Abstract
Background
It is well known physical activity (PA) plays a role in the prevention of type 2 diabetes (T2D). However, the extent to which PA may impact T2D risk among different race-ethnic groups is unknown. Therefore, the purpose of this study was to systematically examine the effect modification of race-ethnicity on PA and T2D.
Methods
PubMed and Embase databases were systematically searched through June 2016. Study assessment for inclusion was conducted in three phases: 1) title review (N= 13,022), 2) abstract review (N=2,200), and 3) full text review (N=265). A total of 27 studies met the inclusion criteria and were used in the analysis. Relative This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/jdb.12574 risks (RRs) and 95% confidence intervals (CIs) were extracted and analyzed using the Comprehensive Meta-Analysis software. All analyses used a random-effects model.
Results
A significant protective summary RR, comparing the most active group to the least active PA group, was found for non-Hispanic White (RR 0.71, 95% CI 0.60–0.85), Asians (RR 0.76, 95% CI 0.67–0.85), Hispanics (RR 0.75, 95% CI 0.64–0.89), and American Indians (RR 0.73, 95% CI 0.60–0.88). The summary effect for non-Hispanic Blacks (RR 0.91, 95% CI 0.76–1.08) was non-significant.
Conclusions
The results of this study indicate that PA (comparing most to least active groups) provides significant protection from T2D with the exception of non-Hispanic Blacks. The results also indicate a need for race-ethnicity specific reporting of RRs in prospective cohort studies that incorporate multi-ethnic samples.
Keywords: Race, ethnicity, diabetes, risk, physical activity
INTRODUCTION
The prevalence of type 2 diabetes among adults in the United States (U.S.) is estimated to be anywhere from 9.3–14.5%, depending upon the dataset and diagnostic criteria 1, 2. Furthermore, by the year 2050, the type 2 diabetes prevalence in the U.S. is projected to reach upwards of 21–33% 3. However, substantial race-ethnic disparities exist in the prevalence of type 2 diabetes. At 15.9%, American Indian/Alaskan Natives have the highest estimated prevalence of type 2 diabetes 1. The next highest prevalence rates are found among Non-Hispanic Blacks (NHB, 13.2%), Hispanics (12.8%), Asians (9.0%) and those who are non-Hispanic White (NHW,7.6%)1. Race-ethnic disparities in the prevalence of type 2 diabetes are expected to persist4: the projected 50 year increase in prevalence of type 2 diabetes will be highest among NHBs, followed by Hispanics and other ethnicities compared to NHW.
Physical activity (PA) is an important component of type 2 diabetes prevention initiatives5 and has been shown to reduce the risk of type 2 diabetes 6. However, the extent of type 2 diabetes protection associated with PA has yet to be fully examined in regards to effect modification by race-ethnicity. A narrative review by Gill et al. 7 suggested varying thresholds of PA protection against type 2 diabetes may across race-ethnic groups. Moreover, the authors also suggested that the current U.S. Department of Health and Human Services (DHHS) uniform guideline of 150 min moderate-intensity aerobic PA per wk 8 may not provide equal protection against developing type 2 diabetes across race-ethnic groups.
The 2008 Physical Activity Guidelines Committee Report 9, indicates significant need to further understand the effects of PA on diabetes risk among ethnically-diverse populations. While meta-analyses have examined and established a clear inverse relationship between PA and type 2 diabetes risk 6, 10, to our knowledge, no meta-analysis has assessed effect modification of this relationship by race-ethnicity. Therefore, the purpose of this systematic review and meta-analysis was to compile the evidence from prospective cohort studies on potential effect modification of the aerobic PA and type 2 diabetes risk relationship by race-ethnic groups.
METHODS
Data sources and searches
Systematic searches of the literature were independently conducted in PubMed and Embase by two authors (W.R.B. and E.C.F.). A modified version of the search criteria employed by Aune et al. 6 was used: (physical activity OR exercise OR sports OR walking OR biking OR running OR fitness OR exercise test OR inactivity OR sedentary activity) AND diabetes AND (case–control OR retrospective OR cohort OR cohorts OR prospective OR longitudinal OR follow-up OR cross-sectional OR trial) AND (ethnic OR ethnic group OR race OR racial group). Furthermore studies included in another meta-analysis we screened as well 6. Standard guidelines for conducting and reporting meta-analyses were followed 11.
Study Selection
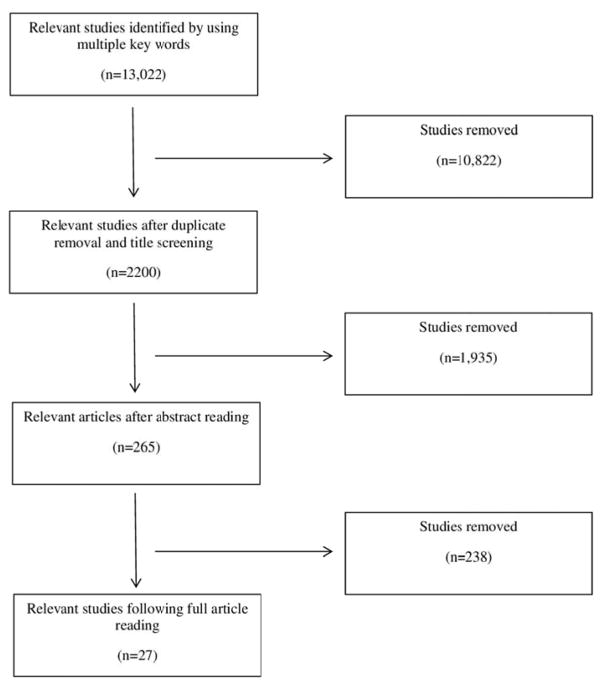
Research articles were examined for the following eligibility criteria: human participants who were without type 2 diabetes at the start of the studies and were adults (≥18 years) at the time of follow-up; assessed aerobic-based PA; published or available in English; were prospective cohort studies; assessed and reported the race-ethnicity specific relative risks (RR) for type II diabetes; adjusted risk estimates for age; and allowed for the determination of a most versus least physically active group. The article screening process is presented in Figure 1. A total of 27 individual articles met the full eligibility criteria 12–38. Race-ethnic groups identified and used in the analyses were NHW, NHB, Asian, Hispanic, and American Indian.
Figure 1.
Prisma Flow Chart
Data Extraction and Quality Assessment
Relative risks and 95% confidence intervals (CIs) for diabetes were extracted and entered into Comprehensive Meta-Analysis (CMA) software version 3.0 (Biostat, Englewood, NJ, USA, 2014). Random-effects models were used for all analyses, given that true effects are likely to vary across studies (rather than a fixed-model, which assumes the same value or true effect for all studies) 39. In all studies, the RR estimates extracted were those comparing the highest to the lowest level of PA. A second analysis was conducted among studies 17, 21, 28, 37, 38 that used the current DHHS moderate-intensity aerobic PA guideline of at least 150 min/wk as their demarcation point for PA 8 (i.e., comparing those who met the recommendations to those who did not). Race-ethnic groups utilized in the secondary analysis included all identified previously except American Indians.
Quality assessment was performed using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies 40 which uses a 14 question scale (higher score corresponding to higher quality) to assess quality. All studies were independently rated by two researchers (W.B. and E.F.); all studies were rated as ‘good’ (average Quality Assessment score= 10.9, range 10–11). Since the studies were all of good quality, analyses examining the potential impact of differential study quality on the main RR effects were not conducted.
Data Synthesis and Analysis
Data analysis was conducted using CMA software version 3.0. Heterogeneity was quantified using I2; a descriptive index that estimates the ratio of true variation (heterogeneity) to total variation across the observed effect sizes 39. An overall main effect of PA on type 2 diabetes was calculated for each race-ethnic group. A second analysis was conducted to assess the overall effect of meeting the 2008 DHHS moderate-intensity aerobic PA recommendation on type 2 diabetes risk 8. Significance for the main effects was set at 0.05. Potential publication bias was examined using a funnel plot.
RESULTS
A total of 1,150,574 participants were identified across all studies meeting the inclusion criteria. Table 1 illustrates the characteristics of the individual studies (N=27). Duration of follow-up ranged from two 30 to twenty-eight years 35. Studies were conducted in the U.S. (n=9) and internationally (n=17), with one study conducted in both the U.S. and Canada 30. The method for identifying cases of type 2 diabetes varied by study and included ascertainment by medical records; report of insulin or medication use; 2 hr oral glucose tolerance tests; fasting plasma glucose, or self-report of a physician diagnosis. Potential publication bias was not detected in the funnel plot (supplemental figure 1 [supp. 1.]).
Table 1.
Study characteristics
| Author (ref) | Race | Country | Gender | Age(years) | N | Physical activity measure | Follow-up (years) | Adjustments |
|---|---|---|---|---|---|---|---|---|
| Burchfield, 1995 | Asian | U.S. | M | 45 – 68 | 6,815 | hrs x estimated O2 consumption | 6 | age, BMI, subscapular/triceps skinfold ratio, systolic blood pressure, triglycerides, glucose, hematocrit, parental history of diabetes |
| Burke, 2007 | NHW | Australia | M/F | 15 – 88 | 514 | d/wk | 14 | sex, age, BMI, location |
| Fan, 2015 | Asian | China | M/F | 35 – 74 | 6,348 | MET-hrs | 7.9 | age, sex, geographic region (north or south), educational level (0–6, 7–9, or ≥10 yr), cigarette smoking (never, ever, or current), alcohol consumption (yes or no), BMI, waist circumference |
| Fretts, 2009 | American Indians | U.S. | M/F | 45 – 74 | 1,651 | MET-hrs/wk | 10 | age, study site, sex, education (less than high school, high school, post-high school), cigarette smoking (never, ever, current), alcohol use (never, ever, current), FH of diabetes |
| Fretts, 2014 | American Indians | U.S. | M/F | 18 – 74 | 1,639 | Steps/d | 8 | age, sex, site, education (years), FH of diabetes |
| Honda, 2015 | Asian | Japan | M/F | 30 – 64 | 26,628 | MET-hrs | 5.2 | age, sex, shift work, sleep duration, alcohol consumption, smoking, hypertension, a family history of diabetes, occupational activity, and walking for commuting to and from work |
| Hsia, 2005 | NHW, Asian, Non-Hispanic Black, Hispanic, American Indian | U.S. | F | No range presented | Total: 86,708 NHW: 74,240 Non-Hispanic Black: 6,465 Asian: 2,445 Hispanic: 3,231 American Indian: 327 | MET-hrs/wk | 4 – 8 | alcohol (past/never vs current drinker), education, smoking, hypertension, hypercholesterolemia, dietary fiber (g), percent energy from carbohydrate |
| Hu, 2004 | NHW | Finland | M/F | 45 – 64 | 4,369 | min | 9.4 | age, sex, study year, systolic blood pressure, smoking status, education, BMI |
| James, 1998 | Non-Hispanic Black | U.S. | M/F | 30 – 55 | 916 | Categorized by min into inactive, moderate or strenuous | 5 | age, sex, education, BMI, WHR |
| Joseph, 2016 | NHW, Asian, Non-Hispanic Black, Hispanic | U.S. | M/F | 45 – 84 | Total: 5,348 NHW: 2,277 Non-Hispanic Black: 1,293 Asian: 676 Hispanic: 1,102 | MET-min/wk | 11.1 | age, education, sex, study site, race/ethnicity, occupational status, alcohol use, estimated glomerular filtration rate, other cardiovascular health components |
| Koloverou, 2014 | NHW | Greece | M/F | 32 – 59 | 1,485 | Total min | 10 | age |
| Kriska, 2003 | American Indian | U.S. | M/F | 15 – 59 | 1,728 | MET-hrs/wk | 6 | age, BMI |
| Lee, 2012 | Asian | Korea | M | ≥18 | 675,496 | min/wk | 7.5 | age, smoking status, alcohol intake, hypertension, parental diabetes, baseline glucose, BMI |
| Ma, 2012 | NHW, Asian, Non-Hispanic Black, Hispanic | U.S. | F | 50 – 79 | Total: 158,833 NHW: 133,541 Non-Hispanic Black: 14,618 Asian: 4,190 Hispanic: 6,484 | MET-hrs/wk | 10.4 | age, FH of diabetes, hormone therapy use, study arm, each lifestyle risk factor |
| Nakanishi, 2004 | Asian | Japan | M | 35 – 59 | 2,924 | Daily energy expenditure (kcals) | 7 | age, FH of diabetes, alcohol consumption, cigarette smoking, BMI, weekly energy expenditure on physical exercise, systolic blood pressure, HDL cholesterol, triglycerides |
| Okada, 2000 | Asian | Japan | M | 35 – 60 | 6,013 | d/wk | 59,966 person-years | age, BMI, alcohol consumption, smoking habits, blood pressure, parental history of Type 2 diabetes |
| Panagiotakos, 2008 | NHW | Greece | M/F | >18 | 1,806 | MET-min/wk | 5 | age |
| Shi, 2013 | Asian | China | M | 40 – 74 | 51,464 | METS | 5.4 | age, energy intake, smoking, alcohol consumption, education level, occupation, income FH of diabetes |
| Tonstad, 2013 (subsample of Non-Hispanic Blacks) | Non-Hispanic Black | U.S. and Canada | M/F | ≥30 | 7,160 | d/wk | 2 | age |
| Tsai, 2015 | Asian | Taiwan | M/F | ≥53 | 2,995 | d/wk | 14 | age |
| Villegas, 2006 | Asian | China | F | 40 – 70 | 70,658 | METS | 4.6 | age, kcal/d, education level, income level, occupation, smoking, alcohol, hypertension, chronic diseases |
| Villegas, 2009 | Asian | China | F | 40 – 70 | 62,227 | METs/d | 4.6 | age, WHR, BMI, kcal/d, alcohol consumption, smoking, education level, income level, occupation, hypertension |
| Waki, 2005 | Asian | Japan | M/F | 40 – 59 | 28,896 | times/wk engaged in activity | 10 | age |
| Waller, 2010 | NHW | Finland | M/F | ≥18 | 20,487 | MET-hrs/d | 28 | age, BMI |
| Wang. 2010 | American Indians | U.S. | M/F | 45 – 74 | 1,677 | MET-hrs/wk | 7.8 | age, sex |
| Xu, 2012 | Asian | China | M/F | ≥35 | 3,031 | min/wk | 3 | age, sex, residence area, educational attainment, BMI category, cigarette smoking, alcohol drinking, TV viewing, vegetables intake, meat intake, diagnosed hypertension |
| Xu, 2015 | Asian | China | M/F | ≥35 | 4,550 | min/wk | 3 | age, gender, educational attainment, FH/PA, body weight status, cigarette smoking, alcohol drinking, TV viewing, vegetables intake, meat intake, diagnosed hypertension |
U.S. = United States, M = Male, F = Female, MET = Metabolic Equivalent, hr = Hour, BMI = Body Mass Index, WHR = Waist-to-hip ratio, kcal = Kilocalorie, FH = Family History, TV = Television, HDL = High-density lipoprotein cholesterol
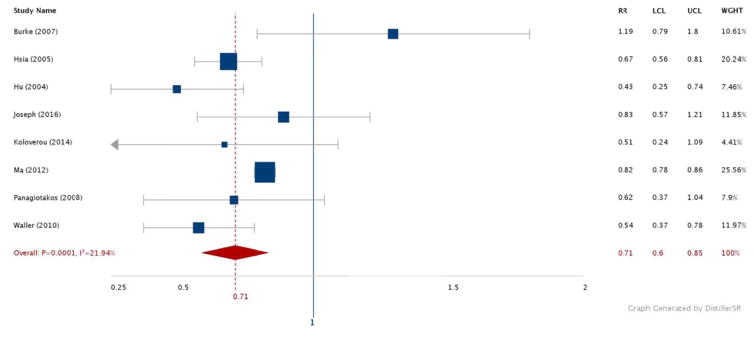
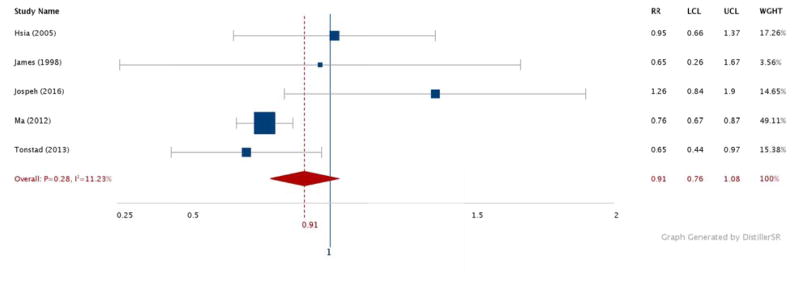
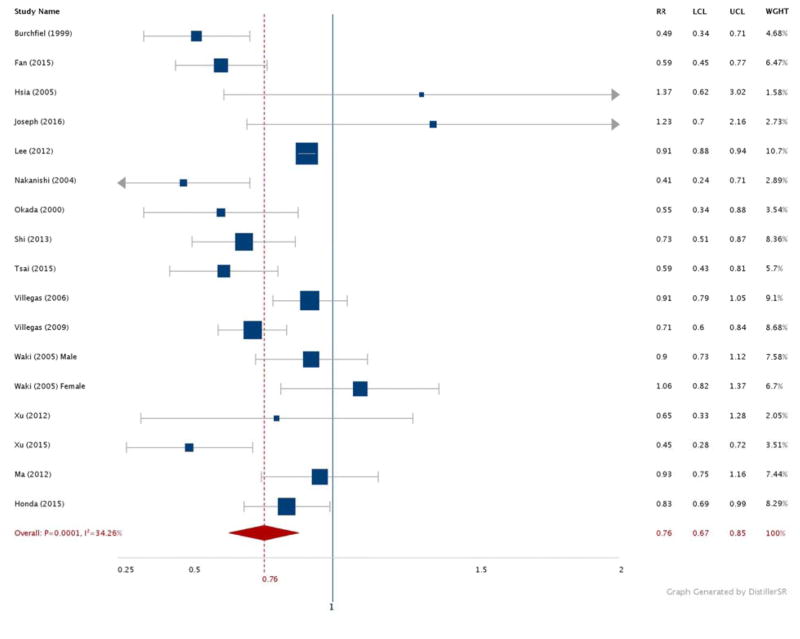
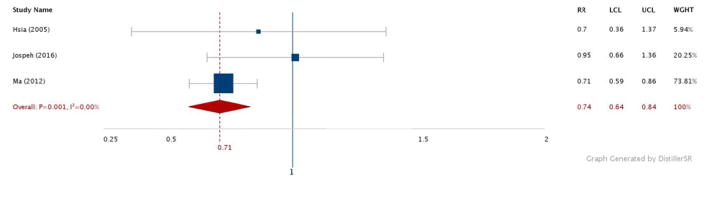
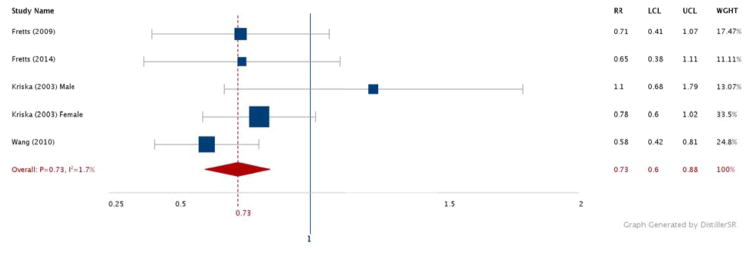
Figures 2–6 show the results comparing the most active to the least active groups among the race-ethnic groups examined (Figure 2: NHW, Figure 3: Asian, Figure 4: Hispanic, Figure 5: American Indian, Figure 6: NHB). Significant, and similar, summary RRs for PA and type 2 diabetes were found for NHWs (RR 0.71, 95% CI 0.60–0.85), Asians (RR 0.76, 95% CI 0.67–0.85), Hispanics (RR 0.74, 95% CI 0.64–0.84), and American Indians (RR 0.73, 95% CI 0.60–0.88). The summary effect for NHBs (RR 0.91, 95% CI 0.76–1.08) did not attain statistical significance.
Figure 2.
Relative risks for diabetes by aerobic physical activity (most vs. least): NHW adults.
N = 238,719. RR: Relative Risk. LCL: Low 95% Confidence Limit. UCL: Upper 95% Confidence Limit. WGHT: Weight
Figure 6.
Relative risks for diabetes by aerobic physical activity (most vs. least): Non-Hispanic Black adults
N = 30,452. RR: Relative Risk. LCL: Low 95% Confidence Limit. UCL: Upper 95% Confidence Limit. WGHT: Weight
Figure 3.
Relative risks for diabetes by aerobic physical activity (most vs. least): Asian adults.
N = 928,319. RR: Relative Risk. LCL: Low 95% Confidence Limit. UCL: Upper 95% Confidence Limit. WGHT: Weight
Figure 4.
Relative risks for diabetes by aerobic physical activity (most vs. least): Hispanic adults.
N = 10,817. RR: Relative Risk. LCL: Low 95% Confidence Limit. UCL: Upper 95% Confidence Limit. WGHT: Weight
Figure 5.
Relative risks for diabetes by aerobic physical activity (most vs. least): American Indian adults.
N = 7,022. RR: Relative Risk. LCL: Low 95% Confidence Limit. UCL: Upper 95% Confidence Limit. WGHT: Weight
In analyses examining the 2008 DHHS moderate-intensity aerobic PA recommendation and diabetes risk by race-ethnicity (data not shown), a suggestion of a significant trend was observed for NHWs (RR 0.75. 95% CI 0.55–1.01, p=0.06) and Asians (RR 0.80, 95% CI 0.64–1.01, p=0.06), but the estimates did not attain statistical significance. The summary effects for meeting the 2008 DHHS moderate-intensity aerobic PA recommendation and diabetes risk among NHBs (RR 1.26, 95% CI 0.84–1.90) and Hispanics (RR 0.95, 95% CI 0.66–1.65) did not attain statistical significance.
Sensitivity analyses were conducted to examine the effect of removing a single study on the overall summary effects by race-ethnic group (data not shown). No significant changes were found to the race-ethnic specific summary RRs with the exception of Hispanics. When removing Hsia et al. 18 from the Hispanic analysis, statistical significance was lost (RR 0.79, 95% CI 0.60–1.03, p=0.09). Furthermore, when removing Ma et al. 25 from the analysis, similar results were found (RR 0.89, 95% CI 0.65–1.22, p=0.46).
DISCUSSION
This systematic review and meta-analysis provides insight into race-ethnic differences in the effect of aerobic PA on type 2 diabetes risk. With the exception of NHBs, a similar magnitude of protection was found comparing the most active to the least active groups, ranging from 24% (among Asians) to 29% (among NHWs). The summary estimate for NHBs, though protective, did not attain statistical significance. The results of this analysis add to the existing literature on PA and type 2 diabetes 6, 10 by demonstrating effect modification by race-ethnicity.
Previous work 7, 41–43 has also found that there may be race-ethnicity specific differences in the volume of aerobic PA necessary to elicit protection against type 2 diabetes. Celis-Morales et al. 41 found that Asian men needed 266 min/wk of moderate aerobic PA in order to achieve similar cardiometabolic profiles (inclusive of fasting glucose and fasting insulin) as NHW men participating in 150 min/wk of moderate aerobic PA. Steinbrecher et al. 42, in a study contrasting race-ethnic specific differences of type 2 diabetes risk with PA, found that moderate intensity PA was associated with lower risk for type 2 diabetes only among NHW males and females. In the same study, Native Hawaiians and Japanese Americans were found to receive no protection from moderate intensity PA. However, vigorous intensity sport activity was associated with lower risk for type 2 diabetes across all race-ethnic groups independent of gender. These results indicate that higher volumes, as well as a higher intensity, may be needed to provide similar protection from type 2 diabetes across various race-ethnic groups.
Among the studies included the current analysis, four studies directly reported the race-specific RRs based upon meeting versus not meeting the current DHHS moderate-intensity aerobic PA recommendation and type 2 diabetes risk. The summary results of these studies suggest a trend towards significant protection against type 2 diabetes for Asians and NHWs, but not NHBs or Hispanics. While these results need to be interpreted with caution, this illustrates a lack of studies specifically examining how meeting the current moderate-intensity aerobic PA guideline relates to type 2 diabetes risk. Previous work in the Diabetes Prevention Program 5 (designed to examine how lifestyle change inclusive of PA, dietary change and weight loss impacts type 2 diabetes risk) indicated that lifestyle intervention, inclusive of 150 min/wk of moderate aerobic PA, significantly reduced the incidence of type 2 diabetes in NHWs, NHBs, Asians, Hispanics and American Indians. It is possible that meeting the recommendations may not have a significant impact on diabetes risk reduction among NHBs without the addition of weight loss and dietary intervention; however more research is needed.
The discussion above, in addition to the results of the current meta-analysis, prompted the current authors to examine/review possible underlying physiological mechanisms that explain the race-ethnic differences in the protective role of PA. Further, the current results warranted specific focus on these mechanisms in NHBs.
Mechanisms proposed
There are several physiological mechanisms proposed that could explain the lack of significance found for NHBs in the current analysis. These include: 1) genetic predisposition, 2) compromised hepatic suppression of endogenous glucose production (EGP) in response to insulin, 3) lower insulin sensitivity (Si) in the peripheral tissues (specifically skeletal muscle) despite similar volumes of aerobic PA, 4) a down regulation of insulin receptors driven by hyperinsulinemia and decreased hepatic insulin clearance, 5) increases in intramuscular adipose tissue (IMAT) and intra-myocellular lipid content (IMCL) deposits, and 6) skeletal muscle fiber type differences.
It is well known that decreases in Si are related to an increased risk for type 2 diabetes 44, 45. Lakoski and colleagues 46, when comparing NHBs to NHWs, found similar changes in the homeostatic model assessment of insulin resistance (HOMA-IR) for every 10 minute increase in MVPA, moderate PA, or vigorous PA. Among NHB and NHW women, Irwin and colleagues 47 reported similar decreases in fasting insulin levels for every 90 MET-min/d change. Another study conducted by Hasson et al. 48 found no differences in the acute improvements in whole-body Si comparing NHBs to NHWs following a single 75 minute bout of exercise at 75% VO2 max. The results of these studies indicate that NHBs and NHW have similar physiological responses in the change in Si from PA. However, similar levels of Si among NHWs and NHBs may not be indicative of similar risk profiles for type 2 diabetes as other factors may contribute.
1. Genetic predisposition
Despite the similar changes in Si in response to PA, NHBs tend to have lower initial Si compared to NHWs 49–51; which may contribute to the lack of significance found in the current study. Further explanation of this potential increased susceptibility to diabetes is associated with the relationship between whole-body Si and insulin response in NHBs compared to NHW and Asians. In a recent systematic review, Kodama et al. 50 calculated and illustrated the relationship between whole-body Si and the acute-insulin response to glucose (AIRg). Specifically in NHBs with normal glucose tolerance (NGT), the authors concluded that due to the relationship found between Si and AIRg, even the slightest decrease in Si results in large nonlinear increases in AIRg which plays a role in the progression towards type 2 diabetes. In contrast, these changes in Si among NHWs and Asians result in a vastly lower magnitude of changes in AIRg. In other words, among NHBs, the slightest decrease in Si results in a large increase in the volume of insulin needed to maintain normal blood glucose. Furthermore, NHBs with similar Si values had significantly higher AIRg compared to NHWs. This relationship was further confirmed in a study by Albu et al. 52 which found higher AIRg in NHBs during an intravenous-glucose-tolerance test independent of Si, visceral adipose tissue, intramuscular adipose tissue (IMAT), subcutaneous adipose tissue, and skeletal muscle mass. Thus Kodama et al. 50 and Albu et al. 52 conclude there is underlying genetic phenotype in NHBs that may predispose them to increased risk to type 2 diabetes reflected by this relationship.
2. Hepatic influence
Previous research examining the potential mechanism behind why NHBs are at a higher risk for type 2 diabetes has focused on the contributions of hepatic glycemic function. Insulin acts primarily on the peripheral tissues but also has inhibitory effects on hepatic endogenous glucose production (EGP) 53, 54. EGP has been shown to be the primary determinant of glucose tolerance 55 as well as a contributor to the development of type 2 diabetes 56. The aforementioned mechanisms may contribute to the lack of significant findings for NHBs in the current meta-analysis. While still controversial, a review by Gaillard et al. 57 suggested that impaired EGP may be a contributing factor to the increased susceptibility of NHBs to type 2 diabetes compared to NHWs. Moreover, in studies that showed similar EGP between NHBs and NHWs, AIRg tended to be higher in NHBs which is indicative of the increased compensation of the pancreatic β-cells to secrete more insulin. Thus, while EGP is linked to increased risk for type 2 diabetes, this proposed mechanism may not be a driving factor as results are equivocal.
3. Peripheral Si
A review by Gaillard et al. 57 showed that while hepatic Si differences between NHBs and NHWs remains uncertain, peripheral Si tends to be lower among NHBs. With the established association between type 2 diabetes risk and peripheral Si; another mechanism to describe the findings of the current study is a diminished response of the skeletal muscle to changes in peripheral Si. Delany et al. 43 revealed that participation in similar volumes of objectively measured PA (via upper extremity activity monitors), peripheral Si (calculated by dividing the rate of glucose disposal by the plasma insulin concentration) was 26% lower in NHBs compared to NHWs in the absence of obesity. Furthermore, hepatic Si as well as EGP was similar between groups. Haffner et al. 58 found lower peripheral Si (quantified by insulin-mediated glucose-disposal) independent of aerobic PA. The results of these studies indicate that aerobic PA may not affect peripheral Si in the same manner that it affects hepatic Si, especially in NHBs, which could explain the lack of significance found in the current analysis. However, more research is warranted to confirm.
4. Hepatic Insulin Clearance
The consistent findings 50, 57 that show NHBs have higher AIRg compared to NHWs even with similar values of Si could also explain the differences seen in peripheral Si. Higher AIRg is related to decreases in hepatic insulin clearance 50, 59. Gaillard et al. 57 suggested that the compensatory hyperinsulinemic response to a diminished hepatic insulin clearance may contribute to the down-regulation of the skeletal muscle insulin receptors. This would directly lead to decreases in peripheral Si. It can also be postulated that this mechanism would manifest independent of aerobic PA as aerobic PA does not seem to impact AIRg in the same way as is does hepatic Si. However, as stated by Gaillard et al. 57 this needs further exploration.
5. Skeletal muscle adiposity
One mechanism that may influence the decreased peripheral Si in NHBs is IMAT and intra-myocellular lipid content (IMCL). IMAT, defined as the adipose tissue found within the muscle fascia, has been linked to a decrease in Si through inhibition of the insulin signaling pathway 60 leading to compromised insulin-stimulated glucose uptake and decreased Si. A recent study by Goedecke et al. 61 showed that, in NHB women, increased IMCL defined as the accumulation of lipid particles within the muscle cell, and muscle fat % (IMAT) in the soleus were significantly correlated with lower Si. NHW women had no relationship between IMCL and Si. It is important to note that dietary habits and PA volume were similar between groups. Albu et al. 52 showed that despite similar overall associations, NHB women had significantly lower Si across volumes of IMAT compared to NHWs. Furthermore, the study participants had similar PA volumes. The results of these studies indicate that independent of PA, the increase in skeletal muscle lipid content may play a role in the decreased peripheral Si observed in NHBs. However, Ingram et al. 62 found conflicting evidence in NHB participants who had no significant correlation between IMCL and decreased peripheral Si; but NHWs did. Study participants were sedentary and no differences were found in regards to PA between groups. While this mechanism may explain differences in peripheral Si and perhaps the lack of significance in the current study, more research is needed to isolate the true effects of skeletal muscle lipid accumulation on Si and type 2 diabetes risk.
6. Skeletal muscle fiber type
Another mechanism that could contribute to lower Si and compromised glucose control 63, is a higher percentage of type II skeletal muscle fibers that can be found among NHBs compared to NHWs. Previous research contrasting race-ethnic groups has suggested a potential predisposition to an increased risk for type 2 diabetes with higher percentages of type II fibers 63, 64. More specfically, among lean, obese or particpants with type 2 diabetes, type I fibers have been shown to have a higher expression of GLUT4, a greater insulin-stimulate glucose disposal rate, higher glucose oxidation rates and higher nonoxidative glucose metabolism compared to type II fibers 65. Interestingly, the expression of proteins specifc to the effects of insulin were similar across fiber types i.e. phosphoregulation. However, the study suggests that there needs to be more research to examine how phosphoregulation itself relates to glucose uptake and similar phosphoregulation might not mediate Si differences between fiber types. These results were futher confirmed by Daugaard et al. 66 who showed that only type I fibers had a significant increase in the expression of GLUT4 compared to both IIa and IIx fibers following two weeks of one-leg knee extensor training at 65% of maximum workload. The authors also conclude that increasing the intensity of the activity could have led to different findings i.e. changes in GLUT4 expression among Type II fibers. Thus, PA at higher intensities may be necessary to elicit the same benefits as those with higher percentages of type I fibers 64. However, more research is needed to confirm this hypothesis especially among NHBs. Nevertheless, among NHBs there is evidence to suggest that the biochemical differences between type I and type II fibers coupled with a higher percentage of type II fibers may help partially explain the findings of the current study.
Limitations
This study is not without limitations. First, all meta-analyses are subject to potential publication bias 39. We attempted to examine potential bias using a funnel plot procedure, which revealed no issues in regards to potential publication bias. Nonetheless, this issue is inherent to the systematic review process. Another limitation is that PA was self-reported in the studies used with the exception of one which used pedometers. Previous literature has shown limitations to using self-reported PA measures which may overestimate time spent in MVPA as well as failure to capture other intensities or sporadic activity 67, 68. Also, the lack of studies specifically examining the relationship between PA and type 2 diabetes using the current DHHS aerobic PA recommendation does not provide enough evidence to clearly draw conclusions. The final limitation relates to inherent issues regarding the definitions of race-ethnicity 69. More specifically, only four of the definitions we used are considered to be race (NHB, NHW, Asian, and American Indian) and one is considered to be ethnicity (Hispanic) 70. Furthermore, there were semi-heterogeneous definitions of “race” and “ethnicity” used across the studies in this analysis. The authors of the current study elected to group study participants into five common race-ethnic groups found in the literature to provide the best insight into the race-ethnic specific relationship between aerobic PA and type 2 diabetes risk.
Future Directions
In the current meta-analysis, there was an evident paucity of prospective cohort studies reporting race-ethnic specific RRs, specifically in NHBs (N=5), Hispanics (N=3), and American Indians (N=5). Sensitivity analyses revealed a complete loss of statistical significance when Hsia et al. 18 or Ma et al. 25 were removed from the model; thus the interpretability of the summary RR for Hispanics is drastically limited by the available literature. Therefore, priorities for future research in this area should include prospective cohort studies examining PA and type 2 diabetes among multi-ethnic cohorts to examine and report risk across race-ethnic groups (rather than using race-ethnicity as a confounding variable). Furthermore, due to the mechanisms discussed previously, it is also evident that specific intensities should be examined as intensity may play a role in reducing risk for type 2 diabetes in NHBs.
Conclusion
In conclusion, with the exception of NHBs, PA plays a significant role in reducing the risk for type 2 diabetes across race-ethnic groups. Furthermore, the current study illustrates the need to continue investigating effect modification of relationship by race-ethnicity, as well as the need to examine the effect modification between the current aerobic PA guideline and type 2 diabetes risk by race-ethnicity. There are several complex, physiological mechanisms that may explain the findings of the current study, which suggest there may be different race-specific thresholds in regards to the minimum aerobic PA needed to significantly reduce the risk for type 2 diabetes. Future studies may lead to a re-examination of the current aerobic PA guideline for potential changes specific to race-ethnicity.
Supplementary Material
Highlights.
There is a significant and similar risk reduction associated with physical activity across race-ethnicity with the exception of non-Hispanic blacks
There are several physiological mechanisms that may explain this finding that are in need of further exploration in the context of physical activity
Acknowledgments
No funding received
Footnotes
Disclosures
None to declare
References
- 1.Centers for Disease Control and Prevention. [Accessed 10 Oct 2016];2014 National Diabetes Statistics Report. 2014 http://www.cdc.gov/diabetes/data/statistics/2014StatisticsReport.html.
- 2.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. Jama-Journal of the American Medical Association. 2015;314:1021–9. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 3.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honeycutt AA, Boyle JP, Broglio KR, et al. A dynamic Markov model for forecasting diabetes prevalence in the United States through 2050. Health Care Manag Sci. 2003;6:155–64. doi: 10.1023/a:1024467522972. [DOI] [PubMed] [Google Scholar]
- 5.Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. New England Journal of Medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. European Journal of Epidemiology. 2015;30:529–42. doi: 10.1007/s10654-015-0056-z. [DOI] [PubMed] [Google Scholar]
- 7.Gill JM, Celis-Morales CA, Ghouri N. Physical activity, ethnicity and cardio-metabolic health: does one size fit all? Atherosclerosis. 2014;232:319–33. doi: 10.1016/j.atherosclerosis.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services. [Accessed 10 Oct 2016];2008 physical activty guidelines for Americans. 2008 http://www.health.gov/paguidelines.pdf.
- 9.Physical Activty Guidelines Advisory Committee. [Accessed 8 Oct 2016];Physical Activty Guidelines Advisory Committee Report. 2008 https://health.gov/paguidelines/report/pdf/committeereport.pdf.
- 10.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30:744–52. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology - A proposal for reporting. Jama-Journal of the American Medical Association. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.Burchfiel CM, Sharp DS, Curb JD, et al. Physical activity and incidence of diabetes: the Honolulu Heart Program. Am J Epidemiol. 1995;141:360–8. doi: 10.1093/aje/141.4.360. [DOI] [PubMed] [Google Scholar]
- 13.Burke V, Zhao Y, Lee AH, et al. Predictors of type 2 diabetes and diabetes-related hospitalisation in an Australian Aboriginal cohort. Diabetes Res Clin Pract. 2007;78:360–8. doi: 10.1016/j.diabres.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Fan S, Chen J, Huang J, et al. Physical activity level and incident type 2 diabetes among Chinese adults. Med Sci Sports Exerc. 2015;47:751–6. doi: 10.1249/MSS.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 15.Fretts AM, Howard BV, Kriska AM, et al. Physical activity and incident diabetes in American Indians: the Strong Heart Study. Am J Epidemiol. 2009;170:632–9. doi: 10.1093/aje/kwp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fretts AM, Howard BV, McKnight B, et al. Life’s Simple 7 and incidence of diabetes among American Indians: the Strong Heart Family Study. Diabetes Care. 2014;37:2240–5. doi: 10.2337/dc13-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honda T, Kuwahara K, Nakagawa T, Yamamoto S, Hayashi T, Mizoue T. Leisure-time, occupational, and commuting physical activity and risk of type 2 diabetes in Japanese workers: a cohort study. BMC Public Health. 2015;15:1004. doi: 10.1186/s12889-015-2362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsia J, Wu L, Allen C, et al. Physical activity and diabetes risk in postmenopausal women. Am J Prev Med. 2005;28:19–25. doi: 10.1016/j.amepre.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Hu G, Lindstrom J, Valle TT, et al. Physical activity, body mass index, and risk of type 2 diabetes in patients with normal or impaired glucose regulation. Arch Intern Med. 2004;164:892–6. doi: 10.1001/archinte.164.8.892. [DOI] [PubMed] [Google Scholar]
- 20.James SAP, Jamjoum LMPH, Raghunathan TEP, Strogatz DSP, Furth EDMD, Khazanie PGP. Physical Activity and NIDDM in African-Americans: The Pitt County Study. Diabetes Care. 1998;21:555–62. doi: 10.2337/diacare.21.4.555. [DOI] [PubMed] [Google Scholar]
- 21.Joseph JJ, Echouffo-Tcheugui JB, Carnethon MR, et al. The association of ideal cardiovascular health with incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Diabetologia. 2016 doi: 10.1007/s00125-016-4003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koloverou E, Panagiotakos DB, Pitsavos C, et al. 10-year incidence of diabetes and associated risk factors in Greece: the ATTICA study (2002–2012) Rev Diabet Stud. 2014;11:181–9. doi: 10.1900/RDS.2014.11.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kriska AM, Saremi A, Hanson RL, et al. Physical activity, obesity, and the incidence of type 2 diabetes in a high-risk population. Am J Epidemiol. 2003;158:669–75. doi: 10.1093/aje/kwg191. [DOI] [PubMed] [Google Scholar]
- 24.Lee DC, Park I, Jun TW, et al. Physical activity and body mass index and their associations with the development of type 2 diabetes in korean men. Am J Epidemiol. 2012;176:43–51. doi: 10.1093/aje/kwr471. [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, Hebert JR, Manson JE, et al. Determinants of racial/ethnic disparities in incidence of diabetes in postmenopausal women in the U.S.: The Women’s Health Initiative 1993–2009. Diabetes Care. 2012;35:2226–34. doi: 10.2337/dc12-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakanishi N, Takatorige T, Suzuki K. Daily life activity and risk of developing impaired fasting glucose or type 2 diabetes in middle-aged Japanese men. Diabetologia. 2004;47:1768–75. doi: 10.1007/s00125-004-1528-y. [DOI] [PubMed] [Google Scholar]
- 27.Okada K, Hayashi T, Tsumura K, Suematsu C, Endo G, Fujii S. Leisure-time physical activity at weekends and the risk of Type 2 diabetes mellitus in Japanese men: the Osaka Health Survey. Diabet Med. 2000;17:53–8. doi: 10.1046/j.1464-5491.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 28.Panagiotakos DB, Pitsavos C, Skoumas Y, Lentzas Y, Stefanadis C. Five-year incidence of type 2 diabetes mellitus among cardiovascular disease-free Greek adults: findings from the ATTICA study. Vasc Health Risk Manag. 2008;4:691–8. doi: 10.2147/vhrm.s2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi L, Shu XO, Li H, et al. Physical activity, smoking, and alcohol consumption in association with incidence of type 2 diabetes among middle-aged and elderly Chinese men. PLoS One. 2013;8:e77919. doi: 10.1371/journal.pone.0077919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis. 2013;23:292–9. doi: 10.1016/j.numecd.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai AC, Lee SH. Determinants of new-onset diabetes in older adults-Results of a national cohort study. Clin Nutr. 2015;34:937–42. doi: 10.1016/j.clnu.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Villegas R, Shu XO, Li H, et al. Physical activity and the incidence of type 2 diabetes in the Shanghai women’s health study. Int J Epidemiol. 2006;35:1553–62. doi: 10.1093/ije/dyl209. [DOI] [PubMed] [Google Scholar]
- 33.Villegas R, Shu XO, Yang G, et al. Energy balance and type 2 diabetes: a report from the Shanghai Women’s Health Study. Nutr Metab Cardiovasc Dis. 2009;19:190–7. doi: 10.1016/j.numecd.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waki K, Noda M, Sasaki S, et al. Alcohol consumption and other risk factors for self-reported diabetes among middle-aged Japanese: a population-based prospective study in the JPHC study cohort I. Diabet Med. 2005;22:323–31. doi: 10.1111/j.1464-5491.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 35.Waller K, Kaprio J, Lehtovirta M, Silventoinen K, Koskenvuo M, Kujala UM. Leisure-time physical activity and type 2 diabetes during a 28 year follow-up in twins. Diabetologia. 2010;53:2531–7. doi: 10.1007/s00125-010-1875-9. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Shara NM, Calhoun D, Umans JG, Lee ET, Howard BV. Incidence rates and predictors of diabetes in those with prediabetes: the Strong Heart Study. Diabetes Metab Res Rev. 2010;26:378–85. doi: 10.1002/dmrr.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu F, Wang Y, Ware RS, et al. Physical activity, family history of diabetes and risk of developing hyperglycaemia and diabetes among adults in Mainland China. Diabetic Medicine. 2012;29:593–9. doi: 10.1111/j.1464-5491.2011.03495.x. [DOI] [PubMed] [Google Scholar]
- 38.Xu F, Wang Y, Ware RS, et al. Joint impact of physical activity and family history on the development of diabetes among urban adults in Mainland China: a pooled analysis of community-based prospective cohort studies. Asia Pac J Public Health. 2015;27:NP372–81. doi: 10.1177/1010539512443700. [DOI] [PubMed] [Google Scholar]
- 39.Bornstein M, Hedges L, Higgins J, Rothstein H. Introduction to Meta-Analysis. Jon Wiley & Sons; United Kingdom: 2009. [Google Scholar]
- 40.National Heart Lung and Blood Institute. [Accessed 15 Novermber 2016];Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2014 https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort.
- 41.Celis-Morales CA, Ghouri N, Bailey MES, Sattar N, Gill JMR. Should Physical Activity Recommendations Be Ethnicity-Specific? Evidence from a Cross-Sectional Study of South Asian and European Men. Plos One. 2013:8. doi: 10.1371/journal.pone.0082568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinbrecher A, Erber E, Grandinetti A, Nigg C, Kolonel LN, Maskarinec G. Physical Activity and Risk of Type 2 Diabetes Among Native Hawaiians, Japanese Americans, and Caucasians: The Multiethnic Cohort. Journal of Physical Activity & Health. 2012;9:634–41. doi: 10.1123/jpah.9.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeLany JP, Dube JJ, Standley RA, et al. Racial differences in peripheral insulin sensitivity and mitochondrial capacity in the absence of obesity. J Clin Endocrinol Metab. 2014;99:4307–14. doi: 10.1210/jc.2014-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonora E, Kiechl S, Willeit J, et al. Population-based incidence rates and risk factors for type 2 diabetes in white individuals: the Bruneck study. Diabetes. 2004;53:1782–9. doi: 10.2337/diabetes.53.7.1782. [DOI] [PubMed] [Google Scholar]
- 45.Boden G. Pathogenesis of type 2 diabetes. Insulin resistance. Endocrinol Metab Clin North Am. 2001;30:801–15. doi: 10.1016/s0889-8529(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 46.Lakoski SG, Kozlitina J. Ethnic differences in physical activity and metabolic risk: the Dallas Heart Study. Med Sci Sports Exerc. 2014;46:1124–32. doi: 10.1249/MSS.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 47.Irwin ML, Mayer-Davis EJ, Addy CL, et al. Moderate-intensity physical activity and fasting insulin levels in women: the Cross-Cultural Activity Participation Study. Diabetes Care. 2000;23:449–54. doi: 10.2337/diacare.23.4.449. [DOI] [PubMed] [Google Scholar]
- 48.Hasson RE, Granados K, Chipkin S, Freedson PS, Braun B. Effects of a Single Exercise Bout on Insulin Sensitivity in Black and White Individuals. Journal of Clinical Endocrinology & Metabolism. 2010;95:E219–E23. doi: 10.1210/jc.2010-0019. [DOI] [PubMed] [Google Scholar]
- 49.Palaniappan LP, Carnethon MR, Fortmann SP. Heterogeneity in the relationship between ethnicity, BMI, and fasting insulin. Diabetes Care. 2002;25:1351–7. doi: 10.2337/diacare.25.8.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36:1789–96. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams SK, Fiscella K, Winters P, Martins D, Ogedegbe G. Association of racial disparities in the prevalence of insulin resistance with racial disparities in vitamin D levels: National Health and Nutrition Examination Survey (2001–2006) Nutr Res. 2013;33:266–71. doi: 10.1016/j.nutres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. American Journal of Clinical Nutrition. 2005;82:1210–7. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26:19–39. [PMC free article] [PubMed] [Google Scholar]
- 54.Girard J. The inhibitory effects of insulin on hepatic glucose production are both direct and indirect. Diabetes. 2006;55:S65–S9. [Google Scholar]
- 55.Bavenholm PN, Pigon J, Ostenson CG, Efendic S. Insulin sensitivity of suppression of endogenous glucose production is the single most important determinant of glucose tolerance. Diabetes. 2001;50:1449–54. doi: 10.2337/diabetes.50.6.1449. [DOI] [PubMed] [Google Scholar]
- 56.Consoli A. Role of Liver in Pathophysiology of Niddm. Diabetes Care. 1992;15:430–41. doi: 10.2337/diacare.15.3.430. [DOI] [PubMed] [Google Scholar]
- 57.Gaillard TR, Osei K. Racial Disparities in the Pathogenesis of Type 2 Diabetes and its Subtypes in the African Diaspora: A New Paradigm. J Racial Ethn Health Disparities. 2016;3:117–28. doi: 10.1007/s40615-015-0121-z. [DOI] [PubMed] [Google Scholar]
- 58.Haffner SM, DAgostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites - The insulin resistance atherosclerosis study. Diabetes. 1996;45:742–8. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 59.Lorenzo C, Hanley AJG, Wagenknecht LE, et al. Relationship of Insulin Sensitivity, Insulin Secretion, and Adiposity With Insulin Clearance in a Multiethnic Population The Insulin Resistance Atherosclerosis Study. Diabetes Care. 2013;36:101–3. doi: 10.2337/dc12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turcotte LP, Fisher JS. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther. 2008;88:1279–96. doi: 10.2522/ptj.20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goedecke JH, Keswell D, Weinreich C, et al. Ethnic differences in hepatic and systemic insulin sensitivity and their associated determinants in obese black and white South African women. Diabetologia. 2015;58:2647–52. doi: 10.1007/s00125-015-3720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ingram KH, Lara-Castro C, Gower BA, et al. Intramyocellular Lipid and Insulin Resistance: Differential Relationships in European and African Americans. Obesity. 2011;19:1469–75. doi: 10.1038/oby.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staiano AE, Harrington DM, Johannsen NM, et al. Uncovering physiological mechanisms for health disparities in type 2 diabetes. Ethn Dis. 2015;25:31–7. [PMC free article] [PubMed] [Google Scholar]
- 64.Ceaser T, Hunter G. Black and White race differences in aerobic capacity, muscle fiber type, and their influence on metabolic processes. Sports Med. 2015;45:615–23. doi: 10.1007/s40279-015-0318-7. [DOI] [PubMed] [Google Scholar]
- 65.Albers PH, Pedersen AJT, Birk JB, et al. Human Muscle Fiber Type-Specific Insulin Signaling: Impact of Obesity and Type 2 Diabetes. Diabetes. 2015;64:485–97. doi: 10.2337/db14-0590. [DOI] [PubMed] [Google Scholar]
- 66.Daugaard JR, Nielsen JN, Kristiansen S, Andersen JL, Hargreaves M, Richter EA. Fiber type-specific expression of GLUT4 in human skeletal muscle - Influence of exercise training. Diabetes. 2000;49:1092–5. doi: 10.2337/diabetes.49.7.1092. [DOI] [PubMed] [Google Scholar]
- 67.Sallis JF, Saelens BE. Assessment of physical activity by self-report: Status, limitations, and future directions (vol 71, pg 1, 2000) Research Quarterly for Exercise and Sport. 2000;71:409. doi: 10.1080/02701367.2000.11082780. [DOI] [PubMed] [Google Scholar]
- 68.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 69.Bhopal R, Donaldson L. White, European, western, Caucasian, or what? Inappropriate labeling in research on race, ethnicity, and health. American Journal of Public Health. 1998;88:1303–7. doi: 10.2105/ajph.88.9.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Executive Office of the President. Office of Management and Budget. Office of Information and Regulatory Affairs. [Accessed 1 November 2016];Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. 1997 https://www.whitehouse.gov/omb/fedreg_1997standards.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.