Abstract
Complex emotional, cognitive and self-reflective functions rely on the activation and connectivity of large-scale neural circuits. These circuits offer a relevant scale of focus for conceptualizing a taxonomy for depression and anxiety based on specific profiles (or biotypes) of neural circuit dysfunction. Here, the theoretical review first outlined the current consensus as to what constitutes the organization of large-scale circuits in the human brain identified using parcellation and meta-analysis. The focus is on neural circuits implicated in resting reflection (“default mode”), detection of “salience”, affective processing (“threat” and “reward”), “attention” and “cognitive control”. Next, the current evidence regarding which type of dysfunctions in these circuits characterize depression and anxiety disorders was reviewed, with an emphasis on published meta-analyses and reviews of circuit dysfunctions that have been identified in at least two well-powered case:control studies. Grounded in the review of these topics, a conceptual framework is proposed for considering neural circuit-defined “biotypes”. In this framework, biotypes are defined by profiles of extent of dysfunction on each large-scale circuit. The clinical implications of a biotype approach for guiding classification and treatment of depression and anxiety is considered. Future research directions will develop the validity and clinical utility of a neural circuit biotype model that spans diagnostic categories and helps to translate neuroscience into clinical practice in the real world.
INTRODUCTION
We are experiencing a paradigm shift in psychiatry and the integration of psychiatry with the neurosciences. This integration is motivated by the search for a model that connects a neurobiological understanding of mental disorder with clinical phenomenology, in order to improve the precision of classification and treatment decisions. Major disorders of mood and anxiety impair the very functions that enable us to live productive and satisfying lives. Each year, an estimated 16 million American adults have at least one episode of major depressive disorder (MDD) and 40 million experience an anxiety disorder[1]. These disorders are the leading causes of disability and lost productivity, with a staggering economic cost of $42-$53 billion per year[2].
CONCEPTUALIZING DEPRESSION AND ANXIETY AS “NEURAL CIRCUIT” DISORDERS
“Brain disorder” is typically used to refer a neurological condition associated with a lesion or degenerative process in discrete brain regions. Mental disorders such as depression and anxiety may not typically have been considered “brain disorders” due to our limited understanding of the organization of human neural circuits and the way in which disorganization of these circuits may disrupt normal cognitive, emotional and self-reflective functions. Now that we have brain imaging techniques with sufficient spatial and temporal resolution to quantify such neural circuit organization in vivo, we have the opportunity to reformulate our neurobiological understanding of mental disorders. This vision is at the heart of NIMH’s goal to “map the connectomes for mental illness” (NIMH’s Strategic Plan, Strategy 1.3 under Objective 1). It is also consistent with the cross-cutting themes of the “Research Domain Criteria” (RDoC) project[3].
The term “neural circuit” has typically referred to how one neuron communicates with another through synaptic connections and transmission[4]. Here, the term “large-scale neural circuit” is used to refer to the macroscale of neural organization. At the macroscale, vast numbers of interconnected neurons constitute anatomical and functional circuits that make up the “connectome” of the brain[5,6]. These vast sets of neurons can be probed by noninvasive brain imaging to visualize the activation and structure of specific regions, the structural communication between regions and functionally correlated regions of activation at rest or during task-evoked situations[7–9]. In brain imaging studies, these macroscale circuits have commonly been referred to as “networks” (for example, the “default mode network”[10,11].
Neural circuits can be considered a pertinent scale of measurement from which to delineate a neurobiology of human mental disorder. Circuits integrate across different levels and measures of brain function, but still reflect the complexity of the brain. Circuits are engaged by specific human cognitive, emotional and self-reflective functions, and offer promise for defining appropriate animal homologues. It is likely that most of the human brain involves multiple parallel circuits that are interdigitated such that each cortical lobe contains components of multiple circuits[12,13]. This organization may have occurred with the expansion of the association cortex in humans relative to non-human primates[12]. Mood and anxiety disorders may be possible maladaptive consequences of this expansion.
METHODS
LITERATURE SEARCH
To search the literature we used PubMedR and PsycINFOR and combined three sets of search terms as follows:
Disorder class. The search was by classes that span the DSM-5 diagnoses of mood and anxiety disorders and also compatible with previous editions of DSM; Depressive Disorders (including Major Depressive Disorder, Disruptive Mood Dysregulation Disorder); Anxiety Disorders (including Agoraphobia, Generalized Anxiety Disorder, Social Anxiety Disorder); Obsessive-Compulsive and Related Disorders; and Trauma-and-Stressor-Related Disorders (including Post-Traumatic Stress Disorder, Unspecified Trauma-and Stressor-Related Disorder). This trans-diagnostic approach also took into account the substantial comorbidity (at least 40%) of mood and anxiety disorders[14]. To consider future implications for the wider transdiagnostic relevance of the research we also included the term “bipolar disorder”.
Neural circuit terms and metrics. “Disorder class” terms were combined with the following neural circuit terms: “neural circuit”, “network”, “functional brain imaging”, “activation”, “connectivity”, “resting” and “task” and “grey matter” and “white matter”.
Specific neural circuits. The focus was on six large-scale circuits identified using the terms “default mode”, “salience”, “affective”, “positive emotion”, “negative emotion”, “threat”, “reward”, “attention”, “frontoparietal”, “cognitive control” and “central executive”.
Searches were restricted to articles written or translated into English from December 1990 to June 30, 2016, consistent with the period in which functional brain imaging has emerged for the study of psychiatric disorder. The focus of this review was on functional activation and connectivity, as reflected in the blood oxygen level-dependent (BOLD) signal of functional magnetic resonance imaging (fMRI) technology. However, studies involving the structural neuroanatomy and white matter connectivity of depression and anxiety were also included as a secondary focus. The reason for this secondary focus was to consider areas of evidence for which depression and/or anxiety are associated with both functional and structural impairments in the same circuit, which may be indicative of a more trait-like biotype,
NEURAL CIRCUIT SELECTION
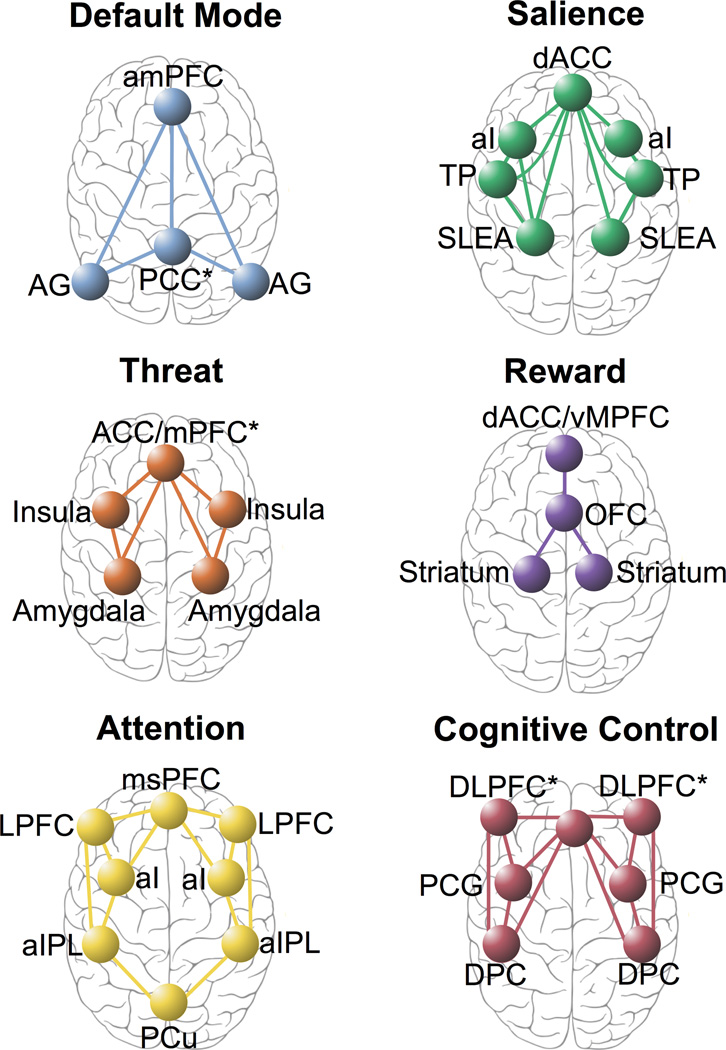
Researchers have identified intrinsic neural circuits that support domain-general processes of self-reflection, salience perception and alertness (Figure 1; [15]) as well as conflict monitoring, attention, sensori-motor, visual and auditory processes (Supplemental Figure 1)[16–24]. The intrinsic architecture has been demonstrated using large-scale functional connectivity analysis of hundreds of brain regions that have been identified using parcellation and meta-analysis and that define major brain systems at rest and across many task-evoked states (e.g., [25]). These circuits may be observed in the task-free state and during task-evoked conditions. During rest the default mode circuit tends to be up-regulated and other circuits, down-regulated[16,25]. Specific task states (such as those designed to probe reactivity to potential threat or reward) engage more specialized functional components of these circuits (e.g.[26–31]) (Figure 1).
Figure 1.
Large-scale intrinsic and task-evoked circuits identified in the extant literature
Abbreviations: ACC, Anterior Cingulate Cortex; AG, Angular Gyrus; aI, Anterior Insula, aIPL, Anterior Inferior Parietal Lobule; amPFC, Anterior Medial Prefrontal Cortex; dACC, Dorsal Anterior Cingulate Cortex; DLPFC*, Dorsolateral Prefrontal Cortex + Anterior Prefrontal Cortex + Inferior Frontal Cortex; DPC, Dorsal Parietal Cortex; Hipp, Hippocampus; LPFC, Lateral Prefrontal Cortex; mPFC, Medial Prefrontal Cortex; msPFC, Medial Superior Prefrontal Cortex; OFC, Orbitofrontal Cortex; PCC, Posterior Cingulate Cortex; PCG, Precentral Gyrus; PCu, Precuneus; SLEA, Sublenticular Extended Amygdala; vMPFC, Ventromedial Prefrontal Cortex
Given the wide scope of the search and the theoretical nature of the review, the focus of interpretation was on areas in which there is a convergence of evidence. Thus, the emphasis was on reviews and meta-analyses and circuit dysfunctions that have been identified in at least two well-powered case:control studies. With this emphasis, we focused on six circuits in particular: default mode, salience, negative affect, positive affect, attention and cognitive control.
RESULTS
The results of this theoretical review are organized as follows:
The current consensus as to what constitutes the organization of large-scale circuits in the human brain identified using parcellation and meta-analysis.
The current evidence regarding which type of dysfunctions in these circuits characterize depression and anxiety disorders was reviewed, with an emphasis on published meta-analyses and reviews of circuit dysfunctions that have been identified in at least two well-powered case:control studies.
Table 1 provides a complementary summary of the extant studies identified by these searches.
Table 1.
A summary of the current knowledge about large-scale neural circuits, their role in human brain organization, functional alterations in these circuits in depression and anxiety, and accompanying structural alterations in depression and anxiety.
| Circuit | Role | Functional alterations in depression and anxiety |
Structural alterations in depression and anxiety |
|---|---|---|---|
|
Default Mode Anterior middle frontal cortex (amPFC), posterior cingulate cortex (PCC) and angular gyrus (AG) [11,32] |
Self- referential thinking at rest [163,164] |
Hypo-connectivity Posterior hypo-connectivity correlated with over- general memory [61] and treatment sensitivity in MDD (for review [62]). mPFC-AG hypo-connectivity in SAD [63] Hyper-connectivity Anterior medial hyper-connectivity in MDD [22,74,163], correlated with rumination in MDD [57,61] and treatment resistance in MDD [62,166,167] Hyper-connectivity of the Default Mode with the Attention circuit in MDD for meta-analysis [74] |
Grey matter Reductions in MDD [58,60] White matter Hypo-connectivity in MDD [59,165] |
|
Salience Anterior cingulate cortex (ACC), anterior insula (aI) and sublenticular extended amygdala (SLEA) [21,168] |
Detecting salient changes [21] |
Hypo-connectivity Amygdala-insula hypo-connectivity in MDD [55] Insula hypo-connectivity in MDD correlated with overall symptom severity [70]; Amygdala- ACC hypo-connectivity in SAD [72] Amygdala hypo-connectivity correlated with avoidance symptoms [71] Hyper-connectivity Hyper-connectivity of the Salience with the Default Mode circuit in MDD [70], correlated with severity of depressive rumination [57] |
|
|
Threat Amygdala, hippocampus dorsal, rostral and subgenual ACC, mPFC and insula [21,168] |
Threat reactivity and regulation [27] |
Altered activation for threat Amygdala hyper-activation for threat faces in MDD, GAD, SAD [95,107] and anxiety traits [87,96] ACC hypo-activation to threat faces in GAD and SAD [40,88,89] and amygdala hypo-activation to threat faces in MDD [77,94,169,170] Hypo-connectivity for threat Amygdala-ACC hypo- connectivity to fear in MDD [94,171], SAD [95] and GAD [40,172] Amygdala hypo-activation to fear/anger is a general predictor of response to antidepressants, and amygdala hyper-activation to sad is a differential predictor of non-response to SNRI antidepressants [77]. |
Grey matter Reduced hippocampal grey matter in MDD and GAD [100,173–176] White matter connectivity Reduced uncinate fasciculus white matter connections in MDD [99] |
|
Reward Ventral striatum, orbitofrontal cortex (OFC) and mPFC regions [28,45] |
Sensitivity to and anticipation of reward stimuli |
Striatal hypo-activation Anhedonic MDD: Striatal hypo-activation for happy faces [110] (for review [102]; for meta-analysis [113]) and monetary tasks [102] (for meta-analysis [101]; mPFC hypo-activation for positively valenced stimuli [111]. Altered frontal activation ACC/MPFC/OFC/ hyper-activation for happy faces [110,111,113,177], reward anticipation and reward outcomes [112] in MDD. |
Grey matter Reduced striatal volume in MDD [104,105] White matter Reduced white matter connectivity in MDD [106] |
|
Attention Medial superior frontal cortices (msPFC), aI, anterior inferior parietal lobule (aIPL) and precuneus (PCu) [46] |
Alertness and sustained attention [47] |
Hypo-connectivity Hypo-connectivity in MDD [55,63,71] Hyper-connectivity Frontoparietal hyper- connectivity with the striatal node of the reward circuit in SAD [72] |
White matter Reduced frontoparietal diffusion centrality in MDD [165] |
|
Cognitive Control Dorsolateral prefrontal cortex (DLPFC), ACC, Precentral gyrus (PCG), dorsal parietal cortex (DPC) [49] |
Working memory and selective attention [49,178] |
Hypo-activation DLPFC/ACC hypo-activation in MDD [114– 117,132,133,179] and in social anxiety [180] and induced anxious mood [118] Hypo-connectivity DLPFC-ACC hypo- connectivity in MDD [132,133] Hyper-activation Hyper-activation in MDD, suggesting compensation to achieve normal cognitive performance [121,124,126,129] |
Grey matter Reduced DLPFC and ACC grey matter in adult MDD [60] and late-life MDD [122]. |
Abbreviations: ACC, Anterior Cingulate Cortex; AG, Angular Gyrus; aI, Anterior Insula, aIPL, Anterior Inferior Parietal Lobule; amPFC, Anterior Medial Prefrontal Cortex; dACC, Dorsal Anterior Cingulate Cortex; DLPFC*, Dorsolateral Prefrontal Cortex + Anterior Prefrontal Cortex + Inferior Frontal Cortex; DPC, Dorsal Parietal Cortex; DSM, Diagnostic and Statistical Manual of Mental Disorders; GAD, Generalized Anxiety Disorder; Hipp, Hippocampus; LPFC, Lateral Prefrontal Cortex; MDD, Major Depressive Disorder; mPFC, Medial Prefrontal Cortex; msPFC, Medial Superior Prefrontal Cortex; OFC, Orbitofrontal Cortex; PCC, Posterior Cingulate Cortex; PCG, Precentral Gyrus; PCu, Precuneus; SAD, Social Anxiety Disorder; SLEA, Sublenticular Extended Amygdala; vMPFC, Ventromedial Prefrontal Cortex
“Default Mode” circuit
The default mode circuit (more typically known as the “default mode network”) is defined by the anterior medial prefrontal cortex (amPFC), posterior cingulate cortex (PCC) and angular gyrus[11,32] (Figure 1; Table 1). This circuit is observed when the brain is at rest under task-free conditions and typically when participants are instructed to reflect on their own spontaneously generated thoughts (Table 1). Independent components analysis suggests that the anterior and posterior regions define sub-networks of the default mode circuit (for review;[33]). This circuit also has a basis in structural white matter connections between the same regions[34,35]. Evidence from a twins samples indicates that the default mode circuit is engaged even during “rest” periods that occur between task stimuli, and this circuit is genetically heritable[35]. The “default mode” is currently listed under the Arousal and Regulatory systems domain of RDoC1.
“Salience” circuit
The “salience” circuit is defined by core nodes in the anterior cingulate cortex (ACC), anterior insula (aI) and sublenticular extended amygdala[19,21] (Figure 1; Table 1). The salience network is implicated in the detection of salient changes in the environment, both interoceptive and external, and signals the need for cognitive control (Table 1, [21]). Increased functioning of this network may result in a maladaptively low threshold to alter cognitive control[36]. The salience circuit is consistent with the RDoC construct of “arousal” listed under the Arousal and Regulatory Systems domain[3]2.
Negative Affect circuit: “Threat”
Affective circuits are robustly activated by biologically salient stimuli such as facial expressions signaling potential threat (fear, anger) and social reward (happy). Affective circuits for processing threat and reward are consistent with the RDoC domains of Negative Valence and Positive Valence systems3.
Threat processing components of the affective circuits comprise the amygdala, hippocampus, insula, and both dorsal and ventral portions of the prefrontal cortex, including the dorsal medial prefrontal cortex (dmPFC) and its dorsal ACC connections, and the ventral mPFC (vMPFC) and its ventral (subgenual and pregenual)-rostral ACC connections ([38,39]; Figure 1; Table 1). The dorsal prefrontal sub-circuit has been preferentially implicated in appraisal and expression of emotion and may be considered an “aversive amplification” sub-network[39] that serves to boost the processing of signals of potential threat [39]. Complementing this function, the ventral sub- circuit has been implicated in automatic regulation of negative emotion[38,40] (for review, [41]; for meta-analysis [38]). These components overlap with components of the salience circuit, and they may both be engaged by the arousal and valence properties of threat stimuli respectively.
These sub-networks may be engaged even in the absence of conscious sensory awareness[27] (for meta-analysis, [38]). In light of their commonly observed co-activation[38], the negative affect circuit might subserve the perception of negative emotion cues and the salience circuit, the arousal aspects of feeling these emotions.
Direct activation of the amygdala and prefrontal regions to which it projects may occur automatically even in the absence of explicit conscious evaluation ([27,42–44] for meta-analysis, [38]). Similar bottom-up amygdala reactivity has been observed for masked presentations of other threat stimuli such as phobic-relevant cues[44].
Positive Affect circuit: “Reward”
Reward processing components of the affective circuits are defined by the striatal nucleus accumbens and ventral tegmental areas (collectively referred to as “the striatum”) and their projections to the orbitofrontal cortex (OFC) and mPFC ([45]; Table 1). These regional components are preferentially engaged by different types of reward processing, including sensitivity to the presence of salient reward stimuli and the anticipation of these stimuli (Table 1). There are also connections between the striatum and the amygdala, consistent with interactions between the processing of threat and reward and of significant stimuli that encompass multiple valences[28].
Attention circuit
The frontoparietal “attention” circuit is defined by nodes in the medial superior frontal cortices, anterior insula, anterior inferior parietal lobule and precuneus[46,47]; Table 1). This circuit is implicated in alertness, sustained attention and the support of recollection ([17,47]; Table 1). It interacts closely with the default mode circuit to configure the switching from resting to task-context processing[47,48]. The attention circuit may be considered relevant to the attention construct listed under the RDoC Cognitive Systems domain4.
Cognitive Control circuit
The “cognitive control” circuit comprises the DLPFC, ACC, dorsal parietal cortex (DPC) and precentral gyrus (Table 1). Together these regions and their interconnectivity are implicated in the support of higher cognitive functions such as working memory and selective attention (for meta-analysis; [49], evidence from convergent neuroimaging methods; [50]). Under task-specific demands the cognitive control circuit is implicated in cognitive flexibility[51].
Types of neural circuit dysfunction underlying depression and anxiety
Three themes emerge from previous research on depression and anxiety. Previous research has focused mainly and appropriately on case:control comparisons of diagnostic groups of mood and anxiety disorder defined by traditional checklists of observed symptoms. These previous studies have also focused on activation within specific brain regions of interest and typically on one imaging modality at a time. While the emphasis has been on regional activation, there has been a recent escalation in structural and functional connectivity investigations of depression and anxiety. This escalating interest in connectivity in part reflects the advances in precision imaging and analysis techniques, including from the Human Connectome Project[7–9,17,52–54].
The findings from previous case:control studies of depression and anxiety tend to be inconsistent, revealing profiles of neural hypo-reactivity and hyper -reactivity, and both hypo-connectivity and hyper-connectivity, in people diagnosed with mood and anxiety disorders compared to their healthy peers.
Default Mode circuit disruptions in depression and anxiety
Several resting state studies have reported on functional over-activation and hyper-connectivity of the default mode circuit in depression[22,55] (for review of meta-analyses; [56]) (Table 1). Hyper-functioning of the default mode circuit in MDD has been associated with higher levels of maladaptive rumination about depressive thoughts and with lower levels of more adaptive self-reflection[56]. Hyper-activation of the frontal and anterior insula cortices in particular has been associated with maladaptive rumination[57]. Anatomical abnormalities might contribute to default mode circuit hyper-function. Structurally, MDD has been associated with decreased regional grey matter connectivity[58] and loss of white matter connectivity[59] within the default mode circuit, particularly within the posterior sub-network. Widespread reductions in grey matter have also been observed across regions of the default mode circuit and in nodes within interacting circuits[60]. Specifically, MDD patients show reduced grey matter volume in ACC and anterior medial regions of the prefrontal cortex and in parietooccpital regions consistent with components of the default mode circuit, as well as in striatal and limbic components of the affective circuits[61].
Other studies have reported evidence for hypo-connectivity of the default mode circuit in MDD that is correlated with clinical indicators of over-general autobiographical memory[61] and some suggestion of treatment sensitivity[62]. Hypo-connectivity of the default mode, specifically involving the MPFC and angular gyrus, has also been observed in social anxiety disorder[63]. This reduction in MPFC-angular gyrus connectivity has been interpreted as a possible neural basis for impairments in the perception of socially relevant emotional states and self-related mental representations[63].
Salience circuit disruptions in depression and anxiety
Studies of the salience circuit in depression and anxiety have focused on insula activation and connectivity in particular.
Insula hyper-reactivity has been observed in MDD under stimulus-evoked conditions of processing sadness and disgust[64,65] (for review[66]) (Table 1). Heightened insula reactivity is positively correlated with severity of depressive symptoms[67], suggesting a bias toward salient and mood-congruent stimuli. Individuals with generalized social anxiety disorder also show exaggerated insula reactivity when attending to salient emotional faces[68]. These functional activation differences might be due in part to structural deficits. For example, MDD patients show a loss of insula gray matter, which is negatively correlated with symptom severity[64].
In regard to functional connectivity, profiles of both hyper- and hypo-connectivity have been observed in depression and anxiety. Insula hypo-connectivity within the salience circuit has been observed in depression, social anxiety disorder and in panic disorder (for review, [33,69]) (Table 1). Insula hypo-connectivity has been inversely associated with symptom severity[33]. In generalized anxiety a weakening of the normal connectivity between the insula and the ACC has been observed, specifically when the patient is required to focus attention on salient emotional faces presented among neutral stimuli (such as shapes)[68].
Hypo-connectivity between the insula and amygdala has also been reported in MDD[55] and correlated with overall symptom severity[70] (for review, [33]; Table 1). Amygdala hypo-connectivity has been more specifically correlated with avoidance symptoms in social anxiety disorder[71]. Correspondingly, hypo-connectivity between the amygdala and ACC has also been observed in social anxiety disorder[72].
Hyper-connectivity between the insula and anterior nodes of the default mode circuit has been positively correlated with symptoms of nervous apprehension[73] and reported in both depression (for review, [33]) and social anxiety disorder (for review, [69]). Dorsal nodes of the salience circuit show both hyper- and hypo-connectivity with the posterior precuneus node of the attention circuit (for meta-analysis, [74]; Figure 2 reflects a profile of hyper-connectivity). The direction of altered connectivity between salience and other circuits may fluctuate with the nature of interoceptive or external events, consistent with the idea that the salience circuit guides how attention is switched flexibly between rest and stimulus-evoked processing.
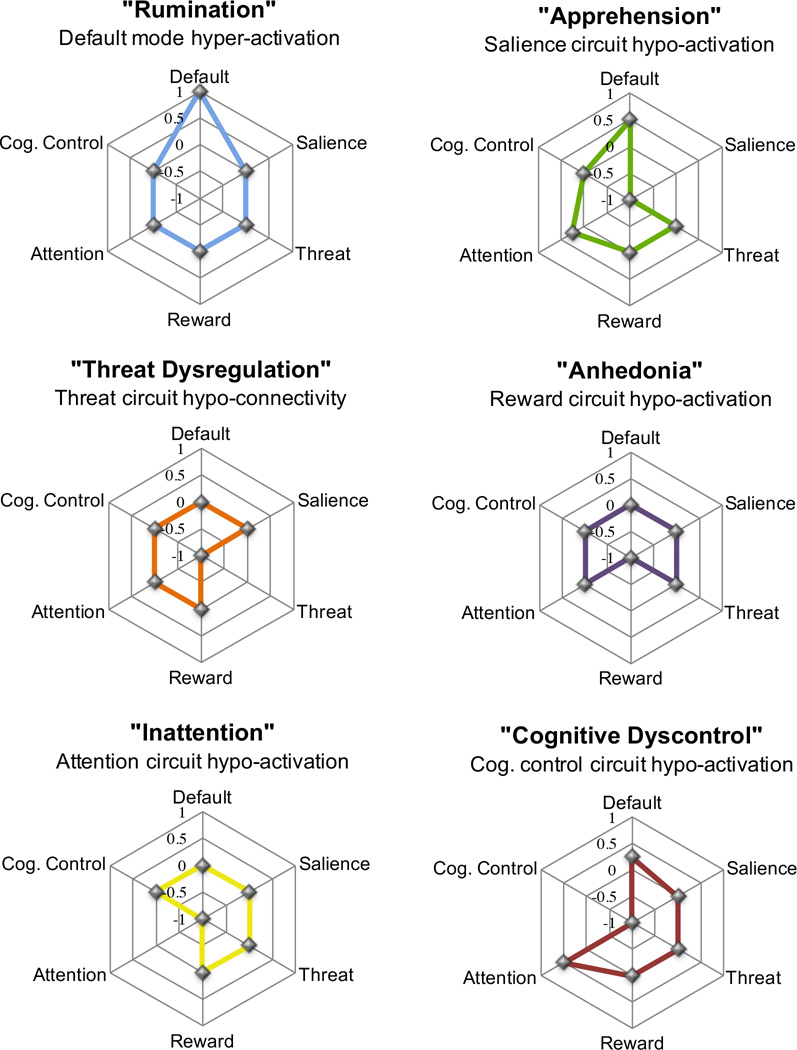
Figure 2.
Types of neural circuit dysfunction underlying phenotypes of depression and anxiety, based on current knowledge. These dysfunctions are described in terms of both the neural circuit involved (e.g., “reward”) and the clinical phenomenology (e.g., “anhedonia”) related to each dsyfunction.
Abbreviations: ACC, Anterior Cingulate Cortex; AG, Angular Gyrus; aI, Anterior Insula, aIPL, Anterior Inferior Parietal Lobule; amPFC, Anterior Medial Prefrontal Cortex; dACC, Dorsal Anterior Cingulate Cortex; DLPFC*, Dorsolateral Prefrontal Cortex + Anterior Prefrontal Cortex + Inferior Frontal Cortex; DPC, Dorsal Parietal Cortex; Hipp, Hippocampus; LPFC, Lateral Prefrontal Cortex; mPFC, Medial Prefrontal Cortex; msPFC, Medial Superior Prefrontal Cortex; OFC, Orbitofrontal Cortex; PCC, Posterior Cingulate Cortex; PCG, Precentral Gyrus; PCu, Precuneus; SLEA, Sublenticular Extended Amygdala; vMPFC, Ventromedial Prefrontal Cortex
Affective circuit disruptions in depression and anxiety
Threat
Altered threat processing, involving amygdala-ACC activation and connectivity have been observed across multiple diagnostic categories (for review [41,75,76]). Amygdala over-reactivity elicited by implicit or non-conscious processing of threat-related stimuli has been reported in current depressive disorder[77–81] (for review, [82]), generalized anxiety disorder[83], generalized social phobia/anxiety disorder[83–85], specific phobia[86], and panic disorder[83,86]. Excessive amygdala activity elicited by masked threat faces has also been associated with a dimension of trait anxiety and with neuroticism in otherwise healthy people[87] consistent with a trait-like phenotype of hyper-reactivity to sources of threat (Table 1). Hypo-activation of the ACC has been observed in generalized anxiety disorder[40,88,89] and generalized social anxiety[89] (Table 1).
While it is commonly presumed that the amygdala is engaged by potential threat, it is also more generally engaged by biologically significant emotion. In addition to the findings for threat, MDD has also been associated with mood-congruent hyper-reactivity of the amygdala evoked by sad faces [90–92].
Alterations in activation may also reflect a reduction in connectivity between the amygdala and subgenual/ventral ACC, observed during implicit processing of threat-related faces in unmedicatd MDD [93,94], generalized social anxiety disorder[95] and generalized anxiety disorder[40]. A lack of connectivity elicited during the conscious evaluation of threat has also been observed between the amygdala and prefrontal regions including the ACC[96], OFC[97], mPFC[98] and [95] for social anxiety disorder (Table 1).
Disruptions in amygdala-ACC functional connectivity might also have a basis in disruptions to white matter connectivity. For example, MDD has been associated with reduction in the uncinate fasciculus white matter connections that support functional communication between the amygdala and ACC [99]. An ongoing state of poor emotion regulation might also contribute to the often-observed loss of hippocampal grey matter in depression and anxiety (for meta-analysis; [100]).
Reward circuit disruptions in depression and anxiety
Across studies hypo-activation of the striatum has been identified as a robust characteristic of at least some patients with depression, especially those who report experiences of anhedonia (for meta-analysis[101]; for review; [102]) (Table 1). Such hypo-activation in depression is elicited not only by primary signals of social reward (such as happy faces) but also by tasks that rely on reward-motivated decision-making [102]. Striatal hypo-activation also characterizes adolescents at risk of depression[103], suggesting that a trait-like disruption to reward circuits may contribute to the development of mood disorder. Consistent with the possibility of a trait-like biotype for altered reward circuitry and anhedonia, depression has also been associated with grey matter loss in the striatum [104,105]. In addition, depression has been associated with increased white matter connectivity in bilateral corticospinal tracts, a structural alteration that might underlie some aspects of the striatal and motor functional disruptions in this disorder [106].
For social reward (happy faces) hypo-activation of the amygdala has also been observed in unmedicated MDD[77], generalized anxiety disorder[107], panic disorder[108] and obsessive compulsive disorder (OCD)[109], and may reflect a further neural characteristic of trans-diagnostic anhedonia. Frontally, in remitted depression, hyper-activation of the OFC, medial prefrontal/midfrontal regions and ACC has also been observed in response to happy faces[110,111], reward outcomes[112] and reward anticipation (for meta-analysis)[113]. Frontal hyper-activation might reflect an adaption accompanying striatal hypo-activation. However, the opposing finding of medial frontal hypo-activation for positive valence processing in anhedonic female patients has also been observed [110,111]
Attention circuit disruptions in depression and anxiety
There has also been relatively little work on disruptions to the frontoparietal attention circuit in depression and anxiety. However, several studies have observed hypo-connectivity within the attention circuit in MDD and in social anxiety[55,63,71]. Such hypo-connectivity within the attention circuit has been correlated with a specific behavioral profile of false alarm errors (e.g., responding to “no go” stimuli as if they are “go” stimuli) in anxiety disorder[36], suggesting a biotype of poor sustained attention and vigilance.
Cognitive control circuit disruptions in depression and anxiety
Dysfunction of the cognitive control circuit may be elicited by tasks that require effortful selective processing of relevant stimuli and inhibition of irrelevant stimuli, such as in a working memory task. Hypo-activation of the DLPFC and dorsal anterior cingulate cortex (dACC) during cognitive tasks, and in stress-induced situations, has been found in depressed patients and in social anxiety [114–117] (Table 1). Induced anxious mood has also been related to persistent DLPFC hypo-activation during working memory performance[118]. Hypo-activity in defining nodes of the cognitive control circuit has been observed in adolescents with depression and found to persist after recovery in adult and later-life depression[117,119–121], suggesting that this type of dysfunction may have a trait-like status. This trait-like status is also suggested by the presence of reductions in grey matter volume of the same DLPFC and ACC regions in younger and older adults with MDD[60,122].
Cognitive control problems in depression may also involve problems suppressing default ruminative thoughts, reflected in positive correlations (rather than anti-correlation) between DLFPC cognitive control regions and posterior cingulate default mode regions[22,123].
Suggesting a second type of cognitive control circuit dysfunction, some depressed patients show hyper- (rather than hypo-) activation of the DLPFC during working memory and executive function tasks. DLPFC hyper-activation has been observed in depression during tasks with an increasing cognitive demand but in the absence of behavioral deficits in performing the task[121,124–129]. In this context, hyper-activation may reflect an attempt at compensation to retain normal cognitive behavior[121,129]. Over-activity in both the rostral and dorsal portions of the ACC[121,125,130]), as well as DLPFC-ACC hyper-connectivity, has also been observed in MDD when participants are performing similarly to controls. Hyper-activation in regions of the cognitive control circuitry has been observed in adolescents with depression[121] and in medicated[121,124,130] and unmedicated[126] individuals with MDD, and it persists in the ACC after remission[131]. Hyper-connectivity of the DLPFC and cingulate has also been observed in MDD during working memory tasks.
Considering clinical translatable profiles of neural circuit dysfunction
Based on the variability of existing findings we might envision a theoretical taxonomy of neural circuit biotypes, each of which may cut across the traditional diagnostic categories of mood and anxiety disorder. One possible explanation for the variability in the extant literature is that multiple types of dysfunction are conflated within the one group average in case: control studies. Because existing studies have understandably focused on particular diagnostic groups it is also unclear which particular types explain dysfunctions at the individual patient level both within and across diagnostic categories. Arguable, a taxonomy based on brain dysfunctions provides us with one cohesive way to identify neurobiologically valid types that may then be mapped onto the specific symptoms that are the expression of these dysfunctions.
In future clinical translational applications, we will likely continue to rely on a system for classifying individual patients. Yet, a classification system may also consider dysfunction (and symptoms) along dimension of severity. For example, based on the existing evidence we might conceptualize six types of circuit dysfunction that contribute to the variability in depression and anxiety (Figure 2). Each of these six types represents a profile of dysfunction defined by the extent of dysfunction on dimensions of activation and connectivity within each neural circuit.
Given evidence from multiple resting state studies that depression (in at least some patients) is characterized by default mode hyper-activation associated with excessive ruminative thought we might consider a “Rumination” type defined by over-engagement of the default mode circuit (Figure 2). The consistent findings regarding the salience circuit, in particular hypo-activation of the insula, suggest a complementary “Apprehension” type defined by over-engagement of the salience circuit and its clinical manifestations in anxious avoidance and stimulus overload (Figure 2). In the threat circuit dysfunctions defined by altered amygdala-ACC activation and connectivity suggest a “Threat Dysregulation” type that may contribute to arousal systems across depression and anxiety diagnoses (Figure 2). The consistent findings of striatal hypo-activation in the complementary reward circuit suggest an addition type characterized clinically by “Anhedonia” and a loss of sensitivity to reward stimuli (Figure 2). In the attention circuit, findings of general hypo-connectivity suggest an additional “Inattention” type that is expressed clinically as hypo-vigilance and loss of alertness such that false alarm errors may occur (Figure 2). Sixth, multiple studies reporting hypo-connectivity of the DLPFC and major regions of the cognitive control circuit suggest a phenotype in which cognitive control over concentration is weakened, suggestive of a “Cognitive Dyscontrol” (Figure 2).
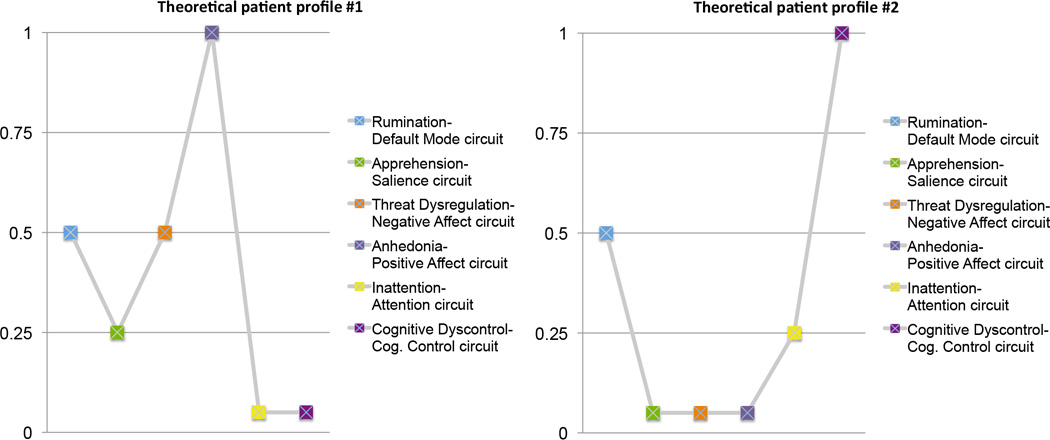
The intention of this theoretical taxonomy is to suggest that a combination of such types is one possible explanation of the heterogeneity and mixed findings within our current knowledge base. This suggestion is certainly not the only possible explanation but it suggests a direction for future research. In this theoretical taxonomy I have considered distinct profiles (or subtypes) of dysfunction without also considering how they might combine within an individual. Very few studies to date have considered multiple circuits and resting as well as task conditions within the same sample of patients, in order to delineate combinations of dysfunctions within and between circuits. To envision a taxonomy suitable for future clinically use we might consider how brain circuit information could guide classification. For example, within each circuit individual patients might be classified based on whether they are at the extremes of hypo- or hyper-activation and hypo- or hyper-connectivity along dimensions of activation and connectivity. To combine information across circuits we might envision a profile taxonomy in which an individual patient is classified according to the degree to which they exhibit dysfunctions on each circuit (for example; Figure 3). Intriguing existing evidence from resting studies suggests that some regions may act as hubs for the combinatorial effect of circuit dysfunction. For example, depressed individuals have been found to show hyper-connectivity between default mode, threat and cognitive control circuits via the dorsal mPFC (“dorsal nexus”)[22]. This dorsal nexus is a potential mechanism by which these networks are “hotwired” and generate a phenotype of multiple symptoms that include rumination, emotional dysregulation and poor cognitive performance. Increasingly detailed information about how circuit dysfunctions map to behavior and symptoms, and ultimately to treatment outcomes, will be essential in testing the clinical utility of taxonomy based on these dysfunctions.
Figure 3.
An illustration of theoretical profiles envisioned for future clinical translation and use of neural circuit dsyfunctions at the individual patient level.
A mechanistic basis for conceptualizing depression and anxiety as neural circuit disorders
Given the weight of available evidence, this review has focused on types of neural circuit dysfunction that characterize the overt expression of depression and anxiety. An important future direction would be to investigate the mechanisms by which pathological neural circuit types develop. A dimensional framework is pertinent for conceptualizing these mechanisms.
Within a dimensional framework, we could consider neural circuit disorders of depression and anxiety as being analogous to systemic illness. Variables of neural activation and connectivity may be considered dimensionally distributed. These variables may also be considered to serve a dual function. On the one hand they contribute to normal variation in brain capacities and on the other they also confer vulnerability to mental disorder. In systemic illness, an analogous variable would be blood pressure. Blood pressure has a fairly wide range of normal distribution. At higher levels, blood pressure may be expressed as hypertension and confer vulnerability to a pathological condition such as ischemic stroke[134]. Although this analogy does not capture the complexity of brain circuits and their interaction, it serves as an illustration for how extremes of hypo- or hyper-activation and activity can produce identifiable failures of function. Observable discontinuities in behavior may occur when neural trait vulnerabilities are coupled with other risk factors, such as environmental hazards (stress, etc.), and pushed to their extreme. To continue the cardiology analogy, observable discontinuities such as stroke may occur when high blood pressure (producing hypertension) is coupled with the effects of other risk factors (stress, obesity etc).
In our current classification system, the DSM, discontinuities in behavior (or function) are categorized according to diagnostic thresholds. To incorporate information on neural circuit dysfunction it will be important to establish metrics as to what constitutes the normative distribution of neural circuit function in healthy individuals, and what thresholds constitute heightened vulnerability through overt disorder and failures of function.
FUTURE CLINICAL DIRECTIONS
As highlighted in this review, advances in brain imaging offer a paradigm shift in how we conceptualize depression and anxiety. Despite these advances, however, we still lack an integrated neural circuit understanding of depression and anxiety that is suited to clinical translation. Several areas of investigation might be pursued in order to accelerate our progress toward incorporating neural circuit information into a clinically viable taxonomy for depression and anxiety. First, it will be important to supplement diagnostically focused studies with trans-diagnostic investigations that consider a wider spectrum of disorders.
For example, additional anxiety disorders such as obsessive compulsive disorder (OCD), trauma disorders such as Post-traumatic Stress Disorder (PTSD) and emotion dysregulation disorders, such as bipolar disorder, share common neural circuit dysfunctions. OCD has been associated with hyper-activation of the default mode circuit (for review, [69,135]) and with altered connectivity of the reward circuit[136]. In PTSD several studies have highlighted disruptions to the resting activation and connectivity of intrinsic default mode, salience and attention (or central executive) circuits (for review, [137,138]). PTSD has also been associated specifically with ACC hypo-activation along with amygdala hyper-activation during task-evoked threat processing, consistent with a threat dysregulation biotype (for review, [139], for meta analysis, [140–142]. The observation of amygdala hyper-activation is common across other disordered emotional states as reviewed above, although the combination of ACC and amygdala disturbances might be specific to trauma states (for meta-analysis, [141]). This profile in PTSD is observed for implicit processing of threat[143,144], and may extend to the salience circuit[144] (for meta-analysis, [140,141]). Threat dysregulation has also been found to characterize bipolar disorder[141]. Bipolar disorder has also been associated with alterations in the intrinsic default mode, salience and attention circuits at rest (for review, [145,146]) and with disruptions to both reward circuits (for review, [147]) and cognitive control circuits (for review, [148]) under task-evoked conditions.
A trans-diagnostic approach will help to characterize the neural circuit dysfunctions that cut across diagnostic boundaries, and define new types that are agnostic to these boundaries. Second, brain imaging studies will also need to consider multiple neural circuits within the same patient samples, the variance explained by between-circuit interactions (e.g., [22,70,72,149]) as well as within circuit dysfunctions, and the effect of resting versus task-evoked conditions. Such an undertaking is likely to be possible only if standardized imaging protocols are used. Standardization will also facilitate data sharing and the viability of future clinical translation[150]. Third, to test the fit of neural circuit taxonomies based on trans-diagnostic, multi-circuit datasets we will need to take advantage of modern computational tools that can handle such multi-dimensional information. The translational relevance of computationally-defined neural circuit types will depend on how well we determine which of these types are clinically (and not just statistically) meaningful. A useful taxonomy would enable us to classify the onset of a disorder, identify its biological cause and select treatment accordingly. Existing evidence points to the likely utility of neural circuit dysfunctions for helping to guide treatment choice. For example, amygdala hyper-reactivity to threat stimuli attenuates in responders to the selective serotonin reuptake inhibitors escitalopram and paroxetine[151,152], while hyper-reactivity to sad faces predicts non-response to venlafaxine[77]. In seminal prediction studies insula hyper-reactivity (assayed by positron emission tomography) has been identified as a differential biomarker of remission on citalopram (versus cognitive behavior therapy)[153,154]. Drugs that bind more selectively to dopamine receptors, such as pramipexole, have antidepressant efficacy [155–157] and modulate striatal function relevant to reward circuit dysfunction [158,159] and anhedonia[160,161]. Neural circuit dysfunctions are also viable biomarkers for guiding treatment selections for emerging new treatments such as neuromodulation. For example, resting state hyper-connectivity within the default mode circuit and hypo-connectivity within the parietofrontal attention circuit are predictive of response to repetitive Transcranial Magnetic Stimulation (for review[162]).
CONCLUSION
With advances in human neuroimaging we have the tools for in vivo quantification of large-scale neural circuits that govern our core functions of self-reflection, emotional reactivity and regulation, attention and cognitive control. Research with patients experiencing a spectrum of mood and anxiety disorders has identified distinctive disruptions in the activation and connectivity of these circuits. Existing knowledge is based understandably on group average data within diagnostic categories, typically focusing on a particular circuit or on rest or task-evoked conditions. With a foundation in existing knowledge now is the right time to develop an integrative translational approach in which we recruit patients across multiple diagnoses (particularly commonly comorbid diagnoses), multiple circuits and both task-free and task-evoked conditions. By integrating these sources of information we will be in a position to parse the neural circuit dysfunctions that define distinct types of depression and anxiety within and across diagnostic boundaries. To be clinically useful a taxonomy of neural circuit dysfunctions will depend on our capacity to map dysfunctions onto profiles of observable symptoms, and to demonstrate the benefit of using neural circuit information to help guide treatment selections and to improve functional outcomes.
Supplementary Material
Acknowledgments
This work was supported by NIMH GRANT R01MH101496. I acknowledge the contributions to manuscript preparation of Andrea Goldstein-Piekarski, Ph.D. (Stanford, CA, USA), Nowreen Chowdhry, B.Sc. (Stanford, CA, USA), and Katherine Grisanzio, B.Sc. (Stanford, CA, USA),
Footnotes
In addition to the salience circuit, a distinct “cingulo-opercular” circuit has also been defined (Supplementary Figure 1). The circuit is defined by nodes in the amPFC, dorsal ACC, aI/frontal operculum and anterior thalamus37 and is involved in the detection of potential mismatches and conflict21. These regions and functions show overlap with the default mode and salience circuits even though the cingulo-opercular circuit is articulated as a distinct circuit21.ibid.
http://www.nimh.nih.gov/research-priorities/rdoc/negative-valence-systems-workshop-proceedings.shtml and http://www.nimh.nih.gov/research-priorities/rdoc/positive-valence-systems-workshop-proceedings.shtml
Disclosures
LMW has received fees from Brain Resource and Humana in projects not related to the present work.
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Mental Health Findings. Rockville, MD: 2013. [Google Scholar]
- 2.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2010;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 3.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 4.Yuste R. From the neuron doctrine to neural networks. Nat Rev Neurosci. 2015;16(8):487–497. doi: 10.1038/nrn3962. [DOI] [PubMed] [Google Scholar]
- 5.Leergaard TB, Hilgetag CC, Sporns O. Mapping the connectome: multi-level analysis of brain connectivity. Front Neuroinform. 2012;6:14. doi: 10.3389/fninf.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 7.Van Essen DC, Smith SM, Barch DM, et al. The WU-Minn Human Connectome Project: an overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sotiropoulos SN, Jbabdi S, Xu J, et al. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage. 2013;80:125–143. doi: 10.1016/j.neuroimage.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barch DM, Burgess GC, Harms MP, et al. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raichle ME. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015;38(1):150504162358003. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 11.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 13.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 14.Kessler RC, Barber C, Beck A, et al. The World Health Organization Health and Work Performance Questionnaire (HPQ) J. Occup. Environ. Med. 2003:156–174. doi: 10.1097/01.jom.0000052967.43131.51. [DOI] [PubMed] [Google Scholar]
- 15.Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16(7):832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 16.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon EM, Laumann TO, Adeyemo B, et al. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex. 2014;26(1):288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindquist KA, Barrett LF. A functional architecture of the human brain: emerging insights from the science of emotion. Trends in Cognitive Sciences. 2012;16(11):533–540. doi: 10.1016/j.tics.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oosterwijk S, Lindquist KA, Anderson E, et al. States of mind: emotions, body feelings, and thoughts share distributed neural networks. Neuroimage. 2012;62(3):2110–2128. doi: 10.1016/j.neuroimage.2012.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. The Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spreng RN, Sepulcre J, Turner GR, et al. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25(1):74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole MW, Bassett DS, Power JD, et al. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83(1):238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castelli F, Happe F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- 27.Williams LM, Das P, Liddell BJ, et al. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci. 2006;26(36):9264–9271. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White SJ, Coniston D, Rogers R, Frith U. Developing the Frith-Happé animations: a quick and objective test of Theory of Mind for adults with autism. Autism Res. 2011;4(2):149–154. doi: 10.1002/aur.174. [DOI] [PubMed] [Google Scholar]
- 30.Rushworth MFS, Mars RB, Sallet J. Are there specialized circuits for social cognition and are they unique to humans? Curr. Opin. Neurobiol. 2013;23(3):436–442. doi: 10.1016/j.conb.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Touroutoglou A, Andreano JM, Barrett LF, Dickerson BC. Brain network connectivity-behavioral relationships exhibit trait-like properties: Evidence from hippocampal connectivity and memory. Hippocampus. 2015 doi: 10.1002/hipo.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulders PC, van Eijndhoven PF, Schene AH, et al. Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Horn DI, Yu C, Steiner J, et al. Glutamatergic and resting state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula. Frontiers in Systems Neuroscience. 2010:4. doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korgaonkar MS, Ram K, Williams LM, et al. Establishing the resting state default mode network derived from functional magnetic resonance imaging tasks as an endophenotype: A twins study. Hum Brain Mapp. 2014;35(8):3893–3902. doi: 10.1002/hbm.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sylvester CM, Corbetta M, Raichle ME, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35(9):527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raichle ME. The restless brain. Brain Connect. 2011;1(1):3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kober H, Barrett LF, Joseph J, et al. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson OJ, Krimsky M, Lieberman L, et al. The dorsal medial prefrontal (anterior cingulate) cortex-amygdala aversive amplification circuit in unmedicated generalised and social anxiety disorders: an observational study. The Lancet Psychiatry. 2014;1(4):294–302. doi: 10.1016/S2215-0366(14)70305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etkin A, Prater KE, Hoeft F, et al. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167(5):545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 42.Liddell BJ, Brown KJ, Kemp AH, et al. A direct brainstem-amygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage. 2005;24(1):235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Williams LM, Liddell BJ, Kemp AH, et al. Amygdala-prefrontal dissociation of subliminal and supraliminal fear. Hum Brain Mapp. 2006;27(8):652–661. doi: 10.1002/hbm.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlson JM, Reinke KS, Habib R. A left amygdala mediated network for rapid orienting to masked fearful faces. Neuropsychologia. 2009;47(5):1386–1389. doi: 10.1016/j.neuropsychologia.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 45.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199(3):457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon EM, Laumann TO, Adeyemo B, et al. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fornito A, Harrison BJ, Zalesky A, Simons JS. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc. Natl. Acad. Sci. U.S.A.: Acad National Sciences. 2012:12788–12793. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vincent JL, Kahn I, Snyder AZ, et al. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niendam TA, Laird AR, Ray KL, et al. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37(1):343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 51.Roalf DR, Ruparel K, Gur RE, et al. Neuroimaging predictors of cognitive performance across a standardized neurocognitive battery. Neuropsychology. 2014;28(2):161–176. doi: 10.1037/neu0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glasser MF, Sotiropoulos SN, Wilson JA, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcus DS, Harms MP, Snyder AZ, et al. Human Connectome Project informatics: quality control, database services, and data visualization. Neuroimage. 2013;80:202–219. doi: 10.1016/j.neuroimage.2013.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Essen DC, Ugurbil K, Auerbach E, et al. The Human Connectome Project: a data acquisition perspective. Neuroimage. 2012;62(4):2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veer IM, Beckmann CF, van Tol MJ, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010:4. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry. 2015;78(4):224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamilton JP, Furman DJ, Chang C, et al. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh MK, Kesler SR, Hadi Hosseini SM, et al. Anomalous gray matter structural networks in major depressive disorder. Biol Psychiatry. 2013;74(10):777–785. doi: 10.1016/j.biopsych.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korgaonkar MS, Fornito A, Williams LM, Grieve SM. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol. Psychiatry. 2014:567–574. doi: 10.1016/j.biopsych.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 60.Grieve SM, Korgaonkar MS, Koslow SH, et al. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332–339. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu X, Wang X, Xiao J, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71(7):611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 62.Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord. 2014;172c:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qiu C, Liao W, Ding J, et al. Regional homogeneity changes in social anxiety disorder: a resting-state fMRI study. Psychiatry Res. 2011;194(1):47–53. doi: 10.1016/j.pscychresns.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Sprengelmeyer R, Steele JD, Mwangi B, et al. The insular cortex and the neuroanatomy of major depression. J Affect Disord. 2011;133(1–2):120–127. doi: 10.1016/j.jad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Suslow T, Konrad C, Kugel H, et al. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry. 2010;67(2):155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 66.Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1(1):10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee BT, Seong Whi C, Hyung Soo K, et al. The neural substrates of affective processing toward positive and negative affective pictures in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1487–1492. doi: 10.1016/j.pnpbp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 68.Klumpp H, Post D, Angstadt M, et al. Anterior cingulate cortex and insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biol Mood Anxiety Disord. 2013;3:7. doi: 10.1186/2045-5380-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peterson A, Thome J, Frewen P, Lanius RA. Resting-state neuroimaging studies: a new way of identifying differences and similarities among the anxiety disorders? Can J Psychiatry. 2014:294–300. doi: 10.1177/070674371405900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manoliu A, Meng C, Brandl F, et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Frontiers in Human Neuroscience. 2014:7. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao W, Qiu C, Gentili C, et al. Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state FMRI study. PLoS One. 2010;5(12):e15238. doi: 10.1371/journal.pone.0015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arnold Anteraper S, Triantafyllou C, Sawyer AT, et al. Hyper-connectivity of subcortical resting-state networks in social anxiety disorder. Brain Connect. 2014;4(2):81–90. doi: 10.1089/brain.2013.0180. [DOI] [PubMed] [Google Scholar]
- 73.Goldstein-Piekarski A, Williams LM. Characterizing intrinsic and task-specific neural circuit dysfunctions in depression and their behavioral correlates. Nature Neuroscience. (in submission) [Google Scholar]
- 74.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim MJ, Loucks RA, Palmer AL, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams LM, Korgaonkar MS, Song YC, et al. Amygdala Reactivity to Emotional Faces in the Prediction of General and Medication-Specific Responses to Antidepressant Treatment in the Randomized iSPOT-D Trial. Neuropsychopharmacology: Nature Publishing Group. 2015 doi: 10.1038/npp.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang TT, Simmons AN, Matthews SC, et al. Adolescents with major depression demonstrate increased amygdala activation. J Am Acad Child Adolesc Psychiatry. 2010;49(1):42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peluso MA, Glahn DC, Matsuo K, et al. Amygdala hyperactivation in untreated depressed individuals. Psychiatry Res. 2009;173(2):158–161. doi: 10.1016/j.pscychresns.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Surguladze S, Brammer MJ, Keedwell P, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57(3):201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 81.Sheline YI, Barch DM, Donnelly JM, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 82.Jaworska N, Yang XR, Knott V, Macqueen G. A review of fMRI studies during visual emotive processing in major depressive disorder. World J Biol Psychiatry. 2014 doi: 10.3109/15622975.2014.885659. [DOI] [PubMed] [Google Scholar]
- 83.Fonzo GA, Ramsawh HJ, Flagan TM, et al. Common and disorder-specific neural responses to emotional faces in generalised anxiety, social anxiety and panic disorders. Br J Psychiatry. 2015 doi: 10.1192/bjp.bp.114.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stein MB, Goldin PR, Sareen J, et al. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59(11):1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 85.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 86.Killgore WD, Britton JC, Schwab ZJ, et al. Cortico-limbic responses to masked affective faces across ptsd, panic disorder, and specific phobia. Depress Anxiety. 2014;31(2):150–159. doi: 10.1002/da.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Etkin A, Klemenhagen KC, Dudman JT, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44(6):1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 88.Palm ME, Elliott R, McKie S, et al. Attenuated responses to emotional expressions in women with generalized anxiety disorder. Psychol Med. 2011;41(5):1009–1018. doi: 10.1017/S0033291710001455. [DOI] [PubMed] [Google Scholar]
- 89.Blair KS, Geraci M, Smith BW, et al. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry. 2012;72(6):476–482. doi: 10.1016/j.biopsych.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arnone D, McKie S, Elliott R, et al. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. Am J Psychiatry. 2012;169(8):841–850. doi: 10.1176/appi.ajp.2012.11121774. [DOI] [PubMed] [Google Scholar]
- 91.Victor TA, Furey ML, Fromm SJ, et al. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67(11):1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fu CH, Williams SC, Cleare AJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 93.Musgrove DR, Eberly LE, Klimes-Dougan B, et al. Impaired Bottom-Up Effective Connectivity Between Amygdala and Subgenual Anterior Cingulate Cortex in Unmedicated Adolescents with Major Depression: Results from a Dynamic Causal Modeling Analysis. Brain Connect. 2015;5(10):608–619. doi: 10.1089/brain.2014.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matthews SC, Strigo IA, Simmons AN, et al. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008;111(1):13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 95.Prater KE, Hosanagar A, Klumpp H, et al. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety. 2013;30(3):234–241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clauss JA, Avery SN, VanDerKlok RM, et al. Neurocircuitry underlying risk and resilience to social anxiety disorder. Depress Anxiety. 2014;31(10):822–833. doi: 10.1002/da.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sladky R, Hoflich A, Kublbock M, et al. Disrupted Effective Connectivity Between the Amygdala and Orbitofrontal Cortex in Social Anxiety Disorder During Emotion Discrimination Revealed by Dynamic Causal Modeling for fMRI. Cereb Cortex. 2013 doi: 10.1093/cercor/bht279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56(3):881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 99.Steffens DC, Taylor WD, Denny KL, et al. Structural integrity of the uncinate fasciculus and resting state functional connectivity of the ventral prefrontal cortex in late life depression. PLoS One. 2011;6(7):e22697. doi: 10.1371/journal.pone.0022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao YJ, Du MY, Huang XQ, et al. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol Med. 2014;44(14):2927–2937. doi: 10.1017/S0033291714000518. [DOI] [PubMed] [Google Scholar]
- 101.Hamilton JP, Etkin A, Furman DJ, et al. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gotlib IH, Hamilton JP, Cooney RE, et al. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67(4):380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim MJ, Hamilton JP, Gotlib IH. Reduced caudate gray matter volume in women with major depressive disorder. Psychiatry Res. 2008;164(2):114–122. doi: 10.1016/j.pscychresns.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sacchet MD, Prasad G, Foland-Ross LC, et al. Characterizing White Matter Connectivity in Major Depressive Disorder: Automated Fiber Quantification and Maximum Density Paths. Proc IEEE Int Symp Biomed Imaging. 2014;11:592–595. doi: 10.1109/ISBI.2014.6867940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blair K, Shaywitz J, Smith BW, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 2008;165(9):1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ottaviani C, Cevolani D, Nucifora V, et al. Amygdala responses to masked and low spatial frequency fearful faces: a preliminary fMRI study in panic disorder. Psychiatry Res. 2012;203(2–3):159–165. doi: 10.1016/j.pscychresns.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 109.Cannistraro PA, Wright CI, Wedig MM, et al. Amygdala responses to human faces in obsessive-compulsive disorder. Biol Psychiatry. 2004;56(12):916–920. doi: 10.1016/j.biopsych.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 110.Keedwell PA, Andrew C, Williams SC, et al. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58(11):843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 111.Mitterschiffthaler MT, Kumari V, Malhi GS, et al. Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport. 2003;14(2):177–182. doi: 10.1097/00001756-200302100-00003. [DOI] [PubMed] [Google Scholar]
- 112.Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord. 2012;136(3):1126–1134. doi: 10.1016/j.jad.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang WN, Chang SH, Guo LY, et al. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151(2):531–539. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 114.Korgaonkar MS, Grieve SM, Etkin A, et al. Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: first wave results from the iSPOT-D study. Neuropsychopharmacology. 2013;38(5):863–871. doi: 10.1038/npp.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Siegle GJ, Thompson W, Carter CS, et al. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 116.Elliott R, Sahakian BJ, Herrod JJ, et al. Abnormal response to negative feedback in unipolar depression: evidence for a diagnosis specific impairment. J Neurol Neurosurg Psychiatry. 1997;63(1):74–82. doi: 10.1136/jnnp.63.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Elliott R, Baker SC, Rogers RD, et al. Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med. 1997;27(4):931–942. doi: 10.1017/s0033291797005187. [DOI] [PubMed] [Google Scholar]
- 118.Fales CL, Barch DM, Burgess GC, et al. Anxiety and cognitive efficiency: differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci. 2008;8(3):239–253. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- 119.Halari R, Simic M, Pariante CM, et al. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naive adolescents with depression compared to controls. J Child Psychol Psychiatry. 2009;50(3):307–316. doi: 10.1111/j.1469-7610.2008.01972.x. [DOI] [PubMed] [Google Scholar]
- 120.Aizenstein HJ, Butters MA, Wu M, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17(1):30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harvey PO, Fossati P, Pochon JB, et al. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage. 2005;26(3):860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 122.Chang CC, Yu SC, McQuoid DR, et al. Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Res. 2011;193(1):1–6. doi: 10.1016/j.pscychresns.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bartova L, Meyer BM, Diers K, et al. Reduced default mode network suppression during a working memory task in remitted major depression. J Psychiatr Res. 2015;64:9–18. doi: 10.1016/j.jpsychires.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walter H, Wolf RC, Spitzer M, Vasic N. Increased left prefrontal activation in patients with unipolar depression: an event-related, parametric, performance-controlled fMRI study. J Affect Disord. 2007;101(1–3):175–185. doi: 10.1016/j.jad.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 125.Wagner G, Sinsel E, Sobanski T, et al. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biol Psychiatry. 2006;59(10):958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 126.Matsuo K, Glahn DC, Peluso MA, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry. 2007;12(2):158–166. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- 127.Holmes AJ, MacDonald A, 3rd, Carter CS, et al. Prefrontal functioning during context processing in schizophrenia and major depression: an event-related fMRI study. Schizophr Res. 2005;76(2–3):199–206. doi: 10.1016/j.schres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 128.Hugdahl K, Rund BR, Lund A, et al. Brain activation measured with fMRI during a mental arithmetic task in schizophrenia and major depression. Am J Psychiatry. 2004;161(2):286–293. doi: 10.1176/appi.ajp.161.2.286. [DOI] [PubMed] [Google Scholar]
- 129.Fitzgerald PB, Srithiran A, Benitez J, et al. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Hum Brain Mapp. 2008;29(4):490–501. doi: 10.1002/hbm.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rose EJ, Simonotto E, Ebmeier KP. Limbic over-activity in depression during preserved performance on the n-back task. Neuroimage. 2006;29(1):203–215. doi: 10.1016/j.neuroimage.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 131.Schoning S, Zwitserlood P, Engelien A, et al. Working-memory fMRI reveals cingulate hyperactivation in euthymic major depression. Hum Brain Mapp. 2009;30(9):2746–2756. doi: 10.1002/hbm.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vasic N, Walter H, Sambataro F, Wolf RC. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychol Med. 2009;39(6):977–987. doi: 10.1017/S0033291708004443. [DOI] [PubMed] [Google Scholar]
- 133.Holmes AJ, Pizzagalli DA. Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia. 2008;46(12):2904–2913. doi: 10.1016/j.neuropsychologia.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Katritsis DG, Gersh BJ, Camm AJ. Clinical Cardiology: Current Practice Guidelines. OUP Oxford; 2013. [Google Scholar]
- 135.McGovern RA, Sheth SA. Role of the dorsal anterior cingulate cortex in obsessive-compulsive disorder: converging evidence from cognitive neuroscience and psychiatric neurosurgery. J Neurosurg. 2016:1–16. doi: 10.3171/2016.1.JNS15601. [DOI] [PubMed] [Google Scholar]
- 136.Menzies L, Chamberlain SR, Laird AR, et al. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32(3):525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rabellino D, Tursich M, Frewen PA, et al. Intrinsic Connectivity Networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli. Acta Psychiatr Scand. 2015;132(5):365–378. doi: 10.1111/acps.12418. [DOI] [PubMed] [Google Scholar]
- 138.Lanius RA, Frewen PA, Tursich M, et al. Restoring large-scale brain networks in PTSD and related disorders: a proposal for neuroscientifically-informed treatment interventions. Eur J Psychotraumatol. 2015;6:27313. doi: 10.3402/ejpt.v6.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sartory G, Cwik J, Knuppertz H, et al. In search of the trauma memory: a meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD) PLoS One. 2013;8(3):e58150. doi: 10.1371/journal.pone.0058150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2(1):9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hayes JP, Vanelzakker MB, Shin LM. Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front Integr Neurosci. 2012;6:89. doi: 10.3389/fnint.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bryant RA, Kemp AH, Felmingham KL, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29(5):517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bruce SE, Buchholz KR, Brown WJ, et al. Altered emotional interference processing in the amygdala and insula in women with Post-Traumatic Stress Disorder. Neuroimage Clin. 2012;2:43–49. doi: 10.1016/j.nicl.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Langenecker SA, Jacobs RH, Passarotti AM. Current Neural and Behavioral Dimensional Constructs across Mood Disorders. Curr Behav Neurosci Rep. 2014;1(3):144–153. doi: 10.1007/s40473-014-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vargas C, Lopez-Jaramillo C, Vieta E. A systematic literature review of resting state network--functional MRI in bipolar disorder. J Affect Disord. 2013;150(3):727–735. doi: 10.1016/j.jad.2013.05.083. [DOI] [PubMed] [Google Scholar]
- 147.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28(1):7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fernandez-Corcuera P, Salvador R, Monte GC, et al. Bipolar depressed patients show both failure to activate and failure to de-activate during performance of a working memory task. J Affect Disord. 2013;148(2–3):170–178. doi: 10.1016/j.jad.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 149.Sylvester CM, Corbetta M, Raichle ME, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012:527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Siegle GJ. Beyond Depression Commentary: Wherefore Art Thou, Depression Clinic of Tomorrow? Clin Psychol (New York) 2011;18(4):305–310. doi: 10.1111/j.1468-2850.2011.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Godlewska BR, Norbury R, Selvaraj S, et al. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychol Med. 2012;42(12):2609–2617. doi: 10.1017/S0033291712000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ruhe HG, Booij J, Veltman DJ, et al. Successful pharmacologic treatment of major depressive disorder attenuates amygdala activation to negative facial expressions: a functional magnetic resonance imaging study. J Clin Psychiatry. 2012;73(4):451–459. doi: 10.4088/JCP.10m06584. [DOI] [PubMed] [Google Scholar]
- 153.Dunlop BW, Kelley ME, McGrath CL, et al. Preliminary Findings Supporting Insula Metabolic Activity as a Predictor of Outcome to Psychotherapy and Medication Treatments for Depression. J Neuropsychiatry Clin Neurosci. 2015;27(3):237–239. doi: 10.1176/appi.neuropsych.14030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dunlop BW, Mayberg HS. Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin Neurosci. 2014;16(4):479–490. doi: 10.31887/DCNS.2014.16.4/bdunlop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.DeBattista C, Solvason HB, Breen JA, Schatzberg AF. Pramipexole augmentation of a selective serotonin reuptake inhibitor in the treatment of depression. J Clin Psychopharmacol. 2000;20(2):274–475. doi: 10.1097/00004714-200004000-00029. [DOI] [PubMed] [Google Scholar]
- 156.Zarate CA, Jr, Payne JL, Singh J, et al. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry. 2004;56(1):54–60. doi: 10.1016/j.biopsych.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 157.Corrigan MH, Denahan AQ, Wright CE, et al. Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depress Anxiety. 2000;11(2):58–65. doi: 10.1002/(sici)1520-6394(2000)11:2<58::aid-da2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 158.Der-Avakian A, D’Souza MS, Pizzagalli DA, Markou A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry. 2013;3:e297. doi: 10.1038/tp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ishibashi K, Ishii K, Oda K, et al. Binding of pramipexole to extrastriatal dopamine D2/D3 receptors in the human brain: a positron emission tomography study using 11C–FLB 457. PLoS One. 2011;6(3):e17723. doi: 10.1371/journal.pone.0017723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 161.Shelton RC, Tomarken AJ. Can recovery from depression be achieved? Psychiatr Serv. 2001;52(11):1469–1478. doi: 10.1176/appi.ps.52.11.1469. [DOI] [PubMed] [Google Scholar]
- 162.Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord. 2015;172C:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shulman GL, Fiez JA, Corbetta M, et al. Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J Cogn Neurosci. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 165.Qin J, Wei M, Liu H, et al. Abnormal brain anatomical topological organization of the cognitive-emotional and the frontoparietal circuitry in major depressive disorder. Magn Reson Med. 2014;72(5):1397–1407. doi: 10.1002/mrm.25036. [DOI] [PubMed] [Google Scholar]
- 166.de Kwaasteniet BP, Rive MM, Ruhe HG, et al. Decreased Resting-State Connectivity between Neurocognitive Networks in Treatment Resistant Depression. Front Psychiatry. 2015:6. doi: 10.3389/fpsyt.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu M, Andreescu C, Butters MA, et al. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 2011;194(1):39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oosterwijk S, Lindquist KA, Anderson E, et al. States of mind: Emotions, body feelings, and thoughts share distributed neural networks. NeuroImage. 2012;62(3):2110–2128. doi: 10.1016/j.neuroimage.2012.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55(6):578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 170.Thomas KM, Drevets WC, Dahl RE, et al. Amygdala response to fearful faces in anxious and depressed children. Arch. Gen. Psychiatry. 2001:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 171.Musgrove D, Eberly L, Klimes-Dougan B, et al. Impaired Bottom-Up Effective Connectivity Between Amygdala and Subgenual Anterior Cingulate Cortex in Unmedicated Adolescents with Major Depression: Results from a Dynamic Causal Modeling Analysis. Brain Connect. 2015 doi: 10.1089/brain.2014.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Etkin A, Prater KE, Schatzberg AF, et al. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66(12):1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mueller SC, Aouidad A, Gorodetsky E, et al. Gray matter volume in adolescent anxiety: an impact of the brain-derived neurotrophic factor Val(66)Met polymorphism? J Am Acad Child Adolesc Psychiatry. 2013;52(2):184–195. doi: 10.1016/j.jaac.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bossini L, Tavanti M, Calossi S, et al. Magnetic resonance imaging volumes of the hippocampus in drug-naive patients with post-traumatic stress disorder without comorbidity conditions. J Psychiatr Res. 2008;42(9):752–762. doi: 10.1016/j.jpsychires.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 175.Kempton MJ, Salvador Z, Munafo MR, et al. Structural neuroimaging studies in major depressive disorder Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68(7):675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 176.Moon CM, Kim GW, Jeong GW. Whole-brain gray matter volume abnormalities in patients with generalized anxiety disorder: voxel-based morphometry. Neuroreport. 2014;25(3):184–189. doi: 10.1097/WNR.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 177.Keedwell PA, Andrew C, Williams SC, et al. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry. 2005;58(6):495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 178.Chen AC, Oathes DJ, Chang C, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A. 2013;110(49):19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vilgis V, Chen J, Silk TJ, et al. Frontoparietal function in young people with dysthymic disorder (DSM-5: Persistent depressive disorder) during spatial working memory. J Affect Disord. 2014;160:34–42. doi: 10.1016/j.jad.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 180.Koric L, Volle E, Seassau M, et al. How cognitive performance-induced stress can influence right VLPFC activation: an fMRI study in healthy subjects and in patients with social phobia. Hum Brain Mapp. 2012;33(8):1973–1986. doi: 10.1002/hbm.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.