Abstract
Background and objectives
Among the cancers of the urogenital system, bladder cancer is ranked second both in incidence and mortality, and hence, a more accurate estimate of the prognosis for individual patients with non-muscle-invasive bladder cancer (NMIBC) is urgently needed. Prognostic nutritional index (PNI) which is based on serum albumin levels and peripheral lymphocyte count has been confirmed to have prognostic value in various cancers. The aim of this study was to clarify the prognostic value of PNI in patients with NMIBC.
Methods
Data of 329 patients with NMIBC were evaluated retrospectively. Recurrence-free survival (RFS) was assessed using the Kaplan–Meier method, and the equivalences of survival curves were tested by log-rank tests. The univariate and multivariate analyses were performed using the Cox proportional hazards regression model. Discrimination of the nomogram was measured by the concordance index. A p-value of <0.05 was considered statistically significant.
Results
In univariate analysis, age, tumor focality, tumor size, tumor grade, pathological T stage and preoperative PNI were significantly associated with RFS. Multivariate analysis identified PNI as an independent predictor of RFS in patients with NMIBC. According to these independent predictors, a nomogram for the prediction of recurrence was developed.
Conclusion
PNI can be regarded as an independent prognostic factor for predicting RFS in NMIBC. The nomogram could be useful to improve personalized therapy for patients with NMIBC.
Keywords: non-muscle-invasive bladder cancer, prognostic nutritional index, nomogram, prognosis, recurrence-free survival
Introduction
Bladder cancer (BCa) is the 6th most commonly diagnosed cancer in the male population worldwide according to the latest published global cancer statistics.1 The majority of BCa occurs in men, and there is about a 10-fold variation in incidence rates internationally. Among the cancers of the urogenital system, BCa is ranked second both in incidence and mortality. Worldwide, the age-standardized mortality rate (per 100,000 person-years) of BCa was 3.2 among men versus 0.9 among women in 2012.1 Approximately 75% of patients with BCa present with a non-muscle-invasive bladder cancer (NMIBC).2 NMIBC includes Ta (noninvasive papillary carcinoma), T1 (tumor invading subepithelial connective tissue) and CIS (carcinoma in situ: “flat tumor”). The patients usually require follow-up chemotherapy or BCG treatment to prevent the tumor recurrence after surgery.3
Increasing evidence shows that nutritional deficiencies and systemic inflammatory response (SIR) might play important roles in the development and progression of human cancer.4,5 For example, neutrophil-to-lymphocyte ratio which is widely used as a biomarker of SIR has been reported to be associated with worse clinical outcomes in NMIBC.6 The prognostic nutritional index (PNI) which was described by Onodera et al is based on serum albumin levels and peripheral lymphocyte count,7 and several studies have confirmed its prognostic value in various cancers, such as hepatocellular carcinoma, lung cancer, renal cancer, colorectal carcinoma and so on.8–11
The most frequently used prognostic model for individual BCa patients is the European Organization for the Research and Treatment of Cancer-Genito-Urinary Cancer Group (EORTC-GUCG) risk scoring system; however, this system is mainly designed for European population. The nomogram, which is a statistical instrument accounting for numerous variables to predict the outcome of an individual patient, was reported by Hong et al for prediction of recurrence-free survival (RFS) in patients with NMIBC.12,13 However, the nomogram has limitations such as indefinite subgroup and lack of some hematologic indexes. Thus, a more accurate prognostic model for patients with NMIBC is needed.
To our knowledge, the value of PNI in predicting RFS in NMIBC has not been investigated yet. Thus, we decided to clarify the prognostic value of preoperative PNI among patients who had undergone transurethral resection of bladder tumor (TURBT) for NMIBC and establish a nomogram to predict RFS for individual patients after surgery.
Methods
Patients
This retrospective analysis included clinicopathological and follow-up data of 329 patients with NMIBC who had undergone TURBT at the Department of Urology at the Qilu Hospital of Shandong University from January 2008 to December 2013. Patients who met the following conditions were included in this study: (1) full data on preoperative peripheral lymphocyte count and serum albumin level were available; (2) absence of autoimmune disease and cancer in other systems, or received neo-adjuvant chemotherapy and radiotherapy; (3) pathological type was Ta or T1 urothelial carcinoma without CIS; (4) presence of primary NMIBC; (5) distant metastasis was excluded before surgery; (6) received intravesical chemotherapy by epirubicin or pirarubicin postoperatively, the regimen of which was once a week for 6–8 times and then once a month for at least 6 times; and (7) full data on follow-up were available. All clinical investigations in our study were conducted in accordance with the Declaration of Helsinki. This study was approved by the Institutional Ethics Committee of the Qilu Hospital of Shandong University. Written informed consent was obtained from all the patients in this study.
All patients underwent routine hematologic examination, computerized tomography, transabdominal ultrasound, cystoscopy, urinary cytology and/or tissue biopsy for diagnosis of NMIBC before surgery. Pathological T stages were uniformly adjusted according to the 2009 TNM classification which was approved by the Union Internationale Contre le Cancer (7th edition), and tumor grade was assessed based on the 1973 World Health Organization (WHO) classification guidelines.14 Preoperative baseline clinicopathological and laboratory data, such as age, gender, history of smoking, symptoms, tumor size and pathological type, were obtained from the electronic medical records and reviewed. Negative symptoms were defined as having no symptom related to BCa, and was only detected by routine examination, whereas positive symptoms were defined as having gross or microscopic hematuria, urgent urination, dysuresia or flank pain. The tumor size was defined as the longest diameter of the general postoperative pathological specimens. PNI was then calculated with the following formula as previously described: 10× serum albumin levels (g/dL) +0.005× peripheral lymphocyte count (per mm3).7
All patients were regularly followed up with physical examination, routine urine and blood tests, biochemical tests and cystoscopy every 3 months for the first 2 years, every 6 months in the next 3 years and yearly thereafter for more than 5 years, and excretory urograms and/or computed tomography scans were obtained every year for 5 years after TURBT.
RFS rates and EORTC-GUCG risk scoring system
Visible or suspicious lesions were removed by TURBT. Due to limited patients suffering BCa progression in this study, RFS was identified as the end point in this study. RFS was defined as the time interval from the date of the initial TURBT to the date of recurrence which was confirmed by histopathology. The most frequently used prognostic model for an individual patient with NMIBC is the EORTC-GUCG risk scoring system. According to this risk scoring system of disease recurrence and progression, the definitions of risk are as follows: (1) low risk: primary, solitary, Ta, low grade/G1, <3 cm and no CIS; (2) intermediate risk: all tumors not defined in the 2 adjacent categories (between the category of low and high risk); and (3) high risk: a) T1 tumor, b) high grade/G3 tumor, c) CIS, and d) multiple and recurrent and large (>3 cm) Ta G1G2 tumors (all conditions must be present in this point).15
Statistical analysis
The peripheral lymphocyte count, serum albumin level and PNI are shown as the mean and standard deviation (SD). Correlations between categorical variables were evaluated by the Pearson’s chi-square test or Fisher’s exact test in this study. The probable cutoff value for the PNI was determined by applying receiver operating curve (ROC) analysis, and the most optimal cutoff value was used for further analysis. In RFS analysis, the Kaplan–Meier method was used to evaluate the survival rates in different groups, and the equivalences of the survival curves were tested by log-rank tests. Besides, the Cox proportional hazards regression model was applied in univariate and multivariate analyses.
Based on the multivariable model, a nomogram was constructed to predict 1-, 3- and 5-year RFS. The nomogram provides a graphic representation linking an individual patient’s multivariable prognostic factors to the RFS probability of a patient with NMIBC. Discrimination is measured by the concordance index (c-index) which is the area under the curve of an ROC curve. A scale of 1.0 represents perfect predictions, and 0.5 is the equivalent of a coin toss. The calibration of the model is assessed visually with calibration plots. A 45° line indicates perfect calibration – when the predictive value of the model perfectly matches the patient’s actual risk. Any deviation above or below the 45° line indicates underprediction or overprediction, respectively. A 2-sided p-value was used in our analyses, and a p-value of <0.05 was considered to be statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS for Windows, version 23.0; IBM Corporation, Armonk, NY, USA) program and R (version 3.1.2; R Foundation, Vienna, Austria; https://www.r-project.org/).
Results
Baseline clinicopathological characteristics
Of the 367 patients with NMIBC who underwent TURBT at our institution from January 2008 to December 2013, 38 were excluded as they did not meet the inclusion criteria. The mean (SD) follow-up duration was 43.9 (27.1) months. The clinicopathological characteristics of 329 patients are shown in Table 1. Of the total, 262 (79.6%) were male and 67 (20.4%) were female, and the mean (SD) age of the study cohort was 62.90 (12.50) years. Among the patients, 92 (28.3%) had a history of smoking and 259 (78.1%) had positive symptoms. Multifocal tumors were found in 123 (37.4%) patients. Tumors in 61 (18.6%) patients were <1 cm, in 155 (47.1%) between 1 and 3 cm and in 113 (34.3%) >3 cm. Tumor grade was G1 in 55 (16.7%), G2 in 189 (57.5%) and G3 in 85 (25.8%) patients. Pathological T stage was pTa in 247 (75.1%) and pT1 in 82 (24.9%) patients.
Table 1.
Clinicopathological characteristics of 329 patients with NMIBC stratified by EORTC-GUCG risk group
| All patients (n=329) | Low risk (n=31) | Intermediate risk (n=189) | High risk (n=109) | p-value | |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Male/female | 262 (79.6)/67 (20.4) | 23 (74.2)/8 (25.8) | 148 (78.3)/41 (21.7) | 91 (83.5)/18 (16.5) | 0.413 |
| Age (years) | |||||
| Mean ± SD | 62.90±12.50 | 58.32±12.98 | 61.76±12.98 | 66.16±10.75 | 0.001 |
| Median (range) | 63 (28–89) | 66 (28–88) | 63.5 (32–89) | 67 (32–85) | |
| History of smoking, n (%) | |||||
| Yes | 93 (28.3) | 11 (35.5) | 53 (28.0) | 29 (26.6) | 0.622 |
| No | 236 (71.7) | 20 (64.5) | 136 (72.0) | 80 (73.4) | |
| Symptoms, n (%) | |||||
| Positive | 259 (78.1) | 23 (74.2) | 140 (74.1) | 96 (88.1) | 0.014 |
| Negative | 70 (21.9) | 8 (25.8) | 49 (25.9) | 13 (11.9) | |
| Tumor focality, n (%) | |||||
| Unifocal | 206 (62.6) | 31 (100.0) | 107 (56.6) | 68 (62.4) | 0.272 |
| Multifocal | 123 (37.4) | 0 (0.0) | 82 (43.4) | 41 (37.6) | |
| Tumor size, n (%) | |||||
| ≤1 cm | 61 (18.6) | 11 (35.5) | 37 (19.6) | 13 (11.9) | <0.001 |
| 1–3 cm | 155 (47.1) | 20 (64.5) | 84 (44.4) | 51 (46.8) | |
| ≥3 cm | 113 (34.3) | 0 (0.0) | 68 (36.0) | 45 (41.3) | |
| Tumor grade, n (%) | |||||
| G1 | 55 (16.7) | 31 (100.0) | 24 (12.7) | 0 (0.0) | <0.001 |
| G2 | 189 (57.5) | 0 (0.0) | 165 (87.3) | 0 (0.0) | |
| G3 | 85 (25.8) | 0 (0.0) | 0 (0.0) | 85 (100.0) | |
| Pathological T stage, n (%) | |||||
| pTa | 247 (75.1) | 31 (100.0) | 189 (100.0) | 27 (24.8) | <0.001 |
| pT1 | 82 (24.9) | 0 (0.0) | 0 (0.0) | 82 (75.2) | |
Abbreviations: NMIBC, non-muscle-invasive bladder cancer; EORTC-GUCG, European Organization for the Research and Treatment of Cancer; SD, standard deviation.
Stratification of patients by EORTC-GUCG risk scoring system
According to the EORTC-GUCG risk scoring system, 31 patients were regarded to have low risk of recurrence, 189 intermediate risk of recurrence and 109 high risk of recurrence. The relationships between clinicopathological characteristics and EORTC-GUCG risk system are shown in Table 1. Statistically significant differences were noticed in age and symptoms among different risk groups.
Prognostic value of the EORTC-GUCG risk scoring system in predicting RFS in patients with NMIBC
During the follow-up period, 125 of the 329 patients (38.0%) experienced intravesical recurrence: 5 (16.1%) in low-risk group, 61 (32.3%) in intermediate-risk group and 59 (54.1%) in high-risk group. The Kaplan–Meier survival analysis showed that the EORTC-GUCG risk scoring system was significantly associated with RFS, and the patients in the higher-risk group had shorter RFS (p<0.001) (Figure 1).
Figure 1.
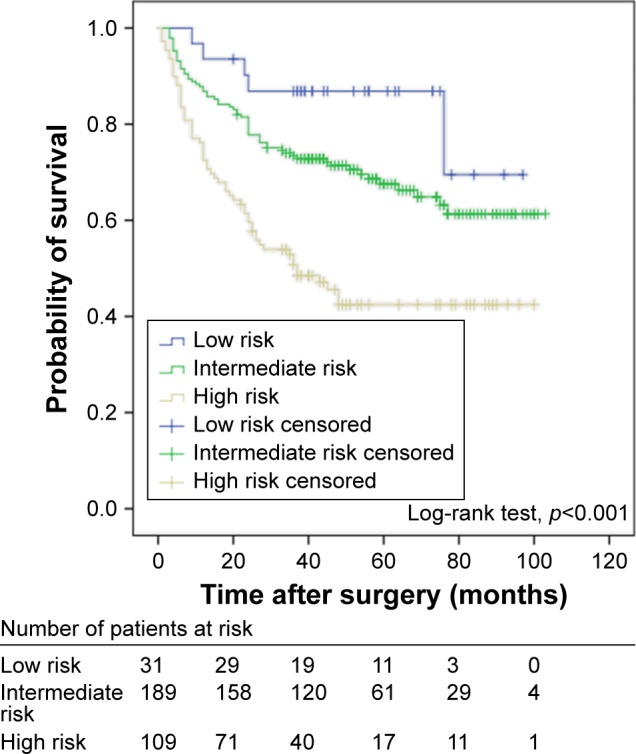
Kaplan–Meier survival curves for recurrence-free survival of all patients with non-muscle-invasive bladder cancer stratified by EORTC-GUCG risk scoring system.
Abbreviation: EORTC-GUCG, European Organization for the Research and Treatment of Cancer.
Prognostic value of the preoperative PNI in predicting RFS in patients with NMIBC
The median (range) preoperative serum albumin level was 43.0 (26.3–54.3) g/L, and the median peripheral lymphocyte count was 1.76 (0.44–4.59) ×109/L. The patients were divided into 2 groups according to the optimal cutoff value of PNI (52.57). Based on this cutoff value, 160 (48.6%) patients were categorized as high-PNI group, while the remaining 169 (51.4%) as low-PNI group.
The relationships between clinicopathological characteristics and PNI are shown in Table 2. Age and tumor grade were found significantly related with preoperative PNI.
Table 2.
Clinicopathological characteristics of 329 patients with NMIBC stratified by PNI
| PNI
|
p-value | ||
|---|---|---|---|
| <52.57 (n=169) | ≥52.57 (n=160) | ||
| Gender, n (%) | |||
| Male/female | 135 (79.9)/34 (20.1) | 127 (79.4)/33 (20.6) | 0.909 |
| Age (years) | |||
| Mean ± SD | 64.45±12.83 | 61.26±11.95 | 0.02 |
| Median (range) | 67 (32–88) | 61 (28–89) | |
| History of smoking, n (%) | |||
| Yes | 52 (30.8) | 41 (25.6) | 0.300 |
| No | 117 (69.2) | 119 (74.4) | |
| Symptoms, n (%) | |||
| Positive | 138 (81.7) | 121 (75.6) | 0.182 |
| Negative | 31 (18.3) | 39 (24.4) | |
| Tumor focality, n (%) | |||
| Unifocal | 101 (59.8) | 105 (65.6) | 0.272 |
| Multifocal | 68 (40.2) | 55 (34.4) | |
| Tumor size, n (%) | |||
| ≤1 cm | 30 (17.7) | 31 (19.4) | 0.382 |
| 1–3 cm | 75 (44.4) | 80 (50.0) | |
| ≥3 cm | 64 (37.9) | 49 (30.6) | |
| Tumor grade, n (%) | |||
| G1 | 27 (16.0) | 28 (17.5) | 0.032 |
| G2 | 88 (52.0) | 101 (63.1) | |
| G3 | 54 (32.0) | 31 (19.4) | |
| Pathological T stage, n (%) | |||
| pTa | 120 (71.0) | 127 (79.4) | 0.079 |
| pT1 | 49 (29.0) | 33 (20.6) | |
| Albumin (g/L) | |||
| Mean ± SD | 40.82±3.30 | 45.07±2.92 | <0.001 |
| Median (range) | 41.4 (26.3–48.2) | 44.8 (37.0–54.3) | |
| Lymphocyte (×109/L) | |||
| Mean ± SD | 1.50±0.42 | 2.22±0.64 | <0.001 |
| Median (range) | 1.49 (0.44–2.89) | 2.16 (0.6–4.59) | |
Abbreviations: NMIBC, non-muscle-invasive bladder cancer; PNI, prognostic nutritional index; SD, standard deviation.
Overall, RFS rate of patients with NMIBC was 82.7% (272/329) at 1 year, 66.1% (205/310) at 3 years and 43.2% (89/206) at 5 years. The mean time of recurrence (SD) was 19.9 (17.3) months. Furthermore, 1-, 3- and 5-year RFS stratified by preoperative PNI were also identified in this study: 1-year RFS rate was 76.9% in low-PNI group and 88.8% in high-PNI group (p=0.004); 3-year RFS rate was 55.4% in low-PNI group and 77.1% in high-PNI group (p<0.001); and 5-year RFS rate was 29.6% in low-PNI group and 58.2% in high-PNI group (p<0.001). The Kaplan–Meier survival analysis showed that preoperative PNI was significantly associated with RFS (p<0.001, Figure 2). Thus, low preoperative PNI could predict poorer RFS.
Figure 2.
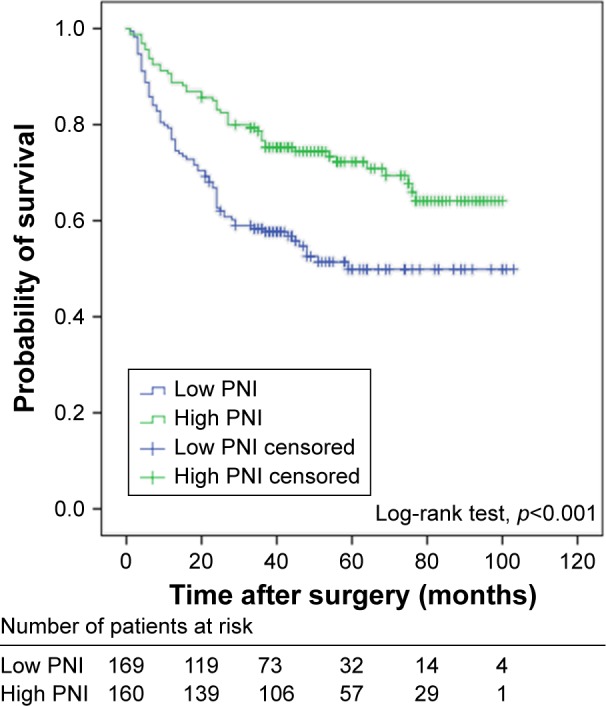
Kaplan–Meier survival curves for recurrence-free survival of patients with non-muscle-invasive bladder cancer stratified by PNI.
Abbreviation: PNI, prognostic nutritional index.
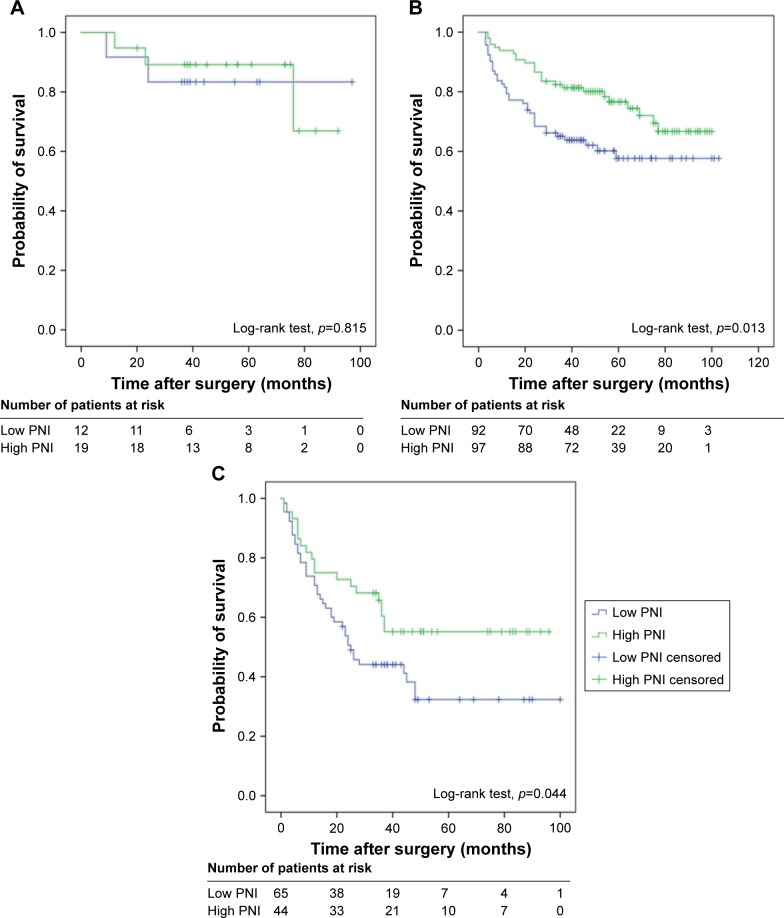
Furthermore, we evaluated whether the PNI in different EORTC-GUCG groups was associated with the RFS. No difference was found in patients of low-risk group for RFS (p=0.815, Figure 3A), but statistically significant difference was found in patients of intermediate-risk group (p=0.013, Figure 3B) and high-risk group (p=0.044, Figure 3C).
Figure 3.
Kaplan–Meier survival curves for different EORTC-GUCG risk groups of patients with non-muscle-invasive bladder cancer stratified by PNI: (A) low risk; (B) intermediate risk; and (C) high risk.
Abbreviations: EORTC-GUCG, European Organization for the Research and Treatment of Cancer; PNI, prognostic nutritional index.
In univariate analysis, no difference for RFS was found in gender, history of smoking and symptoms. Age (<50 or ≥50), tumor focality (unifocal or multifocal), tumor size (≤1, 1–3 or ≥3 cm), tumor grade (G1, G2 or G3), pathological T stage (pTa or pT1) and preoperative PNI (low or high) were significantly associated with RFS (Table 2). To clarify the independent prognostic value of preoperative PNI for RFS, multivariate Cox proportional hazards regression analysis using age, tumor focality, tumor size, tumor grade, tumor stage and preoperative PNI as covariates was performed which revealed that age (hazard ratio [HR] =2.007, 95% confidence interval [CI] 1.036–3.885, p=0.039), tumor focality (HR =1.484, 95% CI 1.039–2.119, p=0.030), tumor grade (G1, G2 or G3; HR =2.603, 95% CI 1.186–5.713 and HR =2.933, 95% CI 1.228–7.003, p=0.015, respectively), tumor stage (pTa or pT1; HR =1.737, 95% CI 1.108–2.724, p=0.016) and PNI (low or high; HR =0.598, 95% CI 0.410–0.870, p=0.007) were independent predictors of RFS in patients with NMIBC (Table 3).
Table 3.
Univariate and multivariate Cox proportional hazards regression analyses of RFS in 329 patients with NMIBC
| Variable | Univariate
|
Multivariate
|
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Gender | ||||
| Male | 1 (reference) | 0.827 | ||
| Female | 1.049 (0.685–1.606) | |||
| Age (years) | ||||
| <50 | 1 (reference) | 0.004 | 1.000 (reference) | 0.039 |
| ≥50 | 2.562 (1.342–4.891) | 2.007 (1.036–3.885) | ||
| History of smoking | ||||
| No | 1 (reference) | 0.833 | ||
| Yes | 1.043 (0.705–1.544) | |||
| Symptoms | ||||
| Positive | 1 (reference) | 0.238 | ||
| Negative | 0.758 (0.478–1.201) | |||
| Tumor focality | ||||
| Unifocal | 1 (reference) | 0.013 | 1.000 (reference) | 0.030 |
| Multifocal | 1.564 (1.100–2.224) | 1.484 (1.039–2.119) | ||
| Tumor size (cm) | ||||
| ≤1 | 1 (reference) | |||
| 1–3 | 1.835 (1.024–3.291) | 0.041 | ||
| ≥3 | 2.477 (1.373–4.466) | 0.003 | ||
| Tumor grade | ||||
| G1 | 1 (reference) | 1.000 (reference) | ||
| G2 | 3.165 (1.455–6.885) | 0.004 | 2.603 (1.186–5.713) | 0.017 |
| G3 | 6.030 (2.727–13.336) | <0.001 | 2.933 (1.228–7.003) | 0.015 |
| Tumor stage | ||||
| pTa | 1 (reference) | <0.001 | 1.000 (reference) | 0.016 |
| pT1 | 2.563 (1.784–3.682) | 1.737 (1.108–2.724) | ||
| PNI | ||||
| <52.57 | 1 (reference) | <0.001 | 1.000 (reference) | 0.007 |
| ≥52.57 | 0.510 (0.354–0.733) | 0.598 (0.410–0.870) | ||
Abbreviations: RFS, recurrence-free survival; NMIBC, non-muscle-invasive bladder cancer; HR, hazard ratio; CI, confidence interval; PNI, prognostic nutritional index.
Nomogram in predicting 1-, 3- and 5-year RFS rates in patients with NMIBC
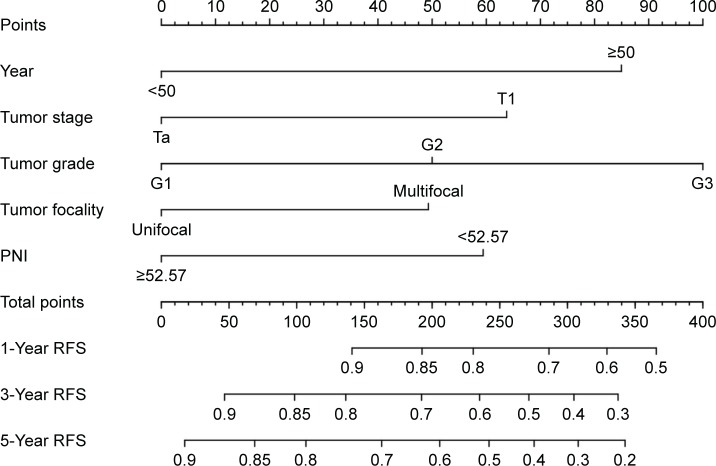
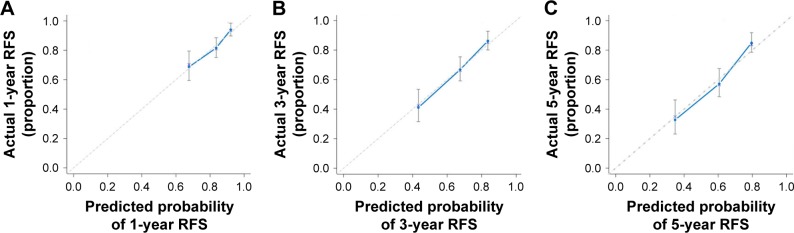
According to the multivariable Cox model, patient’s age, tumor focality, tumor grade, tumor stage and preoperative PNI had a significant effect on RFS. The nomogram can be used to predict the probability of BCa recurrence at 1, 3 and 5 years after primary surgery (Figure 4). The accuracy of this prediction model was relatively high, with a c-index of 0.697. Calibration is another important indicator of the nomogram. The calibration plots showed only a limited deviation from the ideal predictions (Figure 5).
Figure 4.
Nomogram for predicting RFS in patients with non-muscle-invasive bladder cancer.
Abbreviations: RFS, recurrence-free survival; PNI, prognostic nutritional index.
Figure 5.
Calibration curves for internal validation of estimating RFS in patients with non-muscle-invasive bladder cancer: (A) 1-year outcome; (B) 3-year outcome; and (C) 5-year outcome.
Abbreviation: RFS, recurrence-free survival.
Discussion
The results of the present study showed patients stratified by EORTC-GUCG risk scoring system were statistically different in RFS outcomes (p<0.001), which indicated accuracy of our patients’ data. The RFS rates of the low-PNI group were significantly lower than those of the high-PNI group, and our results revealed that PNI was an independent prognostic factor associated with RFS in patients with NMIBC. To the best of our knowledge, this is the first study to investigate the association among PNI, pathological factors, age and RFS outcomes in a cohort of patients with NMIBC and to establish a nomogram using these prognostic factors.
PNI was originally proposed as a marker to predict the nutritional and immunological statuses of cancer patients before gastrointestinal surgery. It was initially reported by Buzby et al, using the following formula: PNI (percent) =158−16× serum albumin level (g/100 mL)−0.78× triceps skinfold (mm)−0.20× serum transferrin level (mg/100 mL)−5.8× cutaneous delayed hypersensitivity reactivity.16 Onodera et al defined PNI as a simple linear predictive model calculated by serum albumin and peripheral lymphocyte count.7 Then, PNI was reported as a prognostic index in various types of human cancers.8–11 However, the optimal cutoff value of PNI to predict the overall survival (OS), cancer-specific survival, progression-free survival and RFS outcomes remains unclear. Jeon et al reported a PNI of 51 for renal carcinoma, and used a minimum p-value approach to identify the cutoff point.10 Mori et al reported a PNI of 50 for non-small-cell lung cancer in their study, and the optimal cutoff value was calculated by ROC curve.17 In the present study, the optimal cutoff value of PNI was 52.57 which was similar to that of patients with breast cancer (52.4).18 The following reasons could cause uncertainty in PNI: first, different tumors had different biological characteristics, and patients’ status was heterogeneous; second, limited number of patients could lead to a bias in cutoff value; and third, different statistical methods could result in different cutoff values.
It is well known that serum albumin level is one of the most widely used markers for evaluating nutritional status, and malnutrition is closely associated with poor prognosis and low quality of life and weakens the human defense mechanisms, including anatomic barriers, cellular and humoral immunity and phagocyte function.19,20 Gregg et al reported nutritional deficiency, as measured by preoperative weight loss, body mass index and serum albumin, was a strong predictor of 90-day mortality and poor OS in patients with BCa who underwent radical cystectomy.21 However, Ataseven et al suggested there was no correlation between hypoalbuminemia and body mass index (BMI) in patients with epithelial ovarian cancer, but hypoalbuminemia could reflect prognosis.20
Another component in PNI is lymphocyte, which plays a fundamental role in the cell-mediated immune response in the formation and progression of tumor.22 The importance of lymphocytes has been clarified in several studies which have shown that lymphocytopenia is associated with poor survival outcome, independent of clinicopathological characteristics in patients with muscle-invasive BCa.23,24 The causes of tumor-induced lymphocytopenia remain to be elucidated in detail, and one potential reason is the result of impaired lymphocyte homeostasis and enhanced lymphocyte apoptosis.25 Meanwhile, tumor cells also express higher levels of proapoptotic molecules such as Fas ligand, resulting in increased destruction of lymphocytes via activation of the extrinsic pathway of apoptosis.26 Thus, lymphocytopenia may be a surrogate marker of tumor-associated immune suppression. Taken together, PNI may serve as an indicator of nutritional status, SIR and immunity, and low PNI may lead to poorer outcome.
Several noteworthy findings were identified in this study. This is the first study to report PNI as an independent prognostic factor and combine age, tumor stage, tumor grade and tumor focality in a single predictor model to evaluate the prognosis of patients with NMIBC followed by intravesical chemotherapy. We also noticed that tumor size was not an independent factor to predict RFS which was included in the EORTC-GUCG risk scoring system, but the accuracy of internal calibrations in the nomogram was high. This could be explained by the limited number of patients.
Nomograms are considered the most accurate model to predict outcomes after surgical treatment, so far.27 By applying points to the 5 variables, we can estimate the probability of recurrence for individual patients easily. Nomograms could guide clinicians in personalized clinical decision-making.28 PNI can be measured at low cost, and routinely and easily in clinical practice, so it is convenient to establish a nomogram using PNI.
The limitation of the present study is inherent to its retrospective design. Moreover, single-institution cohorts might have more complete datasets, yet they might be biased by institutional practice patterns, which can be overcome by the use of multi-institutional or national databases.29 Furthermore, a number of the patients in the present study had shortage of follow-up time. Only 206 patients were involved in the analysis of 5-year OS. Another limitation is that in the present study the nomogram was only internally validated, and external validation should be further investigated with a large multicenter cohort. However, even considering these limitations, the present study suggests PNI as a potential independent prognostic factor for RFS in patients with NMIBC and provides a nomogram for patients with NMIBC who undergo TURBT and are then treated with intravesical chemotherapy to evaluate probability of recurrence.
Conclusion
The present study investigates the value of preoperative PNI as an independent prognostic factor to predict RFS of patients with NMIBC. The serum albumin and peripheral lymphocytes can be measured at low cost, and routinely and easily in clinical practice. Moreover, the nomogram which includes age, tumor stage, tumor grade, tumor focality and PNI could be useful to improve personalized multidisciplinary therapy for patients with NMIBC.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants 81470987 and 81170702 to B. Shi), the Tai Shan Scholar Foundation (to B. Shi), the Science and Technology Development Project of Shandong Province (Grant 2014GSF118054 to B. Shi), Natural Science Foundation of Shandong Province (Grant ZR2014HQ062 to Y. Zhu) and Science Foundation of Qilu Hospital of Shandong University (Grant 2015QLMS28 to B. Shi; Grant 2015QLQN21 to Y. Zhu), Medicine and Health Science Technology Development Project of Shandong Province (Grant 2014WS0138 to Y. Zhu) and Primary Research & Development Plan of Shandong Province (2016GSF201036 to Y. Zhu).
Footnotes
Disclosure
None of the contributing authors have any conflict of interest, including specific financial interests or relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Tsuji S, Chen X, Hancock B, et al. Preclinical evaluation of VAX-IP, a novel bacterial minicell-based biopharmaceutical for nonmuscle invasive bladder cancer. Mol Ther Oncolytics. 2016;3:16004. doi: 10.1038/mto.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ku JH, Kim M, Choi WS, Kwak C, Kim HH. Preoperative serum albu-min as a prognostic factor in patients with upper urinary tract urothelial carcinoma. Int Braz J Urol. 2014;40(6):753–762. doi: 10.1590/S1677-5538.IBJU.2014.06.06. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka N, Kikuchi E, Matsumoto K, et al. Prognostic value of plasma fibrinogen levels in patients with localized upper tract urothelial carci-noma. BJU Int. 2013;111(6):857–864. doi: 10.1111/j.1464-410X.2012.11353.x. [DOI] [PubMed] [Google Scholar]
- 6.Ogihara K, Kikuchi E, Yuge K, et al. The preoperative neutrophil-to-lymphocyte ratio is a novel biomarker for predicting worse clinical outcomes in non-muscle invasive bladder cancer patients with a previous history of smoking. Ann Surg Oncol. 2016;23(Suppl 5):1039–1047. doi: 10.1245/s10434-016-5578-4. [DOI] [PubMed] [Google Scholar]
- 7.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005. Japanese [with English abstract] [PubMed] [Google Scholar]
- 8.Okamura Y, Ashida R, Ito T, Sugiura T, Mori K, Uesaka K. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg. 2015;39(6):1501–1509. doi: 10.1007/s00268-015-2982-z. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu K, Okita R, Saisho S, Maeda A, Nojima Y, Nakata M. Pre-operative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non-small cell lung cancer. World J Surg Oncol. 2015;13:291. doi: 10.1186/s12957-015-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon HG, Choi DK, Sung HH, et al. Preoperative prognostic nutritional index is a significant predictor of survival in renal cell carcinoma patients undergoing nephrectomy. Ann Surg Oncol. 2016;23(1):321–327. doi: 10.1245/s10434-015-4614-0. [DOI] [PubMed] [Google Scholar]
- 11.Tokunaga R, Sakamoto Y, Nakagawa S, et al. Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum. 2015;58(11):1048–1057. doi: 10.1097/DCR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 12.Jehi L, Yardi R, Chagin K, et al. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. 2015;14(3):283–290. doi: 10.1016/S1474-4422(14)70325-4. [DOI] [PubMed] [Google Scholar]
- 13.Hong SJ, Cho KS, Han M, et al. Korean Urological Oncology Society Nomograms for prediction of disease recurrence in patients with primary Ta, T1 transitional cell carcinoma of the bladder. J Korean Med Sci. 2008;23(3):428–433. doi: 10.3346/jkms.2008.23.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babjuk M, Böhle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71(3):447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 15.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC-GUCG risk tables: a combined analysis of 2596 patients from seven EORTC-GUCG trials. Eur Urol. 2006;49(3):466–475. doi: 10.1016/j.eururo.2005.12.031. discussion 475–477. [DOI] [PubMed] [Google Scholar]
- 16.Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–167. doi: 10.1016/0002-9610(80)90246-9. [DOI] [PubMed] [Google Scholar]
- 17.Mori S, Usami N, Fukumoto K, et al. The significance of the prognostic nutritional index in patients with completely resected non-small cell lung cancer. PLoS One. 2015;10(9):e0136897. doi: 10.1371/journal.pone.0136897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohri T, Mohri Y, Shigemori T, Takeuchi K, Itoh Y, Kato T. Impact of prognostic nutritional index on long-term outcomes in patients with breast cancer. World J Surg Oncol. 2016;14(1):170. doi: 10.1186/s12957-016-0920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H, Cho YS, Jung S, Kim H. Effect of nutritional risk at admission on the length of hospital stay and mortality in gastrointestinal cancer patients. Clin Nutr Res. 2013;2(1):12–18. doi: 10.7762/cnr.2013.2.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ataseven B, du Bois A, Reinthaller A, et al. Pre-operative serum albumin is associated with post-operative complication rate and overall survival in patients with epithelial ovarian cancer undergoing cytoreductive surgery. Gynecol Oncol. 2015;138(3):560–565. doi: 10.1016/j.ygyno.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Gregg JR, Cookson MS, Phillips S, et al. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol. 2011;185(1):90–96. doi: 10.1016/j.juro.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Joseph N, Dovedi SJ, Thompson C, et al. Pre-treatment lymphocytopaenia is an adverse prognostic biomarker in muscle-invasive and advanced bladder cancer. Ann Oncol. 2016;27(2):294–299. doi: 10.1093/annonc/mdv546. [DOI] [PubMed] [Google Scholar]
- 24.Ku JH, Kang M, Kim HS, Jeong CW, Kwak C, Kim HH. The prognostic value of pretreatment of systemic inflammatory responses in patients with urothelial carcinoma undergoing radical cystectomy. Br J Cancer. 2015;112(3):461–467. doi: 10.1038/bjc.2014.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2(1):46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 26.Kim R, Emi M, Tanabe K, Uchida Y, Toge T. The role of Fas ligand and transforming growth factor beta in tumor progression: molecular mechanisms of immune privilege via Fas-mediated apoptosis and potential targets for cancer therapy. Cancer. 2004;100(11):2281–2291. doi: 10.1002/cncr.20270. [DOI] [PubMed] [Google Scholar]
- 27.Sonpavde G, Pond GR, Fougeray R, Bellmunt J. Nomogram to predict the benefit from salvage systemic therapy for advanced urothelial carcinoma. BJU Int. 2015;115(6):854–855. doi: 10.1111/bju.12922. [DOI] [PubMed] [Google Scholar]
- 28.Raman JD, Lin YK, Shariat SF, et al. Preoperative nomogram to predict the likelihood of complications after radical nephroureterectomy. BJU Int. 2017;119(2):268–275. doi: 10.1111/bju.13556. [DOI] [PubMed] [Google Scholar]
- 29.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):173–180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]