Abstract
Study Design
In vitro measurements of the oxygen consumption rates (OCR) of human intervertebral disc (IVD) cells.
Objective
To determine the differences in the OCR of nondegenerate and degenerate human annulus fibrosus (AF), nucleus pulposus (NP), and cartilage endplate (CEP) cells at different glucose concentrations.
Summary of Background Data
The avascular nature of the IVD creates a delicate balance between rate of nutrient transport through the matrix and rate of disc cell consumption necessary to maintain tissue health. Previous studies have shown a dependence of OCR for animal (e.g. bovine and porcine) IVD cells on oxygen level and glucose concentration. However, the OCR of nondegenerate human IVD cells compared to degenerate human IVD cells at different glucose concentrations has not been investigated.
Methods
IVD cells were isolated from the AF, NP, and CEP regions of human cadaver spines and surgical samples. The changes in oxygen concentration were recorded when cells were sealed in a metabolic chamber. The OCR of cells was determined by curve fitting using the Michaelis-Menton equation.
Results
Under identical cell culture conditions, the OCR of degenerate human IVD cells was 3-5 times greater than that of nondegenerate human IVD cells. The degenerate IVD cells cultured in low glucose medium (1 mM) exhibited the highest OCR compared to degenerate cells cultured at higher glucose levels (i.e., 5 mM, 25mM), while no significant differences in OCR was found among the nondegenerate IVD cells for all glucose levels.
Conclusions
Considering the significantly higher OCR and unique response to glucose of degenerate human IVD cells, the degeneration of the IVD is associated with a cell phenotypic change related to OCR. The OCR of IVD cells reported in this study will be valuable for understanding human IVD cellular behavior and tissue nutrition in response to disc degeneration.
Keywords: human intervertebral disc, energy metabolism, oxygen consumption rate, disc degeneration, annulus fibrosus, nucleus pulposus, cartilage endplate
Introduction
The intervertebral disc (IVD) is a fibrocartilage structure separating the vertebrae. Differences in biochemical composition and structure distinguish three regions of the disc: annulus fibrosus (AF), nucleus pulposus (NP), and cartilage endplates (CEP). Due to its large avascular nature, critical nutrients (i.e., glucose and oxygen) required for maintaining disc tissue health are supplied by blood vessels at the margins of the disc.1,2 Within the IVD, diffusion mechanisms regulate small nutrient (e.g., oxygen and glucose) supply. The balance between rate of nutrient transport through the matrix and rate of consumption by disc cells determines the local IVD nutrient concentration gradient.3 Poor nutritional supply to the disc is believed to be a key initiator of degeneration where changes in disc morphology, biochemistry, function, and material properties are observed.1
The oxygen gradient is a powerful regulator of IVD cellular activity and can strongly influence rates of extracellular matrix (ECM) synthesis, and possibly cell proliferation.4-6 Oxygen is necessary for cellular activity, although not for survival, as its consumption pathway is unclear.7 Previous studies have shown a dependence of oxygen consumption rates (OCR) for bovine and porcine IVD cells on oxygen level, glucose concentration, and pH level.5,6,8-10 In the porcine IVD, NP cells were shown to have a significantly higher OCR compared to that of AF cells.10 Overall, bovine and porcine IVD cells have been reported to have similarly low OCR compared to articular chondrocytes.5,8,10,11 However, to our knowledge, no study on human AF, NP, and CEP cells has investigated OCR.
Furthermore, there is no study that compares human nondegenerate and degenerate IVD cells in regards to OCR as well as their response to variable glucose levels. Differences in tissue region, level of degeneration, and glucose concentration may contribute to changes in OCR in the human IVD cells. A change in nutrient supply as a consequence of the onset and progression of disc degeneration could drive a change in the IVD cell phenotype. The hypothesis of this study is that IVD degeneration will impact the OCR and response to glucose level due to the distinct cell phenotype of nondegenerate and degenerate human disc cells. Therefore, the objective of this study is to determine the OCR of nondegenerate and degenerate human IVD and examine the differences due to disc degeneration among the AF, NP, and CEP cells at different glucose levels. The investigation will provide a better understanding of the effect of disc degeneration on oxygen consumption in the human IVD as well as compare the human IVD cellular activity to that of animal IVD cells.
Materials and Methods
Tissue preparation
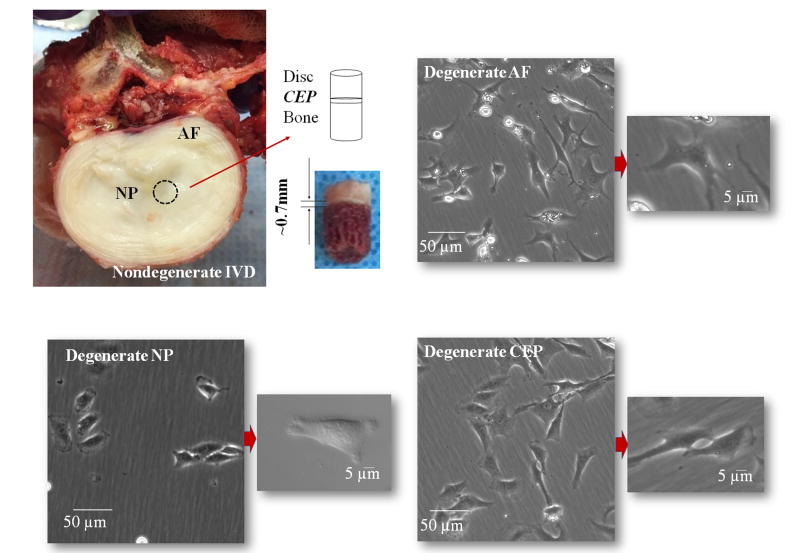
Human IVD tissues (see Table 1 for demographic data; total 8 donors, aged 21-65 years old, Thompson Grade I or II [i.e., nondegenerate])12 consisted of pooled L1 through S1 lumbar motion segments, were collected through an local Organ Procurement Organization within 24 hours of time of death under institutional approval. In addition, to-be-discarded human IVD surgical waste samples from patients undergoing surgery for degenerative disc disease (see Table 1 for demographic data; total 5 patients, aged 43-62 years old, Thompson Grade III or IV [i.e., degenerate])12 were obtained and processed within 2 hours. The degeneration grades of the IVD specimens were assessed by a senior Orthopaedic surgeon. The degenerate disc specimens from discectomy were graded based on both MRI (prior to surgery) and gross anatomy (after surgery). The nondegenerate disc specimens from the cadavers were assessed by gross anatomy during dissection. The samples were carefully separated and pooled into AF, NP, and CEP according to anatomic appearance and location (Figure 1: top left). The transition zone between the AF and NP region was discarded to avoid mixed cell culture populations. All three tissue regions were easily distinguishable due to differences in tissue location, structure, and composition in both the nondegenerate and degenerate tissues samples. For the surgical IVD samples, repeated saline washes were completed to eliminate blood cells carried through to culturing.
Table 1.
Demographic data of human tissue donors for experimental cell studies.
| Subject No. | Gender | Age (Years) | Thompson Grade |
|---|---|---|---|
| 1 (Human cadaver) | M | 65 | II |
| 2 (Human cadaver) | M | 38 | I |
| 3 (Human cadaver) | M | 43 | I |
| 4 (Human cadaver) | F | 51 | II |
| 5 (Human cadaver) | M | 42 | I |
| 6 (Human cadaver) | M | 33 | I |
| 7 (Human cadaver) | M | 21 | I |
| 8 (Human cadaver) | M | 35 | I |
| 9 (Surgical sample) | F | 43 | III-IV |
| 10 (Surgical sample) | F | 54 | IV |
| 11 (Surgical sample) | F | 62 | III-IV |
| 12 (Surgical sample) | F | 62 | IV |
| 13 (Surgical sample) | F | 62 | III-IV |
Figure 1.
Nondegenerate human IVD from lumbar spine with sagittal plug of disc tissue, CEP, and vertebral body (top left). The thin hyaline layer of CEP (∼0.7mm) was carefully separated from the NP and AF (relatively more transparent than the CEP tissue). Light microscopic images of cultured degenerate human IVD cells from AF (top right), NP (bottom left), and CEP (bottom right) regions. There were no visual morphological differences in cultured degenerate and nondegenerate (not shown) human IVD cells.
Cell harvest and culture
Cells were released from AF, NP, and CEP tissues by enzyme digestion in high glucose (25 mM) Dulbecco's Modified Eagle Medium (DMEM; Hyclone) supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 1% antibiotic-antimycotic (Invitrogen) for 24 hours. The enzyme solution contained collagenase II (Worthington Biochemical Corp., Lakewood, NJ) and protease (Sigma Chemical, St. Louis, MO) digestion (AF and CEP: 1 mg/mL collagenase and 0.6 mg/mL protease; NP: 0.5 mg/mL collagenase and 0.3 mg/mL protease). The digestions were strained through a 70 μm filter, washed with phosphate buffered saline, and re-suspended in high glucose DMEM with 10% FBS, 1% penicillin/streptomycin (Gibco Brl), 25 μg/mL ascorbic acid (pH 7.4) and the osmolality was measured within the range of 290–310 mOsmol. All isolated cells were identically cultured in monolayer at 21% O2 and 5% CO2 at 37°C to reduce variability as well as produce sufficient cell numbers for experimentation. The first media change was done less than 72 hours after plating the cells so any residual blood cells were washed away with the medium changes during first passage (P1) primary cell culture, as previously shown in the literature.13 The media was changed every 3 days, and upon reaching 95% confluence (typically within 3 weeks), P1 cells were detached with 0.25% trypsin (Invitrogen) and re-plated at a 1:2 ratio and monolayer cultured to second passage (P2) which was used in experiments. Morphologies of cultured nondegenerate and degenerate human IVD cells from AF, NP, and CEP regions (Figure 1) were examined by a light microscope. Cells were separated into 3 groups and incubated in FBS-free DMEM (pH 7.4) with glucose concentrations of 1, 5, or 25 mM for another 24 hours before the oxygen consumption rate measurements. Glucose levels were chosen based on low levels in the IVD finite element study14 as well as standard glucose culture medium (25 mM). The 24 hour incubation period allowed for cellular glycogen levels to adjust to the low levels of glucose.5
OCR measurement
In the experiment, P2 cell suspensions (3 million/mL) were placed into a 175 μL metabolic chamber (Instech Laboratories, Plymouth Meeting, PA) maintained at 37°C (Figure 2A). The concentration of dissolved oxygen in the culture medium at 37°C and atmospheric pressure was 200 μmol/L (or 21% oxygen level). The chamber was sealed and real time dissolved oxygen concentration in the medium was recorded by a fiber optic oxygen sensor (Ocean Optics, Dunedin, FL) until the oxygen concentration fell to 0.95 μmol/L (0.1% oxygen level). There were no notable changes in glucose concentration, pH of the culture medium, as well as cell viability measured at the end of the experiments.
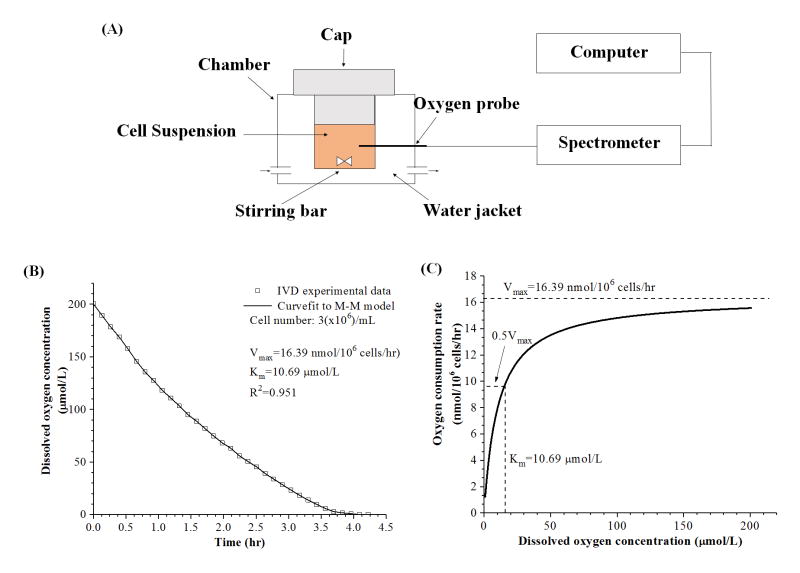
Figure 2.
(A) Schematic of the experimental setup for oxygen consumption rate experiments. AF, NP, and CEP cells were sealed in a water-jacketed metabolism chamber. (B) Characteristic curve of dissolved oxygen concentration (μmol/L) in the metabolism chamber over time (hr). (C) The oxygen consumption rate (nmol/106 cells/hr) was plotted against dissolved oxygen concentration (μmol/L) based on the Michaelis-Menten (M-M) equation with calculated coefficients of Vmax and Km.
Figure 2B shows a typical plot of dissolved oxygen concentration over time in the metabolic chamber. Previous IVD and articular chondrocyte studies have shown the relationship between OCR and oxygen concentration can be expressed using the Michaelis-Menten kinetics equation given by8,10,15:
where R is oxygen consumption rate (nmol/106 cells/hr), Vmax is the maximum oxygen consumption rate (nmol/106 cells/hr), Km is the Michaelis-Menten constant (μmol/L), and C is the oxygen concentration in the chamber (μmol/L). The Vmax is the maximum oxygen consumption rate achieved at high oxygen, and the Km is the oxygen concentration at which the oxygen concentration rate decreases to 50% of the. Vmax Based on the conservation of mass (i.e., oxygen) in the sealed metabolic chamber, the kinetic coefficients of Vmax and Km were determined by curve-fitting the recorded oxygen concentration data in the chamber over time to the Michaelis-Menten equation as previous studies.10,16
Statistical analysis
The OCR outcomes are presented as the mean and standard deviation (SD) from 5 separate OCR experimental runs (n=5) from at least 3 separate cell isolations. Two-way analysis of variance and Tukey's post hoc tests were performed to determine the effects of glucose concentration and disc degeneration on Vmax and Km for each disc tissue region using the SPSS statistics software (SPSS 16.0, IBM, NY).
Results
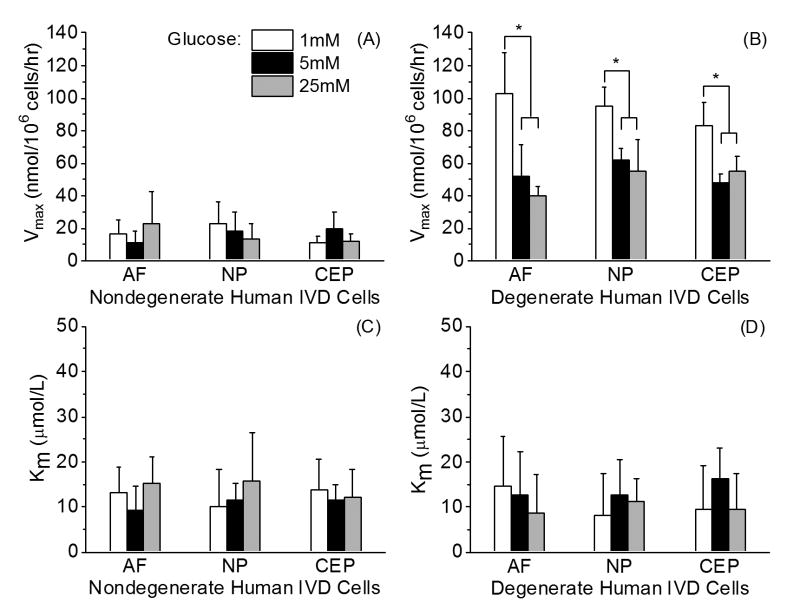
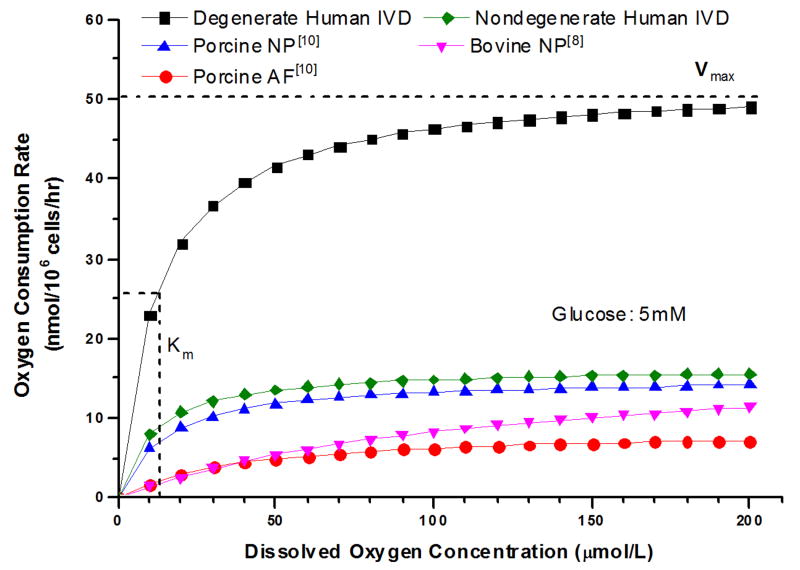
Human IVD cells from three tissue regions can be morphologically distinguished using a transmission light microscope (Figure 1: top right, bottom left, and bottom right). There was no morphological difference between cultured nondegenerate and degenerate human IVD cells. For human nondegenerate and degenerate disc cells, OCR for AF, NP, and CEP decreased with the decrease in oxygen concentration (Figure 2C) in accordance with the Michaelis-Menten kinetics model. Figure 3AB shows the average Vmax and Km for nondegenerate and degenerate AF, NP, and CEP cells at 1 mM, 5 mM, and 25 mM glucose mediums. No significant differences were found in Vmax (16.40±9.78 nmol/106 cells/hr) among the glucose levels (p=0.67) and tissue regions (p=0.102) of the nondegenerate IVD cells. In contrast, significant effects of glucose concentration were found on Vmax of degenerate AF, NP, and CEP with all cells cultured in the lowest glucose medium (1 mM: 93.77±16.83 nmol/106 cells/hr) exhibiting a larger Vmax than those cultured in both higher glucose mediums (5 mM: 53.99±10.77 nmol/106 cells/hr and 25 mM: 48.90±12.03 nmol/106 cells/hr) (p<0.0001). No significant differences were found between tissue region among the Vmax of degenerate IVD cells (p=0.171). As shown in Table 2, for the nondegenerate and degenerate IVD cells, there were no significant differences in Km (12.53±6.16 μmol/L and 11.59±8.49 μmol/L, respectively) between tissue regions (p=0.99; 0.90), glucose concentration (p=0.31; 0.42), as well as grade of degeneration (p=0.49) (Figure 3C and 3D). The average OCR of the degenerate disc cells was significantly higher, approximately 3 times higher, than the nondegenerate disc cells (p<0.0001) and previous porcine10 and bovine8 IVD cell data at 5 mM glucose (Figure 4). The two-way ANOVA interaction between glucose levels and grade of degeneration was significant (p<0.0001).
Figure 3.
Comparison of (A) Vmax and (C) Km among human nondegenerate AF, NP, and CEP cells cultured in DMEM with varying glucose concentrations (n = 5 separate oxygen consumption rate experimental runs for each cell type from three separate cell isolations). Comparison of (B) Vmax and (D) Km among human degenerate AF, NP, and CEP cells cultured in DMEM with varying glucose concentrations (n= 5 separate oxygen consumption rate experimental runs for each cell type from three separate cell isolations). Significant effects due to the glucose level were found for Vmax in all three regions (p<0.0001). Tukey's post hoc: * p<0.05.
Table 2.
Comparison of oxygen consumption rates [mean (standard deviation)] between intervertebral disc (IVD) cells and articular chondrocytes (AC).
| Type of Joint and Species | Subpopulation | Glucose (mM) | Vmax (nmol/106cells/h) | Km (μmol/L) | References |
|---|---|---|---|---|---|
| Bovine AC | * Superficial/Deep | 0.5-5 | 3.2/6.6 | 68/63 | 18 |
|
| |||||
| Bovine AC | 5 | 22.3 | n/a | 8 | |
|
| |||||
| Bovine IVD | NP | 1-5 | 5-10 | n/a | 5 |
| NP | 5 | 14.7 | n/a | 8 | |
|
| |||||
| Porcine IVD | AF | 5 | 17.0 | n/a | 8 |
| NP/Notochord cells | 5 | 90.9 | n/a | 8 | |
|
| |||||
| Porcine IVD | * AF | 1-25 | 6.0 | 35.7 | 10 |
| NP | 1-25 | 11.5 | 6.8 | 10 | |
|
| |||||
| Nondegenerate Human IVD | AF | 1 | 16.41 (8.72) | 13.19 (5.82) | Present study |
| 5 | 11.31 (6.77) | 9.323 (5.32) | |||
| 25 | 23.14 (19.05) | 15.24 (5.79) | |||
| NP | 1 | 23.10 (12.87) | 10.26 (8.09) | ||
| 5 | 18.06 (12.17) | 11.64 (3.44) | |||
| 25 | 13.24 (9.41) | 15.69 (10.93) | |||
| CEP | 1 | 10.75 (3.99) | 13.71 (6.79) | ||
| 5 | 20.05 (10.14) | 11.58 (3.23) | |||
| 25 | 11.55 (4.85) | 12.17 (6.054) | |||
|
| |||||
| Degenerate Human IVD | * AF | 1 | 102.9 (24.97) | 14.60 (11.02) | Present study |
| 5 | 52.09 (19.57) | 12.61 (9.53) | |||
| 25 | 40.16 (5.72) | 8.851 (8.35) | |||
| * NP | 1 | 95.28 (11.02) | 8.182 (9.16) | ||
| 5 | 62.24 (6.57) | 12.58 (8.06) | |||
| 25 | 52.09 (19.57) | 10.50 (6.58) | |||
| * CEP | 1 | 83.12 (14.50) | 9.557 (9.46) | ||
| 5 | 47.65 (6.16) | 16.23 (6.88) | |||
| 25 | 54.46 (10.79) | 11.23 (7.46) | |||
Crabtree effect observed.
Figure 4.
Comparison of the oxygen consumption rate (nmol/106 cells/hr) against dissolved oxygen concentration (μmol/L) based on the Michaelis-Menten equation with calculated coefficients (mean value) of Vmax and Km. Vmax is the maximum oxygen consumption rate (nmol/106 cells/hr) achieved at high oxygen and Km is the dissolved oxygen concentration (μmol/L) at . At 5 mM glucose, the oxygen consumption rate of degenerate human IVD cells from the present study are about 3 times higher than nondegenerate human, porcine10, and bovine8 IVD cells.
Discussion
The effect of degeneration on the OCR of human IVD cells at various glucose concentrations was investigated. The results showed that degenerate IVD cells had a significantly higher OCR and a unique glucose response compared to the nondegenerate IVD cells, suggesting a distinct cellular phenotype may exist in the degenerate IVD. The OCR further suggested that the nondegenerate IVD cells primarily depend on glycolysis, while the energy pathways of degenerate IVD cells may shift towards a more oxidative phenotype. The results of this study support the previous notion that a change in the nutrient environment as a result of the progression of degeneration may lead to changes in IVD cell energy metabolism.1
It is suggested that both IVD cells and articular chondrocytes obtain their energy primarily through the Embden-Meyerhof-Parnas (EMP) pathway of glycolysis, even in the presence of a high oxygen level.9,17 Present findings support a glycolytic metabolic phenotype for nondegenerate human IVD cells. The average Vmax of the nondegenerate human AF, NP, and CEP cells was approximately 10-23 nmol/106 cells/hr. Such low baseline value for Vmax of the nondegenerate human IVD cells is similar to previous studies of bovine and porcine IVD cells (Table 2; Figure 4).5,10 In addition, the low OCR of nondegenerate human IVD cells is not sensitive to the presence of oxygen due to small values of Km (9-16 μmol/L). These results suggest that the glycolysis is the primary pathway for energy metabolism in healthy human IVD. Although its consumption pathway is unclear, oxygen is necessary for cellular activity (e.g., matrix protein synthesis), instead of cell survival.7 The OCR of the nondegenerate human IVD cells were independent of glucose concentration and tissue region. Although the effect of glucose on the OCR of animal IVD cells is inconclusive, the OCR of NP cells was higher than the AF cells in the porcine disc (Table 2).8,10 Since the porcine NP cells are primarily notochordal, differences in OCR may be due to intrinsic tissue differences within that species. Therefore, caution needs to be taken when comparing the OCR results between animal and human IVDs.
The degenerate human IVD cells exhibited a significantly higher Vmax than the nondegenerate IVD cells, approximately 5 times higher at 1 mM glucose level and 3 times higher at 5 mM or 25mM glucose level, as shown in Table 2 and Figure 4. However, no significant difference in Km between nondegenerate and degenerate human IVD cells indicates a similar sensitivity to oxygen, especially at low oxygen levels. Differences in OCR may be resulted from a change in cell behavior due to degeneration. Typically, a higher rate of glycolysis occurs in cells that are cultured in high glucose. A high rate of glycolysis which hinders cellular respiration, described as the Crabtree effect, has been shown in articular chondrocytes11,18,19 and porcine AF cells.10 This study showed a similar effect on degenerate human IVD cells, which demonstrated a significantly lower OCR in the highest glucose mediums (5 mM and 25 mM). While present findings support a glycolytic metabolic phenotype for nondegenerate human IVD cells, the degenerate cells are significantly more metabolically active and may experience a shift in energy pathways to utilize oxidative phosphorylation at low glucose.
Previous studies have established differences between the behaviors and gene expression profiles of nondegenerate versus degenerate human IVD cells in regards to ECM genes and proteolysis, cell proliferation, apoptosis, growth factors, and inflammatory mediators.20-30 Mitochondrial gene expression patterns have also indicated possible changes of mitochondrial function in degenerate human AF cells.31 A change in mitochondrial function related to IVD degeneration could influence the disc cell metabolic phenotype considering the important role the mitochondria plays in regulating cellular respiration through oxidative phosphorylation. Since the degenerate IVD cells exhibited a higher OCR under a physiological glucose concentration (1 mM), it could increase oxygen demand in the disc, and combined with a Crabtree effect could push already low oxygen levels even lower8 which may contribute to the progression of degeneration. Alternatively, the higher OCR of the degenerate IVD cells could be a result of the adaptation to the changed nutrient environment due to vasculature ingrowth in degenerated discs. In future studies, it is of interest to study the coupling of glucose consumption, as well as lactate and ATP production to fully understand the cellular metabolic behavior and the role of oxygen in energy metabolism in human IVD cells.
Human IVD cells may experience possible loss of phenotype when extracted from their ECM as well as during P1 and P2 monolayer culture. This culture method was used due to a low human IVD cell yield obtained after tissue digestion. Studies have examined the effect of high oxygen monolayer expanded in vitro culture of articular chondrocytes and found a cellular induction to an oxidative phenotype and metabolism.32 Due to the maintenance of low OCR of the nondegenerate human IVD cells and identical culture conditions between nondegenerate and degenerate cells in the present study, it appears the culture conditions did not induce an oxidative phenotype in the human IVD cells. Osmolarity ranging from 316 to 600 mOsm was shown to exhibit minimal effects on the OCR of bovine articular chondrocytes.11 In our study, the isotonic osmolarity range of the medium with varying glucose concentrations was within 290 to 310 mOsm. In future studies, it would be beneficial to examine a more physiological hypertonic effect. Due to the scarcity of human disc samples, a future study needs to be conducted to fully understand the role of other factors (e.g., age and sex) on the metabolic phenotype of nondegenerate and degenerate IVD cells.
In summary, the effect of degeneration on the OCR of human IVD cells at various glucose concentrations was investigated. This study showed a significantly higher OCR and unique response to glucose of the degenerate cells compared to the nondegenerate cells under identical culture conditions. The impact of oxygen level on OCR was examined and a quantitative dependent relationship was determined. OCR reported in this study are valuable for understanding the effect of degeneration on human IVD cellular behavior and the differences between human and animal IVD cells. In addition, this data will be valuable for understanding IVD oxygen transport and theoretically predicting the local oxygen concentration in the human IVD. Considering the avascular nature of the IVD and the significantly higher oxygen consumption of the degenerate IVD cells, the nutrient environment in the degenerated disc may be vulnerable to any pathological event which may further disrupt the nutrient supply and promote the progression of IVD degeneration while driving a behavioral change in IVD cell phenotype. These unique nutritional demands of human IVD cells should also be considered in prospective tissue engineering and regeneration approaches for treatment of the degenerated IVD.
Acknowledgments
The authors thank Dr. Simona Baicu at We Are Sharing Hope SC for assisting human spine harvest.
NIH grants AR055775 and DE021134, and a NIH T32 predoctoral fellowship DE017551 and a NSF Graduate Research Fellowship to SEC funds were received in support of this work.
Relevant financial activities outside the submitted work: board membership, consultancy, royalties, expert testimony.
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s).
Level of Evidence: N/A
References
- 1.Huang YC, Urban JP, Luk KD. Intervertebral disc regeneration: do nutrients lead the way? Nat Rev Rheumatol. 2014;10:561–6. doi: 10.1038/nrrheum.2014.91. [DOI] [PubMed] [Google Scholar]
- 2.Grunhagen T, Shirazi-Adl A, Fairbank JC, et al. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42:465–77. vii. doi: 10.1016/j.ocl.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 2004;29:2700–9. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 4.Bibby SR, Fairbank JC, Urban MR, et al. Cell viability in scoliotic discs in relation to disc deformity and nutrient levels. Spine (Phila Pa 1976) 2002;27:2220–8. doi: 10.1097/00007632-200210150-00007. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 5.Bibby SR, Jones DA, Ripley RM, et al. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila Pa 1976) 2005;30:487–96. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 6.Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829–35. doi: 10.1002/jor.1100170607. [DOI] [PubMed] [Google Scholar]
- 7.Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13:695–701. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guehring T, Wilde G, Sumner M, et al. Notochordal intervertebral disc cells: sensitivity to nutrient deprivation. Arthritis Rheum. 2009;60:1026–34. doi: 10.1002/art.24407. [DOI] [PubMed] [Google Scholar]
- 9.Holm S, Maroudas A, Urban JP, et al. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–19. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 10.Huang CY, Yuan TY, Jackson AR, et al. Effects of low glucose concentrations on oxygen consumption rates of intervertebral disc cells. Spine (Phila Pa 1976) 2007;32:2063–9. doi: 10.1097/BRS.0b013e318145a521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heywood HK, Bader DL, Lee DA. Rate of oxygen consumption by isolated articular chondrocytes is sensitive to medium glucose concentration. J Cell Physiol. 2006;206:402–10. doi: 10.1002/jcp.20491. [DOI] [PubMed] [Google Scholar]
- 12.Thompson JP, Pearce RH, Schechter MT, et al. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila Pa 1976) 1990;15:411–5. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Wang F, Wu XT, Zhuang SY, et al. Ex vivo observation of human nucleus pulposus chondrocytes isolated from degenerated intervertebral discs. Asian Spine J. 2011;5:73–81. doi: 10.4184/asj.2011.5.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selard E, Shirazi-Adl A, Urban JP. Finite element study of nutrient diffusion in the human intervertebral disc. Spine (Phila Pa 1976) 2003;28:1945–53. doi: 10.1097/01.BRS.0000087210.93541.23. discussion 53. [DOI] [PubMed] [Google Scholar]
- 15.Zhou S, Cui Z, Urban JP. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 2004;50:3915–24. doi: 10.1002/art.20675. [DOI] [PubMed] [Google Scholar]
- 16.Kuo J, Shi C, Cisewski S, et al. Regional cell density distribution and oxygen consumption rates in porcine TMJ discs: an explant study. Osteoarthritis Cartilage. 2011;19:911–8. doi: 10.1016/j.joca.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee RB, Urban JP. Evidence for a negative Pasteur effect in articular cartilage. Biochem J. 1997;321(Pt 1):95–102. doi: 10.1042/bj3210095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heywood HK, Knight MM, Lee DA. Both superficial and deep zone articular chondrocyte subpopulations exhibit the Crabtree effect but have different basal oxygen consumption rates. J Cell Physiol. 2010;223:630–9. doi: 10.1002/jcp.22061. [DOI] [PubMed] [Google Scholar]
- 19.Otte P. Basic cell metabolism of articular cartilage. Manometric studies. Z Rheumatol. 1991;50:304–12. [PubMed] [Google Scholar]
- 20.Cs-Szabo G, Ragasa-San Juan D, Turumella V, et al. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine (Phila Pa 1976) 2002;27:2212–9. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 21.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 22.Gruber HE, Hoelscher GL, Hanley EN., Jr Annulus cells from more degenerated human discs show modified gene expression in 3D culture compared with expression in cells from healthier discs. Spine J. 2010;10:721–7. doi: 10.1016/j.spinee.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Heathfield SK, Le Maitre CL, Hoyland JA. Caveolin-1 expression and stress-induced premature senescence in human intervertebral disc degeneration. Arthritis Res Ther. 2008;10:R87. doi: 10.1186/ar2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–45. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Maitre CL, Pockert A, Buttle DJ, et al. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35:652–5. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- 27.Park JB, Lee JK, Park SJ, et al. Mitochondrial involvement in fas-mediated apoptosis of human lumbar disc cells. J Bone Joint Surg Am. 2005;87:1338–42. doi: 10.2106/JBJS.D.02527. [DOI] [PubMed] [Google Scholar]
- 28.Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10:R99. doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts S, Caterson B, Menage J, et al. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976) 2000;25:3005–13. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 30.Singh K, Masuda K, Thonar EJ, et al. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine (Phila Pa 1976) 2009;34:10–6. doi: 10.1097/BRS.0b013e31818e5ddd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gruber HE, Watts JA, Hoelscher GL, et al. Mitochondrial gene expression in the human annulus: in vivo data from annulus cells and selectively harvested senescent annulus cells. Spine J. 2011;11:782–91. doi: 10.1016/j.spinee.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Heywood HK, Lee DA. Monolayer expansion induces an oxidative metabolism and ROS in chondrocytes. Biochem Biophys Res Commun. 2008;373:224–9. doi: 10.1016/j.bbrc.2008.06.011. [DOI] [PubMed] [Google Scholar]