Scholar]
- 46.Dym M, Fawcett DW. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod. 1971;4(2):195–215. doi: 10.1093/biolreprod/4.2.195. [DOI] [PubMed] [Google Scholar]
- 47.Gondos B, Zemjanis R. Fine structure of spermatogonia and intercellular bridges in Macaca nemestrina. J Morphol. 1970;131(4):431–46. doi: 10.1002/jmor.1051310406. [DOI] [PubMed] [Google Scholar]
- 48.Braun RE, et al. Genetically haploid spermatids are phenotypically diploid. Nature. 1989;337(6205):373–6. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho AB, et al. Identification of five new genes on the Y chromosome of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(23):13225–30. doi: 10.1073/pnas.231484998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carvalho AB, et al. Y chromosomal fertility factors kl-2 and kl-3 of Drosophila melanogaster encode dynein heavy chain polypeptides. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(24):13239–44. doi: 10.1073/pnas.230438397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonaccorsi S, et al. Y chromosome loops in Drosophila melanogaster. Genetics. 1988;120(4):1015–34. doi: 10.1093/genetics/120.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurst LD, Pomiankowski A. Maintaining Mendelism: might prevention be better than cure? Bioessays. 1991;13(9):489–90. doi: 10.1002/bies.950130910. [DOI] [PubMed] [Google Scholar]
- 53.Telfer WH. Development and physiology of the oocyte-nurse cell syncytium. Advances Insect Physiol. 1975;11:223–319. [Google Scholar]
- 54.Buning J. Germ cell cluster formation in insect ovaries. Int J Insect Morphol & Embryol. 1993;22(2–4):237–253. [Google Scholar]
- 55.Gottanka J, Buning J. Oocytes develop from interconnected cystocytes in the panoistic ovary of Nemoura Sp. (Pictet) (Plecoptera: nemouridae) Int J Insect Morphol & Embryol. 1990;19(5/6):219–225. [Google Scholar]
- 56.Pritsch M, Buning J. Germ cell cluster in the panoistic ovary of Thysanoptera (Insecta) Zoomorphology. 1989;108:309–313. [Google Scholar]
- 57.Park E, et al. Estimation of divergence times in cnidarian evolution based on mitochondrial protein-coding genes and the fossil record. Mol Phylogenet Evol. 2012;62(1):329–45. doi: 10.1016/j.ympev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 58.Burnett AL, et al. A histological and ultrastructural study of germinal differentiation of interstitial cells arising from gland cells in Hydra viridis. J Morphol. 1966;120(1):1–8. doi: 10.1002/jmor.1051200102. [DOI] [PubMed] [Google Scholar]
- 59.Nishimiya-Fujisawa C, Kobayashi S. Germline stem cells and sex determination in Hydra. Int J Dev Biol. 2012;56(6–8):499–508. doi: 10.1387/ijdb.123509cf. [DOI] [PubMed] [Google Scholar]
- 60.Barthel D, Detmer A. The spermatogenesis of Halichondria panicea (Porifera, Demospongiae) Zoomorphology. 1990;110:9–15. [Google Scholar]
- 61.Lanna E, Klautau M. Oogenesis and spermatogenesis in Paraleucilla magna (Porifera, Calcarea) Zoomorphology. 2010;129:249–261. [Google Scholar]
- 62.Dayel MJ, et al. Cell differentiation and morphogenesis in the colony-forming choanoflagellate Salpingoeca rosetta. Dev Biol. 2011;357(1):73–82. doi: 10.1016/j.ydbio.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fairclough SR, et al. Multicellular development in a choanoflagellate. Curr Biol. 2010;20(20):R875–6. doi: 10.1016/j.cub.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu KL, Yamashita YM. Germ cell connectivity enhances cell death in response to DNA damage in the Drosophila testis. eLife. 2017 doi: 10.7554/eLife.27960. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril. 2013;100(5):1180–6. doi: 10.1016/j.fertnstert.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]