Abstract
We aimed to determine in a randomized trial if young adult black, Hispanic, and white men-who-have-sex-with-men (YMSM) are more likely to complete home-based oral fluid rapid HIV self-testing than either mail-in blood sample collection or medical facility/community organization-based HIV testing. Stratified by race/ethnicity, participants were randomly assigned to use a free oral fluid rapid HIV self-test (n=142), a free mail-in blood sample collection HIV test (n=142), or be tested at a medical facility/community organization of their choice (n=141). Of the 425 participants, completion of assigned test (66% oral fluid vs. 40% mail-in blood sample vs. 56% medical facility/community), willingness to refer (36% oral fluid vs. 20% mail-in blood sample vs. 26% medical facility/community), and legitimate referrals (58% oral fluid vs. 43% mail-in blood sample vs. 43% medical facility/community) were greater in the oral fluid rapid HIV self-test than the mail-in blood sample collection HIV test arm, but not the medical facility/community testing arm. There were no differences in assigned test completion by race/ethnicity. Although free home-based oral fluid rapid HIV self-testing showed moderate promise in facilitating HIV testing among black, Hispanic, and white YMSM, it did not lead to greater testing than directing these YMSM to medical facility/community HIV testing venues.
Keywords: HIV testing, men-who-have-sex-with-men, young adult, clinical trial
Introduction
In 2012, the US Food and Drug Administration approved the OraQuick® In-home rapid HIV test (OraSure Technologies, Bethlehem, PA) for oral fluid HIV self-testing.(1) The test’s positive attributes (e.g., convenience and flexibility of testing, oral swab instead of blood sample collection, privacy, rapid provision of results, ready access via stores or the internet, and self-testing) have been hoped to encourage people at higher risk of an HIV infection to be tested, especially those with reduced access to traditional medical/community testing facilities. Although prior studies of US men-who-have-sex-with-men (MSM) indicate that as many as 80% (2, 3) expressed interest in, or willingness to use the test, utilization of this test is believed to be low.
Black, Hispanic, and white young adult (18–24 year-old) men-who-have-sex-with-men (YMSM) in the US appear to be an ideal population for home-based oral fluid rapid HIV self-testing. YMSM typically have lower healthcare access and lower HIV testing rates than other MSM (4–7), yet suffer from increasing HIV incidence rates, high HIV prevalence, and undiagnosed HIV infections. (4, 6, 8–12) Public health groups or researchers have launched campaigns to facilitate oral fluid rapid HIV self-tests to increase testing among MSM.(13) However, it is not known if YMSM are more likely to use this test compared to other HIV testing methods, if it encourages them to be tested sooner, or if they are more likely to encourage other YMSM to use this test. Comparison to currently available testing options is vital in assessing if expressed interest in and perceived advantages of the oral fluid rapid HIV self-test confer greater test utilization in actual practice. It would be particularly useful to examine these questions among YMSM social media users, since social media could be employed to access this hard-to-reach population quickly and confidentially in future HIV testing campaigns.
The primary objective of this randomized trial was to determine if social media-using black, Hispanic, and white YMSM randomly assigned to free home-based, oral fluid rapid HIV self-testing are more likely to complete HIV testing within a three-month period, as compared to those randomly assigned to either free home-based mail-in blood sample collection HIV testing or testing at a medical facility/community organization (rapid or conventional) of their choice. We further assessed assigned test completion by race/ethnicity. The secondary objectives were to compare these three study arms by use of any, another, or no HIV test; time to HIV test completion; and willingness to refer and referrals of other black, Hispanic, or white YMSM to use the same test they had been assigned in the trial. We also compared pre-trial preferences about HIV testing to actual HIV test use and elucidated participant reasons for their HIV test use in the trial.
Patients and Methods
Study design
This randomized trial was conducted from April 2015 to April 2016 among a random sample of 18–24-year-old social media-using black, Hispanic, or white MSM who completed an internet-based anonymous pre-trial survey about their demographic characteristics, HIV testing history, sexual risk-taking behaviors, and HIV testing preferences.(14) Our institutional review board approved the study.
Participant recruitment
1,934 black, Hispanic, and white YMSM solicited via multiple social media platforms completed the pre-trial survey, and 1,128 indicated that they would be interested in being contacted by email about participating in a randomized trial (See supplemental material for description of these platforms). From these pre-trial survey participants, we randomly selected YMSM by race/ethnicity to participate in the trial. Our goal was to enroll 150 black, 150 Hispanic, and 150 white participants. Random selection, email invitations, and subsequent study enrollment continued until the race/ethnicity quotas were filled or no more participants could be recruited. We based our target sample size on the primary objective of determining if completion of oral fluid rapid HIV self-testing was at least a 10% greater than mail-in blood sample collection or medical facility/community organization testing within and across racial/ethnic groups (α=0.05, power>0.80). Study eligibility criteria for the pre-trial survey and trial were: age 18–24 years; English- or Spanish-speaking; self-identified black, Hispanic, or white race/ethnicity; living in the US; ever having had anal sex with another man; and not being HIV infected (per self-report).
Study protocol
Within each racial/ethnic group, we randomly assigned participants into one of three study arms (1:1:1 randomization) using block sizes of six. Participants in the oral fluid rapid HIV self-test and in the mail-in blood sample collection HIV testing study arms were provided with instructions, an internet-based gift card to purchase the assigned test kit, and weblinks to companies from which to purchase their assigned test via the internet. We selected major national companies that included two-day shipping in the purchase price and accepted the gift card. We verified the ordering and delivery processes prior to conducting the trial. YMSM in these two study arms ordered the tests themselves and chose where and when their assigned tests would be delivered. YMSM in the community organization/medical facility testing arm were asked to get tested at a community organization or medical facility of their choice (conventional or rapid HIV testing). They were provided with a weblink to the National HIV and STD testing website (gettested.cdc.gov) to locate testing locations, but were not given a gift card to facilitate testing. YMSM in all three study arms were instructed to contact the study staff to answer questions or resolve difficulties in participating; however, study staff did not encourage participants to be tested and did not offer testing advice or assistance.
Participants were asked to notify study staff after completing their assigned test using a provided weblink. At one-month intervals for up to three months post-enrollment, participants who had not notified us that they were tested were emailed and asked if they had been tested for HIV. The message did not include motivational messages encouraging them to test. After notifying us via the weblink if they were tested, or at the end of the three-month period, participants completed a brief questionnaire where they indicated if, how, and when they were tested for HIV (whether they had done so with their assigned test or another HIV test); to indicate their main reasons for testing or not testing and for using the test they selected; and to answer questions about their testing experience. In addition, participants who used their assigned test were asked if they were willing to refer other YMSM to use the same test. Those who indicated their willingness could refer up to three other 18–24-year-old black, Hispanic, or white YMSM (whether or not concordant with their own racial/ethnic identity) to be invited via email by study staff to undergo the same type of testing they had been assigned. Participants received a $10 internet-based gift card for completing the follow-up questionnaire (irrespective of whether they tested or not), and up to three $5 gift cards for providing email addresses of other YMSM to be contacted about the study. Study staff reviewed the email addresses provided, and removed those who were already in the study, duplicate referrals, or potential fraudulent referrals (e.g., email addresses that were the same as the referring participant). Following this process, study staff emailed the legitimate-appearing referees with an invitation to be screened for study eligibility; those who indicated they met the aforementioned study eligibility criteria were invited to be tested using the same HIV testing method as the person who referred them, and completed the same follow-up process, but were not asked to provide their own referrals.
Data analysis
Recruitment, enrollment, and retention were summarized, and participants were compared by enrollment and retention status using descriptive statistics. An intention-to-treat analysis was performed without imputation for missing values; those not responding to the follow-up questionnaire were counted as not meeting measured outcomes. The proportions of those tested using their assigned test by study arm and by race/ethnicity were calculated along with corresponding 95% confidence intervals (CIs). The proportions of those using any type of HIV test, a test other than which they were assigned, or not undergoing testing; those willing to refer; and testing completion by referees were compared in a similar manner. Median time to testing (in days) was calculated along with 95% bootstrap percentile CIs. The number of referrals, number of legitimate referrals, and number of successful referees were summarized, and the mean number of referrals was calculated along with corresponding 95% CIs. We also compared participants by study arm according to their responses in the pre-trial survey about which type of HIV test they would prefer to use for their next HIV test and the HIV test they actually used during the trial. Proportions and corresponding 95% CIs were calculated. Two-sample tests of binomial proportions or Mann-Whitney U tests were used for pair-wise study arm comparisons, as appropriate. Stated reasons for completing and for recommending the assigned HIV test to others as well as other qualitative responses were summarized.
Results
Participant characteristics
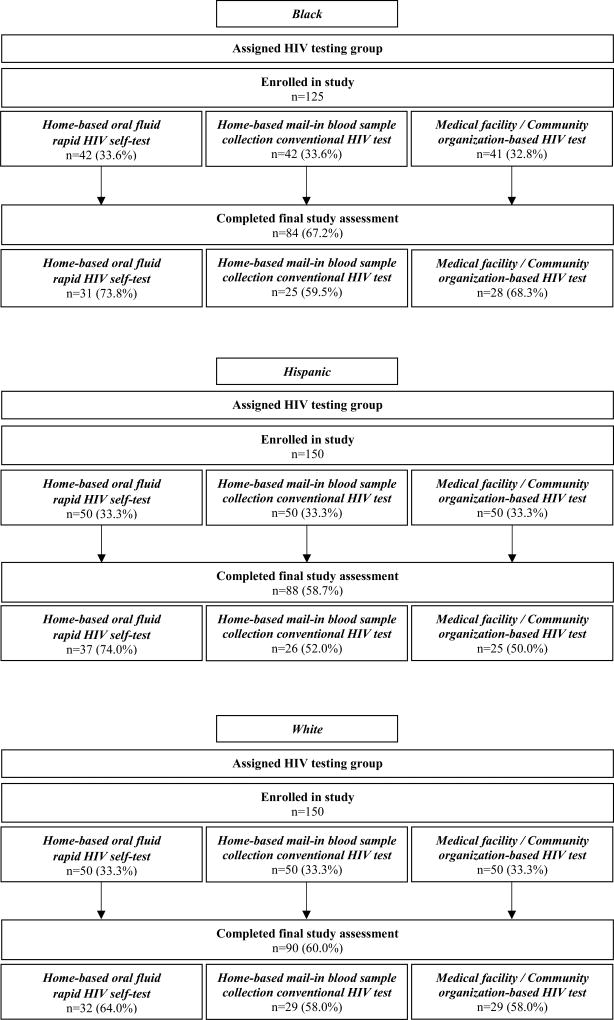
Study enrollment, assignment, and follow-up results are presented in Figure 1. Of the 425 YMSM participants, the median age was 22 years (IQR 21–24); 30% were black, 35% Hispanic, and 35% white; and they lived in 46 of the US states and the District of Columbia. The majority reported condomless anal intercourse with another man within the past six months (67%), and many reported ever having had two or more casual condomless insertive (42%) or receptive (43%) anal sex partners; few had ever injected drugs (2%). Most (83%) had been previously tested for HIV, not during a blood donation. Among these 353 YMSM ever tested for HIV (not during a blood donation), most had their last HIV test at a community organization or medical facility (85%), or used an oral fluid rapid HIV self-test (12%), or a home-based mail-in blood sample collection HIV test (2%). The supplemental material provides comparisons by study arm of participant demographic characteristics, HIV testing history, and HIV risk sexual histories.
Figure 1.
Participant enrollment and follow-up
HIV testing completion and referrals
Of the 425 participants, 59% completed any type of HIV test (54% completed their assigned test, and 5% used a test they were not assigned) and 41% were not tested. Completion of the assigned HIV test was greater in the oral fluid rapid HIV self-test and the community organization/medical facility arms than the mail-in blood sample collection HIV test arm (p<0.01 for both comparisons) (Table 1), but assigned test completion was not greater in the oral fluid rapid HIV self-test than the community organization/medical facility arm (p<0.08). There were no differences by race/ethnicity in completion of assigned HIV test (Table 2).
Table 1.
HIV test completion, time to HIV testing, HIV testing referrals, and referee completion by assigned HIV testing group
|
|
|||
|---|---|---|---|
| Assigned HIV testing group
|
|||
| Home-based oral fluid rapid HIV self-test |
Home-based mail-in blood sample collection conventional HIV test |
Medical facility/Community organization-based HIV test |
|
|
| |||
| n=142 | n=142 | n=141 | |
| HIV testing results | |||
|
| |||
| Completed assigned HIV test, % (95% CI) | 66.2 (58.4, 74.0) | 40.1 (32.1, 48.2) | 56.0 (47.8, 64.2) |
|
| |||
| Completed testing other than assigned HIV test, % (95% CI) | 2.8 (0.1, 5.5) | 11.3 (6.1, 16.5) | 1.4 (0.0, 3.4) |
|
| |||
| Completed any HIV test, % (95% CI) | 69.0 (61.4, 76.6) | 51.4 (43.2, 59.6) | 57.4 (49.3, 65.6) |
|
| |||
| Did not complete any HIV test, % (95% CI) | 31.0 (23.4, 38.6) | 48.6 (40.4, 56.8) | 42.6 (34.4, 50.7) |
|
| |||
| Time to HIV testing* | |||
|
| |||
| Days to complete assigned HIV test, median (95% CI)† | 14.0 (11.0, 17.0) | 17.0 (15.0, 22.0) | 17.0 (11.0, 26.0) |
|
| |||
| Days to complete test other than assigned HIV test, median (95% CI)† | 59.0 (52.0, 72.0) | 12.0 (3.0, 38.0) | 31.0 (3.0, 59.0) |
|
| |||
| Days to complete any HIV test, median (95% CI)† | 14.0 (11.0, 18.0) | 17.0 (13.0, 21.0) | 17.0 (11.0, 26.0) |
|
| |||
| Referral results | |||
|
| |||
| Agreed to refer, % (95% CI) | 35.9 (28.0, 43.8) | 19.7 (13.2, 26.3) | 26.2 (19.0, 33.5) |
|
| |||
| Made at least one referral, % (95% CI) | 33.8 (26.0, 41.6) | 19.7 (13.2, 26.3) | 24.1 (17.1, 31.2) |
|
| |||
| Made at least one legitimate referral‡, % (95% CI) | 28.9 (21.4, 36.3) | 18.3 (11.9, 24.7) | 22.0 (15.1, 28.8) |
|
| |||
| Referral numbers | |||
|
| |||
| Number of referrals | 105 | 61 | 79 |
|
| |||
| Average number of referrals, mean (95% CI) | 0.7 (0.5, 0.9) | 0.4 (0.3, 0.6) | 0.6 (0.4, 0.7) |
|
| |||
| Number of legitimate referrals‡ | 82 | 57 | 71 |
|
| |||
| Average number of legitimate referrals‡, mean (95% CI) | 0.6 (0.4, 0.7) | 0.4 (0.2, 0.6) | 0.5 (0.3, 0.7) |
|
| |||
| n=17 referees | n=14 referees | n=10 referees | |
|
| |||
| Responses by legitimate referees‡ | |||
|
| |||
| Completed referred HIV test, % (95% CI) | 70.6 (48.9, 92.2) | 57.1 (31.2, 83.1) | 70.0 (41.6, 98.4) |
|
| |||
| Completed any HIV test, % (95% CI) | 82.4 (64.2, 100.0) | 57.1 (31.2, 83.1) | 70.0 (41.6, 98.4) |
Participants who had negative time to HIV test completion were assigned a time to testing of zero
Confidence intervals were calculated using bootstrap percentiles
Legitimate referrals were determined by comparing referral vs. referee email addresses
CI = confidence interval
Table 2.
HIV test completion by assigned HIV testing group and race/ethnicity
|
|
|||
|---|---|---|---|
| Race/Ethnicity | |||
|
| |||
| Assigned HIV testing group | Black | Hispanic | White |
| Home-based oral fluid rapid HIV self-test | n=42 | n=50 | n=50 |
|
| |||
| % (95% CI) | % (95% CI) | % (95% CI) | |
|
| |||
| Completed assigned HIV test | 69.0 (55.1, 83.0) | 70.0 (57.3, 82.7) | 60.0 (46.4, 73.6) |
|
| |||
| Home-based mail-in blood sample collection conventional HIV test | n=42 | n=50 | n=50 |
|
| |||
| % (95% CI) | % (95% CI) | % (95% CI) | |
|
| |||
| Completed assigned HIV test | 31.0 (17.0, 44.9) | 38.0 (24.5, 51.5) | 50.0 (36.1, 63.9) |
|
| |||
| Medical facility/Community organization-based HIV test | n=41 | n=50 | n=50 |
|
| |||
| % (95% CI) | % (95% CI) | % (95% CI) | |
|
| |||
| Completed assigned HIV test | 63.4 (48.7, 78.2) | 54.0 (40.2, 67.8) | 52.0 (38.2, 65.8) |
CI = confidence interval
Time to completing assigned or any HIV test was similar across study arms. Willingness to refer (p<0.01), making at least one referral (p<0.03), and average number of legitimate referrals (p<0.04) were greater in the oral fluid rapid HIV self-test than the mail-in blood sample collection HIV test arm. Participation by referees was similar across study arms (21% for oral fluid rapid HIV self-test, 25% for mail-in blood sample collection, and 14% for the community organization/medical facility arm (p<0.46). Other referral outcomes did not differ among the three study arms. Most referees completed their assigned test.
In terms of HIV testing process measures, eighteen participants in the oral fluid rapid HIV self-test arm underwent testing with another person present, and two received assistance from another person for testing. Eight in the mail-in blood sample collection arm underwent testing with another person present, seven reported problems with testing (confusing directions, difficulty obtaining the blood sample, trouble with shipping), and three called the company’s help line for assistance. One person in the oral fluid rapid HIV self-test and one in the community organization/medical facility arm reported having a positive HIV test result.
HIV testing in trial vs. pre-trial preferences and reasons for testing and referral
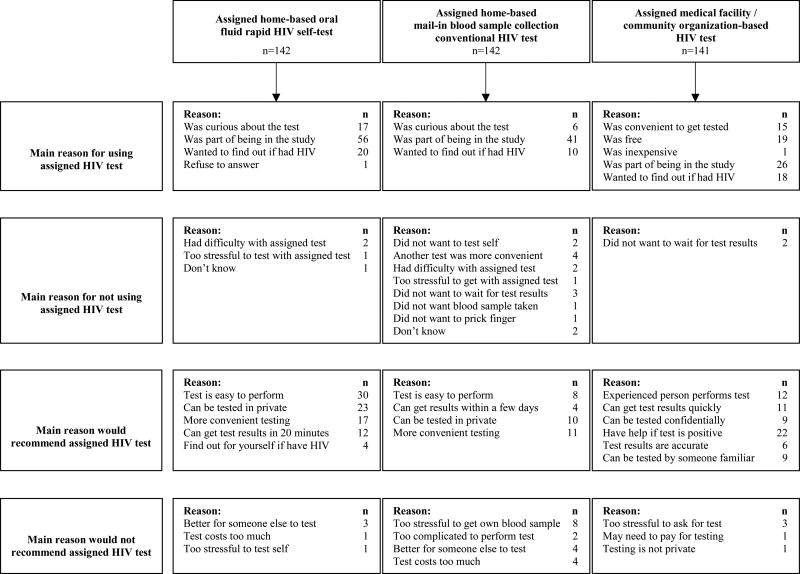
When asked in the pre-trial survey which test they would prefer for their next HIV test, 68% of the 425 YMSM selected HIV testing at a community organization/medical facility, 25% the oral fluid rapid HIV self-test, and 4% the mail-in blood sample collection HIV test. Table 3 displays a comparison of HIV testing use during the trial and pre-trial HIV testing preferences vs. HIV testing assignment in the trial. For the oral fluid rapid HIV self-test arm, test completion despite not being assigned to a preferred test was high; however, over one-quarter of participants assigned to this test and for whom it was not their preferred test did not undergo testing. For the mail-in blood sample collection HIV test arm, over 40% deferred testing when this was not their preferred test. For the oral fluid rapid HIV self-test arm (Table 4), compliance with the trial requirements was reported most often as the reason for completing the assigned test. Testing simplicity, privacy, convenience and availability of rapid results were cited as reasons for recommending this test to others.
Table 3.
Comparisons of HIV testing preferences in pre-trial survey versus HIV testing behavior in trial by assigned HIV testing group
|
|
|||
|---|---|---|---|
| Assigned HIV testing group
|
|||
| Home-based oral fluid rapid HIV self-test |
Home-based mail-in blood sample collection conventional HIV test |
Medical facility/Community organization-based HIV test |
|
|
| |||
| n=142 | n=142 | n=141 | |
| HIV test preferred in pre-trial survey/HIV test taken in trial | % (95% CI) | % (95% CI) | % (95% CI) |
|
| |||
| Preferred assigned HIV test / Tested with assigned HIV test | 16.2 (10.1, 22.3) | 0.7 (0.0, 2.1) | 39.0 (31.0, 47.1) |
|
| |||
| Did not prefer assigned HIV test / Tested with assigned HIV test | 48.6 (40.4, 56.8) | 39.4 (31.4, 47.5) | 16.3 (10.2, 22.4) |
|
| |||
| Preferred assigned HIV test / Did not test with assigned HIV test | 7.7 (3.3, 12.1) | 3.5 (0.5, 6.6) | 29.8 (22.2, 37.3) |
|
| |||
| Used mail-in blood sample: 0.0 | Used rapid: 0.7 | Used rapid: 0.7 | |
| Used community/medical: 0.7 | Used community/medical: 0.0 | Used mail-in blood sample: 0.0 | |
| Did not test: 7.0 | Did not test: 2.8 | Did not test: 29.1 | |
|
| |||
| Did not prefer assigned HIV test / Did not test with assigned HIV test | 26.1 (18.8, 33.3) | 52.8 (44.6, 61.0) | 13.5 (7.8, 19.1) |
|
| |||
| Used mail-in blood sample: 0.7 | Used rapid: 4.9 | Used rapid: 0.7 | |
| Used community/medical: 1.4 | Used community/medical: 5.6 | Used mail-in blood sample: 0.0 | |
| Did not test: 23.9 | Did not test: 42.3 | Did not test: 12.8 | |
Note: 9 participants did not state their preferred HIV test, and are omitted from the table.
CI = confidence interval
Table 4.
Stated reasons for HIV testing behavior in trial and HIV testing recommendations
Discussion
This randomized trial provides insight into the yield of a free home-based oral fluid rapid HIV self-testing campaign that could be directed towards social media-using black, Hispanic, and white YMSM in the US. In a similar approach as our investigation, researchers and public health officials recently reported results of internet-based distribution of oral fluid rapid HIV self-testing programs in the US. (13) Rosengren, et al. used the social media site Grindr™ during a one-month period in 2014 to advertise free oral fluid rapid HIV self-tests in Los Angeles, CA, to adult black or Hispanic MSM.(15) For their feasibility demonstration study, there were 333 test requests, 74% for tests to be mailed to the recipients, 17% for vouchers, and 8% for vending machine codes. Other researchers have described the feasibility of distributing these tests to MSM or other populations through community organizations, direct outreach, or other non-internet-based means in the US (16, 17) and other countries (18, 19).
In contrast to the promising results of these feasibility studies and outreach campaigns, HIV test completion, time to testing, and referrals of others for oral fluid rapid HIV self-testing was not greater than asking YMSM to undergo HIV testing at community organizations/medical facilities in this study. We were surprised that oral fluid rapid HIV self-testing, despite its perceived advantages, ready availability, and previous research indicating interest in the test, was not embraced more strongly than community organization/medical facility testing. On the other hand, consistent with how infrequently it is used, it was apparent that the mail-in blood sample collection HIV test was not a favored testing methodology by YMSM in this sample.
How can this investigation’s results be interpreted in light of the need to increase HIV testing among black, Hispanic, and white YMSM? One interpretation is that perceived barriers of the oral fluid rapid self-test (e.g., concerns about its accuracy and reticence to self-test) make YMSM apprehensive in using it. If true, interventions to build confidence in its accuracy and ease of use as well, such as through testing demonstrations and testimonials on the internet or at clinical or non-clinical settings, might be helpful. As YMSM consumers become more accustomed to the test, apprehension might decrease. Another interpretation is that community organization/medical facility testing is viewed as the accepted standard for testing by YMSM, perhaps because of the other services offered there, support provided during testing, or its notoriety in the community. If true, perceived concerns about these venues inhibiting HIV testing among this population are not accurate. If this explanation is correct, our investigation also indicates that YMSM can be encouraged via the internet to seek testing in their community with good results and lower costs to them. Interventions instead could be directed at facilitating testing at these locations, perhaps instead of distributing oral fluid rapid HIV self-testing to YMSM. On the other hand, there is no one-size-fits-all approach to HIV testing, and the results of this trial cannot be deemed definitive. Access, availability, confidence, convenience, cost, reputation, and other tangible and intangible considerations drive testing behavior. In a more optimistic view, the study results might suggest that home-based oral fluid rapid HIV self-testing might be at least as acceptable to YMSM as being asked to test in their local communities. If testing access and inconvenience override ability to seek testing through traditional means, then oral fluid rapid HIV self-testing might be a good alternative. Even though research can indicate how public health efforts and expenditures might be directed optimally, multiple options for testing need to be readily available. In addition, the costs of having testing available in community organizations, given their fixed costs, might be greater than providing for funding for home-based HIV testing. Further larger-scale investigations comparing testing utilization internet-based approaches among this higher HIV risk population are needed to guide public policy.
As part of this study, we also investigated if a chain-referral process could be generated that would enable additional black, Hispanic, or white YMSM to be tested. Such a chain could capitalize on social media and social networking (online or offline relationships) to reach other YMSM who might not participate in research or public health campaigns. However, only 20% of referees enrolled in the study and some referrals were fraudulent. Fraudulent referrals have been a problem observed in other internet-based HIV studies (20, 21), perhaps due to the financial incentives involved. On a positive note, 66% of referees used their assigned test, which is a similar proportion as those enrolled in the trial, albeit a much smaller sample. Future HIV testing campaigns will need to examine methods to: reduce or mitigate fraudulent referrals, particularly if incentives are involved; encourage referrals; and persuade referees to be tested. Future approaches might consider direct referrals by campaign participants, instead of through an intermediary, and developing interventions that promote referrals and referee participation.
This investigation had several limitations. Despite enrolling a diverse sample of black, Hispanic, and white YMSM, we cannot verify that the observed results definitively represent those that would be obtained by studies involving other YMSM in the US. We do believe that using multiple social media platforms to recruit participants across the US and randomly selecting participants for the trial assisted with promoting the validity of the findings. As expected for anonymous internet-based studies, follow-up was not optimal, which could have affected the observed results. Also, larger sample sizes could have identified differences not detected statistically in this investigation. We also relied on self-report of HIV testing completion, which could be inaccurate. However, we would not expect self-reporting of test completion to differ by HIV testing methodology. The study’s sample size precluded meaningful sub-analyses by factors such as sexual self-identity, socioeconomic and environmental/community-related influences, reasons for testing, self-perceived or reported risk behaviors—whether or not these potential factors were assessed as part of the study. Future larger studies on this topic should investigate the potential influence of factors on HIV testing behavior because they might further impact the design and implementation of interventions to increase HIV testing among this higher HIV risk population.
In conclusion, free, home-based oral fluid rapid HIV self-testing showed moderate promise in facilitating HIV testing among black, Hispanic, and white YMSM in the US recruited through social media, but was not greater than directing these men to use traditional community organization/medical facility HIV testing venues. Further investigation is needed to determine if additional interventions can be developed to increase HIV testing utilization with any of the existing modalities and how public health campaigns should be directed for this higher HIV risk population.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institute of Nursing Research (R21 NR023869).
Footnotes
Findings from this investigation were presented at the 9th IAS Conference on HIV Science (IAS 2017) in Paris, France, July 23–26, 2017.
ClinicalTrials.gov Identifier: NCT02369627
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest: Dr. Rosenberger previously was a consultant for Online Buddies, Inc. Dr. Mayer has received unrestricted research grants from Gilead Sciences and ViiV Healthcare. Drs. Bauermeister, Clark, Liu, and Merchant declare no conflicts of interest.
References
- 1.McNeil DG. Rapid HIV home test wins federal approval. The New York Times. 2012 Jul 3; 2012. [Google Scholar]
- 2.Figueroa C, Johnson C, Verster A, Baggaley R. Attitudes and Acceptability on HIV Self-testing Among Key Populations: A Literature Review. AIDS Behav. 2015;19(11):1949–65. doi: 10.1007/s10461-015-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carballo-Diéguez A, Frasca T, Dolezal C, Balan I. Will Gay and Bisexually Active Men at High Risk of Infection Use Over-the-Counter Rapid HIV Tests to Screen Sexual Partners? Journal of Sex Research. 2012;49(4):379–87. doi: 10.1080/00224499.2011.647117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margolis AD, Joseph H, Belcher L, Hirshfield S, Chiasson MA. 'Never Testing for HIV' Among Men Who Have Sex with Men Recruited from a Sexual Networking Website, United States. AIDS Behav. 2011 doi: 10.1007/s10461-011-9883-4. [DOI] [PubMed] [Google Scholar]
- 6.Vital Signs: HIV Infection, Testing, and Risk Behaviors Among Youths - United States. MMWR Morb Mortal Wkly Rep. 2012;61(47):971–6. [PubMed] [Google Scholar]
- 7.Oster AM, Johnson CH, Le BC, Balaji AB, Finlayson TJ, Lansky A, et al. Trends in HIV prevalence and HIV testing among young MSM: five United States cities, 1994–2011. AIDS Behav. 2014;18(Suppl 3):S237–47. doi: 10.1007/s10461-013-0566-1. [DOI] [PubMed] [Google Scholar]
- 8.Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–9. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trends in HIV/AIDS diagnoses among men who have sex with men--33 states, 2001–2006. MMWR Morb Mortal Wkly Rep. 2008;57(25):681–6. [PubMed] [Google Scholar]
- 10.Subpopulation estimates from the HIV incidence surveillance system--United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(36):985–9. [PubMed] [Google Scholar]
- 11.Pathela P, Braunstein SL, Schillinger JA, Shepard C, Sweeney M, Blank S. Men Who Have Sex With Men Have a 140-Fold Higher Risk for Newly Diagnosed HIV and Syphilis Compared With Heterosexual Men in New York City. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e318230e1ca. [DOI] [PubMed] [Google Scholar]
- 12.Torrone EA, Bertolli J, Li J, Sweeney P, Jeffries WLt, Ham DC, et al. Increased HIV and Primary and Secondary Syphilis Diagnoses Among Young Men-United States, 2004–2008. J Acquir Immune Defic Syndr. 2011;58(3):328–35. doi: 10.1097/QAI.0b013e31822e1075. [DOI] [PubMed] [Google Scholar]
- 13.Estem KS, Catania J, Klausner JD. HIV Self-Testing: a Review of Current Implementation and Fidelity. Current HIV/AIDS reports. 2016;13(2):107–15. doi: 10.1007/s11904-016-0307-y. [DOI] [PubMed] [Google Scholar]
- 14.Merchant RC, Clark MA, Liu T, Rosenberger JG, Romanoff J, Bauermeister J, et al. Preferences for oral fluid rapid HIV self-testing among social media-using young black, Hispanic, and white men-who-have-sex-with-men (YMSM): implications for future interventions. Public health. 2017;145:7–19. doi: 10.1016/j.puhe.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosengren AL, Huang E, Daniels J, Young SD, Marlin RW, Klausner JD. Feasibility of using GrindrTM to distribute HIV self-test kits to men who have sex with men in Los Angeles, California. Sexual health. 2016 doi: 10.1071/SH15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marlin RW, Young SD, Bristow CC, Wilson G, Rodriguez J, Ortiz J, et al. Piloting an HIV self-test kit voucher program to raise serostatus awareness of high-risk African Americans, Los Angeles. BMC public health. 2014;14:1226. doi: 10.1186/1471-2458-14-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods WJ, Lippman SA, Agnew E, Carroll S, Binson D. Bathhouse distribution of HIV self-testing kits reaches diverse, high-risk population. AIDS care. 2016;28(Suppl 1):111–3. doi: 10.1080/09540121.2016.1146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choko AT, MacPherson P, Webb EL, Willey BA, Feasy H, Sambakunsi R, et al. Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study. PLoS Med. 2015;12(9):e1001873. doi: 10.1371/journal.pmed.1001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pant Pai N, Behlim T, Abrahams L, Vadnais C, Shivkumar S, Pillay S, et al. Will an unsupervised self-testing strategy for HIV work in health care workers of South Africa? A cross sectional pilot feasibility study. PLoS One. 2013;8(11):e79772. doi: 10.1371/journal.pone.0079772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauermeister J, Pingel E, Zimmerman M, Couper M, Carballo-Dieguez A, Strecher VJ. Data Quality in web-based HIV/AIDS research: Handling Invalid and Suspicious Data. Field methods. 2012;24(3):272–91. doi: 10.1177/1525822X12443097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teitcher JE, Bockting WO, Bauermeister JA, Hoefer CJ, Miner MH, Klitzman RL. Detecting, preventing, and responding to "fraudsters" in internet research: ethics and tradeoffs. The Journal of law medicine & ethics : a journal of the American Society of Law Medicine & Ethics. 2015;43(1):116–33. doi: 10.1111/jlme.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.