Abstract
Prenatal care providers are advised to evaluate maternal weight at each regularly scheduled prenatal visit, monitor progress toward meeting weight gain goals, and provide individualized counseling if significant deviations from a woman’s goals occur. Today, nearly 50% of women exceed their weight gain goals with overweight and obese women having the highest prevalence of excessive weight gain. Risks of inadequate weight gain include low birth weight and failure to initiate breastfeeding whereas the risks of excessive weight gain include cesarean deliveries and post-partum weight retention for the mother and large for gestational age infants, macrosomia, and childhood overweight or obesity for the offspring. Prenatal care providers have many resources and tools they can use to incorporate weight and other health behavior counseling into their routine prenatal practices. Because many women are motivated to improve their health behaviors, pregnancy is often considered the optimal time to intervene for issues related to eating habits and physical activity so as to prevent excessive weight gain. Gestational weight gain is a potentially modifiable risk factor for a number of adverse maternal and neonatal outcomes and meta-analyses of randomized controlled trials report that diet or exercise interventions during pregnancy can help reduce excessive weight gain. However, health behavior interventions for gestational weight gain have not significantly improved other maternal and neonatal outcomes and have limited effectiveness in overweight and obese women.
Keywords: Gestational weight gain, health behavior interventions, motivational interviewing, pregnancy, perinatal outcomes
Introduction
“How much weight should I gain this pregnancy?” is a question that many women ask their prenatal care providers today. The answer to this important question has changed dramatically over the past century. In the 1950s, one recommendation was for women to limit their weight gain to 10–14 pounds so as to avoid complications such “toxemia” at a time when 10% of women with eclampsia died.1 In recognition of the high neonatal and infant mortality rates in the US during the 1960s, the Committee on Maternal Nutrition highlighted the positive association between weight gain and birth weight and increased the weight gain goal to 20–25 pounds for all women.2 The 1990 “Nutrition During Pregnancy” document also supported increased weight gain so as to optimize birth weight, but also provided specific recommendations according to a woman’s pre-pregnancy weight and restricted weight gain in women with higher pre-pregnancy weights.3 Now, amidst an obesity epidemic, the focus is on meeting, not exceeding the weight gain goals so as to avoid complications such as cesarean delivery and macrosomia.4
This article reviews weight gain goals, the risks of not meeting weight goals, and health behavior management options to achieve optimal weight gain in pregnancy. Regardless of a woman’s pre-pregnancy weight, the American Congress of Obstetricians and Gynecologists (ACOG) currently recommends that providers measure a woman’s height and weight at the first prenatal visit in order to calculate a body mass index (BMI) and then counsel women on gestational weight gain (GWG) goals and the need to limit excessive GWG to achieve optimal pregnancy outcomes.5 According to the “Guidelines for Perinatal Care,” health care providers should also evaluate maternal weight at each regularly scheduled visit, monitor progress toward meeting GWG goals, and provide specific individualized counseling if significant deviations from goals occur.6
How is Gestational Weight Gain Measured and Assessed?
Ideally, total GWG is calculated as the difference between the weight at the first and last prenatal visit just prior to delivery. Yet in practice, often times these measurements vary such that a self-reported pre-pregnancy weight is used to calculate total GWG. Additional challenges arise when prenatal care begins after the first trimester. In these situations, one recommendation is to use the self-reported pre-pregnancy weight to calculate both total GWG and GWG goals. Although women typically underestimate their weight by 5 pounds, >80% of women remained in the same BMI category in one study of self-reported weight in reproductive age women.7 In another study that evaluated GWG from a self-reported pre-pregnancy and a measured first trimester weight value, the proportions of women gaining below, at, and above the guidelines was the same from either approach.8 Use of self-reported weight introduces bias into GWG measurements, but it appears to be a small difference and no other practical solutions are available. In terms of the final pregnancy weight measurement, women can also be re-weighed at the time of delivery, especially if several weeks have passed since a weight measurement. In general, all weight and height measurements should be performed in light clothing without shoes. In epidemiological studies that examine the relationship between GWG and pregnancy outcomes, typical measurements of GWG include the total value (e.g., kg or lbs.), an average rate in the 2nd or 3rd trimester (e.g., kg or lbs. /week), or an adequacy ratio [ratio of total observed GWG to the Institute of Medicine (IOM) guidelines based on the pre-pregnancy BMI and gestational age at delivery].
The routine measurement of weight during pregnancy in the United States is in contrast to the prenatal care practices in other developed countries whose national guidelines do not recommend routine weighing during pregnancy.9–11 For example, the National Institute for Health and Clinical Excellence (NICE) guidelines in the United Kingdom do not recommend repeated weighing during pregnancy as a matter of routine, unless clinical management can be influenced or if nutrition is a concern.9 Furthermore, there are no national guidelines for GWG in the United Kingdom. Routine weighing is not only considered an acceptable practice in the United States, it is inexpensive, widely available, and perhaps the first opportunity for providers to start the discussion of healthy behaviors and GWG and their relationships to pregnancy outcomes.
What are the Gestational Weight Gain Guidelines?
The current GWG guidelines are based on the IOM 2009 document, “Weight Gain during Pregnancy – Reexamining the Guidelines.”(Table 1)4 The primary differences between the initial IOM 1990 guidelines and the current ones are (1) the use of World Health Organization (WHO) categories instead of the Metropolitan Life Insurance Company’s ideal weight-for-height standards for the BMI categories, (2) ranges of GWG rates for the second and third trimesters, and (3) specific goals for women with a pre-pregnancy BMI ≥ 30 kg/m2. The goals for women with obesity are now 11–20 lbs. instead of “at least 15 pounds”. The GWG guidelines for twin gestations are considered provisional because the evidence to support them was more limited compared to singletons. (Table 2) In addition, there are no specific guidelines for other subpopulations such as adolescents, women of short stature, racial-ethnic minorities, and women with higher classes of obesity. Of note, the guidelines are based on observational data of associations between GWG and maternal and neonatal outcomes. For twin gestations, the guidelines reflect the 25–75th% interquartile range of cumulative weight gain among women who delivered twins weighing ≥ 2500 g at 37–42 weeks. The guidelines also assume that all women gain 1.1–4.4 lbs. (0.5–2 kg) in the first trimester.
Table 1.
2009 Gestational weight gain guidelines (Institute of Medicine, 2009)4
| Pre-pregnancy BMI | Total weight gain at term | Rate of weight gain in the 2nd and 3rd trimester; Mean (range) |
|---|---|---|
|
| ||
| Underweight | 12.5–18 kg | 0.51 (0.44–0.58) kg/week |
| (<18.5 kg/m2) | 28–40 lbs. | 1 (1–1.3) lbs./week |
|
| ||
| Normal weight | 11.5–16 kg | 0.42 (0.35–0.50) kg/week |
| (18.5–24.9 kg/m2) | 25–35 lbs. | 1 (0.8–1) lbs./week |
|
| ||
| Overweight | 7–11.5 kg | 0.28 (0.23–0.33) kg/week |
| (25.0–29.9 kg/m2) | 15–25 lbs. | 0.6 (0.5–0.7) lbs./week |
|
| ||
| Obese | 5–9 kg | 0.22 (0.17–0.27) kg/week |
| (≥ 30.0 kg/m2) | 11–20 lbs. | 0.5 (0.4–0.6) lbs./week |
BMI: body mass index
Table 2.
| Pre-pregnancy BMI | Total weight gain (pounds) | Total weight gain (kg) |
|---|---|---|
| Underweight(<18.5 kg/m2) | Insufficient information | Insufficient information |
| Normal weight(18.5–24.9 kg/m2) | 17–25 | 37–54 |
| Overweight(25.0–29.9 kg/m2) | 14–23 | 31–50 |
| Obese(≥ 30.0 kg/m2) | 11–19 | 25–42 |
Guidelines for twin gestations are considered provisional and based upon the interquartile (25th to 75th percentiles) range of cumulative weight gain among women who delivered twins who weighed ≥ 2,500 g at 37–42 weeks gestation.60
What are the Components of Weight Gain?
Energy intake and energy expenditure typically determine energy balance. Energy requirements increase in pregnancy by approximately 200, 300, and 400 kcal/day in the 1st, 2nd, and 3rd trimesters, respectively, but these values vary depending on the BMI, as determined by studies that evaluate basal metabolic rate by calorimetry, total energy expenditure by doubly labeled water, and individual physical activity.12,13 Furthermore, a recent systematic review of energy intake and GWG suggests that women report smaller daily increases in caloric intake (475 kJ/day or 113kcal/day) during pregnancy and propose that the current guidelines may be promoting excessive GWG.14 In a typical pregnancy characterized by 25 pounds or 11 kg total GWG and delivery at 40 weeks, the products of conception (placenta, fetus, amniotic fluid) comprise approximately 35% of the total GWG.15 In general, GWG is comprised of water, protein, or fat in the fetus, placenta, uterus, and amniotic fluid, maternal blood volume, mammary gland, and maternal adipose tissue. The minimal amount of GWG required for fetal growth and deposits of maternal energy for postpartum lactation is estimated at 8 kg. The first trimester weight gain of 1.1–4.4 lbs. is attributed to early placental development and expansion of maternal blood volume, not fat deposits. Fetal growth is nearly uniform until the mid-portion of the second trimester regardless of age, race, and fetal sex, but then other factors including GWG account for the final determinant of birthweight.
Epidemiology and Trends in Gestational Weight Gain in the United States
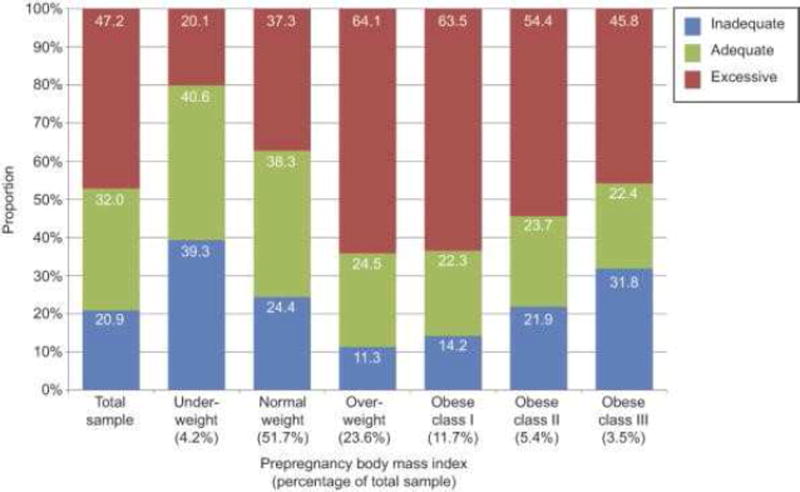
Deputy et al reported on GWG adequacy in a study using the Pregnancy Risk Assessment Monitoring System (PRAMS) for women who had full-term singleton deliveries from 28 states. Pre-pregnancy BMI was a self-reported value and total GWG was obtained from the birth certificate files. After weighting, the final sample size represented approximately 30% of births in the United States from 2010–2011. In their analysis, 20.9% and 47.2% of all women had inadequate or excessive GWG, respectively.16 (Figure 1) Overweight and obese class I (BMI 30–34.9 kg/m2) women had the highest prevalence of excessive GWG (64.1% and 63.5%, respectively). Although underweight women were least likely to exceed GWG goals (aOR 0.50, 95%CI 0.40–0.61), class II and III obese women had higher odds for both excessive GWG (aOR 2.31, 95%CI 1.94–2.75; aOR 2.07, 95%CI 1.63–2.62, respectively) and inadequate GWG (aOR 1.25, 95% CI 1.01–1.55; aOR 1.86, 95% CI 1.45–2.36, respectively) compared to normal weight women. Given that the prevalence of overweight and obese women aged 20–39 years is 58.5% according to NHANES data from 2011–2012 and that overweight and obese women have the highest prevalence of excessive GWG, the trends in excessive GWG are predicted to continue.17
Figure 1.
Prevalence of Gestational Weight Gain Adequacy by Pre-pregnancy Body Mass Index.16
There are known racial-ethnic variances in weight among reproductive age women with the highest prevalence of overweight among non-Hispanic blacks (80.0%) followed by Hispanics (69.5%), whites (55%) and Asians (26.2%), also according to NHANES data from 2011–2012.17 Although minority women are more likely to enter a pregnancy as overweight or obese compared to whites, in general, Hispanic and black women are more likely to have inadequate GWG and less likely to have excessive GWG compared to whites.18–20 For example, in a study of birth records from 2007–2010 in Colorado, Hispanic and black women had an increased odds for inadequate GWG (aOR 1.08, 95% CI 1.04–1.11; aOR 1.12, 95% CI 1.06–1.19, respectively) and decreased odds for excessive GWG (aOR 0.84, 95% CI 0.81–0.86; aOR 0.96, 95% CI 0.90–1.00, respectively) compared to non-Hispanic whites.21 Deputy et al also examined racial-ethnic associations with GWG, but noted that the odds varied by BMI. For example, the odds for inadequate GWG were primarily found in black, Hispanic, and Asian normal weight women (aOR 1.45, 95%CI 1.19–1.77; aOR 1.29, 95%CI 1.07–1.56; aOR 1.31, 95% CI 1.08–1.58, respectively) compared to normal weight white women, but not in other BMI categories.16
In terms of parity, nulliparas typically gain more weight than multiparas. For example, normal weight, overweight, and obese nulliparas had higher odds for excessive GWG compared to multiparas (aOR 1.31, 95% CI 1.18–1.46, aOR 1.24, 95% CI 1.04–1.48, aOR 1.26, 95% CI 1.03–1.54).16 WIC or Medicaid enrollment, first trimester prenatal care, alcohol consumption during pregnancy, pre-pregnancy depression, and partner abuse were not associated with excessive GWG in any BMI category in the same study.16 The IOM conceptual framework for the GWG guidelines includes other social, environmental, and maternal factors, many of which have not been studied or inadequately studied, as potentially associated with either inadequate or excessive GWG.4
Risks of Inadequate and Excessive Weight Gain
The initial IOM 1990 guidelines for GWG (“Nutrition During Pregnancy”) emphasized the importance of meeting the GWG goals so as to prevent the well-described association between inadequate GWG and small for gestational age infants with relative risks approaching 2–3.3 Several epidemiologic studies consistently show a linear and direct relationship between GWG and fetal growth; however, other factors including maternal BMI influence this relationship.22 There are also well-described associations between inadequate weight gain and perinatal mortality. In a study of 100,000 records from the National Center for Health Statistics from linked birth/infant death data files in 2002, infants born to women with inadequate weight gain had an increased odds for infant death up to one year after birth (OR 2.23, 95% CI 1.84–2.70) compared to women with normal weight gain.23 Other reported risks of inadequate GWG include a failure to initiate breastfeeding; however the relationship between low GWG and preterm birth is mixed.4 Part of the difficulty in studying the association between preterm birth and GWG is that the total GWG of a term gestation cannot be compared with that of a preterm birth, thus GWG rates or adequacy ratios are used instead. The relationship between GWG and preterm birth has been summarized as having a U-shaped distribution with inadequate and excessive GWG increasing the risk of preterm birth among underweight and normal weight women.4
In the two decades since the first IOM GWG guidelines were written, several changes occurred in the demographic of women in the United States. This includes women having pregnancies at older ages, more frequent occurrences of medical complications such as chronic hypertension and pre-gestational diabetes, greater racial-ethnic diversity, increasing GWG overall regardless of the pre-pregnancy BMI, and the national epidemic of obesity. As such, the updated guidelines now focus on meeting, but not exceeding the goals due to the risks of excessive GWG. The maternal risks of excessive GWG include cesarean deliveries and post-partum weight retention.4 Associations between excessive GWG and gestational diabetes and preeclampsia have been reported, but the evidence for these associations is more limited. Other proposed long-term metabolic consequences of excessive GWG for women include type 2 diabetes, cardiovascular disease, and metabolic syndrome.24 Most importantly, the weight gain that occurs during the first pregnancy, especially if it is excessive and not lost after delivery, is likely to influence future pregnancy outcomes. In this sense, it is important to target nulliparas in our counseling about avoiding excessive GWG.
The neonatal risks of excessive GWG include large for gestational age infants and macrosomia. Other reported risks of excessive GWG include low five-minute Apgar scores, seizures, hypoglycemia, polycythemia, and meconium aspiration syndrome.25,26 Of greater concern are the reported associations between excessive GWG and long-term outcomes such as childhood overweight or obesity.27 Several theories also suggest that in utero nutrition may impact chronic diseases such as diabetes, hypertension, and other metabolic diseases later in life in the offspring. This research is evolving, but providers should consider that maternal nutrition during pregnancy may have life-long consequences for the offspring including neurocognitive outcomes.28–30
Because overweight and obese women have the greatest prevalence of excessive GWG and already enter a pregnancy at risk for adverse outcomes, we may assume that it is most important for these women to meet the GWG goals. However, women of all BMI categories encounter excess morbidity when GWG is excessive. Swank et al evaluated the categorical change in BMI as a function of pre-pregnancy BMI and several pregnancy outcomes from a database of women who delivered singleton gestations in California. Although overweight women had the highest odds of developing gestational hypertension or preeclampsia with excessive change in BMI, normal weight women also had a nearly three-fold increased risk for gestational hypertension or preeclampsia compared to women with no change in BMI.31 In similar types of analysis from the same database in California, the adjusted odds for cesarean delivery and macrosomia (birthweight >4000g) also increased as change in BMI increased for all BMI categories.32,33 Of note, normal weight women had the greatest odds for macrosomia with excessive change in BMI (aOR 3.85, 95% CI 3.59–4.13) and a greater than two-fold odds for cesarean delivery compared to no change in BMI.32,33
It is difficult to discern which factor, pre-pregnancy BMI or GWG is associated with greater risk for adverse outcomes when excessive GWG occurs in women with obesity. For example, pre-pregnancy obesity increases the risks for preeclampsia, gestational diabetes, cesarean deliveries, and abnormal fetal growth.34 Excessive GWG also is associated with the same risks. Magriples et al studied the relationship between BMI and GWG on birth outcomes in low-risk women who were 14–25 years old, and predominantly nulliparous and non-Hispanic black. In multivariate analysis, pre-pregnancy BMI was a greater predictor of cesarean delivery than GWG (aOR for cesarean delivery in obese women 2.30, 95% CI 1.48–3.58 and aOR for cesarean delivery with excessive GWG 1.51, 95% CI 0.98–2.33).35 The interaction of pre-pregnancy weight and GWG was examined by Abrams and Laros from deliveries in the 1980’s whereby low GWG was defined as <7 kg and excessive GWG defined as >20kg. There was a progressively stronger correlation between GWG and birthweight in underweight (pre-pregnancy BMI <18 kg/m2), ideal weight (pre-pregnancy BMI 19–22 kg/m2), and moderately overweight women (pre-pregnancy BMI 23–28 kg/m2). In contrast, for women with a pre-pregnancy BMI > 28 kg/m2, there was no correlation between GWG and birthweight, suggesting that pre-pregnancy weight was a stronger predictor of birth weight than GWG at higher BMIs.36 Knowing the relationships between pre-pregnancy BMI, GWG, and pregnancy outcomes would not only better inform our GWG guidelines, but also help design optimal interventions for GWG according to a woman’s BMI category.
Is Weight Gain Less than the Guidelines Appropriate for Women with a Higher BMI?
Conventional wisdom is that pregnancy is a time for weight gain so as to meet maternal and fetal needs. The current GWG guidelines have one range of values for all women with a pre-pregnancy BMI > 30 kg/m2 suggesting that a women with a BMI of 30 kg/m2 should gain the same weight as one with a BMI of 50 kg/m2. Several observational studies, most of which were published since 2009, describe improved maternal outcomes such as cesarean deliveries, preeclampsia, and operative vaginal deliveries, in obese women who either gain less than the GWG guidelines or who lose weight during a pregnancy.37–44 However, in some, but not all studies, inadequate GWG (as defined by the 2009 IOM criteria or < 15 lbs. weight gain by the 1990 IOM criteria) or weight loss has been associated with a higher proportion of low birth weight and small for gestational age infants than for women with adequate GWG, though in adjusted analyses the risks were either attenuated or there were no differences in birth weight outcomes in women with GWG lower than the guidelines. 8,45,46 The higher proportions of low birth weight in women with inadequate GWG compared to women with adequate GWG may be attributed to obese women having lower occurrences of low birth weight than normal weight women. In one study from the Swedish Medical Birth Registry, the occurrence of small for gestational age infants in obese women who lost weight (1.7–3.8%) was lower or equal to values typically reported in Sweden (3.6%).42 Additional studies have shown decreased neonatal fat mass, lean mass, and head circumference in overweight or obese women who gained ≤ 5 kg.47 In the majority of these studies, we do not know the circumstances of the weight loss, i.e., whether it was accompanied by maternal illness, a result of intentional dieting, or attributed to improved health behaviors during pregnancy. As noted in Deputy et al, women with class II and III obesity are at greater risk for inadequate GWG and it is possible that weight loss partially contributes to this finding. Given the lack of high-level evidence to support low GWG or weight loss during pregnancy, until further studies are available, women with a pre-pregnancy BMI ≥ 30 kg/m2 should aim to gain a total of 11–20 pounds during pregnancy.
Practical Approaches to Assist Providers and Patients in Meeting Gestational Weight Gain Goals
Motivational interviewing
The ACOG recommends motivational interviewing, a patient-centered counseling style for eliciting behavior change by having patients explore and resolve ambivalence about behavior change, as an approach to achieve positive health outcomes for patients who have alcohol, tobacco, or weight management issues.48 In contrast to typical provider-patient interactions whereby sound and logical advice is given and often resisted, motivational interviewing aims to help patients identify the thoughts and feelings that cause them to continue “unhealthy” behaviors and help them develop new thought patterns to assist in behavior change. The four core skills with motivational interviewing include asking open-ended questions, affirmations, reflections, and summaries. For example, instead of a provider stating, “At only 32 weeks, you have exceeded your weight gain goals. This increases your risk for cesarean delivery and high birthweight,” an alternate approach to the problem is to say, “I notice your weight gain is high. Tell me about your diet and exercise this pregnancy.” Additional examples of core motivational interviewing skills are given in Table 3. Studies show that when a patient is allowed to talk and the provider actively listens and reflects back to the patient what they hear, no more than 3 minutes are added to the encounter.49 There is also evidence to support the efficacy of motivational interviewing in GWG interventions. A systematic review and meta-analysis of trials designed to limit GWG evaluated the theoretical and intervention components of each trial. Of the trials that had a theory for the behavior change, motivational interviewing was cited as one of four strategies (also among provision of information, behavioral self-monitoring, and provision of rewards contingent on successful behavior) as key for successful GWG interventions.50 Resources for providers to learn more about motivational interviewing include textbooks, videos, journal articles, and workshops.(Reference list below)
Table 3.
Examples on how to use Motivational Interviewing Core Skills with Gestational Weight Gain76
| Core Skill | Definition | Example |
|---|---|---|
|
| ||
| Open-ended questions | Open-ended questions keep the conversation going. One word answers such as “yes” or “no” are not possible. The question explores ambivalence (about GWG) and further analyzes the discrepancy (about meeting or not meeting GWG goals). | What concerns you about your weight gain? |
| Has your weight gain caused any problems this pregnancy? | ||
| Tell me about your family member’s/friend’s experience with weight gain in her pregnancy. | ||
| What eating habits or physical activities work well for you? | ||
| How do you think your family/friends hinder your progress in meeting your weight gain goals? | ||
|
| ||
| Affirmations | Affirmations are statements that acknowledge effort, values, skills, and strengths. They accentuate the positive aspects of the goal behavior. | You really care about your health and your fetus’ health. |
| Despite all the changes going on during this pregnancy, you are still finding time to exercise. | ||
| Despite feeling discouraged about your weight gain, you aren’t giving up. | ||
| That must have been a challenge… | ||
|
| ||
| Reflections | Simple reflections let the patient know that you understood what they said. | You’re not concerned about your weight gain. |
| You’re frustrated because your weight gain is higher/lower than it should be. | ||
| Double sided reflections state both sides of the argument that the patient makes. | On the one hand you are more tired this pregnancy and need to rest, but on the other hand you want to stay active so that you remain healthy too. | |
| Reflections that change focus alter the topic from one that the patient is unwilling to discuss at that time to something else. | This is not something you want to talk about right now. Do you want to talk about other things in your life that are interfering with your goals? | |
|
| ||
| Summaries | Summarizing highlights the most important parts of the conversation. | You mentioned that you…as well as… So, this is what you’ve said so far… What do you think you want to do? Did I leave anything out? |
Dispelling myths
Many women believe that pregnancy means “eating for two,” and consequently they nearly double their caloric intake. There are several online resources that can assist providers in determining daily calories during pregnancy. One example is the calculator at from the Pennington Biomedical Research Center (www.pbrc.edu/research-and-faculty/calculators/gestational-weight-gain/) which estimates energy needs during each trimester required to achieve GWG goals. The only required input variables are maternal age, height, and weight at conception. Calories and GWG goals are presented on the same template and women can use this visual tool for caloric intake guidance.
Women typically decrease their activity levels during pregnancy. The ACOG recommends 30 minutes or more of moderate exercise per day on most, if not all, days of the week for pregnant women, in the absence of medical or obstetric contraindications.51 Women can continue to derive the health-related benefits of exercise (e.g., decreased risk of cardiovascular disease and diabetes) during pregnancy. Providers can counsel women that it is safe to begin or continue most forms of exercise, although they may need to adjust their regimens to avoid dehydration and fatigue, especially in the third trimester. Furthermore, a recent systemic review and meta-analysis of RCTs of exercise in women of normal weight showed that occurrences of preterm birth were similar (4.5% vs. 4.4%, RR 1.01, 95%CI 0.68–1.50) in the arm exposed to 35–90 minutes 3–4 times per week of aerobic exercise compared to the control arm. More importantly, women in the exercise arm had higher occurrences of vaginal delivery and lower occurrences of gestational diabetes, and no differences in low birthweight, thus giving providers and patients additional evidence on the safety and benefits of exercise during pregnancy.52
Goal setting, GWG plots, and Electronic Medical Records
Goals can be motivational and goal setting has shown promise in promoting diet and physical activity behavior change in non-pregnant individuals. The overall strategy of goal setting has also been reviewed in the context of health behavior interventions that aimed to prevent excessive GWG through modification of diet and/or physical activity. Among interventions reporting positive results for GWG, a combination of individualized diet and physical activity goals, self-monitoring, and performance feedback indicators were active components of the intervention.53 Using this information, providers can set activity goals for their patients by recommending 30 minutes of daily physical activity, encouraging women to monitor their own activity with logs or step counts calculated by pedometers or activity monitoring devices, and then giving them feedback on their progress.
With respect to GWG, prenatal care providers should know the GWG goals and be clear in their communication about GWG goals so that women know the expectations and that they do not vary from provider to provider at different prenatal care visits. For many women, a plot of GWG over successive prenatal visits with the upper and lower boundaries formed by the 2009 IOM guidelines may provide a useful visual for their GWG progress. Many electronic medical records (EMR) have the capability of creating these graphs specific to each woman’s BMI, but paper versions are also accessible. (http://www.cdph.ca.gov/pubsforms/forms/Pages/MaternalandChildHealth.aspx) These graphs also highlight that a smaller amount of weight gain occurs in the first trimester and that weight gain increases at a greater rate in the second and third trimesters.
Lindberg et al tested the effectiveness of an intervention to improve the consistency and accuracy of GWG counseling with “best practice alerts” built into an EMR. The alert provided individualized total GWG goals, GWG goals per week of gestation, a scripted template for provider counseling and documentation in a “smart set”, and a patient education handout. According to a retrospective chart review before and after the intervention, the rate of GWG counseling in agreement with the 2009 IOM guidelines improved (2.6% before vs. 51% after, p<0.001) among obstetrician-gynecologists, family medicine physicians, and certified nurse midwives.54 These results were encouraging, but additional research is needed to determine how interfaces with the EMR and GWG counseling can translate into improved patient outcomes.
Problem list
Providers often hesitate to identify weight as a problem. Like blood pressure, weight is a data point collected at nearly every prenatal visit, and it should not be neglected. As an example, listing obesity as a problem in the medical record has been shown to significantly increase the likelihood (92.9% versus 56.6%, P<0.001) that the provider will take action at follow-up visits.55 The same may also be true for low or excessive GWG, both of which have ICD-10 codes for documentation and billing purposes.
Multidisciplinary approach
Weight management outside of pregnancy typically involves a multidisciplinary approach with teams of nutritionists, exercise physiologists, medical subspecialists, and health coaches.56 A similar approach is recommended in pregnancy with nutritional advice offered to all women and additional counseling provided when GWG is low or excessive, where these resources are available.
Health Behavior and Lifestyle Interventions for Gestational Weight Gain
Pregnancy is a time when women may be motivated to improve their health behaviors, it is often considered the optimal time to intervene not only for issues related to substance use such as tobacco and alcohol cessation, but also related to eating habits and physical activity so as to prevent excessive GWG. GWG is a potentially modifiable risk factor for a number of adverse maternal and neonatal outcomes. Pregnancy is not a time for weight loss medications or bariatric surgery, so the emphasis of interventions to date are on health behaviors and lifestyle changes such as dietary control and exercise.
Depending on the individual circumstances, the improvements in health behaviors including diet and exercise habits are paramount to achieving GWG goals and deserve greater emphasis during pregnancy as weight gain may have several components and may not be equivalent among women who consume the same diet. Nonetheless, several randomized controlled trials of enhanced GWG counseling, special diets (e.g., low-glycemic index, diabetic, low-calorie, low-fat, low-carbohydrate) and exercise (e.g., walking, dancing, aerobics) or both dietary and exercise combinations have been reported either in individual or group sessions with varying doses of intervention. The most recent Cochrane review on the topic of the effectiveness and safety of diet or exercise or both interventions for preventing excessive GWG describes 49 randomized controlled trials (RCTs) with 11,444 women. Based on high-quality evidence, diet or exercise reduced the frequency of excessive GWG by 20% (RR 0.8, 95% CI 0.73–0.87), while only minimally increasing the risk for inadequate GWG (RR 1.14, 95% CI 1.02–1.27) based on moderate-quality evidence.57
Criticisms of health behavior interventions for GWG include attenuated effects in overweight and obese women and that many women still exceed the GWG in these RCTs. For example, in a RCT of a low-glycemic index diet during pregnancy to prevent macrosomia as the primary outcome, normal weight women had a reduction in excessive GWG (15% vs. 26%, p=0.02), yet obese women did not experience the same benefit (60% vs. 57%, p=0.8) compared to the control group.58 Unfortunately, most of the health behavior interventions either designed specifically for overweight or obese women or that included overweight and obese women in the study design among women of all BMI categories have not demonstrated a significant impact on GWG.58–67 Notable exceptions include two more recent RCTs. The LiP (Lifestyle in Pregnancy) study offered dietary guidance, free membership in a fitness center, and personal coaching. Obese women had reduced mean GWG compared to the control group (7.0 vs. 8.6 kg, p=0.01), but there were no differences in excessive GWG (35.4% vs. 46.6%, p=0.06) and the infants in the intervention group had higher birth weights.68 The Treatment of Obese Pregnant Women (TOP) study randomized obese women to a physical activity intervention with or without a dietary intervention and found that the physical activity intervention decreased GWG by a mean of 1.38 kg (p=0.04) in a multivariate analysis and that women were more likely to meet GWG goals (49–55% intervention arms vs. 37% control, p=0.01).69
It is hypothesized that health behavior interventions in overweight or obese women are less likely to influence GWG due to the physiological adaptations that occur in a normal pregnancy (i.e., decreased insulin sensitivity), the short time period between initiation of lifestyle changes and delivery, and difficulties in significantly increasing physical activity as pregnancy progresses.70 It is also possible that overweight or obese women require a more intensive or higher dose of a health behavior program to influence GWG or other pregnancy outcomes compared to normal weight women. The health behavior or lifestyle changes need to occur earlier for overweight and obese women, ideally prior to pregnancy and in conjunction with weight loss prior to pregnancy in order to achieve both GWG goals and optimal pregnancy outcomes.
When the results of multiple health behavior intervention studies have been examined cumulatively in meta-analyses, the interventions have not significantly influenced other perinatal outcomes such as such as cesarean delivery, preterm birth, macrosomia, shoulder dystocia, hypoglycemia, hyperbilirubinemia, and birth trauma.57,71–74 The limitations of these trials point to the need for more research.75 If the efforts of intensive health behavior interventions during pregnancy can improve GWG, yet have no influence on other perinatal outcomes, then another causative link needs to be evaluated to justify ongoing efforts to control GWG. However, long-term outcomes such as maternal weight 5 or 10 years after participating in an intervention or adult-onset outcomes of the offspring are needed to evaluate whether benefits really exist.
Conclusion
Significant changes have occurred in the guidelines for GWG goals over the past century. GWG remains a complex, yet critical variable in prenatal care management as it is one of the few modifiable risk factors for adverse perinatal outcomes and aberrations have the potential to influence short and long-term maternal and offspring health. Given the increasing prevalence and negative consequences of excessive GWG, preventing excessive GWG is becoming increasingly important for prenatal care providers and patients. Several studies regarding GWG and perinatal outcomes have been published since the 2009 IOM guidelines and it is likely that this evidence will be systematically reviewed and updated guidelines provided, especially for high risk women such as those with obesity. Future research regarding GWG should include measurements of psychosocial determinants of GWG along with the more commonly measured nutrition and exercise variables. The design of health behavior interventions for overweight or obese women deserves greater focus with respect to their content and timing so as to improve GWG and other outcomes in these women and their offspring.
Acknowledgments
This article was supported by Grant Number K23HD076010 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health.(MAK) The funding source had no involvement in the literature review, writing the report, or decision to submit for publication
References for Motivational Interviewing
Books
- Miller WR, Rollnick S. Motivational interviewing: preparing people for change. 2. New York (NY): Guilford Press; 2002. [Google Scholar]
- Rollnick S, Miller WR, Butler C. Motivational interviewing in health care: helping patients change behavior. New York (NY): Guilford Press; 2008. [Google Scholar]
Videos
- Miller WR. Motivational interviewing. Albuquerque (NM): University of New Mexico; 1989. Available from the author at the Department of Psychology, University of New Mexico, Albuquerque, NM, 87131, (505) 277-4121. [Google Scholar]
- Hester RK, Handmaker NS. Motivating pregnant women to stop drinking. Albuquerque (NM): Behavior Therapy Associates; 1997. Available from Behavior Therapy Associates, 9426 Indian School Road NE, Suite 1, Albuquerque, NM, 87112, (505) 345-6100. [Google Scholar]
Online
- http://www.motivationalinterviewing.org/about_mint Motivational Interviewing Network of Trainers (MINT) is an international organization of trainers in motivational interviewing. Their central interest is to improve the quality and effectiveness of counseling and consultations with clients about behavior change. There is a calendar of available 2 day training sessions, some of which are offered on-line.
- http://www.healthsciences.org/Motivational-Interviewing-Health-Coach-Training Health Sciences Institute Motivational Interviewing Training for Health Care
Journals
- Burton AM, Agne AA, Lehr SM, Davis NJ, Willett LL, Cherrington AL. Training residents in obesity counseling: incorporating principles of motivational interviewing to enhance patient centeredness. J Grad Med Educ. 2011;3(3):408–411. doi: 10.4300/JGME-03-03-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen K, Humaidan P, Sorensen LH, Alsbjerg B, Ravn P. Motivational interviewing: a part of the weight loss program for overweight and obese women prior to fertility treatment. Gynecol Endocrinol. 2013;29(9):839–842. doi: 10.3109/09513590.2013.808326. [DOI] [PubMed] [Google Scholar]
- Lindhardt CL, Rubak S, Mogensen O, et al. Healthcare professionals experience with motivational interviewing in their encounter with obese pregnant women. Midwifery. 2015;31(7):678–684. doi: 10.1016/j.midw.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Rollnick S, Butler CC, Kinnersley P, Gregory J, Mash B. Motivational interviewing. BMJ. 2010;340:c1900. doi: 10.1136/bmj.c1900. [DOI] [PubMed] [Google Scholar]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
References
- 1.Ferguson JH, Keaton AG. Special leaflets for use in controlling toxemia and excessive weight gain in pregnancy. Am J Public Health Nations Health. 1950;40(2):194–200. doi: 10.2105/ajph.40.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Research Council (U.S.) Maternal nutrition and the course of pregnancy. Washington: National Academy of Sciences; 1970. Committee on Maternal Nutrition. [Google Scholar]
- 3.Institute of Medicine. Nutrition during pregnancy. Washington, DC: 1990. [Google Scholar]
- 4.Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: 2009. [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists. ACOG Committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol. 2013;121(1):210–212. doi: 10.1097/01.aog.0000425668.87506.4c. [DOI] [PubMed] [Google Scholar]
- 6.Guidelines for perinatal care. 7. Washington, D.C: 2012. American Academy of Pediatrics, American College of Obstetricians and Gynecologists. [Google Scholar]
- 7.Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern Child Health J. 2007;11(2):137–144. doi: 10.1007/s10995-006-0157-0. [DOI] [PubMed] [Google Scholar]
- 8.Johnson J, Clifton RG, Roberts JM, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obstet Gynecol. 2013;121(5):969–975. doi: 10.1097/AOG.0b013e31828aea03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute for Health and Clinical Excellence. Weight management before, during, and after pregnancy. NICE public health guidance. 2010 Jul;:27. [Google Scholar]
- 10.Institute of Obstetricians and Gynaecologists Royal College of Physicians of Ireland. Obesity and Pregnancy Clinical Practice Guideline. Guideline No.2 June 2011, revised June 2013. [Google Scholar]
- 11.The Australian and New Zealand College of Obstetricians and Gynaecologists. C-Obs 49 Management of Obesity in Pregnancy. 2013 [Google Scholar]
- 12.Butte NF, Wong WW, Treuth MS, Ellis KJ, O’Brian Smith E. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am J Clin Nutr. 2004;79(6):1078–1087. doi: 10.1093/ajcn/79.6.1078. [DOI] [PubMed] [Google Scholar]
- 13.Thomas DM, Navarro-Barrientos JE, Rivera DE, et al. Dynamic energy-balance model predicting gestational weight gain. Am J Clin Nutr. 2012;95(1):115–122. doi: 10.3945/ajcn.111.024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jebeile H, Mijatovic J, Louie JC, Prvan T, Brand-Miller JC. A systematic review and metaanalysis of energy intake and weight gain in pregnancy. Am J Obstet Gynecol. 2016;214(4):465–483. doi: 10.1016/j.ajog.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 15.Pitkin RM. Nutritional support in obstetrics and gynecology. Clin Obstet Gynecol. 1976;19(3):489–513. doi: 10.1097/00003081-197609000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol. 2015;125(4):773–781. doi: 10.1097/AOG.0000000000000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krukowski RA, Bursac Z, McGehee MA, West D. Exploring potential health disparities in excessive gestational weight gain. J Womens Health (Larchmt) 2013;22(6):494–500. doi: 10.1089/jwh.2012.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Gallagher AE, Carta CM, Torres ME, Moran R, Wilcox S. Racial differences in gestational weight gain and pregnancy-related hypertension. Ann Epidemiol. 2014;24(6):441–447. doi: 10.1016/j.annepidem.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt KJ, Alanis MC, Johnson ER, Mayorga ME, Korte JE. Maternal pre-pregnancy weight and gestational weight gain and their association with birthweight with a focus on racial differences. Matern Child Health J. 2013;17(1):85–94. doi: 10.1007/s10995-012-0950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawlak MT, Alvarez BT, Jones DM, Lezotte DC. The effect of race/ethnicity on gestational weight gain. J Immigr Minor Health. 2015;17(2):325–332. doi: 10.1007/s10903-013-9886-5. [DOI] [PubMed] [Google Scholar]
- 22.Galjaard S, Pexsters A, Devlieger R, et al. The influence of weight gain patterns in pregnancy on fetal growth using cluster analysis in an obese and nonobese population. Obesity (Silver Spring) 2013;21(7):1416–1422. doi: 10.1002/oby.20348. [DOI] [PubMed] [Google Scholar]
- 23.Davis RR, Hofferth SL. The association between inadequate gestational weight gain, infant mortality among U.S infants born in 2002. Matern Child Health J. 2012;16(1):119–124. doi: 10.1007/s10995-010-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilmore LA, Klempel-Donchenko M, Redman LM. Pregnancy as a window to future health: Excessive gestational weight gain and obesity. Semin Perinatol. 2015;39(4):296–303. doi: 10.1053/j.semperi.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedderson MM, Weiss NS, Sacks DA, et al. Pregnancy weight gain and risk of neonatal complications: macrosomia, hypoglycemia, and hyperbilirubinemia. Obstet Gynecol. 2006;108(5):1153–1161. doi: 10.1097/01.AOG.0000242568.75785.68. [DOI] [PubMed] [Google Scholar]
- 26.Stotland NE, Cheng YW, Hopkins LM, Caughey AB. Gestational weight gain and adverse neonatal outcome among term infants. Obstet Gynecol. 2006;108(3 Pt 1):635–643. doi: 10.1097/01.AOG.0000228960.16678.bd. [DOI] [PubMed] [Google Scholar]
- 27.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196(4):322, e321–328. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau C, Rogers JM, Desai M, Ross MG. Fetal programming of adult disease: implications for prenatal care. Obstet Gynecol. 2011;117(4):978–985. doi: 10.1097/AOG.0b013e318212140e. [DOI] [PubMed] [Google Scholar]
- 29.Jensen ET, van der Burg JW, O’Shea TM, et al. The Relationship of Maternal Prepregnancy Body Mass Index and Pregnancy Weight Gain to Neurocognitive Function at Age 10 Years among Children Born Extremely Preterm. J Pediatr. 2017 doi: 10.1016/j.jpeds.2017.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hochner H, Friedlander Y, Calderon-Margalit R, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation. 2012;125(11):1381–1389. doi: 10.1161/CIRCULATIONAHA.111.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swank ML, Caughey AB, Farinelli CK, et al. The impact of change in pregnancy body mass index on the development of gestational hypertensive disorders. J Perinatol. 2014;34(3):181–185. doi: 10.1038/jp.2013.168. [DOI] [PubMed] [Google Scholar]
- 32.Swank ML, Caughey AB, Farinelli CK, et al. The impact of change in pregnancy body mass index on macrosomia. Obesity (Silver Spring) 2014;22(9):1997–2002. doi: 10.1002/oby.20790. [DOI] [PubMed] [Google Scholar]
- 33.Swank ML, Caughey AB, Farinelli CK, et al. The impact of change in pregnancy body mass index on cesarean delivery. J Matern Fetal Neonatal Med. 2014;27(8):795–800. doi: 10.3109/14767058.2013.845657. [DOI] [PubMed] [Google Scholar]
- 34.Gunatilake RP, Perlow JH. Obesity and pregnancy: clinical management of the obese gravida. Am J Obstet Gynecol. 2011;204:106–119. doi: 10.1016/j.ajog.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Magriples U, Boynton MH, Kershaw TS, et al. The impact of group prenatal care on pregnancy and postpartum weight trajectories. Am J Obstet Gynecol. 2015;213(5):688. doi: 10.1016/j.ajog.2015.06.066. e681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrams BF, Laros RK., Jr Prepregnancy weight, weight gain, and birth weight. Am J Obstet Gynecol. 1986;154(3):503–509. doi: 10.1016/0002-9378(86)90591-0. [DOI] [PubMed] [Google Scholar]
- 37.Bianco AT, Smilen SW, Davis Y, Lopez S, Lapinski R, Lockwood CJ. Pregnancy outcome and weight gain recommendations for the morbidly obese woman. Obstet Gynecol. 1998;91(1):97–102. doi: 10.1016/s0029-7844(97)00578-4. [DOI] [PubMed] [Google Scholar]
- 38.Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynaecol Obstet. 2006;93(3):269–274. doi: 10.1016/j.ijgo.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstet Gynecol. 2007;110(4):752–758. doi: 10.1097/01.AOG.0000278819.17190.87. [DOI] [PubMed] [Google Scholar]
- 40.Beyerlein A, Schiessl B, Lack N, von Kries R. Optimal gestational weight gain ranges for the avoidance of adverse birth weight outcomes: a novel approach. Am J Clin Nutr. 2009;90(6):1552–1558. doi: 10.3945/ajcn.2009.28026. [DOI] [PubMed] [Google Scholar]
- 41.Flick AA, Brookfield KF, de la Torre L, Tudela CM, Duthely L, Gonzalez-Quintero VH. Excessive weight gain among obese women and pregnancy outcomes. Am J Perinatol. 2010;27(4):333–338. doi: 10.1055/s-0029-1243304. [DOI] [PubMed] [Google Scholar]
- 42.Blomberg M. Maternal and neonatal outcomes among obese women with weight gain below the new Institute of Medicine recommendations. Obstet Gynecol. 2011;117(5):1065–1070. doi: 10.1097/AOG.0b013e318214f1d1. [DOI] [PubMed] [Google Scholar]
- 43.Oken E, Kleinman KP, Belfort MB, Hammitt JK, Gillman MW. Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am J Epidemiol. 2009;170:173–180. doi: 10.1093/aje/kwp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kominiarek MA, Hibbard J. Gestational weight gain and obesity: is 20 pounds too much? J Womens Health. 2011;20:1405. doi: 10.1016/j.ajog.2013.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bodnar LM, Siega-riz AM, Simhan HN, Himes KP, Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. Am J Clin Nutr. 2010;91:1642–1648. doi: 10.3945/ajcn.2009.29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vesco KK, Sharma AJ, Dietz PM, et al. Newborn size among obese women with weight gain outside the 2009 Institute of Medicine recommendation. Obstet Gynecol. 2011;117(4):812–818. doi: 10.1097/AOG.0b013e3182113ae4. [DOI] [PubMed] [Google Scholar]
- 47.Catalano PM, Mele L, Landon MB, et al. Inadequate weight gain in overweight and obese pregnant women: what is the effect on fetal growth? Am J Obstet Gynecol. 2014;211(2):137. doi: 10.1016/j.ajog.2014.02.004. e131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.American College of Obstetricians and Gynecologists. Motivational interviewing: a tool for behavior change. 2009 Committee opinion no. 423. [Google Scholar]
- 49.Beckman HB, Frankel RM. The effect of physician behavior on the collection of data. Ann Intern Med. 1984;101(5):692–696. doi: 10.7326/0003-4819-101-5-692. [DOI] [PubMed] [Google Scholar]
- 50.Hill B, Skouteris H, Fuller-Tyszkiewicz M. Interventions designed to limit gestational weight gain: a systematic review of theory and meta-analysis of intervention components. Obes Rev. 2013;14(6):435–450. doi: 10.1111/obr.12022. [DOI] [PubMed] [Google Scholar]
- 51.ACOG Committee Opinion No. 650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet Gynecol. 2015;126(6):e135–142. doi: 10.1097/AOG.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 52.Di Mascio D, Magro-Malosso ER, Saccone G, Marhefka GD, Berghella V. Exercise during pregnancy in normal-weight women and risk of preterm birth: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2016;215(5):561–571. doi: 10.1016/j.ajog.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Brown MJ, Sinclair M, Liddle D, Hill AJ, Madden E, Stockdale J. A systematic review investigating healthy lifestyle interventions incorporating goal setting strategies for preventing excess gestational weight gain. PLoS One. 2012;7(7):e39503. doi: 10.1371/journal.pone.0039503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindberg SM, Anderson CK. Improving gestational weight gain counseling through meaningful use of an electronic medical record. Matern Child Health J. 2014;18(9):2188–2194. doi: 10.1007/s10995-014-1467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McArtor RE, Iverson DC, Benken D, Dennis LK. Family practice residents' identification and management of obesity. Int J Obes Relat Metab Disord. 1992;16(5):335–340. [PubMed] [Google Scholar]
- 56.Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, Dalle Grave R. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. 2016;9:37–46. doi: 10.2147/DMSO.S89836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;6:CD007145. doi: 10.1002/14651858.CD007145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. BMJ. 2012;345:e5605. doi: 10.1136/bmj.e5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callaway LK, Colditz PB, Byrne NM, et al. Prevention of gestational diabetes: feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care. 2010;33(7):1457–1459. doi: 10.2337/dc09-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr. 2010;91(2):373–380. doi: 10.3945/ajcn.2009.28166. [DOI] [PubMed] [Google Scholar]
- 61.Hui A, Back L, Ludwig S, et al. Lifestyle intervention on diet and exercise reduced excessive gestational weight gain in pregnant women under a randomised controlled trial. BJOG. 2012;119(1):70–77. doi: 10.1111/j.1471-0528.2011.03184.x. [DOI] [PubMed] [Google Scholar]
- 62.Jeffries K, Shub A, Walker SP, Hiscock R, Permezel M. Reducing excessive weight gain in pregnancy: a randomised controlled trial. Med J Aust. 2009;191(8):429–433. doi: 10.5694/j.1326-5377.2009.tb02877.x. [DOI] [PubMed] [Google Scholar]
- 63.Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstetrics and gynecology. 2009;113(2 Pt 1):305–312. doi: 10.1097/AOG.0b013e318195baef. [DOI] [PubMed] [Google Scholar]
- 64.Jackson RA, Stotland NE, Caughey AB, Gerbert B. Improving diet and exercise in pregnancy with Video Doctor counseling: a randomized trial. Patient education and counseling. 2011;83(2):203–209. doi: 10.1016/j.pec.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 65.Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. Am J Clin Nutr. 2011;93(4):772–779. doi: 10.3945/ajcn.110.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(11):1494–1502. doi: 10.1038/sj.ijo.0802130. [DOI] [PubMed] [Google Scholar]
- 67.Dodd JM. Dietary and lifestyle advice for pregnant women who are overweight or obese: the LIMIT randomized trial. Ann Nutr Metab. 2014;64(3–4):197–202. doi: 10.1159/000365018. [DOI] [PubMed] [Google Scholar]
- 68.Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jorgensen JS. The LiP (Lifestyle in Pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care. 2011;34(12):2502–2507. doi: 10.2337/dc11-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Renault KM, Norgaard K, Nilas L, et al. The Treatment of Obese Pregnant Women (TOP) study: a randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol. 2014;210(2):134. doi: 10.1016/j.ajog.2013.09.029. e131–139. [DOI] [PubMed] [Google Scholar]
- 70.Catalano P, deMouzon SH. Maternal obesity and metabolic risk to the offspring: why lifestyle interventions may have not achieved the desired outcomes. Int J Obes (Lond) 2015;39(4):642–649. doi: 10.1038/ijo.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanentsapf I, Heitmann BL, Adegboye AR. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth. 2011;11:81. doi: 10.1186/1471-2393-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell F, Johnson M, Messina J, Guillaume L, Goyder E. Behavioural interventions for weight management in pregnancy: a systematic review of quantitative and qualitative data. BMC Public Health. 2011;11:491. doi: 10.1186/1471-2458-11-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG. 2010;117(11):1316–1326. doi: 10.1111/j.1471-0528.2010.02540.x. [DOI] [PubMed] [Google Scholar]
- 74.Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med. 2012;10:47. doi: 10.1186/1741-7015-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flynn AC, Dalrymple K, Barr S, et al. Dietary interventions in overweight and obese pregnant women: a systematic review of the content, delivery, and outcomes of randomized controlled trials. Nutr Rev. 2016;74(5):312–328. doi: 10.1093/nutrit/nuw005. [DOI] [PubMed] [Google Scholar]
- 76.Miller WR, Rollnich S. Motivational interviewing: preparing people for change. 2. New York: Guilford Press; 2002. [Google Scholar]