Abstract
Planar cell polarity (PCP) proteins are implicated in a variety of morphogenetic processes including embryonic cell migration and potentially cancer progression. During zebrafish gastrulation, the transmembrane protein Vang-like 2 (VANGL2) is required for PCP and directed cell migration. These cell behaviors occur in the context of a fibrillar extracellular matrix (ECM). While it is thought that interactions with the ECM regulate cell migration, it is unclear how PCP proteins such as VANGL2 influence these events. Using an in vitro cell culture model system, we previously showed that human VANGL2 negatively regulates membrane type-1 matrix metalloproteinase (MMP14) and activation of secreted matrix metalloproteinase 2 (MMP2). Here, we investigated the functional relationship between VANGL2, integrin αvβ3, and MMP2 activation. We provide evidence that VANGL2 regulates cell surface integrin αvβ3 expression and adhesion to fibronectin, laminin, and vitronectin. Inhibition of MMP14/MMP2 activity suppressed the cell adhesion defect in VANGL2 knockdown cells. Furthermore, our data show that MMP14 and integrin αv are required for increased proteolysis by VANGL2 knockdown cells. Lastly, we have identified integrin αvβ3 as a novel VANGL2 binding partner. Together, these findings begin to dissect the molecular underpinnings of how VANGL2 regulates MMP activity and cell adhesion to the ECM.
Keywords: VANGL2, MMP14, MMP2, integrins, extracellular matrix, adhesion
1. Introduction
Planar cell polarity (PCP) refers to the polarized organization of cellular structures, such as actin, in relation to the plane of a tissue [1]. This concept was originally used to describe the polarization of cuticular structures of the insect epidermis [2]. In the fly, a highly conserved core module of six PCP proteins was identified and shown to regulate PCP in multiple epithelial tissues including the wing and eye [1]. The core PCP genes are van gogh, prickle, dishevelled, frizzled, flamingo, and diego [3–5]. The vertebrate van gogh ortholog, vang-like 2 (vangl2), was first identified in 2001 when it was found to be the genetic defect in Loop-tail mouse mutants [6, 7]. Here, recessive mutations in vangl2 result in severe neural tube defects associated with abnormal morphogenesis of the floor plate neuroectoderm [6, 7]. Humans with mutations in VANGL1 or VANGL2 also develop neural tube closure defects [8]. Accumulating data also suggest a role for VANGL proteins during tumor progression and invasion [9, 10]. In zebrafish, vangl2 was identified as the defective gene in trilobite mutant embryos noted for having a strong convergence and extension gastrulation phenotype [11, 12]. Underlying convergence and extension of embryonic tissues are a variety of cell behaviors including directed migration and mediolateral intercalation [13–15]. Loss of Vangl2 protein function in trilobite mutant embryos produces rounder cells that migrate dorsally along indirect or meandering trajectories [11]. Zebrafish gastrulation cell movements occur in the context of a fibrillar extracellular matrix (ECM) network [16]. Knockdown of zebrafish fibronectin using antisense morpholino oligonucleotides produces a convergence and extension phenotype similar to, but weaker than, the vangl2/trilobite mutant phenotype [16]. Data from the frog gastrula have demonstrated that an intact fibronectin ECM is necessary for the formation of polarized membrane protrusions that drive mediolateral cell intercalation [17]. However, despite the long recognized role for fibronectin during vertebrate gastrulation [18–20], the functional relationship between the ECM and PCP proteins such as VANGL2 is unclear.
Previously, we provided evidence that fibronectin is a substrate for membrane type-1 matrix metalloproteinase (Mmp14) in the zebrafish gastrula [16, 21]. Matrix metalloproteinases (MMPs) cleave ECM substrates to control a variety of processes including cell adhesion, migration, and invasion [22]. Our work identified Mmp14 as a regulator of PCP during gastrulation [23] and we showed that similar to vangl2, mmp14 also exhibits a strong genetic interaction with glypican4 [23, 24]. Glypican4 is a cell-surface proteoglycan thought to function as the co-receptor for Wnt11/Wnt5b-dependent non-canonical PCP signaling [25–27]. These developmental studies did not provide evidence for a mechanistic relationship between zebrafish Vangl2 and MMP-dependent proteolysis of ECM proteins. Using human HT-1080 fibrosarcoma cells (a fibroblast-like cell type that expresses PCP proteins and exhibits a high level of MMP14/MMP2-dependent invasiveness [28]), we showed that human VANGL2 regulates MMP14 and activation of the secreted MMP2 zymogen [29, 30]. MMP2 activation is initiated at the cell surface by MMP14 cleavage of the 68 kDa pro-enzyme to produce a 64 kDa intermediate form of MMP2 followed by autocatalysis and production of the 62 kDa mature enzyme [31–33]. Our results demonstrated that siRNA-mediated knockdown of human VANGL2 increases MMP2 activation and cleavage of ECM substrates [29, 34] suggesting that the normal function of VANGL2 is to inhibit or restrict MMP2 activity. Significantly, these data were relevant to zebrafish embryonic development. We found that vangl2/trilobite mutant embryos have decreased levels of fibronectin protein due to increased MMP proteolytic activity [30]. Moreover, injection of mmp14 antisense morpholinos into vangl2/trilobite mutant embryos partially suppresses the convergence and extension phenotype [30]. Notably, our data from HT-1080 cells also showed that loss of VANGL2 function both increases the number of invadopodia membrane protrusions and decreases the number of paxillin positive focal adhesions [30, 34]. The ability of VANGL2 to impact these events suggests that one function of this transmembrane PCP protein might be to regulate cell-ECM interactions underlying processes such as adhesion and membrane protrusive activity. Yet, it remains unknown molecularly how VANGL2 function influences MMP2 activity and whether this actually affects cell adhesion to ECM proteins.
It is thought that proteolytic cleavage of proteins such as fibronectin by MMP14 or MMP2 alters cellular interactions with the ECM and subsequently behaviors like migration [35, 36]. Integrins are key regulators of cell adhesion to the ECM and play important roles during assembly of fibrillar ECM proteins [37]. Integrins themselves can also influence the activation of secreted MMP2 [38, 39]. The integrin αvβ3 heterodimer in particular was shown to physically interact with MMP14 and MMP2 [40–42]. The concept that integrins and MMP14 can cooperate to increase MMP2 activation provides an attractive mechanism to regulate cell-ECM interactions underlying cell polarity and migration. Therefore, we hypothesized that VANGL2's ability to regulate MMP2 activation and ECM protein cleavage might somehow involve or require integrin αvβ3. In this report, we used HT-1080 cells to examine the functional relationship between human VANGL2, integrin αvβ3, and MMP2 activation. Our data show that VANGL2-dependent regulation of MMP14/MMP2 activity and cell surface integrin αvβ3 expression influence cell adhesion to the ECM. The ability of VANGL2 to regulate MMP2 activation requires both integrin αv and MMP14. We further show that integrin αvβ3 is a novel VANGL2 binding partner. Together, our results support a model whereby VANGL2 acts to inhibit or limit MMP2 proteolytic activity while promoting integrin mediated cell-ECM adhesion.
2. Materials and methods
2.1. Cell culture and MMP inhibition
Human fibrosarcoma HT-1080 cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and used throughout this study (cells were only used below passage 25). Cells were cultured in DMEM media (4.5 g/L glucose, L-glutamine, and sodium pyruvate) containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO) and antibiotics (100 units/mL penicillin G sodium and 100 μg/mL streptomycin). Cells were incubated at 37°C in the presence of 5% CO2. MMP inhibition was achieved by incubating cells 48 hrs in 10 μM of the broad-spectrum MMP inhibitor GM6001 (EMD Millipore, Billerica, MA).
2.2. siRNA transfection and knockdown
Cells were seeded overnight in 6- or 12-well plates and transfected at 40–50% confluence DharmaFECT 4 lipid reagent (GE Dharmacon, Lafayette, CO). The siRNA pools were used at 100 nM while the single siRNAs were used at 25 nM. The following siRNAs were used in this study: Dharmacon human VANGL2 (L-010581) and integrin αv (L-004565) ON-TARGETplus SMARTpool siRNA, Dharmacon human integrin αv single siRNAs (J-004565-08 and J-004565-10), Ambion (Thermo Fisher Scientific, Waltham, MA) human MMP14 Silencer (ID#104074) and Silencer Select (ID#s8877) siRNA, and Dharmacon Non-Targeting #2 ON-TARGETplus SMARTpool Control siRNA (D-001810). For all siRNA experiments, cells were transfected for 4 days and protein knockdown was confirmed using western blot (see below for procedure and antibodies). The MMP14 Silencer siRNA (see Supplementary Fig. 1) and the integrin αv siRNA pool (see Supplementary Fig. 2) were used throughout this study.
2.3. Plasmid DNA transfection and overexpression
Expression plasmids containing full length human VANGL2 and the control plasmids were used in this study. For the adhesions assays, VANGL2/pcDNA3 was used and for co-immunoprecipitation experiments, GFP-VANGL2/pCS2 was used. The control plasmids were pcDNA3 and pEGFPN1. Cells were seeded in 6- or 12-well plates and transfected at 80–90% confluence using Lipofectamine 3000 reagent (Thermo Fisher Scientific) following the manufacturer's instructions. For all plasmid overexpression experiments, cells were transfected for 48 hrs and protein expression was verified using western blot (see below for procedure and antibodies).
2.4. Co-immunoprecipitation and antibodies
Cells used for co-immunoprecipitation experiments had their proteins extracted using a Tris lysis buffer (50 mM Tris-HCL, 150 mM NaCl, 0.1% Triton-X, pH 7.5) and protein concentrations were determined using the BCA assay. Lysates at a concentration of 200–300 μg were treated or untreated with 3–10 μg of antibodies [integrin αvβ3 (MAB1976), EMD Millipore; VANGL2 (21492-1-AP), Proteintech, Rosemont, IL. Lysates were incubated with antibodies for 2 hrs at 4°C then added to prepared protein G magnetic beads (Thermo Fisher Scientific) or anti-GFP mAb-magnetic beads (Medical & Biological Laboratories CO., LTD, Nagoya, Japan). Beads and lysates were incubated overnight at 4°C with rotation. Beads were washed with Tris lysis buffer and then denatured using Laemmli sample buffer containing β-mercaptoethanol at 95°C for 6 minutes.
2.5. Western blot and antibodies
Protein obtained from co-immunoprecipitation experiments and whole-cell lysates were separated by 10% SDS-PAGE under reducing conditions and transferred to nitrocellulose or PVDF membranes using a Trans-Blot Turbo (Bio-Rad, Hercules, CA). Non-specific binding to membranes was blocked with TBS-Tween (50 mM Tris, pH 7.4, 150 mM NaCl, 0.1% Tween-20) containing 0–5% non-fat milk depending on the antibody used and membranes were incubated overnight at 4°C with primary antibody in block solution. Membranes were then incubated with peroxidase-conjugated affinity-purified donkey anti-rabbit, anti-mouse, or anti-rat secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). TrueBlot anti-rabbit or anti-mouse IgG HRP secondary antibodies (Rockland Antibodies & Assays, Limerick, PA) were used for certain western blots. Blots were developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) and imaged using a UVP GelDoc-It Imaging System (Upland, CA). In each experiment blots were stripped at room temperature for 15 minutes using 25 mM glycine and 1% SDS (pH 2.0) and re-probed using a GAPDH antibody. Our western blot primary antibodies were [MMP14 (1:1000, MAB3328), EMD Millipore; VANGL2 (1:1000, MABN750), EMD Millipore; integrin αv (1:1000, ab17975), Abcam, Cambridge, MA), integrin β1 (1:1000, ab30394), Abcam; integrin α5 (1:1000, #4705), Cell Signaling Technology, Danvers, MA); integrin β3 (1:1000, #13166), Cell Signaling Technology; Src Antibody Sampler Kit (1:1000, 2107 non-phospho Src Y527; 1:1000, 2102 non-phospho Src Y416; 1:1000, 2105 phospho-Src Y527; 6943 phospho-Src Y416, Cell Signaling Technology); paxillin (1:1000, ab32084), Abcam; phospho-paxillin Y118 (1:1000, #2541), Cell Signaling Technology; GFP (1:1000, A10262), Thermo Fisher Scientific; and GAPDH-HRP (1:2000, GTX627408-01), GeneTex, Inc., Irvine, CA].
2.6. ECM adhesion assays
Millicoat Cell Adhesion Strips pre-coated with human fibronectin (ECM101), human laminin (ECM103), or human vitronectin (ECM102) were reconstituted with room temperature calcium and magnesium free PBS (pH 7.4) 15 minutes prior to use as directed by the manufacturer (EMD Millipore). Next, the strips were seeded with ~5x105 cells per well. Cells used in this assay were detached from their multiwell plates using a non-enzymatic cell dissociation buffer and were suspended in serum-free DMEM containing 10 mM CaCl2 and 10 mM MgCl2. Cells were allowed to adhere to the strips for 1 hr in a 37°C incubator with 5% CO2. The strips were then washed with room temperature PBS containing calcium and magnesium and stained with 0.2% crystal violet solution. The stain was solubilized using 0.1 M NaH2PO4 solubilization buffer and absorbance determined at 560 nm using a microplate reader.
2.7. Gelatin zymography
Cells were seeded in 12-well plates and transfected with siRNAs as described above. On day 3 post-transfection the cells were washed twice using 37°C pre-warmed PBS and 230 μl of serum free DMEM was added to each well followed by an additional 24 hr incubation period. The media was removed from the wells and centrifuged briefly to remove cell debris. The supernatant was mixed 1:1 with Novex 2X Tris-Glycine SDS Sample Buffer (Thermo Fisher Scientific) and incubated for 5 minutes at room temperature. Samples were run on Novex 10% Zymogram Gels (EC6175, Thermo Fisher Scientific). Gels were washed for 30 minutes each in Novex Renaturing Buffer and Novex Developing Buffer with an additional overnight incubation at 37°C in fresh developing buffer. Gels were rinsed with distilled water three times for 10 minutes each and developed using Novex SimplyBlue Safestain.
2.8. Biotinylation
Cell were seeded in 6-well plates and transfected with siRNAs as described above. On day 4 post-transfection, the cell surface proteins were labeled with biotin as previously described [43]. Briefly, the cells were washed with ice-cold SBS (Sorensen Buffer: 14.7 mM KH2PO4, 2 mM Na2HPO4, and 120 mM Sorbitol, pH 7.8) and then incubated for 15 minutes. The SBS was removed and replaced with 1 mg/ml EZ-Link Sulfo-NHS-LC-Biotin (Thermo Fisher Scientific) and the plates were incubated for 10 minutes followed by two washes with SBS. Non-biotinylated controls were also performed. Next, SBS containing 100 mM glycine was added to the wells and the plates incubated for 20 minutes followed by three more washes with SBS. The samples were kept on ice throughout the entire procedure. Protein was solubilized using RIPA lysis buffer containing a protease inhibitor cocktail and quantified using the BCA assay. Streptavidin M-280 Dynabeads (Thermo Fisher Scientific) were used as directed by the manufacturer to bind biotinylated proteins in whole cell lysates. The proteins were denatured using Laemmli sample buffer containing β-mercaptoethanol at 95°C for 5 minutes.
2.9. Fluorescent gelatin degradation
For fluorescent gelatin degradation, the QCM Gelatin Invadopodia Assay (ECM670, Thermo Fisher Scientific) was used following the manufacturer's instructions. Here, cells were transfected for 3 days with NT, VANGL2, integrin αv, or integrin αv/VANGL2 siRNA prior to seeding on the chamber slides for an overnight incubation. Cells were labeled with phalloidin and DAPI as instructed. Images were taken at 40x magnification. Individual degradation foci were manually counted from each image using ImageJ software and averaged. When multiple foci contacted each other to form larger amorphous degradation spots they were counted as a single foci.
2.10. Statistical analysis and data presentation
All experimental groups were treated equally and quantified using blind analysis. Each experiment was repeated 3–5 times in duplicate, triplicate, or more. Densitometry was performed on western blots using UVP Visionworks software. For western blots, the experimental bands were normalized using GAPDH as a control. Graphing and statistical analyses were performed using Prism 6. The two-tailed unpaired Student's t-test was used for statistical analysis on all data sets. Graphical data shown are the mean values with standard deviations. The standard deviation for control samples was obtained by dividing individual control values by the average of the sum of all control values acquired for each experiment. Except for Fig. 6G and H, graphical results are expressed as percent of control. These numbers were obtained by dividing the individual experimental values by the average of the control values and multiplying by one hundred. Western blot and fluorescent images shown are representative of the entire experiment.
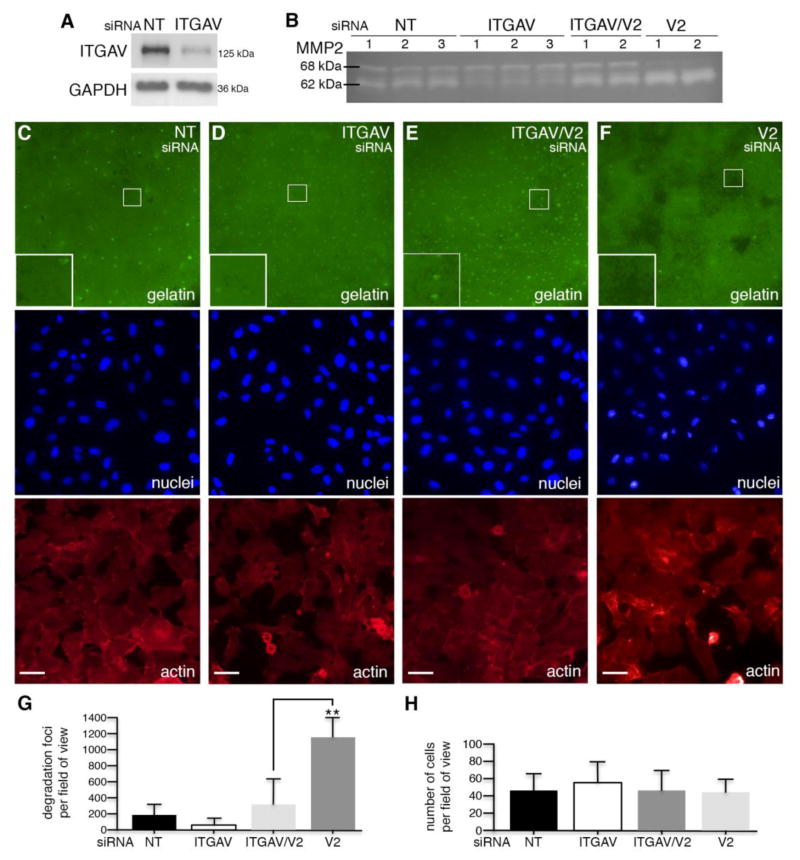
Fig. 6.
Integrin αv is required for VANGL2-dependent regulation of MMP2. (A) Western blot analysis of non-targeting control (NT) and integrin αv (ITGAV) siRNA transfected cells. The ITGAV protein level was reduced 48% compared to control. (B) Gelatin zymography analysis of control, ITGAV, ITGAV/VANGL2 (V2), and V2 siRNA transfected cells. The numbers 1, 2, and 3 on the zymogram represent results from an experiment performed in either triplicate or duplicate. (C–F) Representative images of fluorescent gelatin, DAPI-labeled nuclei, and phalloidin-labeled actin from control, ITGAV, ITGAV/V2, and V2 siRNA transfected cells. Large white boxes in lower left corner of the gelatin images are a magnification of the small white-boxed regions. Focal gelatin degradation is observed as black spots. Scale bars are 20 μm. (G) Quantification of the number of degradation foci per 40x field of view. (H) Quantification of the number of cells per 40x field of view. Significant differences are marked as follows: **p < 0.001.
3. Results
3.1. VANGL2 regulates cell adhesion to ECM proteins
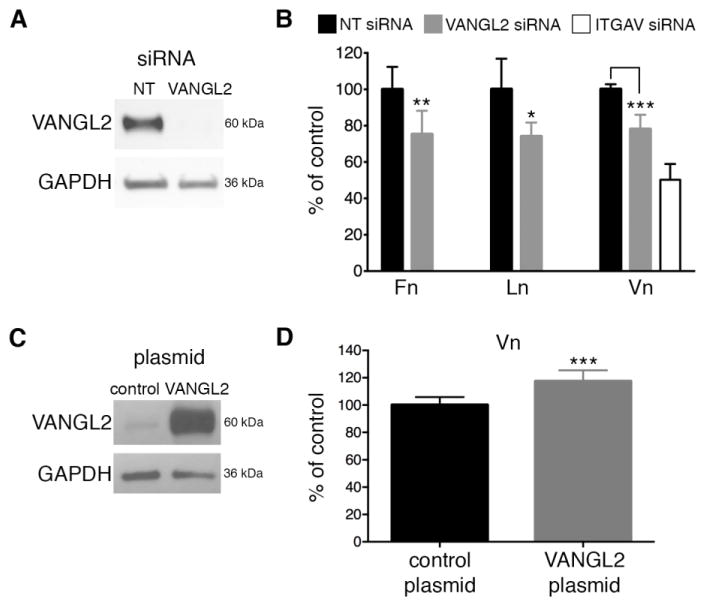
Our previous work demonstrated that loss of VANGL2 function in HT-1080 cells results in increased MMP14/MMP2 enzymatic activity, ECM cleavage, and cell invasion [29, 30, 34]. Our limited data suggested that VANGL2 might affect MMP activity at sites of cell-ECM interactions including focal adhesions and invadopodia [30, 34]. To determine whether VANGL2 is specifically required for cell adhesion to the ECM, we performed adhesion assays using fibronectin, laminin, and vitronectin. These ECM proteins were chosen because they bind integrin αvβ3. For all VANGL2 knockdown experiments in this study we used a previously validated ON-TARGETplus SMARTpool of siRNA [29, 30, 34]. After using siRNA to knockdown VANGL2 expression (Fig. 1A), HT-1080 cells were plated 1 hour on well plates pre-coated with the different ECM proteins. Adherent cells were stained with crystal violet and quantified using a spectrophotometer. Compared to control siRNA transfected cells, VANGL2 knockdown cells exhibited reduced adhesion to all three ECM proteins (Fig. 1B). These results suggest that VANGL2 can function to promote cell adhesion to the ECM. To determine whether indeed overexpression of VANGL2 protein promotes cell-ECM adhesion, HT-1080 cells were transfected with control vector or pcDNA3 containing the human VANGL2 gene. First, we confirmed that our transfection procedure resulted in increased VANGL2 protein expression compared to control cells (Fig. 1C). Next, adhesion assays to vitronectin, the major integrin αvβ3 binding partner, were performed as described above. Our data show that an increase in VANGL2 protein expression promotes cell adhesion to vitronectin (Fig. 1D). Together, these data demonstrate that changes in VANGL2 protein expression levels can regulate cell adhesion to certain ECM proteins.
Fig. 1.
VANGL2 regulates cell-ECM adhesion. (A) Western blot analysis of non-targeting control (NT) and VANGL2 (V2) siRNA transfected HT-1080 cells. (B) Quantification of cell adhesion to fibronectin (Fn), laminin (Ln), and vitronectin (Vn) by NT, VANGL2, and integrin αv (ITGAV) siRNA transfected cells. ITGAV knockdown was included in the vitronectin assays for reference (see Supplementary Fig. 2 for ITGAV siRNA validation). (C) Western blot of control pcDNA3 plasmid and VANGL2/pcDNA3 transfected cells showing VANGL2 protein overexpression. At the low exposure shown endogenous VANGL2 protein expression is barely discernible in the control cells. (D) Quantification of cell adhesion to vitronectin in control and VANGL2/pcDNA3 transfected cells. Significant differences are marked as follows: *p < 0.05; **p < 0.001; ***p < 0.0001.
3.2. Loss of VANGL2 affects cell surface integrin αvβ3 expression
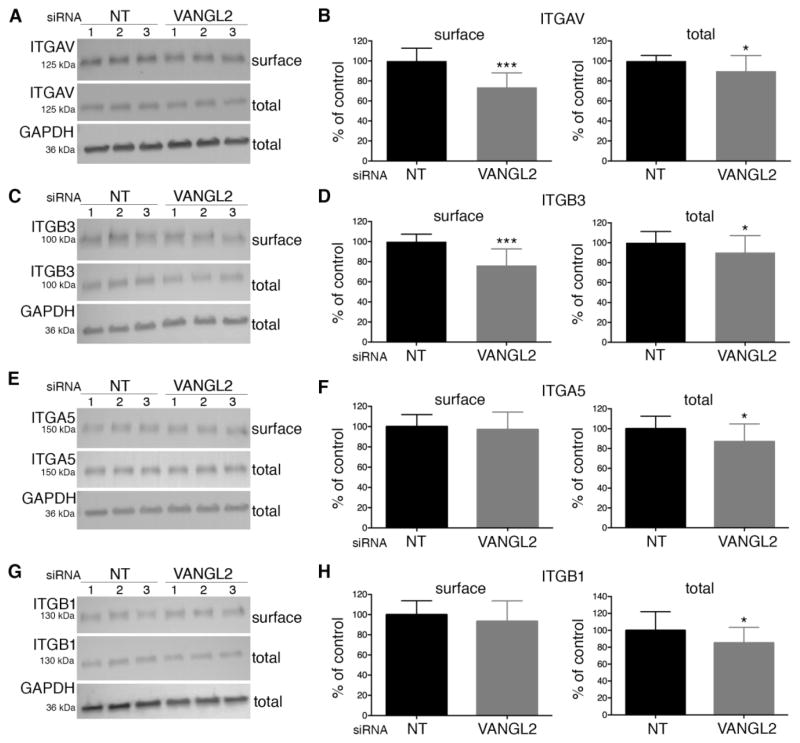
Our observation that VANGL2 is required for proper cell adhesion to fibronectin, laminin, and vitronectin suggested that VANGL2 might regulate the expression of integrin αvβ3 receptors. We first quantified total and cell surface integrin αvβ3 protein expression in control and VANGL2 siRNA transfected cells. Integrin α5β1 was included in these experiments for comparison because it can also function as a receptor for fibronectin and laminin. To assess cell surface protein expression, cells were first biotinylated and biotin-labeled proteins were recovered using magnetic streptavidin beads. The expression of individual integrin proteins was quantified from western blots using densitometry and normalization to GAPDH. The data show a reduction in the cell surface levels of both integrin αv and integrin β3 (Fig 2A–D) but not integrin α5β1 (Fig. 2E–H). By contrast, loss of VANGL2 function had less of an effect on the total protein levels of either integrin αvβ3 or integrin α5β1 (Fig. 2A–H). These results suggest that VANGL2, either directly or indirectly, influences the quantity of plasma membrane associated integrin αvβ3.
Fig. 2.
Integrin expression in VANGL2 knockdown HT-1080 cells. Western blot analyses and quantification of cell surface and total integrin protein expression in non-targeting control (NT) and VANGL2 siRNA transfected cells. (A,B) integrin αv (ITGAV). (C,D) integrin β3 (ITGB3). (E,F) integrin α5 (ITGA5). (G,H) integrin β1 (ITGB1). The numbers 1, 2, and 3 on each blot represent results from an experiment performed in triplicate. Significant differences are marked as follows: *p < 0.05; ***p < 0.0001.
3.3. Loss of VANGL2 does not significantly influence SRC activation or paxillin phosphorylation
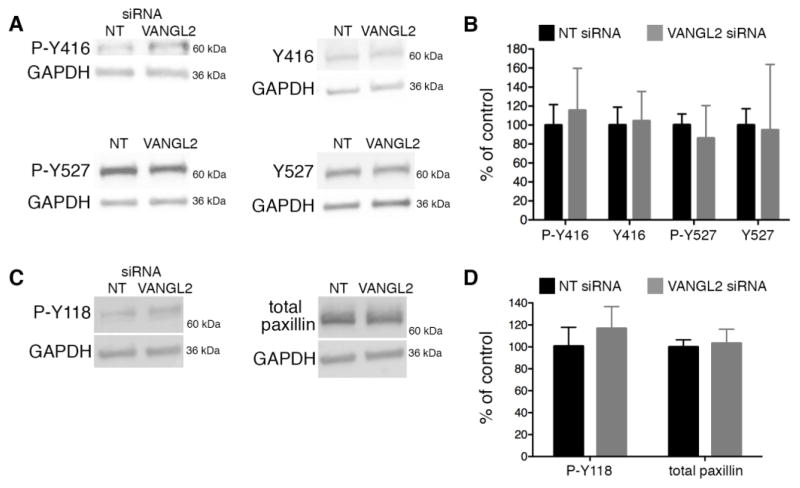
Outside-in integrin signaling pathways can be triggered by ECM components and growth factors in the extracellular environment. The reduction in cell surface integrin αvβ3 expression in VANGL2 knockdown cells suggests a reduced capacity to stimulate such pathways. However, our previous work suggested that VANGL2 might promote integrin signaling as indicated by increased focal adhesion kinase (FAK; also called PTK2) phosphorylation at tyrosine 397 (Y397) and a decreased number of paxillin-positive focal adhesions in VANGL2 knockdown cells [30]. To address this further, we assessed both SRC activation and phosphorylation of paxillin. SRC is recruited to phosphorylated Y397 FAK at focal adhesions where it phosphorylates several proteins including paxillin (Y118) [44–46]. FAK also phosphorylates paxillin [47, 48]. Paxillin is a focal adhesion-associated cytoplasmic protein whose phosphorylation influences both the assembly and turnover of focal adhesions [49]. To assess SRC activation, we used antibodies that recognize both the phosphorylated and non-phosphorylated forms of SRC at positions Y416 and Y527. SRC auto-phosphorylation at Y416 is required for full activation while phosphorylation at Y527 produces a closed protein conformation and kinase inhibition [50–52]. Compared to controls, VANGL2 siRNA transfected cells had minimal differences in the levels of phosphorylated Y416 SRC (p value = 0.1744) and phosphorylated Y527 SRC (p value = 0.1010) (Fig. 3A,B). We next quantified the levels of phosphorylated Y118 and total paxillin in control and VANGL2 knockdown cells. Here, loss of VANGL2 function caused a marginal increase in the level of phosphorylated paxillin (p value = 0.0671) (Fig. 3C,D). Because the above results were not significantly different compared to the controls (when rejecting the H0 at p > 0.05), we conclude that VANGL2 knockdown has little to no impact on SRC activation and paxillin Y118 phosphorylation. Further data are needed to determine how VANGL2 function regulates FAK and the turnover of focal adhesions.
Fig. 3.
Src and paxillin activation in VANGL2 knockdown HT-1080 cells. Western blot analysis of Src and paxillin activation status in non-targeting control (NT) and VANGL2 (V2) siRNA transfected cells. Experiments were repeated five times and representative blots are shown. (A) P-Y416 (activated Src), Y416 negative control (non-phosphorylated), P-Y527 (inhibited Src), Y527 negative control (non-phosphorylated). (B) Quantification of panel (A) data. (C) P-Y118 (activated paxillin), total paxillin control. (D) Quantification of panel (C) data.
3.4. Inhibition of MMP14/MMP2 activity rescues ECM adhesion in VANGL2 knockdown cells
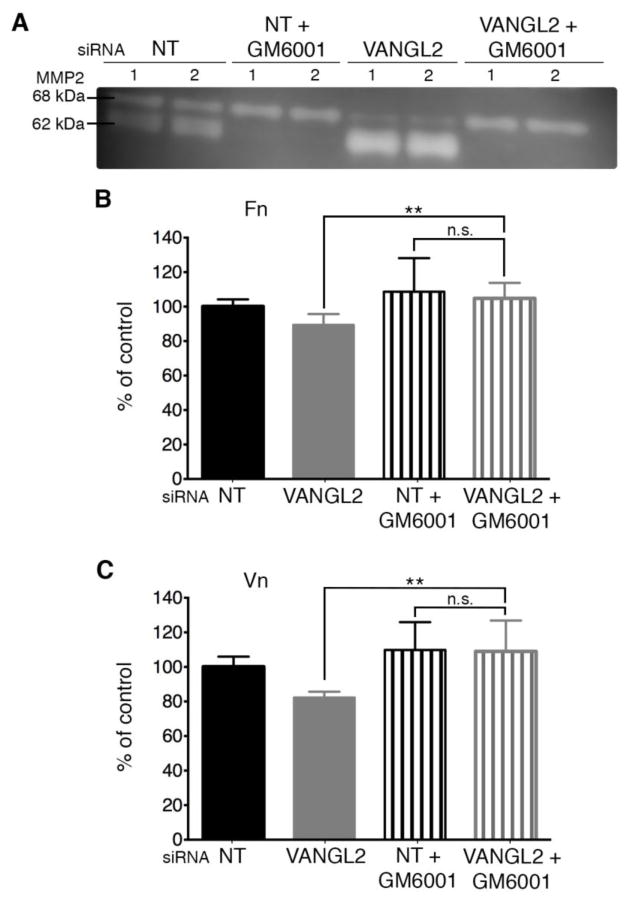
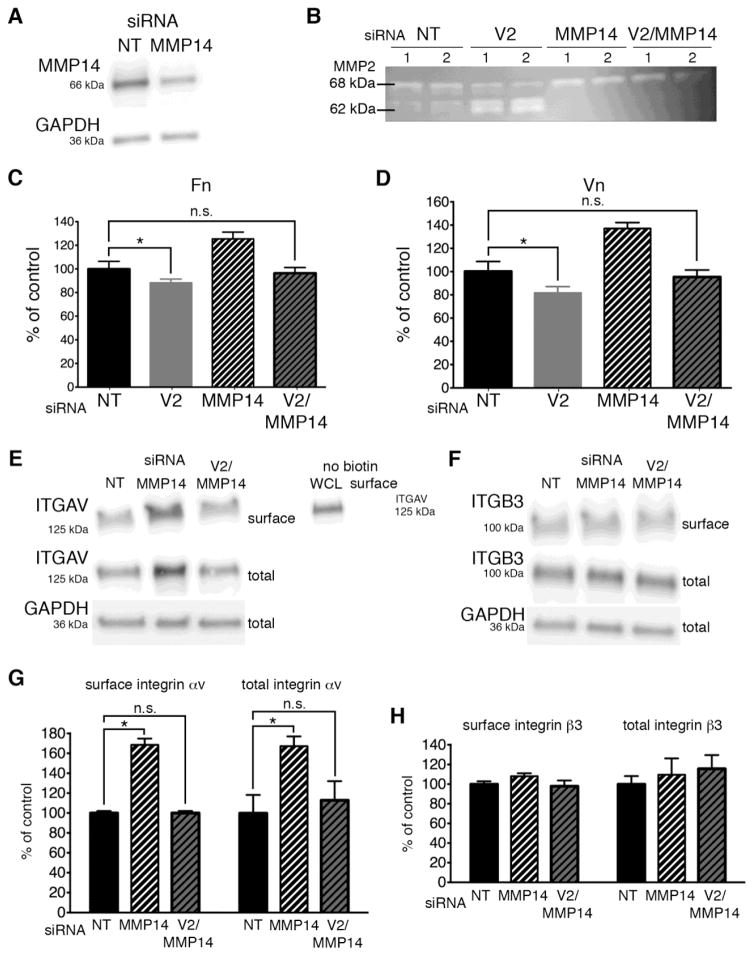
Our data support the notion that VANGL2 function is required for normal cell-ECM adhesion (Fig. 1) and we have shown that VANGL2 protein knockdown disrupts cell surface integrin αvβ3 expression (Fig. 2A–D). However, it is unclear whether loss of adhesion in VANGL2 knockdown cells is due primarily to reduced integrin expression or increased MMP activity, or if these two events are related. To determine the contribution of MMP proteolytic activity to the ECM adhesion phenotype of VANGL2 knockdown cells, we first used the broad-spectrum MMP inhibitor GM6001 (also called galardin or ilomastat) [53]. GM6001 inhibits several MMPs having inhibitory constants in the nanomolar range for MMP1, MMP2, MMP3, MMP7, MMP8, MMP9, MMP12, MMP14, and MMP26 [53]. Applied at 10 μm, this drug prevents increased MMP2 activation in VANGL2 knockdown cells as indicated by gelatin zymography (Fig. 4A). After GM6001 treatment, ECM adhesion assays were performed as described above. The data show that inhibition of MMP activity in VANGL2 knockdown cells rescued the ECM adhesion phenotype to both fibronectin and vitronectin (Fig. 4B,C; laminin was not tested). Because MMP14 is the primary protein responsible for cleavage and activation of the secreted MMP2 zymogen [31–33], it follows that increased MMP2 activation in VANGL2 knockdown cells requires MMP14. First, we confirmed the specificity of two MMP14 siRNAs by comparing their ability to reduce MMP14 protein expression and inhibit MMP2 activation (Fig. 5A and Supplementary Fig. 1). Next, we used a combination of VANGL2 and MMP14 siRNAs and gelatin zymography to assess MMP2 activation in knockdown cells. Our findings show that indeed MMP14 knockdown completely inhibited production of the intermediate (64 kDa) and mature (62 kDa) forms of MMP2 in VANGL2 siRNA transfected cells (Fig. 5B). With this data in hand, we next determined whether, similar to GM6001 drug treatment, MMP14 knockdown could rescue the VANGL2 reduced cell-ECM adhesion phenotype. The results show that unlike VANGL2 knockdown cells, HT-1080 cells co-transfected with both VANGL2 and MMP14 siRNAs exhibited adhesion to fibronectin and vitronectin comparable to controls (Fig. 5C,D). Notably, when transfected with MMP14 siRNA, we observed that these cells had increased cell-ECM adhesion compared to controls. This result is consistent with data showing that MMP enzymatic activity is negatively correlated with cell-ECM adhesion [36, 40] and could be due to an increased amount of ECM available for binding to the MMP14 siRNA treated cells. However, it is also possible that reduced MMP14 activity and/or increased ECM regulate the cell surface expression of integrin αv [35]. Therefore, we performed biotinylation experiments to compare how MMP14 and VANGL2/MMP14 knockdown affect cell surface and total integrin αvβ3 protein levels. The data show that MMP14 siRNA transfected cells had significantly more integrin αv than control cells, but close to normal levels of integrin β3 (Fig. 5E–H). By contrast, VANGL2/MMP14 double knockdown cells had normal expression of both integrin αv and integrin β3 (Fig. 5E–H). These data show that VANGL2-dependent regulation of cell surface integrin αv expression correlates with cell-ECM adhesion. Whether VANGL2 function impacts integrin expression directly or through effects on MMP14/MMP2 proteolytic activity and ECM cleavage is unclear.
Fig. 4.
MMP activity is required for decreased cell-ECM adhesion in VANGL2 knockdown HT-1080 cells. (A) Gelatin zymography analysis of non-targeting control (NT) and VANGL2 siRNA transfected cells untreated or treated with GM6001. The 68 kDa pro-enzyme and 62 kDa mature protein are indicated. The numbers 1 and 2 on the zymogram represent results from an experiment performed in duplicate. (B,C) Quantification of cell adhesion to fibronectin (Fn) and vitronectin (Vn) by control and VANGL2 siRNA transfected cells untreated or treated with GM6001. Data are shown as percent of the NT non-drug treated control. Significant differences between untreated VANGL2 siRNA transfected cells and drug treated VANGL2 siRNA transfected cells are marked as follows: **p < 0.001; not significant (n.s.).
Fig. 5.
MMP14 and integrin αv are necessary for VANGL2-dependent regulation of MMP2. (A) Western blot analysis of non-targeting control (NT) and MMP14 siRNA transfected HT-1080 cells. The MMP14 protein level was reduced 48.9% compared to control. (B) Gelatin zymography analysis of control, VANGL2 (V2), MMP14, and V2/MMP14 siRNA transfected cells. The numbers 1 and 2 on the zymogram represent results from an experiment performed in duplicate. The 68 kDa pro-enzyme and 62 kDa mature protein are indicated. (C,D) Quantification of cell adhesion to fibronectin (Fn) and vitronectin (Vn) by NT, V2, MMP14, and V2/MMP14 siRNA transfected cells. (E) Western blot analysis of biotinylated cell surface and total integrin αv (ITGAV) in NT, MMP14, and V2/MMP14 siRNA transfected cells. Western blot analysis of non-biotinylated cell surface protein sample demonstrating that streptavidin magnetic beads do not directly bind ITGAV. WCL, whole cell lysate. (F) Western blot analysis of biotinylated cell surface and total integrin β3 (ITGB3) in NT, MMP14, and V2/MMP14 siRNA transfected cells. (G,H) Quantification of protein levels from data shown in panels (E) and (F). Significant differences are marked as follows: *p < 0.05; not significant (n.s.).
3.5. VANGL2-dependent regulation of MMP2 activity requires integrin αv
It has been established that integrin αvβ3 is also a regulator of MMP14 and MMP2 activation in multiple cell types [54, 55]. Therefore, we tested whether integrin αv is required for the MMP2 activation phenotype in VANGL2 knockdown cells. The integrin αv siRNAs were validated by protein knockdown and inhibition of cell adhesion to vitronectin (Fig. 6A and Supplementary Fig. 2). Gelatin zymography was then used to assess MMP2 activation in single and VANGL2/integrin αv double siRNA transfected cells. Our results show that reduction of integrin αv protein expression suppressed MMP2 activation in VANGL2 knockdown cells as indicated by both a decrease in the amount of 62 kDa mature enzyme and a concomitant increase in the level of 68 kDa pro-enzyme (Fig. 6B). From our previous work, we know that VANGL2 regulates the formation and degradative capacity of invadopodia [34]. Therefore, to corroborate the zymography data at the cellular level, we tested whether integrin αv knockdown would suppress the proteolytic activity of VANGL2 knockdown cells plated on fluorescently labeled gelatin. We compared control, integrin αv, and VANGL2 siRNA transfected cells with VANGL2/integrin αv double knockdown cells. The data show that while VANGL2 knockdown alone significantly increases the number of degradation foci produced by HT-1080 cells, knockdown of both VANGL2 and integrin αv resulted in focal degradation of gelatin similar to control cells (Fig. 6C–H). These data demonstrate that integrin αv is required for VANGL2 to influence MMP2 activation.
3.6. Integrin αv regulates cell surface MMP14 expression
How might integrin αv regulate MMP2 activation in HT-1080 cells? It is thought that integrin αvβ3 binding of ECM proteins can control the internalization of cell surface MMP14 [42]. Therefore, we hypothesized that integrin αv expression is required for maintenance of MMP14 expression at the plasma membrane. To determine this, we used western blot to assess both total and cell surface levels of MMP14 in control and integrin αv siRNA transfected cells. As in Fig. 2, biotin-labeled cell surface proteins were recovered using magnetic streptavidin beads prior to MMP14 western blot analysis. Our results show that integrin αv knockdown causes a modest reduction in cell surface but not total MMP14 protein expression (Fig. 7A,B). These findings suggest that in HT-1080 cells integrin αv function maintains cell surface MMP14 expression perhaps by inhibiting its internalization. Our previous data suggested that VANGL2 function promotes endocytosis of MMP14 [30]; therefore, integrin αv and VANGL2 have opposing effects on the expression of plasma membrane MMP14.
Fig. 7.
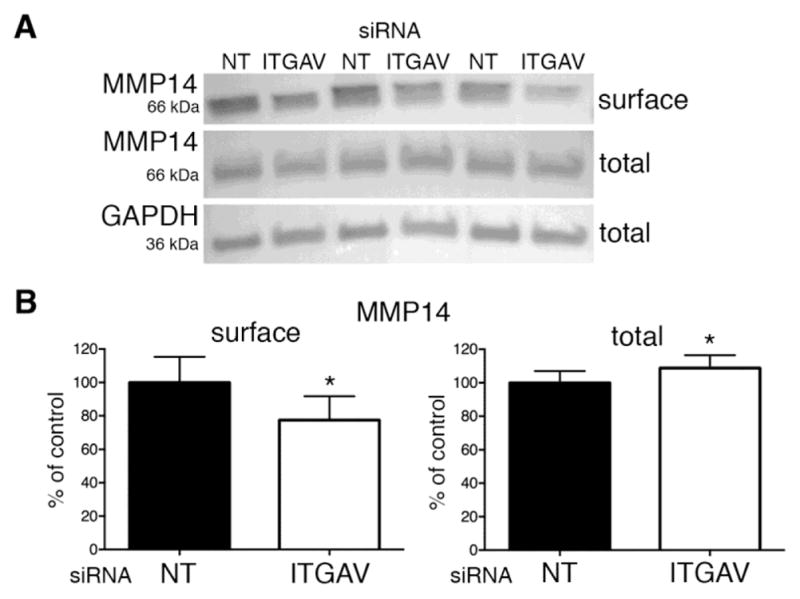
Integrin αv regulates cell surface MMP14 expression. (A,B) Western blot analysis and quantification of biotinylated cell surface and total MMP14 protein expression in non-targeting control (NT) and integrin αv (ITGAV) siRNA transfected cells. The results shown are from an experiment performed in triplicate. Significant differences are marked as follows: *p < 0.05.
3.7. VANGL2 binds integrin αvβ3
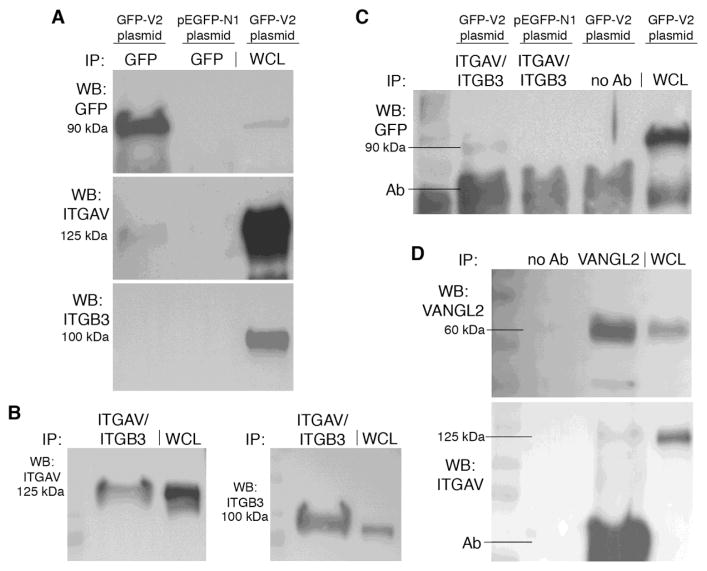
Integrin αvβ3 is a known binding partner for MMP14 and a regulator of MMP2 activation [40–42]. Since our data show that integrin αv is required for VANGL2-dependent regulation of MMP2, we tested whether integrin αvβ3 is also a VANGL2 binding partner. In these experiments, we used co-immunoprecipitation methods to detect interactions between 1) the endogenously expressed integrin αv and integrin β3 proteins and transfected VANGL2, 2) the endogenous integrin αvβ3 heterodimer and transfected VANGL2, and 3) the endogenous integrin αv and endogenous VANGL2 proteins. First, we overexpressed a GFP-VANGL2 expression plasmid in HT-1080 cells and performed co-immunoprecipitation experiments using anti-GFP mAb-magnetic beads (see Supplementary Fig. 3 for further bead validation). Under these conditions we consistently precipitated a band corresponding to endogenous integrin αv (Fig. 8A). We were unable to detect an interaction between transfected GFP-VANGL2 and endogenous integrin β3 (Fig. 8A). Next, using a validated integrin αvβ3 antibody that recognizes the endogenously expressed heterodimer protein (Fig. 8B), we were able to co-immunoprecipitate GFP-VANGL2 (Fig. 8C). Last, we asked whether the endogenous VANGL2 and integrin αv proteins formed a complex. We confirmed that VANGL2 protein could be precipitated from HT-1080 cells using an antibody recognizing the VANGL2 C-terminal cytoplasmic domain (Fig. 8D). We then found that this VANGL2 antibody could indeed precipitate endogenous integrin αv protein (Fig. 8D). The above results suggest that VANGL2 physically associates with the integrin αvβ3 heterodimer possibly through direct binding of the integrin αv protein.
Fig. 8.
VANGL2 binds integrin αvβ3. (A) Western blots (WB) demonstrating that the anti-GFP mAb-magnetic beads immunoprecipitated (IP) GFP-VANGL2 (GFP-V2) and integrin αv (ITGAV) but not integrin β3 (ITGB3). WCL, whole cell lysate. (B) Western blot showing that the integrin αvβ3 antibody precipitates endogenously expressed ITGAV and ITGB3 proteins. (C) Western blot showing endogenous integrin αvβ and GFP-V2 co-immunoprecipitation from GFP-V2/pCS2 but not pEGFP-N1 control plasmid transfected cells. The no antibody control was used to assess non-specific binding of proteins to the protein G beads. (D) Western blots validating VANGL2 antibody for use in immunoprecipitation of endogenously expressed VANGL2 protein and showing co-immunoprecipitation of endogenously expressed VANGL2 and integrin αv. In panels (C) and (D) the background caused by the precipitation antibody (Ab) is indicated.
4. Discussion
Vertebrate VANGL2 is linked to a large number of processes at both the cellular and tissue levels [56]. However, it has often proven difficult to determine mechanistically how this transmembrane protein functions to elicit such a wide range of effects. This might be due in part to VANGL2 being a scaffolding-like protein whose function is to bind and thus bring together multiple proteins. In this study we have used a cell culture model system to interrogate VANGL2 function as it relates to cellular interactions with the ECM. Our previous data from this model identified a novel relationship between VANGL2 and the regulation of MMP2 enzymatic activity [29, 30, 34]. Significantly, these findings were also relevant to zebrafish Vangl2 function during embryonic development [21, 30]. Here we have extended our cell culture studies to determine how VANGL2 influences cell adhesion to ECM proteins and whether VANGL2-dependent regulation of MMP2 activation involves integrin αv.
4.1. VANGL2 interacts with integrin αv and MMP14 to control MMP2 activation
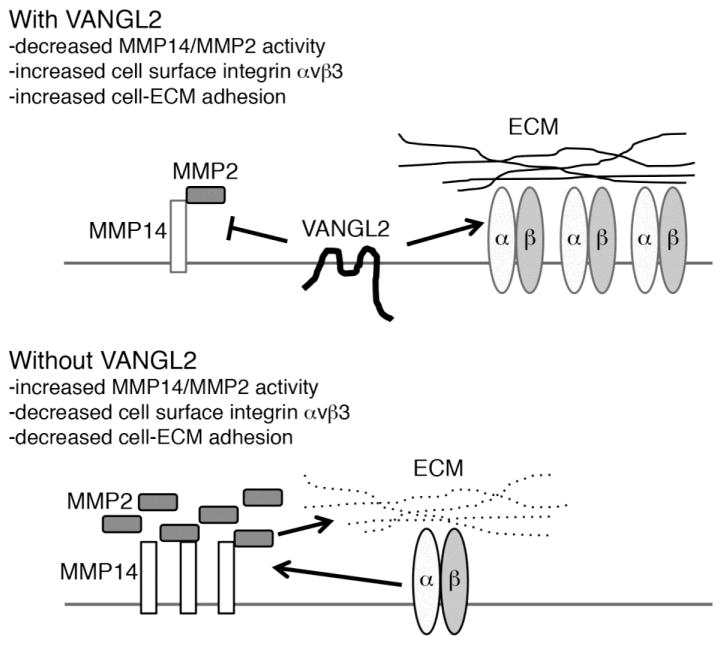
While VANGL2 protein is detected at both the plasma membrane and within intracellular vesicles [30], it is thought to exert its effects at the cell surface. Here, we report that VANGL2-dependent regulation of MMP2 activation requires both integrin αv and MMP14. The requirement for MMP14 makes sense because this cell surface enzyme is the major activator of the secreted MMP2 zymogen. Multiple studies have also linked integrin αvβ3 with both the regulation of MMP14 and the activation of MMP2 [38–42]. In addition to its ability to bind these two MMPs, integrin αvβ3 was shown to cooperate with MMP14 during MMP2 pro-domain cleavage and activation [41]. Cell-ECM interactions mediated by integrin αvβ3 can also regulate MMP14 endocytosis and thus MMP2 activation [42]. Reciprocally, MMP14 cleavage of fibronectin was shown to promote integrin α5β1 endocytosis [35]. Thus, there is a complex interdependent relationship between MMP14, MMP2, and integrin function that we propose can be influenced by VANGL2 (Fig. 9). VANGL2 is a four pass transmembrane protein with short extracellular and intracellular loops and larger N- and C-terminal domains that extend into the cytoplasm [57]. Because of its structure, VANGL2 is unlike classical tetraspanin proteins noted for their large extracellular loop and short cytoplasmic domains [58]. However, it is interesting that similar to VANGL2, tetraspanins have been shown to bind certain integrins [59] and regulate the amount of cell surface MMP14 expression and MMP2 activation [60]. How does VANGL2 regulate MMP14 and subsequently MMP2 activation? From our previous work we know that VANGL2 knockdown HT-1080 cells have defective MMP14 endocytosis and increased activation of MMP2 [30]. Thus it is possible that through direct or indirect physical binding, VANGL2 promotes endocytosis of plasma membrane MMP14. While mechanistic details are lacking, increasing evidence suggests that VANGL2 functions as a regulator of endocytic protein trafficking. In addition to MMP14, VANGL2 was shown to promote endocytosis of N- and E-cadherins in a manner requiring the small GTPase Rab5 [61]. Our finding that integrin αv is necessary for the MMP2 activation phenotype of VANGL2 knockdown cells and the identification of integrin αvβ3 as a VANGL2 binding partner might also be suggestive of a mechanism to regulate MMP14. It is possible that integrin αv is directly required for MMP14-mediated MMP2 activation or that integrin αv-dependent cell-ECM adhesion is required to stabilize MMP14 at the cell surface. We show that VANGL2 influences the amount of integrin αvβ3 at the plasma membrane suggesting that it might regulate integrin endocytosis either directly or indirectly through effects on MMP activity and ECM degradation. It was shown that MMP14 could cleave the 125 kDa integrin αv heavy chain to produce a 115 kDa protein and promote adhesion to vitronectin [62, 63]. Our data do not support the notion that VANGL2 knockdown results in cleavage of cell surface integrin αv. It is perhaps notable that Scribble, another membrane associated polarity protein, was found to bind integrin α5 and control its plasma membrane trafficking [64]. Further data are needed to determine how VANGL2 might affect integrin αvβ3 localization and function. An attractive hypothesis is that VANGL2 regulates the ability of integrin αv to switch between functioning as an MMP2 activator and an ECM adhesion protein (Fig. 9).
Fig. 9.
Model of VANGL2-dependent regulation of cell-ECM adhesion. When VANGL2 is present at the plasma membrane it promotes cell-ECM adhesion through inhibition of MMP14/MMP2 activity and positive regulation of integrin αvβ3 expression. Without VANGL2, increased MMP14/MMP2 activity and decreased integrin αvβ3 expression inhibits cellular adhesion to the ECM. It remains unclear whether VANGL2 regulates integrin αvβ3 expression directly or through effects on MMP14/MMP2 activity and cleavage of ECM proteins.
4.2. VANGL2 functions as a regulator of cell adhesion to the ECM
The ability of PCP proteins to influence ECM structure was first demonstrated in frog where it was found that overexpression of Vangl2 (called Strabismus at that time), Prickle, or Frizzled7 disrupts the assembly of fibrillar fibronectin during gastrulation [65]. In this context, PCP protein overexpression caused fibril assembly to be disorganized and not localized correctly along the surface of the mesoderm. It was suggested that PCP proteins regulate polarized fibronectin deposition but the underlying molecular mechanism was not determined. In homozygous loop-tail/vangl2 mutant mice, it was demonstrated that fibronectin protein is reduced in the subepicardial space adjacent to Vangl2 expressing myocardium [66]; however, it is unclear whether this phenotype is related to increased MMP activity. Our work showing that VANGL2 is capable of regulating MMP-dependent proteolysis suggests a mechanism whereby this PCP protein might influence both fibronectin assembly and cell-ECM adhesion. The concept that proteolytic cleavage of ECM proteins regulates cell-ECM adhesion has been documented in the literature [35, 36, 67]. Our data now show that indeed loss of human VANGL2 reduces cell adhesion to fibronectin, laminin, and vitronectin. A major cell surface receptor for these three ECM proteins is integrin αvβ3. Reduced cell-ECM adhesion in VANGL2 knockdown cells correlates with reduced cell surface integrin αv expression.
However, it is unknown whether VANGL2 regulates integrin αv directly or indirectly through MMP14/MMP2 activity and cleavage of ECM proteins. Integrin signaling as indicated by SRC activation and paxillin phosphorylation remained largely unaffected after loss of VANGL2 function. In migrating cells, matrix cleavage by proteases must be coordinated with integrin-mediated cell adhesion [68, 69]. Cell migration speed and directional persistence are both affected by MMP proteolytic activity and its effect on matrix organization [68]. We propose that VANGL2 functions to inhibit or limit cell surface MMP14/MMP2 proteolytic activity and promote integrin-mediated cell-ECM adhesion (Fig. 9). How might these data be relevant to our understanding of PCP and embryonic cell migration? During zebrafish gastrulation, Vangl2 is proposed to regulate PCP in cells engaged in convergence and extension movements [11]. One of these movements is the directed migration of lateral ectodermal and mesodermal cells to the dorsal embryonic axis. As discussed above, our work has already shown that similar to the human protein, zebrafish Vangl2 controls Mmp activity and fibronectin assembly during gastrulation [21, 30]. Knockdown of the Vangl2 binding partner Prickle1a also produces zebrafish embryos with reduced fibronectin [21]. Furthermore, inhibition of Mmp14 expression causes an increase in fibronectin protein expression [21, 23] indicating that this ECM protein is an Mmp14/Mmp2 substrate in the zebrafish embryo. These data suggest that increased ECM protein cleavage and defective cell-ECM interactions are responsible for at least part of the vangl2/trilobite mutant phenotype. From work on the frog gastrula we know that proper integrin-fibronectin interactions are required to support formation of polarized membrane protrusions [17]. Disruption of the balance between MMP-mediated ECM cleavage and cell-ECM adhesion would likely impact the formation and/or polarity of membrane protrusions required for directed cell migration. Indeed, we know that loss of Vangl2 function causes gastrula cells to migrate along indirect trajectories, a phenotype consistent with improper membrane protrusive activity. We propose that the ability of Vangl2 to influence cell-ECM adhesion provides a mechanism to regulate polarized cell behaviors underlying the directed migration of zebrafish gastrula cells. Regarding the potential role of VANGL2 during tumor cell migration and invasion, our previous and current data suggest an inhibitory role for VANGL2 expression. Loss of VANGL2 function however would support MMP2-dependent tumor cell invasion through the ECM [29, 30, 34]. Published data suggest that VANGL2 might also suppress the growth of certain types of tumors [70, 71]. Interestingly, increased expression of the VANGL2 homologue VANGL1 has been linked to tumor progression and increased malignancy [72, 73]. While both VANGL2 and VANGL1 regulate neural tube closure [74], only VANGL2 is required for PCP during zebrafish gastrulation [75]. Clearly, much remains to be learned about these transmembrane proteins and their functions during embryonic development and cancer progression.
Supplementary Material
Validation of MMP14 siRNA using western blot and gelatin zymography. (A) Western blot comparison of MMP14 expression in non-targeting control (NT) and individual MMP14 siRNA1 (Silencer Select) and siRNA2 (Silencer) transfected HT-1080 cells. (B) Zymogram showing MMP2 activity in siRNA transfected cells. Lanes on zymogram match lanes on western blots. The results from an experiment performed in triplicate are shown. (C) Western blot comparing MMP14 expression in MMP14 siRNA and VANGL2 (V2)/MMP14 siRNA transfected cells. (D) Western blot comparing VANGL2 expression in VANGL2 (V2) siRNA and V2/MMP14 siRNA transfected cells.
Validation of integrin αv (ITGAV) siRNA using western blot and a vitronectin adhesion assay. (A) Western blot comparison of ITGAV expression in non-targeting control (NT) and ITGAV siRNA transfected HT-1080 cells. The individual siRNA's labeled 1 (J-004565-08) and 2 (J-004565-10) are components of the four-siRNA mixture that constitutes the ITGAV ON-TARGETplus SMARTpool. (B) Quantification of cell adhesion to vitronectin (Vn) in NT, siRNA1, siRNA2, and ON-TARGETplus SMARTpool ITGAV siRNA transfected cells. (C) Western blot comparing ITGAV expression in ITGAV siRNA and VANGL2 (V2)/ITGAV siRNA transfected cells. (D) Western blot comparison of VANGL2 expression in VANGL2 (V2) siRNA and V2/ITGAV siRNA transfected cells. Significant differences compared to the NT control cells are marked as follows: **p < 0.001; ***p < 0.0001.
Effectiveness of anti-GFP mAb-magnetic beads for immunoprecipitation. Western blot showing immunoprecipitation (IP) of both GFP-VANGL2 (GFP-V2) and GFP from cells transfected with GFP-V2/pCS2 and pEGFP-N1 expression plasmids, respectively. Whole cell lysates (WCL) were used as positive controls.
Highlights.
VANGL2 regulates cell adhesion to ECM proteins.
VANGL2 function influences cell surface integrin αvβ3 expression.
Inhibition of MMP activity rescues the adhesion defect in VANGL2 knockdown cells.
Integrin αv is required for VANGL2-dependent MMP2 activation.
VANGL2 binds integrin αvβ3.
Acknowledgments
This study was funded by a grant to J.R.J. from the National Institutes of Health (GM102356). Certain laboratory equipment items used to conduct this research were provided by Middle Tennessee State University. J.R.J. also acknowledges support from the Molecular Biosciences PhD Program.
Abbreviations
- ECM
extracellular matrix
- Fn
fibronectin
- ITGAV
integrin αv
- ITGB3
integrin β3
- Ln
laminin
- MMP2
matrix metalloproteinase 2
- MMP14
membrane type-1 matrix metalloproteinase
- PCP
planar cell polarity
- VANGL2
Vang-like 2
- Vn
vitronectin
Footnotes
Conflicts of interest None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nubler-Jung K, Bonitz R, Sonnenschein M. Cell polarity during wound healing in an insect epidermis. Development. 1987;100:163–170. doi: 10.1242/dev.100.1.163. [DOI] [PubMed] [Google Scholar]
- 3.Strutt D, Strutt H. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev Biol. 2007;302:181–194. doi: 10.1016/j.ydbio.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong LL, Adler PN. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J Cell Biol. 1993;123:209–221. doi: 10.1083/jcb.123.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009 doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 7.Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–2601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- 8.De Marco P, Merello E, Piatelli G, Cama A, Kibar Z, Capra V. Planar cell polarity gene mutations contribute to the etiology of human neural tube defects in our population. Birth Defects Res A Clin Mol Teratol. 2014;100:633–641. doi: 10.1002/bdra.23255. [DOI] [PubMed] [Google Scholar]
- 9.Hatakeyama J, Wald JH, Printsev I, Ho HY, Carraway KL., 3rd Vangl1 and Vangl2: planar cell polarity components with a developing role in cancer. Endocr Relat Cancer. 2014;21:R345–356. doi: 10.1530/ERC-14-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jessen JR. Noncanonical Wnt signaling in tumor progression and metastasis. Zebrafish. 2009;6:21–28. doi: 10.1089/zeb.2008.0571. [DOI] [PubMed] [Google Scholar]
- 11.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solnica-Krezel L, Stemple DL, Mountcastle-Shah E, Rangini Z, Neuhauss SC, Malicki J, Schier AF, Stainier DY, Zwartkruis F, Abdelilah S, Driever W. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development. 1996;123:67–80. doi: 10.1242/dev.123.1.67. [DOI] [PubMed] [Google Scholar]
- 13.Jessen JR, Solnica-Krezel L. Morphogenetic cell movements shaping the zebrafish gastrula. In: Mlodzik M, editor. Planar cell polarization during development. Elsevier Press; San Diego: 2005. pp. 131–165. [Google Scholar]
- 14.Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:R213–228. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Solnica-Krezel L, Cooper MS. Cellular and genetic mechanisms of convergence and extension. Results Probl Cell Differ. 2002;40:136–165. doi: 10.1007/978-3-540-46041-1_8. [DOI] [PubMed] [Google Scholar]
- 16.Latimer A, Jessen JR. Extracellular matrix assembly and organization during zebrafish gastrulation. Matrix Biol. 2010;29:89–96. doi: 10.1016/j.matbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Davidson LA, Marsden M, Keller R, Desimone DW. Integrin alpha5beta1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr Biol. 2006;16:833–844. doi: 10.1016/j.cub.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Boucaut JC, Darribere T. Fibronectin in early amphibian embryos. Migrating mesodermal cells contact fibronectin established prior to gastrulation. Cell Tissue Res. 1983;234:135–145. doi: 10.1007/BF00217407. [DOI] [PubMed] [Google Scholar]
- 19.Duband JL, Thiery JP. Appearance and distribution of fibronectin during chick embryo gastrulation and neurulation. Dev Biol. 1982;94:337–350. doi: 10.1016/0012-1606(82)90352-9. [DOI] [PubMed] [Google Scholar]
- 20.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 21.Dohn MR, Mundell NA, Sawyer LM, Dunlap JA, Jessen JR. Planar cell polarity proteins differentially regulate extracellular matrix organization and assembly during zebrafish gastrulation. Dev Biol. 2013;383:39–51. doi: 10.1016/j.ydbio.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coyle RC, Latimer A, Jessen JR. Membrane-type 1 matrix metalloproteinase regulates cell migration during zebrafish gastrulation: evidence for an interaction with non-canonical Wnt signaling. Exp Cell Res. 2008;314:2150–2162. doi: 10.1016/j.yexcr.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Marlow F, Zwartkruis F, Malicki J, Neuhauss SC, Abbas L, Weaver M, Driever W, Solnica-Krezel L. Functional interactions of genes mediating convergent extension, knypek and trilobite, during the partitioning of the eye primordium in zebrafish. Dev Biol. 1998;203:382–399. doi: 10.1006/dbio.1998.9032. [DOI] [PubMed] [Google Scholar]
- 25.Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–2138. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- 26.Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol. 2009;20:986–997. doi: 10.1016/j.semcdb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 28.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantrell VA, Jessen JR. The planar cell polarity protein Van Gogh-Like 2 regulates tumor cell migration and matrix metalloproteinase-dependent invasion. Cancer Lett. 2010;287:54–61. doi: 10.1016/j.canlet.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Williams BB, Cantrell VA, Mundell NA, Bennett AC, Quick RE, Jessen JR. VANGL2 regulates membrane trafficking of MMP14 to control cell polarity and migration. J Cell Sci. 2012;125:2141–2147. doi: 10.1242/jcs.097964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada A, Bellocq JP, Rouyer N, Chenard MP, Rio MC, Chambon P, Basset P. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci U S A. 1995;92:2730–2734. doi: 10.1073/pnas.92.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 33.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 34.Williams BB, Mundell NA, Dunlap JA, Jessen JR. The planar cell polarity protein VANGL2 coordinates remodeling of the extracellular matrix. Commun & Int Biol. 2012;5:1–4. doi: 10.4161/cib.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi F, Sottile J. MT1-MMP regulates the turnover and endocytosis of extracellular matrix fibronectin. J Cell Sci. 2011;124:4039–4050. doi: 10.1242/jcs.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takino T, Watanabe Y, Matsui M, Miyamori H, Kudo T, Seiki M, Sato H. Membrane-type 1 matrix metalloproteinase modulates focal adhesion stability and cell migration. Exp Cell Res. 2006;312:1381–1389. doi: 10.1016/j.yexcr.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Schwarzbauer JE, DeSimone DW. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seftor RE, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJ. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci U S A. 1992;89:1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seftor RE, Seftor EA, Stetler-Stevenson WG, Hendrix MJ. The 72 kDa type IV collagenase is modulated via differential expression of alpha v beta 3 and alpha 5 beta 1 integrins during human melanoma cell invasion. Cancer Res. 1993;53:3411–3415. [PubMed] [Google Scholar]
- 40.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 41.Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 42.Galvez BG, Matias-Roman S, Yanez-Mo M, Sanchez-Madrid F, Arroyo AG. ECM regulates MT1-MMP localization with beta1 or alphavbeta3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J Cell Biol. 2002;159:509–521. doi: 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remacle A, Murphy G, Roghi C. Membrane type I-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J Cell Sci. 2003;116:3905–3916. doi: 10.1242/jcs.00710. [DOI] [PubMed] [Google Scholar]
- 44.Glenney JR, Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989;108:2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanner SB, Reynolds AB, Vines RR, Parsons JT. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990;87:3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds AB, Kanner SB, Bouton AH, Schaller MD, Weed SA, Flynn DC, Parsons JT. SRChing for the substrates of Src. Oncogene. 2014;33:4537–4547. doi: 10.1038/onc.2013.416. [DOI] [PubMed] [Google Scholar]
- 47.Bellis SL, Miller JT, Turner CE. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J Biol Chem. 1995;270:17437–17441. doi: 10.1074/jbc.270.29.17437. [DOI] [PubMed] [Google Scholar]
- 48.Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- 50.Cooper JA, Gould KL, Cartwright CA, Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986;231:1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- 51.Johnson LN, Noble ME, Owen DJ. Active and inactive protein kinases: structural basis for regulation. Cell. 1996;85:149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- 52.Patschinsky T, Hunter T, Esch FS, Cooper JA, Sefton BM. Analysis of the sequence of amino acids surrounding sites of tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1982;79:973–977. doi: 10.1073/pnas.79.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grobelny D, Poncz L, Galardy RE. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry. 1992;31:7152–7154. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- 54.Hofmann UB, Westphal JR, Van Kraats AA, Ruiter DJ, Van Muijen GN. Expression of integrin alpha(v)beta(3) correlates with activation of membrane-type matrix metalloproteinase-1 (MT1-MMP) and matrix metalloproteinase-2 (MMP-2) in human melanoma cells in vitro and in vivo. Int J Cancer. 2000;87:12–19. doi: 10.1002/1097-0215(20000701)87:1<12::aid-ijc3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 55.Hofmann UB, Westphal JR, Waas ET, Becker JC, Ruiter DJ, van Muijen GN. Coexpression of integrin alpha(v)beta3 and matrix metalloproteinase-2 (MMP-2) coincides with MMP-2 activation: correlation with melanoma progression. J Invest Dermatol. 2000;115:625–632. doi: 10.1046/j.1523-1747.2000.00114.x. [DOI] [PubMed] [Google Scholar]
- 56.Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iliescu A, Gravel M, Horth C, Apuzzo S, Gros P. Transmembrane topology of mammalian planar cell polarity protein Vangl1. Biochemistry. 2011;50:2274–2282. doi: 10.1021/bi101767a. [DOI] [PubMed] [Google Scholar]
- 58.Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- 59.Yanez-Mo M, Barreiro O, Gonzalo P, Batista A, Megias D, Genis L, Sachs N, Sala-Valdes M, Alonso MA, Montoya MC, Sonnenberg A, Arroyo AG, Sanchez-Madrid F. MT1-MMP collagenolytic activity is regulated through association with tetraspanin CD151 in primary endothelial cells. Blood. 2008;112:3217–3226. doi: 10.1182/blood-2008-02-139394. [DOI] [PubMed] [Google Scholar]
- 60.Schroder HM, Hoffmann SC, Hecker M, Korff T, Ludwig T. The tetraspanin network modulates MT1-MMP cell surface trafficking. Int J Biochem Cell Biol. 2013;45:1133–1144. doi: 10.1016/j.biocel.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 61.Nagaoka T, Inutsuka A, Begum K, Bin hafiz K, Kishi M. Vangl2 regulates E-cadherin in epithelial cells. Sci Rep. 2014;4:6940. doi: 10.1038/srep06940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deryugina EI, Ratnikov BI, Postnova TI, Rozanov DV, Strongin AY. Processing of integrin alpha(v) subunit by membrane type 1 matrix metalloproteinase stimulates migration of breast carcinoma cells on vitronectin and enhances tyrosine phosphorylation of focal adhesion kinase. J Biol Chem. 2002;277:9749–9756. doi: 10.1074/jbc.M110269200. [DOI] [PubMed] [Google Scholar]
- 63.Ratnikov BI, Rozanov DV, Postnova TI, Baciu PG, Zhang H, DiScipio RG, Chestukhina GG, Smith JW, Deryugina EI, Strongin AY. An alternative processing of integrin alpha(v) subunit in tumor cells by membrane type-1 matrix metalloproteinase. J Biol Chem. 2002;277:7377–7385. doi: 10.1074/jbc.M109580200. [DOI] [PubMed] [Google Scholar]
- 64.Michaelis UR, Chavakis E, Kruse C, Jungblut B, Kaluza D, Wandzioch K, Manavski Y, Heide H, Santoni MJ, Potente M, Eble JA, Borg JP, Brandes RP. The polarity protein Scrib is essential for directed endothelial cell migration. Circ Res. 2013;112:924–934. doi: 10.1161/CIRCRESAHA.112.300592. [DOI] [PubMed] [Google Scholar]
- 65.Goto T, Davidson L, Asashima M, Keller R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr Biol. 2005;15:787–793. doi: 10.1016/j.cub.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 66.Phillips HM, Hildreth V, Peat JD, Murdoch JN, Kobayashi K, Chaudhry B, Henderson DJ. Non-cell-autonomous roles for the planar cell polarity gene Vangl2 in development of the coronary circulation. Circ Res. 2008;102:615–623. doi: 10.1161/CIRCRESAHA.107.160861. [DOI] [PubMed] [Google Scholar]
- 67.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13:3546–3559. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaman MH, Matsudaira P, Lauffenburger DA. Understanding effects of matrix protease and matrix organization on directional persistence and translational speed in three-dimensional cell migration. Ann Biomed Eng. 2007;35:91–100. doi: 10.1007/s10439-006-9205-6. [DOI] [PubMed] [Google Scholar]
- 69.Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dyberg C, Papachristou P, Haug BH, Lagercrantz H, Kogner P, Ringstedt T, Wickstrom M, Johnsen JI. Planar cell polarity gene expression correlates with tumor cell viability and prognostic outcome in neuroblastoma. BMC Cancer. 2016;16:259. doi: 10.1186/s12885-016-2293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piazzi G, Selgrad M, Garcia M, Ceccarelli C, Fini L, Bianchi P, Laghi L, D'Angelo L, Paterini P, Malfertheiner P, Chieco P, Boland CR, Bazzoli F, Ricciardiello L. Van-Gogh-like 2 antagonises the canonical WNT pathway and is methylated in colorectal cancers. Br J Cancer. 2013;108:1750–1756. doi: 10.1038/bjc.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cetin GO, Toylu A, Atabey N, Sercan Z, Sakizli M. Downregulation of VANGL1 inhibits cellular invasion rather than cell motility in hepatocellular carcinoma cells without stimulation. Genet Test Mol Biomarkers. 2015;19:283–287. doi: 10.1089/gtmb.2015.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MacMillan CD, Leong HS, Dales DW, Robertson AE, Lewis JD, Chambers AF, Tuck AB. Stage of breast cancer progression influences cellular response to activation of the WNT/planar cell polarity pathway. Sci Rep. 2014;4:6315. doi: 10.1038/srep06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merello E, Mascelli S, Raso A, Piatelli G, Consales A, Cama A, Kibar Z, Capra V, Marco PD. Expanding the mutational spectrum associated to neural tube defects: literature revision and description of novel VANGL1 mutations. Birth Defects Res A Clin Mol Teratol. 2015;103:51–61. doi: 10.1002/bdra.23305. [DOI] [PubMed] [Google Scholar]
- 75.Jessen JR, Solnica-Krezel L. Identification and developmental expression pattern of van gogh-like 1, a second zebrafish strabismus homologue. Gene Expr Patterns. 2004;4:339–344. doi: 10.1016/j.modgep.2003.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of MMP14 siRNA using western blot and gelatin zymography. (A) Western blot comparison of MMP14 expression in non-targeting control (NT) and individual MMP14 siRNA1 (Silencer Select) and siRNA2 (Silencer) transfected HT-1080 cells. (B) Zymogram showing MMP2 activity in siRNA transfected cells. Lanes on zymogram match lanes on western blots. The results from an experiment performed in triplicate are shown. (C) Western blot comparing MMP14 expression in MMP14 siRNA and VANGL2 (V2)/MMP14 siRNA transfected cells. (D) Western blot comparing VANGL2 expression in VANGL2 (V2) siRNA and V2/MMP14 siRNA transfected cells.
Validation of integrin αv (ITGAV) siRNA using western blot and a vitronectin adhesion assay. (A) Western blot comparison of ITGAV expression in non-targeting control (NT) and ITGAV siRNA transfected HT-1080 cells. The individual siRNA's labeled 1 (J-004565-08) and 2 (J-004565-10) are components of the four-siRNA mixture that constitutes the ITGAV ON-TARGETplus SMARTpool. (B) Quantification of cell adhesion to vitronectin (Vn) in NT, siRNA1, siRNA2, and ON-TARGETplus SMARTpool ITGAV siRNA transfected cells. (C) Western blot comparing ITGAV expression in ITGAV siRNA and VANGL2 (V2)/ITGAV siRNA transfected cells. (D) Western blot comparison of VANGL2 expression in VANGL2 (V2) siRNA and V2/ITGAV siRNA transfected cells. Significant differences compared to the NT control cells are marked as follows: **p < 0.001; ***p < 0.0001.
Effectiveness of anti-GFP mAb-magnetic beads for immunoprecipitation. Western blot showing immunoprecipitation (IP) of both GFP-VANGL2 (GFP-V2) and GFP from cells transfected with GFP-V2/pCS2 and pEGFP-N1 expression plasmids, respectively. Whole cell lysates (WCL) were used as positive controls.