Abstract
Background
C3 glomerulopathy (C3G) defines a group of rare complement-mediated kidney diseases with a shared underlying pathophysiology: dysregulation of complement in the fluid phase and glomerular microenvironment. Dysregulation can be driven by autoantibodies to C3 and C5 convertases.
Study Design
Case series
Setting & Participants
168 C3G patients (dense deposit disease, 68; C3 glumerulonephritis, 100) selected from our C3G bio-bank.
Outcomes
Patient-purified IgGs were tested for C4 nephritic factors (C4Nefs). These autoantibodies recognize C4b2a, the C3 convertase of the classical pathway of complement.
Measurements
C4Nefs were detected using a modified hemolytic assay.
Results
C4Nefs were identified in 5 patients, 4 of whom had C3 glomerulonephritis. The C4Nefs were associated with dysregulation of C3 and C5 convertases and they appear to stabilize these convertases in a dose-dependent manner. The C4Nefs also appear to protect C4b2a from decay mediated by soluble CR1 and C4 binding protein (C4BP). The stabilizing activity of the autoantibodies was further demonstrated by using heat treatment to inactivate complement. C4Nefs were not detected in 150 patients with another complement-mediated kidney disease, atypical hemolytic uremic syndrome. They were also absent in 300 apparently healthy controls.
Limitations
In addition to C4Nefs, two patients had positive findings for other autoantibodies: one patient also had autoantibodies to factor H; the other patient also had autoantibodies to C3bBb (C3Nefs).
Conclusions
The finding of C4Nefs in a small percentage of C3G patients highlights the challenge in identifying autoantibodies that drive complement dysregulation and underscores the complexity of the autoantibody repertoire that can be identified in these patients.
Index words: C3 glomerulopathy (C3G), dense deposit disease (DDD), C3 glomerulonephritis, C3 nephritic factors (C3Nefs), C4 nephritic factors (C4Nefs), complement-mediated renal disease, complement dysregulation, kidney biopsy, autoantibodies, immune deposits, case series
C3 glomerulopathy (C3G) is a term encompassing a group of extremely rare kidney diseases characterized by predominant immune deposits of complement component C3 visible by immunofluorescence (IF) microscopy of kidney biopsy speciem from a patient with active glomerular disease. Although the complete spectrum of pathology is yet to be determined, two major disease subgroups of C3G are recognized based on differences on electron microscopy (EM). The first subgroup, dense deposit disease (DDD), is characterized on EM by exceptionally dense ‘sausage-shaped’ deposits in the lamina densa of the glomerular basement membrane (GBM). The second subgroup, C3 glomerulonephritis (C3GN), covers the remaining C3G lesions and is defined by mesangial, sub-epithelial and/or sub-endothelial lesions that are typically discontinuous and much less dense than the deposits of DDD. A variety of glomerular patterns are seen on light microscopy (LM) and as such a specific pattern of injury visiable by LM is not a part of the disease definition. 1
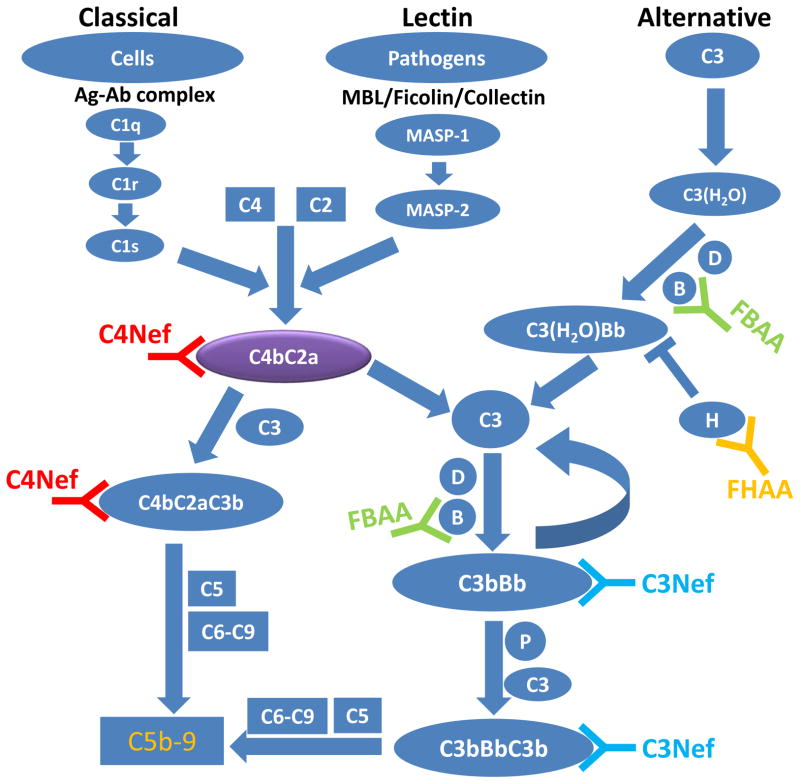
A large number of animal and human studies corroborate the role of the alternate pathway (AP) of complement in the pathogenesis of C3G. A brief review of the major components of complement activity is warranted to understand the underlying pathology. The three initiating pathways of complement—the classical, lectin and alternative—each lead to the generation of C3 and C5 convertases, which are serine proteases that sustain complement activity. They cleave C3 or C5, respectively, to amplify either the initiating or terminal pathways of complement. Control is provided by a number of regulators of complement activation so that unintended host damage is prevented (Figure 1).
Figure 1.
The complement cascade. A simplified schematic of the alternative, lectin and classical pathways of complement. Each of these initiating pathways leads to the generation of C3 convertases (C3bBb or C4b2a) and C5 convertases (C3bBbC3b or C4b2aC3b). C5 convertases cleave C5 and to trigger the generation of membrane attack complex or C5b-9. The presence of C4Nef and/or C3Nef stabilizes C3 convertases, resulting in hyperactivity of the alternative and terminal pathways.(MASP, mannose-associated serine protease; P, properdin; MAC, membrane attack complex; C3Nef, C3 nephritic factor; C4Nef, C4 nephritic factor; FHAA, Factor H autoantibody; FBAA, Factor B autoantibody).
C3G is caused by impaired control of complement in the fluid phase and glomerular microenvironment. The disease is heralded by glomerular deposition of complement protein C3, C3b cleavage products and terminal complement proteins, and is driven by genetic and/or acquired factors. For example, rare and/or novel genetic variants in complement genes are identified in 20%–40% of C3G patients and in endemic areas such as Cyprus or in familial cases of C3GN, complex fusion proteins of the complement factor H–related (CFHR) genes are often seen. 2–8 C3G patients are also enriched for specific genetic haplotypes or ‘risk’ alleles that increase the odds of developing C3G. 9, 10
Of the acquired drivers of disease, the best studied are autoantibodies to the C3 convertase of the AP, C3bBb. Known as C3 nephritic factors (C3Nefs), these autoantibodies increase the half-life of this typically short-lived protein complex. The consequence is more prolonged C3 convertase activity, which in turn leads to consumption of serum C3. C3Nefs are reported in up to 80% of patients with DDD and 50% of patients with C3GN. 11, 12 In C3G patients who had negative test results for C3Nefs, we sought other potentially relevant autoantibodies and focused on C4b2a, which is the C3 convertase of the classical and lectin pathways. Autoantibodies to this convertase are known as C4Nefs. We studied sera and plasma from 168 C3G patients selected from our C3G bio-bank to determine the prevalence of C4Nefs in C3G and to investigate the potential role of these autoantibodies in the pathogenesis of disease.
METHODS
Study Design
Patients enrolled in our C3G biobank from 2010 through 2014 with biopsy-confirmed C3G were eligible to participate in this study if sufficient sera and plasma were available for the proposed complement assays. All biopsies were reviewed in a multi-disciplinary forum as described. 4 Each patient provided informed consent, which was approved by the Institutional Review Board of Carver College of Medicine at the University of Iowa.
Genomic DNA, Serum, and Plasma Isolation
Genomic DNA, serum and plasma were isolated from whole blood using standard techniques. 4, 13 As a source of genomic DNA, the buffy coat was isolated and DNA was extracted using the Gentra Puregene Kit (Qiagen Inc., Valencia, CA). Serum was prepared from whole blood allowed to clot in plain (untreated) tubes for 45min and then centrifuged for 10min at 1000g. Plasma was separated from whole blood drawn in ethylenediaminetetraacetic acid (EDTA) tubes and immediately centrifuged for 10min at 1000g. All samples were kept at −80°C; sera and plasma were stored as single-use 50- to 200-μl aliquots in screw-top microcentrifuge tubes that were not refrozen after thawing. 13 Comparative data were obtained from 150 patients with another complement-mediated kidney disease, atypical hemolytic uremic syndrome. Normative data were generated from 300 apparently healthy blood donors.
Reagents and Buffers
Sheep erythrocytes (E) and hemolysin were purchased from Colorado Serum (Denver, CO). Cells were sensitized and used within one week. Complement component C2 was purchased from Complement Technology, Inc (Tyler, TX). Pooled normal human serum was obtained from Innovative Research (Novi, MI). TTHA (triethylenetetramine-N,N,N′,N″,N‴,N‴-hexa-acetic acid) was obtained from Sigma-Aldrich (St Louis, MO) and prepared as a 0.1 M stock solution.
Isotonic veronal-buffered saline (gelatin veronal buffer [GVB]) with 5 mM barbital, 145 mM NaCl and 0.1% gelatin (pH=7.3±0.1 [standard deviation]) was used to prepare the following buffers: 1) T buffer: dextrose GVB buffer with 150 mM calcium chloride, 10 mM TTHA and 2.5% glucose; 2) +buffer: dextrose GVB+ buffer with half isotonic GVB supplemented with 3 mM calcium chloride plus 2.5% dextrose; 3) ++buffer: dextrose GVB++ buffer with half isotonic GVB supplemented with 0.5 mM calcium chloride and 0.15 mM magnesium chloride plus 2.5% dextrose; 4) D buffer: GVB containing 10 mM EDTA.
Complement Assays
Initiating Pathways of Complement
The integrity of the classical and alternative pathways was analyzed by hemoltyic titration CH50 14; and ELISA (Wieslab ELISA kit, AP330; Euro Diagnostica AB, Malmö, Sweden), respectively.
Antibodies Studies
IgGs were purified using a Melon gel column (Thermo Fisher Scientific, Inc, Waltham, MA) or protein G (SeraCare, Gaithersburg, MD).
C4Nefs
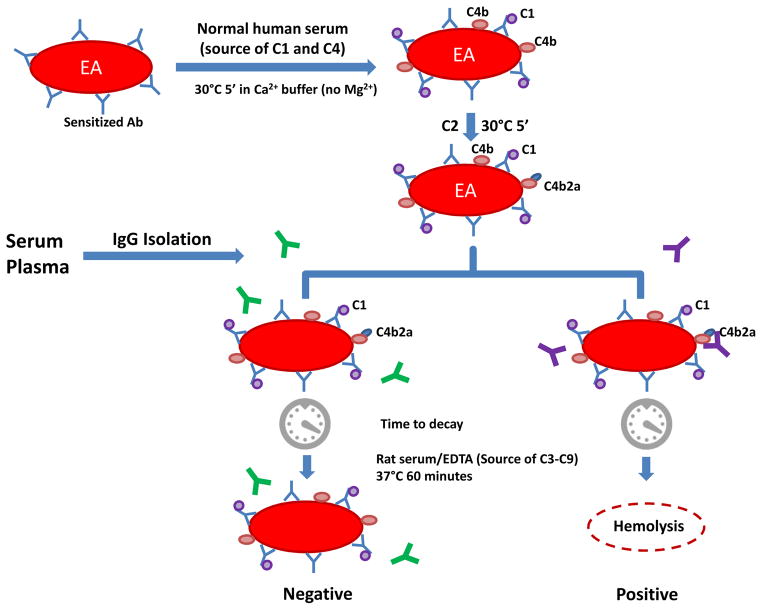
The C4Nef assay was used to measure the ability of patient-derived IgG to stabilize preformed C4b2a. To obtain intermediate EAC1-4b cells, antibody-bearing erythrocytes (EA cells at 1×109/ml) were incubated with pooled normal human serum in T buffer at 30°C for 5min. (Note: in the absence of Mg2+, classical pathway activity stops following the deposition of C1 and C4b; C2 does not bind because this step requires Mg2+.) Cells were washed with + buffer and re-suspended to the starting volume in ++ buffer. To form C4b2a, purified C2 was added and incubated at 30°C for 5min. The required amount of C2 was determined by titration to yield 63%–78% hemolysis (i.e., average number of hemolytic sites [Z] on each cell is 1; Z is calculated as −ln[1-%hemolysis]).
Hemolysis was measured using 30μl of C4b2a-coated sheep erythrocytes (5×108/ml) with increasing aliquots of patient-purified IgG, supplemented with GVB-EDTA buffer to a total volume of 250μl. Cells were allowed to decay at 30°C for 0, 7.5, 15 and 22.5min. Residual C4b2a was measured at each time point by transferring a 50μl aliquot of the mixture into a well of a microplate pre-filled with rat serum (1:19 in diluted GVB-EDTA buffer). After 1h at 37°C, 150μl of ice-cold GVB-EDTA was added and the sample was centrifuged at 1000g for 10 min. Hemolysis was recorded as a measure of the optical density of the supernatant at 415nm (Figure 2).
Figure 2.
C4Nef assay. Antibody-sensitized sheep erythrocytes (EA) are used as index cells to generate EAC1C4b by incubating pooled normal human serum in presence of TTHA (a strong magnesium chelator) and calcium. After washes, EAC1C4b2a (C3 convertase of the classical pathway) is made by adding purified C2. To test for the presence of C4b2a autoantibodies (C4Nefs), patient-purified IgG is added and cells are allowed to decay for different periods of time (7.5,15, 22.5 and 30 min). After each time point, 50μl of the cell mixture is removed. An equal volume of rat-EDTA serum (1:19 dilution) is added as a source of complement proteins C3 through C9. To stop the reaction after 1hr, 150 μl of cooled EDTA-GVB buffer is added. Non-lysed cells are removed by centrifugation and hemoglobin in supernatant (200 μl) is measured at OD415.
Dose response curves were calculated for samples positive for C4Nefs.
Other Autoantibodies
Autoantibodies to C3bBb (C3Nefs), factor H (FHAA) and factor B (FBAA) were measured as described. 11, 15, 16
Complement Biomarkers
Direct sandwich ELISA (detection antibody directly coupled with horseradish peroxidase) was used to measure Ba, Bb, C3c, C4a and soluble C5b-9 (sC5b-9) (Quidel Corporation, San Diego, CA, Hycult Biotech, Plymouth Meeting, PA). Indirect sandwich ELISA (biotinylated detection antibody followed by streptavidin-conjugated horseradish peroxidase) was used for C2, properdin and factor B (Abcam, Cambridge, MA; Hycult Biotech, Plymouth Meeting, PA). Results were interpreted using a microplate reader at wavelength of 450 nm (Bio-Rad Life Science, Hercules, CA) and calculated by Microsoft Excel or by four-parameter logistic regression (MyAssays Add-In For Microsoft Excel [MyAssays Ltd]). R2 exceeded 0.98 for each ELISA assay. Total C3, C4 and C5 were measured by radial immunodiffusion (RID, The Binding Site, Birmingham, UK).
Genetic Analysis
The coding exons and intron-exon boundary junctions of multiple complement genes (C3, CFH, CFI, MCP, CFD, CFB, DGKE) were screened for genetic variants using targeted genomic enrichment with massively parallel sequencing as previously described.4
RESULTS
Clinical Features
The demographic and clinical parameters of the 168 C3G patients are provided in Table 1. Five patients had test results positive for C4Nefs (Table 2).
Table 1.
Demographics for C3G study cohort
| C3 GN (n=100) | DDD (n=68) | |
|---|---|---|
| Sex | ||
| Male | 56 (56) | 30 (44.1) |
| Female | 44 (44) | 38 (55.9) |
| Age | ||
| Mean, y | 31.7 | 26.1 |
| Interquartile Range, y | 18.6 – 44.4 | 17.3 – 30.0 |
| Age at diagnosis | ||
| Mean, y | 26.3 | 18.6 |
| Range, y | 13.0 – 38.0 | 10.0 – 22.5 |
| Race/ethnicity | ||
| Caucasian | 85 (85%) | 66 (97%) |
| Asian | 11 (11%) | 2 (3%) |
| African American | 4 (4%) | 0 (0%) |
| C3NeF positive | 38 (38%) | 49 (72%) |
| FHAA positive | 5 (5%) | 3 (4%) |
| FBAA positive | 3 (3%) | 2 (3%) |
| C3NeF + FHAA positive | 2 (2%) | 1 (2%) |
| C3NeF + FBAA positive | 2 (2%) | 1 (2%) |
| FHAA + FBAA positive | 1 (1%) | 0 (0%) |
| C4NeF positive | 2 (2%) | 1 (2%) |
| C4NeF + C3NeF positive | 1 (1%) | 0 (0%) |
| C4NeF + FHAA positive | 1 (1%) | 0 (0%) |
| C3 <0.44 g/L | 40 (40%) | 33 (49%) |
| C3 0.4–0.9 g/L | 33 (33%) | 21 (31%) |
| C3 >0.9 g/L | 27 (27%) | 14 (21%) |
| Low C4 <0.15 g/L | 0 (0%) | 0 (0%) |
| Serum Creatinine >1.2 mg/dL | 56 (56%) | 31 (46%) |
| Proteinuria | 96 (96%) | 67 (99%) |
| Hematuria | 78 (78%) | 46 (68%) |
| Dialysis & Transplant | 15 (15%) | 19 (28%) |
Note: Unless otherwise indicated, values are given as number (percentage).
Abbreviations: C3G, C3 glomerulopathy; C3NeF, C3 nephritic factor; C4NeF, C4 nephritic factor; DDD, dense deposit disease; FHAA, Factor H autoantibody; FBAA, Factor B autoantibody; GN, glomerulonephritis
Table 2.
Clinical characteristics of the 5 C4NeF-positive patients
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Year of birth/sex | 1992/F | 1991/M | 1996/F | 1984/M | 2001/F |
| Age at diagnosis (y) | 19 | 22 | 14 | 11 | 11 |
| Duration of disease (y) | 6 | 4 | 7 | 22 | 5 |
| Race/Ethnicity | Asian | Asian | Caucasian | Caucasian | Caucasian |
| Presentation | |||||
| Scr (mg/dL) | 0.5 | 1.8 | 1 | 0.5 | 0.56 |
| Nephrotic syndrome | No | Yes | Yes | Yes | Yes |
| Proteinuria | 0.5 g/d | 5 g/g | 5.7 g/g | 4.8 g/g | 8.66 g/g |
| Hematuria | Yes | Yes | Microscopic | Yes | Yes |
| Edema | No | Yes | Yes | Yes | Yes |
| Blood pressure status | Hypertensive | Hypertensive | Normal | Hypertensive | Hypertensive |
| Preceding infection | Unknown | Yes | Unknown | Unknown | None |
| Last followup | |||||
| Scr (mg/dL) | 0.6 | 2.3 | 0.77 | 1.8 | 0.67 |
| UPCR (g/g) | 3.3 | 0.8 | 4.3 | 2.9 | 0.17 |
| Hematuria | Yes | Yes | Microscopic | Yes | Negative |
| Edema | No | No | Yes | Yes | None |
| Blood pressure | Hypertensive | Normal | Hypertensive | Normal | Normal |
UPCR, urinary protein-creatinine ratio; Scr, serum creatinine
Patient 1
This 25-year-old East Asian woman presented with asymptomatic proteinuria and hematuria discovered on routine physical examination in 2010. Persistent proteinuria and hematuria were confirmed 3 months later during a visit for an upper respiratory tract infection. At that time, her serum creatinine was 0.5 mg/dL (correspondign to an eGFR of 167 mL/min/1.73 m2 as calculated by the 4-variable MDRD [Modication of Diet in Renal Disease] Study equation), C3 and CH50 were low, and C4 was normal. A kidney biopsy in 2012 showed C3 granular staining (3+) in mesangial and glomerular capillary wall; no other immunoreactant was detected. EM showed abundant large electron-dense mesangial and hump-shaped subepithelial deposits with small membranous and subepithelial deposits. The patient was diagnosed with C3GN and started on an angiotensin receptor blocker and a lipid lowering agent. At the time of writing, C4Nef titres had not changed and her kidney function was stable with a serum creatinine of 0.6 mg/dL (eGFR, 135 mL/min/1.73 m2).
Patient 2
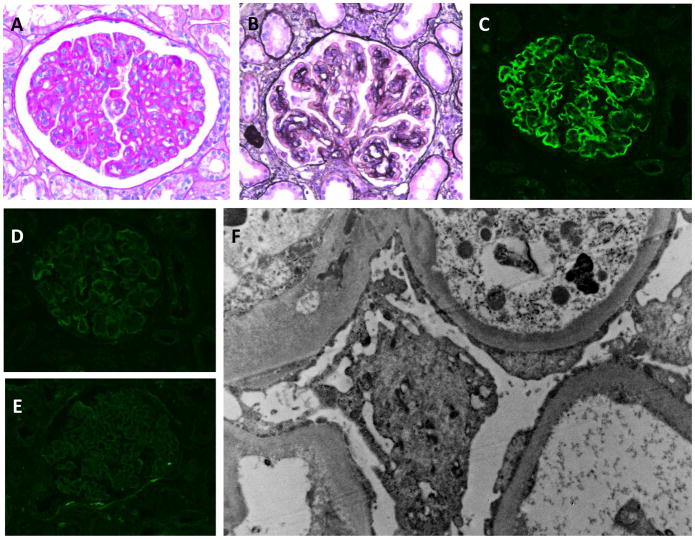
This 26-year-old Asian (Pakistani) man presented at age 22 years with nephrotic syndrome (proteinuria, 10 g/24 h; albumin, 1.5 g/dL), anasarca and decreased kidney function (serum creatinine, 1.8 mg/dL; eGFR, 47 mL/min/1.73 m2). His presentation was preceded by an upper respiratory tract infection several months prior. Serology findings for ANA, ANCA and antistreptolysin O (ASO) were negative; serum levels of C3 were undetectable, while C4 was normal. Kidney biopsy findings were consistent with C3GN (Figure 3). Treatment included an angiotensin receptor blocker, steroids (for 6 months) and mycophenolate mofetil, with a gradual decrease in proteinuria (current random urine protein-creatinine ratio [UPCR], 0.83 g/g). At the time of writing, C4Nef titres had not changed and his current serum creatinine was 2.3mg/dL (eGFR, 38 mL/min/1.73 m2) with the patient 15 pounds lighter (edema free), representing fairly stable kidney function.
Figure 3.
Kidney biopsy from Patient 2. A) PAS staining shows a membranoproliferative pattern with mesangial and capillary loop hypercellularity. B) GBM duplication with deposition of hyaline material is seen on the Masson trichrome stain (×40). C–E) IF findings are positive for C3 (C) but negative for C4d (D) and all other immunoreactants (IgG is shown). F) EM shows diffuse effacement of podocyte foot processes and thickening of the GBM by a combination of subendothelial deposits, basement membrane duplication and mesangial cell interposition.
Patient 3
This 21-year-old Caucasian woman presented at 14 years of age with a several-month history of lower extremity edema. The initial workup revealed nephrotic syndrome with a serum albumin of 1.5 g/dL, a low C3 and a normal C4. Results of viral studies were negative (hepatitis and HIV) and anti-DNA autoantibodies and C3 nephritic factor assays were negative. Result of testing for ANA was weakly positive (signal at a titer of 1:40). Treatment was initiated with diuretics and an angiotensin-converting enzyme inhibitor, and six months later, a kidney biopsy was done. Four of 21 glomeruli were noted to be globally sclerotic, with mesangial hypercellularity of the remaining glomeruli. Multiple segments of the GBM showed segmental duplication. IF results were diffusely positive (3+) for C3 with 1+ focal segmental reactions to IgG, IgM, IgA and C1q. On EM, electron-dense deposits were present on both the subendothelial and subepithelial sides on the GBM. Findings were consistent with C3GN and prednisone therapy was initiated. At the time of writing, the patient was off treatment and had mild persistent proteinuria.
Patient 4
This 33-year-old Caucasian man presented at age 11 years with scrotal edema and nephrotic-range proteinuria (UPCR, 4.8 g/g). Serum creatinine was 0.5 mg/dL (eGFR, 116 mL/min as calculated by the Schwartz equation) and C3 was low. A kidney biopsy in 2006 showed membranoproliferative glomerulonephritis with an exudative pattern, IF findings of 3+ positivity for C3 and 1+ segmental glomerular positivity for IgM. On EM, there were ‘sausage-shaped’ intramembranous electron-dense deposits, subepithelial hump-shaped deposits, and large rounded mesangial deposits most consistent with DDD. When last seen in 2012, the patient remained on angiotensin receptor blockers and had a serum creatinine of 1.8 mg/dL (eGFR, 46 mL/min) and a UPCR of 2.9 g/g.
Patient 5
This 16-year-old girl was diagnosed at age 11 years with nephrotic syndrome. She presented with nephrotic syndrome and was started on high dose steroids by her pediatrician for a presumed diagnosis of minimal change disease. The pediatric nephrology department was consulted after 6 weeks of steroid therapy because the spot UPCR remained elevated at 9.34 g/g. Further testing showed that both C3 and C4 were decreased, at 0.12 g/L and 0.05 g/L, respectively. CH50 was 0, and testing for anti DNase B antibodies, an antinuclear antibody panel and ASO titers all had negative findings. The serum creatinine remained stable at 0.52mg/dL (eGFR, 115 mL/min). High-dose steroids were continued for 8 weeks. The patient was medication compliant and became cushingoid, but continued to have nephrotic-range proteinuria, prompting a percutaneous native kidney biopsy. The biopsy showed membranoproliferative glomerulonephritis with hump-like deposits and predominant staining for C3 on IF. There was minimal interstitial fibrosis, and only a few crescents were identified. Marked GBM thickening and large subendothelial deposits were resolved on EM, and a diagnosis of C3GN was made. Steroids were tapered and tacrolimus was initiated, with gradual resolution of the nephrotic-range proteinuria over several months. The 12-hour tacrolimus level has been maintained at 5–7 ng/ml; if the tacrolimus trough dropped below this level, the patient would become nephrotic. At the time of writing, serum creatinine and UPCR were 0.67 mg/dL (eGFR, 101 mL/min) and 0.17 g/g, respectively.
Complement Assays
Antibody Studies
C4NeFs
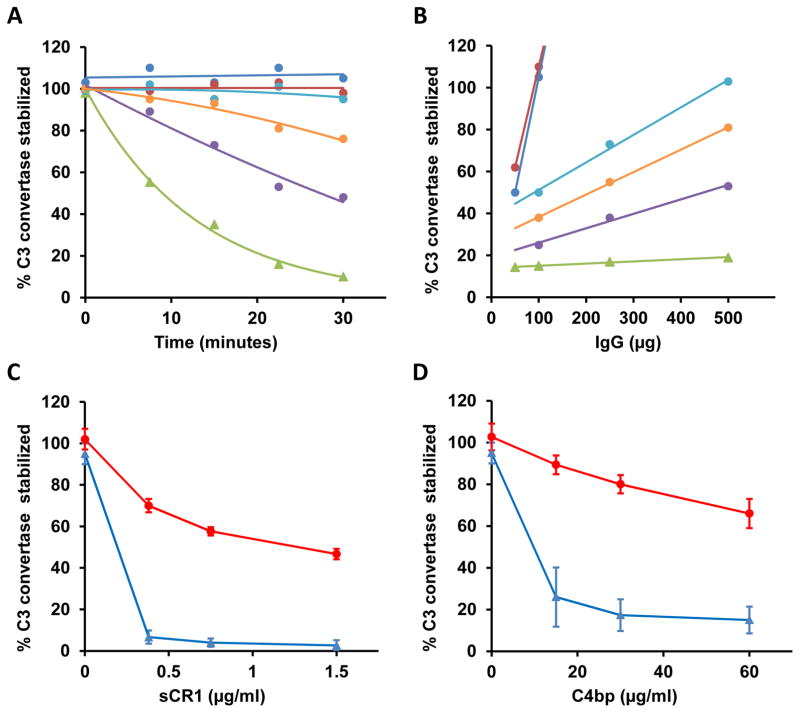
5 patients with C3G had test findings positive for C4Nefs (Figure 4a; Table 2). The C4b2a stabilizing effect of these autoantibodies varied but in each patient was dose dependent (Figure 4b). CR1 and C4BP accelerate the decay of C4b2a. C4Nefs protected C4b2a from this decay (Figures 4c and 4d). Heat treatment to inactivate complement in patient sera and plasma (incubation at 56°C for 30 min) abolished all lytic activity, consistent with an interaction between C4Nef autoantibodies and C4b2a. No patients with atypical hemolytic uremic syndrome and no controls had test findings positive for C4Nefs.
Figure 4.
C4 nephritic factors. A) In each of 5 patients, purified IgGs (500μg) had varying ability to prolong the half-life of C4b2a and cause hemolysis. B) The effect was dose dependent (50μg to 500μg of IgG) confirming the ability of these autoantibodies to stabilize C4b2a (green line, pooled normal IgG; patient-purified IgGs: dark blue, patient 1; red, patient 2; purple, patient 3; light blue, patient 4; orange, patient 5). C) and D) In addition, the C4Nefs protected C4b2a from decay by soluble CR1 (C) and C4 Binding Protein (C4BP) (D) (red, patient 1; blue, control).
Other Autoantibodies
Patients 2, 3 and 4 had results positive for only C4Nefs. Patients 1 and 5 also had findings positive for factor H autoantibodies and C3Nefs, respectively (Table 3).
Table 3.
Autoantibodies, complement assays and genetic variants in the 5 C4NeF-positive patients
| Patient No. | Diagnosis | Date of Testing | C4NeF (ref <20%) | IFE (ref <7.5%) | C3NeF (C3CSA) (ref <20%) | C3NeF (C3CSAP) (ref <20%) | FHAA | FBAA | Hemolytic Assayf (ref <3%) | APFA (ref 50%~130%) | CH50 (ref 30~70 u/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C3 GN | 08/2013 | >100% | 14% | Neg | Neg | Pos (titer of 1:200) | Neg | normal | 0.4% | 7 |
| 2 | C3 GN | 01/2014 | >100% | 11% | Neg | Neg | Neg | Neg | 7% | 0.6% | 10 |
| 3 | C3 GN | 03/2013 | 53% | Neg | Neg | Neg | Neg | Neg | normal | 1.7% | <5 |
| 4 | DDD | 01/2012 | >100% | Neg | Neg | Neg | Neg | Neg | normal | 14% | 25 |
| 5 | C3 GN | 01/2013 | 81% | Neg | 67% | 46% | Neg | Neg | normal | 0 | 6 |
Note:Normal reference (ref) ranges are listed in parentheses of column headings
Abbreviations and definitions: C4NeF, C4 nephritic factor; IFE, immunofixation electrophoresis; C3NeF (C3CSA), C3 nephritic factor measured by C3 convertase stabilizing assay without properdin; C3NeF (C3CSAP), C3 nephritic factor measured by C3 convertase stabilizing assay with properdin; FHAA, factor H autoantibodies; FBAA, factor B autoantibodies; APFA, alternative pathway functional assay; Neg, negative; DDD, dense deposit disease; GN, glomerulonephritis; Pos, positive
Hemolytic assay using non-sensitized sheep erythrocytes
No genetic variants were detected by examining exons and intron-exon boundary junctions of multiple complement genes (C3, CFH, CFI, MCP, CFD, CFB, DGKE)
Complement Biomarkers
All patients had evidence of ongoing complement activity. Initiating pathway activity was reflected by low levels of C3 (0.3 ± 0.23 [reference range, 0.9–1.8] g/L) and elevated levels of its cleavage product, C3c (2.5 ± 0.6 [reference range, <2)] mg/L). Terminal pathway activity was reflected by low levels of complement protein C5 (40.4 ± 13.4 [reference range, 55–125] mg/L) and in 4 patients, reduced levels of properdin (9.6 ± 1 [reference range, 11–33] mg/L). Consistent with ongoing complement dysregulation and terminal pathway activity, soluble C5b-9 (2.5 ± 1.8 [reference range, <0.3] mg/L) was elevated (Table 4).
Table 4.
Biomarker profiling for the 5 C4Nef-positive patients
| Patient No. | C3, g/L (ref 0.9–1.8) | C3c, mg/L (ref < 2) | C4, g/L (ref 0.15–0.47) | C4a, mg/L (ref <0.72) | C2, mg/L (ref 34–50) | fB, mg/L (ref 155–250) | Ba, mg/L (ref <1.2) | Bb, mg/L (ref <1.2) | C5, mg/dL (ref 55–125) | P, mg/L (ref 11–33) | sC5b-9, mg/L (ref <0.3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | <0.1 | 2.3 | 0.18 | 0.17 | 35.3 | 200 | 0.3 | 0.9 | 39.5 | 9.3 | 1.6 |
| 2 | 0.25 | 2.0 | 0.45 | 0.78 | 27.1 | 171 | 2.0 | 2.0 | 40.2 | 8.4 | 2.8 |
| 3 | 0.4 | 2.7 | 0.24 | 0.71 | 38.2 | 163 | 1.2 | 2.0 | 35.5 | 10.3 | 1.9 |
| 4 | 0.65 | 2.1 | 0.34 | 1.22 | 35.7 | 158 | 1.4 | 1.3 | 61.7 | 21.3 | 0.8 |
| 5 | 0.11 | 3.4 | 0.19 | 0.25 | 35.9 | 225 | 0.7 | 1.1 | 24.9 | 10.5 | 1.9 |
Note: Date of testing as for Table 3; normal reference (ref) values are listed under each biomarker C4NeF, C4 nephritic factor
Complement Function
Test findings of classical and alternative pathway activity were low in all patients, consistent with consumption of complement proteins (CH50, 10.6 ± 8.3 [reference range, 30–90] U/mL); alternative pathway functional assay, 3.1% ± 6.1% [reference range, 50%–150%]; Table 3).
Genetic Analysis
No rare or novel variants in complement genes were identified in C4Nef-positive patients (Table 2).
DISCUSSION
The term C3G was proposed in 2013 to designate a group of kidney diseases that share an underlying feature defined on kidney biopsy by IF - the predominance of complement component C3, exceeding any other immuno-reactant by at least two orders of magnitude. This abundant and preferential deposition of C3 reflects the unifying pathophysiology of C3G, which is fluid-phase dysregulation of the complement cascade. The drivers of dysregulation and their impact are highly variable and account for the spectrum of clinical phenotypes and biomarker profiles that are seen. 17–20
To provide greater insight into the pathophysiology of C3G, in a large cohort of C3G patients we have sought to define the genetic and functional characteristics of the complement system in order to correlate complement perturbations with the underlying kidney biopsy (i.e. DDD vs C3GN) and clinical course of disease. A complete evaluation includes: 1) genetic testing for rare and novel variants in complement genes, including detection of copy number variants and genomic rearrangements over the complete CFHR genetic interval; 2) autoantibody screening for acquired drivers of disease like C3Nefs and factor H autoantibodies; 3) assessing complement function by triggering the initiating pathways of complement activation; and 4) measuring serum levels of complement proteins and their cleavage products. The result is a comprehensive picture of complement activity with a number of built-in concordance checks to offer assurances that an accurate assessment of ongoing complement activity is generated. In the majority of C3G patients, we are able to identify drivers of disease. 11, 12 In patients without obvious disease drivers, further studies are completed.
By following this strategy, we have found that C4Nefs are present in ~3% of C3G patients. Like C3Nefs, C4Nefs prolong the half-life of a C3 convertase, C4b2a. C4b2a is generated following activation of the classical or lectin pathways. That triggering of either of these pathways can lead to C3G is consistent with our evolving understanding of this disease and its relationship to post-infectious glomerulonephritis (PIGN). 21 The development of PIGN is characterized by the deposition of immune complexes in the glomerulus following an antecedent infection. Occasional evolution of this pathology into isolated C3-only deposition is consistent with dysregulation of complement in a small subset of PIGN patients and ‘switching’ to an AP-driven process. 22–24 Indeed, the subepithelial humps resolved by EM in our C4Nef-positive patients support the transformation of PIGN to C3G. However, it is currently unclear why particular patients develop C4Nefs.
The stabilizing effects of C4Nefs vary considerably, are dose-dependent, and abrogate normal decay of the convertase mediated by CR1 and C4BP (Figure 4). Biomarker analysis of complement proteins and split products in C4Nef patients paints a picture of dysregulation of both C4b2a and C4b2aC3b/C3bBbC3b. Serum levels of C3, C5 and properdin are low, while C3c and soluble C5b-9 are elevated; both alternative pathway functional assay and CH50 are depressed. None of C4Nef patients had been treated with eculizumab, a C5 antibody that prevents activity of the terminal complement pathway, but based on high serum levels of sC5b-9, we believe that eculizumab treatment would be beneficial to this subgroup of patients.
Our findings are in agreement with data reported by Blom and colleagues, who identified C4Nefs in one of 13 patients with C3G. 25 C4Nefs have also been associated with PIGN, systemic lupus erythematosus, membranoproliferative glomerulonephritis and meningococcal disease. 26–30 Interestingly, in the last example, serum C3 was undetectable over a 2-year followup period but there was no report of renal involvement.
In summary, a small percentage of C3G patients are positive for C4Nefs. We have shown that these autoantibodies to C4b2a have a dose-dependent effect on the stabilization of C4b2a and protect this convertase from CR1- and C4BP-mediated decay. Their presence was associated with predicted perturbations in serum levels of complement proteins and their cleavage products. The clinical course in these five patients was variable, and although none was dialysis dependent at the time of writing, two patients did have chronic kidney disease. These patients underscore the complexity of C3G and further refine our understanding of this group of rare kidney diseases.
Acknowledgments
We thank the patients whose participation made this study possible.
Support: This research was supported in part by NIDDK R01-110023 to Drs Smith and Nester. The funding agency had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research area and study design: YZ, VF-B, RJHS; data acquisition:YZ, NCM, FCF, WL, AK GC-F, DS, AA, SS; data analysis and interpretation: YZ, CMN, RJHS; supervision or mentorship: RJHS. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Peer Review: Evaluated by two external peer reviewers, a Pathology Editor, an Associate Editor, and Editor-in-Chief Feldman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pickering MC, D’Agati VD, Nester CM, et al. C3 glomerulopathy: consensus report. Kidney Int. 2013;84(6):1079–1089. doi: 10.1038/ki.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Q, Manzke M, Hartmann A, et al. Complement Factor H-Related 5-Hybrid Proteins Anchor Properdin and Activate Complement at Self-Surfaces. J Am Soc Nephrol. 2016;27(5):1413–1425. doi: 10.1681/ASN.2015020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao X, Ghossein C, Tortajada A, et al. Familial C3 glomerulonephritis caused by a novel CFHR5-CFHR2 fusion gene. Mol Immunol. 2016;77(9):89–96. doi: 10.1016/j.molimm.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Bu F, Borsa NG, Jones MB, et al. High-Throughput Genetic Testing for Thrombotic Microangiopathies and C3 Glomerulopathies. J Am Soc Nephrol. 2016;27(4):1245–1253. doi: 10.1681/ASN.2015040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gale DP, de Jorge EG, Cook HT, et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376(9743):794–801. doi: 10.1016/S0140-6736(10)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik TH, Lavin PJ, Goicoechea de Jorge E, et al. A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol. 2012;23(7):1155–1160. doi: 10.1681/ASN.2012020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medjeral-Thomas NR, O’Shaughnessy MM, O’Regan JA, et al. C3 glomerulopathy: clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol. 2014;9(1):46–53. doi: 10.2215/CJN.04700513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tortajada A, Yébenes H, Abarrategui-Garrido C, et al. C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest. 2013;123(6):2434–2446. doi: 10.1172/JCI68280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrera-Abeleda MA, Nishimura C, Frees K, et al. Allelic variants of complement genes associated with dense deposit disease. J Am Soc Nephrol. 2011;22(8):1551–1559. doi: 10.1681/ASN.2010080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris CL, Heurich M, Rodriguez de Cordoba S, Morgan BP. The complotype: dictating risk for inflammation and infection. Trends Immunol. 2012;33(10):513–521. doi: 10.1016/j.it.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Meyer NC, Wang K, et al. Causes of alternative pathway dysregulation in dense deposit disease. Clin J Am Soc Nephrol. 2012;7(2):265–274. doi: 10.2215/CJN.07900811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Servais A, Noël LH, Roumenina LT, et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82(4):454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Nester CM, Martin B, et al. Defining the complement biomarker profile of C3 glomerulopathy. Clin J Am Soc Nephrol. 2014;9(11):1876–1882. doi: 10.2215/CJN.01820214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer MM. Complement and complement fixation. In: Kabat E, Mayer MM, editors. Experimental Immunochemistry. Springfield, IL: CC Thomas; 1961. pp. 133–240. [Google Scholar]
- 15.Dragon-Durey MA, Loirat C, Cloarec S, et al. Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16(2):555–563. doi: 10.1681/ASN.2004050380. [DOI] [PubMed] [Google Scholar]
- 16.Strobel S, Zimmering M, Papp K, Prechl J, Józsi M. Anti-factor B autoantibody in dense deposit disease. Mol Immunol. 2010;47(7–8):1476–1483. doi: 10.1016/j.molimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Xiao X, Pickering MC, Smith RJ. C3 glomerulopathy: the genetic and clinical findings in dense deposit disease and C3 glomerulonephritis. Semin Thromb Hemost. 2014;40(4):465–471. doi: 10.1055/s-0034-1376334. [DOI] [PubMed] [Google Scholar]
- 18.Barbour TD, Ruseva MM, Pickering MC. Update on C3 glomerulopathy. Nephrol Dial Transplant. 2016;31(5):717–725. doi: 10.1093/ndt/gfu317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q, Wiesener M, Eberhardt HU, et al. Complement factor H-related hybrid protein deregulates complement in dense deposit disease. J Clin Invest. 2014;124(1):145–155. doi: 10.1172/JCI71866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zipfel PF, Skerka C, Chen Q, et al. The role of complement in C3 glomerulopathy. Mol Immunol. 2015;67(1):21–30. doi: 10.1016/j.molimm.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Sethi S, Fervenza FC, Zhang Y, et al. Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin J Am Soc Nephrol. 2011;6(5):1009–1017. doi: 10.2215/CJN.07110810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandhu G, Bansal A, Ranade A, et al. C3 glomerulopathy masquerading as acute postinfectious glomerulonephritis. Am J Kidney Dis. 2012;60(6):1039–1043. doi: 10.1053/j.ajkd.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Sethi S, Fervenza FC, Zhang Y, et al. Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int. 2013;83(2):293–299. doi: 10.1038/ki.2012.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasto J, Kaplan BS, Russo P, et al. Streptococcal infection as possible trigger for dense deposit disease (C3 glomerulopathy) Eur J Pediatr. 2014;173(6):767–772. doi: 10.1007/s00431-013-2245-7. [DOI] [PubMed] [Google Scholar]
- 25.Blom AM, Corvillo F, Magda M, et al. Testing the Activity of Complement Convertases in Serum/Plasma for Diagnosis of C4NeF-Mediated C3 Glomerulonephritis. J Clin Immunol. 2016;36(5):517–527. doi: 10.1007/s10875-016-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halbwachs L, Leveillé M, Lesavre P, Wattel S, Leibowitch J. Nephritic factor of the classical pathway of complement: immunoglobulin G autoantibody directed against the classical pathway C3 convetase enzyme. J Clin Invest. 1980;65(6):1249–1256. doi: 10.1172/JCI109787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daha MR, Van Es LA. Modulation of complement by autoimmune antibodies isolated from sera of patients with membranoproliferative glomerulonephritis and systemic lupus erythematosus. Neth J Med. 1982;25(7):202–207. [PubMed] [Google Scholar]
- 28.Tanuma Y, Ohi H, Watanabe S, Seki M, Hatano M. C3 nephritic factor and C4 nephritic factor in the serum of two patients with hypocomplementaemic membranoproliferative glomerulonephritis. Clin Exp Immunol. 1989;76(1):82–85. [PMC free article] [PubMed] [Google Scholar]
- 29.Ohi H, Yasugi T. Occurrence of C3 nephritic factor and C4 nephritic factor in membranoproliferative glomerulonephritis (MPGN) Clin Exp Immunol. 1994;95(2):316–321. doi: 10.1111/j.1365-2249.1994.tb06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller EC, Chase NM, Densen P, Hintermeyer MK, Casper JT, Atkinson JP. Autoantibody stabilization of the classical pathway C3 convertase leading to C3 deficiency and Neisserial sepsis: C4 nephritic factor revisited. Clin Immunol. 2012;145(3):241–250. doi: 10.1016/j.clim.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]