Abstract
Objectives
Osteoarthritis (OA) is associated with inflammation, chronic pain, functional limitations, and psychosocial distress. High Omega-3 (n-3) polyunsaturated fatty acids (PUFAs) are associated with lower levels of inflammatory mediators, anti-nociception, and adaptive cognitive/emotional functioning. High Omega-6 (n-6) PUFAs are associated with inflammation, nociception, and psychological distress. While findings related to n-3 supplementation in knee OA are mixed, consideration of the n-6:n-3 ratio and additional outcome measures may provide improved understanding of the potential relevance of these fatty acids in OA. Based on recommended and typical ranges of the n-6:n-3 ratio, we hypothesized that in adults with knee pain, those with a high n-6:n-3 ratio would have greater pain/functional limitations, experimental pain sensitivity, and psychosocial distress compared to those with a low n-6:n-3 ratio.
Methods
A cross-sectional investigation of clinical and experimental pain and physical and psychosocial functioning was completed in 167 adults ages 45–85 meeting knee OA screening criteria. Blood samples were collected and the plasma n-6:n-3 PUFA ratio determined. Quartile splits were computed and low (n=42) and high (n=41) ratio groups were compared.
Results
The high ratio group reported greater pain and functional limitations, (all p’s<0.04), mechanical temporal summation (hand and knee, p<0.05), and perceived stress (p=0.008) but not depressive symptoms.
Discussion
In adults with knee pain, a high n-6:n-3 ratio is associated with greater clinical pain/functional limitations, experimental pain sensitivity, and psychosocial distress compared to a low ratio group. Findings support consideration of the n-6:n-3 PUFA ratio and additional clinical endpoints in future research efforts.
Keywords: Osteoarthritis, Pain, Functioning, Essential Fatty Acids, Omega-6:Omega-3 Ratio
Introduction
Knee osteoarthritis (OA) represents a leading cause of disability worldwide, with current lifetime risk exceeding 40% and prevalence on the rise due to increasing longevity and obesity in the U.S [1]. Knee OA is associated with inflammation, chronic pain, and psychosocial distress [2, 3]. Evidence suggests that in addition to joint-specific inflammation, systemic inflammation is also involved in the pathogenesis of OA [4]. Due to cardio-intestinal risks, standard anti-inflammatory treatments such as NSAIDs have significant limitations in addition to minimal effectiveness, a common issue with other pain medications [5]. Hence, alternative safe and effective approaches to reduce inflammation and pain and improve function are needed [5].
Omega-3 (n-3) poly unsaturated fatty acids (PUFA) represents a group of essential fatty acids. Commonly recognized n-3 PUFAs include alpha linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), there is also docosapentaenoic acid (DPA) in addition to a few others [6]. Higher levels of n-3 PUFAs are associated with lower levels of inflammation and pain in conditions such as rheumatoid arthritis, inflammatory bowel disease, dysmenorrhea, and neuropathy [7–9]. Additionally, recent findings indicate that SPMs, specialized pro-resolving mediators, compounds derived from essential PUFAs that serve as anti-inflammatory mediators, promoting regeneration, recovery, resolution of inflammatory process and return to homeostasis across numerous systems [7]. A recent clinical trial comparing low versus high dose n-3 PUFAs (fish oil supplementation) in adults with symptomatic knee osteoarthritis demonstrated reduced clinical pain and increased functioning, at year one for both groups, but at year two the low dose group showed the greatest benefit. Additionally, there were no significant changes in cartilage volume loss between the two groups [10].
Beyond affecting the OA joint, systemic inflammatory processes can directly influence pain processing peripherally and centrally, contributing toward central sensitization [11, 12]. Importantly, n-3 PUFAs have peripheral and central anti-inflammatory and anti-nociceptive properties [6–9, 13]. The central anti-inflammatory properties are reflected by the ability of n-3 PUFAs to increase neuroplasticity and neurogenesis and in their positive association with cognition and emotional functioning [6, 8, 14–16]. Omega-3 PUFAs are believed to confer neuroprotective effects and reduced neuroinflammation via metabolism to eicosanoids and docosanoids [6, 14].
While n-3 PUFAs are essential in maintaining optimal health outcomes, omega-6 (n-6) PUFAs are equally important. Omega-6 PUFAs are comprised of arachidonic acid (AA), linoleic acid (LA), docosapentaenoic acid (DPA) among others [6]. Omega-6 PUFAs contribute to a number of necessary pro-inflammatory functions including the generation of prostaglandins which are derived from AA. Prostaglandins are vital to the inflammatory process and associated with the two cyclooxygenase isoforms (COX-1 and COX-2) by which their biosynthesis is blocked by non-steroidal anti-inflammatory drugs [17]. High levels of n-6 PUFAs are associated with inflammation, chronic pain, and cognitive impairment and psychological distress [9, 18]. Consideration of the n-6 and n-3 PUFAs balance appears to have clinical and functional relevance. A recommended n-6:n-3 PUFA ratio ranges from 2:1 to 5:1 [6, 19, 20]. Unfortunately, n-6 PUFA intake is excessive in the Western diet resulting in significantly higher ratios in the range of 15:1 or greater [6, 13, 20]. A high n-6:n-3 PUFA ratio was associated with inflammation and depression in older adults [21]. Additionally, an intervention targeting increasing n-3 PUFAs and decreasing n-6 PUFAs demonstrated improved quality of life and functioning, as well as decreased distress and headache frequency and severity in individuals with chronic headaches [22, 23]. Evaluating the n-6:n-3 PUFA ratio provides information regarding metabolism, absorption, and relative balance and may help elucidate the role of these fatty acids in OA-related pain and function [13, 20]. We extend previous findings by investigating the associations between the n-6:n-3 PUFA ratio and clinical pain, experimental pain sensitivity, and physical and psychosocial functioning in middle-aged and older adults with knee pain related to or at risk for OA. Based on recommended and typical n-6:n-3 PUFA ranges [6, 20], we evaluated a low ratio group compared to a high ratio group and hypothesized: in individuals with knee pain, those with a high n-6:n-3 PUFA ratio would have greater 1) clinical pain and functional limitations, 2) experimental pain sensitivity, and 3) psychosocial distress compared to those with a low n-6:n-3 PUFA ratio.
Materials and Methods
Study Design
Participants were recruited for a cross-sectional study from the communities surrounding the University of Florida (UF) and the University of Alabama at Birmingham (UAB) from Jan 2010 to Dec 2012. Participants involved in the current investigation are from a larger study entitled Understanding Pain and Limitations in Osteoarthritic Disease (UPLOAD). All procedures were reviewed and approved by the UF and UAB Institutional Review Boards.
Participants
A total of 167 individuals, ages 45–85 who screened positive for symptomatic knee OA were included in the investigation [24]. A description of the screening, inclusion, and exclusion criteria can be found in Table 1 and are previously reported [25, 26]. Participants completed a health assessment session and a quantitative sensory testing session on separate days. The health assessment included a review of health history, current treatment, physical exam, and knee radiographs. The measures and procedures described below are limited to those included in the current analysis.
Table 1.
Inclusion/exclusion criteria
|
Exclusion Criteria
|
|
|
General Inclusion Criteria
|
|
|
Inclusion Criteria for Knee OA Pain
|
|
Western Ontario and McMaster Universities Index of Osteoarthritis (WOMAC) and the
Graded Chronic Pain Scale (GCPS)
Clinical Pain and Functioning Measures
Participants completed self-report measures of clinical pain and functional limitations including the Western Ontario and McMaster Universities Index of Osteoarthritis (WOMAC) [27] and the Graded Chronic Pain Scale (GCPS) [28]. Physical function was assessed with the Short Physical Performance Battery (SPPB) [29, 30].
Experimental Pain Measures
The current analysis evaluated pressure and punctate mechanical quantitative sensory testing procedures. Both have demonstrated robust differences between individuals with versus without OA-related knee pain. Additionally, mechanical temporal summation provides a measure of central sensitization, a key question in this investigation [25, 31]. Pressure pain sensitivity was assessed on the most symptomatic knee (i.e., index knee) and the ipsilateral forearm with a handheld digital pressure algometer (Algomed, MEDOC, Ramat Yishai, Israel). The algometer was applied at a constant rate of 30 kilopascals (kPa) per second until the participant pressed a button when the sensation “first became painful.” The amount of pressure (kPa) that first produced a painful sensation was recorded.
Punctate stimuli and temporal summation of pain were evaluated with a nylon monofilament (Touchtest Sensory Evaluator 6.65), calibrated to bend at 300g of pressure. Testing sites order was randomized and included the patella of the index knee and the dorsum of the ipsilateral hand. Participants were instructed to provide a verbal rating of pain following a single contact (i.e., 1st Trial) and following a series of 10 contacts (i.e., 10th trial), which were provided at a rate of 1 contact per second. Verbal ratings were on scale of 0 (no pain sensation) to 100 (the most intense pain sensation imaginable). The procedure was completed twice at each site. For each site, verbal ratings for the single and multiple contacts were averaged, and a change score (e.g., rating at 10th trial – rating at 1st trial) was calculated as an index of temporal summation.
Psychosocial Measures
Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression scale, CES-D which measures symptoms of depression over the past week [32]. Perceived stress was measured with the Perceived Stress Scale, PSS [33]. Trait positive and negative affect was assessed with the Positive and Negative Affect Schedule, PANAS [34]. Optimism was measured with the Life Orientation Test-Revised, LOT-R [35].
Omega-6 and Omega-3 Fatty Acid Analysis
A blood sample was collected at the conclusion of the second study session and placed on ice. It was centrifuged at 4°C for 10 minutes at 3000 rpm. Plasma was pipetted (980 µl) into a 1.5 mL cryovial and 10 µl of 5 mmol Butylated hydroxytoluene (BHT) and 10 µl 5 mmol Diethylenetriaminepentaacetic acid (DTPA) were added for a final volume of 1 mL. The sample was mixed gently by inversion and placed in a -80°C freezer.
Sample Preparation
Samples were prepared by aliquoting 100 µL of sample or calibrator into a microcentrifuge tube and adding 10 µL of internal standard (10 µg/mL DHAd5, 3 µg/mL EPAd5, 10µg/mL AAd8, 5µg/mL ALAd14, 20µg/mL LAd4 prepared in 3:1 acetonitrile:methanol with 100 g/L Butylated hydroxyl toluene, BHT). Next, 300 µL of ethanol containing 100 g/L BHT was added to each vial, rapidly mixed using a vortex, then cooled at 4C for 10 min followed by centrifugation at 20,000 rcf for 10 min to pellet the protein. The supernatant was transferred to a clean microcentrifuge tube and dried down for 1 hour using a vacuum dryer. The dried sample was dissolved in 50 µL of 1:1 acetonitrile:methanol containing 0.1% acetic acid, mixed, centrifuged at 20,000 rcf for 5 min and transferred to a 96 well plate. The injection volume for LC-MS/MS analysis was 5 µL. All labeled internal standards and unlabeled standards were obtained from Cayman Chemical (Ann Arbor, MI). All solvents used were of LC/MS grade and obtained from FisherScientific (Fairlawn, NJ).
LC-MS/MS Analysis
Analysis was performed by liquid chromatography tandem mass spectrometry (LC-MS/MS) using an Accela 1250 UHPLC, Accela Open autosampler and TSQ Quantum Access (ThermoScientific, San Jose, CA). The mass spectrometer was operated in negative heated electrospray (HESI) with 3000 V spray voltage, 50C HESI probe temperature, 50 arb sheath gas, 20 arbitrary units auxiliary gas, 300C capillary temperature, and position D for distance of the HESI probe. Selected reaction monitoring (SRM) was used for detection of DHA, EPA, and AA while single ion monitoring (SIM) was used for ALA and LA. DPA n-6 and DPA n-3 among other PUFAs were not quantified as they were not part of the LC-MS/MS assay and were not validated. All parameters for SRM and SIM are described in Table 1. Separation was achieved with gradient elution on a Thermo Hypersil aQ column (100 mm × 2.1mm, 1.7 µm, ThermoScientific) at a flow rate of 300 µL/min and column temperature of 40C. Mobile phase A was 1mM Ammonium acetate, mobile phase B was 0.1% acetic acid in acetonitrile, and mobile phase C was 0.1% acetic acid in methanol. The gradient started at 25% A, 65% B and 10% C from 0–0.25 min, increased to 90% B and 10% C from 0.25–2 min, held constant from 2–4 min, returned to initial conditions from 4–4.5 min and equilibrated from 4.5–6.5 min. Percent totals were obtained for n-6 and n-3 and then the ratio was computed. See the Supplemental Table for the response factors for LC-MS/MS.
Data Analysis
Quartile splits were completed to create a low ratio group and high ratio group that reflected recommended and typical n-6:n-3 PUFA ratio ranges [6, 19, 20]. Descriptive analyses for continuous and categorical variables were conducted and group differences in those variables were tested using either ANOVA F-test or Chi-square test. Linear regression models were used to conduct model based tests for comparing the low versus high ratio group difference adjusting for age, ethnicity, gender and site variables. The model-based means of outcomes in the two groups are computed with the 95% confidence interval and ANCOVA F-test and p-value for testing the group difference with covariates in the model. Since the SPPB scales are limited in range and non-normally distributed, non-parametric analyses were also completed.
Smoking status, exercise, and body mass index (BMI), were explored as additional covariates in the model by 1) completing a partial correlation analyses with n-6:n-3 PUFA ratio controlling for primary covariates (age, sex, ethnicity, and study site), if significant, then 2) partial correlations were completed with outcome variables controlling for primary covariates (age, sex, ethnicity, and study site). The only variable that showed a significant relationship with n-6:n-3 PUFA ratio was smoking status (BMI r = .214, p = 0.060; exercise r = −.203, p = 0.074; and smoking status r = .241, p = 0.034). Smoking status and BMI were also significantly correlated (r = −.257, p = 0.023). A partial correlation was then completed for smoking status and outcome variables with primary covariates in the model. Smoking status was significantly correlated with the PANAS negative affect score (r = .262, p = 0.023) and included in a secondary analysis of this outcome measure. Additional analyses were also completed stratifying by ethnicity to better understand possible ethnic group differences. The data analysis was conducted using SAS 9.4.
Results
Quartile Grouping: n-6:n-3 Ratio
Table 2 displays the unadjusted and adjusted means for each individual n-6 and n-3 PUFA by the low and high n-6:n-3 PUFA ratio groups. The n-6:n-3 PUFA ratio for the low and high groups based on the quartile splits (25th% and 75th%) were in the recommended (≤ 5) and typical (>5) ranges respectively.
Table 2.
Unadjusted/Adjusted means for each PUFA for low & high n-6:n-3 PUFA ratio groups
| Low Ratio n = 42 |
High Ratio n = 41 |
Group Difference | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | F-value | p-value | |||
| Lower Bound |
Upper Bound |
Lower Bound |
Upper Bound |
|||||
| Omega-3 | ||||||||
| DHA% | 6 | 5.14 | 6.67 | 2 | 1.09 | 2.64 | 54.48 | <.0001 |
| 6.31 | 5.32 | 7.30 | 2.13 | 0.99 | 3.27 | 48.76 | <.0001 | |
| ALA% | 10 | 9.34 | 11.33 | 7 | 6.14 | 8.16 | 20.14 | <.0001 |
| 9.67 | 8.38 | 10.95 | 6.66 | 5.18 | 8.14 | 14.91 | 0.0002 | |
| EPA% | 1 | 1.04 | 1.38 | 0 | 0.15 | 0.50 | 49.99 | <.0001 |
| 1.19 | 0.96 | 1.41 | 0.36 | 0.09 | 0.62 | 36.99 | <.0001 | |
| Sum% | 17 | 16.35 | 18.56 | 9 | 8.23 | 10.46 | 105.43 | <.0001 |
| 17.17 | 15.73 | 18.61 | 9.14 | 7.48 | 10.80 | 84.45 | <.0001 | |
| Omega-6 | ||||||||
| AA% | 8 | 6.58 | 9.40 | 4 | 2.71 | 5.56 | 14.7 | 0.0002 |
| 9.76 | 8.13 | 11.39 | 5.85 | 3.97 | 7.73 | 15.66 | 0.0002 | |
| LA% | 75 | 72.63 | 76.48 | 87 | 84.58 | 88.47 | 75.53 | <.0001 |
| 73.07 | 70.72 | 75.42 | 85.01 | 82.30 | 87.72 | 70.20 | <.0001 | |
| Sum% | 83 | 81.45 | 83.65 | 91 | 89.54 | 91.77 | 105.38 | <.0001 |
| 82.84 | 81.40 | 84.28 | 90.86 | 89.20 | 92.52 | 84.43 | <.0001 | |
| n-6:n-3 Ratio | ||||||||
| n-6:n-3 ratio | 5 | 4.65 | 5.38 | 10 | 9.47 | 10.21 | 337.73 | <.0001 |
| 5.08 | 4.61 | 5.56 | 9.92 | 9.37 | 10.47 | 280.96 | <.0001 | |
Adjusted (italics) and unadjusted (bold), controlling for ethnicity, age, sex, and study site yielded similar findings.
Demographic and Biobehavioral Factors
Of the 167 participants in the study, 53% were African American, 47% were non-Hispanic white adults, 29% were men and 71% were women. Demographic characteristics of the low ratio (n=42) and high ratio (n=41) groups are presented in Table 3. The high ratio group included a greater proportion of African Americans and smokers and had a greater body mass index (BMI) compared to the low ratio group (all p’s < 0.01). A greater proportion of individuals in the low ratio group reported supplementing with some form of n-3 PUFA product (n=9) compared to the high ratio group (n=2). No differences were observed for age, sex, employment, marital status, exercise frequency, and Kellgren Lawrence scores [36].
Table 3.
Demographic/bio-behavioral measures by low and high n-6:n-3 PUFA ratio groups
| Low Ratio | High Ratio | Statistics | P-value | ||
|---|---|---|---|---|---|
| Demographic Measures | n = 42 | n = 41 | |||
| Age | 59.3 (7.5) | 56.8 (6.9) | t=1.56 | 0.123 | |
| Race | χ2 = 7.69 | 0.006 | |||
| African Americans | 40.5% | 70.7% | |||
| Non-Hispanic Whites | 59.5% | 29.3% | |||
| Gender | χ2 = 1.56 | 0.212 | |||
| Female | 73.8% | 61.0% | |||
| Male | 26.2% | 39.0% | |||
| Employment | χ2 = 1.04 | 0.309 | |||
| Employed | 47.6% | 36.6% | |||
| Not employed | 52.4% | 63.4% | |||
| Marital Status | χ2 = 1.52 | 0.218 | |||
| Married | 50.0% | 36.6% | |||
| Not married | 50.0% | 63.4% | |||
| BMI [kg/m2 (sd)] | 29.1 (6.0) | 34.4 (9.4) | t=−3.04 | 0.003 | |
| Smoking | χ2 =12.72 | 0.002 | |||
| Non-smoker | 61.9% | 51.2% | |||
| Former smoker | 33.3% | 14.6% | |||
| Current smoker | 4.8% | 34.1% | |||
| Exercise frequency | χ2 =4.35 | 0.114 | |||
| <1 time per week | 19.0% | 40.0% | |||
| 1 to 3 times per week | 54.8% | 40.0% | |||
| >= 4 times per week | 26.2% | 20.0% | |||
| Omega-3 supplement | 21.0% | 5.0% | Χ2 = 3.61 | 0.046 | |
|
| |||||
| Low Ratio | High Ratio | Statistics | p-value | ||
| n=42 | n=41 | ||||
|
| |||||
| Kellgren Lawrence Score | Χ2 = 4.995 | 0.082 | |||
| KL0/KL1 | 57.1% | 56.1% | |||
| KL2 | 19.0% | 4.9% | |||
| KL3/KL4 | 23.8% | 39.0% | |||
Kellgren Lawrence scores determined based on reported criteria [36].
Clinical Pain and Functioning
As noted in Table 4, the high ratio group had higher scores (greater clinical pain and functional limitations) for the WOMAC total score (p = 0.011) and associated subscales (all p’s<0.04), and slightly lower physical functioning scores on the SPPB chair stand (p = 0.001) and total (p = 0.008) scores indicated by non-parametric analysis. Group differences were not indicated for the GCPS pain intensity and interference score.
Table 4.
Clinical, functional, experimental, and psychosocial measures men and confidence intervals by low and high n-6:n-3 PUFA ratio groups
| Low Ratio n = 42 |
95% CI | High Ratio n = 41 |
95% CI | F Value |
P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| WOMAC Total | 23.93 | 17.56 | 30.30 | 33.97 | 26.62 | 41.32 | 6.76 | 0.011 | |
| Pain | 5.08 | 3.74 | 6.41 | 6.76 | 5.23 | 8.30 | 4.38 | 0.040 | |
| Stiffness | 2.60 | 1.91 | 3.28 | 4.08 | 3.29 | 4.88 | 12.8 | 0.001 | |
| Physical Function | 16.26 | 11.48 | 21.03 | 23.12 | 17.60 | 28.64 | 5.61 | 0.020 | |
| GCPS Pain Intensity | 40.26 | 32.20 | 48.32 | 47.04 | 37.73 | 56.36 | 1.92 | 0.170 | |
| Interference | 31.77 | 20.60 | 42.94 | 39.69 | 26.79 | 52.59 | 1.37 | 0.246 | |
| SPPB Total | 10.50 | 9.73 | 11.27 | 10.00 | 9.11 | 10.89 | 1.17 | 0.284* | |
| Chair | 3.19 | 2.79 | 3.60 | 2.71 | 2.24 | 3.17 | 3.94 | 0.051* | |
| Walking | 3.52 | 3.16 | 3.89 | 3.66 | 3.24 | 4.09 | 0.4 | 0.527 | |
| Balance | 3.78 | 3.45 | 4.11 | 3.63 | 3.24 | 4.01 | 0.63 | 0.431 | |
|
| |||||||||
| Experimental Pain | |||||||||
|
| |||||||||
| Pressure Med Knee | 372.04 | 312.97 | 431.12 | 337.32 | 269.09 | 405.55 | 0.94 | 0.336 | |
| Lateral-Knee | 358.81 | 300.85 | 416.77 | 370.13 | 303.19 | 437.07 | 0.1 | 0.748 | |
| Forearm | 269.73 | 222.61 | 316.86 | 261.09 | 206.67 | 315.52 | 0.09 | 0.763 | |
| Punctate 1st Tap Hand | 4.15 | −0.99 | 9.30 | 7.99 | 2.04 | 13.93 | 1.51 | 0.223 | |
| 1st Tap Knee | 8.73 | 2.39 | 15.08 | 12.88 | 5.55 | 20.21 | 1.16 | 0.285 | |
| Change Hand | 8.98 | 2.44 | 15.52 | 17.59 | 10.03 | 25.14 | 4.71 | 0.033 | |
| Change Knee | 16.63 | 10.16 | 23.09 | 25.04 | 17.57 | 32.51 | 4.6 | 0.035 | |
|
| |||||||||
| Psychological Measures | |||||||||
|
| |||||||||
| CES-D | 6.63 | 3.86 | 9.41 | 7.02 | 3.94 | 10.10 | 0.06 | 0.815 | |
| PSS | 11.75 | 9.32 | 14.17 | 15.68 | 12.99 | 18.37 | 7.48 | 0.008 | |
| PANAS | Positive | 38.24 | 35.05 | 41.44 | 35.65 | 32.10 | 39.20 | 1.85 | 0.178 |
| Negative | 12.16 | 10.24 | 14.07 | 14.65 | 12.52 | 16.77 | 4.75 | 0.033 | |
| LOTR | 18.10 | 16.43 | 19.77 | 16.99 | 15.14 | 18.85 | 1.23 | 0.270 | |
Adjusted for ethnicity, age, sex, and study site.
Abbreviations: Western Ontario and McMaster Universities Index of Osteoarthritis (WOMAC), Graded Chronic Pain Scale (GCPS), Short Physical Performance Battery (SPPB), Center for Epidemiologic Studies Depression (CES-D), Perceived Stress Scale (PSS), Positive and Negative Affect Scale (PANAS).
Mann-Whitney U Test (SPPB - Total Score, p = 0.001 and Chair Stand, p = 0.008)
Experimental Pain Sensitivity
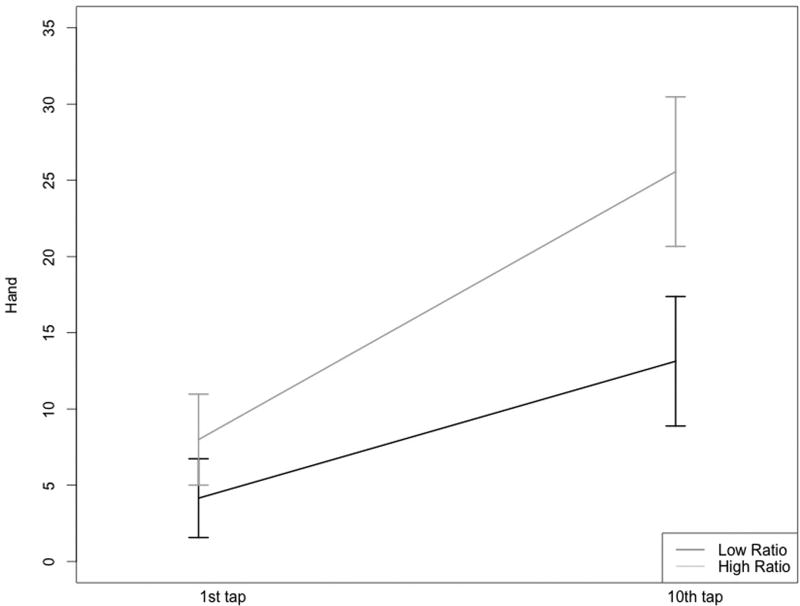
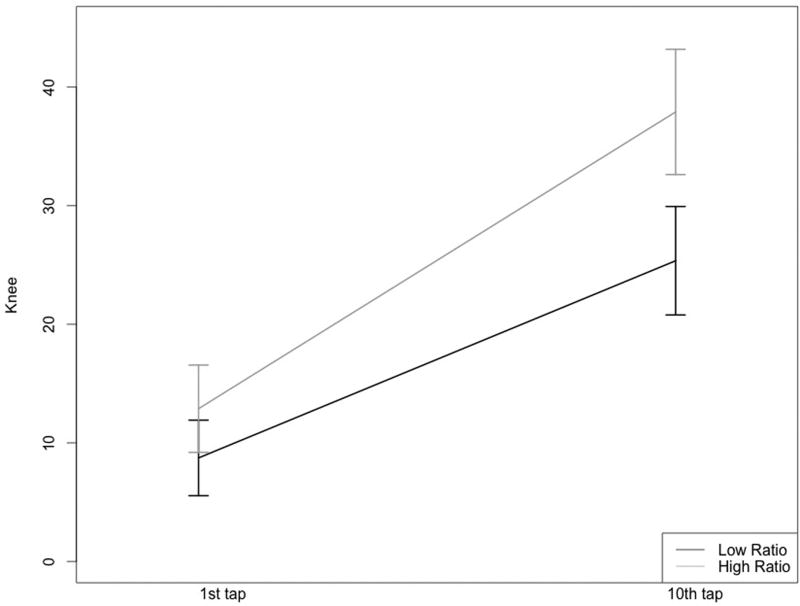
While pressure pain thresholds (PPTh) were similar between the two groups (all p’s > 0.10), a significant group difference emerged for punctate sensitivity at the knee and hand (Table 4). Specifically, participants in the high ratio PUFA group reported greater pain intensity following a series of ten mechanical taps and a greater change score (10th tap – 1st tap), reflecting mechanical temporal summation (Figures 1A & B).
Figures 1A & 1B.
Mechanical temporal summation at the hand and knee for the low and high n-6:n-3 PUFA ratio groups
Low ratio group, n=42; High ratio group, n=41
Psychosocial Functioning
The high ratio group had higher scores for perceived stress (PSS) and negative affect (PANAS), but not for depressive symptoms (CES-D), optimism (LOTR), or positive affect (PANAS), Table 4. Secondary analysis of PANAS Negative Affect was completed with smoking status (current smoker – yes/no) as a covariate in the model, although group differences were no longer significant, the trend continued to show lower scores in the low ratio (mean = 13.2) compared to the high ratio group (mean = 15.2, [p = 0.086]).
Additional Analyses
As the low and high n-6:n-3 PUFA ratio groups differed by ethnicity, the n-6:n-3 PUFA ratio and selected measures are presented in Table 5 stratified by ethnic group. Although interpretation is limited due to small sample sizes, the low and high ratio group differences were similar across ethnic groups, but differences in the WOMAC and PSS appeared somewhat larger for the African American group compared to the non-Hispanic white group.
Table 5.
Mean and confidence interval for the low and high n-6:n-3 PUFA ratio groups by ethnicity across clinical, experimental, and psychosocial measures
| Low Ratio (n = AA -17 and NHW - 25) |
High Ratio (n = AA -29 and NHW -12) |
Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI
|
Mean | 95% CI
|
||||||
| Ethnicity | Lower Bound |
Upper Bound |
Lower Bound |
Upper Bound |
F Value | P-value | |||
| n-6:n-3 PUFA ratio | AA | 5.30 | 4.63 | 5.97 | 9.64 | 9.05 | 10.23 | 124.03 | <.0001 |
| NHW | 5.44 | 4.54 | 6.34 | 10.87 | 9.74 | 12.00 | 166.93 | <.0001 | |
| WOMAC | AA | 24.14 | 15.73 | 32.56 | 41.27 | 33.90 | 48.65 | 12.29 | 0.001 |
| NHW | 26.56 | 14.61 | 38.51 | 29.24 | 14.24 | 44.24 | 0.23 | 0.634 | |
| Mechanical TS - Knee | AA | 26.01 | 15.41 | 36.61 | 32.78 | 23.50 | 42.07 | 1.21 | 0.277 |
| NHW | 2.78 | −7.11 | 12.67 | 12.67 | −0.19 | 24.63 | 4.19 | 0.049 | |
| PSS | AA | 10.74 | 7.44 | 14.03 | 16.08 | 13.43 | 18.73 | 8.39 | 0.006 |
| NHW | 13.02 | 8.04 | 17.99 | 15.21 | 8.96 | 21.45 | 0.89 | 0.352 | |
Adjusted for age, sex, and study site.
Abbreviations: AA= African Americans, NHW = non-Hispanic whites, and TS = temporal summation
Discussion
Our findings suggest that in adults with knee pain, a low plasma n-6:n-3 PUFA ratio is associated with lower levels of knee pain symptoms, improved physical functioning, lower levels of temporal summation of mechanical pain, and less psychosocial distress compared to those with a high plasma n-6:n-3 PUFA ratio. This is the first study to investigate the relationship of n-6:n-3 PUFA ratio with an expanded array of outcome variables including experimental pain sensitivity and psychosocial functioning in individuals screened for OA-related knee pain. Findings encourage the consideration of the n-6:n-3 PUFA ratio and additional clinical endpoints in future research and when evaluating applicability to clinical care.
n-6:n-3 Ratio
Our high ratio group had an average n-6:n-3 PUFA ratio of 9.92, almost twice the recommended ratio of less than 5 [6, 17, 20]. The low ratio group had an average n-6:n-3 PUFA ratio of 5.08, near the top end of the recommended ratio level. There is evidence that diet [6] and supplementation with n-3 PUFAs can increase n-3 PUFA levels and lower the n-6:n-3 PUFA ratio [13, 15]. Although characterized by significant variability in product type and frequency of consumption, we found that more individuals from the low ratio group used some form of n-3 PUFA supplementation, 21%, compared to 5% in the high ratio group. Additionally, a person with an n-6:n-3 ratio below 5 is likely eating different foods compared to someone with a high ratio and also likely living a healthier lifestyle, further investigations exploring these relationships are needed [19,20].
Clinical Pain
Hill and colleagues recently reported that knee OA patients receiving low dose n-3 PUFA supplementation showed greater decreases in WOMAC pain and function scores over a two-year period compared to patients randomized to high dose supplementation [10]. Although cross-sectional, our findings suggest that individuals with higher n-3 PUFAs relative to n-6 PUFAs endorsed lower levels of pain, stiffness, and functional limitations (WOMAC). Additionally, we extend previous findings by including an objective measure of functioning, the SPPB, which has been associated with clinical pain [25, 37]. One of the challenges with the SPPB in our sample is the limited range and non-normal distribution of scores, which dictated use of a non-parametric analysis. The analysis revealed poorer performance on the SPPB chair stand and total scores in the high versus low ratio group. However, the non-parametric approach prohibits controlling for possible confounding variables.
Experimental Pain
Prior studies of n-3 PUFA levels and n-3 PUFA supplementation in knee OA have focused on the peripheral anti-inflammatory benefits specific to joint health [10, 38]. Less investigated are the benefits of n-3 PUFAs in protecting peripheral and central nervous system functioning, including potential influences on nociceptive processing. Early preclinical and clinical findings suggest fatty acids play a role in the onset and suppression of pain by way of peripheral and central mechanistic influences [9]. We add to this developing body of literature by demonstrating that individuals with a high n-6:n-3 PUFA ratio show greater mechanical temporal summation, a measure of central sensitization found to be predictive of OA-related clinical pain [25].
Psychosocial
The relationships between low levels of n-3 PUFAs and high levels of n-6 PUFAs on cognitive and emotional functioning have been reported across populations and conditions [15, 16, 21]. Chronic pain is associated with increased negative affect, depression, anxiety, and psychosocial distress [39, 40]. Supplementation of n-3 PUFAs in medical students resulted in decreased anxiety, improved performance and lower n-6:n-3 PUFA ratios [15], and increasing n-3 PUFAs and decreasing n-6 PUFAs was associated with improved function in adults with chronic headaches [22]. Although a cross-sectional study, we found that a high n-6:n-3 PUFA ratio is associated with greater levels of perceived stress and a trend for greater negative affect among individuals with knee pain.
Additional Considerations
Health disparities in OA-related knee pain, functional limitations, and environmental/social stress have been consistently reported, with African Americans experiencing higher levels of pain, functional limitations, and environmental/social stress compared to non-Hispanic whites [41]. Our findings highlight some potentially important opportunities. Although the low and high ratio group differed by ethnicity, with more African Americans in the higher ratio group and more non-Hispanic whites in the low ratio group, the mean n-6:n-3 PUFA ratio in each group did not significantly differ by ethnicity. Interpretation of post hoc analyses warrants caution given that the sample sizes are small, however, the pattern of findings was similar across ethnic groups, though n-6:n-3 PUFA ratio group differences for WOMAC and PSS appeared larger for African Americans, i.e., lower levels of pain and perceived stress for the low ratio group. Importantly, targeting a low n-6:n-3 PUFA ratio may have benefits regardless of ethnicity.
Limitations and Future Directions
Since the study was cross-sectional, to permit causal interpretations, a prospective intervention study is needed with a similar array of comprehensive measures including clinical, functional, experimental pain, and psychosocial. As this was an initial investigation of the n-6:n-3 PUFA ratio, future investigations will benefit from inclusion of additional individual PUFAs such as DPA n-6 and DPA n-3. The incorporation of other functional measures such as walking speed, which has significant predictive utility in healthy aging and functioning, and well-validated quality of life measures would further enhance our understanding of the contributions of the n-6:n-3 PUFA ratio in individuals with OA-related knee pain. While excluding individuals taking frequent opioids, the current investigation did not include an evaluation of NSAIDs, other pain medications, and/or psychotropics. Future investigations would benefit from inclusion of pain medications in the analyses to determine the influence on n-3 PUFAs, n-6 PUFAs and overall PUFA ratio and the comprehensive array of pain and functioning measures in order to improve understanding of potential clinical applicability. Additionally, deciphering the relationships between BMI, smoking status, and the n-6:n-3 PUFA ratio warrant further investigations. While we controlled for some obvious confounders, the n-6:n-3 PUFA ratio may represent a proxy for other health-related variables that were not measured or included in the model, such as diet and other lifestyle factors. Further research is needed to better understand the relationship between diet, lifestyle behaviors and the n-6:n-3 PUFA ratio. Finally, replication of findings in studies with larger sample sizes, varying OA severity, and balanced gender groups will improve the interpretability of the relevance of the n-6:n-3 PUFA ratio to pain and functioning associated with OA-related knee pain. In general, a greater proportion of women do experience knee OA pain, however, a greater representation of men will allow for the investigation of possible sex differences.
Conclusions
Our findings provide evidence that a lower n-6:n-3 PUFA ratio is associated with clinical, nociceptive, physical, and psychosocial functioning in individuals with knee pain. The results of the current study highlight the importance of 1) considering the n-6:n-3 PUFA ratio in addition to evaluating n-6 and/or n-3 PUFA measures independently, and 2) including a comprehensive range of clinical, functional, and psychosocial outcome measures in future investigations. First, the n-6:n-3 PUFA ratio provides an easy to understand metric reflecting the n-6 and n-3 PUFA balance. Second, evaluating a broad array of clinical and functional outcome measures will enhance our understanding of the role of PUFAs specific to pain and promote the identification of possible treatments for individuals with or at risk for symptomatic knee osteoarthritis.
Supplementary Material
Acknowledgments
We would like to express appreciation to the UPLOAD participants, to the UF and UAB research teams for assisting with data collection, and to John Marks, DHSc for reviewing, providing feedback, and assisting with the final version of this manuscript. Special thanks also to Cynthia Garvan, PhD for providing biostatistical assistance.
Sources of Funding
The current study was supported by the National Institutes of Health/National Institute on Aging (R37AG033906), the University of Florida Clinical and Translational Science Institute (UL1TR000064 and KL2TR000065), and the University of Alabama at Birmingham Center for Clinical and Translational Science Institute (UL1TR000165). KTS is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, K23AR062099; CK by the National Institute on Dental and Craniofacial Research, K99DE022368; YCA by the National Institute on Aging, K01 AG048259; and EJB by the NIA K99AG052642. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
Dr. Fillingim reports grants from Pfizer, personal fees from Algynomics, outside the submitted work. All other authors report no competing interests.
References
- 1.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013:1–19. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Creamer P, Lethbridge-Cejku M, Hochberg MC. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology. 2000;39:490–496. doi: 10.1093/rheumatology/39.5.490. [DOI] [PubMed] [Google Scholar]
- 3.Kauppila A, Kyllonen E, Mikkonen P, et al. Disability in end-stage knee osteoarthritis. Disability and Rehabilitation. 2009;31:370–380. doi: 10.1080/09638280801976159. [DOI] [PubMed] [Google Scholar]
- 4.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenbaum CC, O'Mathuna DP, Chavez M, et al. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16:32–40. [PubMed] [Google Scholar]
- 6.Crupi R, Marino A, Cuzzocrea S. n-3 fatty acids: role in neurogenesis and neuroplasticity. Curr Med Chem. 2013;20:2953–2963. doi: 10.2174/09298673113209990140. [DOI] [PubMed] [Google Scholar]
- 7.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horowitz S. Omega -3 fatty acids for disease prevention and treatment. Alternative and Complimentary Therapies. 2014;20:191–196. [Google Scholar]
- 9.Tokuyama S, Nakamoto K. Unsaturated fatty acids and pain. Biol Pharm Bull. 2011;34:1174–1178. doi: 10.1248/bpb.34.1174. [DOI] [PubMed] [Google Scholar]
- 10.Hill C, March L, Aitken D, et al. Fish oil in knee osteoarthritis: A randomised clinical trial of low dose versus high dose. Ann Rheum Dis. 2015;0:1–7. doi: 10.1136/annrheumdis-2014-207169. [DOI] [PubMed] [Google Scholar]
- 11.Katz EJ, Gold MS. Inflammatory hyperalgesia: A role for the C-fiber sensory neuron cell body? Journal of Pain. 2006;7:170–178. doi: 10.1016/j.jpain.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Lluch E, Torres R, Nijs J, et al. Evidence for central sensitization in patients with osteoarthritis pain: A systematic literature review. Eur J Pain. 2014;18:1367–1375. doi: 10.1002/j.1532-2149.2014.499.x. [DOI] [PubMed] [Google Scholar]
- 13.Kiecolt-Glaser JK, Belury MA, Andridge R, et al. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun. 2012;26:988–995. doi: 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zainuddin MS, Thuret S. Nutrition, adult hippocampal neurogenesis and mental health. Br Med Bull. 2012;103:89–114. doi: 10.1093/bmb/lds021. [DOI] [PubMed] [Google Scholar]
- 15.Kiecolt-Glaser J, Belury M, Andridge R, et al. Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain, Behavior, and Immunity. 2011;25:1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson J, Ijiom N, Harris W. Omega-3 fatty acids and cognitive function in women. Womens Health. 2010;6:119–134. doi: 10.2217/whe.09.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Arterioscl Throm Vas. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frasure-Smith N, Lespée F, Julien P. Major depression is associated with lower Omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55:891–896. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Laye S. What do you eat? Dietary omega 3 can help to slow the aging process. Brain Behav Immun. 2013;28:14–15. doi: 10.1016/j.bbi.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomedicine & Pharmacotherapy. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 21.Kiecolt-Glaser JK, Belury MA, Porter K, et al. Depressive symptoms, omega-6:omega-3 fatty acids, and inflammation in older adults. Psychosom Med. 2007;69:217–224. doi: 10.1097/PSY.0b013e3180313a45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsden C, Faurot K, Zamora D, et al. Targeted alterations in dietary n-3 and n-6 fatty acids improve life functioning and reduce psychological distress among patients with chronic headache: a seconday analysis of a randomized trial. Pain. 2015;156:587–596. doi: 10.1097/01.j.pain.0000460348.84965.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsden C, Faurot K, Zamora D, et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: A randomized trial. Pain. 2013;154:2441–2451. doi: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roux CH, Saraux A, Mazieres B, et al. Screening for hip and knee osteoarthritis in the general population: predictive value of a questionnaire and prevalence estimates. Ann Rheum Dis. 2007 doi: 10.1136/ard.2007.075952. [DOI] [PubMed] [Google Scholar]
- 25.King C, Sibille K, Goodin B, et al. Experimental pain sensitivity differs as a function of clinical pain severity in symptomatic knee osteoarthritis. Osteoarthritis and Cartilage. 2013;21:1243–1252. doi: 10.1016/j.joca.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glover TL, Goodin BR, Horgas AL, et al. Vitamin D, race, and experimental pain sensitivity in older adults with knee osteoarthritis. Arthritis Rheum. 2012;64:3926–3935. doi: 10.1002/art.37687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 28.Von Korff M, Ormel J, Keefe FJ, et al. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 29.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 30.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suokas AK, Walsh DA, McWilliams DF, et al. Quantitative sensory testing in painful osteoarthritis: A systematic review and meta-analysis. Osteoarthritis Cartilage. 2012 doi: 10.1016/j.joca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Journal of Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 33.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 34.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality & Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 35.Carver CS, Scheier MF, Segerstrom SC. Optimism. Clin Psychol Rev. 2010 doi: 10.1016/j.cpr.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eggemont L, Bean J, Guralnik J, et al. Comparing pain severity versus pain location in the MOBILIZE Boston study: chronic pain and lower extremity function. Journal of Gerontology Series A:Biological Sciences and Medical Sciences. 2009;64:763–770. doi: 10.1093/gerona/glp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker K, Matthan N, Lichtenstein A, et al. Associations of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis and Cartilage. 2012;20:382–387. doi: 10.1016/j.joca.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fillingim RB. Concise encyclopedia of pain psychology. Binghamton, NY: Haworth Press; 2005. [Google Scholar]
- 40.Chapman C, Tuckett R, Song C. Pain and stress in a systems perspective: Reciprocal neural, endocrine, and immune interactions. J Pain. 2008;9:122–145. doi: 10.1016/j.jpain.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green C, Anderson K, Baker T, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Medicine. 2003;4:277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.