Abstract
Introduction
Low back pain and vertebral endplate abnormalities are common conditions within the population. Subclinical infection caused by indolent pathogens can potentially lead to these findings, with differentiation between them notably challenging from a clinical perspective. Progressive infection of the intervertebral disc has been extensively associated with increasing low back pain, with Propionibacterium acnes specifically implicated with in relation to sciatica. The main purpose of this study is to identify if the presence of an infective pathogen within the intervertebral disc is primary or is a result of intraoperative contamination, and whether this correlates to low back pain.
Methods and analysis
An open prospective cohort study will be performed. Subjects included within the study will be between the ages of 18 and 65 years and have a diagnosis of lumbar disc herniation requiring open decompression surgery. Excised herniated disc fragments, muscle and ligamentum flavum samples will be collected during surgery and sent to microbiology for tissue culture and pathogen identification. Score questionnaires for pain, functionality and quality of life will be given preoperatively and at 1, 3, 6 and 12 months postoperatively. A MRI will be performed 12 months after surgery for analysis of Modic changes and baseline comparison. The primary endpoint is the rate of disc infection in patients with symptomatic degenerative disc disease. The secondary endpoints will be performance scores, Modic incidence and volume.
Ethics and dissemination
This study was approved by our Institutional Review Board and was only initiated after it (CAAE 65102617.2.0000.0071). Patients agreeing to participate will sign an informed consent form before entering the study. Results will be published in a peer reviewed medical journal irrespective of study findings. If shown to be the case, this would have profound effects on the way physicians treat chronic low back pain, even impacting health costs.
Trials registration number
NCT0315876; Pre-results.
Keywords: spine, magnetic resonance imaging, diagnostic microbiology, propionibacterium acnes, low back pain
Introduction
Low back pain and vertebral endplate abnormalities are common conditions within the population, with Modic et al 1 2 reporting endplate abnormality rates of up to 6% within the population and 46% of patients complaining of low back pain.3 Modic type I changes are described as vertebral bone marrow oedema related to acute low back pain.4 When Modic changes are detected, there is a 4.5-times higher incidence of non-specific low back pain on presentation.1 2
Subclinical infection caused by low-virulence pathogens can potentially lead to vertebral endplate abnormalities, which are identified through MRI. Differentiation between subclinical infection and Modic changes may be notably difficult, given the paucity of examination findings.5 6 Additionally, subclinical infections can be associated with increasing low back pain.7 Albert et al 8 reported 61 patients who had undergone surgical treatment for lumbar disc herniation, with 46% of cases showing a positive culture. The same authors also reported that 80% of the patients with a positive culture for anaerobic pathogens presented with Modic type I changes at the adjacent vertebra after a 2-year follow-up. This is in stark contrast to only 44% of patients with negative culture. Some studies demonstrated the presence of low-virulence pathogens in intervertebral disc tissue cultures,6–10 with the most common causative organism reported as Propionibacterium acnes.
Chronic low back pain and Modic type I changes have been treated with antibiotics for up to 100 days with superior outcomes in comparison with placebo treatment according to Albert et al.7 In this study, patients were treated with amoxicillin/clavulanate (500 mg/125 mg)7 following another study where P. acnes was linked to sciatica.8 However, Carricajo et al 11 suggest that the presence of P. acnes in the intervertebral discs is due to either external surgical or laboratory contamination. Within their study, they detected positive disc cultures in only 3.7% of 54 patients. Further, the same group demonstrated that samples of spinal muscle and ligamentum flavum had positive cultures in 14.8% of cases that had negative disc cultures. Reinforcing the findings of this study, Rigal et al 12 analysed a sample of 313 patients undergoing video-assisted or retroperitoneal anterior approach, and found only 6 cases of positive cultures. No correlation between infection and degeneration of the intervertebral disc was found. Contrastingly, Rollason et al,13 in a study of genotype characterisation, observed that P. acnes cultured from disc samples surgically resected from 64 patients with disc herniation were different from those normally colonising the skin, suggesting that this variant of the P. acnes bacterium could be related to low back pain. A systematic review performed by Urquhart et al 14 concluded that there is moderate evidence of a relationship between positive P. acnes cultures with Modic type I changes and low back pain, although the evidence was not substantive. The group concluded that new studies should be conducted to determine whether pathogens within the vertebral disc arise from external contamination or if they are truly implicated in the development of chronic back pain (low back pain for at least 3 months).
Hypothesis and objectives
We hypothesise that lumbar disc herniation is related to subclinical infection of the intervertebral disc.
Our primary endpoint of this study is to identify whether the presence of a pathogen within the intervertebral disc is primary or if it is a result of intraoperative contamination.
The secondary endpoints are to analyse clinical prognostic factors in patients and the diagnosis of infection. The study also proposes to analyse the relationship between radiological changes (Modic I and II) and infection.
Justification and scientific challenges
If there is a confirmation of a relationship between subclinical pathogens, lumbar disc herniation and Modic changes with non-specific chronic back pain, this will change the way this disease process is managed and improve treatment costs and patient outcomes.
Previously published studies that report a strong correlation between P. acnes and low back pain and/or disc herniation are almost entirely from the same study group.5–8 Few studies have questioned their results and those that have only presented small sample groups and inadequate statistical methodologies.11 For this prospective cohort study, we previously calculated the minimum number of subjects needed for adequate statistical analysis. Aside from outlining a specific culturing method for P. acnes, this project addresses molecular analysis and clinical outcomes follow-up in a single study. This provides a complexity and significance that have not been achieved in previously published studies on the topic.
Methods and analysis
This study protocol is registered at Clinicaltrials.gov under NCT0315876 (Pre-results) (https://clinicaltrials.gov/ct2/show/NCT03158766?term=NCT03158766&rank=1).
Study design
An open prospective cohort study will be performed at a single centre, (Hospital Israelita Albert Einstein (HIAE)) taking 1 year for recruiting, and ending 1 year after inclusion of last patient. Patients’ data will be collected with a specific form created for this study. Patients will be summoned for a new MRI of the lumbar spine 1 year after their surgical procedure.
All included patients will go through further treatment of 10 sessions of postoperative physical therapy. They will be instructed to maintain learnt exercises in their residences. Pain medications will not be controlled and will follow attending physician prescriptions.
Population
Patients will be consecutively included in the study.
Inclusion criteria
Inclusion criteria were as follows: subjects between 18 and 65 years of age; both genders; diagnosis of lumbar disc herniation and are undergoing open decompression surgery (microdiscectomy). Indication for surgery is sciatica caused by disc herniation compression of a lumbar nerve root failing conservative treatment for at least 6 weeks or ongoing neurological deficit. Patients with a history of previous spinal injection will not be excluded from the study. Patients willing and able to go through all phases of clinical investigation will be included. An informed consent form (ICF) must be signed.
Exclusion criteria
Exclusion criteria were as follows: patients with previous lumbar disc surgery at the same level at any point of life; patients undergoing chemotherapy; patients with any immune deficiency; patients previously submitted to disc injection and/or discography; patients submitted to previous endoscopic disc surgery; patients with fusion performed at the same stage of decompression surgery; patients with any other infection within the last 6 months or usage of antibiotics within the last 2 months; patients with incomplete specific form or data; and decline to participate or sign the ICF.
Patient enrolment in the study
Evaluation of patient eligibility will be carried out by the main investigator or by a coinvestigator. Both study coordinators will perform an interview with a candidate patient about his/her willingness to participate in the study. They will be responsible for confirming their eligibility in relation to the inclusion and exclusion criteria; and, if the patient accepts, the investigator will explain all study details and read along the ICF. Any questions regarding the objectives of the study, involved procedures, risks/benefits and confidentiality will be resolved. Patients accepting to participation will date and sign the ICF. A copy of the form will be attached to the patient’s medical record, and another will be provided to the patients themselves. After the ICF is properly signed, the patient will undergo an interview to complete the initial demographic data and pretreatment forms. If the patient is unable to sign the written ICF, the investigator will vocally explain the study and the patient will provide oral consent in the presence of a witness that will sign the ICF. Patient recruitment will be carried out for 24 months, so that 95 patients shall be included (details of estimated n reported at sample size determination).
Patient allocation
Patients will undergo surgery according to surgeons’ preference. Attending surgeons will determine chosen operative technique according to their experience and preference.
Blinding
Neither the patient or attending physician will have access to the results of tissue cultures. The radiologist who will analyse the imaging studies of performed MRIs will also be blinded to the patient data and laboratory results. A blinded investigator will analyse pain and function scores.
Early stopping of participation in the study
Patients will be excluded from the study on:
Withdrawal of ICF.
Death.
Patient selection flaw identified (incompatible eligibility criteria).
Lost to follow-up.
Patient presents with clinical symptoms of infection, inclusive of severe lumbar or radicular pain, fever with no other detected foci, abnormal erythrocyte sedimentation rate (ESR)/C-reactive protein (CRP)/leucogram, altered imaging studies that lead to interruption of the blinding of the results of culture exams.
For each excluded case, the reason and circumstance for the withdrawal will be detailed. Patient data collected until that point of the study will be included at final analysis.
Selected endpoints
Primary endpoint
Rate of intervertebral disc infection
The primary objective of this study was to consider the incidence of intervertebral disc infection by any type of low virulence pathogen, with consequent Modic changes and chronic low back pain. Thus, the calculation of the incidence of infection in lumbar disc herniations will be performed.
Incidence of infection rate (IIR) will be calculated as follows:
Secondary endpoint
Low back pain
Intensity of low back pain and limitation for daily activities of patients with and without infection will be analysed through the numeric rating score (NRS) system applied at time of patient recruitment and 1, 3, 6 and 12 months after surgical procedure (table 1). The clinically significant threshold will be considered to be an increase of 30% of baseline lumbar pain at the first postoperative month. NRS and the visual analogue scale (VAS) have good correlation and are equally sensitive to quantify postoperative pain.15 Compared with VAS, NRS is easier to manage and codify, furthermore, less mistakes occur during data insertion.16 Otherwise, it is easier to complete16 and preferred by patients.17
Table 1.
Chronogram of included patients
| Screening/ Recruiting |
Postop 4 weeks±7 days | Postop 3 months±14 days | Postop 6 months±21 days | Postop 12 months±30 days | |
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | |
| ICF | X | ||||
| Check including and excluding criteria | X | ||||
| Collect demographic data | X | ||||
| Investigate medical history/complete enrolment form | X | ||||
| Lab screening, ESR, CRP, leucogram | X | X | X | X | |
| Apply NRS tool | X | X | X | X | X |
| Apply Oswestry questionnaire | X | X | X | X | X |
| Apply EQ-5D questionnaire | X | X | X | X | X |
| Physical therapy | X | ||||
| MRI | X |
CRP, C-reactive protein; EQ-5D, European quality of life five dimensions; ESR, erythrocyte sedimentation rate; ICF, informed consent form; NRS, numeric rating scale; Postop, postoperative.
Quality of life
Quality of life at the end of 1 year for both infected and uninfected groups, with and without Modic changes, will be analysed through the validated Portuguese version of the EuroQol (EQ-5D) questionnaire. This measurement tool will be applied at timing of patient recruitment, and 1, 3, 6 and 12 months after surgery.
EQ-5D is a self-completing standardised tool containing five items (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). Compared with the Short-Form 36 (SF-36) questionnaire, EQ-5D is a shorter and faster form to analyse.
Function
Function will be quantified through the Portuguese version of the Oswestry Disability Index (ODI) for lumbar pain that will be applied at time of recruitment and 1, 3, 6 and 12 months after surgery.
Modic incidence
Insurgent Modic changes in patients will be analysed 1 year after surgery, as well as its relationship with the presence or the absence of infection.
Incidence of Modic (IM) changes will be calculated for the infection group (IM infec) and for the total group (IM total) as follows:
Volume and size of Modic changes: additional imaging analysis
Quantification of sizing will be done by two radiologists with expertise in musculoskeletal diagnosis. All images will be analysed in sagittal T1-weighted, T2-weighted and FAT-T2-weighted sequences of the lumbar spine in the DICOM format. Modic volume will be measured according to Wang et al.18 Three sagittal slices of the lumbar spine will be considered: midsagittal slice; left pedicle parasagittal slice and right pedicle parasagittal slice. The parameters examined to quantify Modic changes will include measures of ratios of the region affected by Modic changes to the entire corresponding vertebral body, including maximal width ratio, maximal height ratio and area ratio. Vertebral body changes will be classified accordingly to Modic changes type I, II and III.1 2 Soft tissues around the vertebra, such as disc, muscles and ligaments, will also be analysed. Data will be collected for the presence of vertebral or disc oedema, and the presence of disc hydration or not. Disc degeneration will be collected as: normal; degeneration with height preservation and degeneration with loss of height. Preoperative and 12-month postoperative-acquired MRI studies will be compared. Both superior and inferior disc endplates will be evaluated and compared. Preoperative study will be taken as baseline for comparative purposes.
Adverse effects
Fail of surgical treatment (recurrence, instability, need for reoperation and so on), need for additional physical therapy sessions, superficial infection, drainage, deep venous thrombosis and any other possible adverse event that may show up will be included as well.
Study stages
Sample collection
Included patients will undergo standard fashion general anaesthesia and prepped with chlorhexidine solution. Intravenous antibiotic prophylaxis will be administered within first hour before skin incision, according to the standard protocol of HIAE Infection Control Committee published at the hospital Pharmaceutics Manual.
Preoperative blood sample will be collected for leucogram, ESR and CRP. Same laboratory tests will be repeated at 1, 6 and 12 months time point (table 1).
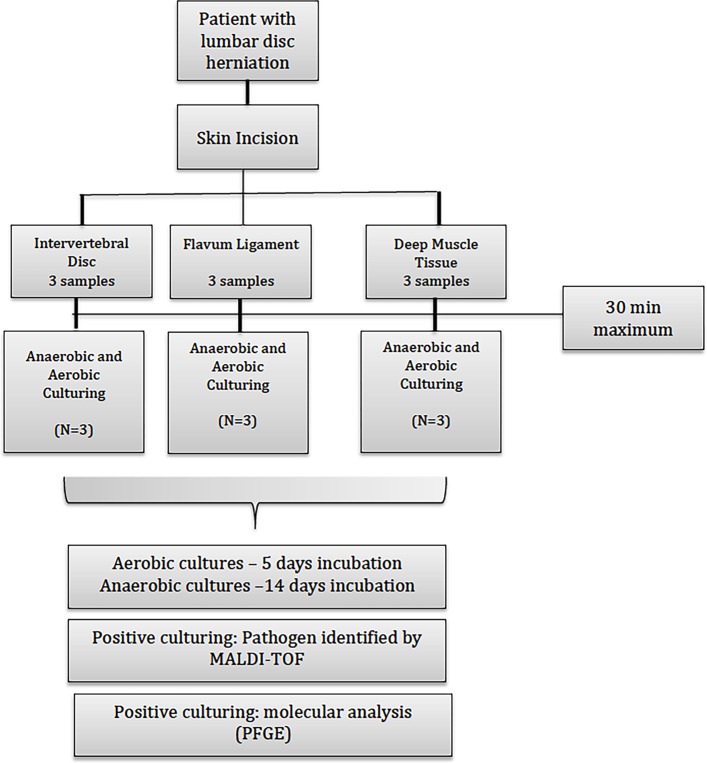
The excised herniated disc fragment will be immediately sent to microbiology laboratory analysis in a universal sterile container (screw cap tube) in no >30 min to be processed as follows. The same process will be applied to samples of deep muscle and ligamentum flavum at the end of the surgical procedure. Three cultures of the intervertebral disc will be done, as well as three of the ligamentum flavum and three of the multifidus muscle for each patient. The flowchart of this stage is described in figure 1. Search for pathogens protocol is similar to the one proposed by Levy et al,19 although due to the avascular nature of the intervertebral disc, we will inoculate the sonication fluid (after sample concentration) in blood cultures bottles (automated system) to improve the recovery of pathogens and reduce contamination.
Figure 1.
Flowchart of collected clinical samples that will be sent to culture analysis. MALDI-TOF, matrix-assisted laser desorption ionisation–time-of-flight; PFGE, pulsed field gel electrophoresis.
Search for pathogens
The herniated intervertebral disc will be split equally into three fragments of 2×2×5 mm and flattened in a laminar flow cabinet until a homogeneous material is achieved. The same process will be carried out for the samples of ligamentum flavum and multifidus. Tissues will be cultured in specific growth medium and incubated according to the respective culture:
Similar criteria used by the Infectious Diseases Society of America (IDSA) to detect joint replacement infection will be adopted, which is the recommendation on at least two positive tissue cultures by the same pathogen to confirm diagnosis of infection.20 This same criterion was already adopted in a study to characterise isolated pathogens in herniated intervertebral discs.14
A: aerobic culture
For aerobic cultures, samples will be cultured in 5% sheep blood agar, chocolate agar and MacConkey agar plate, and will be placed in a 35°C incubator (CO2 atmosphere) for 5 days. If a positive bacteria culture is detected in the plate, the colony will be identified by MALDI-TOF (matrix-assisted laser desorption ionisation–time-of-flight) Microflex LT (Bruker Daltonics/BD). Sensitivity profile will be performed when needed, according to Clinical Laboratory Standards Institute (CLSI) recommendations.
B: anaerobic culture
For anaerobic culture, tissue will be cultured in a Thioglicolate tube and incubated in an incubator at 35°C for up to 14 days. If turbidity occurs at the Thioglicolate medium, material will be cultured in anaerobic blood agar and incubation at 35°C will be done in an anaerobic atmosphere. After growing of colonies, identification will be done by MALDI-TOF and, when necessary, a sensitivity test to antibiotics will be performed according to CLSI recommendations.
C: histological analysis
Anatomic pathology analysis of the remaining fragment of the herniated disc (2×2×5 mm) will be completed. The sample will be transported in a universal container with tamponated formalin (10%), followed by dehydration in alcohol diafanized in xylol and inclusion in paraffin (60°C–65°C), which will be stained in H&E and Gram staining solutions.
H&E: histological cuts of 4 µm will be performed, followed by clearing with xylol for 10 min twice, embedding with alcohol under increasing concentrations and stained with haematoxylin for 5 min, running water for 5 min, eosin for 1 min and running water for 2 min followed by assembly of a glass microscope slide with Entellan.
Gram: another slide will be embedded with crystal violet for 1 min, running water for 1 min followed by lugol for another 1 min and additional wash with running water. Unstaining of the slide will be done with alcohol 95% for 10 s followed by running water and then, stain with fuchsine for 30 s, running water wash again, drying and slide assembly with Entellan.
D: molecular analysis of pathogens
Positive cultures that present aerobic or anaerobic pathogens culturing, will be isolated and stowed refrigerated in −80°C freezer for posterior molecular analysis.
Molecular typing will be performed through pulsed field gel electrophoresis (PFGE) technique of isolated samples according to the protocol described by Oprica et al 21 using Spe-I restriction enzyme and Bionumerics software for analysis of results.
Questionnaires
Scores for pain (NRS), function (ODI) and quality of life (EQ-5D) will be self-assessed and applied before surgery and at 1, 3, 6 and 12 months time points. All questionnaires will be collected by a employee not involved in the study. Follow-up clinic visits will be at 1, 3, 6 and 12 months after surgical procedure, with acceptance deviation of 7, 14, 21 and 28 days, respectively (table 1).
Imaging studies
MRI studies will be performed in Siemens or General Electric 1.5T devices. Studies in 3.0T devices will not be done due to higher frequency of artefacts generated by chemical shift, which would modify measurements of Modic changes.
The following sequences will be used:
Sagittal cut: fast Spin-Echo T1-weighted and T2-weighted sequences with fat suppression or short tau inversion recovery (STIR) instead of T2, according to our institution protocol established for all examinations.
Coronal cut: same sequence imaging will be used for measurements.
At follow-up, contrasted MRI studies of patients will be done as an institution established protocol for postoperative patients, otherwise, it will not interfere with the study protocol.
Lumbar disc herniation will be diagnosed through MRI in eligible patients. Included patients will be submitted to new MRI 12 months after surgery.
Two radiologists with expertise in musculoskeletal MRI will independently classify and perform measurements, and divergences will be solved blindly by common opinion.
Confounding variables
Data on the following confounding variables will be collected: age, gender, alcohol intake, smoking, body mass index (BMI), spinal injections with corticoid within 6 months before surgery, usage of oral corticoids up to 3 months before surgery and diabetes.
As this information may change over time, data will be considered at time of last assessment before surgery.
A: alcohol intake
It is categorised as follows: none or sporadically (<1 glass/day); light intake (1–2 glass/day); moderate/heavy intake (3 or more glasses/day) and not assessed.22
B: smoking
It is categorised as follows: ex-smokers; smoker; non-smoker and not assessed.23
C: BMI
It is categorised as follows: underweight (<18.5); normal weight (18.5–25); overweight (25-30); obese (>30) and not assessed or not available.24
Ethics and dissemination
Some authors suggest that subclinical infection of the intervertebral disc is one of the causes of chronic low back pain unresponsive to treatment, besides promoting Modic type I changes on MRI. Although there are studies reporting relative success on treatment of well-selected patients, there is still uncertainty as to whether these patients were actually infected or not.
We hope to define the accurate incidence of subclinical infection of the intervertebral disc with disc herniation and provide data and possibly an answer to this present gap in the literature.
Statistical planning
Rate of subclinical infection (or Modic change) will be obtained by the ratio between number of positive cultures from surgical samples and total number of patients, and estimates will follow 95% CIs. After infection cases are identified, we will investigate if there is an association between detected infection and patient outcomes by logistic regression models for Modic changes, ordinal logistic regression for Modic volume and size, and linear regression or general linear models for numeric outcomes, such as low back pain, quality of life and function. All models will consider confounding variables such as smoking and alcohol intake, diabetes, corticosteroids injection, BMI, gender and age. Results will be presented as effects estimates such as OR or mean ratio, 95% CIs and P values. Study dropout cases, for any reason, will be considered for final analysis.
Sample size calculation
Sample size was calculated to estimate the incidence of subclinical infection in patients with lumbar disc herniation. Considering that the rate of infection lies around 46%,3 we need to observe a minimum of 95 patients to achieve a 95% CI with 10% absolute accuracy.
The necessary sample size required for analysis of the secondary endpoints will depend on the observed rate of cases with subclinical infection in our study sample. If the observed rate is too small, then an increase in the number of included patients will be needed. To better evaluate this, the sample size calculation will be revisited by the time we have reached half of initially planned sample size (48 patients).
Supplementary Material
Footnotes
Contributors: NA: conception and design of the work, data collection, data analysis and interpretation, and drafting the article. DEM: conception and design of the work, data collection, data analysis and interpretation, critical revision of the article and final approval of the version to be published. MW: data collection, critical revision of the article and final approval of the version to be published. MF: critical revision of the article and final approval of the version to be published. FGM: conception and design of the work and final approval of the version to be published. AMD: conception and design of the work, data collection, data analysis and interpretation. LAR and DCBS: design of the work, data analysis and interpretation, and data collection. ASY, LMRR, MDVM and JRP: data collection and critical revision of the article. ENKF: design of the work and data analysis. ML: conception of the work, critical revision of the article and final approval of the version to be published.
Funding: This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grant number 2016/15830-7).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Ethics Committee of Hospital Israelita Albert Einstein.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 1988;166:193–9. 10.1148/radiology.166.1.3336678 [DOI] [PubMed] [Google Scholar]
- 2. Modic MT, Masaryk TJ, Ross JS. Imaging of degenerative disk disease. Radiology 1988;168:177–86. [DOI] [PubMed] [Google Scholar]
- 3. Jensen TS, Karppinen J, Sorensen JS, et al. Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J 2008;17:1407–22. 10.1007/s00586-008-0770-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Toyone T, Takahashi K, Kitahara H, et al. Vertebral bone-marrow changes in degenerative lumbar disc disease. An MRI study of 74 patients with low back pain. J Bone Joint Surg Br 1994;76:757–64. [PubMed] [Google Scholar]
- 5. Albert HB, Kjaer P, Jensen TS, et al. Modic changes, possible causes and relation to low back pain. Med Hypotheses 2008;70:361–8. 10.1016/j.mehy.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 6. Ohtori S, Koshi T, Yamashita M, et al. Existence of pyogenic spondylitis in Modic type 1 change without other signs of infection: 2-year follow-up. Eur Spine J 2010;19:1200–5. 10.1007/s00586-010-1358-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albert HB, Sorensen JS, Christensen BS, et al. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J 2013;22:697–707. 10.1007/s00586-013-2675-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albert HB, Lambert P, Rollason J, et al. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J 2013;22:690–6. 10.1007/s00586-013-2674-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stirling A, Worthington T, Rafiq M, et al. Association between sciatica and Propionibacterium acnes. Lancet 2001;357:2024–5. 10.1016/S0140-6736(00)05109-6 [DOI] [PubMed] [Google Scholar]
- 10. Agarwal V, Golish SR, Alamin TF. Bacteriologic culture of excised intervertebral disc from immunocompetent patients undergoing single level primary lumbar microdiscectomy. J Spinal Disord Tech 2011;24:397–400. 10.1097/BSD.0b013e3182019f3a [DOI] [PubMed] [Google Scholar]
- 11. Carricajo A, Nuti C, Aubert E, et al. Propionibacterium acnes contamination in lumbar disc surgery. J Hosp Infect 2007;66:275–7. 10.1016/j.jhin.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 12. Rigal J, Thelen T, Byrne F, et al. Prospective study using anterior approach did not show association between Modic 1 changes and low grade infection in lumbar spine. Eur Spine J 2016;25:1000–5. 10.1007/s00586-016-4396-5 [DOI] [PubMed] [Google Scholar]
- 13. Rollason J, McDowell A, Albert HB, et al. Genotypic and antimicrobial characterisation of Propionibacterium acnes isolates from surgically excised lumbar disc herniations. Biomed Res Int 2013;2013:1–7. 10.1155/2013/530382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Urquhart DM, Zheng Y, Cheng AC, et al. Could low grade bacterial infection contribute to low back pain? A systematic review. BMC Med 2015:13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth 2008;101:17–24. [DOI] [PubMed] [Google Scholar]
- 16. Ritter PL, González VM, Laurent DD, et al. Measurement of pain using the visual numeric scale. J Rheumatol 2006;33:574–80. [PubMed] [Google Scholar]
- 17. Gagliese L, Weizblit N, Ellis W, et al. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain 2005;117:412–20. 10.1016/j.pain.2005.07.004 [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Videman T, Niemeläinen R, et al. Quantitative measures of modic changes in lumbar spine magnetic resonance imaging: intra- and inter-rater reliability. Spine 2011;36:1236–43. 10.1097/BRS.0b013e3181ecf283 [DOI] [PubMed] [Google Scholar]
- 19. Levy O, Iyer S, Atoun E, et al. Propionibacterium acnes: an underestimated etiology in the pathogenesis of osteoarthritis? J Shoulder Elbow Surg 2013;22:505–11. 10.1016/j.jse.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 20. Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013;56:1–10. 10.1093/cid/cis966 [DOI] [PubMed] [Google Scholar]
- 21. Oprica C, Emtestam L, Lapins J, et al. Antibiotic-resistant Propionibacterium acnes on the skin of patients with moderate to severe acne in Stockholm. Anaerobe 2004;10:155–64. 10.1016/j.anaerobe.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 22. Alcohol drinking. IARC Working Group, Lyon, 13-20 October 1987. IARC Monogr Eval Carcinog risks to humans;1988:1–378. [PMC free article] [PubMed] [Google Scholar]
- 23. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog risks to humans 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 24. Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007;335:1134 10.1136/bmj.39367.495995.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.