Abstract
Context
The progression of β-cell function in newly diagnosed adolescents with type 2 diabetes mellitus (T2DM) is not well documented.
Objective
We hypothesized that at the time of diagnosis with T2DM, adolescents would have impaired β-cell function as demonstrated by the disposition index (calculated as: insulin secretion adjusted for insulin sensitivity), and this would be followed by a rapid decline of function despite standard medical management.
Setting and Design
39 adolescents with recently diagnosed T2DM, and 32 obese adolescent controls with normal glucose tolerance had acute insulin response to glucose (AIRg), HOMA-IR, and disposition index (DI) measured serially over 2 years.
Results
In the adolescent T2DM group, fasting glucose increased over 2 years (p=0.04), while DI was impaired at baseline, and showed an overall relative decline of 25% per year. The mean HbA1c remained below 8% (64 mmol/mol). Differences were observed between the T2DM and control adolescents in the way DI changed over time (p=0.02).
Conclusions
β-cell function in adolescents with recently diagnosed T2DM was impaired with no improvement of β-cell function over the two years of study despite stable HbA1c, BMI markers of insulin sensitivity and standard treatment of hyperglycemia.
Introduction
The pathophysiology of T2DM in adolescents and adults is characterized by peripheral and hepatic insulin resistance with impaired β-cell function (1–3). In adults, β-cell impairment worsens progressively during the years prior to diagnosis (4, 5), and this continues over the course of disease independent of treatment (6, 7). However in comparison to adults, the natural history of insulin secretion in adolescents is less clear. We have reported results from a small number of adolescents describing a rapid and severe decline of glucose-stimulated insulin secretion in the 1–3 years prior to onset of diabetes (8). These findings support β-cell failure as central to the development of T2DM in youth, consistent with the model proposed for adults (9–11). However, it is not clear whether insulin secretion is comparably impaired in diabetic adolescents at the time of diagnosis, nor has the capacity for β-cell recovery following treatment initiation of adolescents with T2DM been determined. There are few studies that assess β-cell function longitudinally in adolescents with newly diagnosed T2DM. The Treatment Options for type 2 Diabetes in Adolescent and Youth (TODAY) (12) trial was a prospective evaluation of glucose metabolism over 4 years in adolescent subjects with T2DM randomized to metformin alone, metformin and rosiglitazone, or metformin and lifestyle changes. In the TODAY study there was a yearly 20–35% decline per year in β-cell function as measured by insulin secretion corrected for insulin sensitivity during oral glucose tolerance test (OGTT). This rate of decline is similar to other longitudinal reports in adolescent subjects with established T2DM (13), and is greater than the ~10% annual rate of decline described in adults (6). These findings raise the possibility that the natural history of β-cell dysfunction in adolescents and adults differ, but comparison is limited because of differences in the stage of diabetes and in the methods used to measure insulin secretion.
The pre-morbid state is necessarily shorter in adolescents, suggesting a more rapid rate of β-cell decline than in adults (8). We hypothesized that near to the time of T2DM diagnosis, adolescents would have impaired β-cell function, followed by a rapid decline of insulin secretion. To test this hypothesis, we assessed β-cell function using the disposition index (calculated as: measured insulin secretion in response to intravenous (IV) glucose adjusted for insulin sensitivity (1/HOMA)) in adolescents with newly diagnosed T2DM, as well as in age- and weight appropriate adolescents without diabetes. The adolescents had repeated studies over 2 years.
Research Design and Methods
Subjects
The study population consisted of 39 adolescents with newly diagnosed T2DM (within 2.1 ± 2.6 [mean ± standard deviation] months of diagnosis) and 32 adolescents of comparable weight, and of similar distribution for race, sex and pubertal status with normal glucose tolerance. Adolescents with T2DM were identified near diabetes diagnosis by review of outpatient OGTT data or at the time of in-patient hospitalization and subsequently asked to participate in the study. Inclusion criteria were as follows: plasma glucose measurements that meet the American Diabetes Association criteria for diabetes, not being ketosis prone, negative for islet cell antibody titers (glutamic acid decarboxylase, IA-2 and insulin antibodies) and having biochemical or physical evidence of insulin resistance (elevated fasting c-peptide [>4.0 ng/ml], fasting insulin [>20 mcIU/ml] or acanthosis nigracans). Individuals were excluded if they were pregnant or had transient forms of hyperglycemia (steroid or stress induced hyperglycemia). Adolescent control subjects were recruited from a CCHMC Obesity Program. Inclusion criteria for the adolescent control subjects consisted of the presence of puberty (> Tanner II breast development for girls, >4 ml testicular volume for boys as determined by a pediatric endocrinologist), BMI greater than the 99th percentile for age and sex, and normal glucose tolerance as determined by standard 75 gram OGTT using American Diabetes Association criteria (14). Non-diabetic adolescent controls were excluded if they had known chronic medical problems, transient forms of hyperglycemia (steroid or stress induced hyperglycemia) or were pregnant, or were taking medications that alter glucose tolerance. Power calculation was based on a difference in Acute Insulin Response (AIR) greater than 15% to be clinically important. To demonstrate greater than 15% difference between groups with 80% power and two-tailed α-value of 5% the study would require 25 subjects in each group.
Written informed consent was obtained according to the guidelines of the Cincinnati Children’s Hospital Institutional Review Board from all subjects or their parents. Assent was obtained from study participants <18 years old. Age, sex, race, family history of diabetes, medical history and medication use were collected. Baseline data from these cohorts has been reported previously (15), as has some of the longitudinal data from the control subjects (8). The current analysis includes 32 of the 37 control adolescent subjects from the latter manuscript; the five who were taking metformin at baseline were excluded.
Following their initial assessment, all adolescent participants were invited to have testing of β-cell function at 6 months, 1 year, and 2 years post enrollment.
Study Procedures
After an overnight fast, all subjects were admitted to the Clinical and Translational Research Center (CTRC) at the Cincinnati Children’s Hospital Medical Center. Subjects taking glucose lowering medication discontinued the drug 3 days before β-cell function testing. Long-acting insulin (glargine) was held for a minimum of 36 hours and short-acting insulins were held for a minimum of 11 hours prior to study procedures. All female participants had a urine pregnancy test performed prior to testing. Body weight was measured with a digital scale to the nearest 0.1 kg, and height was measured with a stadiometer to 0.1 cm, at each visit.
β-cell function tests
Intravenous Glucose Tolerance Tests (IVGTT) were performed to assess insulin secretion (AIR) and insulin sensitivity (1/HOMA-IR). This test was chosen because it has a good risk assessment profile, short in duration and provides an accurate assessment of first phase inulin response to IV glucose. Indwelling venous catheters were placed in forearms, one for infusion of IV dextrose and the other for blood sampling. The arm designated for blood draw was wrapped in a heating pad to maintain stable blood flow. Following fasting blood sampling at −10, −5 and 0 minutes, subjects received an intravenous bolus of glucose (0.3 grams/kg, max dose 40 grams) over 0.5 minutes; blood was sampled at 2, 4, 6, 8, 10, 12, 15, 18, 21, 25, and 30 minutes after glucose administration. Blood samples were immediately placed on ice, and then centrifuged to separate plasma within an hour of collection. Plasma was separated and stored at −80°C.
Biochemical measurements
Glucose measurements were made at the time of study on whole blood using a YSI glucose analyzer (Yellow Springs Instrument, Yellow Springs, OH). Each sample was run in duplicate and the average used for analysis. Plasma insulin was measured by radioimmunoassay (RIA) as previously described (1). This assay uses a guinea pig anti-insulin serum that does not distinguish between proinsulin and fully processed insulin. Hemoglobin A1c (HbA1c) was determined using a standard method (1) and results are reported as percent of total hemoglobin.
Calculations and Statistical Analysis
Fasting glucose and insulin levels were calculated as the mean of three samples taken before glucose administration. The acute insulin responses to glucose (AIRg) were computed as the respective areas under the curves, above the mean of the immediately preceding baseline values, using concentrations at 2, 4, 6, 8, and 10 minutes after the stimulus administration. Riemann sums based on the midpoint of each 2 minute interval, were used to calculate the area under the curve. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the online Oxford calculator (www.dtu.ox.ac.uk/homa). The disposition index (DI) was defined as the product of insulin secretion to glucose (AIRg) and insulin sensitivity (1/HOMA-IR); this method of computing DI has been validated for adolescents previously (8).
Data were analyzed using SAS®, version 9.3 (SAS Institute, Cary, NC). Body mass index (BMI) Z-scores were calculated from heights and weights collected at each study visit using the SAS program (SAS Institute, Inc., Cary, NC) based on the Centers for Disease Control and Prevention (CDC) growth charts (URL:httpP://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm) (16). Continuous baseline data were summarized as mean ± standard deviation, while categorical data were summarized as frequency counts with percentages. For the baseline comparisons, Chi-square and Fisher’s exact tests were used as appropriate for comparison of categorical variables. T-tests were used to compare age and BMI between the two adolescent groups. Due to skewness, log10 transformations were applied to DI, AIRg, fasting glucose, fasting insulin, and HOMA-IR values for analysis. Generalized linear models with repeated measures were used to assess these log10 metabolic values over time with Tukey-Kramer adjustments for multiple comparisons. HbA1c values were analyzed using a generalized linear model with repeated measures to compare the adolescent diabetes group over time. Due to the distributions of data, geometric means with geometric standard deviations are presented in Table 2 and geometric means with geometric standard errors are presented in the graphs for the outcome variables. Individual study tests where the insulin response for AIRg dropped ≥ 100 pmol/L below baseline within a two minute interval were designated as biologically implausible and not included in the analysis (2/337 tests). Also, if there were negative AIRg and DI values they were set to missing (n=12). In the event that a single value was missing for a 2-minute interval, then the mid-point of the values on either side of the missing value was used for calculation purposes (n=7 study tests). Statistical significance was set a priori at α=0.05. Rate of change difference was assessed by examination of the interaction term of time by group in the model.
Table 2.
Longitudinal Assessment
| Baseline | 6 Months | 1 Year | 2 Year | P-value** | ||
|---|---|---|---|---|---|---|
|
Adolescent Control Total N=32 |
N=32 (100%) | N=31 (97%) | N=28 (88%) | N=27 (84%) | ||
| Fasting glucose, mg/dL | 85.4 (1.07) | 89.1 (1.07) | 88.7 (1.12) | 87.6 (1.10) | 0.09 | |
| Fasting insulin, μU/mL | 32.4 (1.61) | 27.0 (1.79) | 25.6 (1.94) | 21.8 (1.87) | 0.04 | |
| HOMA-IR | 3.6 (1.58) | 3.1 (1.67) | 3.0 (1.83) | 2.7 (1.79) | 0.09 | |
| AIRg, pmol/L x min | 14812 (1.78) n=31 | 13135 (2.05) n=30 | 12742 (2.09) n=26 | 9774 (2.40) n=26 | 0.02 | |
| Disposition index | 4088 (1.65) n=31 | 4032 (1.80) n=30 | 4228 (1.84) n=26 | 3650 (2.19) n=26 | 0.50 | |
| BMI Z-score * | 2.30 (0.44) | 2.28 (0.46) | 2.27 (0.52) | 2.32 (0.50) | 0.74 | |
|
Adolescent T2DM Total N=39 |
N=39 (100%) | N=34 (87%) | N=29 (74%) | N=24 (62%) | ||
| Fasting glucose, mg/dL | 116.1 (1.30) | 128.0 (1.50) | 142.9 (1.44) | 146.8 (1.45) | 0.04 | |
| Fasting insulin, μU/mL | 39.3 (1.67) | 42.0 (1.87) n=31 | 33.3 (1.95) n=22 | 34.9 (1.74) n=15 | 0.36 | |
| HOMA-IR | 4.7 (1.57) | 5.3 (1.69) n=30 | 4.5 (1.79) n=22 | 4.7 (1.71) n=15 | 0.50 | |
| HbA1c * | 7.9 (2.3) n=36 | 6.9 (1.4) n=29 | 7.3 (2.3) n=28 | 7.3 (2.6) n=23 | 0.10 | |
| AIRg, pmol/L x min | 2201 (4.16) n=31 | 2100 (4.17) n=28 | 2771 (2.89) n=15 | 1091 (6.00) n=14 | 0.06 | |
| Disposition index | 453 (3.53) n=31 | 395 (5.01) n=27 | 503 (2.84) n=15 | 234 (5.80) n=14 | 0.046 | |
| BMI Z-score * | 2.43 (0.35) | 2.42 (0.41) | 2.39 (0.46) | 2.36 (0.42) | 0.02 |
Data presented as Geometric Mean (Geometric standard deviation) or for BMI and HbA1c as *Mean (standard deviation) [geometric standard deviations are a multiplicative factor whereas the untransformed standard deviation is additive]
P-values from testing variables individually for each group over 2 years
A sub-analysis was performed to compare those adolescents with T2DM who were initially treated with insulin to those who were not in order to examine potential differences in progression of β-cell function.
Results
Study Cohorts
The baseline demographic and clinical characteristics of the two study groups are shown in Table 1. The two adolescent groups were well paired and did not significantly differ for sex, race, BMI and family history of diabetes. The group with T2DM was about one year older. All participants were Tanner IV or greater. Seventeen of the 39 adolescent T2DM subjects were diagnosed by OGTT and managed with metformin alone; 22 presented with significant hyperglycemia and during inpatient admission exogenous insulin therapy was commenced. The mean duration of diabetes at time of enrollment was 2.1 ± 2.6 months in the adolescents with T2DM.
Table I.
Baseline Characteristics
| Adolescent Control N=32 |
Adolescent T2DM N=39 |
P-value | |
|---|---|---|---|
|
| |||
| Age, years | 14.3 (2.0) | 15.4 (2.5) | 0.03 |
| Sex, n (% male) | 14/32 (44%) | 15/39 (38%) | 0.65 |
| Race, n (% white) | 10/32 (31%) | 14/39 (36%) | 0.68 |
| BMI Z-score | 2.3 (0.4) | 2.4 (0.4) | 0.16 |
| Duration of diabetes, months | Not applicable | 2.1 (2.6) [0.0, 14.4] | - |
| Family history, n (% yes) | 30/32 (94%) | 30/32 (94%) | 1.00 |
| Insulin usage, n (% yes) | 0/32 (0%) | 22/39 (56%) | - |
| Oral anti-T2DM agent, n (% yes) | 0/32 (0%) | 31/39 (79%) | - |
Data presented as mean (standard deviation) or frequency (%). For diabetes duration, range [minimum, maximum] is also reported.
The glycemic characteristics of adolescents with recently diagnosed T2DM not initially treated with insulin (n=17) were: fasting glucose, 143.7 ± 5.5 mg/dl (n=3); 2 hour OGGT glucose, 236.3 ± 62.7mg/dl (n=7); random glucose 301.6 ± 101.3 (n=5) and HbA1c 7.8 ± 2.0% (n=15). In comparison the adolescents with T2DM treated with insulin from the time of diagnosis (n=22) had the following glycemic profiles: fasting glucose 157.0 mg/dl (n=1), 2 hour OGTT glucose, 244.0 ± 11.3 mg/dl (n=2), random glucose 382.5 ± 102.9 mg/dl (n=19), and HbA1c 12.0 ± 2.4% (n=20).
No subjects in the adolescent control group developed diabetes over the two year period as documented by fasting blood glucose values below 126 mg/dl. In only 8 of 118 encounters over the two year period did a subject have a fasting blood glucose >100 mg/dl. Other clinical markers of glycemic status such as HbA1c% were not consistently collected in the adolescent control group.
For those who were lost to follow up, there were no major differences in the adolescent controls that remained in the study (n=27) as compared to those who dropped out (n=5). More of the adolescents with T2DM who did not remain in the study for all 2 years (n=15) were on insulin. However, these differences were not statistically significant. Details may be found in the supplemental Table.
β-cell function and metabolic parameters
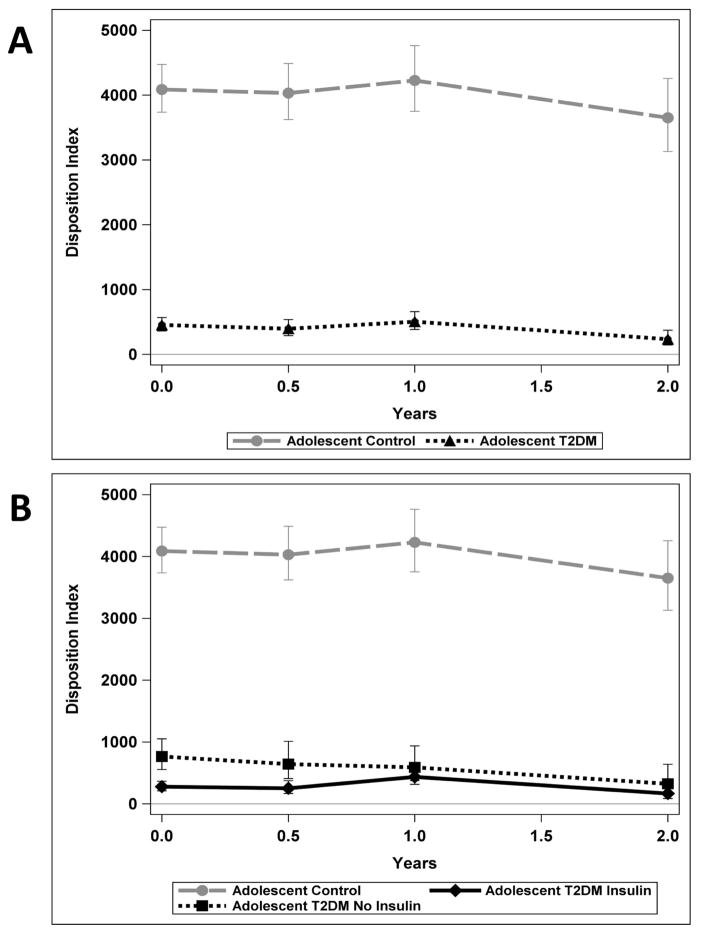
Metabolic variables for each group over the period of observation are shown in Table 2 and DI is also shown in Figure 1. DI for the two groups (adolescent control and T2DM) in which analyses were performed are shown in Figure 1A and then secondarily the adolescents with T2DM are visually portrayed as two groups based on baseline treatment with or without insulin in Figure 1B (3 groups total displayed).
Figure 1(A & B).
Metabolic testing over time of the Adolescent Controls (circles) and Adolescents with T2DM (triangles) for Disposition Index (DI) is shown in 1A. In 1B the Adolescents with T2DM are shown as 2 groups, T2DM adolescents with initial insulin treatment (diamonds) and adolescents with T2DM not on initial insulin treatment (squares).
At the initiation of the study, the adolescent T2DM subjects had significantly higher fasting glucose (p<0.0001) and HOMA-IR (p=0.01) levels than the adolescent controls, and substantially reduced AIRg (p<0.0001) and DI (p<0.0001). At baseline, the subjects within the T2DM adolescent group who required insulin treatment at the time of diagnosis had lower, AIRg (p=0.06, not significant) and DI (p=0.02) than the adolescents with T2DM not treated with insulin, consistent with our previous results (15).
In the adolescents with T2DM, fasting insulin (p=0.36), HOMA-IR (p=0.50), HbA1c (p=0.10), BMI (p=0.08) and AIRg (p=0.06) did not have a statistically significant change over the 2 years of follow-up. However, in this group fasting glucose did increase over the 2 years (p=0.04). DI was impaired at baseline and declined overall 25% per year over the 24 month study period. Among the adolescent controls there were significant decreases in fasting insulin (p=0.04) and AIRg (p=0.02). Within the adolescent control group, the roughly proportional changes in AIRg and HOMA-IR resulted in DI remaining stable with no statistically significant change over time (p=0.50).
Longitudinal examination of the adolescents with and without T2DM over two years
Difference in the rates of change of insulin secretion, glucose and BMI between the two adolescent groups was assessed by the interaction of time and group. DI (p=0.02) changed differently over time in the control adolescents compared to the T2DM adolescents (Table 2; Figure 1A). Specifically there were differences between the T2DM adolescents and control adolescents for DI at 6 months (p<0.0001), at year 1 (p<0.0001), and at 2 years (p<0.0001). There was no statistically significant difference in the rate of change over time between adolescent T2DM and adolescent controls for fasting glucose, fasting insulin, HOMA-IR, AIRg and BMI. Controlling for race or BMI did not alter conclusions drawn regarding any of the metabolic parameters.
Discussion
We present a prospective two year longitudinal assessment of β-cell function in adolescents with newly diagnosed T2DM demonstrating profound reduction of insulin secretion compared to adolescent controls that persisted over time despite standard treatment of hyperglycemia. Importantly, adolescents with newly diagnosed T2DM have severe β-cell dysfunction as represented by DI at ~10% of the level of weight-matched controls. Despite treatment with metformin or insulin, maintenance of glycemic parameters near target for diabetes and progression through late puberty, the adolescent T2DM group did not regain β-cell function, and had overall 25% per year decline in β-cell function over the entire first 2 years of treatment. These findings provide new insights into the pathogenesis of T2DM in adolescents, indicating that the impairment of insulin secretion in affected youth is neither a more modest version of the typical findings in adults, nor recoverable with standard treatment.
The adolescent control group displayed expected metabolic changes described with pubertal progression including improved insulin sensitivity and a compensatory decrease in AIRg resulting in the DI remaining relatively constant (17, 18). In comparison, the adolescents with T2DM, had significantly impaired β-cell function compared to the control group, as measured by AIRg and calculated DI, and persistent insulin resistance, independent of whether they received insulin or an oral anti-hyperglycemic agent.
It has been previously demonstrated that among adults with T2DM, progression of the disease involves worsening β-cell function with progressively worse compensation for insulin resistance (19). We observed a similar trend in our adolescents with T2DM. Previous studies of adolescents with diabetes also noted relatively rapid deterioration of β-cell function over time without significant changes of insulin sensitivity in the absence of weight or BMI change (3, 13, 20). After a median of 20 months since diabetes diagnosis, youth with T2DM lost on average 20% per year of their first-phase insulin response or c-peptide secretion measured with the hyperglycemic clamp (13). The TODAY study data demonstrated a 20–35% decline in β-cell function per year measured with the oral disposition index (12). In addition, the TODAY study reported the inability to stop or reverse this progressive deterioration in β-cell function (12), consistent with our data. Adult studies such as ADOPT (A Diabetes Outcome Progression Trial) reported rates of β-cell decline of 7–11% per year as measured by insulinogenic index, and in the UK Prospective Diabetes Study the estimated rate of decline was 7% per year using the HOMA-β index (19, 21).
Unlike other adolescent longitudinal studies, our report has a control group. Having an adolescent control group allows one to appreciate the profound difference in β-cell reserve between those with and without T2DM. Both the TODAY study and the smaller study by Bacha et al. describe a rate of decline of β-cell function of 12–20% after a diabetes diagnosis. We too document this decline without changes in BMI or insulin sensitivity. The majority of β-cell function decline was observed between year one and year two post-diabetes diagnosis. Whether these changes are clinically relevant when compared to non-diabetic obese adolescents is debatable. Regardless, there is no improvement in β-cell function despite standard medical management and the maintenance of good glycemic control. The IVGTT allows for the evaluation of AIR is a direct measure of the first phase insulin responce and not a surrogate. AIRg is also a sensitive marker for the development of T2DM since this function is lost early in the disease process (9). Our population included adolescents with diabetes who were treated with insulin and may be a more severely affected group. The use of HOMA modeling is not as precise a measure of insulin sensitivity, but is reproducible in children and adolescents (22) supporting use in longitudinal studies. As previously published (8), the combination of AIRg and HOMA-IR to estimate DI provided sufficient measure to allow assessment of β-cell function differences between the two study groups. This is consistent with other studies that used alternative methods to compute DI and found this to be predictive of β-cell failure in youth (12, 23).
There are several relevant limitations to our study. It is possible that exogenous insulin use contributed to the plasma measures, although there was no difference in fasting insulin values between the adolescents who received insulin and those who did not (p=0.78). Second, preservation of β-cell function has been described with insulin therapy (24) and could confound the comparison of insulin secretion between the adolescents. There was improvement in the progression of β-cell function between adolescents with T2DM given insulin and those treated with oral agents but this improvement was not sustained and the decline in β-cell function was equivalent from year 1 to year 2. Finally, our study may be limited in statistical power due to subject attrition. The retention percentage for the adolescents with T2DM was 62% after 24 months. This retention rate is in line with other publications following adolescents with T2DM longitudinally, specifically, the TODAY study which reported similar retention of 63–66% with their adolescent T2DM groups.
In summary, adolescents with newly diagnosed T2DM have profound impairment of β-cell function that does not recover over a two year observational period despite decent glycemic control and no significant change in fasting insulin and HOMA-IR. Despite the severe abnormalities at baseline, we documented an overall 25% decline per year of β-cell function over the 24 month study period. It would be important to assess the effects of improved insulin sensitivity (such as weight loss) on β-cell function in an adolescent T2DM group to help determine if the impairments of β-cell function are permanent.
Acknowledgments
We would like to thank Kay Ellis, Clinton Elfers and Brianne Reedy for their technical support of this study. In addition, we are grateful to the nurses at the Cincinnati Children’s Hospital CTRC for their skill in performing these studies. Declaration of Competing Interests of those acknowledged: Nothing to declare. Funding was primarily through a K23 Award to DE (NIH 5K23DK070775-03). This work has been presented at the American Diabetes Association 70th Scientific Session as an oral presentation.
Funding: NIH 5K23DK070775-03 (to DE) and R01DK57900 (to DD) and USPHS Grant #UL1 RR026314 from the National Center for Research Resources, NIH.
Abbreviations
- T2DM
Type 2 Diabetes Mellitus
- HOMA-IR
Homeostatic model assessment of insulin resistance
- I
Insulin
- AIRg
Acute insulin response to glucose
- DI
Disposition Index
- BMI
Body mass index
- HbA1c
Glycosylated hemoglobin percentage
- IQR
Interquartile range
Footnotes
Disclosure Statement: DE, LH, JK, DA have nothing to declare.
Author Contributions: DE wrote the original protocol, recruited subjects, performed procedures, participated in data analysis and wrote the manuscript. JK and LH performed the data analysis, production of tables and figures, contributed to discussion and extensively reviewed and edited the manuscript. DA reviewed and edited the original protocol, participated in data analysis, contributed to discussion and reviewed and edited the manuscript.
Implication and Contribution
This manuscript documents the severe impairment of β-cell function at diabetes presentation in the adolescent form of T2DM. Despite standard medical management, stable HbA1c%, BMI and measures of insulin sensitivity there was no significant improvement of β-cell function over this 2 year study period.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elder DA, Prigeon RL, Wadwa RP, Dolan LM, D’Alessio DA. Beta-cell function, insulin sensitivity, and glucose tolerance in obese diabetic and nondiabetic adolescents and young adults. J Clin Endocrinol Metab. 2006;91:185–91. doi: 10.1210/jc.2005-0853. [DOI] [PubMed] [Google Scholar]
- 2.Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R. Beta-cell function across the spectrum of glucose tolerance in obese youth. Diabetes. 2005;54:1735–43. doi: 10.2337/diabetes.54.6.1735. [DOI] [PubMed] [Google Scholar]
- 3.Gungor N, Arslanian S. Progressive beta cell failure in type 2 diabetes mellitus of youth. J Pediatr. 2004;144:656–9. doi: 10.1016/j.jpeds.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 4.van Haeften TW, Dubbeldam S, Zonderland ML, Erkelens DW. Insulin secretion in normal glucose-tolerant relatives of type 2 diabetic subjects. Assessments using hyperglycemic glucose clamps and oral glucose tolerance tests. Diabetes Care. 1998;21:278–82. doi: 10.2337/diacare.21.2.278. [DOI] [PubMed] [Google Scholar]
- 5.Burns N, Finucane FM, Hatunic M, Gilman M, Murphy M, Gasparro D, et al. Early-onset type 2 diabetes in obese white subjects is characterised by a marked defect in beta cell insulin secretion, severe insulin resistance and a lack of response to aerobic exercise training. Diabetologia. 2007;50:1500–8. doi: 10.1007/s00125-007-0655-7. [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study Group UK UK prospective diabetes study 16: overview of 6 years of theapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249–58. [PubMed] [Google Scholar]
- 7.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 8.Elder DA, Hornung LN, Herbers PM, Prigeon R, Woo JG, D’Alessio DA. Rapid deterioration of insulin secretion in obese adolescents preceding the onset of type 2 diabetes. J Pediatr. 2015;166:672–8. doi: 10.1016/j.jpeds.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn SE. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:4047–58. doi: 10.1210/jcem.86.9.7713. [DOI] [PubMed] [Google Scholar]
- 10.Ferrannini E, Mari A. Beta cell function and its relation to insulin action in humans: a critical appraisal. Diabetologia. 2004;47:943–56. doi: 10.1007/s00125-004-1381-z. [DOI] [PubMed] [Google Scholar]
- 11.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90:493–500. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]
- 12.Group TS. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes Care. 2013;36:1749–57. doi: 10.2337/dc12-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacha F, Gungor N, Lee S, Arslanian SA. Progressive deterioration of beta-cell function in obese youth with type 2 diabetes. Pediatr Diabetes. 2013;14:106–11. doi: 10.1111/j.1399-5448.2012.00915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes A. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 15.Elder DA, Herbers PM, Weis T, Standiford D, Woo JG, D’Alessio DA. beta-cell dysfunction in adolescents and adults with newly diagnosed type 2 diabetes mellitus. J Pediatr. 2012;160:904–10. doi: 10.1016/j.jpeds.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 17.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50:2444–50. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 18.Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res. 2006;60:759–63. doi: 10.1203/01.pdr.0000246097.73031.27. [DOI] [PubMed] [Google Scholar]
- 19.Viberti G, Kahn SE, Greene DA, Herman WH, Zinman B, Holman RR, et al. A diabetes outcome progression trial (ADOPT): an international multicenter study of the comparative efficacy of rosiglitazone, glyburide, and metformin in recently diagnosed type 2 diabetes. Diabetes Care. 2002;25:1737–43. doi: 10.2337/diacare.25.10.1737. [DOI] [PubMed] [Google Scholar]
- 20.Saad R, Gungor N, Arslanian S. Progression from normal glucose tolerance to type 2 diabetes in a young girl: longitudinal changes in insulin sensitivity and secretion assessed by the clamp technique and surrogate estimates. Pediatr Diabetes. 2005;6:95–9. doi: 10.1111/j.1399-543X.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabet Med. 1998;15:297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 22.Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care. 2004;27:314–9. doi: 10.2337/diacare.27.2.314. [DOI] [PubMed] [Google Scholar]
- 23.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32:335–41. doi: 10.2337/dc08-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chon S, Oh S, Kim SW, Kim JW, Kim YS, Woo JT. The effect of early insulin therapy on pancreatic beta-cell function and long-term glycemic control in newly diagnosed type 2 diabetic patients. Korean J Intern Med. 2010;25:273–81. doi: 10.3904/kjim.2010.25.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]