Abstract
Several studies have suggested that Wnts might contribute to skin fibrosis in systemic sclerosis (SSc) by affecting the differentiation of pluripotent dermal cells. We tested C-82, a therapeutic that inhibits canonical Wnt signaling by blocking the interaction of cAMP Response Element-Binding Protein with β-Catenin and inhibiting Wnt-activated genes. We utilized previously unreported trial design, formulating C-82 for topical application and conducting a placebo controlled, double-blinded clinical trial in which patients with diffuse cutaneous SSc were treated with C-82 or placebo on opposite forearms. C-82 compared to placebo treated forearms did not show any clinical effect. Skin biopsies performed before and after treatment showed a very weak trend toward improvement in the C-82 treated skin of biomarkers of local skin disease, THBS1 and COMP. However, on microarray analysis C-82 treatment strongly upregulated two clusters of genes that correlate negatively with the severity of SSc skin disease. These clusters are highly associated with metabolism and one gene, PLIN2, expressed only by sebocytes and subcutaneous fat cells. These changes in gene expression strongly support a role for Wnts in differentiation of pluripotent cells into profibrotic fibroblasts, and the potential for C-82 with longer treatment to promote fat regeneration in SSc skin.
Keywords: scleroderma, Wnt, β-catenin, fibrosis, adipogenesis
INTRODUCTION
Most patients with diffuse cutaneous systemic sclerosis (SSc) develop skin fibrosis, leading to hand contractures and other disabling symptoms (Man et al., 2017). Skin fibrosis is driven by persistent myofibroblast accumulation, activation and survival. Although the origin of dermal myofibroblasts is controversial, recent studies suggest that they can originate from adipocyte progenitor cells residing within the dermal white adipose tissue (Chia et al., 2016, Marangoni et al., 2015). Adipocyte differentiation into myofibroblasts is modulated by variety of signals including TGF-β and Wnts, and appears to be reversible (Plikus et al., 2017).
Wnts are a family of 19 closely related cytokines originally discovered for their wide ranging effects during development and carcinogenesis (Clevers and Nusse, 2012). Engagement of the LRP5/6 receptors by Wnt ligands leads to receptor phosphorylation and consequent binding of Axin. In this “canonical” signaling pathway, Axin acts as a scaffold for the destruction complex that regulates cytoplasmic levels of β-catenin. Recruitment of the destruction complex to Wnt receptors blocks β-catenin ubiquitination, and accumulation of cytoplasmic phosphorylated β-catenin, which then translocates to the nucleus and complexes with members of the T cell factor (TCF)/lymphoid enhancer factor family of transcription factors to activate target gene transcription (Stadeli et al., 2006). To generate a transcriptionally active complex, β-catenin recruits the transcriptional co-activator and acetyltransferase proteins, cyclic AMP response element-binding protein (CBP) and p300 (Hecht et al., 2000, Takemaru and Moon, 2000). CBP and p300 are closely related, but show partially distinct patterns of gene activation (Ma et al., 2005). Of note, previous studies have demonstrated that p300/CBP expression is up-regulated in the fibrotic skin in patients with SSc as well as in mouse models of scleroderma (Ghosh et al., 2013). Moreover, p300/CBP is recruited to COL1A1 gene promoter upon fibroblast activation, and plays a key role in fibrotic responses (Bhattacharyya et al., 2005).
While the pleiotropic functions of the β-catenin/Wnt pathways have been extensively investigated in the context of development and carcinogenesis, recent studies increasingly implicate Wnts in fibrotic processes, including systemic sclerosis (Bastakoty and Young, 2016, Edeling et al., 2016). The expression and role of Wnts in skin fibrosis has been examined in several settings. Lesional skin in patients with SSc shows evidence of activated Wnt/β-catenin signaling (Akhmetshina A. et al., 2012, Wei et al., 2012). Additional studies have shown that blocking Wnt proteins using a transgenic mouse overexpressing Dickkopf-related protein 1 (DKK1), an endogenous Wnt inhibitor, ameliorates skin fibrosis in mice (Akhmetshina A et al., 2012). The idea that Wnts are profibrotic is also supported by studies showing that Wnt3 upregulates expression of connective tissue growth factor (CTGF) and smooth muscle actin, a marker for myofibroblasts (Lemaire et al.). Overexpression of β-catenin in the skin results in dermal fibrosis and loss of intradermal fat (Beyer et al., 2013, Hamburg and Atit, 2012, Mastrogiannaki et al., 2016). Moreover, transgenic mice conditionally overexpressing Wnt10b in the adipose tissue develop cutaneous lipoatrophy accompanied by replacement of the dermal adipose tissue by a collagen-rich scar (Wei et al.). These studies suggest that the Wnt/β-catenin pathways regulate cell fate decisions determining whether adipocytic progenitor cells within the dermis differentiate into fat-forming adipocytes, or fibrosis-promoting myofibroblasts (Weiet al., 2012). A similar mechanism was shown in studies of aging of muscle stem cells, indicating that Wnts lead to differentiation into a fibrogenic phenotype (Brack et al., 2007). Transgenic mice overexpressing Wnt10b develop skin fibrosis independent of TGF-β. This finding is consistent with earlier studies showing that TGF-β, by downregulating DKK1, enhances the effect of endogenous Wnt(s) on fibroblasts (Beyer et al., 2012). Together these observations implicate Wnt/β-catenin signaling in pathological cutaneous fibrosis and loss of dermal adipose tissue, and suggest that inhibiting Wnt/β-catenin signaling might mitigate skin fibrosis in SSc.
Library screening identified ICG-001 as a small molecule that potently inhibits canonical Wnt pathway activation in a variety of cell types (Eguchi et al., 2005, Emami et al., 2004, Henderson et al., 2010, Maet al., 2005). It blocks Wnt signaling by interacting with the binding protein of cAMP response element-binding protein CREB (CBP). Binding to CBP blocks the interaction of CBP with β-catenin without affecting the interaction of β-catenin with p300, inhibiting a subset of Wnt-activated genes (Maet al., 2005). ICG-001 mitigates fibrosis in rodent models of pulmonary, renal and skin fibrosis. In cultured cells, ICG-001 prevents TGFβ signaling and epithelial-mesenchymal transition in pulmonary alveolar epithelial cells, blocking induction of smooth muscle actin (SMA) (Zhou et al., 2012). ICG-001 also inhibits Wnt-mediated signaling by miR-154, a microRNA induced by TGFβ in normal human lung fibroblasts, and increased in patients with idiopathic pulmonary fibrosis (Milosevic et al., 2012). In the unilateral ureteral obstruction model of renal fibrosis, ICG-001 ameliorates renal interstitial fibrosis and suppressed renal expression of fibronectin, collagen I, collagen III, alpha-SMA, PAI-1, fibroblast-specific protein-1, Snail1, and Snail2 (Hao et al., 2011). ICG-001 also inhibits bleomycin and adenoviral TGFbR1-induced models of skin fibrosis (Beyeret al., 2013).
C-82 is the active metabolite of PRI-724, a Wnt signaling pathway inhibitor with increased affinity compared to its predecessor ICG-001. Similar to ICG-001, PRI-724 inhibits recruitment of beta-catenin to its coactivator, CBP (NCI, 2014). PRI-724 blocks carbon tetrachloride and bile duct ligation induced liver fibrosis in mice (Osawa et al., 2015). C-82 has not been previously administered in any form including topical in humans. However, PRI-724, the pro-drug of C-82, has been administered intravenously by continuous infusion in small groups of oncology patients and as single dose given orally in healthy volunteers. We performed a human trial of C-82 in order to evaluate the effect of β-catenin/CBP inhibition in skin fibrosis in patients with SSc. Based on previous studies showing minimal systemic absorption of C-82 when applied to the skin in minipigs, we administered C-82 in a topical formulation. Patients were randomized to receive C82 or placebo cream applied to opposite forearms for 4 weeks. This innovative clinical trial design, which has not previously been used in SSc, allowed each subject to serve as his/her own control. Skin biopsies before and after treatment enabled a comprehensive evaluation of the effect of inhibiting β-catenin/CBP inhibition on gene expression in the skin of SSc patients. The results showed that prolonged topical administration of C-82 was very well tolerated in SSc subjects. C-82 treated skin showed upregulated expression of a distinct cluster of genes associated with adipocyte and sebocyte metabolism. Supporting a potential efficacy to Wnt inhibition in treatment of SSc, these same genes were found to be correlate negatively the MRSS, suggesting that upregulation of these genes is a marker for normalization of skin fibrosis and amelioration of subcutaneous adipose tissue loss in SSc. The results from this pilot study suggest that blockade of cutaneous Wnt/β-catenin signaling using a topically administered small molecule promotes regeneration of adipocytes and sebocytes in the fibrotic skin in patients with SSc.
RESULTS
Study Subjects
Patients were recruited from Boston University School of Medicine (Boston), Feinberg School of Medicine, Northwestern University (Chicago) and Hospital for Special Surgery (New York) (see Flow Chart Figure 1). All patient had early diffuse SSc with a median duration 8 months (median from first non-Raynaud’s), and moderately severe skin disease (median MRSS 27) (Table 1).
Figure 1. CONSTORT flow diagram.
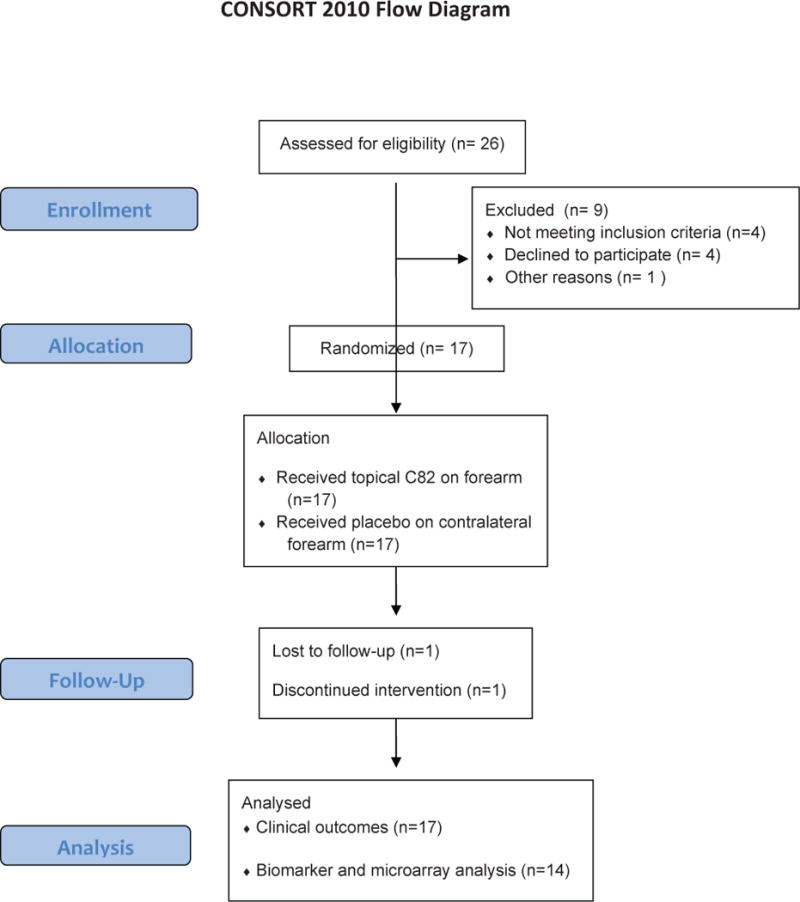
Diagram illustrates screening, enrollment, allocation of study participants and analysis.
Table 1.
Baseline demographic and clinical characteristics of study participants (n=17)
| Characteristic | Value | |
|---|---|---|
| Age yr. | ||
| Mean (SD) | 53.82 (13.15) | |
| Median (Range) | 53 (38–83) | |
| Sex F (M) | 11 (6) | |
| MRSS | ||
| Mean (SD) | 26.71 (8.64) | |
| Median (Range) | 27 (12–41) | |
| Disease Duration* | ||
| Mean (SD) | 6.71 (4.16) | |
| Median (Range) | 8 (1–12) | |
| ILD (no. [%]) | 6 (35.29) | |
Months from first non-Raynaud’s disease manifestation
Clinical outcomes
Study physicians evaluated the MRSS including the local skin score on each forearm at baseline and after 4 weeks of treatment. No significant change at week 4 compared to baseline was seen in either measurement (Table 2A). Patient and physician evaluation of forearms tightness, hardness and pain showed no difference between baseline and 4 weeks in either C-82-treated or placebo-treated arms (Supplementary Table 1).
Table 2A.
Change in MRSS at Day 28 after enrollment compared to baseline according to treatment assignment
| Change in MRSS score | Placebo (n=17) | C-82 (n=17) | X2 (P value) |
|---|---|---|---|
| Improvement | 4 | 4 | 1.18 (P>0.55) |
| The same | 12 | 10 | |
| Worsening | 1 | 3 |
Adverse Events
C-82 treatment was well tolerated, and adverse events thought to be possibly or likely related to treatment were mild. Skin redness, irritation or rash occurred more commonly in C-82 (n=3) compared to placebo (n=1) treated arms (Supplementary Table 2). One severe adverse event occurred during the study (pneumothorax), which was unrelated to treatment.
Biomarker outcomes for local skin disease
In previous studies we showed that in SSc skin expression of two genes, COMP and THBS1, correlate strongly with local skin scores (Rice et al., 2016). In light of these data, we compared expression of these genes in skin biopsies from placebo- and C-82-treated forearms obtained prior to and following 4 weeks of treatment. Expression of both COMP and THBS1 genes showed little change in skin biopsies from the placebo-treated arms. In contrast, both COMP and THBS1 showed a trend toward decreased expression in skin biopsies from the C-82 treated arms, although these changes were not statistically significant (Table 2B).
Table 2B.
Changes in Levels of Skin Biomarkers (i.e., COMP and THBS1) at Day-28 after enrollment from that at baseline according to treatment assignment
| Biomarkers (log value)* | Placebo (n=16) | C-82 (n=14) | P value |
|---|---|---|---|
| Mean of Change (SD) | Mean of Change (SD) | ||
| COMP | −0.062 (0.614) | −0.345 (0.808) | >0.283 |
| THBSI | 0.078 (0.541) | −0.130 (0.496) | >0.285 |
| Local Skin Score | Placebo (n=17) | Treatment (n=17) | P value |
| Mean of Change (SD) | Mean of Change (SD) | ||
| −0.176 (0.529) | −0.059 (0.659) | 0.766 |
Subject 107 had missing value for biomarkers on 28-day after enrollment of the study. Subject 301 had missing value for biomarkers at baseline of the study. Subject ID=304 was excluded from biomarker analysis prior to data lock due to an outlier value.
Global gene expression changes
In order to characterize changes in global gene expression associated with treatment, biopsies from placebo- versus C-82-treated forearms were analyzed by t-stochastic neighbor embedding (t-SNE). T-SNE is a relatively new methodology for dimensional reduction of large datasets (van der Maaten and Hinton, 2008). We analyzed the microarray data by t-SNE to better understand the relationship between gene expression in different study patients, to show the relationship between gene expression skin biopsies from opposite arms, and to detect changes in global gene expression associated with C-82 treatment. Gene expression in the two arms was globally similar at baseline, and did not change in a consistent manner following treatment with either placebo or C-82 (Supplementary Figure 1).
Adipocyte and metabolic genes altered in C-82 treated skin
Gene expression was tested in an unbiased manner by comparing baseline to week 4 changes in the placebo- versus C-82-treated arms by microarray analysis. We did not detect altered gene expression when comparing individual genes, after correction for multiple comparisons. However, clustering genes showing a nominal p-value<0.05 revealed a discrete cluster of genes that were upregulated in C-82-treated but not placebo treated skin (Figure 2). This gene cluster prominently included two genes characteristic of adipocytes: PLIN2, a marker of early adipocyte differentiation (Jiang and Serrero, 1992), and PLIN3, a related family member that coats lipid droplets (Wolins et al., 2001). These genes were significantly associated with the gene ontology terms cellular lipid, isoprenoid, cholesterol and long chain fatty-acyl-CoA biosynthetic processes (Figure 3). These are processes common, though not restricted, to adipocytes (Fujimoto et al., 2006, Lobo et al., 2009, Tilvis et al., 1982). Genes showing increased expression in C-82-treated skin include both proteins coating lipid droplets and proteins involved in synthesizing lipid components of lipid droplets (Table 3, (D’Aquila et al., 2015, Kimmel and Sztalryd, 2016, Schneider et al., 2016)). Sebocyte-regulated genes were particularly well represented, possibly because we were able to identify gene expressed by these cells using data from a recent single cell RNA-seq experiment (Joost et al., 2016)).
Figure 2. Hierarchical clustering of gene expression changes.
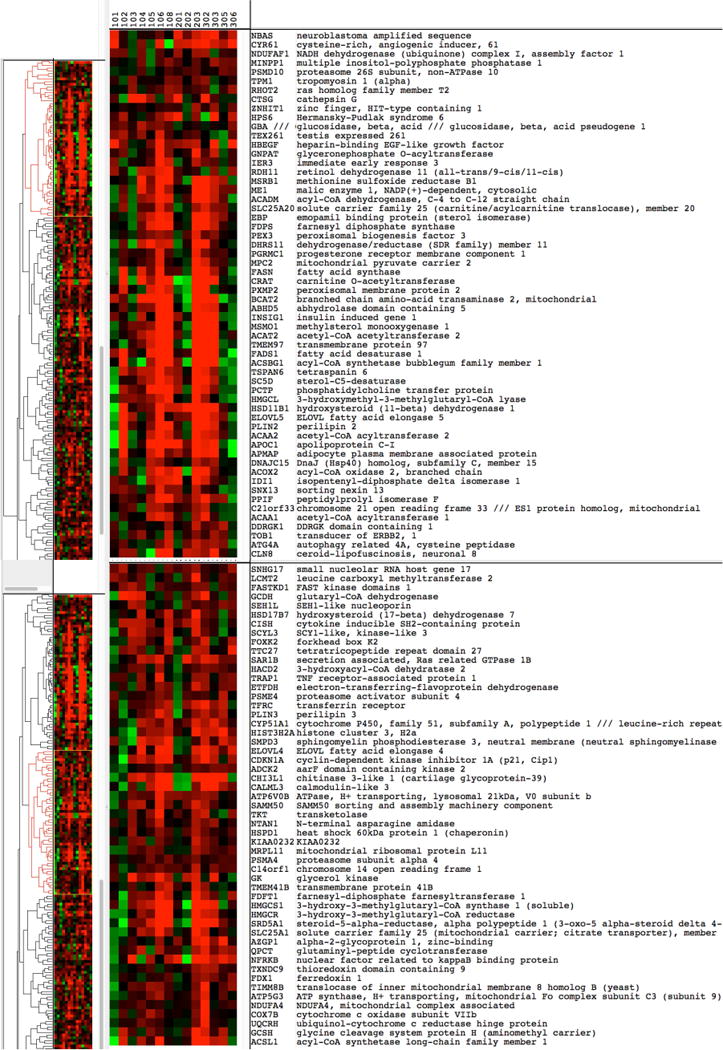
The cluster includes genes that were found to be differentially expressed in C-82 treated skin compared to placebo. Clustering by gene is unsupervised and by patient is supervised. Gene symbols are shown to the right. Red indicates increased, green decreased gene expression (fold change in C-82 treated skin compared to placebo).
Figure 3. GO biological process.
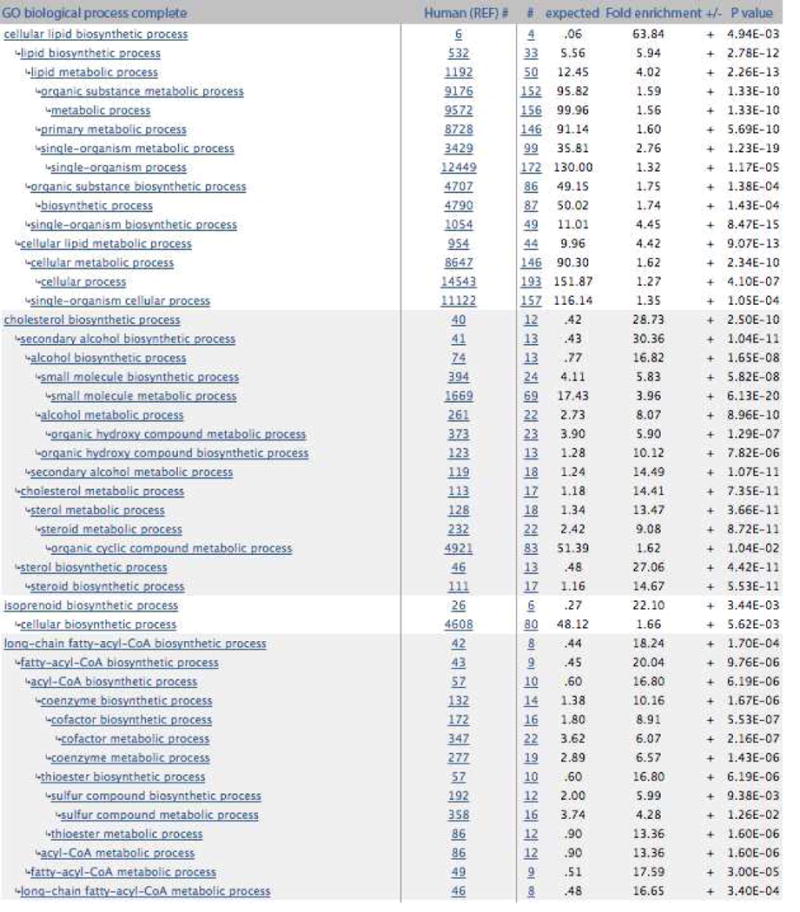
Gene Ontology biological processes enrichment analysis was performed on the cluster of 212 upregulated genes were found to be differentially expressed in the C-82 treated skin. Results show that these genes are associated with cellular, cholesterol, isoprenoid and long chain fatty-acyl-CoA biosynthetic processes commonly observed in adipocytes
Table 3.
Genes from clusters in Figure 2 associated with adipocytes and sebocytes
| Genes expressed by adipocytes | Genes expressed by sebocytes | Gene symbol | Genes expressed by adipocytes | Genes expressed by sebocytes | Gene symbol | Genes expressed by adipocytes | Genes expressed by sebocytes | Gene symbol |
|---|---|---|---|---|---|---|---|---|
| + | NBAS | − | TSPAN6 | + | + | PLIN3 | ||
| − | CYR61 | + | SC5D | − | CYP51A1//LRRD1 | |||
| + | NDUFAF1 | + | PCTP | + | HIST3H2A | |||
| − | MINPP1 | ++ | HMGCL | + | SMPD3 | |||
| + | PSMD10 | + | − | HSD11B1 | ++ | ELOVL4 | ||
| + | TPM1 | + | ++ | ELOVL5 | + | ++ | CDKN1A | |
| + | RHOT2 | ++ | ++ | PLIN2 | + | ADCK2 | ||
| − | CTSG | + | ++ | ACAA2 | − | CHI3L1 | ||
| + | ZNHIT1 | ++ | APOC1 | + | CALML3 | |||
| − | HPS6 | ++ | APMAP | − | ATP6V0B | |||
| − | GBA // GBAP1 | + | DNAJC15 | ++ | SAMM50 | |||
| ++ | TEX261 | + | ACOX2 | ++ | TKT | |||
| + | HBEGF | + | + | IDI1 | + | NTAN1 | ||
| ++ | GNPAT | ++ | SNX13 | ++ | HSPD1 | |||
| ++ | IER3 | + | PPIF | − | KIAA0232 | |||
| ++ | RDH11 | − | C21orf33 | ++ | MRPL11 | |||
| + | MSRB1 | − | ACAA1 | ++ | PSMA4 | |||
| ++ | ME1 | ++ | DDRGK1 | − | C14orf1 | |||
| ++ | ACADM | + | TOB1 | − | GK | |||
| ++ | SLC25A20 | + | ATG4A | + | TMEM41B | |||
| + | + | EBP | + | CLN8 | ++ | FDFT1 | ||
| + | + | FDPS | − | SNHG17 | ++ | HMGCS1 | ||
| ++ | PEX3 | − | LCMT2 | ++ | HMGCR | |||
| − | DHRS11 | − | FASTKD1 | + | SRD5A1 | |||
| ++ | PGRMC1 | + | GCDH | + | ++ | SLC25A1 | ||
| ++ | MPC2 | + | SEH1L | − | AZGP1 | |||
| ++ | FASN | + | HSD17B7 | − | QPCT | |||
| ++ | CRAT | + | CISH | + | NFRKB | |||
| − | PXMP2 | − | SCYL3 | + | TXNDC9 | |||
| ++ | BCAT2 | + | FOXK2 | + | FDX1 | |||
| + | ABHD5 | + | TTC27 | ++ | TIMM8B | |||
| + | ++ | INSIG1 | ++ | SAR1B | ++ | ATP5G3 | ||
| − | MSMO1 | − | HACD2 | ++ | NDUFA4 | |||
| + | ++ | ACAT2 | + | TRAP1 | ++ | COX7B | ||
| ++ | TMEM97 | + | ++ | ETFDH | ++ | UQCRH | ||
| ++ | + | FADS1 | + | PSME4 | + | GCSH | ||
| ++ | ACSBG1 | + | TFRC | ++ | + | ACSL1 |
(−) Not expressed
(+) Highly expressed
(++) Exclusively expressed
C-82 did not affect markers of epidermal differentiation
In order to examine any potential effect of C-82 on epidermal cells, including cells in the hair follicle we examined changes in gene expression by all of the keratin (KRT) and keratin associated protein (KRTAP) genes found on the microarrays. None of these genes showed consistent changes in gene expression associated with C-82 treatment (Supplementary Figure 2).
C-82 regulated biomarkers of SSc skin disease
In previous studies we identified genes upregulated in SSc skin that correlated positively with the MRSS (Rice et al., 2016, Rice et al., 2015b). C-82 treatment was associated with up-regulated expression of a cluster of genes. We reasoned that if these genes reflect pathogenic processes in SSc, such as loss of dermal adipose tissue, their expression would correlate negatively with the MRSS. To examine this premise, we evaluated the correlation between baseline expression of the C-82-regulated gene clusters (shown in Figure 2) and the MRSS. Remarkably, a majority of genes upregulated in C-82 treated skin correlated negatively with the MRSS (Table 4, Supplementary Table 3). Thus, genes in the C-82-regulated cluster are potential markers for the severity of skin disease.
Table 4.
Genes correlating most highly with the MRSS at baseline from clusters in Figure 2 (full listing of genes in Supplemental Table 3)
| GENE SYMBOL | GENE NAME | Correlation (R)* | Correlation p-value** | Paired t-test p-value*** |
|---|---|---|---|---|
| CTSG | cathepsin G | −0.6393 | 0.0003 | 0.0393 |
| PSMD10 | proteasome 26S subunit, non-ATPase 10 | −0.6043 | 0.0007 | 0.0543 |
| C14orf1 | chromosome 14 open reading frame 1 | −0.5924 | 0.0009 | 0.0033 |
| NDUFA4 | NDUFA4, mitochondrial complex associated | −0.5707 | 0.0015 | 0.0365 |
| GCSH | glycine cleavage system protein H (aminomethyl carrier) | −0.5617 | 0.0019 | 0.0509 |
| HSD17B7 | hydroxysteroid (17-beta) dehydrogenase 7 | −0.5419 | 0.0029 | 0.0218 |
| HSD11B1 | hydroxysteroid (11-beta) dehydrogenase 1 | −0.5363 | 0.0033 | 0.0110 |
| HACD2 | 3-hydroxyacyl-CoA dehydratase 2 | −0.5297 | 0.0037 | 0.0275 |
| MPC2 | mitochondrial pyruvate carrier 2 | −0.4766 | 0.0103 | 0.0359 |
| ETFDH | electron-transferring-flavoprotein dehydrogenase | −0.4758 | 0.0105 | 0.0225 |
| UQCRH | ubiquinol-cytochrome c reductase hinge protein | −0.4618 | 0.0134 | 0.0683 |
| COX7B | cytochrome c oxidase subunit VII b | −0.4576 | 0.0144 | 0.0648 |
| ME1 | malic enzyme 1, NADP(+)-dependent, cytosolic | −0.4557 | 0.0148 | 0.0066 |
| HIST3H2A | histone cluster 3, H2a | −0.4406 | 0.0190 | 0.0303 |
| DHRS11 | dehydrogenase/reductase (SDR family) member 11 | −0.4405 | 0.0190 | 0.0684 |
| CISH | cytokine inducible SH2-containing protein | −0.4374 | 0.0199 | 0.0034 |
| TKT | transketolase | −0.4371 | 0.0200 | 0.0348 |
| TSPAN6 | tetraspanin 6 | −0.4333 | 0.0213 | 0.0183 |
| PEX3 | peroxisomal biogenesis factor 3 | −0.4183 | 0.0268 | 0.0128 |
| ACSL1 | acyl-CoA synthetase long-chain family member 1 | −0.4041 | 0.0329 | 0.0671 |
| RHOT2 | ras homolog family member T2 | −0.4029 | 0.0335 | 0.0336 |
| TMEM41B | transmembrane protein 41B | −0.3990 | 0.0354 | 0.0549 |
| PGRMC1 | progesterone receptor membrane component 1 | −0.3908 | 0.0398 | 0.0215 |
| NDUFAF1 | NADH dehydrogenase (ubiquinone) complex I, assembly factor 1 | −0.3840 | 0.0437 | 0.0550 |
| FDFT1 | farnesyl-diphosphate farnesyltransferase 1 | −0.3822 | 0.0447 | 0.0393 |
| PLIN2# | perilipin 2 | −0.2454 | 0.2081 | 0.0140 |
| PLIN3# | perilipin 3 | 0.0876 | 0.6575 | 0.0567 |
Pearson’s correlation between expression of the gene and the baseline MRSS
P-value of the correlation between the gene and the baseline MRSS
Nominal P-value for the difference between the change from baseline to 4 weeks, comparing C-82 to placebo treated skin
PLIN2 and PLIN3 are included here because they are well-recognized markers of lipid droplets
PLIN2 expression by cells producing lipid droplets
To gain further insight into the potential roles of cutaneous adipose tissue and adipocytes in the C-82 response, we examined PLIN2 expression in normal and SSc skin biopsies. By immunohistochemistry, we found that PLIN2+ cells were located primarily in the sebaceous glands, and less intensely, within the intradermal fat layer (Supplementary Figure 3). Because the presence of dermal fat and sebaceous glands is highly variable in skin biopsies, most biopsies not showing sebaceous glands, it was not feasible to compare PLN2 staining on C-82 versus placebo treated skin.
DISCUSSION
We carried out a clinical trial to investigate of the effect of Wnt/β-catenin blockade in fibrotic skin disease. The results of this short-term, controlled clinical trial show that C-82 was well tolerated, and exerted regulatory effects on the expression of a specific cluster of genes within lesional skin. Two linked observations strongly support a potential role for C-82 as an anti-fibrotic for SSc skin. First, C-82 treated skin showed upregulated expression of a cluster of genes highly associated with metabolic processes and adipogenesis. Second, many of these genes at baseline were highly inversely correlated with the MRSS, suggesting that their downregulation reflects an important aspect of SSc skin disease pathogenesis. Thus, C-82 treatment returned the expression of these genes toward a normal level. These observations provide support for the premise that blocking Wnt/-catenin signaling might be a strategy for mitigating skin fibrosis in SSc, and provide fresh insights into the role of reversible preadipocyte-myofibroblast conversion in skin fibrosis and SSc.
The genes upregulated by C-82 suggest an important effect of β-catenin inhibition on adipocyte differentiation, an effect predicted from in vitro and murine studies of β-catenin regulation of adipogenesis in skin (Mastrogiannakiet al., 2016). Wnts block differentiation of mesenchymal progenitor/preadipocytes into adipocytes (Gesta et al., 2007, Ross et al., 2000). In murine skin, stabilization of β-catenin in the ventral mesoderm leads to skin fibrosis (Hamburg and Atit, 2012), and a similar phenotype can be induced by expression restricted to Dlk1+ fibroblasts residing in the lower dermis (Mastrogiannakiet al., 2016). Adipocytes/preadipocytes have further been mapped as progenitor cells of profibrotic myofibroblasts using the adipocyte specific promoter, adiponectin (Marangoniet al., 2015). Thus, we hypothesize that by relieving the inhibitory effect of Wnts/β-catenin on adipogenesis, C-82 treatment might have permitted adipocyte differentiation of mesenchymal progenitor cells within the fibrotic dermis, and with time might divert preadipocytes or other mesenchymal stem cells from a profibrotic to an adipocyte differentiation pathway.
In contrast to studies with mice, where β-catenin signaling in the skin was blocked, in the present study we did not see an effect of β-catenin inhibition on hair follicle formation (Rognoni et al., 2016). Wnt/β-catenin signaling plays key roles in hair follicle development and maturation (Hsu et al., 2014). In mice Wnt/β-catenin deletion in dermal papilla blocks hair follicle formation. (Enshell-Seijffers et al., 2010) and transient β-catenin overexpression activates hair growth associated with proliferation of cell in the outer root sheath (Van Mater et al., 2003). While hair growth over treated areas was not formally assessed clinically, no change in genes associated with hair follicle cycling was observed as we did not see any evidence for consistent changes in genes associated with epidermal (KRT) or hair shaft proteins (KRTAP). These results do not exclude C-82 effects on rare epidermal or hair follicle progenitor cells, since these might be hard to detect analyzing whole skin mRNA.
Although we are uncertain which cell type(s) are being most affected by topical C-82, it is notable that in addition to adipocytes, PLIN2 and PLN3 are highly expressed by sebocytes (Dahlhoff et al., 2013). Inhibiting β-catenin in mouse epidermis by transgenic overexpression of ΔNLef, leads to sebaceous gland hyperplasia (Niemann et al., 2002); and β-catenin deleted bulge hair follicle stem cells show sebaceous gland enlargement upon hair plucking to induce stem cell differentiation (Lien et al., 2014). These observations suggest that inhibition of β-catenin activity by C-82 treatment might also be predicted to expand dermal sebaceous glands.
There was no change in MRSS or local skin score from baseline to 4 weeks in either placebo- or C-82-treated arms. Local skin score is not likely a sensitive measure for change of skin disease and has not been previously validated for such a purpose. Other measures such as ultrasound or durometry might be more sensitive to change and should be compared to gene expression and local skin score as outcomes in future studies of local skin disease (Kissin et al., 2006). The lack of treatment effect on fibrotic gene expression or clinical measures of skin fibrosis is likely to reflect the short duration of the trial. Considering the postulated effect of C-82 blockade of β-catenin signaling of diverting mesenchymal cells from a profibrotic to an adipogenic phenotype within the fibrotic skin, a longer duration of treatment might be required to affect fibrosis. However, the alterations in gene expression associated with C-82 treatment signaling provide a strong surrogate for a likely clinical effect on fibrosis if drug treatment is extended. This is supported by the inverse correlation of these genes with the MRSS, indicating that many of the upregulated genes are biomarkers for SSc skin disease. It is also supported by the trend, albeit weak, toward decreased expression of genes previously described to be markers for TGFβ-regulated fibrotic disease, THBS1 and COMP. Thus, we would anticipate that Wnts and/or their signaling pathway therapeutics require longer duration treatment to see clinical effects on fibrosis.
Modulation of Wnts and β-catenin have been the focus of several therapeutics and related clinical trials, as reviewed recently (Kahn, 2014). PRI-724, a pro-drug of C-82, has been given to patients by continuous intravenous infusion for solid tumors (El-Khoueiry et al., 2013, Ko et al., 2016) with acceptable safety and a suggestion that Survivin/BIRC5 might serve as a biomarker of its activity. However, we did not see any change in Survivin levels in the skin of C-82 treated SSc patients (not shown).
Skin gene expression on opposite arms from the same patient was similar. This reinforces the utility for testing drug efficacy more generally in SSc using topical approach. This trial design allowed paired comparison between active and placebo and thus reduced the sample size needed for the trial by more than a factor of two. Several features of this innovative treatment design merit consideration. First, absorption of drug across the skin might be dramatically altered in fibrotic skin, although this issue has not been previously addressed for any drug. Anecdotal observations suggest that it takes far longer for subcutaneously administered local anesthesia to anesthetize the skin before biopsy, suggesting that fibrotic skin may inhibit drug diffusion through the dermis. Although C-82 absorption was shown to reach therapeutic concentrations in vitro in normal skin explant culture (H. Dittrich, data not shown), we cannot be sure of how effectively C-82 penetrated SSc patient skin, particularly into the deep dermis, likely the critical site for altered mesenchymal stem adipogenesis in SSc skin. Thus, it is possible that the drug did not achieve sufficient concentrations in the subcutaneous tissues in some patients. Second, there is a theoretical concern that systemically absorbed drug might affect clinical disease and gene expression. Studies in guinea pigs indicated that C-82 absorption into the systemic circulation is minimal (peak concentration of C-82 found in the plasma dosing at 8 mg/kg/day was 0.27 to 1.20 ng/ml), well below the IC50 (~150 nM, 87 ng/ml). Since in our study subjects received ~16-fold lower dose (~0.50 mg/kg/day), we anticipated undetectable levels of drug in plasma, precluding the possibility of effect on the contralateral, placebo-treated arm.
Despite the apparent effect of C-82 on gene expression, we did not detect any consistent systemic adverse effects potentially attributable to treatment. Several patients experienced some skin irritation, redness or rash that was more common in C-82 than placebo treated arms.
In summary, results from this short-term proof-of-principle clinical trial show that blockade of β-catenin signaling in the skin using topical C-82 is associated with a rise in expression of genes involved in lipid metabolism that are reduced in SSc skin. Although short-term treatment did not result in clinically detected efficacy, the changes in gene expression suggest that longer and/or systemic treatment might reverse fat loss and fibrosis in SSc skin.
MATERIALS AND METHODS
Study Design
Each recruiting center Boston University School of Medicine, Northwestern Feinberg School of Medicine and Hospital for Special Surgery) obtained Institutional Review Board approval for the study, and all patients gave written informed consent. Declaration of Helsinki protocols were followed. The clinical trial was registered on clinicaltrials.gov # NCT02349009, date of registration January 18, 2015.
Protocol
The study population consisted of men and women at least 18 years of age with diffuse cutaneous SSc as defined by the American College of Rheumatology (ACR). Subjects were required to be within 3 years of first non-Raynaud’s disease manifestation, show evidence of progressive disease (increase in MRSS > 5 units in past 6 months), and to have a local forearm skin score ≥2.
Assignment
The study design was a 1:1 active treatment: placebo, randomized double-blind trial, evaluating the effect of topical C-82 versus placebo given daily for 4 weeks. The study drug was a 0.5% topical gel preparation of C-82. Study subjects were randomized by SAS random number generator to apply the study medication daily for 4 weeks to either the right or left forearm.
Masking
All subjects applied placebo (i.e., a gel without C-82) to the contralateral forearm.
Participant flow-Skin biopsies (two 3 mm punch biopsies) were taken from the mid-forearm of both arms at the screening visit and permitted to heal 2–4 weeks before starting treatment. After four weeks of study drug administration, skin biopsies (two 3 mm biopsies) were taken from both mid-forearms at a site 1–2 cm from the initial biopsy site. Safety assessments extended to 4 weeks after the final application of study drug.
Analysis
The predefined primary study outcome was the change in skin biomarkers, THBS1 and COMP, at day 28 compared to the screening visit in the C-82 treated forearm skin compared to the change in the placebo treated forearm skin. Adverse events were characterized for likely or possible relationship to study drug. The following exploratory outcomes were evaluated: 1) change in patient assessment of skin tightness, hardness and pain from the screening visit and day 0 to days 7 and 28 in the C-82-treated and placebo-treated forearms; 2) change in Physician Local Skin Assessment of skin thickness hardness and tethering; 3) change in Wnt-responsive and microarray gene expression at day 28 compared to the screening visit in the C-82-treated skin compared to the screening visit in placebo-treated skin. Analyses were carried out after data lock by investigators blinded to treatment arms for all clinical and biomarker outcomes, except microarray studies.
Nanostring
Skin tissue RNAs were purified and analyzed on a custom nanostring as previously described (Rice et al., 2015a).
Microarray
Skin tissue RNA was analyzed using Affymetrix HG-U133A2.0 microarray chips and data were normalized using the MAS 5.0 algorithm as previously described (Riceet al., 2015a). Microarray quality control was obtained by using the ArrayAnalysis quality control module (www.arrayanalysis.org) (Eijssen et al., 2013) A total of 19,221 probes were obtained after removing probes that were absent on all arrays. Probes that had a measured expression value of <100 were filtered out, this resulted in 10,079 probes. The resulting probes were then collapsed to 7,055 unique gene symbols by keeping those with the highest average expression. R computing software Version 3.2.3 was used for the processing and normalization of microarrays using the R package “affy” Version 1.48.0. (de Hoon et al., 2004) and Java Treeview were utilized to cluster and visualized gene expression. T-SNE was run on filtered data using the R package “Rtsne” version 0.11. All microarray data have been deposited in the GEO database (accession GSE94340).
Changes in gene expression
Expression values were log2 scaled and for each arm the difference between Pre and Post-Treatment was calculated in order to assess whether there were gene expression changes between time points. A paired t-test was then performed from the resulting deltas to determine which changes were statistically significant.
We performed biological process enrichment analysis on the upregulated genes (212 genes), of the 500 most highly statistically significantly regulated genes comparing C-82 to placebo treated skin (p-values ranging from 0.0002–0.068), through the Gene Ontology Consortium database (http://geneontology.org/) using the PANTHER Overrepresentation Test Version 11.1 (released 2016-10-24) (Gene Ontology, 2015). Genes associated adipocytes were identified using BioGPS (Wu et al., 2013) and genes associated with sebocytes identified using online single cell RNA-seq data (Joostet al., 2016).
Statistical analysis
We classified the change in MRSS at day-28 after enrollment from that at the day of screening into three groups: Improved (i.e., change of MRSS score <0), the same (i.e., change of MRSS score =0), or worse (i.e., change of MRSS score >0). We examined the effect of treatment (i.e., C82 vs. placebo) on change in MRSS using Chi-square test. We took the same approach to assess the effect of treatment on changes of several measurement of local skin either reported by patients or assessed by physicians. Wilcoxon matched-pairs signed ranked test was used to investigate changes in the local skin score between the placebo and treatment groups.
Immunohistochemistry
Immunostaining for PLIN2 was carried out on formalin fixed, paraffin embedded sections. After antigen retrieval in Citrate buffer pH 6.0, sections were incubated with anti-PLIN2 (1:500 dilution, LSBio, LS-B4850) for two hours, washed and detected using HRP polymer (DAKO, K5361).
Supplementary Material
Supplemental Figure 1. Gene skin expression t-SNE. Shows the similarity in skin gene expression within participants.
Supplemental Figure 2. Hierarchical clustering of KRT and KRTAP expression changes. The cluster includes all KRT and KRTAP genes detected by microarray analysis, showing the change in gene expression at 4 weeks compared to baseline. Clustering by gene is unsupervised and by patient is supervised. Gene symbols are shown to the right. Red indicates increased, green decreased gene expression (fold change in C-82 treated skin compared to placebo). The uncorrected p-value of change comparing placebo to treatment is shown to the far right.
Supplemental Figure 3. Immunohistochemical staining of PLIN2. Skin from a patient with SSc before treatment showing expression of PLIN2 in sebaceous glands (left panel) and subcutaneous fat (right panel)
Acknowledgments
Funding for this work was provided by PRISM Biolab. The work in this manuscript was also supported by National Institutes of Arthritis Musculoskeletal and Skin Disease grants: Scleroderma Center of Research Translation (1P50AR060780) to R.L.; and Department of Defense Award (PR110276) to J.V. and R. L.
Dr. Lafyatis has received consulting fees from PRISM Biolab, Merck, Bristol Myers Squibb, Biocon, Formation, Genentech/Roche, UCB and Sanofi; and grant support from Elpidera, Regeneron. Dr. Dittrich has received consulting fees from PRISM Pharma Co., Ltd.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Work was carried out in Boston, MA; Chicago, IL; and New York, NY
Conflict of Interest
The other authors have declared no conflict of interests.
References
- Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nature Communications. 2012 doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, et al. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nature communications. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastakoty D, Young PP. Wnt/beta-catenin pathway in tissue injury: roles in pathology and therapeutic opportunities for regeneration. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2016;30(10):3271–84. doi: 10.1096/fj.201600502R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer C, Reichert H, Akan H, Mallano T, Schramm A, Dees C, et al. Blockade of canonical Wnt signalling ameliorates experimental dermal fibrosis. Annals of the rheumatic diseases. 2013;72(7):1255–8. doi: 10.1136/annrheumdis-2012-202544. [DOI] [PubMed] [Google Scholar]
- Beyer C, Schramm A, Akhmetshina A, Dees C, Kireva T, Gelse K, et al. beta-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Annals of the rheumatic diseases. 2012;71(5):761–7. doi: 10.1136/annrheumdis-2011-200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Ghosh AK, Pannu J, Mori Y, Takagawa S, Chen G, et al. Fibroblast expression of the coactivator p300 governs the intensity of profibrotic response to transforming growth factor beta. Arthritis Rheum. 2005;52(4):1248–58. doi: 10.1002/art.20996. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science (New York, NY) 2007;317(5839):807–10. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Chia JJ, Zhu T, Chyou S, Dasoveanu DC, Carballo C, Tian S, et al. Dendritic cells maintain dermal adipose-derived stromal cells in skin fibrosis. The Journal of clinical investigation. 2016;126(11):4331–45. doi: 10.1172/JCI85740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- D’Aquila T, Sirohi D, Grabowski JM, Hedrick VE, Paul LN, Greenberg AS, et al. Characterization of the proteome of cytoplasmic lipid droplets in mouse enterocytes after a dietary fat challenge. PloS one. 2015;10(5):e0126823. doi: 10.1371/journal.pone.0126823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhoff M, Camera E, Picardo M, Zouboulis CC, Chan L, Chang BH, et al. PLIN2, the major perilipin regulated during sebocyte differentiation, controls sebaceous lipid accumulation in vitro and sebaceous gland size in vivo. Biochimica et biophysica acta. 2013;1830(10):4642–9. doi: 10.1016/j.bbagen.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20(9):1453–4. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- Edeling M, Ragi G, Huang S, Pavenstadt H, Susztak K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nature reviews Nephrology. 2016;12(7):426–39. doi: 10.1038/nrneph.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi M, Nguyen C, Lee SC, Kahn M. ICG-001, a novel small molecule regulator of TCF/beta-catenin transcription. Medicinal chemistry. 2005;1(5):467–72. doi: 10.2174/1573406054864098. [DOI] [PubMed] [Google Scholar]
- Eijssen LM, Jaillard M, Adriaens ME, Gaj S, de Groot PJ, Muller M, et al. User-friendly solutions for microarray quality control and pre-processing on ArrayAnalysis.org. Nucleic acids research. 2013;41:W71–6. doi: 10.1093/nar/gkt293. Web Server issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khoueiry AB, Ning Y, Yang D, Cole S, Kahn M, Zoghbi M, et al. A phase I first-in-human study of PRI-724 in patients (pts) with advanced solid tumors. J Clin Oncol. 2013;31 suppl; abstr 2501. [Google Scholar]
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proceedings of the National Academy of Sciences of the United States of America. 2004;101(34):12682–7. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Developmental cell. 2010;18(4):633–42. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y, Onoduka J, Homma KJ, Yamaguchi S, Mori M, Higashi Y, et al. Long-chain fatty acids induce lipid droplet formation in a cultured human hepatocyte in a manner dependent of Acyl-CoA synthetase. Biological & pharmaceutical bulletin. 2006;29(11):2174–80. doi: 10.1248/bpb.29.2174. [DOI] [PubMed] [Google Scholar]
- Gene Ontology C. Gene Ontology Consortium: going forward. Nucleic acids research. 2015;43:D1049–56. doi: 10.1093/nar/gku1179. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Bhattacharyya S, Lafyatis R, Farina G, Yu J, Thimmapaya B, et al. p300 is elevated in systemic sclerosis and its expression is positively regulated by TGF-beta: epigenetic feed-forward amplification of fibrosis. The Journal of investigative dermatology. 2013;133(5):1302–10. doi: 10.1038/jid.2012.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburg EJ, Atit RP. Sustained beta-catenin activity in dermal fibroblasts is sufficient for skin fibrosis. The Journal of investigative dermatology. 2012;132(10):2469–72. doi: 10.1038/jid.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, He W, Li Y, Ding H, Hou Y, Nie J, et al. Targeted inhibition of beta-catenin/CBP signaling ameliorates renal interstitial fibrosis. Journal of the American Society of Nephrology: JASN. 2011;22(9):1642–53. doi: 10.1681/ASN.2010101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. The EMBO journal. 2000;19(8):1839–50. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, et al. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14309–14. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nature medicine. 2014;20(8):847–56. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HP, Serrero G. Isolation and characterization of a full-length cDNA coding for an adipose differentiation-related protein. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(17):7856–60. doi: 10.1073/pnas.89.17.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost S, Zeisel A, Jacob T, Sun X, La Manno G, Lonnerberg P, et al. Single-Cell Transcriptomics Reveals that Differentiation and Spatial Signatures Shape Epidermal and Hair Follicle Heterogeneity. Cell systems. 2016;3(3):221–37 e9. doi: 10.1016/j.cels.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. Can we safely target the WNT pathway? Nature reviews Drug discovery. 2014;13(7):513–32. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel AR, Sztalryd C. The Perilipins: Major Cytosolic Lipid Droplet-Associated Proteins and Their Roles in Cellular Lipid Storage, Mobilization, and Systemic Homeostasis. Annual review of nutrition. 2016;36:471–509. doi: 10.1146/annurev-nutr-071813-105410. [DOI] [PubMed] [Google Scholar]
- Kissin EY, Schiller AM, Gelbard RB, Anderson JJ, Falanga V, Simms RW, et al. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum. 2006;55(4):603–9. doi: 10.1002/art.22093. [DOI] [PubMed] [Google Scholar]
- Ko AH, Chiorean EG, Kwak EL, Lenz H-J, Nadler PI, Wood DL, et al. Final results of a phase Ib dose-escalation study of PRI-724, a CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with advanced pancreatic adenocarcinoma (APC) as second-line therapy after FOLFIRINOX or FOLFOX. J Clin Oncol. 2016;34 suppl; abstr e15721. [Google Scholar]
- Lemaire R, Farina G, Bayle J, Dimarzio M, Pendergrass SA, Milano A, et al. Antagonistic effect of the matricellular signaling protein CCN3 on TGF-beta- and Wnt-mediated fibrillinogenesis in systemic sclerosis and Marfan syndrome. The Journal of investigative dermatology. 2010;130(6):1514–23. doi: 10.1038/jid.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Polak L, Lin M, Lay K, Zheng D, Fuchs E. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nature cell biology. 2014;16(2):179–90. doi: 10.1038/ncb2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo S, Wiczer BM, Bernlohr DA. Functional analysis of long-chain acyl-CoA synthetase 1 in 3T3-L1 adipocytes. The Journal of biological chemistry. 2009;284(27):18347–56. doi: 10.1074/jbc.M109.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Nguyen C, Lee KS, Kahn M. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene. 2005;24(22):3619–31. doi: 10.1038/sj.onc.1208433. [DOI] [PubMed] [Google Scholar]
- Man A, Correa JK, Ziemek J, Simms RW, Felson DT, Lafyatis R. Development and validation of a patient-reported outcome instrument for skin involvement in patients with systemic sclerosis. Annals of the rheumatic diseases. 2017 doi: 10.1136/annrheumdis-2016-210534. [DOI] [PubMed] [Google Scholar]
- Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis & rheumatology. 2015;67(4):1062–73. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiannaki M, Lichtenberger BM, Reimer A, Collins CA, Driskell RR, Watt FM. beta-Catenin Stabilization in Skin Fibroblasts Causes Fibrotic Lesions by Preventing Adipocyte Differentiation of the Reticular Dermis. The Journal of investigative dermatology. 2016;136(6):1130–42. doi: 10.1016/j.jid.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic J, Pandit K, Magister M, Rabinovich E, Ellwanger DC, Yu G, et al. Profibrotic role of miR-154 in pulmonary fibrosis. American journal of respiratory cell and molecular biology. 2012;47(6):879–87. doi: 10.1165/rcmb.2011-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCI. 2014 http://www.cancer.gov/drugdictionary?cdrid=696436.
- Niemann C, Owens DM, Hulsken J, Birchmeier W, Watt FM. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development (Cambridge, England) 2002;129(1):95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- Osawa Y, Oboki K, Imamura J, Kojika E, Hayashi Y, Hishima T, et al. Inhibition of Cyclic Adenosine Monophosphate (cAMP)-response Element-binding Protein (CREB)-binding Protein (CBP)/beta-Catenin Reduces Liver Fibrosis in Mice. EBioMedicine. 2015;2(11):1751–8. doi: 10.1016/j.ebiom.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, et al. Regeneration of fat cells from myofibroblasts during wound healing. Science (New York, NY. 2017;355(6326):748–52. doi: 10.1126/science.aai8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. The Journal of clinical investigation. 2015a;125(7):2795–807. doi: 10.1172/JCI77958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice LM, Stifano G, Ziemek J, Lafyatis R. Local skin gene expression reflects both local and systemic skin disease in patients with systemic sclerosis. Rheumatology. 2016;55(2):377–9. doi: 10.1093/rheumatology/kev335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice LM, Ziemek J, Stratton EA, McLaughlin SR, Padilla CM, Mathes AL, et al. A longitudinal biomarker for the extent of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis & rheumatology. 2015b;67(11):3004–15. doi: 10.1002/art.39287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E, Gomez C, Pisco AO, Rawlins EL, Simons BD, Watt FM, et al. Inhibition of beta-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development (Cambridge, England) 2016;143(14):2522–35. doi: 10.1242/dev.131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–3. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Zhang S, Li P. Lipid droplets and associated proteins in the skin: basic research and clinical perspectives. Archives of dermatological research. 2016;308(1):1–6. doi: 10.1007/s00403-015-1599-2. [DOI] [PubMed] [Google Scholar]
- Stadeli R, Hoffmans R, Basler K. Transcription under the control of nuclear Arm/beta-catenin. Current biology: CB. 2006;16(10):R378–85. doi: 10.1016/j.cub.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. The Journal of cell biology. 2000;149(2):249–54. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilvis R, Kovanen PT, Miettinen TA. Metabolism of squalene in human fat cells. Demonstration of a two-pool system. The Journal of biological chemistry. 1982;257(17):10300–5. [PubMed] [Google Scholar]
- van der Maaten L, Hinton G. Visualizing Data using t-SNE. Journal of Machine Learning Research. 2008;9:2579–605. [Google Scholar]
- Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of beta -catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes & development. 2003;17(10):1219–24. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Fang F, Lam AP, Sargent JL, Hamburg E, Hinchcliff ME, et al. Wnt/b-cateninsignaling is hyperactivated in systemic sclerois and induces smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 2012 doi: 10.1002/art.34424. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Melichian D, Komura K, Hinchcliff M, Lam AP, Lafyatis R, et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum. 2011;63(6):1707–17. doi: 10.1002/art.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolins NE, Rubin B, Brasaemle DL. TIP47 associates with lipid droplets. The Journal of biological chemistry. 2001;276(7):5101–8. doi: 10.1074/jbc.M006775200. [DOI] [PubMed] [Google Scholar]
- Wu C, Macleod I, Su AI. BioGPS and MyGene. info: organizing online, gene-centric information. Nucleic acids research. 2013;41:D561–5. doi: 10.1093/nar/gks1114. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Liu Y, Kahn M, Ann DK, Han A, Wang H, et al. Interactions between beta-catenin and transforming growth factor-beta signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP) The Journal of biological chemistry. 2012;287(10):7026–38. doi: 10.1074/jbc.M111.276311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Gene skin expression t-SNE. Shows the similarity in skin gene expression within participants.
Supplemental Figure 2. Hierarchical clustering of KRT and KRTAP expression changes. The cluster includes all KRT and KRTAP genes detected by microarray analysis, showing the change in gene expression at 4 weeks compared to baseline. Clustering by gene is unsupervised and by patient is supervised. Gene symbols are shown to the right. Red indicates increased, green decreased gene expression (fold change in C-82 treated skin compared to placebo). The uncorrected p-value of change comparing placebo to treatment is shown to the far right.
Supplemental Figure 3. Immunohistochemical staining of PLIN2. Skin from a patient with SSc before treatment showing expression of PLIN2 in sebaceous glands (left panel) and subcutaneous fat (right panel)


