Abstract
Aims and objectives
To explore and synthesize current research to assess the state of science about the relationship between sleep disturbance and glycemic control in adults with type 2 diabetes (T2DM).
Background
Sleep disturbance is suggested a risk factor for T2DM. Diabetes alone is a leading cause of death, but when coupled with sleep disturbance poses additional health risks. However, little is known about the relationship between sleep disturbance and glycemic control in people with overt diabetes.
Design
An integrative review.
Methods
Whittemore and Knafl's methodology guided this integrative review. Original studies published before Oct. 2016 were identified through systematic searches of seven databases using terms: diabet*; sleep or insomnia; glycem* or glucose or A1C or HbA1c or sugar; and their combinations. The matrix and narrative synthesis were employed to organize and synthesize the findings, respectively. The Crowe Critical Appraisal Tool was used to evaluate the study quality.
Results
A total of 26 studies were identified; 17 of which reported significant relationships between sleep measures and glycemic control. In 13 studies sleep duration was associated with glycemic control in both linear (n=2) and nonlinear (n=3) relationships, however 8 studies reported no significant relationships. Sleep quality was significantly related to glycemic control in 14 of 22 studies. Nine studies found no relationship between any measure of sleep and glycemic control.
Conclusions
There is strong evidence supporting the relationship between sleep quality and glycemic control but further examination of the relationship between sleep duration and glycemic control is warranted. Sleep disturbance, particularly impaired sleep quality, could potentially influence glycemic control in adults with T2DM.
Relevance to clinical practice
Nurses who treat patients with diabetes should include assessment of sleep, education for healthy sleep, and referral for treatment of sleep disturbance in order to maximize the potential for achieving good glycemic control.
Keywords: diabetes, glycemic control, integrative review, sleep disturbance, symptom
Introduction
In the U.S., 9.3% of the population has diabetes (Center for Disease Control and Prevention 2014); globally, 592 million (Guariguata et al. 2014) people are projected to have diabetes by 2035. Over 90% of all cases of diabetes are type 2 diabetes (T2DM) (Center for Disease Control and Prevention 2014). Parallel with the high prevalence of diabetes is the occurrence of sleep disturbance. Sleep disturbance differs from sleep disorder, which is a disease that requires strict criteria for diagnosis. Sleep disturbance is a symptom that can be caused by various physical and psychological factors and may be experienced by anyone. To date, there is no consistent theoretical definition of sleep disturbance. Sleep disturbance is most commonly characterized as poor sleep quality or abnormal sleep duration. Thus, for the purpose of clarity and consistency, we operationalized sleep disturbance in this review as poor sleep quality or abnormal sleep duration.
The prevalence of sleep disturbance is increasing at an alarming rate, particularly in patients with T2DM. It was reported that 39.4% and 55.0% of them have short sleep duration (< 6.5 h per night) (Ohkuma et al. 2014) and poor sleep quality (Luyster & Dunbar-Jacob 2011). Evidence indicates that sleep disturbance and glucose regulation form a cycle through multiple pathophysiological pathways (Reutrakul & Van Cauter 2014). Sleep disturbance is associated with higher risk of T2DM (Cappuccio et al. 2010). However, despite growing concern regarding the effects of sleep disturbance on health, few studies have examined the relationship between sleep disturbance and diabetes-related health outcomes, particularly glycemic control in people who already have T2DM.
Glycemic control is the regulation and maintenance of the blood glucose levels within the normal range and is best evaluated by the combination of blood glucose and Glycated hemoglobin A1c (HbA1c) (American Diabetes Association 2017). HbA1c is an indicator of the overall glucose level for the past 2 to 3 months and has been widely used as the “gold standard” for glycemic control (Lenters-Westra et al. 2013). In people with T2DM, glycemic control is paramount for maintaining health and reducing the risks of diabetes complications, including retinopathy, neuropathy, and nephropathy (American Diabetes Association 2017). Glycemic control may be affected by multiple biological, psychological, and behavioral factors; sleep plays a potential role among these factors. Recently, Lee and colleagues (Lee et al. 2016) conducted a meta-analysis of 20 studies and examined the impact of sleep duration and sleep quality on glycemic control in people with T2DM. It was reported that short and long sleep duration, as well as poor sleep quality, was related to an increased HbA1c. The evidence provided by Lee and colleagues is compelling, however, there have been new publications since their review, and independent reviews of similar topics by different teams may be useful in adding to the evidence and expanding the science (Siontis et al. 2013). Therefore, we independently reviewed the evidence from current studies, aiming to expand and strengthen our knowledge in the relationship between sleep disturbance and glycemic control in people with T2DM.
Aims
The aim of this integrative review was to explore and synthesize current evidence to determine whether sleep disturbance, defined as poor sleep quality or abnormal sleep duration, is related to glycemic control in adults with T2DM. The findings will address research and methodological gaps and provide further evidence about the relationship between sleep and diabetes.
Methods
Design
Unlike a meta-analysis, which requires homogeneity in the measurement of the construct of interest and similar conceptual hypothesis addressed in the primary sources (Cooper 1998), an integrative review allows for the synthesis of both observational and experimental studies, thereby providing a comprehensive understanding of a topic of interest (Whittemore & Knafl 2005). Thus, Whittemore and Knafl's methodology (Whittemore & Knafl 2005) for integrative review was employed to enhance the validity of this review.
Inclusion and exclusion criteria
This integrative review included original studies that were conducted in adults with T2DM. The study population was restricted to T2DM adults because physiological, social, and behavioral pathways influencing both sleep and glycemic control are different in adults and children, as well as in type 1 diabetes and T2DM. Based on our definition of sleep disturbance, the variables of interest included sleep quality or duration. Similarly, the other inclusion criteria is that physiological indicator of glycemic control (e.g., blood glucose or HbA1c) should be available. Additionally, only studies published in English were included. Review papers and abstracts presented at scientific conferences were excluded. Pregnancy-related physiological and psychological changes might confound the relationship between sleep and glycemic control. Thus, studies conducted in people with gestational diabetes were excluded. Studies focused on sleep architecture, sleep stages, or sleep pattern was excluded. Sleep architecture is the basic structural organization of normal sleep and can be classified into non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep (Colten & Altevogt 2006). Sleep stages are distinctive stages of normal sleep, and sleep pattern is an individual's wake/sleep schedule as well as nap behaviors (National Sleep Foundation 2017). These three sleep measures have different pathophysiological characteristics as sleep quality or duration. Studies exclusively investigating obstructive sleep apnea, restless leg syndrome, or periodic leg movement disorder were excluded.
Search strategies
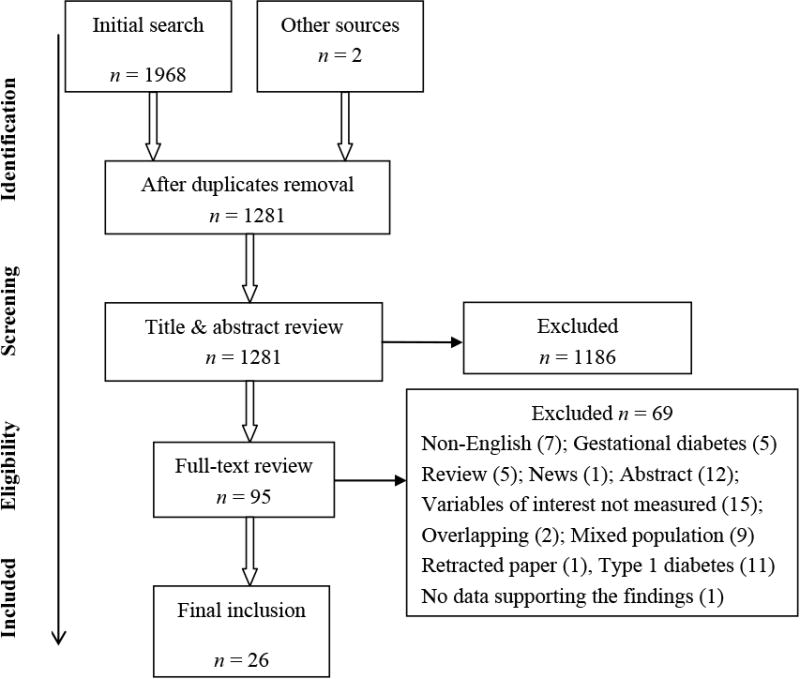
A systematic search was conducted in October 2016 following the PRISMA guidelines (Figure 1) (Moher et al. 2010). There was no restriction on the year of publication. Three search terms were used: (1) diabet*; (2) sleep OR insomnia; (3) glycem* OR glucose OR A1C OR HbA1c OR sugar. Insomnia was used as a search term, as the definition is closely related to the definition of sleep disturbance in this review. Combinations of the three terms were applied to the following electronic databases: PubMed, CINAHL, PsycINFO, Web of Science, and ProQuest. The Cochrane and Annual Review of Public Health were also searched to find relevant reviews where reference lists could provide additional original studies. Initial screening was conducted by reading titles and abstracts; candidates for reviewing were then read through the full text. Ancestry searching was performed to find additional studies.
Figure 1. PRISMA flow diagram for systematic search.
Quality appraisal
The Crowe Critical Appraisal Tool (CCAT) (Crowe 2013) was used to evaluate the quality of each study from eight aspects (e.g., design, sampling, and data collection). It has a global score ranging from 0 to 40. Higher scores indicate higher quality. No cut-off point of the appraisal score is suggested as an indicator for exclusion (Whittemore & Knafl 2005). Thus, all of the eligible papers were included in this review.
Data abstraction and synthesis
A table matrix was used for efficient and reliable abstraction of pertinent information. Data were abstracted, coded, and tabulated into the matrix. Constant comparison was conducted to identify the relationships between sleep disturbance and glycemic control. Similar patterns were clustered under the same themes, and discrepancies between studies were compared. A pooled meta-analysis is unlikely to be done due to the heterogeneity of the primary sources (Whittemore & Knafl 2005). Thus, the narrative synthesis was conducted to summarize and explain the findings.
Results
Search outcomes
The initial search identified 1,968 studies. Duplications and any that did not meet the inclusion criteria were removed. A full-text review of the 95 articles resulted in 24 eligible papers after further exclusion (Figure 1). When multiple articles using the same dataset were available, only the most recent version was included in the review. Two additional papers were retrieved through ancestry searching of the bibliographies of the 24 articles. Searching of Cochrane and Annual Review of Public Health resulted in no eligible studies. Thus, the final number of studies included is 26.
Study quality
The overall CCAT score ranged from 25 to 39 (Table 1), indicating varying levels of study quality. Specifically, the four dissertation work (Giacinto 2016, Kwan 2013, Moehling 2016, Tannas 2012) scored generally high (35-39) because much of the data necessary for the quality appraisal could be retrieved. Examining the eight individual categories indicated that the included studies typically scored high on the introduction and ethical components. However, the quality of other categories such as design and sampling varied from 1 to 4. Although all eligible studies, regardless of the CCAT score, were included, the scoring provided comprehensive evaluation about the methodological soundness of each one.
Table 1. Characteristic and findings of the included studies (n = 26).
| Author (Year), country | Design & sample source (size) | Measurement | Findings | CCAT Score |
|---|---|---|---|---|
| Giacinto (2016), U.S. | Cross-sectional Nationwide community (3180) | Women's health Initiative Insomnia Rate Scale: sleep quality; Self-reported sleep duration (short < 6h, normal 6-9h, long > 9h); Concurrent measure: HbA1c (poor ≥ 7.0%) |
|
37 |
| Lecube (2016), Spain | Case-control Clinic (135 patients and 45 controls) | PSQI: sleep quality (poor > 5); Medical record: HbA1c and FBG |
|
36 |
| Moehling (2016), U.S. | Cross-sectional Clinic (194) | PSQI: sleep quality; Concurrent measure: HbA1c |
|
35 |
| Tanik (2015), Turkey | Cross-sectional Clinic (122) | PSQI: sleep quality; Concurrent measure: FBG |
|
26 |
| Keskin (2015), Turkey | Cross-sectional Clinic (575) | PSQI: sleep quality (poor ≥ 5); Concurrent measure: FBG and HbA1c (poor ≥ 6.5%) |
|
29 |
| Czech (2015), U.S. | Cross-sectional Clinic (86) | One item from Patient Health Questionnaire-9: sleep disturbance (quality and duration); Medical record: HbA1c |
|
33 |
| Jennum (2015), U.S. | Quasi-experimental NA (26) | PSG: sleep quality (awakenings); Continuous glucose monitoring |
|
38 |
| Reutrakul (2015), Thailand | Cross-sectional Clinic (210) | PSQI (modified): sleep quality; Self-reported: sleep duration; Medical record: HbA1c |
|
35 |
| Nefs (2015), Netherland | Cross-sectional Nationwide (361 out of the 628 participants were T2DM) | PSQI: sleep quality (poor > 5); Self-reported: HbA1c (poor > 7%) |
|
36 |
| Cooper (2015), UK | Cross-sectional Nationwide (391) | Self-reported and movement sensor data: sleep duration; Concurrent measure: HbA1c |
|
37 |
| Yoda (2015), Japan | Cross-sectional Hospital (63) | EEG: sleep duration, sleep quality (REM latency); Concurrent measure: FBG, HbA1c (poor > 9%; average 7-9%; good < 7%) |
|
25 |
| Osonoi (2015), Japan | Cross-sectional Hospital (724) | PSQI: sleep quality (good < 6; average 6-8; poor > 8); Concurrent measure: FBG and HbA1c |
|
32 |
| Ohkuma (2014), Japan | Cross-sectional Hospital (4402) | Self-reported sleep duration (< 5.5 h, 5.5-6.4 h, 6.5-7.4 h, 7.5-8.4 h, > 8.5 h); Concurrent measure: HbA1c and FBG |
|
34 |
| Cho (2014), Korea | Cross-sectional Hospital (614) | PSQI: sleep quality; Self-reported sleep duration; Medical record: HbA1c, FBG, 2h-BG |
|
28 |
| Mahmood (2013), Ireland | Cross-sectional Clinic (114) | PSQI: sleep quality (poor > 5) and sleep duration (short < 6 h; average 6-8 h; long > 8 h); Concurrent measure: HbA1c, FBG |
|
33 |
| Kwan (2013), HongKong | Two-phase: cross-sectional and quasi-experimental Clinic (548/60) | PSQI: sleep quality; Actigraphy (7 days): sleep duration and sleep efficiency; Concurrent measure: HbA1c |
Phase 1:PSQI scores were related to HbA1c (r = 0.127-0.301, p <0.05)
|
39 |
|
||||
| Tsai (2013), Taiwan | Cross-sectional Clinic (46) | PSQI: sleep quality (good ≤ 5; average 6-8; poor > 8); Concurrent measure: HbA1c (poor ≥ 7%) |
|
35 |
| Tannas (2012), U.S. | Quasi-experimental Community (9) | PSQI: sleep quality; Sleep diary and actigraphy: sleep duration; Concurrent measure: HbA1c and blood glucose |
|
37 |
| Rajendran (2012), India | Cross-sectional Medical center (120) | PSQI: sleep quality (poor ≥ 5); Medical record: FBG, 2h-BG, HbA1c (poor ≥ 7.0%) |
|
31 |
| Jain (2012), U.S. | Cross-sectional Clinic (81) | NA: insomnia; Concurrent measure: HbA1c, FBG |
|
28 |
| Yagi (2011), Japan | Cross-sectional Clinic (270) | PSQI: sleep quality (poor ≥ 5.5); Concurrent measure: HbA1c, FBG |
|
35 |
| Knutson (2011), U.S. | Cross-sectional Nationwide (40 out of the 571 participants with T2DM) | Actigraphy (3 days): sleep duration and sleep quality (fragmentation and insomnia); PSQI: sleep quality; Medical record: FBG |
|
35 |
| Garfinkel (2011), Israel | RCT NA (36) | Actigraphy (3 days): sleep duration, sleep quality (efficiency, latency, wake after sleep onset) Concurrent measure: HbA1c, FBG |
|
34 |
| Trento (2008), Italy | Cross-sectional NA (47) | Actigraphy (3 days): sleep duration and quality (efficiency and fragmentation); NA: HbA1c |
|
29 |
| Williams (2007), U.S. | Cross-sectional Nationwide (935) | self-reported sleep duration (≤ 5 h, 6 h, 7 h, 8 h, ≥ 9 h); Medical record: HbA1c |
|
35 |
| Knutson (2006), U.S. | Cross-sectional Hospital (161) | Modified PSQI: sleep quality (poor > 5) and sleep duration (weekly average); Medical record: HbA1c |
|
38 |
Notes: NA-not available
Study characteristics
The articles were published between 2006 and 2016. As is shown in Table 1, the studies were conducted in various countries, including U.S., Japan, Italy, and Netherland. Among the 26 studies, a majority of the studies used a cross-sectional design. A total of 13,757 participants were recruited. Within individual studies, the sample size ranged from 9 to 4,402. Five studies were nationwide (Cooper et al. 2015, Giacinto 2016, Knutson et al. 2011, Nefs et al. 2015, Williams et al. 2007), and the remaining recruited participants from clinics or hospitals. Both objective and subjective methods were used to measure sleep disturbance such as actigraphy and Pittsburgh Sleep Quality Index (PSQI). Glycemic control was primarily measured by HbA1c or blood glucose (Table 1).
Participant characteristics
In the studies reporting participant age range, the average age was between 26.3 and 66.6 years old. Two studies only recruited women (Tannas 2012, Williams et al. 2007); the remaining included both genders. The average diabetes duration ranged from 5.4 years (Jennum et al. 2015) to 18.1 years (Yagi et al. 2011). The average BMI ranged from 24.7 kg/m2 (Yagi et al. 2011) to 37.9 kg/m2 (Knutson et al. 2011). Overall, glycemic control across studies, as measured by HbA1c (7.0% to 8.9%), was higher than recommended by the American Diabetes Association (American Diabetes Association 2017), except two studies [6.7% (Jennum et al. 2015) and 6.9% (Keskin et al. 2015)].
Relationship between sleep disturbance and glycemic control
Findings regarding the relationship between sleep disturbance and glycemic control were inconsistent across studies. Three main patterns were identified and thus were clustered under the following three themes: sleep disturbance unrelated to glycemic control; inconclusive relationships between sleep duration and glycemic control; and sleep quality related to glycemic control.
Sleep disturbance unrelated to glycemic control
Nine studies found no significant associations between sleep disturbance and glycemic control (Cho et al. 2014, Cooper et al. 2015, Garfinkel et al. 2011, Jain et al. 2012, Moehling 2016, Rajendran et al. 2012, Tannas 2012, Williams et al. 2007, Yagi et al. 2011). Among the nine studies, seven used a cross-sectional design and the sleep measures included only self-reported sleep duration or sleep quality. One of the remaining two studies was a randomized controlled trial examining the relationship between glycemic control and objective sleep, however, the sample included only 36 adults with T2DM and insomnia (Garfinkel et al. 2011). In the other quasi-experimental study (Tannas 2012), Tannas recruited only nine participants and did not include a control group.
Inconclusive relationships between sleep duration and glycemic control
Five studies explored the nonlinear (U-shaped) relationship between sleep duration and glycemic control. Compared with patients who slept 6.5-7.4 h, patients with shorter or longer sleep duration tended to have higher levels of fasting glucose and HbA1c (P trend value<0.01) (Ohkuma et al. 2014). Cooper and colleagues (Cooper et al. 2015) also reported that the lowest HbA1c level tended to be in those who slept 7-8h, although the trend was not significant. Compared to normal sleep duration (6-9h), long sleep duration (over 9h) increased the likelihood of having poor glycemic control (OR=0.76, P=0.038) (Giacinto 2016). In contrast, the U-shaped relationship between self-reported sleep duration and HbA1c was not found in two studies (Mahmood et al. 2013, Williams et al. 2007).
In the eight studies that examined the linear relationship between sleep duration and glycemic control, six did not find a significant association. HbA1c or fasting glucose was not related to sleep duration measured by objective EEG (Yoda et al. 2015), actigraphy (Knutson et al. 2011, Kwan 2013, Trento et al. 2008), or self-reported questionnaire (Cho et al. 2014, Giacinto 2016). Only in two studies was the relationship between sleep duration and HbA1c significant. Specifically, HbA1c negatively correlated with sleep duration (r = -0.168 - -0.17, P < 0.05) (Knutson et al. 2006, Reutrakul et al. 2015), and shorter sleep duration explained additional 2.8% of the variance in HbA1c, after controlling for covariates (Reutrakul et al. 2015).
Sleep quality related to glycemic control
Fourteen of the 22 studies examining the relationship between sleep quality and glycemic control found a significant association. Sleep quality has been objectively measured by EEG, PSG, or actigraphy. Objective sleep latency (r = -0.342 - -0.292, P < 0.05) (Yoda et al. 2015) and sleep efficiency (r = -0.29, P = 0.047) (Trento et al. 2008) was negatively correlated with glycemic control. Greater sleep fragmentation was associated with higher fasting glucose (β = 0.089, P < 0.05) (Knutson et al. 2011) and HbA1c (r = 0.31, P = 0.031) (Trento et al. 2008). Similarly, sleep quality operationalized as nocturnal awakening was related to hypoglycemia (Jennum et al. 2015). Sleep quality was also subjectively measured by self-reported questionnaires such as PSQI, and similar findings have been reported. Subjective poor sleep quality was correlated with poorer glycemic control (r = 0.14-0.30, P < 0.05) (Keskin et al. 2015, Knutson et al. 2006, Kwan 2013, Mahmood et al. 2013, Tanik et al. 2016, Tsai et al. 2012), although the relationship became nonsignificant after adjustment for confounders in two studies (Mahmood et al. 2013, Osonoi et al. 2015). People with poor sleep quality had poor glycemic control (P < 0.05) (Lecube et al. 2016, Nefs et al. 2015). Sleep disturbance also contributed unique variance in HbA1c (β = 0.043 – 0.27, P < 0.05) (Czech et al. 2015, Knutson et al. 2006) or posed as a risk factor for poor glycemic control (OR=6.94, P = 0.050) (Tsai et al. 2012).
Discussion
This review addresses the relationship between sleep disturbance and glycemic control in adults with T2DM and builds on the recent review by Lee and colleagues (Lee et al. 2016). In this review, we systematically examined 26 studies. We found that sleep quality is related to glycemic control in people with T2DM. However, evidence supporting a significant relationship between sleep duration and glycemic control is not strong.
Our finding of the significant relationship between sleep quality and glycemic control is consistent with current evidence. Lee et al. reported that poor sleep quality was associated with an increased HbA1c (weighted mean difference = 0.35%; 95% CI = 0.12, 0.58) in people with T2DM. A similar pattern was also observed in people with type 1 diabetes; good sleep quality was related to lower HbA1c (mean difference = −0.19%; 95% CI = −0.30, −0.08) (Reutrakul et al. 2016). The mechanisms underlying the relationship between sleep quality and glycemic control remain unclear. Reutrakul and Van Cauter proposed physiological pathways involved in the detrimental effect of sleep disturbance on metabolism. For instance, short sleep duration and poor sleep quality might cause decreased brain glucose utilization, which leads to hyperglycemia. An alteration in appetite-regulating hormones, including ghrelin and leptin, caused by sleep disturbance might also play a role (Reutrakul & Van Cauter 2014). Additionally, Larcher and colleagues suggest there is a behavioral mechanism linking sleep disturbance and diabetes. Sleep disturbance likely increases calorie intake, decreases energy expenditure, and leads to impaired decision-making (e.g., unhealthy food choice and sedentary behaviors), which will ultimately increase the risk of T2DM or poor glycemic control (Larcher et al. 2015).
There is little evidence of a significant association between sleep duration and glycemic control. Lee and colleagues reported that, compared to normal sleep duration, both short and long sleep duration were related to an increased HbA1c (weighted mean difference 0.23% and 0.13%, respectively); suggesting a U-shaped curve (Lee et al. 2016). Our findings differed from those of Lee et al., in that there was no strong evidence in support of a relationship between sleep duration and glycemic control. The inconsistent findings could be explained by lack of standard classifications for short, normal, and long sleep duration between studies. Sleep duration measurement could also explain the inconsistency in findings. In the review by Lee et al., most studies measured sleep duration subjectively, while in our review, we included studies that used both subjective and objective measures of sleep duration.
Variances in the quality of the primary sources included in this review could explain the inconsistent findings regarding the relationship between sleep disturbance and glycemic control. The studies' CCAT scores ranged from 25 to 39, indicating much variability in the quality, particularly the two dimensions included in the CCAT: study design and sampling.
Study design
Study design elements within CCAT, including the inclusion of confounders and measurement (Crowe 2013), may help to explain the inconsistent findings. The choice of confounding variables or lack thereof may underestimate or overestimate the relationship between sleep disturbance and glycemic control. Patient demographics (e.g., age and gender) and diabetes-related factors (e.g., diabetes duration) have been related to glycemic control (de Pablos-Velasco et al. 2014), and therefore might need to be adjusted. The potential confounding effect of these variables was further demonstrated in a current study where a significant U-shaped relationship between sleep duration and glycemic control was reported. When age, gender, and diabetes duration were controlled, the relationship was no longer significant (Kim et al. 2013). Similarly, in our review, the relationship between sleep and glycemic control lost significance after controlling for confounders in several studies (Mahmood et al. 2013, Nefs et al. 2015, Osonoi et al. 2015). There is little concensus regarding choice of control variables when examining the relationship between sleep and diabetes. Future research might help to clarify potential diabetes and non diabetes-related factors that may confound these relationships.
Measures of sleep varied across studies, yet psychometric properties of the instruments were rarely reported. Lack of evidence for validity and reliability could also weaken the reported associations between sleep disturbance and glycemic control. Additionally, the discrepancy between subjective and objective measures of sleep disturbance might account for the inconsistency between study findings. When sleep quality was objectively measured (Jennum et al. 2015, Knutson et al. 2011, Trento et al. 2008, Yoda et al. 2015), poor sleep quality was associated with poor glycemic control. In contrast, subjectively measured sleep quality was not related to glycemic control in several studies (Cho et al. 2014, Nefs et al. 2015, Rajendran et al. 2012, Reutrakul et al. 2015, Yagi et al. 2011). Objective and subjective measures of sleep provide different and unique portrayals of an individual's sleep (Landry et al. 2015). People might have misperceptions of their sleep time. Thus, their subjective report of nightly sleep does not necessarily align with objective measures, such as PSG (Bathgate et al. 2016). Significant relationships between glycemic control and subjectively measured sleep disturbance might be missed if only objective measures are used. However, in the clinical setting, the subjective interpretation of sleep disturbance may affect patients' daily lives. Thus, any conclusion regarding the relationship between sleep disturbance and glycemic control must account for variations in measures. Future research using both objective and subjective sleep measures will enable us to compare their respective association with glycemic control.
Sampling
Variations in sampling protocols, such as sample size, could also account for the inconsistent findings. Frequently, statistical significance is a result of a large sample size (Ellis 2009). In studies that found a significant non-linear relationship between glycemic control and sleep duration, the significance could be explained by the large sample sizes; each included over 2,000 participants (Giacinto 2016, Ohkuma et al. 2014). Meanwhile, low statistical power, such as inadequate sample size, can undermine the likelihood of detecting the significance (Button et al. 2013). For instance, in the experimental study that included nine participants (Tannas 2012), the study is very likely underpowered, and therefore the significant relationship between sleep disturbance and glycemic control could have been missed. In view of the studies included in this review, many did not address the adequacy of the sample size. Therefore, interpretation of the findings from each study needs to take into consideration the sample size.
Variability in the inclusion and exclusion criteria across studies, particularly diabetic neuropathy, could explain the inconsistent findings. Diabetic neuropathy is a progression pattern of sensory loss due to diabetes-related metabolic and neurodegenerative changes. It has been suggested as an independent risk factor for sleep disturbance (Öztürk et al. 2015). In this review, the inclusion of participants with diabetic neuropathy varied. For instance, one study only recruited participants without neuropathy (Trento et al. 2008). In contrast, the other study only included those with painful neuropathy (Tanik et al. 2016). This variation did not make a clear and consistent impact on the findings regarding the relationship between sleep disturbance and glycemic control. However, the effect of sleep disturbance on glycemic control differed when participants were stratified by the number of complications of diabetes, including painful neuropathy (Knutson et al. 2006). This further suggests that variations in the inclusion of people with diabetic complications need to be accounted for when examining the relationship between sleep disturbance and glycemic control.
Limitations
In this integrative review, we systematically reviewed current evidence regarding the relationship between sleep disturbance and glycemic control in adults with T2DM. Using a narrative synthesis, we added new evidence to our current understanding of the relationship between sleep and diabetes and shedded more light on an important health issue that is mostly under-researched. Nevertheless, this review has several limitations. Although we conducted an exhaustive search, the gray literature was not fully captured, as non-English papers and unpublished reports were not included. All eligible studies were included in the analysis regardless of the quality due to a paucity of existing studies. This was somewhat mitigated by the use of systematic quality appraisal, which informed us of the strength, weakness, and overall quality of each study. This enabled us to weigh and report the strength of evidence. Aditionally, the inclusion of all studies regardless of the quality score present us the state of science in this particular research area. Another limitation is the lack of standardization of sleep disturbance measurement. Objective and subjective measures evaluate different aspects of sleep. However, most of the studies used only subjective or objective sleep assessment. In addition, the adequacy of the sample size was not addressed, which might have underestimated or overestimated the relationship. Finally, the causality between sleep disturbance and glycemic control cannot be determined as most of the study used a cross-sectional design. Reciprocal relationships between sleep disturbance and glycemic control have been proposed. It is also possible that glycemic control could affect sleep.
Conclusion
The prevalence of T2DM and sleep disturbance is increasing. The role sleep plays in diabetes development remains to be examined. This review provided further evidence for the importance of sleep in the diabetes population. Strong evidence supports a significant relationship between sleep quality and glycemic control. Nevertheless, the relationship between sleep duration and glycemic control needs to be further investigated. Future research using a more rigorous design would shed more lights on this topic. Specifically, these studies should include a power analysis and use a combination of subjective and objective sleep assessment. Research designed to test the causal relationship between sleep disturbance and glycemic control would contribute more to this area of investigation.
What does this paper contribute to the wider global clinical community?
Identifies that sleep disturbance, particularly impaired sleep quality, is related to glycemic control in people with type 2 diabetes.
Demonstrates the importance of healthy sleep and the need for conducting sleep-related assessment and intervention in people with diabetes.
Lends evidence to current clinical practice regarding the necessity of incorporating sleep-related self-care into the overall self-care repertoire.
Relevance to Clinical Practice.
Both T2DM and sleep disturbance are major public issues that have brought great health and economic burdens. National initiatives, such as Healthy People 2020, have set clear objectives for sleep health, yet the importance of healthy sleep, especially in people with T2DM, remains underappreciated. This review adds to current evidence and reveals the possible adverse effect of sleep disturbance, particularly poor sleep quality, on glycemic control in people with T2DM. There is a need to increase nurses' awareness of the importance of sleep in diabetes development. Sleep disturbance includes not only impaired sleep quality and loss of sleep but also longer sleep duration, which is often overlooked. Thus, the complexity of sleep disturbance needs to be underscored in health care professionals so that they can provide sleep-related education for patients. In clinical practice, nurse practitioners need to pay attention to patients' complaints about their sleep and make referrals if necessary. This review provides further evidence for the incorporation of routine sleep assessment and education, which may help to change current diabetes education guidelines. Good sleep quality can reduce HbA1c by 0.35%, which can be translated to 3% and 5% reduction in death and microvascular complications, respectively (Lee et al. 2016). Thus, collaborations among clinicians, nurses, and patients are needed to better manage sleep disturbance.
Acknowledgments
The authors thank Kevin Grandfield, Publication Manager for the UIC Department of Biobehavioral Health Science, for editorial assistance.
Funding: This project received no specific grant from any funding agency.
Footnotes
Conflicts of interest: No conflict of interest has been declared by the authors.
References
- American Diabetes Association. Standards of medical care in diabetes-2017. Diabetes Care. 2017;40:S11–S48. [Google Scholar]
- Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037–1045. doi: 10.5665/sleep.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafò MR. Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. [accessed Feburary 24th 2017];National diabetes statistics report. 2014 Available at: https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
- Cho EH, Lee H, Ryu OH, Choi MG, Kim SW. Sleep disturbances and glucoregulation in patients with type 2 diabetes. Journal of Korean Medical Science. 2014;29:243–247. doi: 10.3346/jkms.2014.29.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colten HR, Altevogt BM. Sleep physiology. In: Colten Hr, Altevogt BM., editors. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. National Academies Press; Washington (DC): 2006. pp. 33–53. [PubMed] [Google Scholar]
- Cooper AJ, Westgate K, Brage S, Prevost AT, Griffin SJ, Simmons RK. Sleep duration and cardiometabolic risk factors among individuals with type 2 diabetes. Sleep Medicine. 2015;16:119–125. doi: 10.1016/j.sleep.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Cooper HM. Synthesizing Research: A guide for Literature Reviews, 3rd edn (Cooper HM ed.) Sage; Thousand Oaks (CA): 1998. The data analysis stage; pp. 104–156. [Google Scholar]
- Crowe M. Crowe Critical Appraisal Tool (CCAT) form: Version 1.4. [accessed March 27th 2016];2013 Available at: https://conchra.com.au/wp-content/uploads/2015/12/CCAT-form-v1.4.pdf.
- Czech SJ, Orsillo SM, Pirraglia PA, English TM, Connell AJ. Association between specific depression symptoms and glycemic control among patients with comorbid type 2 diabetes and provisional depression. Primary Care Companion for CNS Disorder. 2015;17:1–19. doi: 10.4088/PCC.14m01754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pablos-Velasco P, Parhofer KG, Bradley C, Eschwege E, Gönder-Frederick L, Maheux P, Wood I, Simon D. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: Data from the PANORAMA study. Clinical Endocrinology. 2014;80:47–56. doi: 10.1111/cen.12119. [DOI] [PubMed] [Google Scholar]
- Ellis PD. Thresholds for interpreting effect sizes. [accessed March 4th 2017];2009 Available at: http://www.polyu.edu.hk/mm/effectsizefaqs/thresholds_for_interpreting_effect_sizes2.html.
- Garfinkel D, Zorin M, Wainstein J, Matas Z, Laudon M, Zisapel N. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes, Metabolic Syndrome, and Obesity. 2011;4:307–313. doi: 10.2147/DMSO.S23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacinto RE. Psychosocial, sociocultural, and biobehavioral correlates of glycemic control among individuals with diabetes in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) University of California; San Diego: 2016. p. 135. [Google Scholar]
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Jain SK, Kahlon G, Morehead L, Lieblong B, Stapleton T, Hoeldtke R, Bass PF, 3rd, Levine SN. The effect of sleep apnea and insomnia on blood levels of leptin, insulin resistance, IP-10, and hydrogen sulfide in type 2 diabetic patients. Metabolic Syndrome and Related Disorder. 2012;10:331–336. doi: 10.1089/met.2012.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennum P, Stender-Petersen K, Rabol R, Jorgensen NR, Chu PL, Madsbad S. The impact of nocturnal hypoglycemia on sleep in subjects with type 2 diabetes. Diabetes Care. 2015;38:2151–2157. doi: 10.2337/dc15-0907. [DOI] [PubMed] [Google Scholar]
- Keskin A, Unalacak M, Bilge U, Yildiz P, Guler S, Selcuk EB, Bilgin M. Effects of sleep disorders on hemoglobin A1c Levels in type 2 diabetic patients. Chinese Medical Journal (English) 2015;128:3292–3297. doi: 10.4103/0366-6999.171415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BK, Kim BS, An SY, Lee MS, Choi YJ, Han SJ, Chung YS, Lee KW, Kim DJ. Sleep duration and glycemic control in patients with diabetes mellitus: Korea National Health and Nutrition Examination Survey 2007-2010. Journal of Korean Medical Science. 2013;28:1334–1339. doi: 10.3346/jkms.2013.28.9.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Archive of Internal Medicine. 2006;166:1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: The Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34:1171–1176. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan YM. Feasibility study of a randomized controlled trial protocol to examine the effectiveness of auriculotherapy (AT) in improving sleep condition and glycaemic control in clients with type 2 diabetes. The Chinese University of Hong Kong; HongKong: 2013. p. 205. [Google Scholar]
- Landry GJ, Best JR, Liu-Ambrose T. Measuring sleep quality in older adults: A comparison using subjective and objective methods. Frontiers in Aging Neuroscience. 2015;7:166. doi: 10.3389/fnagi.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher S, Benhamou PY, Pepin JL, Borel AL. Sleep habits and diabetes. Diabetes & Metabolism. 2015;41:263–271. doi: 10.1016/j.diabet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Lecube A, Sanchez E, Gomez-Peralta F, Abreu C, Valls J, Mestre O, Romero O, Martinez MD, Sampol G, Ciudin A, Hernandez C, Simo R. Global assessment of the impact of type 2 diabetes on sleep through specific questionnaires: A case-control study. PLoS ONE. 2016;11:e0157579. doi: 10.1371/journal.pone.0157579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: A systematic review and meta-analysis. Sleep Medicine Reviews. 2016;31:91–101. doi: 10.1016/j.smrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Lenters-Westra E, Schindhelm RK, Bilo HJ, Slingerland RJ. Haemoglobin A1c: Historical overview and current concepts. Diabetes Research and Clinical Practice. 2013;99:75–84. doi: 10.1016/j.diabres.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Luyster FS, Dunbar-Jacob J. Sleep quality and quality of life in adults with type 2 diabetes. The Diabetes Educator. 2011;37:347–355. doi: 10.1177/0145721711400663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood WAW, Yusoff MSD, Behan LA, Di Perna A, Tun TK, McDermott J, Sreenan S. Association between sleep disruption and levels of lipids in Caucasians with type 2 diabetes. International Journal of Endocrinology. 2013;2013:1–8. doi: 10.1155/2013/341506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehling JA. Effect of temporal distribution of food intake and chronotype on glycemic control in subjects with type 2 diabetes. Rush University; Chicago: 2016. p. 157. [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. International Journal of Surgery. 2010;8:336–341. [Google Scholar]
- National Sleep Foundation. Sleeptionary-Definitions of common sleep terms. [accessed Feburary 24th 2017];2017 Available at: https://sleepfoundation.org/sleeptionary.
- Nefs G, Donga E, van Someren E, Bot M, Speight J, Pouwer F. Subjective sleep impairment in adults with type 1 or type 2 diabetes: Results from Diabetes MILES-The Netherlands. Diabetes Research and Clinical Practice. 2015;109:466–475. doi: 10.1016/j.diabres.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Ohkuma T, Fujii H, Iwase M, Ogata-Kaizu S, Ide H, Kikuchi Y, Idewaki Y, Jodai T, Hirakawa Y, Nakamura U, Kitazono T. U-shaped association of sleep duration with metabolic syndrome and insulin resistance in patients with type 2 diabetes: The Fukuoka Diabetes Registry. Metabolism-Clinical and Experimental. 2014;63:484–491. doi: 10.1016/j.metabol.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Osonoi Y, Mita T, Osonoi T, Saito M, Tamasawa A, Nakayama S, Someya Y, Ishida H, Kanazawa A, Gosho M, Fujitani Y, Watada H. Poor sleep quality is associated with increased arterial stiffness in Japanese patients with type 2 diabetes mellitus. BMC Endocrine Disorders. 2015;15:1–7. doi: 10.1186/s12902-015-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öztürk ZA, Yesil Y, Kuyumcu ME, Savas E, Uygun Ö, Sayiner ZA, Kepekçi Y. Association of depression and sleep quality with complications of type 2 diabetes in geriatric patients. Aging Clinical and Experimental Research. 2015;27:533–538. doi: 10.1007/s40520-014-0293-0. [DOI] [PubMed] [Google Scholar]
- Rajendran A, Parthsarathy S, Tamilselvan B, Seshadri KG, Shuaib M. Prevalence and correlates of disordered sleep in southeast asian indians with type 2 diabetes. Diabetes and Metabolism Journal. 2012;36:70–76. doi: 10.4093/dmj.2012.36.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul S, Siwasaranond N, Nimitphong H, Saetung S, Chirakalwasan N, Ongphiphadhanakul B, Thakkinstian A, Hood MM, Crowley SJ. Relationships among sleep timing, sleep duration and glycemic control in type 2 diabetes in Thailand. Chronobiology International. 2015;32:1469–1476. doi: 10.3109/07420528.2015.1105812. [DOI] [PubMed] [Google Scholar]
- Reutrakul S, Thakkinstian A, Anothaisintawee T, Chontong S, Borel AL, Perfect MM, Janovsky CS, Kessler R, Schultes B, Harsch IA. Sleep characteristics in type 1 diabetes and associations with glycemic control: Systematic review and meta-analysis. Sleep Medicine. 2016;23:26–45. doi: 10.1016/j.sleep.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: Implications for risk and severity of diabetes. Annals of the New York Academy of Sciences. 2014;1311:151–173. doi: 10.1111/nyas.12355. [DOI] [PubMed] [Google Scholar]
- Siontis KC, Hernandez-Boussard T, Ioannidis JPA. Overlapping meta-analyses on the same topic: Survey of published studies. BMJ. 2013;347:f4501. doi: 10.1136/bmj.f4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanik N, Sarp U, Ucar M, Celikbilek A, Balbaloglu O, Ak H, Atalay T, Arik HO, Okyay MY, Inan LE. Pain, depression and sleep disorders in patients with diabetic and nondiabetic carpal tunnel syndrome: A vicious cycle. Arquivos De Neuro-Psiquiatria. 2016;74:207–211. doi: 10.1590/0004-282X20160020. [DOI] [PubMed] [Google Scholar]
- Tannas CL. Type 2 diabetes and insomnia: Impact on metabolic control. Wayne State University; Detroit: 2012. p. 165. [Google Scholar]
- Trento M, Broglio F, Riganti F, Basile M, Borgo E, Kucich C, Passera P, Tibaldi P, Tomelini M, Cavallo F, Ghigo E, Porta M. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetologica. 2008;45:225–229. doi: 10.1007/s00592-008-0047-6. [DOI] [PubMed] [Google Scholar]
- Tsai YW, Kann NH, Tung TH, Chao YJ, Lin CJ, Chang KC, Chang SS, Chen JY. Impact of subjective sleep quality on glycemic control in type 2 diabetes mellitus. Family Practice. 2012;29:30–35. doi: 10.1093/fampra/cmr041. [DOI] [PubMed] [Google Scholar]
- Whittemore R, Knafl KA. The integrative review: updated methodology. Journal of Advanced Nursing. 2005;52:546–553. doi: 10.1111/j.1365-2648.2005.03621.x. [DOI] [PubMed] [Google Scholar]
- Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007;30:1233–1240. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- Yagi A, Nishio Y, Ugi S, Kawai H, Uzu T, Imai M, Yamada N, Okawa M, Kashiwagi A, Maegawa H. The role of sleep disturbance and depression in patients with type 2 diabetes. Diabetology International. 2011;2:79–85. [Google Scholar]
- Yoda K, Inaba M, Hamamoto K, Yoda M, Tsuda A, Mori K, Imanishi Y, Emoto M, Yamada S. Association between poor glycemic control, impaired sleep quality, and increased arterial thickening in type 2 diabetic patients. PLoS ONE. 2015;10:1–12. doi: 10.1371/journal.pone.0122521. [DOI] [PMC free article] [PubMed] [Google Scholar]


