Abstract
Purpose
Mild cognitive impairment (MCI) is associated with a higher risk of dementia and is becoming a topic of interest for pharmacological and nonpharmacological interventions. With advances in technology, computer-based cognitive exercises are increasingly integrated into traditional cognitive interventions, such as cognitive training. Another type of cognitive intervention involving technology use is cognitive engagement, consisting of involving participants in highly motivational and mentally challenging activities, such as learning to use a form of new digital technology. This study examined the feasibility and acceptability of a computerized cognitive stimulation (CCS) program and a computerized cognitive engagement (CCE) program, and then compared their effects in older adults with MCI.
Patients and methods
In this randomized study, data from 19 MCI patients were analyzed (n=9 in CCS and n=10 in CCE). The patients attended a group weekly session for a duration of 3 months. Assessments of cognitive and psychosocial variables were conducted at baseline (M0) and at the end of the programs (M3).
Results
All of the participants attended the 12 sessions and showed a high level of motivation. Attrition rate was very low (one dropout at M3 assessment). At M3, the CCS participants displayed a significant improvement in part B of the Trail Making Test (TMT-B; p=0.03) and self-esteem (p=0.005), while the CCE participants showed a significant improvement in part A of the Trail Making Test (TMT-A; p=0.007) and a higher level of technology acceptance (p=0.006). The two groups did not differ significantly (p>0.05) in cognitive and psychosocial changes after the intervention. However, medium effect sizes (Cohen’s d=0.56; 95% CI =−0.43:1.55) were found on the free recall, favoring the CCS group, as well as on TMT-A (d=0.51; 95% CI =−0.48:1.49) and technology acceptance (d=−0.65; 95% CI =−1.64:0.34), favoring the CCE group.
Conclusion
Both interventions were highly feasible and acceptable and allowed improvement in different aspects of cognitive and psychosocial functioning in MCI subjects.
Keywords: cognitive intervention, mild cognitive impairment, tablet computers, technology
Introduction
The number of people living with dementia has been estimated to be 35.6 million worldwide (around 0.5% of the world’s total population). In Europe, the 2009 World Alzheimer Report forecasted an increase of 40% in the number of people suffering from dementia during the 20-year period from 2010 to 2030.1 Older adults with mild cognitive impairment (MCI) have a higher risk of developing dementia (10%–15% per year), compared to the general older population (1%–2% per year).2,3 However, while some individuals with MCI progress to dementia, others may improve over time toward normal cognitive functioning or stay stable.4,5 In the absence of effective treatment for neurodegenerative diseases, MCI is becoming a topic of interest for pharmacological and nonpharmacological interventions, in order to prevent further cognitive decline.
Recent studies have taken into serious consideration the importance of neuropsychological interventions to maintain or improve cognitive abilities in dementia.6,7 Among these interventions, cognitive stimulation (CS) is used as a global approach in responding to memory complaints in the elderly, as well as a complementary approach to pharmacological treatments in Alzheimer’s disease (AD).8 It emerged in 1980s in the context of care for patients with AD, with a twofold objective: 1) to optimize cognitive functioning such as attention, executive functions, memory strategies, perception, memory, visuospatial skills and so on and 2) to intervene on psychosocial factors, in order to enhance motivation, self-confidence, emotional balance, self-esteem and self-valorization, which are also known to influence cognition.9–11 Unlike cognitive training and cognitive rehabilitation, CS sessions are usually held in a group, in which participants are involved in a range of cognitive exercises, the contents of which aim to enhance individuals’ overall cognitive and social functioning.12 Benefits of CS on cognitive functioning, mood, quality of life and well-being have been largely reported in patients suffering from dementia13–17 and MCI.18 The UK guidelines on dementia recommend that patients with mild/moderate dementia should be given the opportunity to participate in a structured group CS program.19
With advances in technology, computer-based programs and games are increasingly integrated into traditional cognitive interventions, mostly in cognitive training programs in which a participant is engaged in a set of standard tasks, tapping into several cognitive domains, with a range of levels of difficulty. Several studies have shown that computerized cognitive training has the potential to enhance global and select domain cognition and positively impact psychosocial functioning in older adults with MCI.20–26 Another type of cognitive intervention for older adults is cognitive engagement, consisting of motivating and mentally stimulating activities, such as learning to use a form of new digital technology. In fact, acquiring new skills and being involved in novel learning experiences such as using a computer or a tablet-PC, may improve episodic memory and processing speed in healthy older adults.27,28 To the best of our knowledge, no studies compare the effects of a cognitive engagement program and of a computerized CS program in older adults with MCI. In this pilot study, we aimed to test the feasibility and the acceptability of such programs and to explore the effects of these two types of cognitive interventions in patients with MCI.
Patients and methods
Design
We designed a randomized single-blind study conforming to Consolidated Standards of Reporting Trials criteria for pilot and feasibility studies.29 This study was conducted from December 2014 to July 2015 in Broca hospital. Patients were assigned to either a computerized CS (CCS) group or a computerized cognitive engagement (CCE) group with a simple computerized randomization procedure.
Participants
Participants were recruited from the memory clinic in Broca hospital. They previously underwent a comprehensive geriatric evaluation including biological analyses, a physical examination and standardized neuropsychological assessment. All the participants read and signed the consent form before the randomization.
Inclusion and exclusion criteria
All participants were community-dwelling older adults, meeting the MCI criteria according to Petersen et al.30,31 They were aged 60 or over; had a Mini-Mental Status Examination32 (MMSE) score >24; reported a subjective memory complaint, preferably corroborated by an informant; performed at/or below 1.5 standard deviations (SDs) from the mean for age and education on more than one of the neuropsychological tests; had preserved or minimal impairments in functional abilities and absence of dementia.
The exclusion criteria were 1) psychiatric and neurologic disorders (eg, bipolar disorder, schizophrenia, stroke, Parkinson’s disease, epilepsy and so on), 2) history of alcohol or other substance abuse, and 3) sensory and/or motor deficits affecting the use of a tablet-PC.
Intervention
The two groups (CCS and CCE) attended one group session per week (5–7 participants) for 3 months (12 sessions in total). Each session lasted 90 minutes and was conducted by a trained neuropsychologist blinded to assessment. In the first session, the participants introduced themselves. They were given explanations about the outline of the program. They were trained to use some basic functions of a tablet-PC.
Computerized cognitive stimulation
The CCS program was designed to stimulate several cognitive domains with computerized cognitive exercises and social interactions among participants. Each session was conducted as follows:
Presentation of the day’s program, recall of the last session and discussion (15 minutes).
Cognitive exercises on tablet with a short break between exercises (60 minutes).
Feedback and group discussion about the session (15 minutes).
Computerized cognitive exercises were selected from the Institution version of KODRO (Altera-Group, Paris, France), a web-based platform which provided several applications (ie, appointment and event reminding, cognitive games, communication, entertainment, videos and a library, and so on) tailored to older adults. We selected KODRO for its large content of playful and ecological cognitive exercises.
We used three devices to deliver cognitive exercises:
An iPAD was used by the neuropsychologist who selected a set of cognitive exercises, the difficulty level of which was adapted to the overall group level. At the end of each session, neuropsychologists could monitor participants’ performances, thanks to automatic statistical analyses of each participant’s success and failure rates for each exercise. Therefore, for the following session, he/she could set the exercises with the most adapted difficulty level, according to the overall group performance.
A TV screen was linked to the iPAD and to participant’s android tablet-PC. It displayed the date at the beginning of the session and the instructions for each exercise, allowing the participants to refer to them if necessary.
An android tablet-PC was used by each participant. It was also linked to the iPAD used by the neuropsychologist who launched cognitive exercises. It displayed feedback (good, very good, excellent) at the end of each set of exercises to encourage participants.
Computerized cognitive engagement
The CCE program was designed to train participants to use a tablet-PC and stimulate social interactions among participants. Each session was conducted as follows:
Presentation of the day’s program, recall of the last session and discussion (15 minutes).
Discovery of a variety of available applications (60 minutes).
Feedback and group discussion about the session (15 minutes).
In contrast to the CCS program, the CCE participants were involved in a casual atmosphere, while the content was preprogrammed. A specific topic was defined for each session, and participants were invited to explore different applications relating to this. For example, for the theme “compensating for memory problems”, participants discovered the calendar and learned to schedule an appointment in it. During the sessions, participants were also invited to suggest a theme and the neuropsychologist showed the applications associated to the theme.
Outcome measures
Feasibility and acceptability outcomes
The main focus of this study was feasibility of recruitment, retention and acceptability of intervention procedures.
Recruitment rates were calculated with the number of actual eligible and consenting participants divided by the total number of candidates suggested by clinicians.
Attrition rates were calculated thanks to the number of cases of withdrawal or dropouts before the postintervention assessment.
- The acceptability of the interventions was assessed through:
- Session attendance rates.
- Levels of motivation regarding interest in participating in this study and in future interventions like this. We asked participants to indicate their motivation levels by choosing one of the ranked options on a 7-point Likert scale (not at all =0 to very motivated =6). We also interviewed participants about the reasons behind their motivation to participate in the study.
Cognitive and psychosocial outcomes
The participants completed baseline (M0) and postintervention (M3) assessments tapping into several cognitive and psychosocial domains. These were carried out by an experienced neuropsychologist blinded to the intervention.
-
Cognitive outcomes
Cognitive measures were selected from the neuropsychological battery of tests used in Broca hospital’s memory clinic. - Psychosocial outcomes
- Anxiety and depression symptoms were assessed with the Goldberg anxiety and depression scales.39
- Quality of life was assessed using the quality of life scale for older French people [Echelle de Qualité de Vie adpatée aux Personnes Agées].44
- Acceptance of information and communication technologies (ICT) was evaluated with the Technology Acceptance Questionnaire.45
Analysis
Sample size calculation was not performed for this pilot study. Baseline characteristics were described using means and SD or percentages.
Feasibility and acceptability outcomes were reported descriptively, and 95% CI was calculated for the recruitment rates.
Cognitive and psychosocial outcomes were reported by mean (SD) or median and range. For each group, comparisons of outcomes between baseline and postintervention (M3) assessments were performed using paired Student’s t-tests or Wilcoxon signed-rank tests. Changes between baseline (M0) and M3 were compared between groups using Student’s t-tests or Wilcoxon–Mann–Whitney tests (in case of non-normal distribution).
Given this study’s small sample size, there was a high probability of finding nonsignificant differences when comparing the two groups. Therefore, to assess the magnitude and direction of intervention outcome, effect sizes (ESs) were computed in the form Cohen’s d with a 95% confidence interval (CI) where appropriate. ES can be considered small (d=0.2), medium (d=0.5) or large (d=0.8).46
The significance level used for all statistical tests was set at 0.05. Analyses were carried out using R statistical software version 3.1.2.60
Ethics approval
This study was approved by the ethics committee of Paris Descartes University Institutional Review Board under the instituional review board number 20161300001072. The National Clinical Trial number is NCT03195829.
Results
Feasibility and acceptability
Recruitment rate
The recruitment period lasted about 5 months (from December 2014 to April 2015), and the interventions were conducted from April to July 2015. Of the 53 patients referred by geriatricians and neuropsychologists, 33 were excluded, yielding 20 patients eligible to take part in this study (14 females and 6 males). Figure 1 presents the flow diagram showing patients’ attrition from the screening phase to the postintervention assessment.
Figure 1.
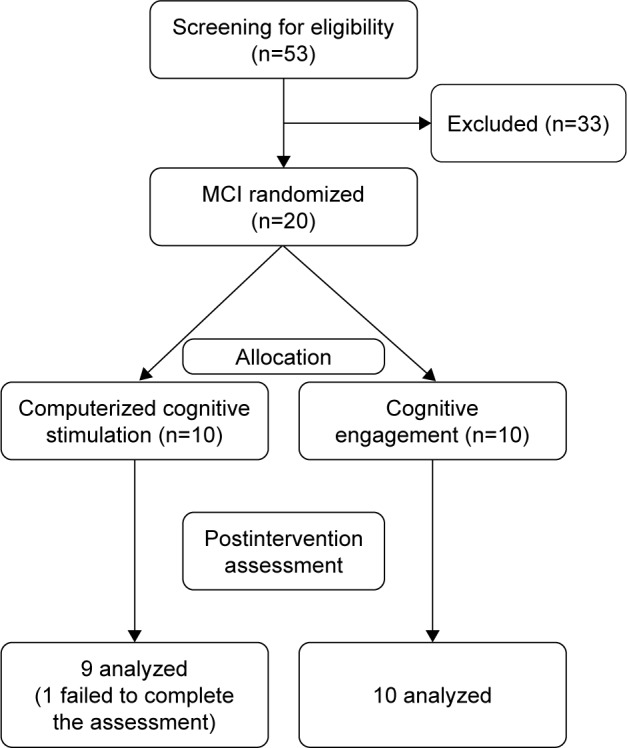
Flow diagram showing patients’ attrition from the screening phase to postintervention assessment.
Abbreviation: MCI, mild cognitive impairment.
The recruitment rate was 38% (n=20/53; 95% CI: 25%–52%).
Of those ineligible, 19 (57.6%) did not meet the inclusion criteria, 8 (24.2%) were unavailable, 4 (12.1%) refused to participate in the study and 2 (6.1%) were unreachable.
Ten participants were allocated to the CCS group and ten to the CCE group. One participant in the CCS group did not perform the M3 assessment, yielding 19 subjects for the final analyses. Table 1 presents the demographic characteristics of the sample. There was no significant group difference in age, education, sex and MMSE score (p>0.05).
Table 1.
Demographic characteristics of the participants
| Characteristics | CCE group (n=10) | CCS group (n=10) |
|---|---|---|
| Age (y), mean (SD) | 78.2 (7.0) | 75.2 (6.4) |
| Women, % (n) | 60.0 (6) | 70.0 (7) |
| MMSE score, mean (SD) | 27.4 (2.0) | 27.7 (1.9) |
| Education, college degree or higher, % (n) | 44.4 (4) | 60.0 (6) |
Abbreviations: CCE, computerized cognitive engagement; CCS, computerized cognitive stimulation; MMSE, Mini-Mental State Examination; y, years.
Attrition
None of the participants discontinued the intervention. Only one participant in the CCS group did not perform the M3 assessment for medical reasons (surgery), resulting in 19 subjects for the final analyses.
Acceptability of the interventions
The small sample size did not allow us to assess the difference in acceptability between men and women. However, acceptability for both interventions was very high. All participants in both groups attended the 12 sessions.
On a 7-point Likert scale assessing the level of motivation to participate in this study and in a future intervention, participants in both groups showed high levels of motivation before the interventions: for the CCS group, median =6, ranging from 3 to 6; for the CCE group, median =6, ranging from 4 to 6. The high level of motivation was maintained after the interventions: for the CCS group, median =6, ranging from 4 to 6; for the CCE group, median =5.5, ranging from 4 to 6.
The main motivations to participate in the study were wishing to resist the onset of AD and overcoming loneliness. Verbatim quotes concerning the participants’ motivations, as cited by the participants, were translated from French to English by the authors, and are shown in Box 1.
It is worth mentioning that during the last session of both CCS and CCE, participants reported that sessions conducted in a group format were very engaging and stimulating. We noted that social ties were created between some of the participants throughout the sessions, and some of them shared other leisure activities together outside the sessions (eg, going to restaurant or watching movies). A majority of the participants in both groups found the intervention too brief and experienced a feeling of frustration at the end of it. They expressed a desire to continue the intervention on a weekly basis. It can also be noted that 8 out of the 20 participants bought the same tablet-PC which they had been familiarized with, just after the intervention.
Box 1. The examples of motivation to participate in the study.
“I realize that I forget a lot of things, and with all I hear about Alzheimer’s disease this worries me very much, so I do crossword puzzles but I’m not always motivated at home, that’s why I’m here.”
“It’s the same as this woman (fear of having Alzheimer’s disease), and I do not often go out, so it gives me a reason to leave home and see and meet people.”
“My aunt had Alzheimer’s disease, and I’m worried about the slightest oversight.”
“I have persistent memory problems and it’s scary.”
“I am here to meet people because my children work abroad and I am alone. At least I am sure that because I will be coming regularly, I will be able to exchange more easily here than elsewhere.”
“I like to participate in research and make myself useful.”
“My grandchildren bought me a tablet-PC and I want to learn how to use it.”
Note: The participants’ comments were translated by the authors from French to English for inclusion in this paper.
Cognitive outcomes
Table 2 presents the cognitive scores of both the intervention groups at M0 and M3.
Table 2.
Cognitive outcomes at baseline and postintervention: change from baseline (M3–M0) in each group and comparison between two groups
| Cognitive measures | CCS group (n =9), mean (SD)/median (range)
|
CCE group (n =10), mean (SD)/median (range)
|
Group comparison for change M3–M0
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M0 | M3 | M3–M0 | p-value | M0 | M3 | M3–M0 | p-value | Cohen’s d (95% CI) | p-value | |
| MMSE | 27 (2) | 28 (1.4) | 1 (2.1) | 0.20 | 27.7 (1.9) | 27.8 (1.5) | 0.1 (2) | 0.88 | 0.44 (−0.54:1.42) | 0.35 |
| VST | 6.9 (2.3) | 7.4 (2.3) | 0.6 (1.5) | 0.30 | 7.2 (1.8) | 8.3 (2.1) | 1.1 (1.7) | 0.06 | −0.34 (−1.32:0.63) | 0.46 |
| RL/RI-16 FR | 22.8 (10.7) | 25.1 (10) | 2.3 (3.7) | 0.09 | 26.6 (8.7) | 26.3 (7.5) | −0.3 (5.4) | 0.87 | 0.56 (−0.43:1.55) | 0.23 |
| TMT-A | 52.1 (18.8) | 47 (22.8) | −5.1 (9.3) | 0.14 | 50.8 (18.3) | 41.1 (12.3) | −9.7 (8.8) | 0.007** | 0.51 (−0.48:1.49) | 0.29 |
| TMT-B | 135.7 (65.6) | 135.7 (65.6) | −24.2 (42.2) | 0.12 | 112 (19.8) | 101.5 (29.2) | −10.5 (20.9) | 0.15 | −0.42 (−1.4:0.56) | 0.4 |
| TMT-B-Err | 2 (0:9) | 1 (0:3) | −1 (−8:0) | 0.03* | 1 (0:5) | 1 (0:2) | −0.5 (−4:2) | 0.34 | – | 0.31 |
| Backward digit span | 4 (3:5) | 4 (3:5) | 0 (−1:1) | 1.00 | 4 (3:5) | 4 (2:5) | 0 (−1:1) | 0.77 | – | 0.74 |
| Phonemic verbal fluency | 19.6 (9.7) | 22.4 (8) | 2.9 (7.5) | 0.28 | 22.1 (6.4) | 22.5 (5.9) | 0.4 (4.5) | 0.78 | −0.41 (−1.39:0.57) | 0.4 |
| Categorical verbal fluency | 24.2 (3.8) | 25.8 (6) | 1.6 (3.5) | 0.22 | 27 (8) | 27.4 (7.4) | 0.4 (6.3) | 0.85 | 0.22 (−0.75:1.2) | 0.62 |
Notes:
p<0.05;
p<0.01.
Abbreviations: CCE, computerized cognitive engagement; CCS, computerized cognitive stimulation; M0, baseline; M3, the end of the programs; MMSE, Mini-Mental State Examination; RL/RI-16 FR, free recall of free and cued recall test; SD, standard deviation; TMT-A, trail making test part A; TMT-B, trail making test part B; TMT-B-Err, trail making test B errors; VST, visuospatial memory test.
CCS group
Comparison between pre- and postintervention assessments showed a trend toward an improvement of the sum of three free recalls as assessed by the RL/RI-16 (p=0.09) and a significant error reduction in the TMT-B. No intervention effect was observed on other cognitive variables.
CCE group
Comparison between pre- and postintervention assessments showed a significant time reduction in the completion of the TMT-A (p=0.007), indicating an improvement in processing speed. A trend toward improvement was observed for the visuospatial memory test (p=0.06). Other cognitive performances remained unchanged.
Group comparison for cognitive changes from baseline
Comparison between groups for cognitive change from the baseline did not reveal any significant differences on all measures. However, a medium ES was observed on TMT-A (d=0.51; 95% CI: −0.48:1.49; p=0.29), favoring the CCE group, and on the free recall of RL/RI-16 test (d=0.56; 95% CI: −0.43:1.55; p=0.23), favoring the CCS group.
Psychosocial outcomes
Table 3 presents the psychosocial measures scores for both the intervention groups at M0 and M3.
Table 3.
Psychosocial outcomes at baseline and postintervention: change from baseline (M3–M0) in each group and comparison between two groups
| Psychosocial measures | CCS group (n =9), mean (SD)/median (range)
|
CCE group (n =10), mean (SD)/median (range)
|
Group comparison for psychosocial change
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M0 | M3 | M3–M0 | p-value | M0 | M3 | M3–M0 | p-value | Cohen’s d (95% CI) | p-value | |
| Motivation | 6 (3:6) | 6 (4:6) | 0 (−1:2) | 0.57 | 6 (4:6) | 5.5 (4:6) | 0 (−2:1) | 0.85 | – | 0.62 |
| TAQ | 70.3 (40.9) | 79 (31.8) | 8.7 (23) | 0.29 | 69.3 (26.2) | 92 (26.7) | 22.7 (20.3) | 0.006** | −0.65 (−1.64:0.34) | 0.18 |
| Depression | 4.6 (2.9) | 4.4 (3) | −0.1 (1.6) | 0.84 | 4 (2.2) | 3.4 (2) | −0.6 (2.7) | 0.50 | 0.22 (−0.76:1.19) | 0.64 |
| Anxiety | 5.1 (1.7) | 4.7 (1.6) | −0.4 (1.4) | 0.38 | 4.6 (2.2) | 4.7 (1.6) | 0.1 (1.9) | 0.87 | −0.32 (−1.3:0.66) | 0.49 |
| Self-esteem | 29.8 (4.4) | 32.7 (3.8) | 2.9 (2.3) | 0.005** | 26.7 (6) | 30.2 (4.7) | 3.5 (5.6) | 0.08 | −0.14 (−1.11:0.83) | 0.76 |
| EQVPA | 10 (3.8) | 10.1 (2.8) | 0.1 (2.3) | 0.89 | 10.2 (1.3) | 10.5 (2.1) | 0.3 (1.6) | 0.58 | −0.1 (−1.07:0.87) | 0.87 |
| CDS | 19.3 (5.5) | 19.4 (5) | 0.1 (3.8) | 0.93 | 23.3 (8) | 22.8 (6.3) | −0.5 (6.4) | 0.81 | 0.12 (−0.86:1.09) | 0.80 |
Note:
p<0.01.
Abbreviations: CCE, computerized cognitive engagement; CCS, computerized cognitive stimulation; CDS, cognitive difficulties scale; EQVPA, quality of life scale for older French people [Echelle de Qualité de Vie adpatée aux Personnes Agées]; M0, baseline; M3, the end of the programs; SD, standard deviation; TAQ, technology acceptance questionnaire.
CCS group
A significant improvement in self-esteem was found postintervention (p<0.005), with an enhancement from a low (M=29.8) to a medium (M=32.7) self-esteem score. Quality of life, technology acceptance, memory complaints, anxiety and depression remained unchanged postintervention.
CCE group
At M3, a significant improvement in technology acceptance scores was found (p=0.006), indicating greater ICT acceptance.
Self-esteem showed a trend toward improvement (p=0.08), but the mean score at M3 remained within the ranks of a low self-esteem score. Quality of life, memory complaints, anxiety and depression remained unchanged.
Group comparison for psychosocial changes from baseline
Comparison of psychosocial intervention effects between the two groups did not reveal any significant differences in all measures. However, we found a medium intervention ES, favoring the CCE group with regards to the technology acceptance measure (d=−0.65; 95% CI: −1.64:0.34; p=0.18).
Discussion
The objective of this study was to examine the feasibility and acceptability and to compare the effects of a CCS program and a CCE program on both cognitive and psychosocial functioning in individuals with MCI. Our findings indicated that both interventions were highly feasible and acceptable in individuals with MCI. For both interventions, we observed improvements in different aspects of cognitive and psychological functioning at postintervention assessment.
Both interventions were highly feasible, as indicated by participants’ perfect attendance, the very low dropout rate throughout the study and the lack of reports of adverse effects. Both programs were highly acceptable and appreciated by participants, as they met their needs to overcome cognitive difficulties and loneliness/social isolation. Additionally, participants showed high levels of motivation, maintained throughout the 12 sessions. They also expressed a desire to continue participating in the program.
The CCS intervention was designed to stimulate several cognitive domains with computerized cognitive exercises, using a tablet-PC and social interactions among participants. At postintervention assessment, we observed a significant error reduction in the TMT-B, suggesting the improvement of inhibitory control and mental flexibility. This finding is not surprising, as several computerized cognitive exercises in the program required the participants to focus on targeted stimuli, while ignoring distractors. In addition, the participants showed a trend toward an improvement of free recall. Optimal free recall performance involves a number of memory strategies, such as semantic processing, visual imagery, categorization, association and organization,47,48 which were trained by several exercises in the CCS program. This finding is consistent with previous studies showing positive effects on free recall performance of various cognitive training programs in subjects with MCI.48,49 The CCS program also showed a beneficial effect on participants’ self-esteem. During the group sessions, participants were encouraged to interact and discuss with others. Discussions were led by the neuropsychologist animating the group on a variety of themes, such as cognitive complaints, strategies to compensate for cognitive difficulties, leisure activities and so on. Participants could experience and express their emotions in a supportive atmosphere. They were also encouraged to help each other when encountering difficulties during cognitive exercises. A sense of togetherness or group cohesiveness was created during the intervention, which might contribute to promote self-esteem.50 These findings are also consistent with previous studies showing the benefits of CS therapy on cognitive functioning and well-being in patients suffering from dementia in different cultures.51–55
The CCE intervention was designed to train participants to use a tablet-PC, in order to discover different kinds of applications and stimulate social interactions among participants. At postintervention assessment, participants showed a significant time reduction in the completion of the TMT-A, indicating an improvement in processing speed. A trend toward improvement was also observed for the visuospatial memory test. Our findings concur with those of two recent studies training healthy older adults to use a tablet-PC, showing a positive intervention effect on processing speed27,28 and episodic memory.28 Regarding the psychosocial intervention effects of the CCE, significant improvement on technology acceptance scores suggests greater ICT acceptance. Participants in the CCE group were trained in the use of a tablet-PC by the neuropsychologist, playing the part of a caring and sensitive trainer, capable of bringing adequate support to participants. It has been reported that adequate training in the use of ICT has a positive impact not only on ICT skills, but also on attitudes toward ICT, such as reduced anxiety, more perceived usefulness, improved self-efficacy, increased interest and so on. These factors contribute to greater ICT acceptance.56,57 Participants also showed a trend toward self-esteem improvement, probably due to group cohesiveness, as created during the group sessions. This was also the case for the CCS group.
Both CCS and CCE interventions engaged subjects with MCI in mentally challenging activities, which have the potential to maintain cognitive abilities and to reduce cognitive decline.58 In the CCS group, participants were required to concentrate on a variety of computerized exercises tapping into different cognitive domains, while in the CCE group, participants learned new skills with regards to tablet-PC use, which may stimulate several cognitive abilities, such as executive functions, memory and reasoning.59 These two different intervention approaches could thus have positive impacts on different aspects of cognition. Compared to CCE, the CCS group showed a medium ES on free recall performance, as the participants were trained to use mnemonic strategies to perform computerized cognitive exercises. The CCE group showed a medium ES in promotion of ICT acceptance, as the participants learned to use a form of new digital technology and discovered its usefulness in their daily life. This intervention allows participants to keep up-to-date with a society rich in and reliant on ICT. Finally, in this study, we could not explain why the CCE group showed a medium ES on processing speed, as compared with the CCS group. This issue could be addressed in a future study.
Limitation
The main limitation of the study was the small sample size, making it difficult to interpret the findings and possibly prohibiting many effects from emerging. However, this study was exploratory in nature, allowing us to adjust the study protocol (eg, increasing the number of sessions per week, adding a 6-month follow-up to investigate long-term effects and so on) for a future study.
Conclusion
This study showed high feasibility and acceptability and beneficial effects of two cognitive intervention programs in older adults with MCI. The CCS program had a potential to improve episodic memory, which seems to be a promising approach to delay the onset of dementia. The cognitive engagement program could have the potential to improve processing speed and ICT acceptance, offering newer perspectives and an interesting approach to bridge the digital divide and to promote social inclusion.
Acknowledgments
The authors thank KODRO for generously providing them with access to the software for this thesis work. The authors would like to thank the participants for their collaboration in this project. Leila Djabelkhir was awarded a doctoral scholarship from the Fondation des Gueules Cassées. [Foundation of Broken Jaws].
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wimo A, Jönsson L, Bond J, Prince M, Winblad B, International AD. The worldwide economic impact of dementia 2010. Alzheimer’s Dement. 2013;9(1):1–11.e13. doi: 10.1016/j.jalz.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Farias S, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66(9):1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Stevens J, Ganguli M, Tangalos EG, Cummings JL, DeKosky S. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review) Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 5.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on mild cognitive impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 6.Cavallo M, Zanalda E, Johnston H, Bonansea A, Angilletta C. Cognitive training in a large group of patients affected by early-stage Alzheimer’s disease can have long-lasting effects: a case-control study. Brain Impairment. 2016;17(2):182–192. [Google Scholar]
- 7.Cavallo M, Hunter EM, van der Hiele K, Angilletta C. Computer-ized structured cognitive training in patients affected by early-stage Alzheimer’s disease is feasible and effective: a randomized controlled study. Arch Clin Neuropsychol. 2016;31(8):868–876. doi: 10.1093/arclin/acw072. [DOI] [PubMed] [Google Scholar]
- 8.De Rotrou J, Wenisch E, Cantegreil I, et al. La stimulation cognitive. NPG Neurologie-Psychiatrie-Gériatrie. 2006;6(34):17–18. [Google Scholar]
- 9.De Rotrou J. Méthodologie pour une stimulation psychologique des fonctions cognitives. Paper presented at: Démences du sujet âgé et environnement: actes du 2e colloque, les; 1985. [Google Scholar]
- 10.De Rotrou J. Stimulation et rééducation de la memoire. Gazette Médicale de France. 1989;96(18):49–53. [Google Scholar]
- 11.De Rotrou J. Stimulation et éducation cognitives. Gérontologie et Société. 2001;(2):175–192. [Google Scholar]
- 12.Clare L, Woods RT. Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer’s disease: a review. Neuropsychol Rehabil. 2004;14(4):385–401. [Google Scholar]
- 13.Breuil V, De Rotrou J, Forette F, et al. Cognitive stimulation of patients with dementia: preliminary results. Int J Geriatr Psychiatry. 1994;9(3):211–217. [Google Scholar]
- 14.Spector A, Thorgrimsen L, Woods B, et al. Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia. Br J Psychiatry. 2003;183(3):248–254. doi: 10.1192/bjp.183.3.248. [DOI] [PubMed] [Google Scholar]
- 15.Orrell M, Spector A, Thorgrimsen L, Woods B. A pilot study examining the effectiveness of maintenance cognitive stimulation therapy (MCST) for people with dementia. Int J Geriatr Psychiatry. 2005;20(5):446–451. doi: 10.1002/gps.1304. [DOI] [PubMed] [Google Scholar]
- 16.Knapp M, Thorgrimsen L, Patel A, et al. Cognitive stimulation therapy for people with dementia: cost-effectiveness analysis. Br J Psychiatry. 2006;188(6):574–580. doi: 10.1192/bjp.bp.105.010561. [DOI] [PubMed] [Google Scholar]
- 17.Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;(2):CD005562. doi: 10.1002/14651858.CD005562.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Wenisch E, Cantegreil-Kallen I, De Rotrou J, et al. Cognitive stimulation intervention for elders with mild cognitive impairment compared to normal aged subjects: preliminary results. Aging Clin Exp Res. 2007;19(4):316. doi: 10.1007/BF03324708. [DOI] [PubMed] [Google Scholar]
- 19.National Collaborating Centre for Mental H. National institute for health and clinical excellence: guidance . Dementia: A NICE-SCIE Guideline on Supporting People with Dementia and Their Carers in Health and Social Care. Leicester, UK: The British Psychological Society and The Royal College of Psychiatrists; 2007. [PubMed] [Google Scholar]
- 20.Fiatarone Singh MA, Gates N, Saigal N, et al. The study of mental and resistance training (SMART) study resistance training and/or cognitive training in mild cognitive impairment: a randomized, double-blind, double-sham controlled trial. J Am Med Dir Assoc. 2014;15(12):873–880. doi: 10.1016/j.jamda.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Gagnon LG, Belleville S. Training of attentional control in mild cognitive impairment with executive deficits: results from a double-blind randomised controlled study. Neuropsychol Rehabil. 2012;22(6):809–835. doi: 10.1080/09602011.2012.691044. [DOI] [PubMed] [Google Scholar]
- 22.Oskoei AS, Nejati V, Ajilchi B. The effectiveness of cognitive rehabilitation on improving the selective attention in patients with mild cognitive impairment. J Behav Brain Sci. 2013;3:474–478. [Google Scholar]
- 23.Coyle H, Traynor V, Solowij N. Computerized and virtual reality cognitive training for individuals at high risk of cognitive decline: systematic review of the literature. Am J Geriatr Psychiatry. 2015;23(4):335–359. doi: 10.1016/j.jagp.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Hill NTM, Mowszowski L, Naismith SL, Chadwick VL, Valenzuela M, Lampit A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am J Psychiatry. 2016;174(4):329–340. doi: 10.1176/appi.ajp.2016.16030360. [DOI] [PubMed] [Google Scholar]
- 25.Jak AJ, Seelye AM, Jurick SM. Crosswords to computers: a critical review of popular approaches to cognitive enhancement. Neuropsychol Rev. 2013;23(1):13–26. doi: 10.1007/s11065-013-9226-5. [DOI] [PubMed] [Google Scholar]
- 26.Gates N, Valenzuela M. Cognitive exercise and its role in cognitive function in older adults. Curr Psychiatry Rep. 2010;12(1):20–27. doi: 10.1007/s11920-009-0085-y. [DOI] [PubMed] [Google Scholar]
- 27.Vaportzis E, Martin M, Gow AJ. A tablet for healthy ageing: the effect of a tablet computer training intervention on cognitive abilities in older adults. Am J Geriatr Psychiatry. 2017;25(8):841–851. doi: 10.1016/j.jagp.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan MY, Haber S, Drew LM, Park DC. Training older adults to use tablet computers: does it enhance cognitive function? Gerontologist. 2016;56(3):475–484. doi: 10.1093/geront/gnu057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thabane L, Hopewell S, Lancaster GA, et al. Methods and processes for development of a CONSORT extension for reporting pilot randomized controlled trials. Pilot Feasibility Stud. 2016;2(1):25. doi: 10.1186/s40814-016-0065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 31.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 32.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Reitan RM. Trail Making Test: Manual for Administration and Scoring. Tucson, AZ: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- 34.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 35.Wechsler D. Wechsler Adult Intelligence Scale – Fourth Edition (WAIS-IV) San Antonio, TX: Pearson; 2008. [Google Scholar]
- 36.Grober E, Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol. 1987;3(1):13–36. [Google Scholar]
- 37.Van der Linden M, Coyette F, Poitrenaud J, et al. L’épreuve de rappel libre/rappel indicé à 16 items (RL/RI-16) Solal; 2004. [Google Scholar]
- 38.De Rotrou J, Forette F, Hervy MP, et al. The cognitive efficiency profile: description and validation in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 1991;6(7):501–509. [Google Scholar]
- 39.Goldberg D, Bridges K, Duncan-Jones P, Grayson D. Detecting anxiety and depression in general medical settings. BMJ. 1988;297(6653):897–899. doi: 10.1136/bmj.297.6653.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNair DM, Kahn RJ. Self-assessment of cognitive deficits scale. In: Crook T, Ferris S, Bartus R, editors. Assessment in Geriatric Psychopharmacology. New Canaan, CT: Mark Powley Associates; 1983. pp. 137–143. [Google Scholar]
- 41.Poitrenaud J, Israel L, Barrere H, Le Roc’h K. Version française de l’échelle de difficulties cognitive de McNair et Khan. In: Michel BF, Derouesne C, Gely-Nargeot MC, editors. de la plainte mnésique à la maladie d’Alzheimer. Marseille: Solal; 1997. pp. 159–177. [Google Scholar]
- 42.Vallieres EF, Vallerand RJ. Traduction et validation canadienne-française de l’échelle de l’estime de soi de Rosenberg. Int J Psychol. 1990;25(2):305–316. [Google Scholar]
- 43.Rosenberg M, Black SR, Self-Esteem W. The Urban School Child. Washington, DC: American Sociological Association; 1971. [Google Scholar]
- 44.Petit S, Bergua V, Peres K, Bouisson J, Koleck M. Élaboration et validation d’une échelle de qualité de vie adaptée aux personnes âgées (EQVPA) Gériatrie et Psychologie Neuropsychiatrie du Vieillissement. 2014;12(4):379–386. doi: 10.1684/pnv.2014.0510. [DOI] [PubMed] [Google Scholar]
- 45.Brangier E, Hammes S. Elaboration et validation d’un questionnaire de mesure de l’acceptation des technologies de l’information et de la communication basé sur le modèle de la symbiose humain-technologie-organisation [Development and validation of a questionnaire to measure the acceptance of the technologies of information and communication based on the model of the human-technology-organization symbiosis] In: Brangier E, Kolski C, Ruault JR, editors. L’humain comme acteur de performance des systèmes complexes. Actes du congrès Ergo’IA 2006 [The human as an actor of performance of complex systems. Acts of Congress Ergo’ AI 2006] Bidart: Estia Innovation éditeur; 2006. pp. 71–78. French. [Google Scholar]
- 46.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 47.Bellezza FS, Richards DL, Geiselman RE. Semantic processing and organization in free recall. Mem Cognit. 1976;4(4):415–421. doi: 10.3758/BF03213198. [DOI] [PubMed] [Google Scholar]
- 48.Herrera C, Chambon C, Michel BF, Paban V, Alescio-Lautier B. Positive effects of computer-based cognitive training in adults with mild cognitive impairment. Neuropsychologia. 2012;50(8):1871–1881. doi: 10.1016/j.neuropsychologia.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Belleville S, Gilbert B, Fontaine F, Gagnon L, Ménard E, Gauthier S. Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: evidence from a cognitive intervention program. Dement Geriatr Cogn Disord. 2006;22(5–6):486–499. doi: 10.1159/000096316. [DOI] [PubMed] [Google Scholar]
- 50.Chao SY, Liu HY, Wu CY, et al. The effects of group reminiscence therapy on depression, self esteem, and life satisfaction of elderly nursing home residents. J Nurs Res. 2006;14(1):36–45. doi: 10.1097/01.jnr.0000387560.03823.c7. [DOI] [PubMed] [Google Scholar]
- 51.Aguirre E, Hoare Z, Streater A, et al. Cognitive stimulation therapy (CST) for people with dementia–who benefits most? Int J Geriatr Psychiatry. 2013;28(3):284–290. doi: 10.1002/gps.3823. [DOI] [PubMed] [Google Scholar]
- 52.Paddick SM, Mkenda S, Mbowe G, et al. Cognitive stimulation therapy as a sustainable intervention for dementia in sub-Saharan Africa: feasibility and clinical efficacy using a stepped-wedge design. Int Psychogeriatr. 2017;29(6):979–989. doi: 10.1017/S1041610217000163. [DOI] [PubMed] [Google Scholar]
- 53.Yamanaka K, Kawano Y, Noguchi D, et al. Effects of cognitive stimulation therapy Japanese version (CST-J) for people with dementia: a single-blind, controlled clinical trial. Aging Ment Health. 2013;17(5):579–586. doi: 10.1080/13607863.2013.777395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spector A, Orrell M, Woods B. Cognitive Stimulation Therapy (CST): effects on different areas of cognitive function for people with dementia. Int J Geriatr Psychiatry. 2010;25(12):1253–1258. doi: 10.1002/gps.2464. [DOI] [PubMed] [Google Scholar]
- 55.Spector A, Gardner C, Orrell M. The impact of cognitive stimulation therapy groups on people with dementia: views from participants, their carers and group facilitators. Aging Ment Health. 2011;15(8):945–949. doi: 10.1080/13607863.2011.586622. [DOI] [PubMed] [Google Scholar]
- 56.Wu YH, Damnée S, Kerhervé H, Ware C, Rigaud AS. Bridging the digital divide in older adults: a study from an initiative to inform older adults about new technologies. Clin Interv Aging. 2015;10:193–201. doi: 10.2147/CIA.S72399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodward AT, Freddolino PP, Wishart DJ, et al. Outcomes from a peer tutor model for teaching technology to older adults. Ageing Soc. 2012;33(8):1315–1338. [Google Scholar]
- 58.Ackerman PL, Kanfer R, Calderwood C. Use it or lose it? Wii brain exercise practice and reading for domain knowledge. Psychol Aging. 2010;25(4):753–766. doi: 10.1037/a0019277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park DC, Gutchess AH, Meade ML, Stine-Morrow EAL. Improving cognitive function in older adults: nontraditional approaches. J Gerontol Ser B. 2007;62(Special_Issue_1):45–52. doi: 10.1093/geronb/62.special_issue_1.45. [DOI] [PubMed] [Google Scholar]
- 60.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5(3):299–314. [Google Scholar]


