Abstract
The terminalis nerve (TN) is in a class of cranial nerves that plays important roles in animal development, physiology and behavior. Here, we report a study on the characterization of voltage-activated ionic currents in GnRH-containing TN cells in zebrafish. The experiments were performed using acutely dissociated TN cells from the transgenic zebrafish Tg (GnRH-3::GFP). In the transgenic zebrafish, the TN cells express GFP under the transcriptional control of the zebrafish GnRH-3 promoter. In all of the GnRH-containing TN cells examined, we recorded both low-voltage activated (LVA) and high-voltage activated (HVA) calcium current (ICa). The characteristics of the ICa were similar to those described in other zebrafish cell types. However, the distribution patterns of the currents in the GnRH-containing TN cells were different in comparison to the distribution of the currents in other cell types. In addition, we characterized TTX-sensitive sodium current (INa) and 4AP-sensitive and TEA-resistant potassium current (IK). The characteristics of voltage-activated INa and IK in the GnRH-containing TN cells were similar to those described in other zebrafish cell types. Together, the data from this study revealed the electrophysiological properties of the GnRH-containing TN cells, thereby providing insight on the regulatory mechanisms of TN-signaling in animal physiology.
Keywords: zebrafish, transgene, voltage-activated current, terminalis nerve, olfactory bulb
1. Introduction
The terminalis nerve (TN) is in a class of cranial nerves that has been described in all vertebrate species (Arey, 1916; Munz et al., 1982; Demski and Northcutt, 1983). Previous studies have demonstrated that the TN plays an important role in animal development, behavior and physiology (for reviews, see Wirsig-Wiehmann et al., 2003; Whitlock, 2004). In fish, the TN cells are located in the olfactory bulbs and/or its nerve tracts (Stell et al., 1984; Chiba et al., 1996). The TN cells project axons into many brain areas, which include the neural retinas (Stell et al., 1984; 1987; Zucker and Dowling, 1987). Recent studies have suggested that TN signaling may play an important role in sensory integration between the olfactory and visual systems. For example, in response to olfactory stimulation zebrafish visual sensitivity (measured by behavioral assays or by electrophysiological recordings) increased by nearly half a log unit (Maaswinkel and Li, 2003; Huang et al., 2005). The integration of olfacto-retinal signals is likely mediated by the olfactoretinal centrifugal pathway, which originates from TN cells in the olfactory bulb and terminates in the inner plexiform layer in the retina (Stell et al., 1984; Behren and Wagner, 2004). In zebrafish, for example, after olfactory bulb-ectomy or drug inhibition of the TN synaptic input to retinal dopaminergic cells, stimulation of olfactory neurons produced no effect on visual sensitivity (Maaswinkel and Li, 2003).
Recently, we generated a transgenic zebrafish line [Tg (GnRH-3::GFP)], which provides a tool for easy identification of the GnRH-containing TN cells in live embryos and in cell culture (Wang et al., 2010). In the transgenic fish, the TN cells express GFP, which is under the transcriptional control of the zebrafish GnRH-3 promoter. Using the transgenic fish, Wang et al (2010) characterized the GnRH-containing TN cell development and axonal projection. They also characterized, to some extent, the physiological properties of the GFP-tagged GnRH-containing TN cells, including the firing patterns of spontaneous and evoked action potentials. They found that the GnRH-containing TN cells express ionotropic glutamate receptors, suggesting that the activity of TN cells may be influenced by signals from the olfactory bulbs.
Whereas the developmental course of TN cells and the potential roles of TN-signaling in zebrafish visual system functions have been documented (Abraham et al., 2008; Wang et al., 2010), the physiological properties of GnRH-containing TN cell membranes (e.g., the expression of voltage-activated ionic currents) remain to be further characterized. In this study, we characterized voltage-activated calcium current (ICa), sodium current (INa) and potassium current (IK) using acutely dissociated GFP-tagged GnRH-containing TN cells from Tg (GnRH-3::GFP) fish. This study provides data on the electrophysiological properties of GnRH-containing TN cells, thereby shedding insight on the mechanisms of TN-signaling in animal physiology.
2. Results
2.1. Voltage-activated calcium currents (ICa) in the GnRH-containing TN cells
In this study, we examined voltage-activated ionic currents from freshly dissociated GFP-tagged TN cells from the olfactory bulbs of adult transgenic animals (between 2–3 months of age). In the transgenic zebrafish [Tg (GnRH-3::GFP)], the TN cells can be readily identified in live embryos and in cell culture (Fig. 1).
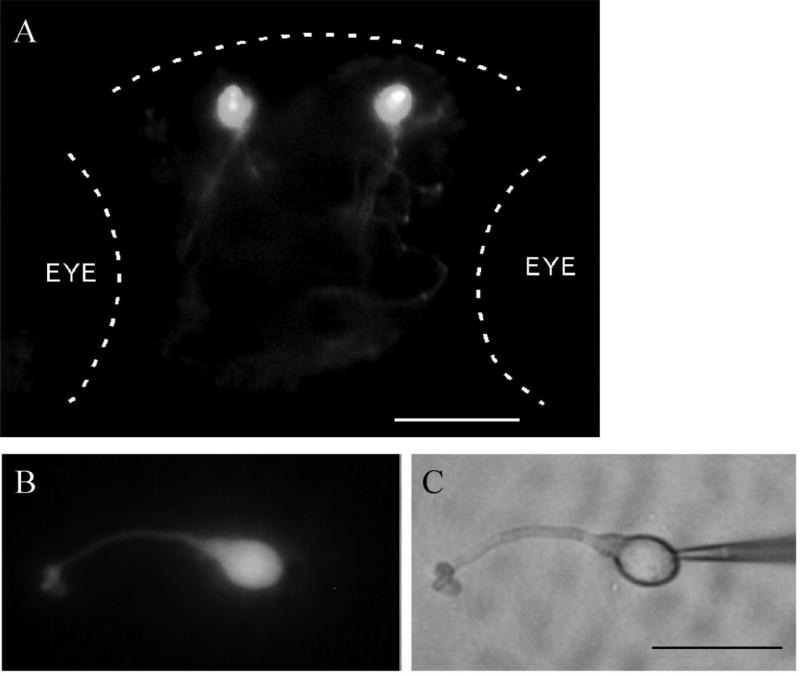
Fig. 1.
Images of a transgenic [Tg(GnRH-3::GFP)] zebrafish embryo and an isolated GFP-tagged GnRH-containing TN cell. A. A fluorescent image of a 4-day-old transgenic embryo that shows the TN cells in the olfactory bulb (dorsal view). Dashed lines outline the head and eyes (Anterior, top). Scale bar: 30 µm. B, C. Fluorescent and bright-field images of an acutely dissociated GFP-tagged GnRH-containing TN cell. Note the recording electrode placed on the right side of the cell. Scale bar: 15 µm.
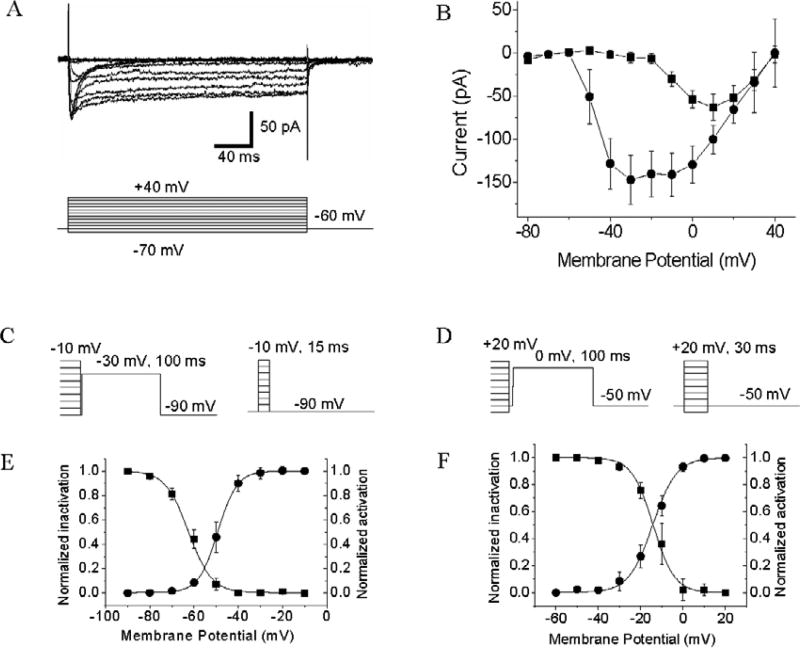
We recorded voltage-activated calcium current (ICa) in dissociated GFP-tagged GnRH-containing TN cells. During the recordings, the cells were perfused with medium that contained TTX and TEA in order to block voltage-activated sodium currents and potassium currents, respectively. All the examined GnRH-containing TN cells (n = 12) express low-voltage activated (LVA) ICa and high-voltage activated (HVA) ICa (Fig. 2A, B). Depolarizing steps from a holding potential of −60 mV elicited a mixture of transient and sustained inward currents, which showed the characteristics of LVA and HVA ICa. With the increase of membrane depolarization, the amplitude of the current increased, until it reached the peak value at about −30 mV and +10 mV (transient and sustained currents, respectively). We measured the activation and steady-state inactivation voltages of LVA and HVA ICa. To measure activation voltages, the membrane potentials were first hyperpolarized or depolarized to a series of test pulses, ranging from −90 mV to −10 mV (LVA ICa) or from −60 mV to +20 mV (HVA ICa). The duration of depolarization varied (15 ms for LVA ICa and 30 ms for HVA ICa) in order to reach the maximum activation. Then the membrane potentials were repolarized to −90 mV and −50 mV, respectively and tail-currents were measured at the peak (Fig. 2C). To measure inactivation voltages, the membrane potentials were first depolarized to a series of 3-sec conditioning pulses, ranging from −90 mV to −10 mV (LVA ICa) or from −60 mV to +20 mV (HVA ICa). Then the membrane potentials were repolarized to −90 mV (LVA ICa) or −50 mV (HVA ICa) for 5 ms and then depolarized −30 mV (LVA ICa) or 0mV (HVA ICa). Peak-currents were measured after the membrane was depolarized to −30 mv or 0 mV, respectively, and were fitted by a standard Boltzmann function (Fig. 2D). Data revealed that the Vhalf of LVA and HVA ICa were −49.27 ± 0.09 mV and −13.52± 0.34 mV, respectively, and the inactivation voltages for LVA ad HVA ICa were −61.46 ± 0.42 mV and −13.34 ± 0.62 mV, respectively (Fig. 2E, F).
Fig. 2.
LVA and HVA ICa recorded from acutely dissociated GFP-tagged GnRH-containing TN cells. A. Voltage-clamp traces of inward ICa. Voltage steps are shown at the bottom of the current traces. B. Voltage-current (I/V) relationship curves of LVA and HVA ICa generated from data collected at the peak (transient ICa, circles) and at the plateau (sustained ICa, squares). Data represent the means ± SE (n = 12). C, D. Protocols for measuring activation and inactivation voltages of LVA and HVA ICa, respectively. E, F. Voltage-dependent activation (circles) and inactivation (squares) of LVA and HVA ICa. Data represent the means ± SE (n = 12).
2.2. Voltage-activated sodium current (INa) in the GnRH-containing TN cells
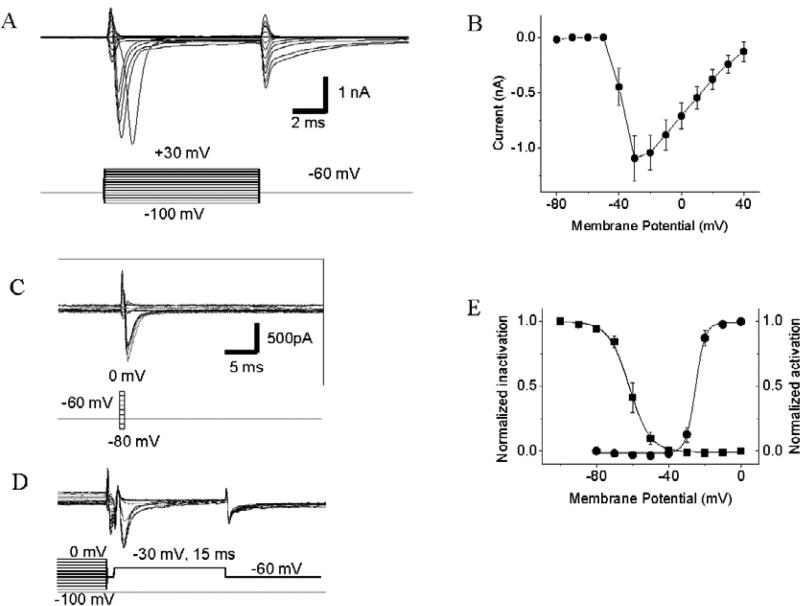
Voltage-activated INa were recorded in all of the GnRH-containing TN cells examined (n = 10) (Fig. 3A, B). When the membrane potential was depolarized from the holding potential of − 60 mV to −50 mV, a small transient inward INa were recorded. With the increase of depolarizing potential, the amplitude of the current increased, until it reached the peak value at about −30 mV. We measured the activation and inactivation voltages of INa in the GnRH-containing TN cells. To measure the activation voltages, the membrane potential was first depolarized to a series of test pulses, ranging from −80 mV to 0 mV. Then the membrane potential was repolarized to −60 mV, by which tail-currents were induced (Fig. 3C). To measure the inactivation voltages, the membrane was first depolarized, ranging from −10 mV to 0 mV. Then the membrane potential was repolarized to −60 mV and then depolarized to −30 mV. Peak-currents were measured after the cell membrane was depolarized to −30 mV (Fig. 3D). The relationships between membrane voltage and current were analyzed off-line and were plotted by the Boltzmann Function (Fig. 3E). Data revealed that the Vhalf of INa activation and inactivation voltages were −24.75 ± 0.94 mV and −61.82 ± 1.14 mV, respectively.
Fig. 3.
Voltage-activated INa recorded from acutely dissociated GFP-tagged GnRH-containing TN cells. A. Traces of transient inward INa. Voltage steps are shown at the bottom of the current traces. B. Voltage-current (I/V) relationship curve of INa. Data represent the means ± SE (n = 10). C, D. Protocols for measuring activation and inactivation voltages of INa, respectively. E. Voltage-dependent activation (circles) and inactivation (squares) of INa. Data represent the means ± SE (n = 10).
2.3. Voltage-activated potassium current (IK) in the GnRH-containing TN cells
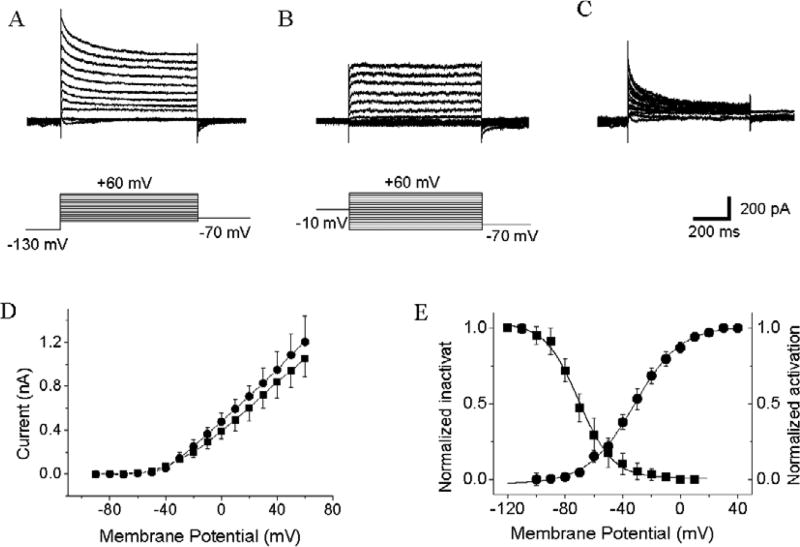
We recorded voltage-activated IK in isolated GnRH-containing TN cells (Fig. 4A–D). In the presence of TEA in the medium, voltage-activated 4AP-sensitive and TEA-resistant IK were observed (n = 15). Depolarizing steps from a holding potential of −70 mV elicited a mixture of transient (4AP-sensitive) and sustained (TEA-resistant) outward currents. When 4AP were added to the medium, the initial transient 4AP-sensitive current was blocked, but TEA-resistant sustained outward IK persisted. Using a standard protocol (Yu and Li, 2005), we measured the activation and inactivation voltages of the IK. Data were plotted by the Boltzmann Function (Fig. 4E). The Vhalf of IK activation and steady-state inactivation voltages were −31.21 ± 1.59 mV and −61.13 ± 2.47 mV, respectively.
Fig. 4.
Voltage-activated 4AP-sensitive and TEA-resistant IK recorded from acutely dissociated GFP-tagged GnRH-containing TN cells. A, B. Traces of transient (4AP-sensitive) and sustained (TEA-resistant) IK. Voltage steps are shown at the bottom of the current traces. C. Transient 4AP-sensitive currents (panel B digitally subtracted from Panel A). D. Voltage-current (I/V) relationship curves of transient (circles) and sustained (squares) IK. Data represent the means ± SE (n = 15). E. Voltage-dependent activation (circles) and inactivation (squares) of IK. Data represent the means ± SE (n = 15).
3. Discussion
Characterization of voltage-activated ionic currents on the GnRH-containing TN cell membrane may provide insight for the roles of TN-signaling in animal physiology (Eisthen et al., 2000; Oka, 2002; Grens et al., 2005). Zebrafish TN cells express different neurotransmitter receptor types, including glutamate receptors (Kiya and Oka, 2003; Wayne et al., 2005; Mousley et al., 2006; Haneda and Oka, 2008; Ramakrishnan and Wayne, 2009; Wang et al., 2010). It is possible that in response to olfactory stimulation, the GnRH-containing TN cells will become activated by glutamate released from the olfactory neurons. Because the GnRH-containing TN cells project axons to the neural retina, it is conceivable that alterations in TN activity may modulate the visual system functions. In fish retinas, the TN axons synapse onto dopaminergic interplexiform cells (DA-IPCs) (Zucker and Dowling, 1988; Li and Dowling, 2000). DA-IPCs are the only cell types that synthesize and release dopamine (Zucker and Dowling, 1987; Yazulla and Zucker, 1988). In a previous study, we demonstrated that in zebrafish, stimulation of olfactory neurons decreases dopamine concentrations in the retina (Maaswinkel and Li, 2003; Puppala et al., 2004). Furthermore, Huang et al (2005) demonstrated that in zebrafish retinas, dopamine inhibits the activity of retinal ganglion cells. Together, we hypothesize that via the olfactoretinal centrifugal pathway, olfactory signals may modify visual function, i.e., by relieving the inhibition of dopamine to retinal ganglion cells. The mechanisms underlying the regulation of TN-signaling and dopamine release in the retina remain to be further studied.
In this study, we examined voltage-activated ionic currents of GFP-tagged GnRH-containing TN cells in transgenic zebrafish [GnRH::GFP]. The rational for conducting this research are two-fold. First, it is important to characterize the TN cells in zebrafish, which has recently become a major model vertebrate for cell biology and genetics. This model provides a tool for better understanding the cellular and genetic make-up of the TN cells and its relevance to animal physiology. Second, all of the previous studies (i.e., in gourami fish) were performed in TN neurons that were identified based on cell morphology. However, the cell types were not confirmed (i.e., not by gene expression profiles). In our study, we recorded GFP-tagged GnRH-containing TN cells from transgenic zebrafish. This is in fact the first study of GnRH-containing TN using transgenic approaches.
We have demonstrated that in the transgenic fish, all the GFP-positive cells expressed GnRH examined by RT-PCR (see Wang et al., 2010). Previously, we demonstrated that in zebrafish the GnRH-containing TN cells fire spontaneous and evoked action potentials (Wang et al., 2010). Bath application of TTX diminished the firings and altered membrane potentials, indicating that GnRH-containing TN cells express voltage-activated sodium channels (Abe and Oka, 2000). We found that the expression profiles of voltage-activated INa in the zebrafish GnRH-containing TN cells are similar to the expression of TTX-sensitive INa reported in dwarf gourami TN cells (Oka, 1995, 1996). We did not attempt to examine TTX-resistant INa in TN cells. In dwarf gourami, different components of IK were expressed in TN cells, which include TEA-sensitive, 4AP-sensitive, and TEA and 4AP-resistant IK (Abe and Oka, 1999). In this study, we characterized 4AP-sensitive and TEA-resistant IK in the zebrafish GnRH-containing TN cells. The IK we observed in zebrafish TN cells was similar to 4AP-sensitive and TEA-resistant IK reported in dwarf gourami TN cells (Abe and Oka, 1999).
We recorded both LVA and HVA ICa. The expression profiles of ICa were different from those previously reported in other zebrafish neural cell types. For example, in isolated zebrafish retinal ganglion cells, only one-third of the cells expressed LVA ICa, whereas HVA ICa was recorded in all the ganglion cells examined (Huang and Li, 2006; see also Haneda and Oka, 2004). We suspect that the differential expression of LVA ICa in the retinal ganglion cells is due to the complexity of the ganglion cells. In the zebrafish retina, for example, more than ten different subtypes of retinal ganglion cells have been identified by morphology, (Mangrum et al., 2002). Thus, it is possible that the distribution patterns of LVA ICa are different among different subtypes of retinal ganglion cells.
Recently, several TN-specific transgenic zebrafish lines have been described (Abraham et al., 2008; Wang et al., 2010; Ramakrishnan et al., 2010). The transgenic fish provide an excellent model for further studies of TN cell development (Abraham et al., 2008), axonal projection (Wang et al., 2010) and physiology (Wang et al., 2010; and the current study). The current study provides a better understanding of GnRH-containing TN cell membrane properties, i.e., voltage-activated ionic currents.
4. Experimental procedures
Electrophysiological recordings were made from isolated GFP-tagged GnRH-containing TN cells from transgenic zebrafish. Cell isolation and culture were performed as previously described (Huang et al., 2005). Briefly, transgenic fish were anesthetized with 0.5% 3-amino benzoic methylester. The olfactory bulbs were isolated and transferred to an enzyme cocktail (20 u/ml papain, 1.0 mg/ml L-cysteine, 0.6 mM EDTA, 140 mM NaCl, 5.0 mM KCl, 0.3 mM NaH2PO4, 0.3 mM Na2HPO4, 10 mM Glucose; pH, 7.4). After 30 min of incubation, the tissue was mechanically dissociated. The resulting cell suspension was plated on plastic culture dishes containing L-15 medium (Invitrogen, NY).
Whole-cell patch clamp recordings were performed using GFP-tagged GnRH-containing TN neurons after the cells were cultured for 2–10 hours. Patch pipettes (1.5 mm outer diameter borosilicate glass; Sutter Instrument, Novato, CA) were pulled on a Brown/Flaming puller (model P-97; Sutter Instrument, Novato, CA). Whole-cell current recording were processed using an Axopatch 200B amplifier (filtered at 3 kHz) and Clampex 8.0 software (Axon Instruments, CA). The experimental solutions were made by modifying the standard solution (Yu and Li, 2005; Huang et al., 2005; Huang and Li, 2006). All drugs and solutions were delivered to the culture media at a rate of 2 ml/minute.
For Ca2+ current recordings, external solution contained (in mM): 85 NaCl, 5.0 KCl, 10 Cs-Acetate, 30 TEA-Cl, 1.0 MgCl2, 10 CaCl2, 10 HEPES, 10 glucose, 0.001 TTX, pH 7.4 adjusted with NaOH. The pipette solution contained (in mM): 40 CsCl, 80 Cs-Acetate, 30 TEA-Cl, 1.0 MgCl2, 5 EGTA, 10 HEPES, 2.0 ATP-Mg, 0.5 GTP-Na, pH 7.2 adjusted with CsOH. For Na+ and K+ current recordings, the external solution contained (in mM): 135 NaCl, 4.0 KCl, 0.5 MgCl2, 2.5 CaCl2, 10 HEPES, 10 D-glucose, 0.005 nifedipine, 0.001 TTX (only added to K+ current recordings), pH 7.4, adjusted with NaOH. The pipette solution for Na+ current measurement contained (in mM): 140 CsCl, 0.5 CaCl2, 5.0 EGTA, 10 HEPES, 2.0 ATP-Mg, 1.0 TEA-Cl, 1 4-AP, pH 7.2 adjusted with CsOH. The pipette solution for K+ current recordings contained (in mM): 140 KCl, 4.0 NaCl, 0.5 CaCl2, 5.0 EGTA, 10 HEPES, 3.0 ATP-Mg, 1 GTP-Na, pH 7.2 adjusted with KOH. TEA-Cl (40 mM) and 4AP (5.0 mM) were added to external solutions to block TEA-resistant and 4AP-sensitive K+ currents, respectively.
Research highlights.
-
*
Patch-clamp GFP-tagged terminalis neurons from transgenic zebrafish
-
*
Terminalis neurons express both low- and high-voltage activated calcium currents
-
*
Terminalis neurons express TTX-sensitive sodium currents
-
*
Terminalis neurons express 4AP-sensitive and TEA-resistant potassium currents
Acknowledgments
The authors thank Dr. Daoqi Zhang for technical advice on the electrophysiological experiments, Aprell Carr and April DeLaPaz for proof reading the manuscript. This work was supported in part by the NIH grant R01EY013147.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe H, Oka Y. Characterization of K+ currents underlying pacemaker potentials of fish gonadotropin-releasing hormone cells. J. Neurophysiol. 1999;81:643–653. doi: 10.1152/jn.1999.81.2.643. [DOI] [PubMed] [Google Scholar]
- Abe H, Oka Y. Modulation of pacemaker activity by salmon gonadotropin-releasing hormone (sGnRH) in terminal nerve (TN)-GnRH neurons. J. Neurophysiol. 2000;83:3196–3200. doi: 10.1152/jn.2000.83.5.3196. [DOI] [PubMed] [Google Scholar]
- Abraham E, Palevitch O, Ijiri S, Du SJ, Gothilf Y, Zohar Y. Early development of forebrain gonadotrophin-releasing hormone (GnRH) neurons and the role of GnRH as an autocrine migration factory. J. Neuroendo. 2008;20:394–405. doi: 10.1111/j.1365-2826.2008.01654.x. [DOI] [PubMed] [Google Scholar]
- Arey LB. The function of the afferent fibers of the optic nerve of fishes. J. Comp. Neurol. 1916;26:213–245. [Google Scholar]
- Behren U, Wagner HJ. Terminal nerve and vision. Microsc. Res. Tech. 2004;65:25–32. doi: 10.1002/jemt.20108. [DOI] [PubMed] [Google Scholar]
- Chiba A, Sohn YC, Honma Y. Distribution of neuropeptide Y and gonadotropin- releasing hormone immunoreactivities in the brain and hypophysis of the ayu, Plecoglossus altivelis (Teleostei) Arch. Histol. Cytol. 1996;59:137–148. doi: 10.1679/aohc.59.137. [DOI] [PubMed] [Google Scholar]
- Demski LS, Northcutt RG. The terminal nerve: a new chemosensory system in vertebrates? Science. 1983;220:435–437. doi: 10.1126/science.6836287. [DOI] [PubMed] [Google Scholar]
- Eisthen HL, Delay RJ, Wirsig-Wiechmann CR. Neuromodulatory effects of gonadotropin releasing hormone on olfactory receptor neurons. J. Neurosci. 2000;20:3947–3955. doi: 10.1523/JNEUROSCI.20-11-03947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grens KE, Greenwood AK, Fernald RD. Two visual processing pathways are targeted by gonadotropin-releasing hormone in the retina. Brain Behav. Evol. 2005;66:1–9. doi: 10.1159/000085043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneda K, Oka Y. Selective modulation of voltage-gated calcium channels in the terminal nerve gonadotropin-releasing hormone neurons of a teleost, the dwarf gourami (Colisa lalia) Endocrinology. 2004;145:4489–4499. doi: 10.1210/en.2004-0353. [DOI] [PubMed] [Google Scholar]
- Haneda K, Oka Y. Coordinated synchronization in the electrically coupled network of terminal nerve gonadotropin-releasing hormone neurons as demonstrated by double patch-clamp study. Endocrinology. 2008;149:3540–3548. doi: 10.1210/en.2008-0299. [DOI] [PubMed] [Google Scholar]
- Huang L, Li L. Differential expression of voltage-activated calcium currents in zebrafish retinal ganglion cells. J. Neurosci. Res. 2006;84:497–504. doi: 10.1002/jnr.20951. [DOI] [PubMed] [Google Scholar]
- Huang L, Maaswinkel H, Li L. Olfactoretinal centrifugal input modulates zebrafish retinal ganglion cell activity: a possible role for dopamine-mediated Ca2+ signaling pathways. J. Physiol. 2005;569:939–948. doi: 10.1113/jphysiol.2005.099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiya T, Oka Y. Glutamate receptors in the terminal nerve gonadotropin-releasing hormone neurons of the dwarf gourami (teleost) Neurosci. Lett. 2003;345:113–116. doi: 10.1016/s0304-3940(03)00503-2. [DOI] [PubMed] [Google Scholar]
- Li L, Dowling JE. Disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant zebrafish. J. Neurosci. 2000;20:1883–1892. doi: 10.1523/JNEUROSCI.20-05-01883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaswinkel H, Li L. Olfactory input increases visual sensitivity in zebrafish: a possible function for the terminal nerve and dopaminergic interplexiform cells. J. Exp. Biol. 2003;206:2201–2209. doi: 10.1242/jeb.00397. [DOI] [PubMed] [Google Scholar]
- Mangrum WI, Dowling JE, Cohen ED. A morphological classification of ganglion cells in the zebrafish retina. Vis. Neurosci. 2002;19:767–779. doi: 10.1017/s0952523802196076. [DOI] [PubMed] [Google Scholar]
- Mousley A, Polese G, Marks NJ, Eisthen HL. Terminal nerve-derived neuropeptide y modulates physiological responses in the olfactory epithelium of hungry axolotls (Ambystoma mexicanum) J. Neurosci. 2006;26:7707–7717. doi: 10.1523/JNEUROSCI.1977-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münz H, Class B, Stumpf WE, Jennes L. Centrifugal innervation of the retina by luteinizing hormone releasing hormone (LHRH)-immunoreactive telencephalic neurons in teleostean fishes. Cell Tissue Res. 1982;222:313–323. doi: 10.1007/BF00213215. [DOI] [PubMed] [Google Scholar]
- Oka Y. Tetrodotoxin-resistant persistent Na+ current underlying pacemaker potentials of fish gonadotrophin-releasing hormone neurones. J. Physiol. 1995;482:1–6. doi: 10.1113/jphysiol.1995.sp020494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y. Characterization of TTX-resistant persistent Na+ current underlying pacemaker potentials of fish gonadotropin-releasing hormone (GnRH) neurons. J. Neurophysiol. 1996;75:2397–2404. doi: 10.1152/jn.1996.75.6.2397. [DOI] [PubMed] [Google Scholar]
- Oka Y. Physiology and release activity of GnRH neurons. Prog. Brain Res. 2002;141:259–281. doi: 10.1016/S0079-6123(02)41098-9. [DOI] [PubMed] [Google Scholar]
- Puppala D, Maaswinkel H, Mason B, Legan SJ, Li L. An in vivo microdialysis study of light/dark-modulation of vitreal dopamine release in zebrafish. J. Neurocytol. 2004;33:193–201. doi: 10.1023/b:neur.0000030694.88653.d6. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan S, Wayne NL. Social cues from conspecifics alter electrical activity of gonadotropin-releasing hormone neurons in the terminal nerve via visual signals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:135–141. doi: 10.1152/ajpregu.00143.2009. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan S, Lee W, Navarre S, Kozlowski DJ, Wayne NL. Acquisition of spontaneous electrical activity during embryonic development of gonadotropin-releasing hormone-3 neurons located in the terminal nerve of transgenic zebrafish (Danio rerio) Gen. Comp. Endocrinol. 2010;168:401–407. doi: 10.1016/j.ygcen.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell WK, Walker SE, Ball AK. Functional-anatomical studies on the terminal nerve projection to the retina of bony fishes. Ann. NY Acad. Sci. 1987;519:80–96. doi: 10.1111/j.1749-6632.1987.tb36288.x. [DOI] [PubMed] [Google Scholar]
- Stell WK, Walker SE, Chohan KS, Ball AK. The goldfish nervus terminalis: a luteinizing hormone-releasing hormone and molluscan cardioexcitatory peptide immunoreactive olfactoretinal pathway. Proc. Natl. Acad. Sci. USA. 1984;81:940–944. doi: 10.1073/pnas.81.3.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen J, Nourizadeh-Lillabadi R, Husebye H, Alestrom P. In silico and in situ characterization of the zebrafish (Danio rerio) gnrh3 (sGnRH) gene. BMC Genomics. 2002;3:25. doi: 10.1186/1471-2164-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huang L, Li X, Li L. Characterization of GFP-tagged GnRH-containing Terminalis Neurons in Transgenic Zebrafish. J. Cell. Physiol. 2010 doi: 10.1002/jcp.22369. In press. [DOI] [PubMed] [Google Scholar]
- Wayne NL, Kuwahara K, Aida K, Nagahama Y, Okubo K. Whole-cell electrophysiology of gonadotropin-releasing hormone neurons that express green fluorescent protein in the terminal nerve of transgenic medaka (Oryzias latipes) Biol. Reprod. 2005;73:1228–1234. doi: 10.1095/biolreprod.105.042721. [DOI] [PubMed] [Google Scholar]
- Whitlock KE. Development of the nervus terminalis: origin and migration. Microsc. Res. Tech. 2004;65:2–12. doi: 10.1002/jemt.20094. [DOI] [PubMed] [Google Scholar]
- Wirsig-Wiechmann CR, Oka Y. The terminal nerve ganglion cells project to the olfactory mucosa in the dwarf gourami. Neurosci. Res. 2002;44:337–341. doi: 10.1016/s0168-0102(02)00150-5. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Zucker CL. Synaptic organization of dopaminergic interplexiform cells in the goldfish retina. Vis. Neurosci. 1988;1:13–29. doi: 10.1017/s0952523800000997. [DOI] [PubMed] [Google Scholar]
- Yu CJ, Li L. Dopamine modulates voltage-activated potassium currents in zebrafish retinal on bipolar cells. J. Neurosci. Res. 2005;82:368–376. doi: 10.1002/jnr.20637. [DOI] [PubMed] [Google Scholar]
- Zucker CL, Dowling JE. Centrifugal fibres synapse on dopaminergic interplexiform cells in the teleost retina. Nature. 1987;330:166–168. doi: 10.1038/330166a0. [DOI] [PubMed] [Google Scholar]