Abstract
Daily acute intermittent hypoxia (dAIH) elicits respiratory plasticity, enhancing respiratory motor output and restoring breathing capacity after incomplete cervical spinal injuries (cSCI). We hypothesized that dAIH-induced functional recovery of breathing capacity would occur after both acute (2 weeks) and chronic (8 weeks) cSCI, but through distinct cellular mechanisms. Specifically, we hypothesized that dAIH-induced breathing recovery would occur through serotonin-independent mechanisms 2wks post C2 cervical hemisection (C2Hs), versus serotonin-dependent mechanisms 8wks post C2Hs. In two independent studies, dAIH or sham (normoxia) was initiated 1 week (Study 1) or 7 weeks (Study 2) post-C2Hs to test our hypothesis. Rats were pre-treated with intra-peritoneal vehicle or methysergide, a broad-spectrum serotonin receptor antagonist, to determine the role of serotonin signaling in dAIH-induced functional recovery. Our data support the hypothesis that dAIH-induced recovery of breathing capacity transitions from a serotonin-independent mechanism with acute C2Hs to a serotonin-dependent mechanism with chronic C2Hs. An understanding of shifting mechanisms giving rise to dAIH-induced respiratory motor plasticity is vital for clinical translation of dAIH as a therapeutic modality.
Keywords: phrenic, long-term facilitation, intermittent hypoxia, plasticity, spinal cord injury, serotonin, adenosine
1. Introduction
Acute intermittent hypoxia (AIH) has emerged as a safe, non-invasive method for enhancing motor function in humans with chronic, incomplete spinal cord injury [SCI; (Dale et al., 2014; Gonzalez-Rothi et al., 2015; Navarrete-Opazo and Mitchell, 2014a)]. Indeed, individuals with chronic SCI (>5 years post-injury) who received a single AIH session consisting of ten 60-90 sec exposures to 10.5% O2 (interspersed with 60 sec of room air at 20.9% O2) displayed a prolonged increase in maximal ankle torque production and enhanced electromyographic (EMG) activity in ankle plantar flexor muscles that persisted for at least an hour (and up to 4 hours) post-treatment (Trumbower et al., 2012). Subsequently, when AIH was repeated over 5 consecutive days (daily, acute intermittent hypoxia; dAIH) and combined with task-specific rehabilitation (locomotor training), additive functional benefits were observed; groups with combined dAIH and locomotor training ambulated longer and faster than groups receiving either treatment individually (Hayes et al., 2014; Navarrete-Opazo et al., 2016a). Improvements in dynamic balance following dAIH have also recently been reported in patients with chronic SCI (Navarrete-Opazo et al., 2016a). Collectively, these studies support dAIH as a promising therapeutic strategy for improving function following SCI, especially in chronic time periods when the prospect of significant functional return remains bleak. Much work remains to fully understand cellular mechanisms leading to AIH and dAIH-induced motor recovery.
Our working knowledge of mechanisms giving rise to AIH-induced motor enhancement originated with rodent studies of AIH-induced plasticity in respiratory motor control, specifically AIH-induced phrenic long-term facilitation [pLTF; (Bach and Mitchell, 1996; Baker and Mitchell, 2000)]. Moderate AIH (3, 5-min episodes of PaO2 = 35-45mmHG; 5 min normoxic intervals) elicits prolonged increases in phrenic neural output lasting hours post-AIH. This form of AIH-induced plasticity requires intermittent serotonin release and serotonin receptor activation on or near phrenic motor neurons (Baker-Herman and Mitchell, 2002; Fuller et al., 2001b; Kinkead and Mitchell, 1999). Downstream signaling in the cellular cascade leading to AIH-induced pLTF requires: ERK MAP kinase signaling (Hoffman et al., 2012), new synthesis and release of brain-derived neurotrophic factor [BNDF; (Baker-Herman et al., 2004)], activation of the high-affinity BDNF receptor TrkB (Baker-Herman et al., 2004; Dale et al., 2016), and downstream activation of a protein kinase C isoform [PKCθ; (Devinney et al., 2015)]. More severe AIH protocols (sAIH; 3, 5-min exposures of PaO2 = 25-30mmHG, 5 min normoxic intervals) also elicit pLTF, although through a unique, adenosine-dependent mechanism (Nichols et al., 2012). With sAIH, extracellular adenosine accumulation activates adenosine 2A receptors on or near phrenic motor neurons (Nichols et al., 2012), leading to activation of phosphatidylinositol 3 (PI3)-kinase/Akt (Golder et al., 2008), EPAC (Fields et al., 2015) and mTORC1 (Dougherty et al., 2015), followed by new synthesis of an immature TrkB isoform (Golder et al., 2008). These parallel intracellular pathways to phrenic motor facilitation interact via mutual inhibition (Hoffman et al., 2010), permitting only one pathway to be expressed at a time (depending on severity of dose) with the other acting as an “anchor” to constrain its expression. This mechanistic interplay imparts flexibility in respiratory control and could be critical for maintaining respiratory function following SCI (Devinney et al., 2013).
Cervical SCI (cSCI) at or above the phrenic motor nucleus (C3-C5) may cause respiratory motor neuron death and disrupt descending neural input to respiratory motor neurons (Nicaise et al., 2012). Such injuries reduce the ability to recruit respiratory muscles, particularly during conditions of increased respiratory demand (Alvarez-Argote et al., 2016). cSCI may also disrupt descending neuromodulatory pathways decreasing neuronal excitability and/or the capacity for adaptive plasticity in surviving motor circuits (Dougherty et al., 2016; Golder and Mitchell, 2005; Saruhashi et al., 1996; Zhou and Goshgarian, 1999). With C2 hemisection (C2Hs), an initial decline and subsequent return of serotonergic innervation within the phrenic motor nucleus over 8 weeks post-injury strongly correlates with ipsilateral expression of AIH-induced pLTF (Golder and Mitchell, 2005). When serotonin or serotonin receptor agonists are pharmacologically administered (Zhou and Goshgarian, 2000; Zimmer and Goshgarian, 2006), or serotonergic producing cells are transplanted below C2Hs lesions (Dougherty et al., 2016), enhanced recovery of respiratory motor output is observed and the capacity to express plasticity is restored. Thus, invoking AIH-induced plasticity with cSCI is most effective with chronic injuries after the level of serotonin has had time to recover (Golder and Mitchell, 2005).
Daily AIH (dAIH; 7 consecutive days; 10 5-min episodes per day; 5-min intervals) initiates functional recovery of breathing capacity in rats when initiated as early as 2 weeks post-C2Hs (Lovett-Barr et al., 2012), although the mechanism of this recovery has not been confirmed. In a recent series of studies, Navarette-Opazo and colleagues demonstrated that dAIH initiated one-week post-C2Hs induces functional recovery by a mechanism that requires adenosine 2A (A2a) receptor activation (Navarrete-Opazo et al., 2015), but reverts to an adenosine-constrained mechanism when initiated 8 weeks post-C2Hs (Navarrete-Opazo et al., 2016b), more like the response in uninjured rats (Navarrete-Opazo and Mitchell, 2014b). Thus, different mechanisms appear to contribute to dAIH-induced functional recovery 1-2 versus 7-8 weeks post-cSCI. However, the role of serotonin receptor activation in this functional recovery has never been reported.
We hypothesized that dAIH-induced functional recovery of breathing capacity with acute (1-2 weeks post) C2Hs is driven by serotonin-independent mechanisms, since serotonin availability below the injury is limited (Golder and Mitchell, 2005). We also hypothesized that serotonin-dependence of dAIH-induced functional recovery would revert to serotonin-dependent mechanisms with chronic (7-8 weeks) C2Hs due to restoration of serotonergic innervation below the injury (Golder and Mitchell, 2005). To test these hypotheses, we synthesized unpublished data from two independent studies exploring the effects of methysergide pretreatment on dAIH induced functional recovery at 1-2 (Study 1) and 7-8 weeks (Study 2) post-C2Hs. Methysergide is a broad-spectrum serotonin receptor antagonist known to cross the blood brain barrier and to block moderate AIH-induced respiratory motor plasticity (Bach and Mitchell, 1996). The collective data from these studies support a temporal shift in mechanisms underlying dAIH-induced recovery of breathing capacity, transitioning from a serotonin-independent mechanism with acute C2Hs, to a serotonin-dependent mechanism with chronic injuries. These findings have important implications for the translation of this promising therapeutic approach.
2. Materials and Methods
All experimental procedures were approved by the Animal Care and Use Committee at the University of Wisconsin-Madison, and conformed to policies in the NIH Guide for the Care and Use of Laboratory Animals. Experiments were performed on 3-5 month-old male Lewis (Charles River colony P06; Study 1) or Sprague Dawley (Harlan colony 211; Study 2) rats. Rats had access to food and water ad libitum and were housed in 12hr light-dark cycles in an AAALAC-accredited animal facility. Experimental designs for each study are presented in Figure 1.
Figure 1. Study design.
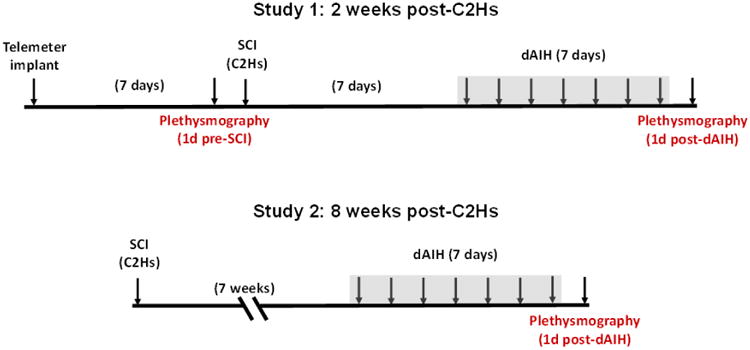
Schematic timeline of Study 1 (2wk C2Hs) and Study 2 (8wk C2Hs). Daily, acute intermittent hypoxia was administered for 7 consecutive days beginning one week post-SCI for Study 1 and 7 weeks post-SCI for Study 2. Intraperitoneal injections of methysergide (4 mg × kg-1) or vehicle were given prior to each dAIH session (i.e. daily for 7 consecutive days).
2.1. Surgical Preparation (Radio telemetry)
In Study 1, rats were instrumented with an abdominal telemeter for continuous monitoring of real-time body temperature during ventilatory assessments (Nakamura et al., 2010). Rats were anesthetized with isoflurane in 100% O2. A sterilized temperature telemeter (Mini-Mitter, Sun River, OR) was inserted into the rat's peritoneal cavity under aseptic conditions. At the end of surgery, triple-antibiotic ointment was applied to incisions, and analgesic (buprenorphine) was administered (0.03 mg × kg-1, s.q.) at 12-h intervals for 48 h post-surgery. Rats were visually monitored and weighed daily, and topical triple-antibiotic ointment was continued twice daily as needed. Rats recovered for 7 days prior to initial pre-SCI ventilatory measures. Rats in Study 2 (8wk post-SCI) did not receive telemeter implants; body temperature was assessed immediately before and after ventilatory measurements as described below.
2.2. Surgical Preparation (C2 hemisection)
For both Studies, spinal hemisections at the second cervical segment (C2Hs) were performed in accordance with previous publications (Dougherty et al., 2012; Keomani et al., 2014; Navarrete-Opazo et al., 2015; Sandhu et al., 2009). Rats were pre-medicated with subcutaneous buprenorphine (0.03 mg/kg), carprofen (Rimadyl, 5 mg/kg) and enrofloxacin (Baytril, 4 mg/kg). Body temperature was maintained between 36.5 and 37.5 °C via heated surgical pad. After tracheal intubation, rats were artificially ventilated (Rodent Ventilator, model 683; Harvard Apparatus, South Natick, MA) with 1.5–2.5% isoflurane in 100% O2. Effective anesthesia was judged by abolition of pedal withdrawal and corneal blink reflexes. Oxygen saturation during surgery was monitored via pulse oximetry (Nonin Medical Inc. Plymouth, MN). After anesthetic induction and pre-operative care, the spinal cord was exposed at C2 via dorsal laminectomy. The dura matter was cut and a left C2Hs performed using a micro-scalpel followed by aspiration. The overlying muscles were sutured with polysorb 3.0 and the skin closed with stainless steel wound clips. Sham surgeries were completed for Study 2 and consisted of cervical laminectomy without spinal injury. Following surgery, animals received buprenorphine (0.03 mg × kg-1, s.q.) and sterile Lactated Ringers solution (5 ml s.q.). Post-surgical care included administration of buprenorphine (0.03 mg × kg-1, s.q.) during the initial 48h post-injury, delivery of Lactated Ringers solution (5 ml × day-1, s.q.) and oral Nutri-cal supplements (1–3 ml, Webster Veterinary, MA, USA) as needed until adequate volitional drinking and eating resumed.
2.3. Daily Acute Intermittent Hypoxia (dAIH)
The protocol for dAIH is described in previous reports (Lovett-Barr et al., 2012) and lasted for 7 days in both Study 1 and Study 2. In Study 1, exposures began 7d post-C2Hs (Fig. 1). For Study 2, dAIH was initiated 7wks post-C2Hs. Rats recovered in their home cages without additional interventions until initiation of dAIH (Fig. 1). Each day for 7 consecutive days, rats were weighed and placed in a Plexiglas tube flushed with a mixture of N2/O2 (4 L × min-1) to attain continuous normoxia or intermittent hypoxia (75 s equilibration; 10 - 5 min episodes of 10.5% O2; 5 min 21% O2 intervals). All rats were pre-treated with an i.p. injection of either methysergide [4mg/kg; (Bach and Mitchell, 1996; Nichols et al., 2014)] or corresponding vehicle (10%DMSO in sterile saline) five minutes prior to the start of each dAIH session. After dAIH, or the equivalent duration of normoxia (110 min) for non-dAIH groups, rats were returned to their cages until the following day. All groups presented as “SCI” alone received daily exposures to normoxia with i.p. vehicle injections as did all non-injured Control groups (pre-injury group in Study 1 and sham SCI groups in Study 2).
2.4. Barometric Plethysmography
Barometric plethysmography [DSI for Study 1 (Navarrete-Opazo et al., 2015); Buxco for Study 2 (Lovett-Barr et al., 2012)] was used to measure breathing in unanesthetized rats following C2Hs and dAIH. Ventilation was measured 24 hrs after dAIH completion; 2wks post-C2Hs in Study 1 and 8wks post-C2Hs in Study 2. Identical protocols were used in both Study 1 and Study 2 and were described previously (Lovett-Barr et al., 2012). Briefly, following a 30 min acclimation period, baseline ventilation was recorded under normoxic conditions (21% O2) for 30 min. Rats were subsequently exposed to a combined hypoxic/hypercapnic gas mixture (7% CO2 in 10.5% O2, balance N2) for 25 mins to assess maximal chemo-reflex stimulated breathing (i.e. MCS). Gas flowed continuously through the chambers at 2L × min-1 to prevent CO2 buildup and allow control of rapidly changing gas concentrations. Chamber pressure, chamber temperature, humidity and body temperature were used to calculate Vt (ml × br-1) and V̇e (ml × min-1) according to the Drorbaugh and Fenn equation (Drorbaugh and Fenn, 1955). In Study 1, telemetric body temperature was recoded in real time during plethysmography and used for calculations. In Study 2, the rats had not been implanted with telemeters prior to C2Hs. Instead, rectal temperatures were taken immediately prior to entering the plethysmograph chambers, and immediately following the Max response. Adjustments were made to Vt and V̇e calculations, with the initial temperature used for air breathing, and the latter temperature used to calculate the Max response. There were no differences in initial rectal temperatures (p=0.392) or final temperatures (p=0.848) across groups. Within each study, data were expressed as absolute units (e.g., breaths × min-1 for frequency), relative to body weight (e.g., ml × min-1 × 100g-1) or relative to values obtained in control rats (%control). “Control” rats were defined as a group of spinally intact rats receiving normoxia (versus dAIH) and intrathecal vehicle injections. For Study 1, plethysmography measures were obtained one day prior to SCI surgeries in all experimental rats and the combined pre-injury group data were used for statistical comparisons (n=22). For Study 2, no pre-injury plethysmography data were collected. Instead, a separate group of rats received sham C2Hs surgeries as described above and these spinally intact rats were used for statistical comparisons. As anticipated, SCI caused a reduction in body weight relative to spinally intact control groups regardless of treatment; all experimental groups weighed less than control groups in Study 1 (p<0.001) and Study 2 (p<0.03). Data were analyzed in 5 min bins using commercially available statistical software (SigmaPlot, Version 11, Systat Software, Inc.) and are presented as means ± 1 SEM. Statistical tests included One-way ANOVA for body weight and temperature values and 2-way RM ANOVA for ventilatory parameters; Fisher's LSD post-hoc test was used to identify statistically significant individual comparisons. Differences were considered significant if p<0.05.
3. Results
3.1. Study 1: 2 weeks post-C2Hs
As reported often in the literature, C2Hs reduced Vt generating capacity with a compensatory increase in respiratory frequency that preserved V̇e during BL (i.e room air) conditions (n=7; Fig. 2A). Exposure to hypoxia/hypercapnic challenge (i.e. MCS) revealed significant reductions in V̇e relative to pre-injury control values (p<0.001; Fig. 2A), confirming persistent respiratory impairment. Reductions in V̇e were characterized by diminished Vt generating capacity during MCS (Fig 2D), while the ability to increase respiratory rate was retained (Fig. 2C). This transition to a rapid, shallow pattern of BL breathing and the reduced capacity to increase Vt during MCS are indicative of disrupted neural input to respiratory motor neurons following C2Hs.
Figure 2. Restoration of ventilation with dAIH 2wks post-C2Hs by serotonin-independent mechanisms.
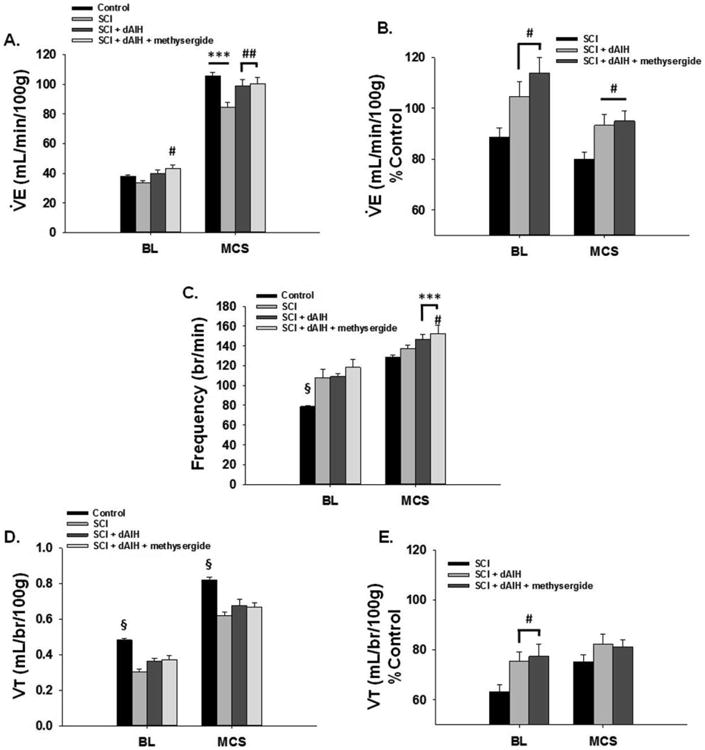
C2Hs caused a reduction in V̇e that was revealed with maximal chemoreceptor stimulated breathing (MCS; hypoxia/hypercapnia). In (A.), rats with C2Hs alone (n=7) showed a significantly diminished MCS response relative to the control group 7d post injury (the control group consisted of averaged plethysmography data from all spinally-intact experimental rats one day prior to C2Hs; n=22). The C2Hs-induced reductions in V̇e were brought back to control levels when dAIH was administered beginning 7d post-injury (n=8). Pre-treatment with methysergide, a broad spectrum serotonin receptor antagonist did not affect the MSC (vs Control; n=7) suggesting dAIH-induced recovery is not serotonin-dependent. In (B.) V̇e data are presented as % Control to further demonstrate enhanced V̇e with dAIH. (C.) All experimental groups showed increased respiratory frequency with BL when compared to controls. dAIH appeared to enhance frequency during MCS, while C2Hs rats showed similar frequency to controls. (D.) C2Hs reduced Vt generating capacity in all experimental groups in BL and with MCS. dAIH (with and without methysergide) had no significant effect on Vt compared to controls. However, when expressed as % Control (E.) dAIH appeared to enhance Vt capacity in BL. #: p<0.05, ##: p<0.01 compared to SCI; ***:p<0.001 compared to control; §:p<0.001 from all other groups within condition.
3.2 dAIH restores ventilation 2wks post-C2Hs by a serotonin-independent mechanism
Reductions in V̇e during MCS were mitigated by dAIH. Indeed, V̇e returned to pre-injury control levels in groups receiving dAIH (n=8), and was significantly higher than C2Hs rats during MCS at 2wks post-injury (p=0.003; Fig. 2A). Daily pre-treatment with methysergide (n=7) had minimal impact on dAIH-induced V̇e recovery (Fig. 2A), suggesting a serotonin-independent mechanism. Rats receiving methysergide + dAIH produced V̇e nearly identical to rats receiving dAIH alone during MCS (p=0.727), but significantly higher than rats with C2Hs during BL (p=0.050) or MCS (p=0.002; Fig. 2A). When V̇e was analyzed as a %pre-injury to determine relative return of respiratory function, dAIH (with and without methysergide) significantly elevated V̇e during BL and MCS relative to C2Hs alone (Fig. 2B).
Recovery of V̇e with dAIH (with and without methysergide) 2wks post-C2Hs occurred primarily through increased respiratory frequency and not enhanced Vt capacity as previously reported. BL frequency in dAIH rats and dAIH + methysergide rats was elevated to a similar extent as C2Hs rats and was significantly higher than pre-injury values (p<0.001; Fig. 2C). With MCS, dAIH groups demonstrated significantly elevated frequency relative to control rats (p<0.001; Fig. 2C) and the frequency in dAIH + methysergide rats was elevated significantly above C2Hs rats (p=0.031; Fig. 2C). Conversely, dAIH did not significantly impact Vt 2wks post-C2Hs (Fig. 2D). dAIH and dAIH + methysergide groups demonstrated similar Vt to C2Hs during BL and MCS and these remained significantly below pre-injury values (p<0.001; Fig. 2D). When Vt was compared as a %difference from pre-injury, dAIH (with and without methysergide) increased Vt relative to C2Hs rats with BL breathing (Fig. 2E). However, this difference in Vt generation was lost during MCS (Fig. 2E).
3.3 Study 2: 8 weeks post-C2Hs
The functional impact of chronic C2Hs on ventilation was similar to that seen at the 2wk time point. In Study 2, rats 8wks post-C2Hs displayed persistent reductions in Vt generating capacity and a compensatory increase in respiratory frequency that sustained V̇e during BL conditions (n=11; Fig. 3A). As with acute C2Hs rats, MCS revealed significant reductions in V̇e versus normoxia treated controls (Sham SCI; n=18; p<0.001; Fig. 3A), confirming persistent respiratory impairment 8 weeks post-C2Hs. Reduced V̇e was characterized by diminished Vt generating capacity with MCS (Fig. 3D), while frequency remained similar to all other groups (Fig. 3C). The persistence of a rapid, shallow pattern with BL breathing and the inability increase Vt with the respiratory challenge 8wks post C2Hs is consistent with previous studies (Fuller et al., 2008; Navarrete-Opazo et al., 2015).
Figure 3. dAIH enhances ventilation 8wks post-C2Hs through a serotonin-dependent mechanism.
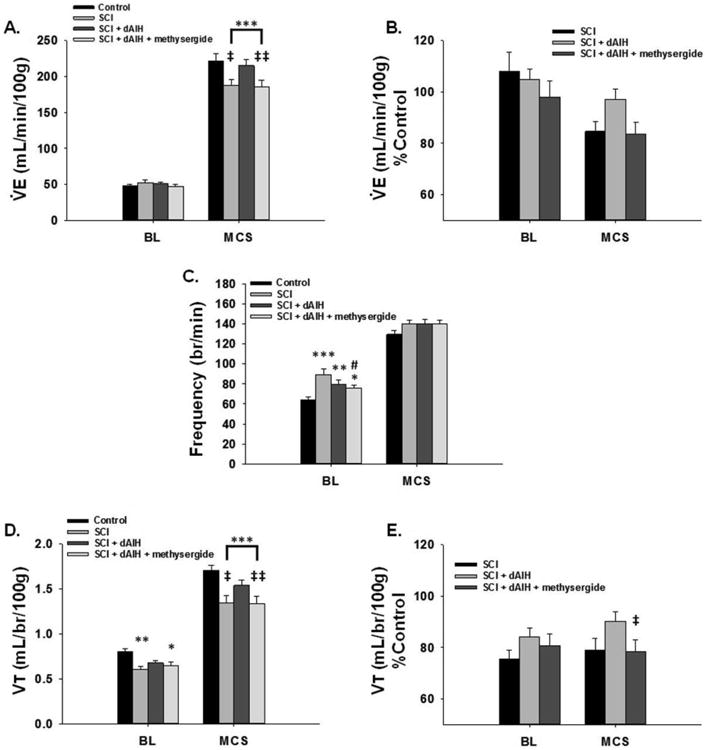
Persistent reductions in V̇e were seen 8wks post-C2Hs with Maximal chemoreceptor stimulated breathing (MCS). In (A.), BL V̇e was similar among groups, but MCS revealed significantly reduced V̇e in C2Hs rats (n=18). dAIH (n=14) restored V̇e to level equivalent with the control group with MCS while pre-treatment with methysergide (n=13) significantly blunted dAIH-induced recovery of V̇e. Changes in V̇e were observable when expressed as %Control, though they did not reach statistical significance (B.). The control group for Study 2 consisted of a unique group of spinally intact rats that received sham SCI surgeries (n=18), daily NX and intrathecal vehicle pre-treatment. Unlike at the 2wk time point, dAIH had minimal effect on respiratory frequency, but significant impact on Vt generation 8wks post-C2Hs. (C.) All C2Hs groups showed significant elevations in frequency with BL breathing, though rats pre-treated with methysergide prior to dAIH displayed reduced frequency relative to C2Hs rats. No differences in frequency were observed between groups during MCS. Vt was reduced in C2Hs rats with BL and MCS relative to controls (D.). dAIH restored Vt generating capacity in each condition to levels similar to controls; significantly above C2Hs rats. Methysergide pre-treatment blunted the dAIH-induced recovery of Vt. Reduced Vt capacity with methysergide was also apparent when Vt was expressed as %Control; dAIH-methysergide rats demonstrated significantly less Vt capacity relative to dAIH treatment alone (E.). #: p<0.05 compared to SCI; *:p<0.05, **:p<0.01, ***:p<0.001 compared to control; ‡:p<0.05, ‡‡: p<0.01 compared to dAIH group.
3.4 dAIH restores ventilation 8wks post-C2Hs
dAIH initiated with chronic C2Hs restored V̇e capacity, although the strategies employed to enhance V̇e differed substantially from acute C2Hs in Study 1. Rats receiving dAIH 7wks post-injury (n=14) showed similar V̇e during BL when compared with control and C2Hs groups (Fig. 3A). But, with MCS, dAIH induced substantial functional recovery such that V̇e was restored to values equivalent to sham controls without hemisection (p=0.516), and was significantly elevated above C2Hs rats (p=0.012; Fig. 3A). This elevated V̇e was also apparent when the data were expressed as %control, although differences were only marginally significant (p=0.085; Fig. 3B). Unlike Study 1, dAIH-induced recovery of V̇e 8wks post-C2Hs resulted from increased Vt capacity and not changes in breathing frequency. Breathing frequency was elevated relative to control rats in all SCI groups during BL, but was similar across all groups during MCS (Fig. 3C). Conversely, dAIH rats generated larger Vt with BL and MCS, similar to control rats during BL (p=0.066) and significantly greater than C2Hs rats during MCS (p=0.016; Fig. 3D) (though still impaired versus controls; p<0.001). When expressed as %control, dAIH-induced improvements in Vt capacity during MCS were also observed, though once again they were only marginally significant (p=0.053; Fig. 3E).
3.5 Serotonin-dependent mechanisms underlie dAIH-induced recovery 8wks post-C2Hs
dAIH-induced recovery of V̇e was abolished by methysergide pretreatment 8wks post-C2Hs (n=13). These data are in stark contrast to the minimal effects of methysergide pre-treatment observed in Study 1, and suggest that recovery of V̇e with dAIH occurs via serotonin-dependent mechanisms 8wks post-C2Hs. In specific, dAIH + methesergide rats showed V̇e similar to C2Hs rats during MCS (p=0.919; Fig. 3A), and were significantly reduced compared to controls (p<0.001) and dAIH (p=0.009; Fig. 3A). When expressed as %control, the reduced V̇e with dAIH + methysergide was again apparent versus dAIH rats, but was only marginally significant (p=0.054; Fig. 3B). The impact of methysergide on V̇e appeared specific to changes in Vt capacity. Methysergide pretreatment reversed dAIH-induced improvements in Vt generation 8wks post-C2Hs, with lesser impact on respiratory frequency. dAIH + methysergide rats continued to show significantly higher BL frequencies versus controls (p=0.022), but were significantly reduced versus C2Hs rats (p=0.028; Fig. 3C). Differences in breathing frequency were no longer apparent during MCS; dAIH + methysergide rats had similar breathing frequency as all other groups (Fig. 3C). Deficits in Vt capacity were observed with BL and MCS when rats were treated with methysergide prior to dAIH. Vt in dAIH + methysergide rats was similar to C2Hs rats in each condition and remained significantly below control rats during BL (p=0.028) and MCS (p<0.001; Fig. 3D). In addition, methysergide blunted Vt generation during MCS significantly below rats receiving dAIH alone (p=0.009; Fig. 3D). This was again apparent when Vt was expressed as %control (p=0.035; Fig. 3E). Collectively, these data suggest that dAIH was effective in restoring V̇e in rats with chronic C2Hs through enhanced Vt generating capacity, an effect mediated through serotonin-dependent mechanisms.
4. Discussion
Taken together, data from these independent studies support the hypothesis that dAIH improves breathing function with acute (2wks post-injury) and chronic (8wks post-injury) C2Hs through distinct cellular mechanisms. Specifically, we confirm that dAIH initiated one week post C2Hs augments V̇e, as previously described (Lovett-Barr et al., 2012; Navarrete-Opazo et al., 2015), and extend these findings to demonstrate that dAIH-induced recovery of V̇e occurs via a serotonin-independent mechanism since methysergide pre-treatment had minimal impact on dAIH-induced recovery; methysergide at this same dose is known to block moderate AIH-induced phrenic long-term facilitation in rats (Bach and Mitchell, 1996). Conversely, when rats were allowed to recover for 7 weeks post-C2Hs, methysergide pre-treatment abolished dAIH-induced recovery of V̇e, suggesting conversion to serotonin-dependent mechanisms. This transition from serotonin-independent to serotonin-dependent mechanisms of dAIH-induced functional recovery parallels our previous reports that dAIH induced functional recovery shifts from an adenosine-dependent [acute; (Navarrete-Opazo et al., 2015)] to an adenosine-constrained mechanism [chronic; (Navarrete-Opazo et al., 2016b)]. An understanding of these changing mechanisms will be of importance for future pre-clinical studies and translational applications of dAIH since something as simple as consumption of caffeine (an A2a antagonist) could greatly affect the impact of dAIH therapy depending on the time post-injury.
In study 1, dAIH-induced improvements in V̇e 2wk post-C2Hs occurred through enhancement of respiratory frequency versus Vt generating capacity in contrast with our previous reports (Lovett-Barr et al., 2012). Indeed, Lovett-Barr et al. (2012) found minimal changes in respiratory frequency and improved Vt during Max respiratory challenge when dAIH was initiated 1wk post-injury. Although the overall impact of dAIH on V̇e was similar to the current study (significantly enhanced versus C2Hs alone), the observed enhancement in respiratory frequency (and not Vt) was a surprise. There are a few considerations for interpretation of these data. First, the plethysmograph used for Study 1 (Data Science International) was different from those used in previous studies (Buxco) (Doperalski et al., 2008; Dougherty et al., 2016; Fuller et al., 2008; Fuller et al., 2006; Lovett-Barr et al., 2012) in order to facilitate simultaneous radio telemetry measures of body temperature. It is possible that differences in gas flow dynamics and/or internal algorithms applied to calculate respiratory measures between plethysmography systems may introduce subtle variability to Vt and V̇e data. However, since volume calibrations were completed for each plethysmograph prior to all experiments, it is unlikely that differences in equipment accounted for the notable differences in respiratory dynamics with dAIH 2wks post-C2Hs observed in Study 1. Also, this same DSI system was used by Navarette-Opazo and colleagues (2015), who reported primary effects of dAIH on Vt. It is more likely that variations in breathing patterns with dAIH represent either genetic variance among colonies and/or strains of experimental rats (Baker-Herman et al., 2010; Golder et al., 2005). Indeed, previous studies of dAIH-induced recovery of breathing function with C2Hs (and Study 2 herein) were completed using Harlan Sprague-Dawley rats (Lovett-Barr et al., 2012); Study 1 was completed using Charles River Lewis rats from colony P06. It is known that hypoxic ventilatory responses (Golder et al., 2005) and expression of respiratory motor plasticity (Baker-Herman et al., 2010) can vary between strains, and even sub-strains (Fuller et al., 2001a) of experimental rats. Genetic factors, such as differences in serotonin or TrkB receptor expression (Baker-Herman et al., 2010) may have contributed to the unique enhancement of respiratory frequency and minimal increase in Vt observed with dAIH in acute C2Hs. Though, unique plasticity in afferent projections to the brainstem rhythm generating circuits (or specifically within rhythm generating circuits) in P06 rats cannot be completely ruled out. Unfortunately, the PO6 colony from Charles River was discontinued prior to the start of Study 2, making direct comparisons between studies untenable.
Despite differences in the respiratory patterns used, net improvements in V̇e at 2wks post-C2Hs with dAIH were similar to previous reports (Lovett-Barr et al., 2012), strengthening the hypothesis that dAIH improves respiratory motor function in acute SCI. To begin discerning mechanisms of V̇e recovery and the specific role for serotonin signaling mechanisms 2wks post-C2Hs, rats were pre-treated with methysergide prior to AIH in each session of the dAIH protocol. Methysergide had minimal impact on dAIH-induced V̇e improvements or on component variables giving rise to V̇e (frequency and Vt). These data support a non-serotonergic mechanism of dAIH-induced functional recovery 2wks post-C2Hs. C2Hs interrupts descending raphe-spinal projections to phrenic and intercostal motor neurons leading to reduced expression of serotonin-dependent plasticity (Dougherty et al., 2016; Golder and Mitchell, 2005). In addition, C2Hs may induce vascular changes that alter blood flow to spinal tissue below the injury, especially during acute stages of recovery (Holtz et al., 1990). Reduced perfusion of spinal tissue in the vicinity of phrenic motor neurons (just caudal to C2Hs) during dAIH may produce more severe hypoxemia for a given level of FIO2 compared to spinal-intact control rats. Repeated exposure to severe hypoxia induces respiratory motor plasticity through adenosine-dependent (versus serotonin-dependent) mechanisms (Nichols et al., 2012). Thus, the combined loss of serotonergic innervation to respiratory motor neurons and possible increased hypoxemia and tissue hypoxia during respiratory challenge may have been sufficient to shift dAIH-induced functional recovery towards adenosine-dependent mechanisms. Nevertheless, we cannot rule out overall strain differences in 5-HT function between P06 and SD rats as another plausible explanation, though recent reports of dAIH effects on breathing function 2wks (Navarrete-Opazo et al., 2015) versus 9 wks post- C2Hs (Navarrete-Opazo et al., 2016b) support a shift from an adenosine-dependent to an adenosine-constrained mechanism, consistent with the proposed shift in serotonin function in this same time frame.
In contrast to the serotonin-independent mechanism of dAIH-induced recovery with acute C2Hs, dAIH with chronic C2Hs appears to rely on serotonergic signaling mechanisms. In study 2, we demonstrated that pre-treating rats with methysergide prior to dAIH with more chronic C2Hs eliminates the functional impact of dAIH. Indeed, dAIH treatment alone enhanced Vt generating capacity to normal levels, whereas methysergide pretreatment reduced Vt to levels similar to C2Hs alone. The mechanistic transition to serotonin-dependent mechanisms with chronic C2Hs is likely due to restoration of descending serotonergic innervation to respiratory motor nuclei over time post-injury. Golder et al. (2005) demonstrated a gradual return of serotonergic terminal density ipsilateral to a C2Hs lesion over 8 weeks, with subsequent return of serotonin-dependent phrenic long-term facilitation ipsilateral to injury following moderate AIH (Golder and Mitchell, 2005). In addition, restoring serotonin signaling via pharmacological means (Hsu and Lee, 2015; Zhou and Goshgarian, 2000; Zimmer and Goshgarian, 2006), or through cell transplants designed to restore serotonin near respiratory motor neurons (Dougherty et al., 2016), enhances respiratory motor output with chronic C2Hs, underscoring the importance of serotonergic signaling for functional respiratory motor recovery.
The return of serotonin-dependent mechanisms with chronic C2Hs may be accompanied by restoration of cross-talk inhibition between serotonergic and adenosinergic pathways to phrenic motor plasticity (Devinney et al., 2013; Hoffman et al., 2010; Hoffman and Mitchell, 2013). Indeed, serotonin and adenosine signaling likely occur simultaneously with dAIH, with one pathway emerging as the dominant mechanism of plasticity, and the other acting as a “brake” (Devinney et al., 2013; Navarrete-Opazo and Mitchell, 2014a). With chronic C2Hs, pre-treatment with an adenosine receptor antagonist prior to dAIH should remove the inhibitory “brake” on the dominant serotonin-dependent pathway, thereby enhancing the impact of dAIH (versus abolishing dAIH-induced recovery as seen with acute C2Hs). Indeed, in rats with chronic C2Hs pre-treated with KW-6002, enhanced recovery of Vt generating capacity and diaphragm motor output are observed following dAIH (Navarrete-Opazo et al., 2016b). Thus, our data are consistent with prior studies, and provide compelling support for shifting mechanisms of dAIH-induced functional recovery of breathing capacity.
The appreciation for shifting mechanisms of dAIH-induced recovery of motor function is vital for translational applications of dAIH to humans with SCI. Rodent studies designed to unravel the complex, evolving mechanisms of intermittent-hypoxia induced plasticity are likely to uncover targets for pharmacological manipulation or rehabilitation-specific co-treatments to maximize the impact of dAIH; perhaps even uncovering methods for driving recovery without the necessity for hypoxic exposures. However, of equal importance is our understanding of conditions that may undermine the effectiveness of dAIH as a therapeutic adjunct. For example, even mild inflammatory states can undermine the expression of AIH-induced plasticity (Huxtable et al., 2011; Vinit et al., 2011), and may impact the magnitude of dAIH-induced recovery in individuals with chronic, incomplete SCI. Something as simple as consuming caffeinated beverages (caffeine is an adenosine receptor antagonist) may undermine the benefits of dAIH on motor recovery with acute SCI whereas the same “pre-treatment” may enhance dAIH-induced functional recovery with chronic SCI. Ultimately, the meaningful clinical application of dAIH hinges upon clear understanding of these complex (and changing) mechanisms.
Highlights.
Daily acute intermittent hypoxia (dAIH) improves breathing function following cervical spinal cord injury (C2 hemisection; C2Hs) in rats.
dAIH-induced recovery of breathing occurs in both acute (2 weeks) and chronic (8 weeks) C2Hs, but through unique cellular mechanisms.
Pre-treatment with methysergide, a broad spectrum serotonin receptor antagonist, prior to dAIH has minimal effect on dAIH-induced recovery in acute C2Hs, suggesting a serotonin-independent mechanisms of action.
However, methysergide reduces dAIH-induced recovery in chronic C2Hs, suggesting a temporal shift to serotonin-dependent mechanisms.
Acknowledgments
Support for this work was provided by grants from: The National Institute of Health (NIH) 5RO1HL069064 (GSM), The Craig H. Neilsen Foundation (BJD, SV) and The Francis Family Foundation (PMM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Argote S, Gransee HM, Mora JC, Stowe JM, Jorgenson AJ, Sieck GC, Mantilla CB. The Impact of Midcervical Contusion Injury on Diaphragm Muscle Function. J Neurotrauma. 2016;33:500–509. doi: 10.1089/neu.2015.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Bavis RW, Dahlberg JM, Mitchell AZ, Wilkerson JER, Golder FJ, MacFarlane PM, Watters JJ, Behan M, Mitchell GS. Differential expression of respiratory long-term facilitation among inbred rat strains. Respiratory Physiology & Neurobiology. 2010;170:260–267. doi: 10.1016/j.resp.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nature Neuroscience. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529 Pt 1:215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology. 2014;29:39–48. doi: 10.1152/physiol.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Fields DP, Devinney MJ, Mitchell GS. Phrenic motor neuron TrkB expression is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. Exp Neurol. 2016 doi: 10.1016/j.expneurol.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, Mitchell GS. Phrenic Long-Term Facilitation Requires PKCtheta Activity within Phrenic Motor Neurons. J Neurosci. 2015;35:8107–8117. doi: 10.1523/JNEUROSCI.5086-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols L, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann N Y Acad Sci. 2013;1279:143–153. doi: 10.1111/nyas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doperalski NJ, Sandhu MS, Bavis RW, Reier PJ, Fuller DD. Ventilation and phrenic output following high cervical spinal hemisection in male vs. female rats. Respir Physiol Neurobiol. 2008;162:160–167. doi: 10.1016/j.resp.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Fields DP, Mitchell GS. Mammalian Target of Rapamycin is required for phrenic long-term facilitation following severe but not moderate acute intermittent hypoxia. J Neurophysiol. 2015 doi: 10.1152/jn.00539.2015. jn 00539 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Gonzalez-Rothi EJ, Lee KZ, Ross HH, Reier PJ, Fuller DD. Respiratory outcomes after mid-cervical transplantation of embryonic medullary cells in rats with cervical spinal cord injury. Exp Neurol. 2016;278:22–26. doi: 10.1016/j.expneurol.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Lee KZ, Gonzalez-Rothi EJ, Lane MA, Reier PJ, Fuller DD. Recovery of inspiratory intercostal muscle activity following high cervical hemisection. Respir Physiol Neurobiol. 2012 doi: 10.1016/j.resp.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Fields DP, Springborn S, Mitchell GS. Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J Neurophysiol. 2015 doi: 10.1152/jn.00374.2015. jn 00374 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics. 2001a;4:175–181. doi: 10.1152/physiolgenomics.2001.4.3.175. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. Journal of Applied Physiology. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001b;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. discussion 2000. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. Journal of Neuroscience. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. Journal of Neuroscience. 2008;28:2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Zabka AG, Bavis RW, Baker-Herman T, Fuller DD, Mitchell GS. Differences in time-dependent hypoxic phrenic responses among inbred rat strains. Journal of Applied Physiology. 2005;98:838–844. doi: 10.1152/japplphysiol.00984.2004. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD. Intermittent hypoxia and neurorehabilitation. J Appl Physiol (1985) 2015;119:1455–1465. doi: 10.1152/japplphysiol.00235.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology. 2014;82:104–113. doi: 10.1212/01.WNL.0000437416.34298.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A(2A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. Journal of Physiology-London. 2010;588:255–266. doi: 10.1113/jphysiol.2009.180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation. Neuroscience. 2013;250:632–643. doi: 10.1016/j.neuroscience.2013.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Nichols L, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol. 2012;113:1184–1193. doi: 10.1152/japplphysiol.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz A, Nystrom B, Gerdin B. Relation between Spinal-Cord Blood-Flow and Functional Recovery after Blocking Weight-Induced Spinal-Cord Injury in Rats. Neurosurgery. 1990;26:952–957. doi: 10.1097/00006123-199006000-00005. [DOI] [PubMed] [Google Scholar]
- Hsu SH, Lee KZ. Effects of serotonergic agents on respiratory recovery after cervical spinal injury. J Appl Physiol (1985) 2015;119:1075–1087. doi: 10.1152/japplphysiol.00329.2015. [DOI] [PubMed] [Google Scholar]
- Huxtable AG, Vinit S, Windelborn JA, Crader SM, Guenther CH, Watters JJ, Mitchell GS. Systemic inflammation impairs respiratory chemoreflexes and plasticity. Respiratory Physiology & Neurobiology. 2011;178:482–489. doi: 10.1016/j.resp.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keomani E, Deramaudt TB, Petitjean M, Bonay M, Lofaso F, Vinit S. A murine model of cervical spinal cord injury to study post-lesional respiratory neuroplasticity. Journal of visualized experiments : JoVE. 2014 doi: 10.3791/51235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. The American journal of physiology. 1999;277:R658–666. doi: 10.1152/ajpregu.1999.277.3.R658. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci. 2012;32:3591–3600. doi: 10.1523/JNEUROSCI.2908-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Olson EB, Jr, Terada J, Wenninger JM, Bisgard GE, Mitchell GS. Sleep state dependence of ventilatory long-term facilitation following acute intermittent hypoxia in Lewis rats. J Appl Physiol (1985) 2010;109:323–331. doi: 10.1152/japplphysiol.90778.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Alcayaga J, Sepulveda O, Rojas E, Astudillo C. Repetitive intermittent hypoxia and locomotor training enhances walking function in incomplete spinal cord injury subjects: A randomized, triple-blind, placebo-controlled clinical trial. J Neurotrauma. 2016a doi: 10.1089/neu.2016.4478. [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Dougherty BJ, Mitchell GS. Enhanced recovery of breathing capacity from combined adenosine 2A receptor inhibition and daily acute intermittent hypoxia after chronic cervical spinal injury. Exp Neurol. 2016b doi: 10.1016/j.expneurol.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol. 2014a;307:R1181–1197. doi: 10.1152/ajpregu.00208.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Vinit S, Dougherty BJ, Mitchell GS. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp Neurol. 2015;266C:1–10. doi: 10.1016/j.expneurol.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo AA, Mitchell GS. Recruitment and plasticity in diaphragm, intercostal and abdominal muscles in unanesthezised rats. J Appl Physiol (1985) 2014b doi: 10.1152/japplphysiol.00130.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise C, Hala TJ, Frank DM, Parker JL, Authelet M, Leroy K, Brion JP, Wright MC, Lepore AC. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Experimental Neurology. 2012;235:539–552. doi: 10.1016/j.expneurol.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Nichols L, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol. 2012;112:1678–1688. doi: 10.1152/japplphysiol.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols L, Johnson RA, Satriotomo I, Mitchell GS. Neither serotonin nor adenosine-dependent mechanisms preserve ventilatory capacity in ALS rats. Respir Physiol Neurobiol. 2014;197:19–28. doi: 10.1016/j.resp.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD. Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol. 2009;169:94–101. doi: 10.1016/j.resp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saruhashi Y, Young W, Perkins R. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp Neurol. 1996;139:203–213. doi: 10.1006/exnr.1996.0094. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26:163–172. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- Vinit S, Windelborn JA, Mitchell GS. Lipopolysaccharide attenuates phrenic long-term facilitation following acute intermittent hypoxia. Respir Physiol Neurobiol. 2011;176:130–135. doi: 10.1016/j.resp.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. Effects of serotonin on crossed phrenic nerve activity in cervical spinal cord hemisected rats. Exp Neurol. 1999;160:446–453. doi: 10.1006/exnr.1999.7213. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. 5-Hydroxytryptophan-induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol. 2000;89:1528–1536. doi: 10.1152/jappl.2000.89.4.1528. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spinal activation of serotonin 1A receptors enhances latent respiratory activity after spinal cord injury. J Spinal Cord Med. 2006;29:147–155. doi: 10.1080/10790268.2006.11753868. [DOI] [PMC free article] [PubMed] [Google Scholar]


