Abstract
Background
Delayed graft function (DGF) is a common complication that impairs allograft function after kidney transplantation. However, the mechanism of DGF remains unclear. Nuclear magnetic resonance (NMR)-based analysis has been widely used in recent times to assess changes in metabolite levels.
Material/Methods
Samples of perfusate from allografts donated after circulatory death were collected prior to transplantation, during static cold storage. 1H-NMR-based metabolomics combined with the statistical methods, orthogonal partial least-squares discriminant analysis (OPLS-DA), and principle-component analysis (PCA), were employed to test different levels of metabolites between the allografts that exhibited DGF and those that exhibited immediate graft function (IGF).
Results
The study population consisted of 36 subjects, 11 with DGF and 25 with IGF. Of the 37 detected and identified metabolites, α-glucose and citrate were significantly elevated in the perfusate of DGF allografts, and taurine and betaine were significantly decreased.
Conclusions
1H-NMR analysis of DGF and IGF perfusates revealed some significant differences in their metabolite profiles, which may help explain the mechanisms of kidney ischemia-reperfusion injury and DGF.
MeSH Keywords: Delayed Graft Function, Kidney Transplantation, Magnetic Resonance Spectroscopy
Background
Delayed graft function (DGF) is usually defined based on the need for dialysis during the first week after kidney transplantation; excluding dialysis for hyperkalemia in the first 24 hours. It is increasingly recognized as a serious complication of kidney transplantation that can adversely affect the survival of the graft and transplant recipients [1,2]. According to one study, recipients who experienced DGF had a 41% greater risk of graft loss at a mean follow-up time of 3.2 years [3]. DGF rates have been increasing in parallel with increased use of donation-after-circulatory-death (DCD) kidneys, therefore it is likely to remain a significant clinical challenge in terms of patient quality of life and long-term allograft survival [4]. Accurate evaluation of allograft quality is essential for surgeons to give a short-term prognosis for recipients and allografts, and thereby determine the appropriate perioperative care. However, there are few biomarkers and evaluation parameters which are routinely applied in clinical practice.
Metabolomics entails studying the metabolite profiles of biological fluids and extracts from cell or tissue, and it has been extensively applied for diagnosis and evaluation of some human pathologies, such as cancer, diabetes, neurological conditions and heart disease [5–8]. Owing to developments in diagnostic instrumentation, many analytical methods are now available for detecting and quantifying metabolites; these include nuclear magnetic resonance (NMR), gas chromatography/mass spectrometry (GC/MS) and liquid chromatography/mass spectrometry (LC/MS). Metabolomic analysis using proton nuclear magnetic resonance (1H-NMR) has shown a high degree of reproducibility, and can non-destructively and non-selectively detect and quantify multiple classes of metabolites. It has therefore become a preferred platform for quantitative spectral acquisition and comprehensive profiling of proton-containing low-molecular-weight metabolites [9].
In the present study, we collected perfusate samples from kidney allografts prior to transplantation, and used 1H-NMR-based metabolomic analysis of these to compare kidneys exhibiting DGF upon transplantation to those exhibiting immediate graft function (IGF; controls).
Material and Methods
Ethics statement
The study protocol was in accordance with the ethical standards of the Declarations of Helsinki and Istanbul. Being limited to donations after circulatory death, the protocol of this study was approved by the local Ethics Committee of the First Affiliated Hospital of Nanjing Medical University, and written informed consent was obtained from all transplant recipients. None of the transplant donors were from a vulnerable population, and written informed consent were received from each of patients.
Sample collection
Adult kidneys acquired by DCD and accepted for transplantation at the kidney transplantation center of the First Affiliated Hospital of Nanjing Medical University between October 2014 and June 2015 were included. All allografts arrived at our transplant center in static cold storage, and demographic and clinical data for the donors were recorded.
Graft perfusion was performed 30 min before the transplantation surgery, with 1 L of hypertonic citrate adenine II solution at 0–4°C and a perfusion pressure of 100 cm H2O. No additional oxygen or glutathione (GSH) was supplied. After repair of the allograft was completed, 15 ml of the perfusate was sampled from the first outflow of the allograft renal vein. After high-speed centrifugation at 3000 rpm for 10 min, the supernatant of the perfusate was transferred to a cryogenic vial and stored at −80°C for further experiments.
Sample preparation and 1H-NMR analysis
We centrifuged the perfusate samples at 12,000 rpm for 10 min prior to NMR analysis. Then, we mixed buffer solution and sodium 3-trimethylsilyl-(2,2,3,3-D4)propionate (150 μL; Sigma-Aldrich, MO, USA) in D2O with 300 μL of supernatant from each perfusate sample. Finally, we collected 550 μL aliquots from the mixtures and transferred into NMR tubes.
AV 500 MHz spectrometer (Bruker, MA, USA) was used to detect 1H-NMR spectra. A transverse relaxation-edited Carr-Purcell-Meiboom-Gill sequence [90-(τ-180-τ)n-acquisition] with a total spin echo delay (2nτ) of 10 ms was employed to decay broad signals. Then, 1H-NMR spectra were measured using the following parameters: spectral width, 7500 MHz; number of sampling points, 32 K; relaxation delay time, 2 s; scanning time, 128 s. Finally, we phased the baseline correction of the spectra manually using the TOPSPIN 3.0 package (Bruker Biospin, Germany). All 1H-NMR spectrograms were obtained from the same spectrometer.
Statistical analysis
Based on shifts corrected by the TSP signal, the spectra of 1H-NMR were aligned into integrated segments with 0.005 ppm widths (4.25–4.7 and 4.73–5.28 ppm). Then, principle component analysis (PCA) and orthogonal partial least-squares discriminant analysis (OPLS-DA) were applied to determine differences in the metabolite patterns of these 2 perfusate groups. In addition, corresponding loading plots were used to provide variable quantities. Taking component indices of subjects in both groups as study factors, SPSS 13.0 was used to process general data (SPSS Inc., IL, USA). The measured data was presented as mean ± standard deviation (SD), and a t-test was conducted for inter-group comparison of individual metabolites. Statistically significant was identified when P<0.05.
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of the subjects are summarized in Table 1. In all, 36 subjects were included: 11 with allografts that showed DGF after kidney transplantation and 25 with allografts showing IGF. There were no significant differences in the characteristics of donors and recipients between the DGF and IGF groups.
Table 1.
Baseline charaterisctics of subjects included in this study.
| IGF group | DGF group | P value | |
|---|---|---|---|
| Number | 25 | 11 | NS |
| Donor information | |||
| Age (years, mean ± SD) | 39.72±2.00 | 40.73±1.01 | NS |
| Male (%) | 92.00 | 90.91 | NS |
| Warm ischemia time (min, mean ±SD) | 2.240±0.24 | 3.455±0.98 | NS |
| Cold ischemia time (hour, mean ±SD) | 9.040±0.48 | 9.273±0.60 | NS |
| Causes of death | NS | ||
| Brain tumor | 1 | 1 | |
| Brain trauma | 20 | 9 | |
| Cerebral hemorrhage | 4 | 1 | |
| Recipient information | |||
| Age (years, mean ±SD) | 37.94±1.93 | 39.15±1.42 | NS |
| Male (%) | 60.00 | 54.55 | NS |
| PRA (%) | 0 | 0 | – |
IGF – immediate graft function; DGF – delayed graft dysfunction; NS – not significant; SD – standard deviation; PRA – panel reactive antibody.
Metabolite profiling
Representative 1H-NMR spectra for perfusate samples from both groups are shown in Figure 1. We assigned and identified 37 metabolites, whose inter-group variation is presented in Table 2.
Figure 1.
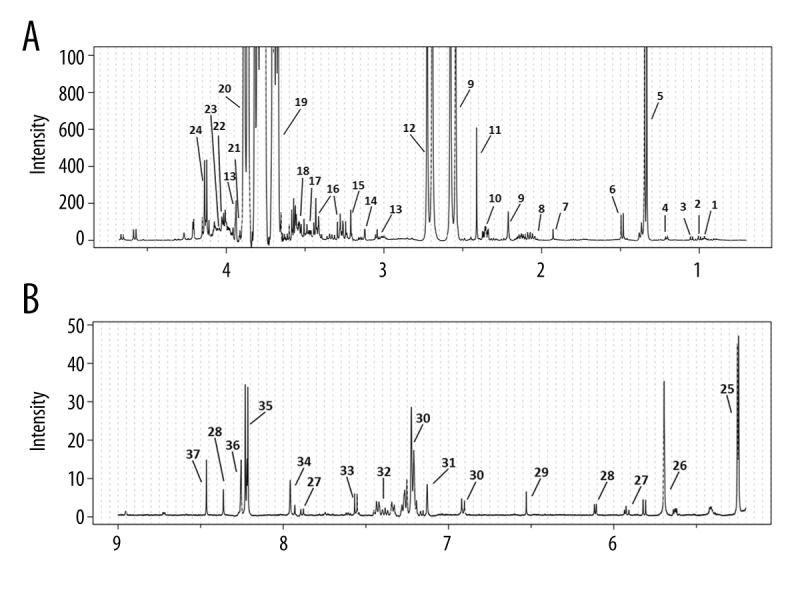
(A, B) Representative 500 MHz 1H-NMR spectra of perfusate samples obtained from renal allografts that exhibited either delayed or immediate function upon transplantation (DGF and IGF, respectively).
Table 2.
Metabolites detected in the perfute of IGF and DGF allograft.
| No. | Metabolites | Chemical shifts (ppm) | Changes in IGF group vs. DGF group |
|---|---|---|---|
| 1 | Isoleucine | 0.88–0.94 | – |
| 2 | Leucine | 0.95–0.98 | – |
| 3 | Valine | 0.99–1.02; 1.03–1.06 | – |
| 4 | 3-hydroxybutyrate | 1.19–1.23 | – |
| 5 | Lactic acid | 1.32–1.38; 4.11–4.14 | Down |
| 6 | Alanine | 1.46–1.52 | Dwon |
| 7 | Acetate | 1.92–1.94 | Down |
| 8 | NAG | 2.05–2.15 | – |
| 9 | GSH | 2.2–2.25; 2.55–2.58; 4.56–4.60 | Down |
| 10 | Glutamate | 2.33–2.39 | Down |
| 11 | Succinate | 2.41–2.42 | Down |
| 12 | Citrate | 2.65–2.75 | Down* |
| 13 | Creatine | 3.03–3.04; 3.92–3.95 | Down |
| 14 | Ethanolamine | 3.11–3.14 | Down |
| 15 | O-Acetylcholine | 3.20–3.23 | Down |
| 16 | Taruine | 3.30–3.32; 3.40–3.45 | Up* |
| 17 | β-glucose | 3.46–3.51 | Up |
| 18 | Glycine | 3.50–3.60 | Up |
| 19 | α-glucose | 3.60–3.88 | Down* |
| 20 | Bet | 3.87–3.90 | Up* |
| 21 | Glycolate | 3.91–3.92 | Up |
| 22 | Isocitrate | 4.01–4.05 | Up |
| 23 | Myo-inositol | 4.06–4.08 | Up |
| 24 | O-Phosphoserine | 4.08–4.10; 4.16–4.20 | Up |
| 25 | Unsaturated lipid | 5.24–5.26 | Down |
| 26 | UDP-galactose | 5.62–5.70 | Up |
| 27 | Uridine | 5.89–5.95; 7.87–7.91 | Up |
| 28 | Inosine | 6.09–6.12; 8.35–8.37 | Up |
| 29 | Sodium fumarate dibasic | 6.52–6.54 | Up |
| 30 | Tyrosine | 6.89–6.92; 7.18–7.28 | Up |
| 31 | Histamine | 7.10–7.13 | Up |
| 32 | Phenylalanine | 7.30–7.46 | Down |
| 33 | Uracil | 7.54–7.57 | Down |
| 34 | Xanthine | 7.92–7.94 | Up |
| 35 | Oxypurinol | 8.20–8.23 | Down |
| 36 | Niacinamide | 8.26–8.28 | Up |
| 37 | Formate | 8.45–8.46 | Down |
DGF – delayed graft function; IGF – immediate graft function; NAG – N-acetyl-beta-D-glucusamidase; GSH – glutathione.
P value <0.05;
P value <0.01.
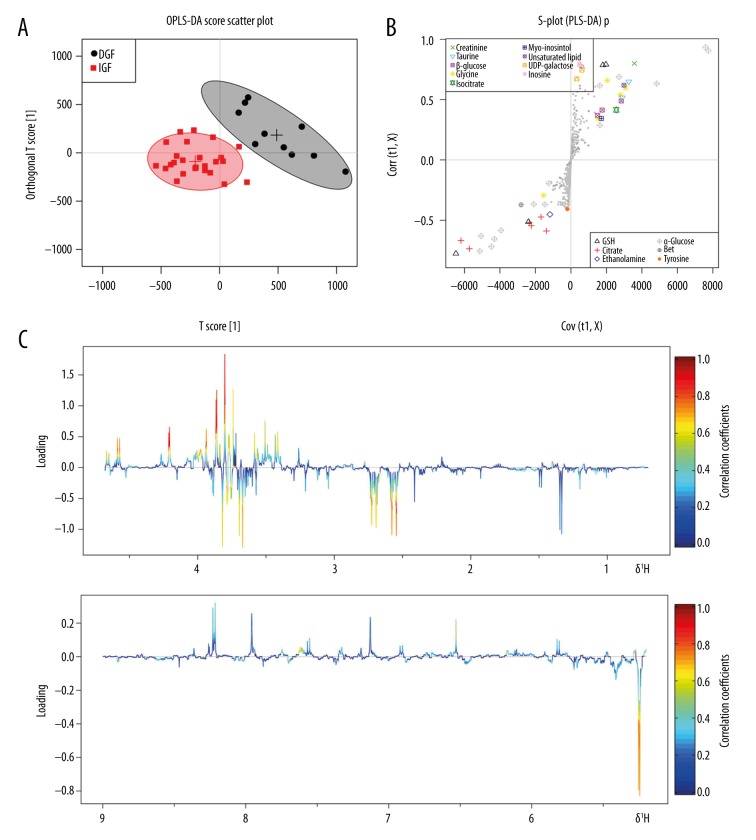
Figure 2A shows an OPLS-DA score plot of the 1H-NMR data, which was used to distinguish between DGF and IGF samples. In the OPLS-DA score plot, each point identifies a sample and each cluster represents a corresponding metabolic pattern. Significant separation was found between DGF and IGF samples (R2Y=0.922; Q2=0.910). The color-coded plot corresponded with coefficient-loading values visualized the influence of each metabolite between the DGF and IGF groups (Figure 2C). Levels of metabolites with positive correlation coefficients were lower in the DGF group than in the IGF group, whereas those with negative correlation coefficients were higher in the DGF group. Combining the coefficient-loading plot and an S-plot from the OPLS-DA analysis (Figure 2B), we found that the levels of 4 metabolites, citrate, α-glucose, betaine and taurine, were significantly different between the 2 groups (p<0.05; Table 2).
Figure 2.
Scores plot, S-plot, and color-coded loadings plot for orthogonal partial least-squares discriminant analysis (OPLS-DA) of 1H-NMR data from renal-allograft perfusates. (A) Scores plot where each point represents 1 perfusate sample. (B) S-plot of perfusate samples. (C) Color-coded loadings plot shows metabolites that differed between the delayed (DGF) and immediate (IGF) graft-function groups (red, higher concentration; blue, lower concentration).
Discussion
DGF is strongly associated with an increased risk of allograft loss after renal transplantation. DGF is becoming increasingly important, as the number of patients awaiting kidney transplantations is growing and there has been a great rise in the rate of donations after circulatory death in recent years in China. The current literature does not contain adequate evidence to provide a comprehensive mechanism for the development of DGF after kidney transplantation. Changes in perfusate compositions during static cold storage (SCS) may represent ongoing cell processes, or products of metabolism or degradation, being released from the kidneys. Metabolic profiling has been used to determine biomarkers for drug safety and efficacy, as well as for disease diagnosis (10). In this study, we applied the 1H NMR-based approach to evaluate the perfusate of allografts with DGF, and found that 4 important endogenous metabolites, citrate, α-glucose, betaine and taurine, were significantly associated with the occurrence of DGF.
Ischemia-reperfusion (IR) injury, which is a multifactorial pathogenesis, plays a central role in the development of DGF in allografts [11]. IR injury is attributed to several processes triggered by the initial deprivation of oxygen and nutrients, along with the concomitant accumulation of metabolic waste products. Further damage is then done as a result of reperfusion products [12,13]. Similar to how gene and protein levels often correlate with the activity of specific biochemical pathways and mechanisms, metabolite levels often correlate with processes such as cell metabolism, tissue oxygenation and oxidative stress, as well as general homeostasis [14]. When compared to those of the IGF group, the DGF perfusates showed a significant increase in their α-glucose concentrations. Since α-glucose is one of the main components of the total glucose content, we inferred that total glucose would also likely have increased in the DGF group; this would indicate more active glucose metabolism in the DGF allografts during SCS. A potential role for glucose metabolism in IR injury is suggested by the results of Chang et al. [15], who found that Dapagliflozin, an antidiabetic inhibitor of sodium/glucose cotransporter 2 (SGLT2), could attenuate IR injury. Moreover, hyperglycemia has been shown to exacerbate kidney IR injury and accelerate renal dysfunction, with several molecular pathways being profoundly affected by the hyperglycemia that occurs prior to renal IR injury [16,17]. However, in the absence of samples and clinical measures from the donors, it was impossible to determine whether the glucose increase was due to IR injury or a prior insult.
Along with increased α-glucose, we found that DGF perfusates exhibited significantly elevated citrate and reduced levels of taurine and betaine. This suggests notable IR injury in the more ischemically damaged DGF allografts. There is evidence that anoxia, ischemia and infarction can lead to rapid loss of high-energy phosphates and accumulation of hydrolysis products, such as citrate, lactate and β-hydroxybutyrate; thus, allografts with higher levels of citrate may have suffered greater IR injury and thus be more susceptible to the occurrence of DGF after transplantation [18–20].
Even more importantly, IR injury could significantly impact osmoregulation, as reflected by the altered levels of osmolytes, like taurine and betaine, that have been demonstrated in both the present study and in previous ones [21,22]. Taurine is a ubiquitous free amino acid which is present in many tissues and is involved in various physiological processes, such as osmoregulation, antioxidant activity and hepatic detoxification [23,24]. There is strong evidence that taurine benefits ischemic reperfused kidneys by exerting a number of cytoprotective effects, such as a purported antioxidant effect, membrane stabilization, cellular osmoregulation and antiapoptotic effects [25,26]. In addition, taurine is known to be an important modulator and regulator of renal function, and it contributes to body-fluid and electrolyte homeostasis. This suggests a critical role for taurine in renal IR injury [27,28]. Like to taurine, the most important physiological roles for betaine are as an osmolyte and a methyl donor [29]. As an osmolyte, betaine protects cells, proteins, and enzymes from environmental stresses, such as high salinity, low water and oxidative attack [30]. Numerous studies have demonstrated the protective role of betaine during IR injury [21,31–33]. In our study, the DGF allografts showed lower taurine and betaine levels, so they may have suffered IR injury due, at least in part, to the loss of taurine and betaine’s protective effects.
In 2014, Guy et al. [34] studied the metabolite profiles of perfusates from 26 cadaveric cases, during hypothermic machine perfusion (HMP) of cadaveric kidneys using NMR spectroscopy. Their results showed that glucose, inosine, leucine and gluconate were significantly different in DGF group, which differed from ours in that the DGF group exhibited significant decreases in glucose, inosine, leucine and gluconate. This discrepancy could be explained by a number of methodological differences between the studies, including the details of measurement instruments, the application of HMP and the use of different preservation solutions.
Although our metabolomic analysis achieved significant results, it had some limitations. First, during SCS and perfusion of the allografts, we applied a hypertonic citrate adenine (HC-A) II solution, which, in China, is the most widely used kidney-preservation solution. HC-A II provides similar efficacy and safety to classical HTK solutions [35], but along with dihydrogen phosphate, hydroxide phosphate, adenine and mannitol, it contains citrate and is therefore a potential confounding factor for the elevated citrate we observed in the DGF perfusates. Second, our sample population was too small owing to the strict inclusion and exclusion criteria used. A large number of subjects were ruled out because of particular details relating to the donation; for example, the recipient exhibited acute rejection or the presence of panel reactive antibodies, the donor was a child, the donor had a viral infection or abnormal blood pressure, the donor had received excessive administration of nephrotoxic drugs. Owing to the small number of eligible subjects, we were unable to evaluate the predictive role of the altered metabolites in the diagnosis of DGF. Therefore, a prospective large-scale study is needed to confirm our conclusions.
Conclusions
In summary, an integrated NMR-based analysis of perfusates during SCS was employed to obtain the metabolite profiles of DGF and IGF allografts. Metabolomic analysis revealed significant increases in α-glucose and citrate levels, and significant decreases in taurine and betaine levels. These metabolomic perturbations may prove useful in explaining the mechanisms of kidney IR injury and DGF. Due to some limitations, larger-scale prospective studies should be conducted to confirm our results and their diagnostic and prognostic power.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the National Natural Science Foundation of China [grant numbers 81570676, 81100532, and 81470981]; the Science and Education Health Project of Jiangsu Province for Important Talent [grant number RC2011055]; the “333 High Level Talents Project” in Jiangsu Province, China [grant numbers BRA2015469 and BRA2016514 (2011 and 2013)]; the Standardized Diagnosis and Treatment Research Program of Key Diseases in Jiangsu Province, China [grant number BE2016791]; the Open Project Program of Health Department of Jiangsu Province, China [grant number JSY-2-2016-099]; the Jiangsu Province Six Talents Peak from Department of Human Resources, Social Security Office of Jiangsu Province, China [grant numbers 2010WSN-56, 2011-WS-033]; the General Program of Health Department of Jiangsu Province, China [grant number H2009907]; and the Priority Academic Program Development of Jiangsu Higher Education Institutions [grant number JX10231801]
References
- 1.Tugmen C, Sert I, Kebabci E, et al. Delayed graft function in kidney transplantation: Risk factors and impact on early graft function. Prog Transplant. 2016;26(2):172–77. doi: 10.1177/1526924816640978. [DOI] [PubMed] [Google Scholar]
- 2.Troppmann C, Gillingham KJ, Benedetti E, et al. Delayed graft function, acute rejection, and outcome after cadaver renal transplantation. The multivariate analysis. Transplantation. 1995;59(7):962–68. doi: 10.1097/00007890-199504150-00007. [DOI] [PubMed] [Google Scholar]
- 3.Yarlagadda SG, Coca SG, Formica RN, Jr, et al. Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24(3):1039–47. doi: 10.1093/ndt/gfn667. [DOI] [PubMed] [Google Scholar]
- 4.Tahir W, Hakeem A, Dawrant M, et al. Early sirolimus conversion as rescue therapy in kidneys with prolonged delayed graft function in deceased donor renal transplant. Transplant Proc. 2015;47(6):1610–15. doi: 10.1016/j.transproceed.2015.04.102. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig C, Ward DG, Martin A, et al. Fast targeted multidimensional NMR metabolomics of colorectal cancer. Magn Reson Chem. 2009;47(Suppl 1):S68–73. doi: 10.1002/mrc.2519. [DOI] [PubMed] [Google Scholar]
- 6.Narath SH, Mautner SI, Svehlikova E, et al. An untargeted metabolomics approach to characterize short-term and long-term metabolic changes after bariatric surgery. PLoS One. 2016;11(9):e0161425. doi: 10.1371/journal.pone.0161425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcinkiewicz-Siemion M, Ciborowski M, Kretowski A, et al. Metabolomics – A wide-open door to personalized treatment in chronic heart failure? Int J Cardiol. 2016;219:156–63. doi: 10.1016/j.ijcard.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Weiss N, Hilaire PB, Colsch B, et al. Cerebrospinal fluid metabolomics highlights dysregulation of energy metabolism in overt hepatic encephalopathy. J Hepatol. 2016;65(6):1120–30. doi: 10.1016/j.jhep.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Sui W, Che W, et al. 1H NMR-based metabolic profiling of human serum before and after renal transplantation. ASAIO J. 2013;59(3):286–93. doi: 10.1097/MAT.0b013e31828e2d9f. [DOI] [PubMed] [Google Scholar]
- 10.Schnackenberg LK, Beger RD. Metabolomic biomarkers: Their role in the critical path. Drug Discov Today Technol. 2007;4(1):13–16. doi: 10.1016/j.ddtec.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Cavaille-Coll M, Bala S, Velidedeoglu E, et al. Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant. 2013;13(5):1134–48. doi: 10.1111/ajt.12210. [DOI] [PubMed] [Google Scholar]
- 12.Abu Jawdeh BG, Rabb H. Delayed kidney allograft function – what does it tell us about acute kidney injury? Contrib Nephrol. 2011;174:173–81. doi: 10.1159/000329395. [DOI] [PubMed] [Google Scholar]
- 13.Kezic A, Spasojevic I, Lezaic V, Bajcetic M. Mitochondria-targeted antioxidants: future perspectives in kidney ischemia reperfusion injury. Oxid Med Cell Longev. 2016;2016:2950503. doi: 10.1155/2016/2950503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberholzer J, Testa G, Sankary H, et al. Kidney transplantation at the University of Illinois at Chicago from 1988–2004. Clin Transpl. 2004:143–49. [PubMed] [Google Scholar]
- 15.Chang YK, Choi H, Jeong JY, et al. Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS One. 2016;11(7):e0158810. doi: 10.1371/journal.pone.0158810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirose R, Xu F, Dang K, et al. Transient hyperglycemia affects the extent of ischemia-reperfusion-induced renal injury in rats. Anesthesiology. 2008;108(3):402–14. doi: 10.1097/ALN.0b013e318164cff8. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Chen H, Wang L, et al. Acute hyperglycemia prevents dexmedetomidine-induced preconditioning against renal ischemia-reperfusion injury. Acta Cir Bras. 2014;29(12):812–18. doi: 10.1590/S0102-86502014001900008. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Bai J, Chen G, et al. A metabolic profiling analysis of the acute hepatotoxicity and nephrotoxicity of Zhusha Anshen Wan compared with cinnabar in rats using (1)H NMR spectroscopy. J Ethnopharmacol. 2013;146(2):572–80. doi: 10.1016/j.jep.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Maloyan A, Eli-Berchoer L, Semenza GL, et al. HIF-1alpha-targeted pathways are activated by heat acclimation and contribute to acclimation-ischemic cross-tolerance in the heart. Physiol Genomics. 2005;23(1):79–88. doi: 10.1152/physiolgenomics.00279.2004. [DOI] [PubMed] [Google Scholar]
- 20.Cai Z, Manalo DJ, Wei G, et al. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation. 2003;108(1):79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- 21.Wettstein M, Haussinger D. Taurine attenuates cold ischemia-reoxygenation injury in rat liver. Transplantation. 2000;69(11):2290–96. doi: 10.1097/00007890-200006150-00012. [DOI] [PubMed] [Google Scholar]
- 22.Wettstein M, Haussinger D. Cytoprotection by the osmolytes betaine and taurine in ischemia-reoxygenation injury in the perfused rat liver. Hepatology. 1997;26(6):1560–66. doi: 10.1053/jhep.1997.v26.pm0009397998. [DOI] [PubMed] [Google Scholar]
- 23.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72(1):101–63. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 24.Chesney RW, Han X, Patters AB. Taurine and the renal system. J Biomed Sci. 2010;17(Suppl 1):S4. doi: 10.1186/1423-0127-17-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaffer S, Azuma J, Takahashi K, Mozaffari M. Why is taurine cytoprotective? Adv Exp Med Biol. 2003;526:307–21. doi: 10.1007/978-1-4615-0077-3_39. [DOI] [PubMed] [Google Scholar]
- 26.Takatani T, Takahashi K, Uozumi Y, et al. Taurine prevents the ischemia-induced apoptosis in cultured neonatal rat cardiomyocytes through Akt/caspase-9 pathway. Biochem Biophys Res Commun. 2004;316(2):484–89. doi: 10.1016/j.bbrc.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 27.Mozaffari MS, Schaffer D. Taurine modulates arginine vasopressin-mediated regulation of renal function. J Cardiovasc Pharmacol. 2001;37(6):742–50. doi: 10.1097/00005344-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Mozaffari MS, Abdelsayed R, Patel C, et al. Differential effects of taurine treatment and taurine deficiency on the outcome of renal ischemia reperfusion injury. J Biomed Sci. 2010;17(Suppl 1):S32. doi: 10.1186/1423-0127-17-S1-S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80(3):539–49. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Roig S, Cavalle-Busquets P, Fernandez-Ballart JD, et al. Low folate status enhances pregnancy changes in plasma betaine and dimethylglycine concentrations and the association between betaine and homocysteine. Am J Clin Nutr. 2013;97(6):1252–59. doi: 10.3945/ajcn.112.054189. [DOI] [PubMed] [Google Scholar]
- 31.Vali L, Stefanovits-Banyai E, Szentmihalyi K, et al. Liver-protecting effects of table beet (Beta vulgaris var. rubra) during ischemia-reperfusion. Nutrition. 2007;23(2):172–78. doi: 10.1016/j.nut.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Niemann CU, Choi S, Behrends M, et al. Mild hypothermia protects obese rats from fulminant hepatic necrosis induced by ischemia-reperfusion. Surgery. 2006;140(3):404–12. doi: 10.1016/j.surg.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol. 2008;23(Suppl 1):S16–24. doi: 10.1111/j.1440-1746.2007.05276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guy AJ, Nath J, Cobbold M, et al. Metabolomic analysis of perfusate during hypothermic machine perfusion of human cadaveric kidneys. Transplantation. 2015;99(4):754–59. doi: 10.1097/TP.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 35.Sui M, Zhang L, Yang J, et al. A new HC-A II solution for kidney preservation: A multi-center randomized controlled trial in China. Ann Transplant. 2014;19:614–20. doi: 10.12659/AOT.892250. [DOI] [PubMed] [Google Scholar]