Abstract
Retinal pigment epithelium (RPE) has been implicated as key source of cholesterol-rich deposits at Bruch’s membrane (BrM) and in drusen in aging human eye. We have shown that serum-deprivation of confluent RPE cells is associated with upregulation of cholesterol synthesis and accumulation of unesterified cholesterol (UC). Here we investigate the cellular processes involved in this response. We compared the distribution and localization of UC and esterified cholesterol (EC); the age-related macular degeneration (AMD) associated EFEMP1/Fibulin3 (Fib3); and levels of acyl-coenzyme A (CoA): cholesterol acyltransferases (ACAT) ACAT1, ACAT2 and Apolipoprotein B (ApoB) in ARPE-19 cells cultured in serum-supplemented and serum-free media. The results were compared with distributions of these lipids and proteins in human donor eyes with AMD. Serum deprivation of ARPE-19 was associated with increased formation of FM dye-positive membrane vesicles, many of which co-labeled for UC. Additionally, UC colocalized with Fib3 in distinct granules. By day 5, serum-deprived cells grown on transwells secreted Fib3 basally into the matrix. While mRNA and protein levels of ACTA1 were constant over several days of serum-deprivation, ACAT2 levels increased significantly after serum-deprivation, suggesting increased formation of EC. The lower levels of intracellular EC observed under serum-deprivation were associated with increased formation and secretion of ApoB. The responses to serum-deprivation in RPE-derived cells: accumulation and secretion of lipids, lipoproteins, and Fib3 are very similar to patterns seen in human donor eyes with AMD and suggest that this model mimics processes relevant to disease progression.
Keywords: Aging, Retinal pigment epithelium, Age-Related Macular Degeneration, Cholesterol, Fibulin 3, Serum deprivation
Introduction
Retinal pigment epithelium (RPE) cells are crucial for retinal homeostasis and visual function [1]. The key homeostatic functions of RPE include phagocytosis of shed photoreceptor outer segments; formation of the blood retinal barrier; transportation of nutrients, water, and ions to the retina and removing metabolic waste from the retina to the choroidal vasculature [2]. The role of RPE in the visual cycle includes absorption of light, protection against photo-oxidation and recycling of all-trans-retinol to 11-cis retinal to maintain the visual cycle [3].
During aging, RPE cells undergo numerous structural changes that typically progress gradually and at varying rates among individuals [4]. These include increased density of residual bodies or undigested outer segments, and an accumulation of lipofuscin pigment and basal deposits on or within BrM [5]. The aging process also results in the accumulation of lipids, formation of druse between the basal lamina of the RPE and inner collagenous layer of BrM, microvilli atrophy, disruption of the basal in-folding, and thickening of the BrM [6, 7].
Studies investigating the accumulation and composition of lipids in BrM/choroid implicate both plasma and local cells as the source of the lipids [6, 8, 9]. RPE especially has been implicated as a secretor of esterified cholesterol (EC)-rich ApoB to BrM [10]. Initially it was hypothesized that ApoB lipoprotein from RPE removes fatty acids released by lysosomal phospholipases after phagocytosis of shed outer segments [11], however, BrM lipoproteins and drusen are more highly enriched in EC and unesterified cholesterol (UC) than outer segment membranes [12]. Thus, the source of lipids found in RPE lipoproteins is not yet clear.
Confluent human-derived ARPE-19 cells express scavenger receptor B-I (SRB-I) for high-density lipoprotein (HDL) [13]. Furthermore, we have shown that ARPE-19 cells also express functional receptors for LDL (LDLR), and respond to serum deprivation by upregulating expression of genes in the lipid and cholesterol pathways as well as accumulating intracellular UC [14]. A similar transcriptional response is also seen in primary human fetal RPE. Serum-deprived ARPE-19 cells also show increased secretion of the extracellular matrix protein Fib3 which is deposited basally in AMD [15, 16]. Accumulating defects in BrM and dysfunction of the choriocapillaris may obstruct nutrient transport and signaling to the RPE and retina. The response to serum deprivation by RPE cells could contribute to upregulated cholesterol synthesis and eventually to the secretion and accumulation of cholesterol and lipids associated with the progression of AMD.
Here, we examine the behavior of ARPE-19 cells under serum deprivation. Our results show that under serum deprived conditions UC is synthesized by ARPE-19 in the endoplasmic reticulum (ER) and accumulates intracellularly, whereas EC lipid droplet accumulation is lower than in cells in serum. The effects of serum deprivation on ARPE-19 cells include increased formation of FM dye-positive membrane vesicles, and increased basal secretion of Fib3. Both the membrane vesicles and Fib3 co-localize with UC. Serum deprivation also increased the expression of ACAT2 and secretion of ApoB lipoprotein from ARPE-19 cells. ACAT2 promotes cholesterol esterification and stimulates cholesteryl ester secretion in ApoB-containing lipoproteins [17], suggesting that the lower levels of intracellular EC under serum-deprivation may be associated with increased formation and secretion of ApoB lipoproteins.
Material and Methods
In vitro RPE cell culture
ARPE-19 cells were purchased from ATCC (ATCC, Manassas, VA, CRL-2302; passage numbers 5–15), were cultured in complete Dulbecco’s Modified Eagle Medium F12 (Gibco Life Technology, Gaithersburg MD; DMEM F12) medium containing 10% fetal calf serum (FCS) and 1% penicillin/streptomycin (100 unit penicillin/100 μg streptomycin per ml), (Invitrogen, San Diego, California, USA). Cells were grown in an incubator at 37°C with 5% CO2 using T25, T75 tissue culture flasks, tissue culture plates or collagen-coated transwell inserts (Corning Primaria plastic culture ware, Thermo Fisher Scientific, Waltham, MA). ARPE-19 cells were authenticated using short tandem repeat (STR) analysis by the cell line authentication service (ATCC). The cell line used has been published previously and the cells were free from contamination. ARPE-19 cells were validated for the expression of the RPE65 and RLBP1 marker genes [18] with PCR of cDNA using the RPE65-specific primers 5′-CCA GAT GCC TTG GAA GAA GA-3′; 5′-CTT GGC ATT CAG AAT CAG GAG-3′ (99 bp amplicon) and the RLBP-specific primers 5′-AGA TCT CAG GAA GAT GGT GGA C-3′; 5′-TGG ATG AAG TGG ATG GCT TT-3′ (72 bp amplicon) [14].
Transwell culture system for ARPE-19 cells
Permeable supports enable cells to grow in a polarized state under more natural conditions and results in cells that are morphologically and functionally better representative of in vivo cells. In this model, cells were grown on collagen-coated 24 mm transwell inserts (Corning Primaria plastic culture ware, Thermo Fisher Scientific, Waltham, MA) for 4 weeks before serum starvation experiments. The permeable membranes generate two compartments, the apical (upper compartment) domain corresponds to the retinal facing side of the RPE monolayer and the basolateral (lower compartment) domain corresponds to the choroidal/BrM facing side of the RPE monolayer.
For live cell imaging, the membrane immersed in culture media was dissected and placed with the cells facing downward on in 100 μl of media in a petri-dish and imaged using a Zeiss LSM 880 microscope with Airyscan (Zeiss, USA). For membrane sections, the membranes with cells fixed in 4% PFA for 15 minutes were glued, cells facing upward, onto a 4mm thick 5% agarose gel. The blocks with attached membrane were cut into 100μm thick sections using a Leica_VT1000S_Vibratome.
Serum deprivation
After reaching confluence in serum supplemented media culture medium was removed and the cells were washed once with serum free medium (SFM) before re-incubating in SFM, DMEMF12 with 1% penicillin/streptomycin (100 units penicillin/100μg streptomycin per ml). Day 0 in all experiments denotes cells that remained in complete culture medium (10% serum) throughout the experiment. Days 1, 3, 5 and 9 represent cells in SFM.
Western blot
ARPE-19 cells washed with 1X phosphate-buffered saline (PBS; KD Medical, Columbia MD: catalog# RGF-3190) were either lysed in RIPA buffer with protease inhibitors (Thermo Fisher Scientific) or culture supernatants were collected. Protein concentrations were measured using a BCA assay kit (Thermo Fisher Scientific). 20μg of total protein was loaded onto 10% SDS-PAGE gel. Gels were run at 80V for 30 min followed by 150V for 60 min. Proteins were transferred to Immobilon-FL polyvinylidene difluoride (PVDF) membrane at 350 mA for 50 min. Secreted protein blots were transferred to 5 ml of Ponceau S staining solution for 5 min, and washed thoroughly with 5% acetic acid solution (v/v) before continuing with blocking. All blots were blocked with 5% bovine serum albumin (BSA) in tris-buffered saline with Tween-20 (TBS/T) for 1h at room temperature then rinsed once in TBS/T. Next the blots were incubated with primary antibodies diluted 1:1000 with TBS/T overnight at 4 °C. Rabbit polyclonal anti-ACAT1 (Abcam), rabbit polyclonal anti-ACAT2 (Abcam) and rabbit anti- EFEMP-1 (Century Biochemicals) were used as primary antibodies. After thorough washes, the membranes were incubated with HRP-conjugated secondary antibodies diluted 1:10000 for 2h in the dark at room temperature. Finally, the membranes were washed in TBS/T 3 times before scanning using LumiGold ECL Western Blotting Detection Kit (VerII; Signagen Laboratories, Ijamsville, MD). The blots shown are representative of at least three biological repeats of each experiment. The β-actin level or Ponceau S stained image was used to normalize the signal from other proteins. The Western blot signals were quantitated using ImageJ software (version 1.45; National Institutes of Health, Bethesda, MD).
Immunofluorescent labeling and staining of cells
ARPE-19 cells cultured on cover slips, chambers or transwell inserts were washed with cold PBS and fixed with 2% paraformaldehyde (PFA) for 10 min, followed by permeabilization with 0.1% Triton-X for 5 min. The samples were blocked with 5% BSA for 30 min at room temperature. Cells were incubated with EFEMP-1(Fib3) (Century Biochemicals) or rabbit polyclonal ZO-1 (Abcam) primary antibody diluted 1:100 for 4h. After washing with PBS, samples were incubated with anti-rabbit 488 or 568 (Thermo Fisher Scientific) secondary antibody diluted 1:100 with PBS and counter stained with DAPI diluted 1:500 in the dark for 1h. FM dye (Thermo Fisher Scientific) was added to live cells for 1 minute at room temperature, Hoechst 33342 for 30 mins at 37°C and CellLight GFP early/late endosomes or, CellLight GFP endoplasmic reticulum (ER) staining (Thermo Fisher Scientific) were added based on manufacture instructions to cells over night at 37°C. After extensive washing, samples were mounted using Prolong Gold (Molecular Probes), and examined by a Zeiss LSM 880 confocal microscope with Airyscan (Zeiss, USA) at 63X magnification.
Filipin and Oil Red O labeling
ARPE-19 cells were grown on a Nunc® Lab-Tek® Chamber (Sigma-Aldrich) in DMEM with 10% serum to confluence. Culture medium was removed, the cells were washed once with SFM, and then re-incubated in SFM. At each time point, the medium was removed, and the cells were washed once with PBS before fixing in 4% PFA for 30 min at room temperature. Fixed cells were washed twice with PBS for 5 min each.
The Oil Red O working solution was 3 parts of Stock Solution to 2 parts water. Cells were incubated with Oil Red O for 10–20 minutes with continuous nutation. Following incubation, the cells were washed 2–5 times with water until no excess stain remained, followed by filipin staining. Desiccated filipin was diluted to 0.05 mg/ml in PBS. Diluted filipin was added to each well in the dark at room temperature for 2h. Then the cells were washed once with PBS, followed by mounting with Prolong Gold (Molecular Probes). Staining was immediately visualized using a FV1000 confocal microscope (Olympus America Inc., Center Valley, PA).
Immunofluorescence staining of human retina sections
Normal and AMD-affected human donor eyes from NDRI (Table 1) were fixed with formalin, washed in PBS and cryoprotected in PBS/sucrose. Eyes were cut and sectioned through the macula. Sections were incubated with ICC buffer (0.5% BSA, 0.2% Tween20, 0.05% sodium azide, in PBS, pH 7.3) for 1 hour at room temperature. Sections were incubated with primary antibodies for EFEMP-1(Fib3) (Century Biochemicals) diluted 1:100, mouse monoclonal Apolipoprotein B (Thermo Fisher Scientific) diluted 1:200 overnight at 4 °C. Following thorough washes with ICC buffer, anti-rabbit 488 and anti-mouse 633 secondary antibodies (Thermo Fisher Scientific) were added for 1 hour at room temperature. Sections were washed extensively with ICC buffer, stained with Oil Red O and filipin as described above, mounted, and imaged by FV1000 confocal microscope (Olympus America Inc., Center Valley, PA).
Table 1.
Donor eyes described in this paper.
| NDRI | Genotype | Description | Age/sex |
|---|---|---|---|
| #0068299 | H/Y | Normal | 76/M |
| #0068536 | H/H | Dry AMD | 78/F |
| #0068280 | Y/Y | Wet AMD | 85/M |
Quantitative PCR
RNA was isolated from cells grown and treated as described in 6-well tissue culture plates using 1ml of Trizol® (#15596-018, Invitrogen, Invitrogen.com) and resuspending the isolated RNA in 10μl DEPC-treated water.
cDNA was prepared from using 6μl of the isolated RNA per sample using ProtoScript® II First Stand cDNA Synthesis Kit (New England BioLabs Inc. Ipswich, MA). Each cDNA sample was diluted to 300μl with H2O.
Quantitative PCR of selected genes was performed using the Roche Universal ProbeLibrary hydrolysis probe system (Roche, Mannheim Germany), the Luna Universal Probe qPCR Master Mix (New England BioLabs Inc. Ipswich, MA), and custom primers (Table 2) (Eurofins MWG|Operon) on an Applied Biosystems ViiA7 System using QuantStudio™ (v1.2) software (Life Technologies, Carlsbad, CA) following manufacturer’s recommendations. Relative expression values were calculated using the RQ (relative quantity) method normalized to the genes ABCF3 and NOL8. RQ = 2(CtDay0 – CtDay) × NF. NF (normalization factor) = (RQRef1 × RQRef2)1/2.
Table 2.
Primers and Probes for QPCR
| Gene | Probe # | Left Primer | Right Primer |
|---|---|---|---|
| ABCF3 | #27 | CCAACAGGGCCTCTCAAGT | CGACCTCTGATTCCTTGTCC |
| NOL8 | #26 | CTGCAGCTCTGACCAGTGAC | CACTGTTTCAACTCTGTAGGTCTCTT |
| ACAT1 | #17 | GATCCCCAAAAAGTGAATATCAA | ATCCTGGCTCCAGACATCC |
| ACAT2 | #40 | CCGGAAGATGTGTCTGAGGT | CACCCACACTGGCTTGTCTA |
Apolipoprotein B (ApoB) secretion ELISA
ARPE-19 cells were grown in 6-well tissue culture plates (Corning Primaria plastic culture ware, Thermo Fisher Scientific, Waltham, MA) with DMEM + 10% serum. When the cells were confluent, the culture medium was removed, and the cells were washed once with serum SFM. Next SFM media was added to the cells to begin the experiment. Once the incubation time in SFM was complete, the culture supernatant was collected and assayed for ApoB using an ELISA assay kit (Human Apolipoprotein B ELISA Kit (APOB) - ab108807) (ABCAM, Cambridge, MA). In brief, 50μL Apolipoprotein B Standards and culture supernatant from each sample were added to an active ApoB-capturing antibody-coated 96-well plate. The plate was incubated at room temperature for 2h. After thorough washing, 50μL of Biotinylated Apolipoprotein B Antibody was added to each well and incubated for 1h. After washing, 50μL of Streptavidin-Peroxidase Conjugate was added to each well and incubated for 30 mins. The wells were washed for a final time, 50μL of Chromogen Substrate was added to each well for ten minutes followed by 50μL of Stop Solution. The absorbance was read at wavelength of 450nm using a Bio-Rad 680 reader (Bio Rad laboratories, Hercules, CA).
Data and statistical analysis
Data were expressed as mean ± SEM with P<0.05 deemed statistically significant. Differences between groups were assessed using either an independent t test or one-way analysis of variance with Dunnett’s or Tukey’s post-hoc tests.
Results
Accumulation of EC and UC in serum deprived ARPE-19
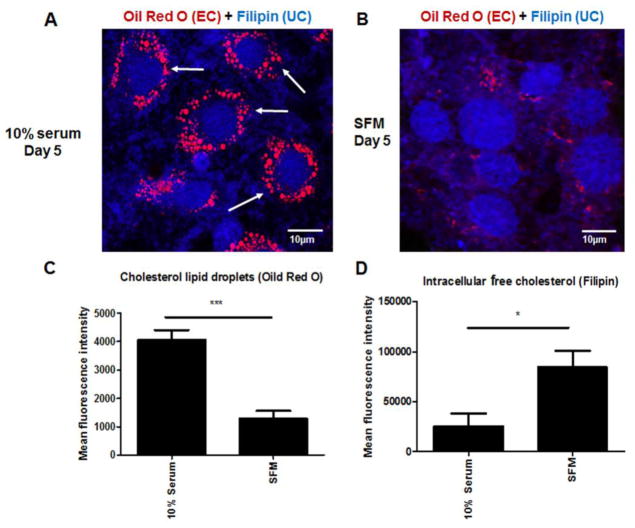
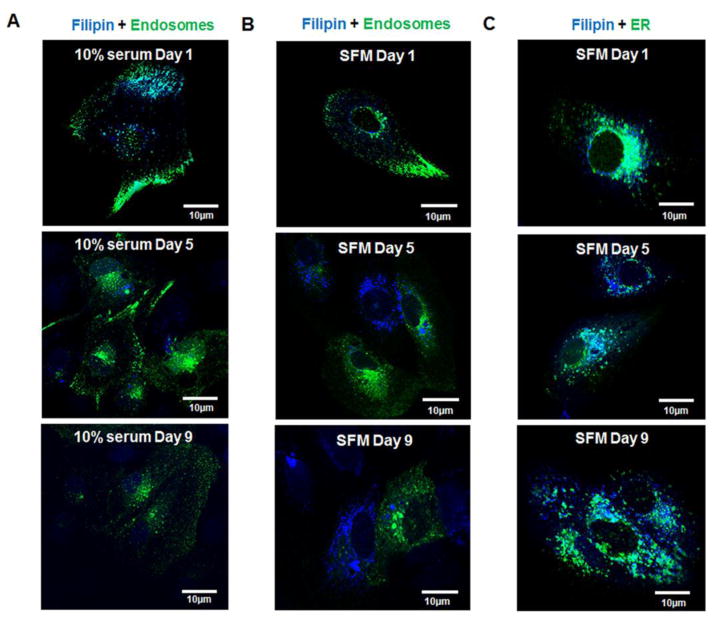
Our earlier findings showed that in ARPE-19 cells cultured in SFM, cholesterol and lipid synthesis pathway genes and proteins were prominently upregulated [14]. Based on these findings, the levels of intracellular EC and UC were investigated. Increased intracellular filipin labeling for UC was seen in cells after 5 days in SFM, compared with cells grown for the same length of time in medium containing 10% serum (Fig 1A–B and D), confirming our previous findings [14]. However, the accumulation of EC droplets was significantly lower in cells after 5 days in SFM compared with cells cultured in 10% serum (Fig 1A–B and C). The EC droplets stained with Oil Red O showed uniform organization around the nuclei in 10% serum cells, but not in SFM. By day 1, cells cultured in 10% serum showed colocalization of filipin with early endosome markers, indicating the movement of free cholesterol into the cells (Fig 2A). Filipin labelling decreased from days 5 to 9 in 10% serum indicating there was no new free cholesterol movement into the cells (Fig 2A). There was little or no colocalization of filipin and early endosomes in cells in SFM at day 1, but filipin labelling continued to increase from day 5 to 9, indicating that these cells were synthesizing UC (Fig 2B). Endoplasmic reticulum (ER) of ARPE-19 cells in SFM showed filipin labelling, suggesting the biosynthesis of cholesterol in these cells was occurring in ER (Fig 2C).
Figure 1. Changes in EC and UC accumulation in serum deprived ARPE-19 cells.
A: Filipin (UC) and Oil Red O (EC droplet) staining of cells in complete growth medium at day 5. B: Filipin (UC) and Oil Red O (EC droplet) staining of cells in SFM at day 5. C: Quantitation of Oil Red O fluorescence intensity in ARPE-19 cells in SFM compared to 10% serum. D: Quantitation of filipin fluorescence intensity in ARPE-19 cells in SFM compared to 10% serum. The fluorescence intensity of filipin and Oil Red O in each cell and background fluorescence from cell-free area was measured using ImageJ software 1.45. The background fluorescence intensity was deducted for data analysis. * P <0.05, *** P <0.001. Data analysed by student T-test. N= 50.
Figure 2. Confocal images showing changes in localization of UC cholesterol in cell organelles during serum deprivation.
A: Filipin (UC) and Endosome staining of cells in 10% medium from day 1–9. B: Filipin (UC) and Endosome staining of cells in SFM from day 1–9. C: Filipin (UC) and Endoplasmic Reticulum (ER) staining of cells in SFM from day 1–9.
Membrane vesicle formation
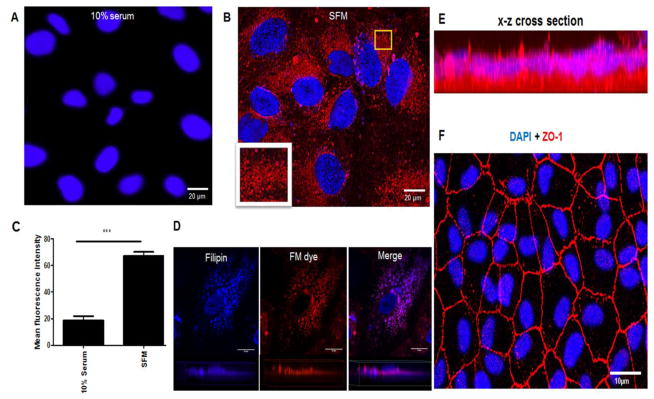
High accumulation of UC is toxic to cells, and cholesterol homeostasis is tightly regulated [19]. We observed that despite increased levels of UC, RPE cells remained viable, suggesting that the increased UC may be packaged for transport out of the cells. Staining with FM dye for membrane vesicles showed that cells in SFM increased production of intracellular vesicles compared to cells in serum (Fig 3A–C). Filipin co-labelling demonstrated colocalization of UC with the membrane vesicles (Fig 3D). Z-series images of polarized RPE cells showed these vesicles located mostly in the basal domains of ARPE-19 cells (Fig 3E). Figure 3F is a 2D image of the polarized ARPE-19 monolayer positive for ZO-1 staining, confirming tight junction formation between cells.
Figure 3. Serum deprivation increased FM dye positive membrane vesicle formation and UC accumulation in ARPE-19 cells.
A: Confocal image of ARPE-19 cells in 10% serum stained with Hoechst 33342 (blue) and FM dye (red). B: Confocal image of ARPE-19 cells in SFM stained with Hoechst 33342 (blue) and FM dye (red). Yellow square highlights the magnified area showing membrane vesicles stained with FM dye. C: Quantitation of FM dye fluorescence intensity in ARPE-19 cells cultured in SFM compared to 10% serum. *** P <0.001. Data analysed by student T-test. N= 50. D: 63x magnification 2D and 3D images of a ARPE-19 cell showing filipin (UC) localization with FM dye. E: Live ARPE-19 cell image of FM dye and Hoechst 33342 on confluent cell monolayer grown on transwells. FM dye was localized mainly in the basal domain as confirmed by the x–z cross-section stack image panel. F: Fixed image of ARPE-19 cell monolayer grown on transwells stained for ZO-1 (red) and DAPI (blue).
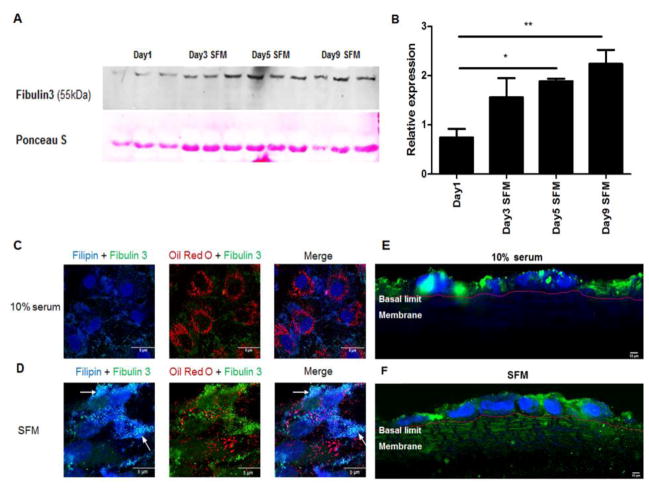
Increased intracellular EFEMP1/Fib3 expression and secretion
Supporting our previous findings [15], we detected increased Fib3 translation and secretion by ARPE-19 cells in SFM (Fig 4A). Levels of secreted Fib3 significantly increased from 5 to 9 days culture in SFM (Fig 4B). Immunofluorescent images of ARPE-19 cells in SFM at day 5 showed that increased accumulation of Fib 3 in distinct granules when compared to cells serum (Fig 4C–D). Furthermore, these Fib3granules showed some co-localization with filipin stain (Fig 4D). Vibratome sections of polarized RPE cells grown on transwells with 10% serum showed Fib3 predominantly in the apical domain (Fig 4E) whereas for cells in SFM, Fib3 was found in both apical and basal domains and in the matrix, indicating basolateral secretion of Fib3 reminiscent of the depositions found in AMD [15, 16] (Fig 4F).
Figure 4. Serum starvation increased intracellular levels of Fib 3 in distinct granules and secreted levels in SFM over time.
A: Western blot showing expression of Fib3 in ARPE-19 cell culture media supernatant after the indicated number of days in serum-free medium. For the bands that showed progressive increases, the blots were scanned and quantitated using ImageJ. Ponceau S staining was used to normalize. B: Quantification of Fib3 in cell culture supernatant day 1–9. *, P < 0.05; **, P < 0.01. One-way ANOVA followed by Tukey’s multiple comparison test. N = 3. C: Filipin, Oil Red O and Fib3 staining of ARPE-19 in 10% serum. D: Filipin, Oil Red O and Fib3 staining of ARPE-19 in SFM. E–F: Immunofluorescence staining of Fib3 and DAPI in polarized ARPE-19 monolayer sections. ARPE-19 cells were grown on transwells, the membranes were embedded on 5% agarose gel and 100 μm sections were made using Leica_VT1000S_Vibratome. E: ARPE-19 in 10% serum. F: ARPE-19 in SFM. The basal limits of the cells and the beginning of the membrane is marked with a red line.
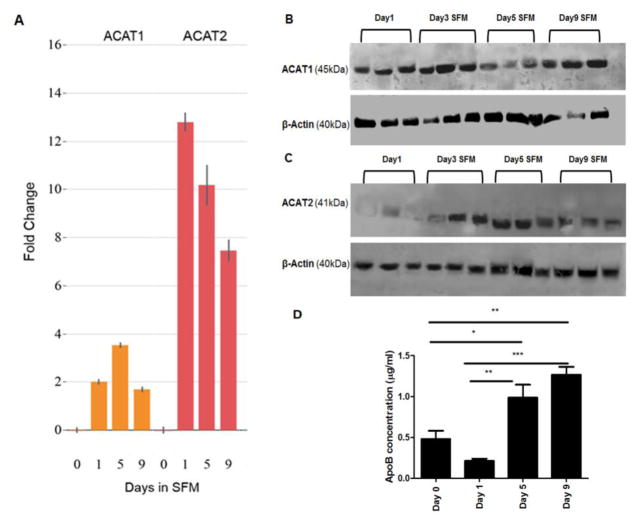
Increased ACAT2 expression and ApoB secretion following serum starvation
Figure 1 shows that ARPE-19 cells cultured in SFM accumulated significantly fewer EC droplets compared to 10% serum cultures. Therefore, we investigated changes in ACAT enzymes. ACATs are located in the ER and form cholesterol esters from cholesterol [20]. The mRNA levels of ACAT2 in particular increased significantly with serum starvation (Fig 5A). ACAT1 protein was expressed stably over several days of serum starvation whereas expression of ACAT2 protein increased from day 3 onward (Fig 5B and 5C). At first sight, the increase in levels of ACAT2 seemed inconsistent with the low levels of Oil Red O stained EC seen with serum starvation. However, increased levels of cellular cholesterol and ACAT2 have previously been shown to increase formation and secretion of EC-containing ApoB [17]. ApoB secretion, assayed by ELISA, showed a significant increase in the supernatants of cells in SFM compared to 10% serum cultures at day 0 (Fig 5D). This suggests that EC produced by the ARPE-19 cells under serum deprived conditions is packaged into nascent ApoB-containing lipoproteins for secretion out of the cells. In contrast, in cells cultured in serum, the EC was predominantly stored as lipid droplets in the cytosol.
Figure 5. Serum deprivation increased ACAT2 expression and secreted ApoB concentrations in ARPE-19 cells.
A: Quantitative PCR analysis of ACAT1 and ACAT2 gene expression in ARPE-19 cells at day 0 (10% serum) and SFM at day 1, day 5 and day 9. Normalized fold change relative to day 0 values measured over the course of serum starvation. All measures normalized to the average values of ABCF3 and NOL8 genes. Error bars represent standard deviation. B–C: Western blots showing expression of ACAT1 (45kDa) and ACAT2 (41kDa) in ARPE-19 cell lysate after the indicated number of days in serum-free medium. Blots are representative of two to three replications each. D: Secreted ApoB concentrations in ARPE-19 cell culture media from day 0 (10% serum) days 1–9 in serum-free medium. Cell supernatants from each treatment were evaluated by specific ApoB ELISA kit *, P < 0.05; **, P < 0.01, ***, P < 0.001. One-way ANOVA followed by Tukey’s multiple comparison test. N = 4.
Localization of UC, EC, Fib3 and ApoB in AMD Eyes
We showed that in ARPE-19 cells in SFM, intra-cellularly synthesized UC colocalized with Fib3 and FM dye-positive membrane vesicles, while EC was secreted with ApoB. We compared these observations with human donor AMD eyes. Flourescent stains and antibodies were used to localize UC, EC, Fib3 and ApoB in donor eyes with either the dry or wet forms of AMD and a normal eye. Eyes were previously genotyped for the Complement Factor H (CFH) 402 variant [15].
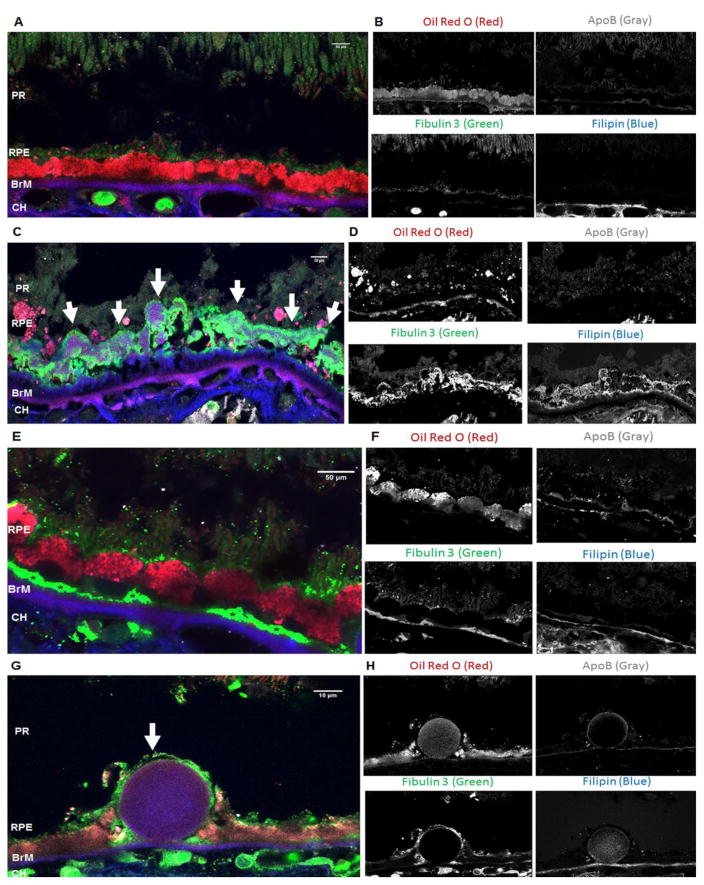
Figure 6A and 6B shows that in the normal eye Fib3 was located in the apical tips of the RPE and was not associated with UC or EC while there was localization of both UC and EC as well as ApoB in BrM. In the eye diagnosed with Geographic atrophy (dry) AMD (#0068536) (Fig 6C and 6D) a large area of soft drusen showed accumulated UC, especially in a dense area directly above BrM, and some deposits of EC. There was also strong Fib3 staining in the soft drusen associated with UC, as seen before. There were no intact RPE cells above the drusen area. There was some colocalization of Oil Red O (EC) and ApoB staining observed in the drusen. Figures 6E and 6F show the beginning of continuous basal deposits below the intact RPE in a different area of the same eye diagnosed with dry AMD.
Figure 6. Patterns of localization of Fib3, EC, UC and ApoB in normal and AMD human eyes.
RPE: retinal pigment epithelium; BrM: Bruch’s membrane; CH: Choroid. A: Section of retina from a normal human H/Y eye (#68299) labeled with Fib3 (green), Oil Red O (red), filipin (blue) and ApoB (grey). B: Individual color channels for Fib3, Oil Red O, filipin and ApoB labelling. Fib3 immunoreactivity is observed in the apical tips of the RPE and in CH whereas filipin labelling is only located in BrM and CH showing no colocalization with Fib3. Oil Red O labels RPE, BrM, CH while ApoB staining is located mainly in the BrM. C: Section from a H/H AMD human donor eye (#68536) with large area of soft drusen deposit (indicated by arrows) labeled with Fib3 (green), Oil Red O (red), filipin (blue) and ApoB (grey). D: Similar section to B showing individual color channels for the different stains. Strong Fib3 immunoreactivity is observed in the druse. Strong filipin labelling is observed in the druse and some signal colocalizes with that of Fib3. Oil red O staining and ApoB are also observed in the druse with some areas of colocalization. E: area of the H/H AMD human donor eye (#68536) showing Fib3 deposition (indicated by *) in basal regions of RPE above the BrM. F: individual color channels of panel E for the different stains. G: Section from a Y/Y AMD human donor eye (#68280) with large hard druse (indicated by the arrow) labeled with Fib3 (green), Oil Red O (red), filipin (blue) and ApoB (grey). H: Similar section to B, D and F showing individual color channels for the different stains. Fib3 immunoreactivity is only observed in the RPE and CH. Filipin and Oil Red O labelling is observed in the drusen and BrM with strong colocalization. ApoB staining is only seen in the RPE some in the BrM. ApoB staining in the BrM is colocalized with both filipin and Oil Red O. Note that the images for individual channels show that sections stained under this protocol show only limited autofluorescence, so that each dye is essentially specific. Phase contrast images of unstained, close sections from the same eyes are shown in Supplement Figure 1.
Strong Fib3 immunolabeling was seen in choroidal regions in the eye diagnosed with wet AMD (#0068280) that was not observed in dry AMD and normal eyes (Figure 6G and 6H). The hard drusen and BrM both had strong staining for both filipin and Oil Red O (Figure 6G and 6H) as previously reported [8, 21]. There was also ApoB localization in BrM with both UC and EC, however there was no Fib3 accumulation within the druse in this form of the disease (Figure 6G and 6H). There was some intact RPE in the drusen area that showed strong staining for Fib3 as did an area above the BrM (Figure 6G and 6H). In all these sections subjected to Oil Red O staining, each fluorescent signal was limited to specific channels, showing that there were only low levels of autofluorescence.
Discussion
AMD is a multifactorial disease that affects the photoreceptors and involves the RPE and BrM [22]. AMD has metabolic, inflammatory and vascular components, resulting in secondary neurodegeneration of the photoreceptors [23, 24]. The vascular insufficiency component of the disease occurs due to recession of the capillary bed and the accumulation of extracellular material between outer retinal cells and their blood supply, therefore AMD is now compared in many ways to atherosclerotic cardiovascular disease [25].
Cholesterol is strongly implicated in the pathogenesis of AMD [26–29]. Studies have established that EC and UC are both significant components of basal linear deposits (BLinD), soft drusen associated with AMD, and comprise >40% of hard druse volume [8, 15, 21, 30]. Large/soft drusen are a major risk factor for AMD progression [31]. It is suggested that soft druse formation occurs through secretion of ApoB/ApoE-containing lipoproteins from RPE that are retained by defective BrM, until a lipid-rich layer forms on BrM’s surface [32]. Previous work done in our laboratory using the serum deprived ARPE-19 model and primary human fetal RPE showed upregulation of cholesterol pathway genes [14], while Zheng et al. [33] showed expression of genes for cholesterol homeostasis in human donor RPE [33].
In the current study, using the same ARPE-19 model under serum deprived conditions, we confirmed the increase in UC compared with cells grown in serum and that ARPE-19 cells began to endogenously synthesize cholesterol in the endoplasmic reticulum. This finding further supports our previous findings that both the precursor and activated form of SREBF2 increased in ARPE-19 cells in SFM [14]. SREBF2 is anchored in the ER, and when activated by proteolysis is translocated to the nucleus where it activates a group of target genes [34, 35]. In most cells, these pathways are activated to maintain levels of cholesterol needed for membrane synthesis in dividing cells. RPE cells, in contrast, upregulate these pathways in response to serum deprivation leading to increased levels of UC rather than homeostasis.
Here we show that UC produced by ARPE-19 cells in SFM localized in membrane vesicles and in distinct granules that colocalized with Fib3. In addition, experiments in polarized and differentiated ARPE-19 cells on transwells showed that Fib3 was secreted basally from the cells in SFM, compared with a predominantly apical location in cell cultures supplemented with serum. Western blots confirmed increased levels of secreted Fib3 from cells in SFM. The serum-deprivation response of ARPE-19 was also associated with increased expression of ACAT2 and increased ApoB secretion, supporting the notion that RPE cells secrete ApoB-particles when RPE cholesterol levels increase [33].
Although these experiments used RPE-derived cells in culture, their results are quite consistent with what can be seen in AMD donor eyes. In particular, we found very similar localization of Fib3, UC, EC and ApoB in AMD donor eyes with soft drusen. While a normal eye showed Fib3 mainly in the apical tips of the RPE, in dry AMD in areas where the RPE was still intact we saw basal deposition of Fib3. In an area of the donor eye with large soft drusen we observed a thick layer of UC directly above BrM, similar to what has previously been described [32]. Above this region there was a large soft drusen containing Fib3 and UC. In contrast to the pattern in the soft drusen, in the eye with wet AMD, presenting prominent hard druse, there was no Fib3 observed in the druse but there were areas with basal Fib3 deposition. Both EC and ApoB staining were observed in soft and hard drusen in different AMD eyes and in BrM, corroborating previous findings [36]. These results show that the serum deprived ARPE-19 model may illustrate how stressed RPE cells could be responsible for the secretion of key components of soft drusen and basal deposits along BrM.
It is conceivable that age or insult-related dysfunction of the choroidal vasculature and BrM could decrease the delivery of key serum components to the RPE. This could trigger an RPE stress response that involves synthesis of lipids and secretion of lipid/protein complexes, particularly including Fib3. This, in itself, could contribute to the “oil-spill” blockage at BrM, adding another step in a cascade of events in AMD progression. So why does RPE have this response if it may actually make things worse in AMD? Two possibilities are suggested. One is that the response is aimed at improving deficient transport mechanisms, perhaps by modifying the fluidity of BrM [37, 38]. The other is that this response has been selected through evolution because of the barrier function of the RPE. Even in a young eye, local dysfunction of the retina/vasculature complex, perhaps through trauma or infection, could lead to RPE death. In the absence of a patch of functioning RPE, this could progress to local breakage of the barrier between the retina and the blood supply, allowing infiltration of the immune system or exposure of RPE and retina cells to blood, either of which could cause widespread damage to the retina. To retard this, RPE in the damaged region could respond by reinforcing BrM with lipids and ECM proteins, including Fib3. While this could result in a local patch of dead RPE and photoreceptors, it might serve to delay wider damage. Indeed, dry AMD is generally a patchy disease, with regions of healthy photoreceptors preserved for some time. Such a mechanism could help preserve the retina in a young individual, but in extreme age, with multiple age-related dysfunctions it might contribute to disease progression.
Conclusions
We have shown that serum-deprivation of RPE cells in culture produces several responses that seem to mimic steps in the AMD cascade. These include specific patterns of synthesis and processing of UC and EC and secretion of the AMD-related protein Fib3. If this same mechanism occurs in at least some forms of AMD, it is possible that supplementation of key factors or nutrients (such as zinc and other factors as suggested by AREDS [39]) could prolong the survival of RPE and thereby delay AMD progression. The serum-deprivation ARPE-19 model provides opportunities for exploring such possibilities.
Supplementary Material
Highlights.
Serum deprivation regulates cholesterol levels in confluent RPE cells.
Serum deprivation leads to specific distributions of cholesterol and Fibulin3.
The patterns of protein and lipid deposition resemble those in AMD eyes.
Acknowledgments
We thank our colleagues Jianguo Fan and Joshua Lerner for help with transwell section preparation.
Funding: This work was supported by the Intramural Program of the National Eye Institute.
Abbreviations
- RPE
Retinal pigment epithelium
- UC
unesterified cholesterol
- EC
esterified cholesterol
- Fib3
EFEMP1/Fibulin3
- AMD
age-related macular degeneration
- ApoB
Apolipoprotein B
- BrM
Bruch’s membrane
- HDL
high-density lipoprotein
- FCS
fetal calf serum
- SFM
serum free medium
- PVDF
polyvinylidene difluoride
- BSA
bovine serum albumin
- PFA
paraformaldehyde
- CFH
Complement Factor H
- ER
endoplasmic reticulum
- BLinD
basal linear deposits
- ACAT
Acyl-coenzyme A (CoA): cholesterol acyltransferases
- SRB-I
scavenger receptor B-I
Footnotes
Conflict of interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dinusha Rajapakse, Section on Molecular Structure and Functional Genomics, National Eye Institute, Building 6 Room 107, National Institutes of Health, Bethesda, MD, USA.
Katherine Peterson, Section on Molecular Structure and Functional Genomics, National Eye Institute, Building 6 Room 107, National Institutes of Health, Bethesda, MD, USA.
Sanghamitra Mishra, Section on Molecular Structure and Functional Genomics, National Eye Institute, Building 6 Room 107, National Institutes of Health, Bethesda, MD, USA.
Graeme Wistow, Section on Molecular Structure and Functional Genomics, National Eye Institute, Building 6 Room 108, National Institutes of Health, Bethesda, MD, USA.
References
- 1.Simo R, Villarroel M, Corraliza L, Hernandez C, Garcia-Ramirez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier--implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724. doi: 10.1155/2010/190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu HZ, Song Z, Fu S, Zhu M, Le YZ. RPE barrier breakdown in diabetic retinopathy: seeing is believing. J Ocul Biol Dis Infor. 2011;4:83–92. doi: 10.1007/s12177-011-9068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiser PD, Golczak M, Palczewski K. Chemistry of the retinoid (visual) cycle. Chem Rev. 2014;114:194–232. doi: 10.1021/cr400107q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yonekawa Y, Kim IK. Clinical characteristics and current treatment of age-related macular degeneration. Cold Spring Harb Perspect Med. 2014;5:a017178. doi: 10.1101/cshperspect.a017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonilha VL. Age and disease-related structural changes in the retinal pigment epithelium. Clin Ophthalmol. 2008;2:413–424. doi: 10.2147/opth.s2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch’s membrane. Investigative ophthalmology & visual science. 2001;42:265–274. [PubMed] [Google Scholar]
- 7.Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye (Lond) 2001;15:384–389. doi: 10.1038/eye.2001.141. [DOI] [PubMed] [Google Scholar]
- 8.Haimovici R, Gantz DL, Rumelt S, Freddo TF, Small DM. The lipid composition of drusen, Bruch’s membrane, and sclera by hot stage polarizing light microscopy. Investigative ophthalmology & visual science. 2001;42:1592–1599. [PubMed] [Google Scholar]
- 9.Rudolf M, Curcio CA. Esterified cholesterol is highly localized to Bruch’s membrane, as revealed by lipid histochemistry in wholemounts of human choroid. J Histochem Cytochem. 2009;57:731–739. doi: 10.1369/jhc.2009.953448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Li CM, Rudolf M, Belyaeva OV, Chung BH, Messinger JD, Kedishvili NY, Curcio CA. Lipoprotein particles of intraocular origin in human Bruch membrane: an unusual lipid profile. Investigative ophthalmology & visual science. 2009;50:870–877. doi: 10.1167/iovs.08-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li CM, Chung BH, Presley JB, Malek G, Zhang X, Dashti N, Li L, Chen J, Bradley K, Kruth HS, Curcio CA. Lipoprotein-like particles and cholesteryl esters in human Bruch’s membrane: initial characterization. Investigative ophthalmology & visual science. 2005;46:2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- 12.Curcio CA, Johnson M, Huang JD, Rudolf M. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. Journal of lipid research. 2010;51:451–467. doi: 10.1194/jlr.R002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. Journal of lipid research. 2008;49:1715–1724. doi: 10.1194/jlr.M700580-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra S, Peterson K, Yin L, Berger A, Fan J, Wistow G. Accumulation of cholesterol and increased demand for zinc in serum-deprived RPE cells. Mol Vis. 2016;22:1387–1404. [PMC free article] [PubMed] [Google Scholar]
- 15.Wyatt MK, Tsai JY, Mishra S, Campos M, Jaworski C, Fariss RN, Bernstein SL, Wistow G. Interaction of complement factor h and fibulin3 in age-related macular degeneration. PLoS One. 2013;8:e68088. doi: 10.1371/journal.pone.0068088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marmorstein LY, Munier FL, Arsenijevic Y, Schorderet DF, McLaughlin PJ, Chung D, Traboulsi E, Marmorstein AD. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13067–13072. doi: 10.1073/pnas.202491599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Temel RE, Hou L, Rudel LL, Shelness GS. ACAT2 stimulates cholesteryl ester secretion in apoB-containing lipoproteins. Journal of lipid research. 2007;48:1618–1627. doi: 10.1194/jlr.M700109-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Experimental eye research. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 19.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 20.Chang TY, Li BL, Chang CC, Urano Y. Acyl-coenzyme A:cholesterol acyltransferases. Am J Physiol Endocrinol Metab. 2009;297:E1–9. doi: 10.1152/ajpendo.90926.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curcio CA, Presley JB, Malek G, Medeiros NE, Avery DV, Kruth HS. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Experimental eye research. 2005;81:731–741. doi: 10.1016/j.exer.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151–171. doi: 10.1146/annurev-genom-090413-025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pikuleva IA, Curcio CA. Cholesterol in the retina: the best is yet to come. Prog Retin Eye Res. 2014;41:64–89. doi: 10.1016/j.preteyeres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, Zack DJ, Arakawa S, Cipriani V, Ripke S, Igo RP, Jr, Buitendijk GH, Sim X, Weeks DE, Guymer RH, Merriam JE, Francis PJ, Hannum G, Agarwal A, Armbrecht AM, Audo I, Aung T, Barile GR, Benchaboune M, Bird AC, Bishop PN, Branham KE, Brooks M, Brucker AJ, Cade WH, Cain MS, Campochiaro PA, Chan CC, Cheng CY, Chew EY, Chin KA, Chowers I, Clayton DG, Cojocaru R, Conley YP, Cornes BK, Daly MJ, Dhillon B, Edwards AO, Evangelou E, Fagerness J, Ferreyra HA, Friedman JS, Geirsdottir A, George RJ, Gieger C, Gupta N, Hagstrom SA, Harding SP, Haritoglou C, Heckenlively JR, Holz FG, Hughes G, Ioannidis JP, Ishibashi T, Joseph P, Jun G, Kamatani Y, Katsanis N, NKC, Khan JC, Kim IK, Kiyohara Y, Klein BE, Klein R, Kovach JL, Kozak I, Lee CJ, Lee KE, Lichtner P, Lotery AJ, Meitinger T, Mitchell P, Mohand-Said S, Moore AT, Morgan DJ, Morrison MA, Myers CE, Naj AC, Nakamura Y, Okada Y, Orlin A, Ortube MC, Othman MI, Pappas C, Park KH, Pauer GJ, Peachey NS, Poch O, Priya RR, Reynolds R, Richardson AJ, Ripp R, Rudolph G, Ryu E, Sahel JA, Schaumberg DA, Scholl HP, Schwartz SG, Scott WK, Shahid H, Sigurdsson H, Silvestri G, Sivakumaran TA, Smith RT, Sobrin L, Souied EH, Stambolian DE, Stefansson H, Sturgill-Short GM, Takahashi A, Tosakulwong N, Truitt BJ, Tsironi EE, Uitterlinden AG, van Duijn CM, Vijaya L, Vingerling JR, Vithana EN, Webster AR, Wichmann HE, Winkler TW, Wong TY, Wright AF, Zelenika D, Zhang M, Zhao L, Zhang K, Klein ML, Hageman GS, Lathrop GM, Stefansson K, Allikmets R, Baird PN, Gorin MB, Wang JJ, Klaver CC, Seddon JM, Pericak-Vance MA, Iyengar SK, Yates JR, Swaroop A, Weber BH, Kubo M, Deangelis MM, Leveillard T, Thorsteinsdottir U, Haines JL, Farrer LA, Heid IM, Abecasis GR. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45:433–439. 439e431–432. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Stambolian D, Edwards AO, Branham KE, Othman M, Jakobsdottir J, Tosakulwong N, Pericak-Vance MA, Campochiaro PA, Klein ML, Tan PL, Conley YP, Kanda A, Kopplin L, Li Y, Augustaitis KJ, Karoukis AJ, Scott WK, Agarwal A, Kovach JL, Schwartz SG, Postel EA, Brooks M, Baratz KH, Brown WL, Brucker AJ, Orlin A, Brown G, Ho A, Regillo C, Donoso L, Tian L, Kaderli B, Hadley D, Hagstrom SA, Peachey NS, Klein R, Klein BE, Gotoh N, Yamashiro K, Ferris F, Iii, Fagerness JA, Reynolds R, Farrer LA, Kim IK, Miller JW, Corton M, Carracedo A, Sanchez-Salorio M, Pugh EW, Doheny KF, Brion M, Deangelis MM, Weeks DE, Zack DJ, Chew EY, Heckenlively JR, Yoshimura N, Iyengar SK, Francis PJ, Katsanis N, Seddon JM, Haines JL, Gorin MB, Abecasis GR, Swaroop A. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010;107:7401–7406. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curcio CA, Johnson M, Huang JD, Rudolf M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Retin Eye Res. 2009;28:393–422. doi: 10.1016/j.preteyeres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 30.Wolter JR, Falls HF. Bilateral confluent drusen. Arch Ophthalmol. 1962;68:219–226. doi: 10.1001/archopht.1962.00960030223013. [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 32.Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011;95:1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng W, Reem RE, Omarova S, Huang S, DiPatre PL, Charvet CD, Curcio CA, Pikuleva IA. Spatial distribution of the pathways of cholesterol homeostasis in human retina. PLoS One. 2012;7:e37926. doi: 10.1371/journal.pone.0037926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bommer GT, MacDougald OA. Regulation of lipid homeostasis by the bifunctional SREBF2-miR33a locus. Cell Metab. 2011;13:241–247. doi: 10.1016/j.cmet.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castoreno AB, Wang Y, Stockinger W, Jarzylo LA, Du H, Pagnon JC, Shieh EC, Nohturfft A. Transcriptional regulation of phagocytosis-induced membrane biogenesis by sterol regulatory element binding proteins. Proc Natl Acad Sci U S A. 2005;102:13129–13134. doi: 10.1073/pnas.0506716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malek G, Li CM, Guidry C, Medeiros NE, Curcio CA. Apolipoprotein B in Cholesterol-Containing Drusen and Basal Deposits of Human Eyes with Age-Related Maculopathy. The American Journal of Pathology. 2003;162:413–425. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pucadyil TJ, Chattopadhyay A. Effect of cholesterol on lateral diffusion of fluorescent lipid probes in native hippocampal membranes. Chemistry and physics of lipids. 2006;143:11–21. doi: 10.1016/j.chemphyslip.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Miersch S, Espey MG, Chaube R, Akarca A, Tweten R, Ananvoranich S, Mutus B. Plasma membrane cholesterol content affects nitric oxide diffusion dynamics and signaling. The Journal of biological chemistry. 2008;283:18513–18521. doi: 10.1074/jbc.M800440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chew EY. Nutrition effects on ocular diseases in the aging eye. Investigative ophthalmology & visual science. 2013;54:Orsf42–47. doi: 10.1167/iovs13-12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.