Abstract
Background
The diagnosis of acute kidney injury (AKI), which is currently defined as a rise in serum creatinine (SCr), provides little information on the condition’s actual etiology. To improve phenotyping of AKI, many urinary biomarkers of tubular injury are being investigated. Since AKI cases are not frequently biopsied, the diagnostic accuracy of SCr and urinary biomarkers for histological acute tubular injury (ATI) is unknown.
Study Design
Cross-sectional analysis from multicenter, prospective cohort
Settings & Participants
Hospitalized deceased kidney donors on whom kidney biopsies were performed at the time of organ procurement for histological evaluation
Predictors
(a) AKI diagnosed by change in SCr during donor hospitalization and (b) urinary biomarkers (neutrophil gelatinase-associated lipocalin [NGAL], liver-type fatty acid-binding protein [L-FABP], interleukin 18 [IL-18], and kidney injury molecule 1 [KIM-1]) measured at organ procurement
Outcome
Histological ATI
Results
Of 581 donors, 98 (17%) had mild ATI and 57 (10%) had severe ATI. Overall, SCr-based AKI had poor diagnostic performance for identifying histological ATI and 49% of donors with severe ATI did not have AKI. The area under the receiver operating characteristic curve (AUROC) of change in SCr for diagnosing severe ATI was 0.58 (95% CI, 0.49–0.67), and for any ATI was 0.52 (95% CI, 0.45–0.58). Compared with SCr, NGAL demonstrated higher AUROC for diagnosing both severe ATI (0.67; 95% CI, 0.60–0.74; P=0.03) and any ATI (0.60; 95% CI, 0.55–0.66; P=0.005). In donors who did not have SCr-based AKI, NGAL levels were higher with increasing severities of ATI (subclinical AKI). However, compared with SCr, AUROCs for ATI diagnosis were not significantly higher for urinary L-FABP, IL-18, or KIM-1 concentrations.
Limitations
Spectrum of AKI etiology in deceased donors may be different from that of general hospitalized population.
Conclusions
SCr and kidney injury biomarkers (L-FABP, IL-18, and KIM-1) lack accuracy for diagnosing ATI in hospitalized deceased donors. While urinary NGAL had slightly higher discrimination for ATI than did SCr, its overall AUROC was still modest.
Keywords: Acute Kidney Injury (AKI), Acute Tubular Injury (ATI), Serum Creatinine (Scr), NGAL, L-FABP, KIM-1, IL-18, Kidney biopsy, Kidney histology, subclinical AKI, kidney injury biomarker, diagnostic performance
Clinically, acute kidney injury (AKI) is defined by a rise in serum creatinine (SCr) concentration, which is a marker of glomerular filtration rate (GFR), or by acute reductions in urine output. Acute tubular injury (ATI) is frequently presumed to be a leading etiology of AKI in many hospital settings.1,2 ATI is a histologically-diagnosed condition and is often associated with progressive chronic kidney disease and end stage renal disease.3,4
SCr-based AKI definitions have several limitations when applied to diagnose ATI.5 First, elevations in SCr are not specific to ATI. The administration of drugs that inhibit tubular secretion of creatinine or inhibit the renin-angiotensin aldosterone system can lead to increases in SCr in the absence of ATI. Hemodynamic reductions in renal blood flow that do not cause structural injury but increase SCr concentrations can also lead to false positive ATI diagnoses (e.g. cardiorenal and hepatorenal syndromes).5–7 Second, SCr can fail to identify some patients who do have ATI – a condition termed “subclinical AKI.”8,9 This can occur when the effects of tubular injury and reduced GFR in some nephrons are compensated for by other non-injured and functioning nephrons via a phenomenon called “renal reserve”. While many investigators and clinicians recognize the limitations of SCr and would prefer a kidney biopsy to confirm ATI, patients with suspected ATI are frequently critically ill and are thus rarely biopsied. Instead, other clinical parameters like fractional excretion of sodium, fractional excretion of urea, serum urea nitrogen to SCr (SUN-SCr) ratio, urine output, and urine microscopy are used in conjunction with elevations in SCr to infer the diagnosis of ATI.
Translational researchers are evaluating urinary proteins that directly assess tubular injury for non-invasive confirmation of ATI. Protein biomarkers currently under consideration include neutrophil gelatinase-associated lipocalin (NGAL), liver-type fatty acid-binding protein (L-FABP), interleukin 18 (IL-18), and kidney injury molecule 1 (KIM-1). Despite the limitations of SCr, however, studies on these biomarkers have used SCr for comparison rather than the gold standard of kidney biopsy-diagnosed ATI.10 This has led to suboptimal biomarker development.5
ATI is common in deceased organ transplant donors,11 who are managed in the intensive care unit (ICU) prior to organ procurement.12 Donor management involves the same critical care provided to other ICU patients. The etiology of and risk factors for AKI in deceased donors are thus similar to those of other ICU patients.13 Moreover, organ procurement clinicians perform kidney biopsies in over half of these donors, and biopsies are often used to help make organ allocation decisions. Thus, the deceased donor setting provides an opportunity to compare urinary tubular injury biomarkers with SCr for ATI confirmed via gold-standard histology.
We hypothesized that urinary biomarkers of kidney injury would have improved discrimination for diagnosing histological ATI compared with SCr. In a prospective, multicenter cohort of deceased organ donors with kidney biopsies performed at the time of organ procurement for transplantation, we compared the accuracy of urinary tubular injury biomarkers (NGAL, L-FABP, IL-18, and KIM-1) with traditional clinical parameters (SCr, fractional excretion of sodium and fractional excretion of urea, SUN:SCr, urine output) for diagnosing histological ATI.
METHODS
Study Design
We have previously described the details of this multicenter, prospective cohort of deceased kidney donors.14,15 Briefly, we collaborated with five organ procurement organizations (OPOs). These OPOs collected donor urine samples as per study protocol at the time of organ procurement between April 2010 and November 2013 from donors whose surrogates had given consent for research. In a subset of donors, the OPOs also obtained wedge biopsies of kidneys to assist with the allocation process. Frozen biopsy sections were reviewed by clinical pathologists at the respective hospitals or by pathologists contracted by the OPO. We excluded donors from this analysis if biopsies were not performed or if biopsy reports did not mention either the presence or absence of ATI.
We obtained donor data from the Organ Procurement and Transplantation Network (OPTN) that were submitted by its members, which has been described elsewhere.16 We reviewed OPO charts for additional donor information not available in the OPTN data system, including procurement kidney biopsy reports, admission donor SCr, serum sodium, SUN, and urine output. In a subset of donors from each participating OPO, we also confirmed the quality and accuracy of OPTN data using these systematic chart reviews. We obtained recipient SCr values from the OPTN database, currently maintained under contract with the United Network for Organ Sharing (UNOS).
We adhered to the ethics principles of the Declaration of Helsinki and obtained institutional review board (IRB) approval from the Data Coordinating Center at Yale (Human Research Protection Program Approval numbers: 0912006086, 0909005694, 0912006085, 0909005696) as well as the respective IRBs and/or scientific review committees for all sites in the study. The clinical and research activities outlined herein are also consistent with the principles outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.17,18 We used de-identified UNOS data for recipient outcomes under an approved waiver of consent.
Exposures
We defined donor AKI as an increase in SCr concentration from admission to the terminal value (change in SCr) by ≥0.3 mg/dl or by ≥50% rise from baseline. Severe AKI was defined as ≥100% rise in SCr. These SCr cut-offs correspond to AKI Network stage 1 or greater and stage 2 or greater, respectively.19,20 Since we did not have dates associated with the SCr, we did not use KDIGO (Kidney Disease: Improving Global Outcomes) AKI definitions. We used the terminal creatinine because it was the closest creatinine to the kidney biopsy. We also used change in SCr using peak SCr during hospitalization for a supplementary analysis. We evaluated fractional excretion of sodium cut-offs of ≥1% and ≥2% and fractional excretion of urea ≥35% for diagnosing ATI.21,22 We also evaluated SUN:SCr ≥20 and urine output ≤0.5 ml/kg/h for diagnosing ATI.19
We measured urine injury biomarkers NGAL, L-FABP, IL-18 and KIM-1 from stored urine samples that were collected from the indwelling catheter tube in the operating room prior to organ procurement. We provide details on assay characteristics in Item S1 (provided as online supplementary material) and in a previous publication.14 In our primary analysis, we did not index biomarkers to urine creatinine given unreliable urine creatinine kinetics in AKI.23 We instead present a supplementary analysis indexing biomarkers to urine creatinine. Since deceased donors experience polyuria, we also present a supplementary analysis adjusting for urine specific gravity to account for urine concentration differences.
Outcomes
Primary Outcome
The primary outcome was histological ATI on procurement kidney biopsy interpretations by pathologists contracted by the OPO. ATI was a 3-level ordinal outcome: No ATI, mild ATI, and severe ATI. We assigned a diagnosis of no ATI if reports specified the absence of ATI; mild ATI if <25% of tubules were affected or if the report specified mild (including terms such as modest or minimal) ATI; and severe ATI if ≥25% of tubules were affected or if the ATI was reported as moderate or severe.11 When kidneys from the same donor had discordant reports of ATI, we categorized the donor and both kidneys as having the higher degree of ATI. We excluded donors for whom reports did not mention presence or absence of ATI in either kidney.
Secondary Outcome
To determine the association of donor ATI, donor SCr-based AKI, and ATI biomarkers with recipient outcomes, we evaluated transplant function at six months post-transplantation as a secondary outcome. We used calculated 6-month eGFR via the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) creatinine equation as previously described and validated.24,25
Statistical Analysis
We reported continuous variables as mean ± SD or median (interquartile range [IQR]), and categorical variables as frequency (percentage). We calculated the kidney donor risk index (KDRI) from donor characteristics as described26,27 and mapped it to the kidney donor profile index (KDPI) relative to all deceased donors from the United States in 2010. For analyses using the KDRI score without SCr, we weighted all of the other KDRI variables as per the actual KDRI score but excluded SCr.
We compared continuous variables between groups using the ANOVA test and categorical variables using the chi-square test. We calculated the area under the receiver operating characteristic curve (AUROC) using logistic regression to model the discrimination of ATI as a function of change in SCr individually and for each biomarker. We performed AUROC assessment by (a) comparing severe ATI with no ATI, and (b) comparing any ATI (mild and severe) with no ATI. We used the De Long test to compare the AUROC of logistic models of each individual biomarker to change in SCr.28 We determined the sensitivity and specificity of biomarkers for ATI diagnosis using the 3rd tertile cut-off value from a previously published cohort of cardiac surgery-related AKI.29 These cut-off values were 81 ng/ml for NGAL, 171 ng/ml for L-FABP, 133 pg/ml for IL-18, and 12 ng/ml for KIM-1.
For transplanted kidneys, we regressed 6-month recipient eGFR as a linear function of donor SCr-based AKI with further adjustment for the KDRI score (excluding SCr). We also regressed 6-month recipient eGFR as a linear function of histological ATI and biomarker level above the cut-off value with further adjustment for KDRI score (excluding SCr) and SCr-based AKI. These analyses were clustered at the donor level. We also performed two subgroup analyses: (a) by traditional clinical parameters in donors with SCr-based AKI as this is the clinical scenario when these tests are used; and (b) by novel biomarker measurement in donors without SCr-based AKI and terminal SCr<1.5 mg/dL for the outcome of subclinical AKI. We used STATA Statistical Software: Release 14 (StataCorp LP, College Station, TX, USA) for all analyses, and all statistical tests were two-sided with a significance level of 0.05.
RESULTS
Study Participants
Of 1634 donors enrolled in the cohort, 905 (55%) had at least one kidney biopsied (Figure S1 and Table S1). Of the 905 donors biopsied, 581 (64%) had a biopsy report that mentioned the presence or absence of ATI and were thus included in this study. Median length of donor hospital stay before organ procurement was 3 (IQR, 2–5) days. Median donor age was 54 years, 268 (46%) were female, 86 (15%) had diabetes, and 230 (40%) had SCr-based AKI. A total of 98 (17%) donors had mild ATI and 57 (10%) donors had severe ATI. There was excellent concordance for ATI diagnosis between biopsy reports for both kidneys from the same donor (30 [5.4%] discordant pairs; κ =0.87; P<0.001; Table S2). There was a higher proportion of female donors with ATI; and donors with ATI were more likely to have urine output <0.5 ml/kg/h (Table 1).
Table 1.
Baseline characteristic of deceased kidney donors by histological ATI
| Characteristic | No ATI | Mild ATI | Severe ATI | P-valueˆ |
|---|---|---|---|---|
| (n=426) | (n=98) | (n=57) | ||
| Age, years | 54 (46–62) | 53 (47–61) | 53 (41–62) | 0.3 |
| Black race | 73 (17%) | 16 (16 %) | 12 (21%) | 0.6 |
| Female sex | 190 (45 %) | 43 (44 %) | 35 (61%) | 0.05 |
| BMI, kg/m2 | 28.5 (24.9–33.8) | 28.3 (24–33.8) | 28.8 (23.9–36.5) | 0.5 |
| Hypertension | 268 (63 %) | 55 (56 %) | 32 (56%) | 0.3 |
| Diabetes | 68 (16 %) | 11 (11 %) | 7 (12%) | 0.4 |
| Cause of death | 0.6 | |||
| Head trauma | 67 (16 %) | 15 (15 %) | 11 (20%) | |
| Anoxia | 138 (34 %) | 30 (31 %) | 24 (43%) | |
| Stroke | 205 (50 %) | 53 (54 %) | 21 (38%) | |
| Other | 2 (0.5 %) | 0 (0 %) | ||
| ECD | 212 (50 %) | 51 (52 %) | 26 (46%) | 0.7 |
| DCD | 60 (14 %) | 9 (9 %) | 8 (14%) | 0.4 |
| KDRI# | 1.6 (1.4–2) | 1.6 (1.3–1.8) | 1.5 (1.3–1.9) | 0.5 |
| KDPI, % | 90 (81–97) | 89 (76–95) | 88 (74–97) | 0.4 |
| Admission Scr, mg/dL | 1 (0.8–1.3) | 1 (0.8–1.3) | 1 (0.8–1.3) | 0.6 |
| Terminal Scr, mg/dL* | 1.1 (0.8–1.7) | 1.1 (0.8–1.6) | 1.2 (0.7–2.3) | 0.1 |
| AKI (≥Stage 1) | 165 (39%) | 36 (37%) | 29 (51%) | 0.2 |
| Severe AKI (≥Stage 2) | 70 (16 %) | 16 (16 %) | 15 (26%) | 0.2 |
| Urine output, ml/kg/h | 1.6 (1–2.9) | 1.5 (1–2.8) | 1.4 (0.7–2.5) | 0.5 |
| Urine output <0.5 ml/kg/h | 38 (9%) | 5 (5%) | 10 (18%) | 0.03 |
| Use of any vasoactive drugs | 334 (78%) | 84 (86%) | 44 (77%) | 0.2 |
| No. of vasoactive agents used | 1 (1–2) | 2 (1–2) | 2 (1–2) | 0.7 |
| No. of kidneys transplanted | 0.9 | |||
| 0 | 121 (28 %) | 30 (31 %) | 17 (30%) | |
| 1 | 71 (17 %) | 18 (18 %) | 9 (16%) | |
| 2 | 234 (55 %) | 50 (51 %) | 31 (54%) |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as median [interquartile range]. Conversion factor for Scr in mg/dL to mol/L, ×88.4.
ATI, acute tubular injury; BMI, body mass index; ECD, expanded criteria donor; DCD, donation after cardiac determination of death; KDRI, kidney donor risk index; KDPI, kidney donor profile index; AKI, acute kidney injury; Scr, serum creatinine
KDRI is calculated using the following variables: age, height, weight, race, history of hypertension or diabetes, cause of death, hepatitis C, DCD, and terminal Scr.
Anova test for continuous variables and chi-square test for categorical variables.
Scr in donors on the day of organ procurement
Serum Creatinine and Other Traditional Clinical Parameters
The AUROC for severe ATI using change in SCr was 0.58 (95% confidence interval [CI], 0.49–0.67); for any ATI it was 0.52 (95% CI, 0.45–0.58; Figure 1). Sensitivity and specificity of AKIN stage I AKI for severe ATI were 51% and 61%, respectively. Sensitivity and specificity of AKIN stage II AKI for severe ATI were 26% and 84%, respectively. Using peak SCr, the AUROC of change in SCr for presence of severe ATI was 0.60 (95% CI, 0.51–0.68) and for presence of any ATI was 0.55 (95% CI, 0.50–0.61).
Figure 1. Performance of delta serum creatinine for histological ATI.
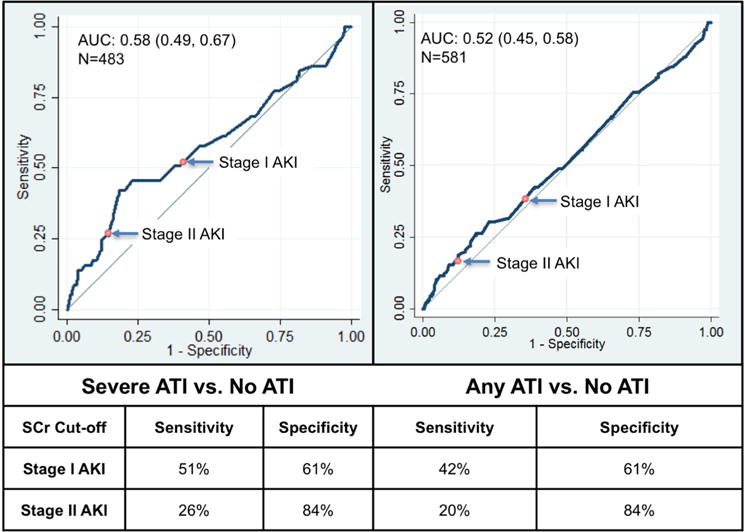
Stage I AKI defined by a SCr rise of ≥0.3 mg/dl or ≥ 50% from baseline; Stage II AKI defined as ≥100% rise in SCr. SCr: serum creatinine, ATI: acute tubular injury, AKI: acute kidney injury.
Any ATI includes mild, moderate and severe ATI. Severe ATI includes moderate and severe ATI.
Among the other traditional tests, fractional excretion of sodium was significantly higher with increasing degrees of ATI, whereas urine output, fractional excretion of urea and SUN-SCr ratio were not significantly different among donors with varying degrees of ATI (Table 2 and Table S3). These traditional tests are often clinically utilized in the setting of SCr-based AKI, and in a subgroup analysis of donors with SCr-based AKI, we found nominally higher fractional excretion of sodium in donors with increasing severities of ATI, although this was not statistically significant (P=0.06, Table S4).
Table 2.
Comparison of traditional and kidney injury biomarker levels between patients varying severities of ATI on kidney biopsy
| Biomarker | No ATI (n=426) | Mild ATI (n=98) | Severe ATI (n=57) | P – valueˆ |
|---|---|---|---|---|
| Traditional Biomarkers | ||||
| ΔScr (mg/dl) | 0.1 (−0.2–0.5) | 0.1 (−0.2–0.4) | 0.3 (−0.1–1.1) | 0.02 |
| Urine Output (ml/kg/h) | 1.6 (1–2.9) | 1.5 (1.1–2.8) | 1.4 (0.7–2.5) | 0.5 |
| FENa (%) | 1.1 (0.4–2.8) | 1.1 (0.5–3.3) | 1.6 (0.4–4.2) | 0.02 |
| FEUrea (%) | 46 (33–57.5) | 47.1 (36–56.2) | 48.9 (33.0–59.2) | 0.8 |
| SUN-Scr ratio | 15.6 (11.8–22) | 16.3 (12.2–23.3) | 14.3 (11.7–26.5) | 0.4 |
| Kidney Injury Biomarkers | ||||
| NGAL (ng/mL) | 45.2 (15–172.1) | 103.8 (14.7–370.2) | 201.5 (34.4–530.3) | <0.001 |
| L-FABP (ng/mL) | 13.8 (4.8–62) | 21.6 (4–78) | 37.8 (7.6–85.6) | 0.04 |
| IL-18 (pg/mL) | 56.8 (23.8–128.4) | 58.6 (22.6–122.6) | 66.3 (30.3–155.74) | 0.7 |
| KIM-1 (ng/mL) | 1.5 (0.7–3.7) | 1.5 (0.9–3) | 1.2 (0.7–2.8) | 0.9 |
Note: Values are given as median [interquartile range]. Conversion factor for Scr in mg/dL to mol/L, ×88.4.
ATI, Acute Tubular Injury; Scr, serum creatinine; FENa, fractional excretion of sodium; FEUrea, fractional excretion of urea; SUN, serum urea nitrogen. NGAL, neutrophil gelatinase-associated lipocalin; L-FABP, liver-fatty acid binding protein; IL-18, interleukin 18; KIM-1, kidney injury molecule 1.
ANOVA test
Tubular Injury Biomarkers
Median urinary NGAL and L-FABP were higher with increasing ATI severity (Table 2). Urine IL-18 and KIM-1 concentrations were not statistically different by ATI severity. Compared with SCr, NGAL had significantly higher AUROC for diagnosing severe and any ATI, at 0.67 (95% CI, 0.60–0.74) versus 0.58 (95% CI, 0.49–0.67; P=0.03) and 0.60 (95% CI, 0.55–0.66) versus 0.52 (95% CI, 0.45–0.58; P=0.005), respectively (Table 3). To account for urine concentration differences, we performed additional analyses accounting for urine creatinine and specific gravity, and found consistent results (Table S5).
Table 3.
Performance indices of kidney injury biomarkers to diagnose histological ATI
| Biomarker | Median [IQR] | Cut-off from45* | Donors above cut-off | Severe ATI vs. No ATI | Any ATI vs. No ATI | ||||
|---|---|---|---|---|---|---|---|---|---|
| C statistic (95% CI)ˆ | P-value@ | Sensitivity, Specificity | C statistic (95% CI) | P-value@ | Sensitivity, Specificity | ||||
| NGAL | 54.2 [16.1–262.9] ng/mL | 81 | 243 (44%) | 0.67 (0.60–0.74) | 0.03 | 53%, 61% | 0.60 (0.55–0.66) | 0.005 | 53%, 61% |
| L-FABP | 16.4 [5.2–67.2] ng/mL | 171 | 71 (13%) | 0.61 (0.54–0.68) | 0.6 | 16%, 88% | 0.56 (0.50–0.61) | 0.3 | 16%, 88% |
| IL-18 | 58.2 [24.1–127.5] pg/mL | 133 | 131 (24%) | 0.54 (0.45–0.62) | 0.5 | 21%, 76% | 0.51 (0.46–0.57) | 0.9 | 21%, 76% |
| KIM-1 | 1.5 [0.7–3.5] ng/mL | 12 | 31 (6%) | 0.47 (0.39–0.56) | 0.1 | 7%, 95% | 0.50 (0.44–0.55) | 0.6 | 7%, 95% |
ATI, Acute Tubular Injury; AUROC, area under receiver operating characteristic curve; CI, confidence interval; Scr, serum creatinine; NGAL, neutrophil gelatinase-associated lipocalin; L-FABP, liver-fatty acid binding protein; IL-18, interleukin 18; KIM-1, kidney injury molecule 1. IQR, interquartile range
Injury biomarker cut-offs derived as highest tertile cut-off values from Coca et al JASN 201429.
De-long test comparing AUROC of each log-transformed ATI biomarker with AUROC of Scr for diagnosing severe and any ATI. AUROC of ΔScr for severe ATI was 0.58 (95% CI, 0.49–0.67) and for any ATI was 0.52 (95% CI, 0.45–0.58)
Note: Any ATI includes mild, moderate and severe ATI. Severe ATI includes moderate and severe ATI.
Subclinical AKI
Over 60% of mild ATI cases (n=59) and 49% of severe ATI cases (n=28) were diagnosed in the subgroup of 326 donors without SCr-based AKI and terminal SCr < 1.5 mg/dL. In this setting of subclinical AKI, the prevalence of mild and severe ATI was 18% and 9%, respectively. In this subgroup, median urinary NGAL concentration was significantly higher in patients with ATI, but L-FABP, IL-18, and KIM-1 were not significantly different by ATI status (Table S6).
Association of Donor Kidney Injury With Recipient eGFR
At least one kidney was transplanted from 413 (71%) donors, which included 40 (70%) of 57 donors with severe ATI, 68 (69%) of 98 donors with mild ATI, and 305 (72%) of 426 donors without ATI (P=0.9). The presence of ATI was borderline associated (P = 0.05) with lower adjusted 6-month recipient eGFR compared with the absence of ATI (eGFRs with severe, mild, and no ATI: 43.2 [95% CI, 38.0–48.3], 46.1 [95% CI, 42.1–50.2], 49.4 [95% CI, 47.5–51.4] ml/min/1.73 m2, respectively; Figure 2). However, SCr-based donor AKI was paradoxically associated with higher adjusted 6-month eGFR (AKI versus no AKI: 50.7 [95% CI, 47.6–53.7] versus 46.7 [95% CI, 44.7–48.7] ml/min/1.73m2, respectively; P=0.04). Compared with donor kidneys with biomarker levels below the cut-off, donor kidneys with NGAL concentrations above the cut-off were associated with nominally lower adjusted 6-month eGFR in recipients (49.7 [95% CI, 47.4–52.0] versus 46.3 [95% CI, 43.5–49.2] ml/min/1.73m2, respectively), although this was not statistically significant (P=0.09). Donor urine L-FABP, IL-18, and KIM-1 levels were not associated with recipient 6-month eGFR.
Figure 2. Association of donor characteristics with 6-month recipient eGFR.
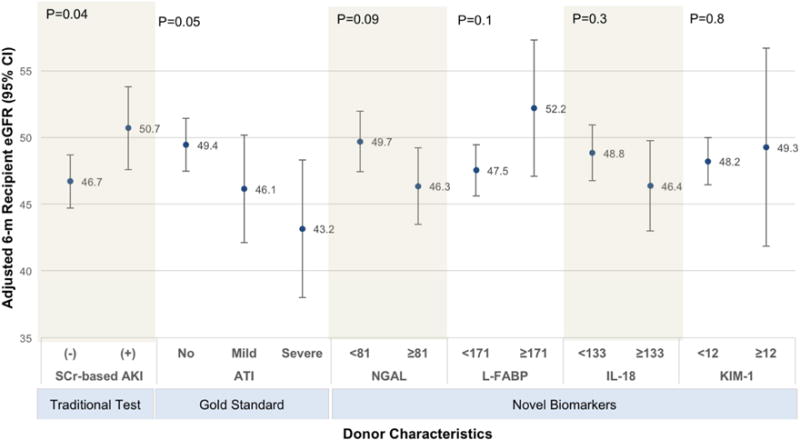
Multivariable regression of donor SCr-based AKI, histological ATI and biomarker-based AKI on 6-month recipient eGFR controlling for KDRI score components (excluding serum creatinine) and SCr-based AKI. For ATI, (+) and (−) indicate presence and absence of ATI, respectively. For SCr, (+) and (−) indicate presence and absence of AKI. Biomarker cut-offs obtained from Coca et. al. JASN 201429 ATI, acute tubular injury; AKI, acute kidney injury; KDRI, kidney disease risk index; eGFR, estimated glomerular filtration rate. Severe ATI includes moderate and severe ATI
DISCUSSION
The currently accepted SCr-based clinical definition of AKI provides little to no information about etiology. Numerous urinary kidney injury biomarkers are being translated in humans after demonstrating promise in experimentally-induced tubular injury in animal models. However, most translational studies have compared these urinary biomarkers with the imperfect “gold-standard” of SCr-based AKI. Only a kidney biopsy can definitively diagnose ATI. In this study, we found that SCr-based AKI lacks discriminatory ability for diagnosing histological ATI, with a modest AUROC and poor sensitivity and specificity at the AKIN definition cutoffs. Other traditional clinical tests, which are routinely used to assess AKI clinically, also lacked discrimination and accuracy for histological ATI. Among the ATI biomarkers, we found that urine NGAL had higher discrimination for ATI compared with SCr; however, the overall AUROC was still quite modest. L-FABP, IL-18, and KIM-1 did not have higher AUROCs than SCr.
Despite the clinical desire to understand etiology, kidney biopsies are not typically performed in most patients with clinically-defined AKI because these patients are frequently critically ill, unable to cooperate with the procedure, and at increased risk for bleeding. Thus, reliable data about the accuracy of SCr for histological ATI in humans are not available. In a preclinical study of ischemia-reperfusion in mice, SCr was shown to have an area under the curve for detecting ATI of 0.67–0.73 and a sensitivity of 20%–56% for detecting various ATI severities.30 While many investigators have evaluated injury biomarkers as tools to overcome the limitations of SCr-based AKI definitions, nearly all have relied on SCr to define kidney injury.5
We tested four of the most promising biomarkers of tubular injury to date. NGAL is a marker of distal tubular injury that is upregulated when tubular cells are structurally damaged and is an early biomarker of various ischemic, septic, or nephrotoxic insults to the kidney.31–34 L-FABP, an intracellular carrier protein, is produced by proximal tubular cells in response to tubulointerstitial damage.35 IL-18, which is produced in proximal tubular cells in response to kidney injury and excreted in urine, has shown promise for diagnosing ATI.36 KIM-1 is expressed in the apical membrane of proximal tubular cells after kidney injury and can be detected in urine.37 NGAL and L-FABP are approved for clinical use for AKI diagnosis in Europe and Asia. In North America, KIM-1 is part of a panel of seven biomarkers that is approved for preclinical use to monitor nephrotoxicity in drug development studies.38,39
Median urinary levels of NGAL and L-FABP were significantly higher in donors with increasing severities of histological ATI. Despite prior data demonstrating associations with various AKI etiologies, L-FABP, IL-18 and KIM-1 did not perform well in our study.40,41 One explanation for our findings, which are discordant with prior studies demonstrating improved accuracy with these injury biomarkers, is our use of histology to determine ATI. Our finding of poor discrimination of injury biomarkers for diagnosing ATI is consistent with the study by Parekh et al,42 in which the authors evaluated histological ATI after ischemia-reperfusion injury in patients undergoing partial nephrectomies for treatment of renal cancer. They reported poor correlation of NGAL, L-FABP, IL-18 and KIM-1 with the degree of renal insult measured by duration of ischemia and by histological ATI.
Our results also inform emerging clinical concepts of “subclinical AKI,” namely high levels of injury biomarker levels in the urine without changes in SCr levels. Subclinical AKI is thought to be an early step in the spectrum of kidney injury and is associated with poor outcomes.9 In the current study, half of the cases of histological ATI occurred in donors without SCr-based AKI. In donors without SCr-based AKI, we found that urine NGAL concentrations were significantly higher in those with histological ATI than those without it.8 Our results are consistent with previous studies that have demonstrated the role of injury biomarkers in diagnosing subclinical AKI. In a meta-analysis of 10 prospective NGAL studies, patients with elevated NGAL concentrations but without a concomitant rise in SCr subsequently required renal replacement therapy more frequently and had greater mortality compared with patients with no elevations in NGAL or SCr.43 In a multicenter cohort of emergency room patients, urine NGAL as well as KIM-1 predicted initiation of dialysis or in-hospital death in patients without increases in SCr.44 In a cardiac surgery cohort, we demonstrated that urinary IL-18 and KIM-1 were independently associated with 3-year mortality in those without SCr-based AKI.29
We evaluated the prognostic value of donor ATI, SCr-based AKI and urinary biomarkers by their association with recipient 6-month eGFR. While histological donor ATI was associated with lower 6-month recipient eGFR, SCr-based donor AKI was paradoxically associated with higher 6-month recipient eGFR. This discordant finding may be attributed to the fact that in addition to ATI, SCr also captures decreased kidney perfusion and other clinical attributes such as hydration status, hemodynamic derangements or muscle breakdown. Since donor kidneys in these last situations do not experience intrinsic kidney damage, they are likely to recover complete function after transplantation, which is less likely to be the case for donor kidneys with overt ATI. In recipients of donor kidneys with NGAL above 81 ng/mL, we also noted slightly lower 6-month eGFR, suggesting a consistent association with the histological diagnosis of structural kidney injury.
This study does have a number of important limitations. First, since kidney biopsies were only performed at the discretion of the OPOs, selection bias could have resulted such that only donor kidneys assumed to have moderate injury were selected for biopsy. Donor kidneys with plausible evidence of milder or more severe injury may have been either transplanted or discarded, respectively, without biopsy. Moreover, due to the limited tissue obtained for evaluation during kidney biopsy and because ATI is often non-uniformly distributed in the kidneys, sampling bias is also a concern. However, we noted agreement in acute tubular injury severity between right and left kidneys in 524 (95%) out of 554 donors in whom both kidneys were biopsied. Second, findings from deceased organ donors may not be generalizable since brain death affects multiple neuroendocrine functions, including reduced antidiuretic hormone levels leading to polyuria,45 which could affect urinary biomarker concentrations. Also, AKI etiologies may differ in donors as compared with hospitalized patients. For example, unlike other hospitalized patients in whom sepsis in an important cause of AKI, deceased donors have near-complete lack of sepsis. Also, donors may receive large volumes of resuscitation fluids and diuretics, which would make urine output and fractional excretion of sodium unreliable as biomarkers of ATI. To address polyuria, we used urine creatinine and specific gravity as covariates in supplementary analyses and found consistent results. Third, the observed associations of SCr and biomarkers with ATI may be inaccurate due to detection bias resulting from lack of exact timing of SCr, which could have led to misclassification of AKI, and lack of longitudinal biomarker measurements, which could have led us to miss the peak biomarker levels. However, for novel biomarkers to be useful in clinical practice, the association of these biomarkers with ATI must persist despite lack of longitudinal data. Fourth, kidney biopsies were read by different attending pathologists at the respective institutions, which could have introduced variability in reporting ATI.46 However, since ATI is commonly observed with kidney biopsy, we believe the biopsy reports had high specificity for the diagnosis. Fifth, when evaluating associations for donor factors with recipient eGFR, it is possible that donor ATI led to selection bias such that only the injured kidneys deemed most likely to perform well were preferentially transplanted. However, we observed similar organ discard rates regardless of ATI. Given the observational nature of our study and despite controlling for KDRI, residual confounding is possible. It is also important to emphasize that our study shows donor histological ATI is associated with lower 6-month recipient eGFR, but the effect size is small and should by no means deter appropriate utilization of donated kidneys with ATI. Finally, we cannot exclude the possibility that some of the statistically significant findings in our observational study could be due to multiple comparisons of biomarkers with ATI.
In conclusion, we found that SCr and urinary kidney injury biomarkers (NGAL, L-FABP, IL-18, and KIM-1) have poor discrimination for diagnosing histological ATI in hospitalized deceased donors. Future clinical studies should focus on evaluating other biomarkers for diagnosis of histological ATI.
Supplementary Material
Table S1: Comparison of donors who were biopsied vs those who were not.
Table S2: Concordance between ATI reports for kidneys from the same donor.
Table S3: Performance indices of traditional biomarkers to diagnose histological ATI.
Table S4: Traditional biomarker levels in donors with Scr-based AKI.
Table S5: Performance indices of injury biomarkers to diagnose histological ATI adjusting for urine creatinine and specific gravity.
Table S6: Biomarker levels in subgroup of donors without AKI but with histological diagnosis of ATI.
Figure S1: Flowchart of patients included for final analysis.
Item S1: Additional methods: Kidney Donor Risk Index and assay characteristics.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Acknowledgments
We are tremendously grateful for the study participation of our collaborators at the following organ procurement organizations: Gift of Life Philadelphia, New York Organ Donor Network, Michigan Organ and Tissue Donation Program, New Jersey Sharing Network, and New England Organ Bank.
Support: This work was supported by the National Institutes of Health (NIH; grant numbers RO1DK-93770 and K24DK090203), a Roche Organ Transplantation Research Foundation Award to Dr Parikh, an award from the American Heart Association to Dr Hall, National Institute of Diabetes and Digestive and Kidney Diseases grant K23-097201 to Dr Wilson, a Yale Kidney O’Brien Center Award (P30DK79337), and the Health Resources and Services Administration (contract no. 234-2005-37011C). Dr. Moledina is supported by T32 training grant (T32DK007276) from the NIH and by the Robert E. Leet and Clara Guthrie Patterson Trust Mentored Clinical Research Award; he and is also a graduate student in the Yale Investigative Medicine PhD Program, which is supported by Clinical and Translational Science Award grant UL1 TR000142 from the National Center for Advancing Translational Science, a component of the NIH. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. These organizations were not involved in study design, analysis, interpretation, or manuscript creation. NGAL assays were donated by Abbott Diagnostics and measured at University of Ireland. L-FABP assays were donated by Sekisui Medical. The companies did not participate in design, analysis, or interpretation of study results. The data reported here have been supplied by UNOS as the contractor for the OPTN. The Health Resources and Services Administration provides oversight to the activities of the OPTN contractor. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A part of this study was presented as an oral presentation at the American Society of Nephrology meeting in Atlanta, Georgia, November 2015.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: DGM, IEH, CRP; data acquisition: IEH, HT-P, PPR, FLW, BS, MDD, CRP; data analysis/interpretation: DGM, IEH, HT-P, PPR, FLW, BS, MDD, FPW, SGC, CRP; statistical analysis: DGM, HT-P; supervision and mentorship: CRP. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Peer Review: Evaluated by 3 external peer reviewers, a statistician, and an Acting Editor-in-Chief.
References
- 1.Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50(3):811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 2.Perazella MA, Coca SG, Hall IE, Iyanam U, Koraishy M, Parikh CR. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clinical journal of the American Society of Nephrology: CJASN. 2010;5(3):402–408. doi: 10.2215/CJN.06960909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009;76(10):1089–1097. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 4.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. Journal of the American Society of Nephrology: JASN. 2012;23(1):13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blantz RC. Pathophysiology of pre-renal azotemia. Kidney Int. 1998;53(2):512–523. doi: 10.1046/j.1523-1755.2003_t01-1-00784.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Fernandez H, Shashaty MG, et al. False-Positive Rate of AKI Using Consensus Creatinine-Based Criteria. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(10):1723–1731. doi: 10.2215/CJN.02430315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haase M, Kellum JA, Ronco C. Subclinical AKI–an emerging syndrome with important consequences. Nature reviews. Nephrology. 2012;8(12):735–739. doi: 10.1038/nrneph.2012.197. [DOI] [PubMed] [Google Scholar]
- 9.Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Critical care. 2012;16(3):313–313. doi: 10.1186/cc11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puthumana J, Ariza X, Belcher JM, Graupera I, Gines P, Parikh CR. Urine Interleukin 18 and Lipocalin 2 are Biomarkers of Acute Tubular Necrosis in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2016.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall IE, Reese PP, Weng FL, et al. Preimplant histologic acute tubular necrosis and allograft outcomes. Clinical journal of the American Society of Nephrology: CJASN. 2014;9(3):573–582. doi: 10.2215/CJN.08270813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood KE, Becker BN, McCartney JG, D’Alessandro AM, Coursin DB. Care of the potential organ donor. The New England journal of medicine. 2004;351(26):2730–2739. doi: 10.1056/NEJMra013103. [DOI] [PubMed] [Google Scholar]
- 13.Cittanova ML, Leblanc I, Legendre C, Mouquet C, Riou B, Coriat P. Effect of hydroxyethylstarch in brain-dead kidney donors on renal function in kidney-transplant recipients. Lancet. 1996;348(9042):1620–1622. doi: 10.1016/s0140-6736(96)07588-5. [DOI] [PubMed] [Google Scholar]
- 14.Reese PP, Hall IE, Weng FL, et al. Associations between Deceased-Donor Urine Injury Biomarkers and Kidney Transplant Outcomes. Journal of the American Society of Nephrology: JASN. 2016;27(5):1534–1543. doi: 10.1681/ASN.2015040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall IE, Schroppel B, Doshi MD, et al. Associations of deceased donor kidney injury with kidney discard and function after transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(6):1623–1631. doi: 10.1111/ajt.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickinson DM, Bryant PC, Williams MC, et al. Transplant data: sources, collection, and caveats. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(Suppl 9):13–26. doi: 10.1111/j.1600-6135.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 17.World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA: the journal of the American Medical Association. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 18.International Summit on Transplant T. Organ T. The Declaration of Istanbul on Organ Trafficking and Transplant Tourism. Clinical journal of the American Society of Nephrology: CJASN. 2008;3(5):1227–1231. doi: 10.2215/CJN.03320708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 21.Esson ML, Schrier RW. Diagnosis and treatment of acute tubular necrosis. Ann Intern Med. 2002;137(9):744–752. doi: 10.7326/0003-4819-137-9-200211050-00010. [DOI] [PubMed] [Google Scholar]
- 22.Carvounis CP, Nisar S, Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62(6):2223–2229. doi: 10.1046/j.1523-1755.2002.00683.x. [DOI] [PubMed] [Google Scholar]
- 23.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. Journal of the American Society of Nephrology: JASN. 2009;20(3):672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potluri VS, Parikh CR, Hall IE, et al. Validating Early Post-Transplant Outcomes Reported for Recipients of Deceased Donor Kidney Transplants. Clinical journal of the American Society of Nephrology: CJASN. 2016;11(2):324–331. doi: 10.2215/CJN.06950615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 27.OPTN/UNOS. A Guide to Calculating and Interpreting KDPI. https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf. Date accessed: Date accessed: July 23, 2017.
- 28.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 29.Coca SG, Garg AX, Thiessen-Philbrook H, et al. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. Journal of the American Society of Nephrology: JASN. 2014;25(5):1063–1071. doi: 10.1681/ASN.2013070742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaidya VS, Ozer JS, Dieterle F, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nature biotechnology. 2010;28(5):478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2009;54(6):1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Devarajan P, Mishra J, Supavekin S, Patterson LT, Steven Potter S. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol Genet Metab. 2003;80(4):365–376. doi: 10.1016/j.ymgme.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. Journal of the American Society of Nephrology. 2003;14(10):2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 34.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. The Lancet. 365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 35.Portilla D, Dent C, Sugaya T, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73(4):465–472. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 36.Parikh CR, Han G. Variation in Performance of Kidney Injury Biomarkers Due to Cause of Acute Kidney Injury. American Journal of Kidney Diseases. 2013;62(6):1023–1026. doi: 10.1053/j.ajkd.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. American journal of physiology. Renal physiology. 2006;290(2):F517–529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 38.Agency EM. Letter of Support for PSTC translational Drug-Induced Kidney Injury (DIKI) biomarkers. 2014 http://www.ema.europa.eu/docs/en_GB/document_library/Other/2014/11/WC500177133.pdf. Accessed 10/21/2015, 2015.
- 39.ngal.com. The NGAL Test™ now launched for diagnostic use in Europe. 2011 http://ngal.com/news/the-ngal-test%E2%84%A2-for-ivd-in-europe/. Accessed 10/21/2015, 2015.
- 40.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 41.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2004;43(3):405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 42.Parekh DJ, Weinberg JM, Ercole B, et al. Tolerance of the human kidney to isolated controlled ischemia. Journal of the American Society of Nephrology: JASN. 2013;24(3):506–517. doi: 10.1681/ASN.2012080786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57(17):1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59(3):246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirschl MM, Matzner MP, Huber WO, et al. Effect of desmopressin substitution during organ procurement on early renal allograft function. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 1996;11(1):173–176. [PubMed] [Google Scholar]
- 46.Liapis H, Gaut JP, Klein C, et al. Banff Histopathological Consensus Criteria for Preimplantation Kidney Biopsies. Am J Transplant. 2017;17(1):140–150. doi: 10.1111/ajt.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Comparison of donors who were biopsied vs those who were not.
Table S2: Concordance between ATI reports for kidneys from the same donor.
Table S3: Performance indices of traditional biomarkers to diagnose histological ATI.
Table S4: Traditional biomarker levels in donors with Scr-based AKI.
Table S5: Performance indices of injury biomarkers to diagnose histological ATI adjusting for urine creatinine and specific gravity.
Table S6: Biomarker levels in subgroup of donors without AKI but with histological diagnosis of ATI.
Figure S1: Flowchart of patients included for final analysis.
Item S1: Additional methods: Kidney Donor Risk Index and assay characteristics.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org


