Abstract
In Parkinson’s disease (PD) patients and animal models of PD, the progressive degeneration of the nigrostriatal dopamine (DA) projection leads to two major changes in the morphology of striatal projection neurons (SPNs), i.e. a profound loss of dendritic spines and the remodeling of axospinous glutamatergic synapses. Striatal spine loss is an early event tightly associated with the extent of striatal DA denervation, but not the severity of parkinsonian motor symptoms, suggesting that striatal spine pruning might be a form of homeostatic plasticity that compensates for the loss of striatal DA innervation and the resulting dysregulation of corticostriatal glutamatergic transmission. On the other hand, the remodeling of axospinous corticostriatal and thalamostriatal glutamatergic synapses might represent a form of late maladaptive plasticity that underlies changes in the strenght and plastic properties of these afferents and the resulting increased firing and bursting activity of striatal SPNs in the parkinsonian state. There is also evidence that these abnormal synaptic connections might contribute to the pathophysiology of L-DOPA-induced dyskinesia. Despite the significant advances made in this field over the past thirty years, many controversial issues remain about the striatal SPN subtypes affected, the role of spine changes in the altered activity of SPNs in the parkinsonisn state and the importance of striatal spine plasticity in the pathophysiology of L-DOPA-induced dyskinesia. In this review, we will examine the current state of knowledge of these issues, discuss the limitations of the animal models used to address some of these questions and assess the relevance of data from animal models to the human diseased condition.
Keywords: Monkey, striatum, dopamine, glutamate, corticostriatal, thalamostriatal
Anatomy, Connections and Cell Types of the Striatum
One of the main neuropathological features of Parkinson’s disease (PD) is the degeneration of the nigrostriatal dopaminergic pathway, which induces complex physiological changes within the basal ganglia (BG) circuitry. The BG is a group of interconnected subcortical nuclei, including the striatum, globus pallidus (GP), substantia nigra (SN), and subthalamic nucleus (STN). The information that flows through the BG circuitry is segregated into motor, associative, and limbic/emotional domains based on their relationships with specific cortical projection areas and the engagement of these regions in various behaviors (Alexander et al. 1986; Lanciego et al. 2012). The GP is formed by two anatomically and functionally separate nuclei, the external (GPe) and internal (GPi) pallidal segments in primates; GP and entopeduncular nucleus (EPN) in rodents. The substantia nigra (SN) comprises two separate nuclei, the GABAergic pars reticulata (SNr) and the dopaminergic pars compacta (SNc). The dopaminergic neurons of the SNc project primarily to the striatum, but also provide significant innervation of other BG nuclei (Smith and Kieval 2000; Garcia-Cabezas et al. 2009) (Rommelfanger and Wichmann 2010), while the SNr and GPi (and EPN) are the output nuclei of the basal ganglia that project to the thalamus and brainstem.
The dorsal striatum, formed by the putamen and caudate nucleus in primates, is mainly innervated by sensorimotor (to post-commissural putamen) and associative (to caudate nucleus and pre-commissural putamen) cortices, respectively, while the ventral striatum (to nucleus accumbens and olfactory tubercle) is the main target of limbic-related inputs from the hippocampus, amygdala and medial prefrontal cortices. Together with the STN, the striatum is the main gateway of extrinsic information from the cerebral cortex to the BG circuits. (Russchen et al. 1985; Alexander et al. 1986; McGeorge and Faull 1987, 1989; Haber et al. 1995; Parent and Hazrati 1995; Mink 1996; Smith et al. 1998; Fudge et al. 2002; Wichmann and DeLong 2003; DeLong and Wichmann 2007; Lanciego et al. 2012; Haynes and Haber 2013; Smith and Wichmann 2015). The striatum also receives a massive glutamatergic innervation from the thalamus. Although the thalamostriatal system originates from most thalamic nuclei, the caudal intralaminar nuclear complex, made up of the centromedian (CM) and parafascicular (Pf) nuclei in primates, is the main source of thalamic afferents to the striatum (Smith et al. 2004, 2009a, 2014, 2016; Galvan et al. 2016). It is noteworthy that the CM/Pf complex undergoes profound neuronal degeneration in PD (Henderson et al. 2000a, b; Halliday 2009) and in chronically MPTP-treated monkeys (Villalba et al. 2014) (see discussion below).
Within the dorsal striatum, the vast majority of neurons are the spiny projection neurons (SPNs) that represent 90–97% of all striatal neurons in rodents (Kemp and Powell 1971a, b, c; Gerfen et al. 1990; Oorschot 1996; Wickens et al. 2007). These GABAergic SPNs are the main targets of extrinsic inputs to the striatum and can be categorized into two main populations: 1. The direct pathway neurons that send their main axonal projections directly to the output nuclei of the BG (i.e. GPi and SNr), and express preferentially the D1 dopamine receptors (D1R) and the neuropeptides substance P (SP) and dynorphin (DYN). 2. The indirect pathway neurons that project preferentially to the GPe, and express D2 dopamine receptors (D2R) and the neuropeptide enkephalin (ENK) (Gerfen et al. 1990; Xu et al. 1994; Sidibe and Smith 1999; Lanciego et al. 2004; Lei et al. 2004, 2013; Surmeier et al. 2007; Galvan and Smith 2011; Gerfen and Surmeier 2011; Huerta-Ocampo et al. 2014; Smith et al. 2014). Although less frequent, some striatal SPNs project to both GPe and GPi/SNr and co-express D1 and D2 DA receptor subtypes (Kawaguchi et al. 1990; Surmeier and Kitai 1993; Hersch et al. 1995; Surmeier et al. 1996; Wu et al. 2000). Striatal SPNs also express D3R, D4R and D5R, albeit to a lower degree than D1R and D2R (Rivera et al. 2002, 2003; Fiorentini et al. 2015). As mentioned above, the STN is also considered as a major entry for cortical information to the BG network (Nambu et al. 2000; DeLong and Wichmann 2010). Because information flows more rapidly to the BG output nuclei via the cortico-subthalamic projection than via the direct and indirect trans-striatal pathways, the trans-subthalamic route is referred to as the hyperdirect pathway of the BG (Nambu et al. 2002; Papa and Wichmann 2015; Smith and Wichmann 2015).
Even though D1R and D2R SPNs display very similar morphological characteristics, the D2R SPNs exhibit increased excitability and harbor a less extensive dendritic tree than D1R cells in mice (Surmeier et al. 2007; Gertler et al. 2008; Kreitzer and Malenka 2008; Fieblinger et al. 2014), and each type of SPNs is differentially modulated by DA in normal and diseased states (Surmeier et al. 2007; Day et al. 2006; Kreitzer and Malenka 2008; Shen et al. 2008; Kreitzer 2009; Fieblinger et al. 2014; Suarez et al. 2014, 2016). The dendritic trees of both populations of striatal SPNs are covered with spines, which are the main targets of glutamatergic inputs from the cerebral cortex and thalamus (Fig. 1a). In rodents, the dendrites of individual SPNs harbor as many as 5000 dendritic spines (Wickens et al. 2007). In addition to their glutamatergic innervation, a subset of striatal spines also receives synaptic inputs from midbrain dopaminergic neurons which frequently terminate onto the neck of the spine or a nearby segment of the dendritic shaft, thereby providing an anatomical substrate for close synaptic interactions between glutamatergic and dopaminergic inputs (Freund et al. 1984; Smith and Bolam 1990; Smith et al. 1994, 2014; Nicola et al. 2000; Wickens et al. 2007; Moss and Bolam 2008). These functional interactions are critical for the development and maintenance of long-term synaptic plasticity of glutamatergic corticostriatal synapses (Nicola et al. 2000; Calabresi et al. 2007; Surmeier et al. 2007, 2010; Gerfen and Surmeier 2011; Picconi et al. 2012).
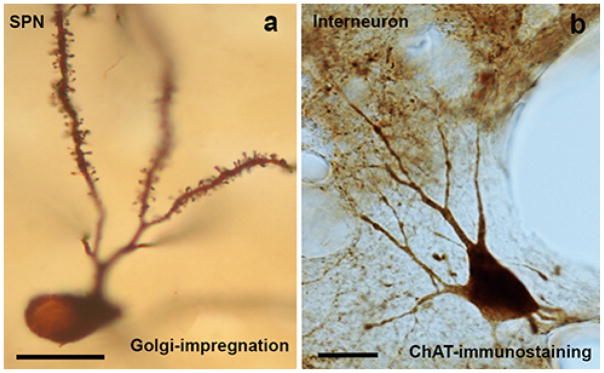
Fig. 1. Light microscopy pictures of a spiny projection neuron (SPN) (a) and an interneuron (b) in dorsal striatum of the non-human primate.
a: Golgi-impregnated striatal SPN. These neurons have ovoid or polygonal cell bodies with a maximum diameter of ≤ 25 μm, and extensive dendritic trees. The somata of the neurons are smooth while the dendrites, except for their most proximal portions, are covered with spines. b: Example of an aspiny striatal cholinergic interneuron. This neuron has been immunostained using specific antibodies against the enzyme choline acetyltransferase (ChAT), a specific marker for cholinergic neurons. These neurons have large cell bodies, with a diameter-size between 30–50 μm, and different morphologies (ovoid, elongated or triangular). The length and ramification of their immunostained dendritic trees vary, and usually branch close to the cell body. Scale bar in a and b: 25 μm.
The aspiny interneurons are far fewer in number, accounting for about 3–10% of the total striatal population in rodents. This proportion is significantly higher in primates, where interneurons account for as much as 24% of the striatal neuron population (Pasik et al. 1976; Graveland and DiFiglia 1985; Roberts et al. 1996; Wu and Parent 2000; Rivera et al. 2002; Tepper and Bolam 2004; Bernacer et al. 2005; 2007; 2012; Oorschot, 2013; Gonzales and Smith 2015). Anatomically, they can be categorized into medium-sized GABAergic cells and large cholinergic neurons (Figure 1b) (Kawaguchi et al. 1995; Bernacer et al. 2007, 2012; Gonzales and Smith 2015). Medium-sized GABAergic interneurons can be further classified histochemically into different subtypes: (a) parvalbumin-, (b) somatostatin-, neuropeptide Y-, and nitric oxide synthase- (c) calretinin- (Tepper and Bolam 2004; Bernacer et al. 2005, 2007, 2012) and (d) tyrosine hydroxylase (TH)-positive (Tepper et al. 2010). This latter subtype is rare in the normal primate striatum, but undergoes an upregulation after striatal DA denervation (Betarbet et al. 1997; Mazloom and Smith 2006; Tepper et al. 2010; Bernacer et al. 2012). L-DOPA treatment further increases the prevalence of these interneurons in rodent models of PD (Espadas et al. 2012).
Structure-function Relationships of Dendritic Spines
The analysis of the structure-function relationships of dendritic spines in synaptic plasticity and brain disorders are active areas of research in modern neuroscience. Dendritic spines were first described by Cajal in 1888 when he was studying Purkinje cells of the avian cerebellum stained by the Golgi method (Cajal 1888). Using the Golgi impregnation, that enabled him the relatively complete staining of the dendritic trees, he noted that the dendrites of Purkinje cells were covered with small protrusions, which he called “espinas” (i.e. “spines”), and proposed the idea that spines would extend the surface of the dendrites, and therefore dramatically increase their capability to receive axons (Cajal 1896). The definitive demonstration of dendritic spines as postsynaptic units came later with electron microscopic studies (Gray 1959). Cajal also proposed that morphological changes in spines could be associated with neuronal function and learning, and compared the size, density and morphology of dendritic spines among different species (Cajal 1891, 1893).
Dendritic spines are highly plastic entities that exhibit a wide spectrum of structural reorganization, from formation to elimination, to more subtle changes in size and shape in normal and diseased conditions. The use of cutting-edge techniques at the electron microscopic level for three-dimensional (3D) reconstructions of individual spines and cellular structures (Harris and Kater 1994; Spacek and Harris 1997; Yuste and Bonhoeffer 2001; Harris et al. 2006; Fiala 2005; DeFelipe 2008; Knott et al. 2008; Merchan-Perez et al. 2009; Smith et al. 2009b; Villalba and Smith 2010; 2011a, b, 2013; Zhang et al., 2010; Villalba et al. 2015, 2016; Cali et al. 2016; Moratalla et al. 2016), single- and two-photon microscopy in conjunction with fluorescent molecular tools (Yuste and Bonhoeffer 2004; Knott and Holtmaat 2008) and neuro-computational methods (Zhang et al. 2010) have been instrumental in advancing knowledge about the plasticity of dendritic spines and the pathophysiology of neuronal networks in various brain disorders (Ingham et al. 1989, 1998; Harris and Kater 1994; Yuste and Bonhoeffer 2001; Fiala et al. 2002; Stephens et al. 2005; Zaja-Milatovic et al. 2005; Deutch et al. 2007; Surmeier et al. 2007; Bourne and Harris 2008; Villalba et al. 2009, 2015, 2016; Villalba and Smith 2010, 2011a, b, 2013; Suarez et al. 2016).
Striatal Spine Loss in Parkinson’s Disease and Animal Models of Parkinsonism
Over the past three decades, it became clear that striatal SPNs undergo complex structural changes in the density, morphology and ultrastructural features of their dendritic spines in animal models of Parkinson’s disease (PD). The first evidence for striatal spine loss in PD came from postmortem analyses of Golgi-impregnated striatal neurons in PD patients, which showed significant atrophy of the dendritic tree and loss of spines on individual SPNs in the diseased condition (McNeill et al. 1988). These findings were later confirmed and extended by various groups using the unilateral 6-hydroxydopamine (6-OHDA) rat model of PD (Ingham et al. 1989). Since then, numerous studies have demonstrated different degrees of spine pruning and plastic changes in striatal SPNs in rodent and monkey models of PD and in postmortem striatal tissue of PD patients (Ingham et al. 1989, 1993, 1998; Anglade et al. 1996; Meshul et al. 1999, 2000; Stephens et al. 2005; Zaja-Milatovic et al. 2005; Day et al. 2006; Deutch et al. 2007; Neely et al. 2007; Solis et al. 2007; Shen et al. 2008; Smith and Villalba, 2008; Villalba et al. 2009, 2015; Villalba and Smith 2010, 2011a, 2013; Garcia et al. 2010; Deutch 2014; Fieblinger et al. 2014; Suarez et al. 2014, 2016; Toy et al. 2014; Gagnon et al. 2017). In PD patients, the severity of spine loss is homogeneous along the proximo-distal extent of dendritic branches (Stephens et al. 2007). Although there is consensus through the literature that striatal spine loss is closely associated with PD, much remains to be known about the importance of such change in the pathophysiology of striatal SPNs and the development of parkinsonian motor symptoms in PD.
Is the Extent of Striatal Spine Loss Related to the Severity of Parkinsonian Motor Symptoms?
Most findings about striatal spine loss in PD come from either postmortem studies of advanced PD patients (McNeill et al. 1988; Stephens et al. 2005; Zaja-Milatovic et al. 2005) or rodent models of parkinsonism with severe nigrostriatal dopaminergic denervation (Ingham et al. 1989, 1998; Day et al. 2006; Deutch et al. 2007; Neely et al. 2007; Fieblinger et al. 2014; Suarez et al. 2014, 2016; Moratalla et al. 2016; Gagnon et al. 2017). Although these studies provide important information about SPNs morphology in animals and patients that display parkinsonian motor symptoms, they do not help determine the course of striatal spine loss in relation to nigrostriatal DA degeneration and the severity of parkinsonian motor signs. We recently addressed this issue in chronically MPTP-treated monkeys, and found that the extent of dendritic spine loss on striatal SPNs is correlated with the degree of nigrostriatal DA denervation, but not with the development or severity of parkinsonian motor symptoms (Smith et al. 2009b; Villalba et al. 2009) (Fig. 2). This was achieved through the analysis of striatal tissue of “motor asymptomatic” MPTP-treated monkeys with partial (~40–50%) striatal DA denervation (Fig. 2a, e, f) (Villalba et al. 2014). Despite the lack of parkinsonian motor signs, SPNs in the post-commissural putamen (i.e., the sensorimotor striatum), one of most severely DA-depleted striatal regions in PD, displayed as much as 50% dendritic spine loss, while in striatal areas with less severe DA depletion, such as the caudate nucleus and nucleus accumbens, the extent of spine loss ranged between 20% to 25% (Smith et al. 2009b; Villalba et al. 2009, 2015; Villalba and Smith 2010, 2011a, 2013) (Fig. 2b–f). Based on these findings, we concluded: 1. Striatal spine loss is an early plastic change that is tightly associated with the degree of nigrostriatal dopaminergic degeneration in parkinsonism. 2. The sole loss of striatal spines in the sensorimotor striatum (i.e. post-commissural putamen) does not account for the development of parkinsonian motor signs in MPTP-treated monkeys. At this stage, the functional significance of early striatal spine loss in response to DA depletion remains unknown. Does it represent a homeostatic response of SPNs to compensate for the changes induced by the lack of dopamine’s modulatory effects on SPNs and their glutamatergic afferents?. If so, it could be considered as a beneficial response that helps maintain normal striatal activity despite progressive striatal dopaminergic denervation during the motor asymptomatic prodromal stage of PD. Alternatively, is the spine pruning the beginning of a pathological process that progressively alters striatal glutamatergic transmission, SPNs activity and striatofugal outflow?. These questions remain unanswered at this point. Future studies that relate parkinsonian motor signs with striatal recordings and ultrastructural analyses of glutamatergic synapses in chronically MPTP-treated monkeys at various stages of striatal dopamine denervation should help further address these issues.
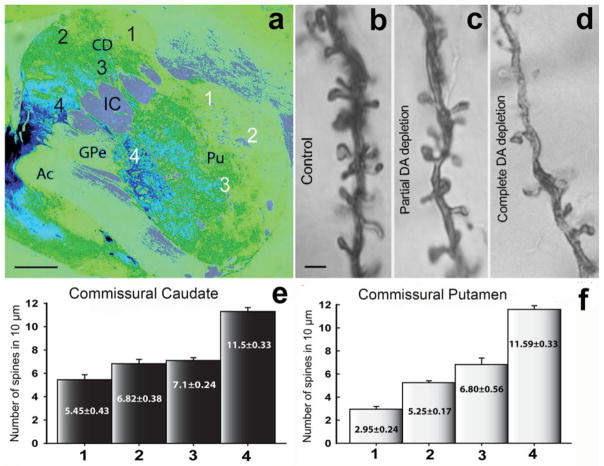
Fig. 2. Tyrosine hydroxylase (TH) immunoreactivity (a) and dendritic spine density in the striatum of partially dopamine depleted MPTP-treated monkeys (b–f).
a: Pseudo-colored image (NIH ImageJ program) of a TH-immunostained section from the monkey commissural striatum. The numbers (1–4) in the caudate (black numbers) and putamen (white numbers) indicate the areas from where the Golgi-impregnated neurons were selected for the dendritic spine analysis showed in e and f. The lowest (complete DA loss) and highest (no DA loss) levels of TH-immunostaining correspond to the numbers 1 and 4, respectively. b–d: Golgi impregnated dendrites (20–30 μm from the soma) from monkey striatal SPNs in control (no DA depletion; 10–12 spines per μm) (b), partial DA depletion (c), and complete DA depletion (4–6 spines per μm) (d). e, f: Histograms showing the dendritic spine density of Golgi-impregnated neurons from commissural striatal areas with different degrees of dopamine depletion. The values in the bars represent the mean of the spine density (mean±SEM) per 10 μm of dendritic length (primary dendrite) from neurons (10 neurons per area) from the corresponding striatal areas indicated by number (1 to 4) and color (black or white) in a. The Abbreviations: Ac: anterior commissure; CD: caudate; IC: internal capsule; Pu: putamen; GPe: Globus pallidus external segment. Scale bars in a: 1 mm, and in b (applies to c and d): 1 μm.
What Subtypes of Striatal SPNs Lose Dendritic Spines in Parkinsonism?
The basic model of direct and indirect pathways introduced in the late 1980s has been instrumental in our understanding of the BG circuitry, the pathophysiology of PD and in the development of new therapeutic approaches for BG-related movement disorders. The imbalance of activity between these two pathways in favor of an increased striatal output from indirect pathway neurons is considered as a key pathophysiological feature of PD (Albin et al. 1989; DeLong 1990; Smith et al. 1998; Wichmann and DeLong 2003, 2007; DeLong and Wichmann 2007). Although there is agreement among investigators that SPNs undergo spine loss in PD and animal models of parkinsonism, there is some controversy as to whether both direct (D1R) and indirect (D2R) striatal SPNs are affected. Thus, various Golgi studies in 6-OHDA-treated rats, MPTP-treated monkeys and PD patients described homogeneous loss of striatal dendritic spines across SPNs without any evidence for selective sparing of a specific subpopulation (McNeill et al. 1988; Ingham et al. 1989; Stephens et al. 2005; Zaja-Milatovic et al. 2005; Smith et al. 2009b; Villalba et al., 2009, 2015; Garcia et al. 2010; Villalba and Smith 2010, 2013). These findings are consistent with data in mice models of parkinsonism induced either by intrastriatal (Suarez et al. 2014, 2016; Moratalla et al. 2016) or medial forebrain bundle (MFB) (Gagnon et al. 2017) injection of 6-OHDA, or systemic treatment with MPTP (Toy et al. 2014), which demonstrated that both D1R and D2R SPNs display significant spine loss in parkinsonism. However, these findings are at odds with other studies showing that D2R striatopallidal neurons, but not D1R striatonigral neurons, selectively lose spines in rodent models of PD. Day et al. (2006) provided the first evidence for this selective spine loss on indirect pathway SPNs in two different animal models of parkinsonism (Day et al. 2006). On one hand, they showed that D2R SPNs displayed ~ 50% spine loss in reserpine-treated BAC D2R mice, whereas no significant spine pruning was found in D1R SPNs of reserpine-treated BAC D1R mice (Day et al. 2006).
The authors further supported these observations with quantitative electron microscopy data showing selective loss of D1R-negative spines (presumed striatopallidal D2R-containing spines) in the dorsal striatum of DA-depleted rats following injection of 6-OHDA in the MFB (Day et al. 2006). This preferential loss of spines on indirect (i.e. D2R-positive) versus direct (i.e. D1R-positive) pathway neurons has also been reported in other studies by the same group and another using 6-OHDA MFB lesion in transgenic BAC D1R and D2R mice (Schuster et al. 2009; Fieblinger et al. 2014). A single nonhuman primate study described a selective decrease in D2R spines accompanied with an increase in the density of D1R spines in the caudate nucleus of cynomolgus monkeys acutely intoxicated with MPTP (Scholz et al. 2008; Schuster et al. 2009). As in PD patients, the dendritic atrophy of both populations of SPNs has been described in some mice models (Fieblinger et al. 2014; Gagnon et al. 2017), but not in others (Suarez et al. 2014).
Although there is no clear explanation for these discrepancies, an important factor to consider in the interpretation of these findings is the lack of details provided by the authors about the extent of thalamic cell loss in their animal models. As mentioned above and discussed in previous studies (Henderson et al. 2000a, b; Smith et al. 2004, 2009a, 2014; Villalba et al. 2014, 2015), the thalamic CM/Pf complex undergoes massive neuronal degeneration in PD. Thus, if striatal spine loss is a dynamic homeostatic process set in motion to compensate for functional changes in SPNs activity induced by the progressive degeneration of the nigrostriatal dopaminergic system (Smith et al. 2009b; Villalba et al. 2009; Fieblinger et al. 2014), we believe that the early breakdown of the glutamatergic thalamostriatal system may be a determining factor for the cellular specificity of striatal spine loss in the parkinsonian state (Villalba et al. 2014, 2015).
Does Degeneration of the CM/Pf Thalamic Complex Contribute to the Pattern of Striatal Spine Loss in PD?
Although there is not a definitive answer to this question, this important issue has been overly neglected in our understanding of the overall network changes that lead to striatal spine plasticity in PD. As mentioned above, the CM/Pf thalamic nuclei undergo severe neuronal loss in PD (Henderson et al. 2000a, b; Halliday 2009; Smith et al. 2014; Villalba et al. 2014). Because the CM/Pf is the main source of thalamic glutamatergic inputs to the striatum, the loss of these thalamic neurons and their corresponding axonal projections to the striatum (Villalba et al. 2013, 2014) is likely to further contribute to the synaptic homeostasis and scaling properties of remaining striatal glutamatergic synapses in the parkinsonian condition. Despite strong evidence for CM/Pf pathology in PD patients, attempts at mimicking such pathology in animal models of parkinsonism have led to variable results. While some authors did not find evidence for Pf degeneration 3 months after unilateral 6-OHDA nigrostriatal dopaminergic lesion (via MFB injection) in rats (Henderson et al. 2005; Kusnoor et al. 2012), other studies, in the same animal model or after systemic administration of MPTP, reported significant Pf cell loss (Aymerich et al. 2006; Sedaghat et al. 2009; Freyaldenhoven et al. 1997). Some authors have also shown that intrastriatal administration of 1-methyl-4-phenylpyridinium ion (MPP+) induces significant Pf cells damage in rats, without any significant impact upon corticostriatal glutamatergic terminals (Ghorayeb et al. 2002).
In nonhuman primates, we have shown that chronically MPTP-treated rhesus monkeys display 40–50% neuronal loss in CM/Pf (Villalba et al. 2014, 2015) and that this neuronal loss impacts upon the relative abundance of vGluT2-positive terminals in the striatum (Villalba et al. 2013). Although the underlying mechanisms remain to be elucidated, it appears that MPTP toxicity might be a more reliable tool to induce CM/Pf neuronal loss and degeneration of the thalamostriatal system in mice and monkeys (Ghorayeb et al. 2002; Villalba et al. 2013, 2014; Smith et al. 2014; Toy et al. 2014). For reasons discussed above, animal studies aimed at assessing the impact of parkinsonism on the anatomical and functional plasticity of glutamatergic axo-spinous synapses in the striatum must be achieved in animal models that display CM/Pf pathology (Villalba et al. 2013, 2014; Smith et al. 2014). However, most rodent studies, except for that of Parker et al. (2016), of PD-related striatal spine plasticity that have been achieved so far did not determine if the animals used in these experiments displayed Pf degeneration (Day et al. 2006; Soderstrom et al. 2010; Zhang et al. 2013; Fieblinger et al. 2014; Suarez et al. 2014, 2016; Parker et al. 2016). Therefore, care must be taken in translating those data to the human diseased condition (PD and L-DOPA-induced dyskinesia) without a reassessment of the anatomical and functional changes reported in those studies in animal models with concomitant lesion of both the glutamatergic CM/Pf-striatal and the dopaminergic nigrostriatal systems.
Does Striatal Spine Loss Affect Corticostriatal and/or Thalamostriatal Glutamatergic Projections in PD?
This is a fundamental question to address if one hopes to understand the functional significance of striatal spine plasticity in PD. Using unbiased stereological synaptic counts, Ingham and colleagues (1998) reported ~20% decrease in the total number of axo-spinous asymmetric synapses in the striatum of 6-OHDA-treated rats (Ingham et al. 1998). However, the sources of the pre-synaptic terminals affected by this synaptic loss were not determined in this early study. We and others have recently addressed this issue in monkey and mice models of PD using vesicular glutamate transporter 1 (vGluT1) or vGluT2 as specific markers of cortical or thalamic terminals, respectively. Thus, findings from our laboratory have shown a significant decrease in the total number of putative glutamatergic terminals (as revealed by asymmetric synaptic specializations) in the putamen of MPTP-treated parkinsonian monkeys (Villalba et al. 2013). However, when labeled cortical (vGluT1-positive) or thalamic (vGluT2-positive) terminals were counted, the relative density of corticostriatal terminals in the putamen and the caudate nucleus was either unchanged or significantly increased (Raju et al. 2008; Villalba et al. 2013) while there was a significant decrease in the prevalence of thalamostriatal terminals in these animals (Raju et al. 2008; Villalba et al. 2013). These findings are consistent with human data showing a slight increase in the amount of vGluT1 protein expression in the putamen of PD patients compared with controls (Kashani et al. 2007). However, these findings are different from those of recent studies showing a profound reduction in the total number of vGluT1-positive terminals in 6-OHDA-treated parkinsonian rats (Zhang et al. 2013; Fieblinger et al. 2014). These studies also demonstrated that the pruning of corticostriatal synapses selectively affected indirect pathway neurons, which displayed decreased intrinsic excitability and reduced cortical excitatory drive after striatal dopamine depletion (Fieblinger et al. 2014; Fieblinger and Cenci, 2015; Suarez et al. 2016). Conversely, D1R-containing direct pathway neurons did not display any significant loss of cortical inputs and exhibited increased excitability in the same model (Fieblinger et al. 2014). Although Fieblinger et al. (2014) did not determine if the morphologic and functional changes of cortical synapses also applied to thalamostriatal glutamatergic afferents, other studies led to controversial results in that regard. On one hand, Zhang et al. (2013) concluded that the number of vGluT2-containing thalamostriatal terminals did not change in 6-OHDA-treated rats (Zhang et al. 2013), while a recent study showed a selective decrease in the strength of thalamic synapses on direct SPNs in 6-OHDA-treated mice (Parker et al. 2016).
Although these data are difficult to reconcile without additional information, it is noteworthy that none of these rodent studies were achieved in animal models that displayed significant thalamic pathology, while our nonhuman primate data were gathered from MPTP-treated monkeys with significant CM/Pf neuronal loss (Villalba et al. 2013, 2014). As discussed above, the lack of thalamostriatal lesion in the rodent models of PD is a concern that must be addressed to assess the significance of findings collected in these animals towards the human parkinsonian state condition. The need for mice PD models with significant Pf cell loss is imperative to reconcile these various data sets.
Striatal Glutamatergic Axo-spinous Synapses Remodeling; Another Form of Plasticity that may Abnormally Drive Direct Pathway Neurons in Advanced Parkinsonian State and L-DOPA-induced Dyskinesia?
As discussed above, morphometric changes in various elements of axo-spinous glutamatergic synapses have been closely linked to the plasticity and altered functional properties of these synapses in normal and pathological condition in various CNS regions (see above). In addition to changes in the number of dendritic spines, the remodeling of glutamatergic axo-spinous synapses in the striatum has also been reported in rodent and monkey models of parkinsonism (Ingham et al. 1993, 1998; Anglade et al. 1996; Meshul et al. 1999, 2000; Smith et al. 2009b; Villalba and Smith, 2010; 2011a; 2013; Zhang et al. 2013; Suarez et al. 2014, 2016; Villalba et al. 2015). Earlier studies in 6-OHDA-treated rats have demonstrated a significant increase in the number of perforated asymmetric synapses in the striatum, a plastic change commonly associated with increased glutamatergic synaptic transmission (Ingham et al. 1993, 1998; Anglade et al. 1996; Meshul et al. 1999, 2000). We recently extended these observations in chronically MPTP-treated parkinsonian monkeys using three dimensional electron microscopy reconstruction of individual corticostriatal and thalamostriatal glutamatergic synapses (Fig. 3a, a.1, a.2, 3b, b.1, b.2). Our data revealed a significant increase in the volume of dendritic spines and the pre-synaptic glutamatergic afferents as well as increases in the area of post synaptic densities (PSD) at both cortical and thalamic glutamatergic synapses in these animals (Fig. 3c, d) (Smith et al. 2009; Villalba and Smith, 2010, 2011a, 2013; Villalba et al. 2015). This study did not provide information about the striatal SPN subtypes that were affected by the remodeling of these axo-spinous synapses. However, a recent study in mice has shown that these changes might be specific to D1R direct pathway neurons (Suarez et al. 2016). Using the intrastriatal 6-OHDA-treated mouse model of PD, these authors have demonstrated that the remaining spines and PSDs of glutamatergic synapses on D1R direct pathway neurons, but not on D2R indirect pathway neurons, undergo significant growth in L-DOPA-treated dyskinetic mice (Darmopil et al. 2009; Murer and Moratalla 2011; Ruiz-DeDiego et al. 2015, 2016; Solis et al. 2015, 2016; Suarez et al. 2016). They also showed that these structural changes were associated with increased strength of corticostriatal glutamatergic synapses on these neurons.
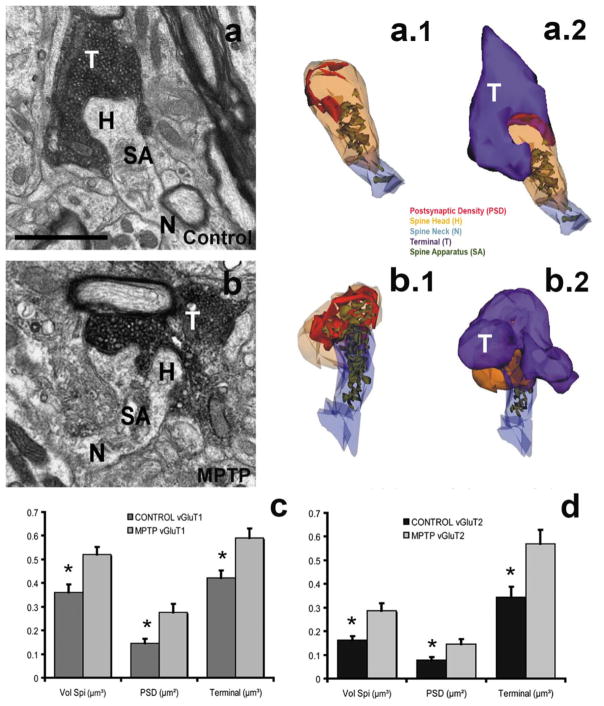
Fig. 3. Glutamatergic terminals and SPNs dendritic spines in the striatum of control and MPTP-treated monkeys.
a, b: Electron micrographs of dendritic spines and their corresponding glutamatergic synapses in the striatum of control (a), and MPTP-treated parkinsonian (b) monkeys. a.1, a.2, b.1, b.2: Three-dimensional reconstructions of vGluT1-positive terminals forming axo-spinous synapses in the striatum of control (a.1; a.2) and MPTP-treated parkinsonian (b.1; b.2) monkeys. c: Histograms comparing the quantitative analysis of morphometric parameters (spine head volume, postsynaptic density area, terminal volume) of 3D-reconstructed axo-spine corticostriatal (vGluT1, c) and thalamostriatal (vGluT2, d) synapses in the striatum of control and parkinsonian (c, d) monkeys. In MPTP-treated parkinsonian monkeys (N=3), the spine volume (Vol Spi, μm3), the areas of the postsynaptic densities (PSDs, μm2) and the volume of the presynaptic terminals (μm3) at corticostriatal (c) and thalamostriatal (d) synapses are significantly increased (*, t-test, P<0.001; SigmaPlot) in MPTP-treated monkeys compared with controls (N=3). Scale bar in a (applies to b): 1μm.
In agreement with results from a previous study (Fieblinger et al. 2014), these authors also showed that L-DOPA treatment could reverse the spine loss on D2R indirect pathway neurons, but that this spine recovery did not induce any significant change in the strength of cortical afferents to indirect pathway neurons. As discussed above, another important observation made by Suarez and colleagues (Suarez et al. 2014, 2016) is that mice models of PD developed through intrastriatal 6-OHDA injection display homogeneous striatal spine loss on both D1R and D2R SPNs. These findings are consistent with data from human PD patients and chronically MPTP-treated monkeys (Villalba and Smith, 2010, 2011b, 2013; Villalba et al. 2015; Singh et al. 2015), but different from other rodent studies showing selective loss of spines on D2R indirect pathway neurons after 6-OHDA administration in the MFB (Day et al. 2006, 2008; Schuster et al. 2009; Fieblinger et al. 2014). Thus, it appears that the effects of DA loss and L-DOPA-induced dyskinesia on striatal spine plasticity are far more complex than a mere selective spine loss of D2R indirect pathway neurons that can be reversed by L-DOPA. Despite the controversy between some of the rodent studies, the human and monkey observations as well as the recent findings from Suarez et al. (2014, 2016) highlight the complex nature of the plastic changes that regulate axo-spinous glutamatergic synapses in parkinsonian and dyskinetic conditions (Suarez et al. 2014, 2016). We suggest that the degeneration or not of the thalamostriatal system from CM/Pf may contribute to some of the discrepant findings presented in these studies. Future rodent studies in this field must pay closer attention to the state of the thalamostriatal system in the animal models used to generate data that can be more closely related to the human disease condition.
Are the Morphology and Functional Properties of both Corticostriatal and Thalamostriatal Glutamatergic Synapses Affected in the Parkinsonian and Dyskinetic States?
Data obtained so far from various animal models only partly address this important issue. In chronically MPTP-treated parkinsonian monkeys that display significant nigrostriatal and thalamostriatal lesions, axo-spinous synapses that involve both corticostriatal (vGluT1-positive) and thalamostriatal (vGluT2-positive) terminals undergo profound synaptic remodeling (Villalba and Smith, 2010, 2011a; Villalba et al. 2013, 2015). However, Zhang et al. (2013) recently reported an aberrant restoration of spines and corticostriatal synapses, without any significant effect on thalamostriatal synapses, after L-DOPA treatment in a mouse model of L-DOPA-induced dyskinesia. It is noteworthy that the state of degeneration of the thalamostriatal system in animals used in this study was not assessed (Zhang et al. 2013). This lack of critical information makes these findings difficult to compare with the monkey and human data gathered from cases with severe thalamostriatal degeneration (Henderson et al. 2000a, b; Villalba and Smith 2010, 2011a; Villalba et al. 2013, 2015). In contrast to the findings of Zhang et al. (2013), a recent study demonstrated that striatal dopamine loss results in a selective decrease in synaptic strength of the thalamostriatal, but not corticostriatal, projections that innervate specifically D1R direct pathway neurons in intra-MFB 6-OHDA-treated mice (Zhang et al. 2013; Parker et al. 2016). Although the authors did not report information about the morphology of SPNs, they highlighted the fact that there was no evidence for Pf cell death in the mice used in their study (Parker et al. 2016). These data differ from other recent ex vivo results showing that the strength of corticostriatal projection on D2R indirect pathway SPNs is reduced in parkinsonian mice (Fieblinger et al. 2014; Suarez et al. 2016). Thus, the small amount and controversial outcome of studies published so far make conclusions difficult to draw about the impact of parkinsonism and LID on corticostriatal versus thalamostriatal synapses. However, there is a significant likelihood that CM/Pf cell death induces structural and functional remodeling of thalamostriatal synapses in PD patients, as was shown in MPTP-treated monkeys (Villalba et al. 2013) thereby reinforcing the fact that rodent models of PD that mimic nigrostriatal and thalamostriatal lesions are needed to further address this issue.
Coming up with a consensus about the possible pathophysiology of these glutamatergic systems in human PD and L-DOPA-induced dyskinesia is of critical importance. Because the striatum is one of the main gateways of extrinsic information to the basal ganglia circuits, a disruption of cortical and/or thalamic inputs to specific or both subsets of striatal SPNs could have significant consequences on the whole basal ganglia-thalamocortical circuitry. Elucidating the exact nature of the plastic changes striatal glutamatergic afferents undergo in diseased states may also help determine the substrate of some electrophysiology studies showing a significant increase in the firing rate and bursting pattern of SPNs in PD patients and animal models of parkinsonism (Calabresi et al. 1993; Liang et al. 2008; Azdad et al. 2009; Suarez et al. 2014, 2016; Fieblinger 2014; Singh et al. 2015; 2016). Although these findings were recently challenged by results of an MPTP-treated monkey study (Deffains et al. 2016), they strongly suggest that abnormal glutamatergic transmission in the mammalian striatum may contribute to the pathophysiology of parkinsonian motor signs (Calabresi et al. 1993; Papa and Chase 1996; Chase et al. 1998; Gubellini et al. 2002; Picconi et al. 2004).
Could Remodeling of Perisynaptic Astrocytes Contribute to Functional Changes of Axo-spinous Glutamatergic Synapses in Parkinsonism?
Astrocytes in the CNS undergo dynamic structural remodeling in response to physiological or pathological changes in synaptic activity (Dervan et al. 2004; Theodosis et al. 2008; Reichenbach et al. 2010; Penzes et al. 2011; Potts et al. 2014; Heller and Rusakov 2015; Singh et al. 2015; Blanco-Suarez et al. 2016; Singh et al. 2016). Astrocytes are involved in the formation and maintenance of glutamatergic synapses and have a crucial role in the turnover and enlargement of spines (Witcher et al. 2007, 2010; Barres 2008; Buard et al. 2010; Pfrieger 2010; Martin et al. 2015). Comparative studies using 3D reconstruction, animal models of PD, as well as human PD, have shown that in response to DA denervation astrocytes in both the striatum and GP occupy a larger volume (Charron et al. 2014). This increase in the volume is mainly due to the reorganization and enlargement of astrocyte processes at the level of asymmetric synapses (Charron et al., 2014), but also to an increase in the number of astrocytes (Dervan et al. 2004; Henning et al. 2008; Charron et al. 2014).
The modifications in astrocytes morphology and in their spatial relationships with glutamatergic synapses in the striatum of PD models, together with the different molecular mechanisms by which astrocytes respond to changes in neuronal activity, suggest that pathological changes in perisynaptic striatal astrocytes might play a key role in triggering and/or contributing to the morphological and functional changes in striatal network plasticity in parkinsonism (Villalba and Smith 2011b). Data from our laboratory have shown that the synaptic remodeling of axo-spinous synapses in MPTP-treated monkeys is accompanied with a significant growth in the extent of glial coverage of striatal glutamatergic synapses in parkinsonian condition (Villalba and Smith 2011b; Villalba et al. 2015). Using EM serial sections and 3D ultrastructural reconstruction, we have demonstrated significant growth in the extent of glial coverage of glutamatergic axo-spinous synapses that undergo remodeling in parkinsonian monkeys (Villalba and Smith 2011b; Villalba et al. 2015). In control animals, perisynaptic astrocytes exhibit an interdigitated finger-like morphology (Fig. 4a, a.1, a.2), while after MPTP treatment there is an expansion of their processes to surround the axo-spinous complexes (Fig. 4b, b.1, b.2), making them much tighter and continuous than in controls (Figures 4a,a.1,a.2). These changes affect both vGluT1- and vGluT2-positive glutamatergic axo-spinous synapses (Villalba and Smith 2011b). These morphological and ultrastructural changes in perisynaptic astrocytes might underlie an active participation of glial processes in structural plasticity of glutamatergic synapses in the striatum, as previously shown in the hypothalamus (Theodosis et al. 2008) and hippocampus (Ventura and Harris 1999; Witcher et al. 2007, 2010). A better understanding of glia-neurons communication at corticostriatal and thalamostriatal synapses in normal and pathological conditions might help to characterize the pathophysiology of striatal glutamatergic afferents in the parkinsonian state.
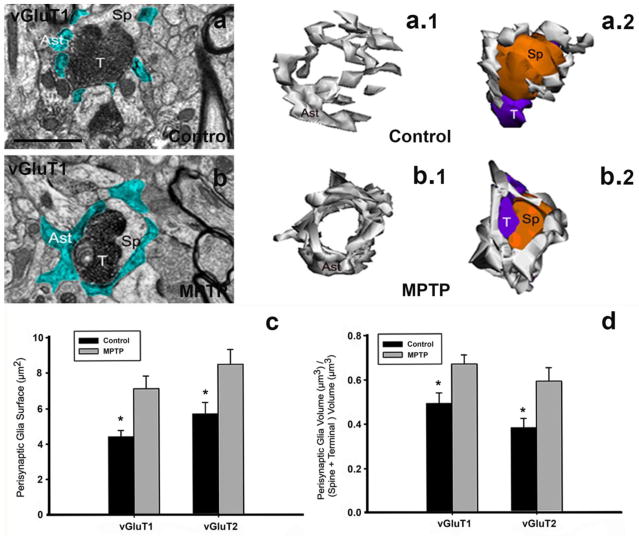
Fig. 4. Perisynaptic astrocytes in striatal axo-spine glutamatergic synapses of control and MPTP-treated monkeys.
a, b: Examples of single electron micrographs (EM) images of perisynaptic astrocytic processes (Ast) in control (a) and MPTP-treated monkey (b). Glial processes have been pseudocolored in blue (a, b). a.1, a.2, b.1, b.2: Three–dimensional reconstruction of axo-spine synapses formed by a vGluT1-positive terminal (T) and a dendritic spine (Sp) in the striatum of control (a.1, a.2) and MPTP-treated parkinsonian (b.1, b.2) monkey. While axo-spinous interfaces in control (a, a.1, a.2) are only partially surrounded by astroglial processes (Ast), those synapses are almost completely wrapped by enlarged astrocyte (Ast) processes in MPTP-animals (b, b.1, b.2). c, d: Quantitative analysis of the perisynaptic glia in striatal glutamatergic axo-spine synapses. c: Comparison of the surface area (mean±SEM) of perisynaptic glia associated with axo-spine synapses formed vGluT1- and vGluT2-immunopositive terminals in control and MPTP-treated monkeys. The surface of the perisynaptic glia is significantly larger (*, t-test; SigmaPlot) in MPTP-parkinsonian monkeys than in control (p=0.017 for vGluT1, and p=0.06 for vGluT2). d: Comparison of the volume of the perisynaptic glia over the total volume of the spine and the vGluT1- or vGluT2-immunoreactive terminal. This ratio is significantly increased in axo-spines synapses from MPTP-treated animals compared with control (*, t-test, p=0.049 for vGluT1 and p=0.028 for vGluT2, SigmaPlot). No significant difference is found between the volume of perisynaptic glia in axo-spine synapses formed for vGluT1- or vGluT2-positive terminals. Number of animals=3 controls and 3 MPTP-treated monkeys. Total number of reconstructed spines=32 (8 per group). Scale bar in a (applies to b): 1μm.
Concluding Remarks
Evidence for pruning and anatomo-functional plasticity of axo-spinous glutamatergic synapses in PD has grown in recent years. Despite these major advances, key issues still remain unanswered or controversial about the progression and significance of these changes towards the pathophysiology and symptomatology of PD. In this review, we discussed these challenges and highlighted some of the main questions that warrant further studies in animal models that better display the pathology of the glutamatergic and dopaminergic systems in PD. Most importantly, we believe that the lack of consideration of thalamic degeneration in most rodent studies that have been recently achieved represents an important limitation that may contribute to some of the discrepancies raised in this review. Because degeneration of intralaminar thalamic nuclei is a key feature of thalamic pathology in human PD, combined with the fact that the degenerated thalamic nuclei are the main sources of the glutamatergic thalamostriatal system, strongly suggest that thalamic degeneration must be induced in animal models used to study plastic changes of glutamatergic transmission in parkinsonism. However, rodent studies published so far that looked at this issue either did not report any information on thalamic pathology or indicated that the thalamus was not affected in the mouse model used in the study.
Altogether, the current state of knowledge gained from postmortem human material, rodent and non-human primate models of PD indicates:
Striatal spine loss is a plastic phenomenon that affects preferentially the dorsal striatum (because DA denervation is more pronounced) in both PD patients and various animal models of parkinsonism.
In chronically MPTP-treated monkeys, striatal spine loss is an early event that is tightly correlated with the extent of striatal dopamine denervation, but not with the severity of parkinsonian motor symptoms. We suggest that this early striatal spine loss might be a homeostatic response to compensate for the progressive breakdown of the regulatory functions of dopamine upon SPNs activity and corticostriatal glutamatergic transmission.
-
At least some of the remaining axo-spinous glutamatergic synapses undergo profound morphological changes consistent with increased synaptic strength in rodent and monkey models of PD. Despite convincing evidence that synaptic remodeling is induced, many important lingering questions remain unanswered about this plastic phenomenon. When does it occur during the course of striatal dopamine denervation?. Does it affect both direct and indirect pathway neurons?. Does it involve both thalamostriatal and corticostriatal glutamatergic synapses?. Is it the main substrate of increased corticostriatal glutamatergic transmission in parkinsonism?. Does it represent a form of maladaptive plasticity that contributes to the development and severity of PD motor symptoms?. Is it worsened by L-DOPA treatment and the development of dyskinesia?.
As discussed in the review, partial information has been gathered about some of these issues, but because of controversial reports, a consensus awaits further studies. In light of findings published so far, we hypothesize that the increased size of spines, pre-synaptic glutamatergic terminals and PSDs is a form of late maladaptive plasticity that affects both corticostriatal and thalamostriatal glutamatergic synapses on D1R direct pathway neurons after severe and prolonged striatal dopaminergic denervation. We suggest that these changes play a critical role in mediating increased glutamatergic transmission which may contribute to the increased firing rate of SPNs in the parkinsonian state.
Some controversy remains about the extent and progression of spine loss on D1R direct vs D2R indirect pathway neurons. Data suggesting that either both populations or only D2R indirect pathway neurons undergo spine loss in parkinsonism have been reported. Because these findings were gathered from different animal models, the underlying substrate of these discrepancies remains unclear. As mentioned above, we believe that this issue must be examined in animal models that display both nigral and early thalamic pathology to better reflect the state of human PD. The selective loss of spines on D2R neurons reported in some rodent studies may illustrate the effect of acute 6-OHDA-induced striatal DA denervation, while early degeneration of the thalamostriatal system combined with progressive nigrostriatal dopaminergic cell loss may affect spine plasticity on both D1R and D2R SPNs. Future studies are needed to directly test this hypothesis.
Based on data gathered from rodent models of PD, of which the state of thalamic pathology remains unknown, L-DOPA reverses striatal spine loss selectively on D2R indirect pathway neurons. These observations must be confirmed in animal models PD that harbor both thalamic and nigral lesions.
In conclusion, striatal spine plasticity is a cardinal and intricate feature of PD that likely contributes to both compensatory homeostatic regulation of SPNs activity during the prodromal “motor asymptomatic” stage of the disease and maladaptive plasticity that may significantly contribute to the pathophysiology of striatal glutamatergic afferents and parkinsonian motor signs in advanced stage of PD.
Acknowledgments
The authors thank Jean-Francois Pare and Susan Jenkins for technical assistance. This work was supported by the NIH grants R01NS083386 (YS) and P50NS098685 (YS) and the Yerkes Primate Center base grant P51OD01113.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander G, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Anglade P, Mouatt-Prigent A, Agid Y, Hirsch E. Synaptic plasticity in the caudate nucleus of patients with Parkinson’s disease. Neurodegeneration. 1996;5:121–128. doi: 10.1006/neur.1996.0018. [DOI] [PubMed] [Google Scholar]
- Aymerich MS, Barroso-Chinea P, Perez-Manso M, Munoz-Patino AM, Moreno-Igoa M, Gonzalez-Hernandez T, Lanciego JL. Consequences of unilateral nigrostriatal denervation on the thalamostriatal pathway in rats. Eur J Neurosci. 2006;23:2099–2108. doi: 10.1111/j.1460-9568.2006.04741.x. [DOI] [PubMed] [Google Scholar]
- Azdad K, Chavez M, Don Bischop P, Wetzelaer P, Marescau B, De Deyn PP, Gall D, Schiffmann SN. Homeostatic plasticity of striatal neurons intrinsic excitability following dopamine depletion. PLoS One. 2009;4:e6908. doi: 10.1371/journal.pone.0006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Bernacer J, Prensa L, Gimenez-Amaya JM. Morphological features, distribution and compartmental organization of the nicotinamide adenine dinucleotide phosphate reduced-diaphorase interneurons in the human striatum. J Comp Neurol. 2005;489:311–327. doi: 10.1002/cne.20616. [DOI] [PubMed] [Google Scholar]
- Bernacer J, Prensa L, Gimenez-Amaya JM. Cholinergic interneurons are differentially distributed in the human striatum. PLoS One. 2007;2:e1174. doi: 10.1371/journal.pone.0001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacer J, Prensa L, Gimenez-Amaya JM. Distribution of GABAergic interneurons and dopaminergic cells in the functional territories of the human striatum. PLoS One. 2012;7:e30504. doi: 10.1371/journal.pone.0030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Turner R, Chockkan V, DeLong MR, Allers KA, Walters J, Levey AI, Greenamyre JT. Dopaminergic neurons intrinsic to the primate striatum. J Neurosci. 1997;17:6761–6768. doi: 10.1523/JNEUROSCI.17-17-06761.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Suarez E, Caldwell AL, Alle NJ. Role of astrocyte-synapse interactions in CNS disorders. J Physiol. 2016 doi: 10.1113/JP270988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buard I, Steinmetz CC, Claudepierre T, Pfrieger FW. Glial cells promote dendrite formation and the reception of synaptic input in Purkinje cells from postnatal mice. Glia. 2010;58:538–545. doi: 10.1002/glia.20943. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, Sancesario G, Bernardi G. Electrophysiology of dopamine-denervated striatal neurons. Implications for Parkinson’s disease. Brain. 1993;116:433–452. [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cali C, Baghabra J, Boges DJ, Holst GR, Kreshuk A, Hamprecht FA, Srinivasan M, Lehvaslaiho H, Magistretti PJ. Three-dimensional immersive virtual reality for studying cellular compartments in 3D models from EM preparations of neural tissues. J Comp Neurol. 2016;524:23–38. doi: 10.1002/cne.23852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SR. Estructura de los centros nerviosos de las aves. Rev Trim Histol Norm Pat. 1888;1:1–10. [Google Scholar]
- Cajal SR. Sur la structure de l’ecorce cerebrale de quelques mammiferes. La Cellule. 1891;7:125–176. [Google Scholar]
- Cajal SR. Neue darstellung vom histologischen bau des centralnervensystem. Arch Anat Enwick. 1893;1893:319–428. [Google Scholar]
- Cajal SR. Las espinas colaterales de las celulas del cerebro tenidas por el azul. Rev Trimest Micrograf. 1896;1:123–136. [Google Scholar]
- Charron G, Doudnikoff E, Canron MH, Li Q, Vega C, Marais S, Baufreton J, Vital A, Oliet SH, Bezard E. Astrocytosis in parkinsonism: considering tripartite striatal synapses in physiopathology? Front Aging Neurosci. 2014;6:258. doi: 10.3389/fnagi.2014.00258. doi.org/10.3389/fnagi.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase TN, Oh JD, Blanchet PJ. Neostriatal mechanisms in Parkinson’s disease. Neurology. 1998;5:S30–35. doi: 10.1212/wnl.51.2_suppl_2.s30. [DOI] [PubMed] [Google Scholar]
- Darmopil S, Martin AB, DeDiego IR, Ares S, Moratalla R. Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol Psychiatry. 2009;66:603–613. doi: 10.1016/j.biopsych.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. The neuroanatomist’s dream, the problems and solutions, and the ultimate aim. Front Neurosci. 2008;2:10–12. doi: 10.3389/neuro.01.018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffains M, Iskhakova L, Katabi S, Haber SN, Israel Z, Bergman H. Subthalamic, not striatal, activity correlates with basal ganglia downstream activity in normal and parkinsonian monkeys. Elife. 2016;5:e16443. doi: 10.7554/eLife.16443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- DeLong M, Wichmann T. Changing views of basal ganglia circuits and circuit disorders. Clin EEG Neurosci. 2010;41:61–67. doi: 10.1177/155005941004100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervan AG, Meshul CK, Beales M, McBean GJ, Moore C, Totterdell S, Snyder AK, Meredith GE. Astroglial plasticity and glutamate function in a chronic mouse model of Parkinson’s disease. Exp Neurol. 2004;190:145–156. doi: 10.1016/j.expneurol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Colbran RJ, Winder DJ. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism Relat Disord. 2007;13(Suppl 3):S251–258. doi: 10.1016/S1353-8020(08)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY. The thorny problem of dyskinesias: dendritic spines, synaptic plasticity, and striatal dysfunction. Biol Psychiatry. 2014;75:676–677. doi: 10.1016/j.biopsych.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espadas I, Darmopil S, Vergano-Vera E, Ortiz O, Oliva I, Vicario-Abejon C, Martin ED, Moratalla R. L-DOPA-induced increase in TH-immunoreactivite striatal neurons in parkinsonian mice:insights into regulation and function. Neurobiol Dis. 2012;48:271–281. doi: 10.1016/j.nbd.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Fiala JC. Reconstruct: a free editor for serial section microscopy. J Microsc. 2005;218:52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- Fieblinger T, Graves SM, Sebel LE, Alcacer C, Plotkin JL, Gertler TS, Chan CS, Heiman M, Greengard P, Cenci MA, Surmeier DJ. Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat Commun. 2014;5:5316. doi: 10.1038/ncomms6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieblinger T, Cenci MA. Zooming in on the small: the plasticity of striatal dendritic spines in L-DOPA-induced dyskinesia. Mov Disord. 2015;30:484–493. doi: 10.1002/mds.26139. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Savoia P, Bono F, Tallarico P, Missale C. The D3 dopamine receptor: From structural interactions to function. Eur Neuropsychopharmacol. 2015;25:1462–1469. doi: 10.1016/j.euroneuro.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Freyaldenhoven TE, Ali SF, Schmued LC. Systemic administration of MPTP induces thalamic neuronal degeneration in mice. Brain Res. 1997;759:9–17. doi: 10.1016/s0006-8993(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110:257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Petryszyn S, Sanchez MG, Bories C, Beaulieu JM, De Koninck Y, Parent A, Parent M. Striatal Neurons Expressing D1 and D2 Receptors are Morphologically Distinct and Differently Affected by Dopamine Denervation in Mice. Sci Rep. 2017;7:41432. doi: 10.1038/srep41432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Smith Y. The primate thalamostriatal systems: Anatomical organization, functional roles and possible involvement in Parkinson’s disease. Basal Ganglia. 2011;1:179–189. doi: 10.1016/j.baga.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Villalba RM, Wichmann T, Smith Y. The thalamostriatal system in normal and diseased states. In: Steiner HZ, Tseng K-Y, editors. Handbook of basal ganglia structure and function. 2. Elsevier; Amsterdam: 2016. [Google Scholar]
- Garcia BG, Neely MD, Deutch AY. Cortical regulation of striatal medium spiny neuron dendritic remodeling in parkinsonism: modulation of glutamate release reverses dopamine depletion-induced dendritic spine loss. Cereb Cortex. 2010;20:2423–2432. doi: 10.1093/cercor/bhp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, Martinez-Sanchez P, Sanchez-Gonzalez MA, Garzon M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cereb Cortex. 2009;19:424–434. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorayeb I, Fernagut PO, Hervier L, Labattu B, Bioulac B, Tison F. A ‘single toxin-double lesion’ rat model of striatonigral degeneration by intrastriatal 1-methyl-4-phenylpyridinium ion injection: a motor behavioural analysis. Neuroscience. 2002;115:533–546. doi: 10.1016/s0306-4522(02)00401-3. [DOI] [PubMed] [Google Scholar]
- Gonzales KK, Smith Y. Cholinergic interneurons in the dorsal and ventral striatum: anatomical and functional considerations in normal and diseased conditions. Ann N Y Acad Sci. 2015;1349:1–45. doi: 10.1111/nyas.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327:307–311. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- Gray EG. Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature. 1959;183:1592–1593. doi: 10.1038/1831592a0. [DOI] [PubMed] [Google Scholar]
- Gubellin P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, Bernardi G, Finazzi-Agro A, Maccarrone M. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci. 2002;22:6900–6907. doi: 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM. Thalamic changes in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S152–155. doi: 10.1016/S1353-8020(09)70804-1. [DOI] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Harris KM, Perry E, Bourne J, Feinberg M, Ostroff L, Hurlburt J. Uniform serial sectioning for transmission electron microscopy. J Neurosci. 2006;26:12101–12103. doi: 10.1523/JNEUROSCI.3994-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes WI, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci. 2013;33:4804–4814. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller JP, Rusakov DA. Morphological plasticity of astroglia: Understanding synaptic microenvironment. Glia. 2015;63:2133–2151. doi: 10.1002/glia.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JM, Carpenter K, Cartwright H, Halliday GM. Degeneration of the centre median-parafascicular complex in Parkinson’s disease. Ann Neurol. 2000a;47:345–352. [PubMed] [Google Scholar]
- Henderson JM, Carpenter K, Cartwright H, Halliday GM. Loss of thalamic intralaminar nuclei in progressive supranuclear palsy and Parkinson’s disease: clinical and therapeutic implications. Brain. 2000b;123:1410–1421. doi: 10.1093/brain/123.7.1410. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Schleimer SB, Allbutt H, Dabholkar V, Abel D, Jovic J, Quinlivan M. Behavioural effects of parafascicular thalamic lesions in an animal model of parkinsonism. Behav Brain Res. 2005;162:222–232. doi: 10.1016/j.bbr.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Henning J, Strauss U, Wree A, Gimsa J, Rolfs A, Benecke R, Gimsa U. Differential astroglial activation in 6-hydroxydopamine models of Parkinson’s disease. Neurosci Res. 2008;62:246–253. doi: 10.1016/j.neures.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Ocampo I, Mena-Segovia J, Bolam JP. Convergence of cortical and thalamic input to direct and indirect pathway medium spiny neurons in the striatum. Brain Struct Funct. 2014;219:1787–1800. doi: 10.1007/s00429-013-0601-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Arbuthnott GW. Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res. 1989;503:334–338. doi: 10.1016/0006-8993(89)91686-7. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Vanmaldegem B, Weenink A, Arbuthnott GW. Morphological-Changes in the Rat Neostriatum after Unilateral 6-Hydroxydopamine Injections into the Nigrostriatal Pathway. Experimental Brain Research. 1993;93:17–27. doi: 10.1007/BF00227776. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci. 1998;18:4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani A, Betancur C, Giros B, Hirsch E, El Mestikawy S. Altered expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in Parkinson disease. Neurobiol Aging. 2007;28:568–578. doi: 10.1016/j.neurobiolaging.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci. 1990;10:3421–3438. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The site of termination of afferent fibres in the caudate nucleus. Philos Trans R Soc Lond B Biol Sci. 1971a;262:413–427. doi: 10.1098/rstb.1971.0104. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The synaptic organization of the caudate nucleus. Philos Trans R Soc Lond B Biol Sci. 1971b;262:403–412. doi: 10.1098/rstb.1971.0103. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: a study with the Golgi method. Philos Trans R Soc Lond B Biol Sci. 1971c;262:429–439. doi: 10.1098/rstb.1971.0105. [DOI] [PubMed] [Google Scholar]
- Knott G, Marchman H, Wall D, Lich B. Serial section scanning electron microscopy of adult brain tissue using focused ion beam milling. J Neurosci. 2008;28:2959–2964. doi: 10.1523/JNEUROSCI.3189-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Kusnoor SV, Bubser M, Deutch AY. The effects of nigrostriatal dopamine depletion on the thalamic parafascicular nucleus. Brain Res. 2012;1446:46–55. doi: 10.1016/j.brainres.2012.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Gonzalo N, Castle M, Sanchez-Escobar C, Aymerich MS, Obeso JA. Thalamic innervation of striatal and subthalamic neurons projecting to the rat entopeduncular nucleus. Eur J Neurosci. 2004;19:1267–1277. doi: 10.1111/j.1460-9568.2004.03244.x. [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med. 2012;2:a009621. doi: 10.1101/cshperspect.a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci. 2004;24:8289–8299. doi: 10.1523/JNEUROSCI.1990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Deng Y, Liu B, Mu S, Guley NM, Wong T, Reiner A. Confocal laser scanning microscopy and ultrastructural study of VGLUT2 thalamic input to striatal projection neurons in rats. J Comp Neurol. 2013;521:1354–1377. doi: 10.1002/cne.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, DeLong MR, Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J Neurosci. 2008;28:7537–7547. doi: 10.1523/JNEUROSCI.1176-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Bajo-Graneras R, Moratalla R, Perea G, Araque A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science. 2015;349:730–734. doi: 10.1126/science.aaa7945. [DOI] [PubMed] [Google Scholar]
- Mazloom M, Smith Y. Synaptic microcircuitry of tyrosine hydroxylase-containing neurons and terminals in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkeys. J Comp Neurol. 2006;495:453–469. doi: 10.1002/cne.20894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill TH, Brown SA, Rafols JA, Shoulson I. Atrophy of medium spiny I striatal dendrites in advanced Parkinson’s disease. Brain Res. 1988;455:148–152. doi: 10.1016/0006-8993(88)90124-2. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization and collateralization of corticostriate neurones in the motor and sensory cortex of the rat brain. Brain Res. 1987;423:318–324. doi: 10.1016/0006-8993(87)90855-9. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Merchan-Perez A, Rodriguez JR, Alonso-Nanclares L, Schertel A, Defelipe J. Counting Synapses Using FIB/SEM Microscopy: A True Revolution for Ultrastructural Volume Reconstruction. Front Neuroanat. 2009;3:Art 18. doi: 10.3389/neuro.05.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshul CK, Emre N, Nakamura CM, Allen C, Donohue MK, Buckman JF. Time-dependent changes in striatal glutamate synapses following a 6-hydroxydopamine lesion. Neuroscience. 1999;88:1–16. doi: 10.1016/s0306-4522(98)00189-4. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Cogen JP, Cheng HW, Moore C, Krentz L, McNeill TH. Alterations in rat striatal glutamate synapses following a lesion of the cortico- and/or nigrostriatal pathway. Exp Neurol. 2000;165:191–206. doi: 10.1006/exnr.2000.7467. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Solis O, Suarez LM. Morphological Plasticity in the Striatum Associated with Dopamine Dysfunction. In: Steiner Heinz, Tseng Kuei Y., editors. Handbook of Basal Ganglia Structure and Function. 2. Elsewier Academic Press; 2016. pp. 755–770. [Google Scholar]
- Moss J, Bolam JP. A dopaminergic axon lattice in the striatum and its relationship with cortical and thalamic terminals. J Neurosci. 2008;28:11221–11230. doi: 10.1523/JNEUROSCI.2780-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer MG, Moratalla R. Striatal signaling in L-DOPA-induced dyskinesia: common mechanisms with drug abuse and long term memory involving D1 dopamine recepor estimulation. Front Neuroanat. 2011;5:51. doi: 10.3389/fnana.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Neely MD, Schmidt DE, Deutch AY. Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons. Neuroscience. 2007;149:457–464. doi: 10.1016/j.neuroscience.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Oorschot DE. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical disector methods. J Comp Neurol. 1996;366:580–599. doi: 10.1002/(SICI)1096-9861(19960318)366:4<580::AID-CNE3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Oorschot D. The percentage of interneurons in the dorsal striatum of the rat, cat, monkey and human: A critique of the evidence. Basal Ganglia. 2013;3:19–24. [Google Scholar]
- Papa SM, Chase TN. Levodopa-induced dyskinesias improved by a glutamate antagonist in Parkinsonian monkeys. Ann Neurol. 1996;39:574–578. doi: 10.1002/ana.410390505. [DOI] [PubMed] [Google Scholar]
- Papa SM, Wichmann T. Interaction between hyperdirect and indirect basal ganglia pathways. Mov Disord. 2015;30:909. doi: 10.1002/mds.26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Parker PR, Lalive AL, Kreitzer AC. Pathway-Specific Remodeling of Thalamostriatal Synapses in Parkinsonian Mice. Neuron. 2016;89:734–740. doi: 10.1016/j.neuron.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasik P, Pasik T, Hamori J. Synapses between interneurons in the lateral geniculate nucleus of monkeys. Exp Brain Res. 1976;25:1–13. doi: 10.1007/BF00237322. [DOI] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW. Role of glial cells in the formation and maintenance of synapses. Brain Res Rev. 2010;63:39–46. doi: 10.1016/j.brainresrev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Rossi S, Bernardi G, Calabresi P. Therapeutic doses of L-dopa reverse hypersensitivity of corticostriatal D2-dopamine receptors and glutamatergic overactivity in experimental parkinsonism. Brain. 2004;127:1661–1669. doi: 10.1093/brain/awh190. [DOI] [PubMed] [Google Scholar]
- Picconi B, Piccoli G, Calabresi P. Synaptic dysfunction in Parkinson’s disease. Adv Exp Med Biol. 2012;970:553–572. doi: 10.1007/978-3-7091-0932-8_24. [DOI] [PubMed] [Google Scholar]
- Potts LF, Wu H, Singh A, Marcilla I, Luquin MR, Papa SM. Modeling Parkinson’s disease in monkeys for translational studies, a critical analysis. Exp Neurol. 2014;256:133–143. doi: 10.1016/j.expneurol.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju DV, Ahern TH, Shah DJ, Wright TM, Standaert DG, Hall RA, Smith Y. Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism. Eur J Neurosci. 2008;27:1647–1658. doi: 10.1111/j.1460-9568.2008.06136.x. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Derouiche A, Kirchhoff F. Morphology and dynamics of perisynaptic glia. Brain Res Rev. 2010;63:11–25. doi: 10.1016/j.brainresrev.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Rivera A, Alberti I, Martin AB, Narvaez JA, de la Calle A, Moratalla R. Molecular phenotype of rat striatal neurons expressing the dopamine D5 receptor subtype. Eur J Neurosci. 2002;16:2049–2058. doi: 10.1046/j.1460-9568.2002.02280.x. [DOI] [PubMed] [Google Scholar]
- Rivera A, Trias S, Penafiel A, Angel Narvaez J, Diaz-Cabiale Z, Moratalla R, de la Calle A. Expression of D4 dopamine receptors in striatonigral and striatopallidal neurons in the rat striatum. Brain Res. 2003;989:35–41. doi: 10.1016/s0006-8993(03)03328-6. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Gaither LA, Peretti FJ, Lapidus B, Chute DJ. Synaptic organization of the human striatum: a postmortem ultrastructural study. J Comp Neurol. 1996;374:523–534. doi: 10.1002/(SICI)1096-9861(19961028)374:4<523::AID-CNE4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Wichmann T. Extrastriatal dopaminergic circuits of the Basal Ganglia. Front Neuroanat. 2010;4:139. doi: 10.3389/fnana.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-DeDiego I, Naranjo JR, Herve D, Moratalla R. Dopaminergic regulation of olfactory type G-protein α subunit expression in the striatum. Mov Disord. 2015;30:1039–1049. doi: 10.1002/mds.26197. [DOI] [PubMed] [Google Scholar]
- Ruiz-DeDiego I, Mellstrom B, Vallejo M, Naranjo JR, Moratalla R. Activation of DREAM (downstream regulatory element antagonistic modulator), a calcium-binding protein, reduces L-DOPA-induced dyskinesias in mice. Biol Psychiatry. 2016;77:95–100. doi: 10.1016/j.biopsych.2014.03.023. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 1985;329:241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- Scholz B, Svensson M, Alm H, Skold K, Falth M, Kultima K, Guigoni C, Doudnikoff E, Li Q, Crossman AR, Bezard E, Andren PE. Striatal proteomic analysis suggests that first L-dopa dose equates to chronic exposure. PLoS One. 2008;3:e1589. doi: 10.1371/journal.pone.0001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster S, Doudnikoff E, Rylander D, Berthet A, Aubert I, Ittrich C, Bloch B, Cenci MA, Surmeier DJ, Hengerer B, Bezard E. Antagonizing L-type Ca2+ channel reduces development of abnormal involuntary movement in the rat model of L-3,4-dihydroxyphenylalanine-induced dyskinesia. Biol Psychiatry. 2009;65:518–526. doi: 10.1016/j.biopsych.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Sedaghat K, Finkelstein DI, Gundlach AL. Effect of unilateral lesion of the nigrostriatal dopamine pathway on survival and neurochemistry of parafascicular nucleus neurons in the rat--evaluation of time-course and LGR8 expression. Brain Res. 2009;1271:83–94. doi: 10.1016/j.brainres.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidibe M, Smith Y. Thalamic inputs to striatal interneurons in monkeys: synaptic organization and co-localization of calcium binding proteins. Neuroscience. 1999;89:1189–1208. doi: 10.1016/s0306-4522(98)00367-4. [DOI] [PubMed] [Google Scholar]
- Singh A, Liang L, Kaneoke Y, Cao X, Papa SM. Dopamine regulates distinctively the activity patterns of striatal output neurons in advanced parkinsonian primates. J Neurophysiol. 2015;113:1533–1544. doi: 10.1152/jn.00910.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Mewes K, Gross RE, DeLong MR, Obeso JA, Papa SM. Human striatal recordings reveal abnormal discharge of projection neurons in Parkinson’s disease. Proc Natl Acad Sci U S A. 2016;113:9629–9634. doi: 10.1073/pnas.1606792113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bennett BD, Bolam JP, Parent A, Sadikot AF. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. J Comp Neurol. 1994;344:1–19. doi: 10.1002/cne.903440102. [DOI] [PubMed] [Google Scholar]
- Smith Y, Shink E, Sidibe M. Neuronal circuitry and synaptic connectivity of the basal ganglia. Neurosurg Clin N Am. 1998;9:203–222. [PubMed] [Google Scholar]
- Smith Y, Kieval JZ. Anatomy of the dopamine system in the basal ganglia. Trends Neurosci. 2000;23(Suppl 10):S28–33. doi: 10.1016/s1471-1931(00)00023-9. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Smith Y, Villalba R. Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Mov Disord. 2008;23(Suppl 3):S534–547. doi: 10.1002/mds.22027. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009a;78:60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Villalba RM, Raju DV. Striatal spine plasticity in Parkinson’s disease: pathological or not? Parkinsonism Relat Disord. 2009b;15(Suppl 3):S156–161. doi: 10.1016/S1353-8020(09)70805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Galvan A, Ellender TJ, Doig N, Villalba RM, Huerta-Ocampo I, Wichmann T, Bolam JP. The thalamostriatal system in normal and diseased states. Front Syst Neurosci. 2014;8:5. doi: 10.3389/fnsys.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Wichmann T. The cortico-pallidal projection: an additional route for cortical regulation of the basal ganglia circuitry. Mov Disord. 2015;30:293–295. doi: 10.1002/mds.26095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Villalba R, Galvan A. The thalamostriatal system and cognition. In: Soghomonian J-J, editor. The basal ganglia, Innovations in cognitive neuroscience. Springer; Switzerland: 2016. pp. 69–85. [Google Scholar]
- Soderstrom KE, O’Malley JA, Levine ND, Sortwell CE, Collier TJ, Steece-Collier K. Impact of dendritic spine preservation in medium spiny neurons on dopamine graft efficacy and the expression of dyskinesias in parkinsonian rats. Eur J Neurosci. 2010;31:478–490. doi: 10.1111/j.1460-9568.2010.07077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis O, Limon DI, Flores-Hernandez J, Flores G. Alterations in dendritic morphology of the prefrontal cortical and striatum neurons in the unilateral 6-OHDA-rat model of Parkinson’s disease. Synapse. 2007;61:450–458. doi: 10.1002/syn.20381. [DOI] [PubMed] [Google Scholar]
- Solis O, Espadas I, Del-Bel EA, Moratalla R. Nitric oxide synthase inhibition decreases L-DOPA-induced dyskinesia and the expression of striatal molecular markers in Pitx3(−/−) aphakia mice. Neurobiol Dis. 2015;73:49–59. doi: 10.1016/j.nbd.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Solis O, Garcia-Sanz P, Herranz AS, Asensio MJ, Moratalla R. L-DOPA reverses the increased free amino acids tissue levels induced by dopamine depletion and rises GABA and tyrosine in the striatum. Neurotox Res. 2016;30:67–75. doi: 10.1007/s12640-016-9612-x. [DOI] [PubMed] [Google Scholar]
- Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens B, Mueller AJ, Shering AF, Hood SH, Taggart P, Arbuthnott GW, Bell JE, Kilford L, Kingsbury AE, Daniel SE, Ingham CA. Evidence of a breakdown of corticostriatal connections in Parkinson’s disease. Neuroscience. 2005;132:741–754. doi: 10.1016/j.neuroscience.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Suarez LM, Solis O, Carames JM, Taravini IR, Solis JM, Murer MGMoratalla R. L-DOPA treatment selectively restores spine density in dopamine receptor D2-expressing projection neurons in dyskinetic mice. Biol Psychiatry. 2014;75:711–722. doi: 10.1016/j.biopsych.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Suarez LM, Solis O, Aguado C, Lujan R, Moratalla R. L-DOPA Oppositely Regulates Synaptic Strength and Spine Morphology in D1 and D2 Striatal Projection Neurons in Dyskinesia. Cereb Cortex. 2016;26:4253–4264. doi: 10.1093/cercor/bhw263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Kitai ST. D1 and D2 dopamine receptor modulation of sodium and potassium currents in rat neostriatal neurons. Prog Brain Res. 1993;99:309–324. doi: 10.1016/s0079-6123(08)61354-0. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Shen W, Day M, Gertler T, Chan S, Tian X, Plotkin JL. The role of dopamine in modulating the structure and function of striatal circuits. Prog Brain Res. 2010;183:149–167. doi: 10.1016/S0079-6123(10)83008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Tecuapetla F, Koos T, Ibanez-Sandoval O. Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat. 2010;4:150. doi: 10.3389/fnana.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- Toy WA, Petzinger GM, Leyshon BJ, Akopian GK, Walsh JP, Hoffman MV, Vuckovic MG, Jakowec MW. Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurobiol Dis. 2014;63:201–209. doi: 10.1016/j.nbd.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Lee H, Smith Y. Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp Neurol. 2009;215:220–227. doi: 10.1016/j.expneurol.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Smith Y. Striatal spine plasticity in Parkinson’s disease. Front Neuroanat. 2010;4:133. doi: 10.3389/fnana.2010.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Smith Y. Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated Parkinsonian monkeys. J Comp Neurol. 2011a;519:989–1005. doi: 10.1002/cne.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Smith Y. Neuroglial plasticity at striatal glutamatergic synapses in Parkinson’s disease. Front Syst Neurosci. 2011b;5:68. doi: 10.3389/fnsys.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Smith Y. Differential striatal spine pathology in Parkinson’s disease and cocaine addiction: a key role of dopamine? Neuroscience. 2013;251:2–20. doi: 10.1016/j.neuroscience.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Wichmann T, Smith Y. Preferential loss of thalamostriatal ove corticostriatal glutamatergic synapses in parkinsonian monkeys. Society of Neuroscience Annual Meeting Abstracts 240.02.2013. [Google Scholar]
- Villalba RM, Wichmann, Smith Y. Neuronal loss in the caudal intralaminar thalamic nuclei in a primate model of Parkinson’s disease. Brain Struct Funct. 2014;219:381–394. doi: 10.1007/s00429-013-0507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Mathai A, Smith Y. Morphological changes of glutamatergic synapses in animal models of Parkinson’s disease. Front Neuroanat. 2015;9:117. doi: 10.3389/fnana.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Pare JF, Smith Y. Three-Dimensional Electron Microscopy Imaging of Spines in Non-human Primates. In: Bocstale EJV, editor. Transmission Electron Microscopy Methods for Understanding the Brain. Springer Science+Business Media; New York: 2016. pp. 81–103. [Google Scholar]
- Wichmann T, DeLong MR. Pathophysiology of Parkinson’s disease: the MPTP primate model of the human disorder. Ann N Y Acad Sci. 2003;991:199–213. doi: 10.1111/j.1749-6632.2003.tb07477.x. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Delong MR. Anatomy and physiology of the basal ganglia: relevance to Parkinson’s disease and related disorders. Handb Clin Neurol. 2007;83:1–18. doi: 10.1016/S0072-9752(07)83001-6. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Arbuthnott GW, Shindou T. Simulation of GABA function in the basal ganglia: computational models of GABAergic mechanisms in basal ganglia function. Prog Brain Res. 2007;160:313–329. doi: 10.1016/S0079-6123(06)60018-6. [DOI] [PubMed] [Google Scholar]
- Witcher MR, Kirov SA, Harris KM. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia. 2007;55:13–23. doi: 10.1002/glia.20415. [DOI] [PubMed] [Google Scholar]
- Witcher MR, Park YD, Lee MR, Sharma S, Harris KM, Kirov SA. Three-dimensional relationships between perisynaptic astroglia and human hippocampal synapses. Glia. 2010;58:572–587. doi: 10.1002/glia.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Richard S, Parent A. The organization of the striatal output system: a single-cell juxtacellular labeling study in the rat. Neurosci Res. 2000;38:49–62. doi: 10.1016/s0168-0102(00)00140-1. [DOI] [PubMed] [Google Scholar]
- Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, Deutch AY, Montine TJ. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology. 2005;64:545–547. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Baron M, Teylan MA, Kim Y, Song Z, Greengard P, Wong ST. A neurocomputational method for fully automated 3D dendritic spine detection and segmentation of medium-sized spiny neurons. Neuroimage. 2010;50:1472–1484. doi: 10.1016/j.neuroimage.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Meredith GE, Mendoza-Elias N, Rademacher DJ, Tseng KY, Steece-Collier K. Aberrant restoration of spines and their synapses in L-DOPA-induced dyskinesia: involvement of corticostriatal but not thalamostriatal synapses. J Neurosci. 2013;33:11655–11667. doi: 10.1523/JNEUROSCI.0288-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]