ABSTRACT
We characterized the tension response of clathrin-mediated endocytosis by using various cell manipulation methodologies. Elevated tension in a cell hinders clathrin-mediated endocytosis through inhibition of de novo coat initiation, elongation of clathrin coat lifetimes and reduction of high-magnitude growth rates. Actin machinery supplies an inward pulling force necessary for internalization of clathrin coats under high tension. These findings suggest that the physical cues cells receive from their microenvironment are major determinants of clathrin-mediated endocytic activity.
KEY WORDS: Clathrin-mediated endocytosis, Membrane tension, Microaspiration, Hypotonic shock, Actin dynamics
Summary: Elevated tension in a cell hinders clathrin-mediated endocytosis through inhibition of de novo coat initiation, elongation of clathrin coat lifetimes, and reduction of high magnitude growth rates.
INTRODUCTION
Clathrin-coated structures bear a major fraction of the endocytic load from the plasma membrane of eukaryotic cells. During formation of an endocytic vesicle, clathrin heterohexamers assemble into a multifaceted cage that is linked to the plasma membrane by clathrin adaptors. Tension on the membrane hinders this process as it increases the energy cost of curvature formation (Sheetz, 2001). Curvature-bearing clathrin-coated pits are replaced by less-dynamic shallow coats when tension is elevated (Saleem et al., 2015). In various cellular contexts, actin dynamics supplements the energy required for formation of clathrin-coated vesicles under high membrane tension (Aghamohammadzadeh and Ayscough, 2009; Boulant et al., 2011; Kaur et al., 2014). However, actin-dependent clathrin-mediated endocytic events have a longer duration than their counterparts taking place at lower tension levels (Boulant et al., 2011). Here, we characterized the regulation of clathrin coat dynamics by membrane tension by using cell manipulation techniques (i.e. microaspiration, cell squeezing and hypo-osmotic swelling) coupled with fluorescence live-cell imaging. Our results show that the density of endocytic clathrin-coated structures on the plasma membrane depends on tension, and actin machinery rescues internalization of clathrin coats under high tension by moving clathrin coats away from the membrane.
RESULTS AND DISCUSSION
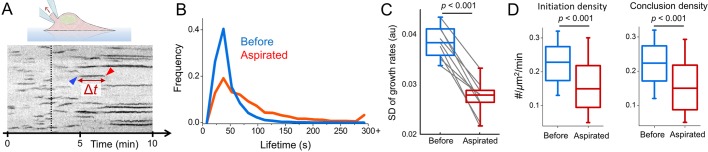
We used three independent approaches to increase the tension on the plasma membrane while monitoring clathrin coat dynamics at the ventral (adherent) surface of cells. Applying negative pressure on the plasma membrane by micropipette aspiration is an effective way to increase tension (Herant et al., 2005; Houk et al., 2012). We detected a significant increase in average clathrin coat lifetime (time elapsed between the origination and conclusion of the coat; 44±23 s versus 88±73 s, mean±s.d., P<0.001; ncells=9, ntraces=40,943; Fig. 1A,B) in BSC1 cells upon microaspiration of the membrane. The impeded clathrin coat dynamics is also observed as gradual disappearance of high-magnitude growth rates in clathrin traces (Movie 1) (Ferguson et al., 2016). As a result, the standard deviation of clathrin growth rate distributions reduced [0.038±0.003 (before aspiration) versus 0.027±0.004 (during aspiration), P<0.001; Fig. 1C]. We also found that increased tension reduced the surface density of clathrin coat initiation and conclusion events (Fig. 1D).
Fig. 1.
Aspiration of the plasma membrane slows down clathrin coat dynamics. (A) Kymograph showing the clathrin activity at the ventral surface of a BSC1 cell expressing AP2–eGFP. Clathrin coat traces elongate gradually upon microaspiration (dashed line; Movie 1). Blue and red arrowheads mark the initiation and conclusion of a clathrin-coated structure, respectively. Δt is its lifetime. (B) Clathrin coat lifetime distributions are shown for nine BSC1 cells imaged before and during microaspiration (ntraces=40,943). (C) For the same nine cells, the standard deviation of the clathrin growth rate distributions are shown in boxplots. Lines connect the standard deviation values obtained from the same cell before and during aspiration. The narrower growth rate distributions indicate slower clathrin coat dynamics. (D) Box plots are the initiation and conclusion densities of clathrin-coated structures before and during aspiration. In the boxplots, the box represents the 25–75th percentiles, and the median is indicated. The whiskers show the 10–90th percentiles. P-values were obtained with a two-tailed t-test.
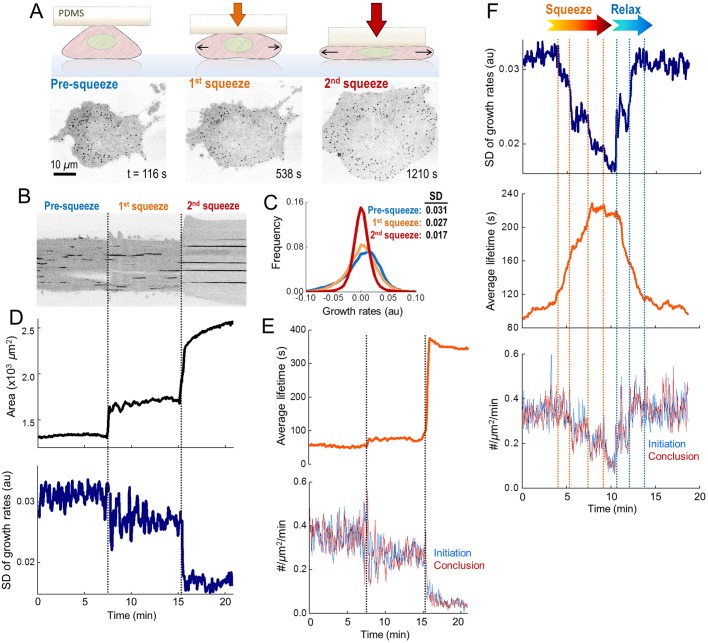
To induce faster changes in plasma membrane tension, we increased the hydrostatic pressure in cells by squeezing them with a micromanipulator-controlled polymer cushion (Fig. 2A,B). We used growth rate distributions obtained from clathrin coat intensity profiles to temporally resolve the fast alterations in endocytic dynamics (Ferguson et al., 2016). In good agreement with the microaspiration experiments, fast dissolution and fast formation phases in the growth rate distributions diminished with the increasing tension, whereas the frequency of the plateau phase increased (Fig. 2C; Fig. S1, Movie 2). Discrete changes in the tension could be resolved as stepwise reduction in the standard deviation of clathrin growth rates (Fig. 2D). Furthermore, the average clathrin coat lifetimes increased while initiation and conclusion densities reduced (Fig. 2B,E). When we relieved the squeezing to verify the viability of cells, we found that the parameters determining clathrin coat dynamics reverted to normal (Fig. 2F; Fig. S2, Movie 3).
Fig. 2.
Cell squeezing induces fast and reversible alterations in clathrin coat dynamics. (A) Cartoon and representative frames of a BSC1 cell are shown at different stages of squeezing (Movie 2). (B) Kymograph showing the temporal evolution of the clathrin traces detected at the ventral surface of the cell shown in A. Dashed lines mark the squeezing steps. (C) For the cell in A, normalized distributions of clathrin growth rates are plotted for different squeezing levels (Fig. S1). The standard deviation of the distribution reduces as the tension increases. (D) For the same cell, the time variation of the ventral surface area (upper) and the standard deviation of the clathrin growth rates (lower). The stepwise changes in these parameters are due to discrete levels of squeezing. (E) The response of the same cell to squeezing is shown as the mean clathrin lifetime (upper) and initiation and conclusion densities (lower). Dashed lines indicate change in squeezing (ntraces=8217). (F) Standard deviation of clathrin growth rates (upper), mean lifetime (middle), and initiation and conclusion densities (lower) from a cell that undergoes increased stepwise squeezing (orange dashed lines) and relaxation (blue dashed lines) (Movie 3) (ntraces=8255).
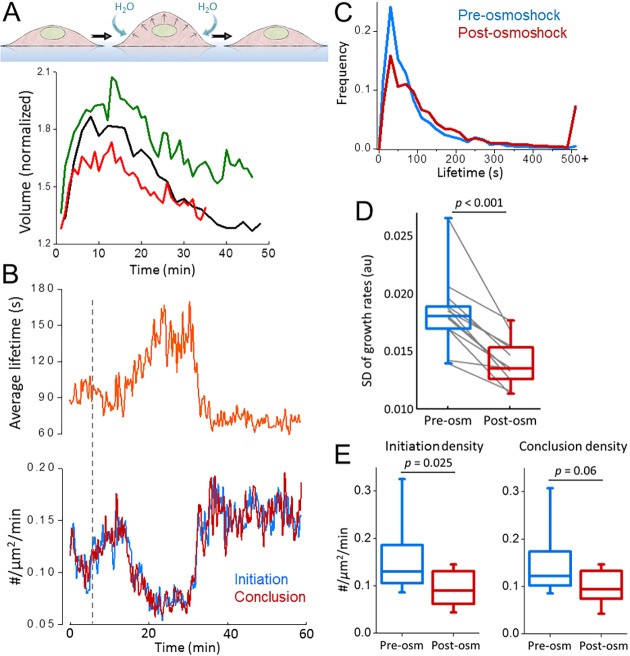
Hypotonic swelling is a straightforward and widely used approach to increase membrane tension (Boulant et al., 2011; Cocucci et al., 2014; Diz-Muñoz et al., 2016). Upon reducing the osmolarity of the imaging medium, we found that the hypotonic swelling takes effect within minutes but the volume of cells converges back to the original values within an hour (Fig. 3A). As expected, the temporary increase of the tension due to stretching of the membrane affected endocytic clathrin coat dynamics only temporarily (Fig. 3B). We found that the average lifetime of clathrin-coated structures increased significantly in SUM159 cells (Aguet et al., 2016) under hypo-osmotic shock (87±86 s versus 161.2±208.1 s, mean±s.d., P<0.001; ncells=12, ntraces=34,113; Fig. 3C). In accordance with the microaspiration and cell squeezing assays, increased tension resulted in reduction of the standard deviation of clathrin growth rates [0.018±0.003 (pre-osmoshock) versus 0.014±0.002 (post-osmoshock), P<0.001; Fig. 3D], and initiation and conclusion densities (Fig. 3E).
Fig. 3.
Hypotonic swelling inhibits clathrin coat dynamics temporarily. (A) Change in the volume (normalized to the initial value) is plotted for three BSC1 cells during hypotonic swelling (i.e. osmoshock). (B) Mean clathrin coat lifetime (upper), and initiation and conclusion densities (lower) are plotted against time for a BSC1 cell treated with hypotonic shock (dashed line). (C) Clathrin lifetime distributions are assembled pre- and post-osmoshock for 12 gene-edited SUM159 cells expressing AP2–eGFP (ntraces=34,113). (D,E) For the same cells, the standard deviation of clathrin growth rates (D) and initiation and conclusion densities of clathrin-coated structures pre- and post-osmotic shock (E) are shown in boxplots. Lines connect the standard deviation values obtained from the same cell pre- and post-osmoshock. In the boxplots, the box represents the 25–75th percentiles, and the median is indicated. The whiskers show the 10–90th percentiles. P-values were obtained with a two-tailed t-test.
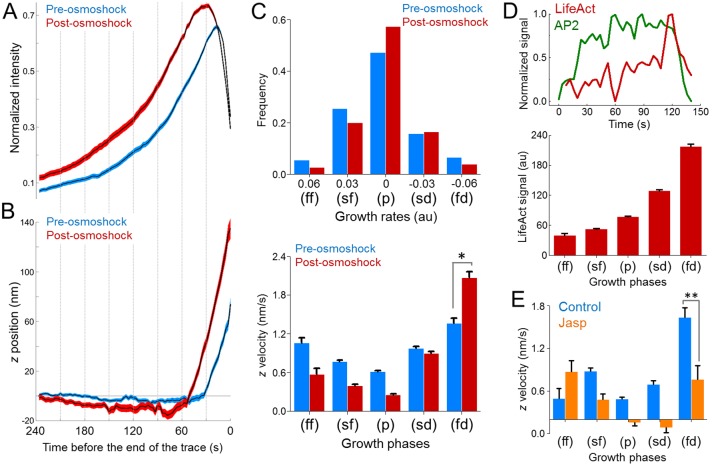
As plasma membrane tension increases, actin polymerization energy becomes indispensable for narrowing of the neck between clathrin-coated pits and the plasma membrane. Therefore, inhibition of the actin machinery arrests clathrin coats prior to the scission phase (Boulant et al., 2011). There are two proposed models for actin-dependent formation of clathrin-coated vesicles under high tension (Hassinger et al., 2017). The first model predicts a vertical force generated by actin polymerization to move the clathrin coat away from the plasma membrane. The second model suggests formation of an actin collar that constricts the neck region directly (Collins et al., 2011). By tracing the three-dimensional (3D) displacement of clathrin-coated structures, we found that coats move ∼100 nm into the cell before uncoating, and the inward displacement is significantly higher when the membrane tension is increased by hypotonic swelling (Fig. 4A,B). We also found that the axial velocity of the inward movement is the highest during the fast dissolution phase of clathrin coats (Fig. 4C). We adapted a master–slave approach (Aguet et al., 2013) to monitor the intensity profiles of clathrin coats (AP2S1–eGFP as the master; hereafter denoted AP2–eGFP) and colocalizing actin filaments (LifeAct–mCherry as the slave), simultaneously. As expected, the LifeAct signal peaked during the later stages of clathrin-coated vesicle formation (Fig. 4D), and the axial velocity detected during the fast dissolution phase reduced significantly when the actin dynamics is inhibited upon jasplakinolide treatment (Fig. 4E). These results indicate that actin polymerization provides the inward force that is required for constriction of the neck under high membrane tension, a mechanism analogous to clathrin-mediated endocytosis in yeast (Aghamohammadzadeh and Ayscough, 2009).
Fig. 4.
Actin dynamics mediate the inward movement of clathrin coats prior to disassembly. (A) Mean±s.e.m. values for of normalized AP2 intensity traces (determined for a SUM159 cell before and after hypotonic swelling; ntraces=3728). The traces are aligned at the end point before averaging. (B) Mean±s.e.m. z displacements are shown for the two trace groups in A. (C) Top, growth rate distributions are assembled for eight SUM159 cells before and after hypotonic swelling (ntraces=30,409). Different growth phases (ff, fast formation; sf, slow formation; p, plateau; sd, slow dissolution; fd, fast dissolution) were determined by quantifying the change in the clathrin coat signal over 12-s-long time windows (Ferguson et al., 2016). Bottom, for the same cells, bar plots show the mean+s.e.m. of the z velocities of the trace fragments (12 s long) that are used to generate the growth rate distributions above. Trace fragments that have the highest z velocity are found in the fast dissolution (fd) phase. (D) Top, representative intensity traces of AP2 (green) and LifeAct (red) fluorescence during the formation of a clathrin-coated vesicle at the ventral surface of a BSC1 cell. Bottom, the relative LifeAct intensity (mean±s.e.m.) colocalizing with clathrin coats is shown for different growth phases (ncells=4, ntraces=28,795). Note that the growth phases are determined by using the master (AP2–eGFP) signal. (E) Bar plots show the z velocities (mean±s.e.m.) corresponding to different growth phases for AP2 traces obtained from BSC1 cells in the absence and presence of jasplakinolide (Jasp) (Control, ncells=7, ntraces=20,204; Jasp, ncells=6, ntraces=35,972). *P<0.0001; **P<0.001 (two-tailed t-test).
In this study, we used quantitative live-cell imaging in combination with diverse cell manipulation techniques to detect the changes in clathrin coat dynamics as cells undergo mechanical perturbations. This powerful approach allowed us to investigate the response of individual cells to mechanical stimuli in real time, rather than making a comparative analysis between different cells. Collectively, our assays reveal an inverse relationship between plasma membrane tension and endocytic clathrin coat dynamics. Increased tension manifests itself as reduced initiation and conclusion densities, elongated lifetime, and a reduced standard deviation of clathrin coat growth rates. These results suggest that the reduced density of clathrin-coated structures observed during mitosis (Aguet et al., 2016) and at the lamellae of migrating cells (Kural et al., 2015) can be a product of increased membrane tension (Fogelson and Mogilner, 2014; Kaur et al., 2014; Lieber et al., 2015; Raucher and Sheetz, 1999). Correspondingly, previously described feedback regulation between membrane tension and membrane-bending proteins in migrating cells (Tsujita et al., 2015) can explain the stark increase in clathrin coat density upon mechanical inhibition of cell protrusion (Movie 4).
Our results show that tension is an effective, fast-acting and reversible regulator of clathrin-mediated endocytosis. To induce hypotonic swelling, we reduced the osmolarity of the imaging medium to 63 mOsm. In a recent study, comparable changes in osmolarity are shown to increase the membrane tension ∼2-fold (Diz-Muñoz et al., 2016). This is within the boundaries of physiologically relevant variances in plasma membrane tension given that spreading of a cell results in an ∼3-fold reduction in membrane tension (Gauthier et al., 2009), and the tension at the apical surface of polarized cells is ∼2.5-fold higher than that at the basal surface (Dai and Sheetz, 1999). Compression of cells by surrounding mechanical cues has been proposed to control tissue morphogenesis at different stages of metazoan development (Desprat et al., 2008; Legoff et al., 2013; Rauskolb et al., 2014). In our cell-squeezing assays, we observed changes in clathrin-mediated endocytic activity even when the relative fold change in the cell area is lower than the levels detected in developmental processes associated with cell compression (Aegerter-Wilmsen et al., 2012) (Fig. S3). Dynamics and organization of the actin cytoskeleton were unperturbed in these assays (Movie 5). These findings suggest that morphological alterations involving mechanical forces within physiological contexts can induce abrupt changes in clathrin coat dynamics. Consequently, mechanoregulation of clathrin-mediated endocytosis can influence related biological processes that are central for development and homeostasis of multicellular organisms, such as signal transduction and cell shape regulation.
MATERIALS AND METHODS
Cell culture, reagents and fluorescence imaging
BSC1 cells stably expressing AP2–eGFP (gift of Steeve Boulant, Department of Infectious Diseases, Virology, Heidelberg University, Germany) were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, penicillin/streptomycin. SUM159 cell gene edited to express AP2–eGFP (Aguet et al., 2016) (gift of Tomas Kirchhausen, Departments of Cell Biology and Pediatrics, Harvard Medical School Boston, MA) were grown in F-12 medium containing 5% fetal bovine serum (FBS), penicillin-streptomycin and hydrocortisone. Transient expression of LifeAct–mCherry (gift of Patrick M. Reeves, Vaccine & Immunotherapy Center, Charlestown, MA) in BSC1 cells stably expressing AP2–eGFP was carried out using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions, and imaging was performed 24–48 h after transfection. The final jasplakinolide (Enzo Life Sciences) concentration used to inhibit actin dynamics was 1 µm.
The fluorescence imaging system is composed of an Eclipse TI-E microscope (Nikon) equipped with a perfect focusing system (PFS), a temperature-controlled chamber, a CSU-W1 spinning disk confocal unit (Yokogawa Electric Corporation), a 100× objective lens (Nikon CFI Plan-Apochromat Lambda, NA 1.45) and an EMCCD camera (iXon DU897 Ultra; Andor Technology). All image acquisition was performed by using NIS Elements software.
Imaging of cultured cells was performed 30 min after plating on glass bottom dishes (Greiner Bio-One) in the case of squeezing cells. The plating time prior to aspiration and osmotic shock experiments was 24 h. All cells were maintained at an ambient temperature of 37°C during imaging. Images were acquired at rate of 0.25–0.5 Hz with a laser exposure of 50–300 ms per frame. Imaging medium was Phenol Red-free L15 (Thermo Fisher Scientific) supplemented with 10% FBS. The snapshots and movies of fluorescence acquisitions are inverted to increase visibility.
Squeezing, micropipette aspiration and osmotic shock
In squeezing experiments, a 15 μl suspension of cells and imaging medium is plated in the middle of the imaging dish, forming a small droplet. A polydimethylsiloxane (PDMS) brick of ∼10 mm×10 mm×2.5 mm is placed on top of the droplet. The dish is capped and the cells are allowed to spread for ∼30 min (at this point cells are not in contact with the PDMS). After spreading, a micromanipulator (Narishige MMO-202ND, Narishige MMN-1) fitted with a rounded glass pipette tip is slowly brought into contact with the PDMS brick from above. The micromanipulator is used to press down on the PDMS while observing the cells under brightfield illumination. Fluorescence acquisitions start after the PDMS brick is brought into contact with the cells. The maximum level of compression is signaled by complete halting of clathrin coat activity. Further imaging is performed at various stages while the compression is released. In the microaspiration experiments, a microinjection system (BRE110/E; Sutter Instrument) was used to control the negative pressure applied on the dorsal surface of cells via a 5–10-μm-thick microneedle.
For osmotic shock experiments, SUM159 cells are cultured on four-well glass bottom plates (Fisher Scientific) and imaged every 3 s. At 5 min after the start of the experiment, 800 µl of ddH2O is added to the 200 µl of imaging medium to induce hypo-osmotic shock. The measured osmolarity level after this dilution is 63 mOsm. The cells are then imaged for another 20 min to study the cellular response. To compare clathrin coat dynamics before and after osmotic shock, two time windows are analyzed: the pre-osmoshock time window consists of the 5 min immediately prior to addition of water, whereas the post-osmoshock time window starts 2.5 min after water addition (to allow time for osmotic shock effects fully take hold) and runs for 5 min. For lifetime analyses, only traces whose mean time-point lies within the window are considered.
For the calculation of the cell volume, we used the 3D time-lapse spinning-disk confocal microscopy acquisitions. A custom MATLAB program was written to allow the user to select the boundary of the cell for each plane in a z-stack (available from the corresponding author upon request). The number of pixels inside of these boundaries was multiplied by the size of the pixels to determine the area of the cell in that stack. The volume was calculated by multiplying this area by the difference in position between the stacks and adding all those values together.
Single-particle tracking
cmeAnalysis software was used for two dimensional (2D) particle tracking (obtained from http://lccb.hms.harvard.edu/software.html) (Aguet et al., 2013). We used exclusionary criteria for the traces that last a single frame or persist consistently in the background without following a characteristic clathrin intensity profile (Ferguson et al., 2016). Selected traces are at least three frames long and contain a sequence which meets statistical criteria for demonstrating a linear increase or decrease in intensity (corresponding to clathrin coat growth and dissolution, respectively). For each group of three or four consecutive intensity points (three for traces <10 frames and four for longer traces), we performed a least-squares fit. Traces that had no fits with an r2 value >0.5 were rejected. Rejected traces were excluded from the calculation of initiation and dissolution densities, growth rate distributions, lifetime distributions and lifetime dipoles.
We used the traces that passed the rejection scheme to determine the average clathrin coat lifetime per frame. In each frame, we added together the lifetime of each trace that exists in that frame, and divided by the number of traces considered. The beginning and end of each trace is considered as an initiation and conclusion event, respectively. For each frame, initiation and conclusion densities (number/µm2/minute) were determined by finding the number of traces that begin and end in that frame, multiplying by the frame length (2–4 s), dividing by the visible cell area (in µm2) and by 60 s.
A custom MATLAB program was used for the master–slave analysis (available from the corresponding author upon request). The traces in the master channel were determined by using the cmeAnalysis software as described above. To quantify the intensity in the slave channel, we determined the average intensity in a 5×5 pixel region around the structure, and then subtracted the background intensity, which was calculated as the average intensity of the outside pixels of the 7×7 pixel region around the structure.
3D traces and growth rate distributions of clathrin coats were determined as described previously (Ferguson et al., 2016). z-velocities are calculated for each 12-s long trace fragments, which were used to determine the corresponding clathrin growth rates.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: J.P.F., S.D.H., C.K.; Methodology: J.P.F., S.D.H.; Software: J.P.F., S.D.H., N.M.W.; Formal analysis: J.P.F., S.D.H., N.M.W.; Investigation: J.P.F., S.D.H., N.M.W., E.A., S.G., T.A.; Resources: C.K.; Writing - original draft: C.K.; Writing - review & editing: C.K.; Visualization: C.K.; Supervision: C.K.
Funding
This work was supported by the National Institutes of Health (R01 AI121124). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.205930.supplemental
References
- Aegerter-Wilmsen T., Heimlicher M. B., Smith A. C., de Reuille P. B., Smith R. S., Aegerter C. M. and Basler K. (2012). Integrating force-sensing and signaling pathways in a model for the regulation of wing imaginal disc size. Development 139, 3221-3231. 10.1242/dev.082800 [DOI] [PubMed] [Google Scholar]
- Aghamohammadzadeh S. and Ayscough K. R. (2009). Differential requirements for actin during yeast and mammalian endocytosis. Nat. Cell Biol. 11, 1039-1042. 10.1038/ncb1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguet F., Antonescu C. N., Mettlen M., Schmid S. L. and Danuser G. (2013). Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev. Cell 26, 279-291. 10.1016/j.devcel.2013.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguet F., Upadhyayula S., Gaudin R., Chou Y.-Y., Cocucci E., He K., Chen B.-C., Mosaliganti K., Pasham M., Skillern W. et al. (2016). Membrane dynamics of dividing cells imaged by lattice light-sheet microscopy. Mol. Biol. Cell 27, 3418-3435. 10.1091/mbc.E16-03-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant S., Kural C., Zeeh J.-C., Ubelmann F. and Kirchhausen T. (2011). Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat. Cell Biol. 13, 1124-1131. 10.1038/ncb2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E., Gaudin R. and Kirchhausen T. (2014). Dynamin recruitment and membrane scission at the neck of a clathrin-coated pit. Mol. Biol. Cell 25, 3595-3609. 10.1091/mbc.E14-07-1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A., Warrington A., Taylor K. A. and Svitkina T. (2011). Structural organization of the actin cytoskeleton at sites of clathrin-mediated endocytosis. Curr. Biol. 21, 1167-1175. 10.1016/j.cub.2011.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J. and Sheetz M. P. (1999). Membrane tether formation from blebbing cells. Biophys. J. 77, 3363-3370. 10.1016/S0006-3495(99)77168-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprat N., Supatto W., Pouille P.-A., Beaurepaire E. and Farge E. (2008). Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell 15, 470-477. 10.1016/j.devcel.2008.07.009 [DOI] [PubMed] [Google Scholar]
- Diz-Muñoz A., Thurley K., Chintamen S., Altschuler S. J., Wu L. F., Fletcher D. A. and Weiner O. D. (2016). Membrane tension acts through PLD2 and mTORC2 to limit actin network assembly during neutrophil migration. PLoS Biol. 14, 1-30. 10.1371/journal.pbio.1002474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. P., Willy N. M., Heidotting S. P., Huber S. D., Webber M. J. and Kural C. (2016). Deciphering dynamics of clathrin-mediated endocytosis in a living organism. J. Cell Biol. 214, 347-358. 10.1083/jcb.201604128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson B. and Mogilner A. (2014). Computational estimates of membrane flow and tension gradient in motile cells. PLoS ONE 9, e84524 10.1371/journal.pone.0084524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier N. C., Rossier O. M., Mathur A., Hone J. C. and Sheetz M. P. (2009). Plasma membrane area increases with spread area by exocytosis of a GPI-anchored protein compartment. Mol. Biol. Cell 20, 3261-3272. 10.1091/mbc.E09-01-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinger J. E., Oster G., Drubin D. G. and Rangamani P. (2017). Design principles for robust vesiculation in clathrin-mediated endocytosis. Proc. Natl. Acad. Sci. USA 114, E1118-E1127. 10.1073/pnas.1617705114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herant M., Heinrich V. and Dembo M. (2005). Mechanics of neutrophil phagocytosis: behavior of the cortical tension. J. Cell Sci. 118, 1789-1797. 10.1242/jcs.02275 [DOI] [PubMed] [Google Scholar]
- Houk A. R., Jilkine A., Mejean C. O., Boltyanskiy R., Dufresne E. R., Angenent S. B., Altschuler S. J., Wu L. F. and Weiner O. D. (2012). Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell 148, 175-188. 10.1016/j.cell.2011.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S., Fielding A. B., Gassner G., Carter N. J. and Royle S. J. (2014). An unmet actin requirement explains the mitotic inhibition of clathrin-mediated endocytosis. Elife 3, e00829 10.7554/eLife.00829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kural C., Akatay A. A., Gaudin R., Chen B.-C., Legant W. R., Betzig E. and Kirchhausen T. (2015). Asymmetric formation of coated pits on dorsal and ventral surfaces at the leading edges of motile cells and on protrusions of immobile cells. Mol. Biol. Cell 26, 2044-2053. 10.1091/mbc.E15-01-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legoff L., Rouault H. and Lecuit T. (2013). A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development 140, 4051-4059. 10.1242/dev.090878 [DOI] [PubMed] [Google Scholar]
- Lieber A. D., Schweitzer Y., Kozlov M. M. and Keren K. (2015). Front-to-rear membrane tension gradient in rapidly moving cells. Biophys. J. 108, 1599-1603. 10.1016/j.bpj.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D. and Sheetz M. P. (1999). Membrane expansion increases endocytosis rate during mitosis. J. Cell Biol. 144, 497-506. 10.1083/jcb.144.3.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb C., Sun S., Sun G., Pan Y. and Irvine K. D. (2014). Cytoskeletal tension inhibits hippo signaling through an ajuba-warts complex. Cell 158, 143-156. 10.1016/j.cell.2014.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M., Morlot S., Hohendahl A., Manzi J., Lenz M. and Roux A. (2015). A balance between membrane elasticity and polymerization energy sets the shape of spherical clathrin coats. Nat. Commun. 6, 6249 10.1038/ncomms7249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P. (2001). Cell control by membrane-cytoskeleton adhesion. Nat. Rev. Mol. Cell Biol. 2, 392-396. 10.1038/35073095 [DOI] [PubMed] [Google Scholar]
- Tsujita K., Takenawa T. and Itoh T. (2015). Feedback regulation between plasma membrane tension and membrane-bending proteins organizes cell polarity during leading edge formation. Nat. Cell Biol. 17, 749-758. 10.1038/ncb3162 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.