Abstract
The modulation of c-di-GMP levels plays a vital role in the regulation of various processes in a wide array of bacterial species. Thus, investigation of c-di-GMP regulation requires reliable methods for the assessment of c-di-GMP levels and turnover. Reversed-phase high-performance liquid chromatography (RP-HPLC) analysis has become a commonly used approach to accomplish these goals. The following describes the extraction and HPLC-base detection and quantification of c-di-GMP from Pseudomonas aeruginosa samples, a procedure that is amenable to modifications for the analysis of c-di-GMP in other bacterial species.
Keywords: bis-(3′,5′)-cyclic dimeric guanosine monophosphate; c-di-GMP; cyclic dinucleotides; reversed phase; ion exchange; high-performance liquid chromatography; biofilm
1. Introduction
Key cellular behaviors in a wide variety of bacteria are regulated via the turnover of bis-(3′,5′)-cyclic dimeric guanosine monophosphate (c-di-GMP) (1, 2). Modulation of c-di-GMP levels involves di-guanylate cyclases (DGCs) catalyzing the condensation of two guanosine-5'-triphosphate (GTP) molecules to form c-di-GMP, and phosphodiesterases (PDEs) driving the hydrolysis of c-di-GMP to guanosine monophosphate (GMP) via the linear intermediate 5'-phosphoguanylyl-(3'–>5')-guanosine (pGpG) (3–6). The delicate balance between c-di-GMP synthesis and breakdown contributes to subtle changes that drive major alterations in bacterial cell physiology. Thus, reliable methods for measuring c-di-GMP levels and turnover are essential to investigate mechanisms of c-di-GMP regulation.
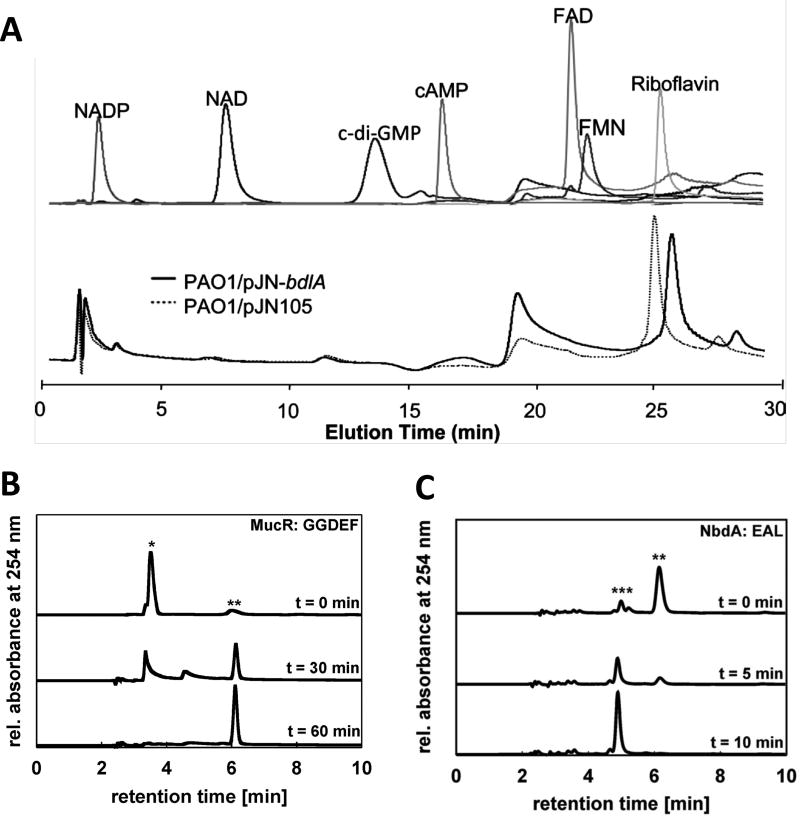
High-performance liquid chromatography (HPLC) was among the methods used in the seminal body of work that originally identified c-di-GMP as a secondary messenger molecule (3, 4, 7). And HPLC is still commonly used today for the separation, detection, and quantitation of c-di-GMP (8–11). It is fairly rapid and does not require extensive sample preparation. The method enables the quantitation of c-di-GMP in the picomolar, or even the femtomolar range, depending on the detection approach used (12–14). Moreover, HPLC has the potential to separate c-di-GMP from other compounds likely present in cellular extracts and/or those that can interfere with proper quantitation of c-di-GMP (Fig. 1). While enabling this separation, HPLC can also detect compounds that are associated with c-di-GMP turnover, including GTP, GMP, and pGpG. Thus, in addition to simple quantitation of total cellular c-di-GMP levels, HPLC analysis also allows for time-course assays of c-di-GMP synthesis or breakdown, and has been successfully used to investigate DGC and PDE enzyme kinetics (Fig. 1) (15–17).
Figure 1. Separation of c-di-GMP and other small molecules by HPLC.
(A) Experiment demonstrating that the Pseudomonas aeruginosa chemotaxis protein BdlA does not bind FAD, FMN, NAD, NADP, cAMP, c-di-GMP or riboflavin. Top panel demonstrates the HPLC elution profiles of FAD, FMN, NAD, NADP, cAMP, c-di-GMP or riboflavin. Bottom panel demonstrates chromatograms of supernatants of purified BdlA (from P. aeruginosa PAO1/pJN-bdlA) or from vector control extracts (PAO1/pJN105). Reproduced from (22) with permission from American Society for Microbiology (ASM). (B) HPLC chromatograms demonstrating c-di-GMP synthesis by P. aeruginosa MucR. Peaks representing GTP (*) and c-di-GMP (**) are assigned. Reproduced from (17) with permission from ASM. (C) HPLC chromatograms showing c-di-GMP hydrolysis by the P. aeruginosa PDE NbdA. Peaks representing c-di-GMP (**) and pGpG (***) are assigned. Reproduced from (17) with permission from ASM. For all experiments, separation was performed on a C18 reversed-phase column, with analytes monitored at 254 nm.
HPLC is a form of column chromatography that pumps at high pressure a sample (analyte) dissolved in a solvent (mobile phase) through a column with an immobilized chromatographic packing material (stationary phase). The properties of the sample and the solvent, as well as the nature of the stationary phase, determine the retention time of the analytes, or how fast they pass through the column. As the sample passes through the column, analytes having the strongest interactions with the stationary phase exit the column the slowest, meaning they exhibit the longest retention times. In contrast, samples demonstrating little interaction with the column material elute quickly and are thus characterized by short retention times. Separation of compounds in a sample can be accomplished via an isocratic elution, where the composition of the mobile phase remains constant, or via a gradient elution, where the mobile phase composition is changed over the course of the separation toward conditions favoring analyte dissociation from the stationary phase. Upon exiting the column, the mobile phase passes through a detection module, such as a fluorimeter or a UV-absorbance detector. Selection of the appropriate detector and monitoring wavelengths is essential for optimizing the sensitivity of HPLC detection. The detector generates a signal correlating to the quantity of analyte emerging from the column, which is then transferred to and recorded by an HPLC control computer program, with the data available for subsequent analysis.
A few variations of HPLC protocols for the detection and quantitation of c-di-GMP have been developed. While studies requiring the separation of cyclic nucleotides and di-nucleotides have preferentially opted for reversed-phase HPLC (12, 18–20), which utilizes a non-polar stationary phase and a moderately polar mobile phase, the detection approach has been more varied. Often, cyclic nucleotides and di-nucleotides are directly monitored by a UV detector module in the 250–275 nm wavelength range (20), with c-di-GMP exhibiting an absorption maximum at 253 nm. More recently, quantitation of c-di-GMP and other cyclic nucleotides has been accomplished by coupling HPLC to tandem mass spectrometry (LC-MS/MS) or matrix-assisted laser desorption/ionization–time-of-flight (MALDI-TOF) analysis for improved detection of the messenger molecules (8, 11, 12, 14).
The following presents a specific variation of the approaches described above: an optimized method for the extraction of c-di-GMP from planktonic and biofilm cells of the opportunistic human pathogen Pseudomonas aeruginosa and subsequent separation and quantitation of c-di-GMP using reversed-phase HPLC with a UV detector module. The procedure has been adapted and modified from (8, 9). While the method has been optimized using P. aeruginosa cells and an Agilent 1100 HPLC system, the procedure is easily adaptable for other organisms and HPLC systems.
2. Materials
Prepare all solutions using nanopure water (18 MΩ-cm ionic purity at 25 °C) and analytical grade reagents, unless indicated otherwise. Filter all solutions using an 0.2 µm filter. Prepare and store all reagents at room temperature, unless indicated otherwise. Follow all appropriate waste disposal regulations when disposing waste materials.
2.1. Extraction of c-di-GMP
Unpolished 1.5 mL microcentrifuge tubes. See Note 1.
Bacterial culture. See Note 2.
Ethanol (190 or 200 proof). Store at −20°C. See Note 3.
Phosphate-buffered saline (PBS): To approximately 800 mL of water add 8.0g NaCl, 0.2g KCl, 2.56g Na2HPO4·7H2O, and 0.24g KH2PO4. Mix until all salts are dissolved. Adjust the pH to 7.4 with HCl. Add water to a total volume of 1L. Store at 4°C.
Refrigerated microcentrifuge set to 4°C and capable of attaining speeds up to 16,000×g.
Heating block set to 100°C.
Vacuum concentrator/Centrifugal evaporator (e.g. SpeedVac).
2.2. HPLC quantitation of c-di-GMP
HPLC system equipped with an autosampler, degasser and UV/Vis detector (e.g. Agilent 1100 HPLC), and connected to computer with HPLC system control software for HPLC peak analysis (e.g.: ChemStation for LC, Agilent Technologies).
HPLC Solvent A: Add 0.385 g ammonium acetate (MS grade) to 500mL of water for a final concentration of 10mM. Do not adjust pH. Store at 4°C until use. See Note 4.
HPLC Solvent B: Add 0.385 g ammonium acetate (MS grade) to 500mL of methanol. Dissolve ammonium acetate salt in methanol. Do not adjust pH. Store at 4°C until use. See Note 4.
Bis-(3'–5')-cyclic diguanylic monophosphate (c-di-GMP) (Bio-log): Dilute with water to a final concentration of 200 pmol/µl. Store at −80°C.
Syringes (1 ml)
HPLC Syringe filters (Hydrophobic PTFE, 0.45 µm)
Reverse-phase C18 column (2.1 × 40 mm, 5 µm)
2.3 Protein quantitation
Refrigerated microcentrifuge set to 4°C and capable of attaining speeds up to 16,000×g.
Protein determination reagents (e.g.: Modified Lowry Assay)
0.5 M Ethylenediaminetetraacetic acid (EDTA) disodium salt solution (pH 8.0): Add 186.1 g of disodium EDTA·2H2O to 800 mL of water. Stir vigorously on a magnetic stirrer. Adjust the pH to 8.0 with NaOH (~20 g of NaOH pellets). See Note 5.
TE buffer: To approximately 800 mL water add 1.21g Tris and 2mL of the 0.5M EDTA (pH 8.0) solution. Mix to allow the Tris to dissolve. Adjust the pH to 8.0 with HCl. Store at 4°C.
Spectrophotometer capable of measuring absorbance at 600 nm.
Sonicator capable of pulsed mode operation and 5W output.
3. Methods
3.1. Extraction of c-di-GMP
Grow bacterial cells to desired growth stage under required experimental conditions. Proceed directly with the extraction, with no waiting periods or incubation of cells on ice, as this may drastically alter the c-di-GMP levels. See Note 2.
Determine the optical density of the bacterial culture at 600 nm (OD600). See Note 6.
Obtain a bacterial culture volume equivalent to 1 ml of OD600 = 1.8 (e.g. if the OD600 = 0.9, spin down 2 ml of culture). See Notes 2 and 7.
Centrifuge (16,000 × g, 2 min, 4°C) the respective culture volume. Discard the supernatant.
Wash the cell pellet with 1 ml ice-cold PBS (16,000 × g, 2 min, 4°C). Discard the supernatant.
Repeat 3.1 step 5.
Resuspend the cell pellet in 100 µl ice-cold PBS and incubate at 100°C for 5 min. See Note 8.
Add ice-cold ethanol (stored at −20 °C until use) to a final concentration of 65% (186 µl of 200 proof ethanol or 217 µl of 190 proof ethanol) and vortex for 15 sec. See Note 8.
Centrifuge sample (16,000 × g, 2 min, 4 °C)
Remove and retain the supernatant containing extracted c-di-GMP in a new microfuge tube. Store the tube with the supernatant on ice until the next step. Retain the cell pellet.
Using the cell pellet, repeat twice the extraction procedure in 3.1 steps 7–10. Pool the supernatants obtained from these repeat extractions into the microfuge tube from 3.1 step 10 (i.e.: supernatants from the repeated three extractions should be combined in one tube). Retain the cell pellet after the final extraction step, and store it at −20 °C until step 3.2.3 step 1.
Dry the combined supernatants (total volume of ~900 µl) using a vacuum concentrator/centrifugal evaporator. The drying time can vary from 3 to 6 hrs. See Note 9.
Following evaporation, a white pellet should be visible. Store this sample, containing the extracted c-di-GMP, at −80°C until 3.2.2 step 1.
3.2. Quantification of c-di-GMP
This procedure has been optimized for the detection of c-di-GMP using an Agilent 1100 HPLC equipped with an autosampler, degasser, pressure regulator, prefilter, and UV/Vis detector set to 253 nm. Separation was carried out using a reverse-phase C18 Targa column (2.1 × 40 mm; 5 µm) and a flow rate of 0.2 ml min−1. Solvents containing methanol and ammonium acetate (see 2.2) were used. The following gradient was used to elute c-di-GMP: 0 to 9 min, 1% B (= 1% Solvent B and 99% Solvent A); 9 to 14 min, 15% B; 14 to 19 min, 25% B; 19 to 26 min, 90% B; 26 to 40 min, 1% B. This gradient resulted in the elution of c-di-GMP at approximately 14–15 min. See Note 10.
Use at least 20 column volumes of Solvent A to equilibrate a newly installed HPLC column, and at least 10 column volumes of Solvent A to re-equilibrate the column between runs.
3.2.1. Generation of a c-di-GMP standard curve
Using the 200 pmol/µl c-di-GMP stock solution, prepare the following standards in nanopure water, preferably by serial dilution: 1, 2, 5, 10 and 20 pmol/µl.
Inject and run on the HPLC 20 µl of the 1 pmol/µl c-di-GMP standard.
Repeat 3.2.1 step 2 for the 2, 5, 10, and 20 pmol/µl c-di-GMP standards.
Inject and run 20 µl of nanopure water as a negative control (0 pmol/µl c-di-GMP).
Use the HPLC analysis software (e.g.: ChemStation) to obtain the peak area for each of the standard concentrations. See Note 11.
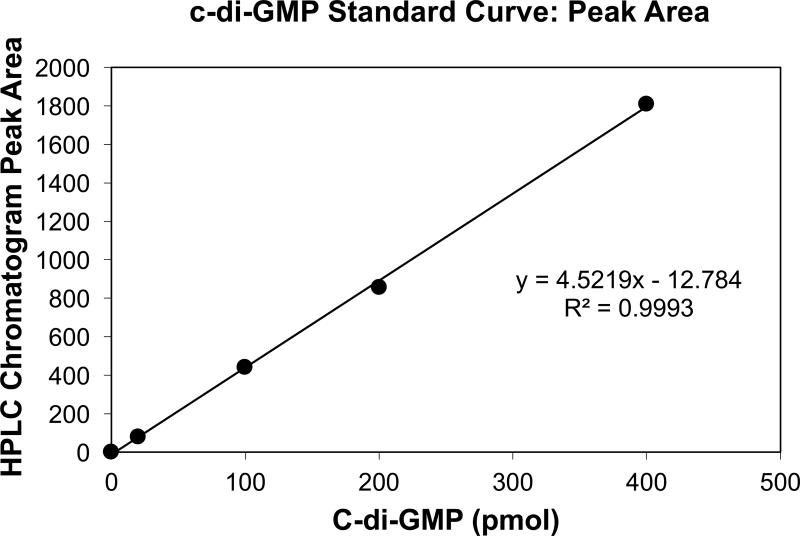
Prepare a standard curve by plotting the c-di-GMP amount in pmol (e.g.: 200 pmol for the 20 µl of the 10 pmol/µl standard) vs the peak areas (see Fig. 2 for an example of a standard curve).
Calculate the linear trendline and the R2 coefficient of the plotted points (see Fig. 2 for an example). See Note 12.
Figure 2. Example of a c-di-GMP HPLC standard curve.
The standard curve was generated by plotting the peak areas obtained following the separation of c-di-GMP standards versus the total c-di-GMP amounts (pmol) in the respective standard samples. 20 µl aliquots of the following c-di-GMP standards (0, 1, 5, 10 and 20 pmo/µl) were separated on a reverse-phase C18 Targa column. Peak areas were obtained using the ChemStation for LC software.
3.2.2. HPLC analysis of extracted c-di-GMP samples
Resuspend the dried extract from 3.1 step 13 in 200 µl nanopure water.
Vortex for 1 min.
Centrifuge the solution at max speed (≥ 16,000 × g) to remove insoluble material.
Carefully draw up the liquid sample into a 1 mL syringe, making sure to leave the pelleted debris in the tube.
Attach a 2 µm syringe filter to the 1 ml syringe with the extract.
Filter the sample supernatant into a new microfuge tube. See Note 13.
Analyze 20 µl per sample using the HPLC program used for the standards above.
Using the HPLC analysis software, determine the area of the c-di-GMP peak detected in the sample, ensuring that the peak is sharp and well-separated from other detected compounds. See Note 14.
Repeat 3.2.2 steps 1–8 for all c-di-GMP samples to be analyzed.
Determine the c-di-GMP amounts using the standard curve established with the commercially available c-di-GMP in 3.2.1.
3.2.3. Normalization of c-di-GMP calculations
Resuspend the cell pellet from 3.1 step 11 in 500 µl TE buffer.
Sonicate the suspension for a total of 2 min using 10 sec bursts at 5 W followed by 15 sec off. Sonication should be carried out on ice. See Note 15.
Determine the protein concentrations using a protein determination assay (e.g.: Modified Lowry Assay).
- Normalize the c-di-GMP levels to total cellular protein levels (i.e. pmol/mg) using the following calculations:
- Total c-di-GMP
- Where Tc-di-GMP is the total c-di-GMP in the extracted sample in pmol, Pc-di-GMP is the fraction of c-di-GMP (20 µl of 200 µl) quantified by the HPLC analysis and calculated in 3.2.2 step 10.
- Total protein
- Where Tprotein is the total cellular protein in the sample subjected to extraction, and Cprotein is the protein concentration determined in 3.2.3 step 3.
- Normalized c-di-GMP
- Where Nc-di-GMP is the cellular c-di-GMP level in pmol normalized per mg of cellular protein
Footnotes
Polished, especially highly polished, microcentrifuge tubes should be avoided during c-di-GMP extraction, to prevent static build up and the migration of powder during the sample drying procedure in a vacuum concentrator/centrifugal evaporator in 3.1 step 12. Such static build up and dry sample powder migration can lead to reduced recovery and quantitation of c-di-GMP.
c-di-GMP can be extracted from cells grown as a planktonic culture or as biofilms. It is important to note, however, that extraction from cells in the early exponential phase may yield c-di-GMP amounts below the limit of detection for HPLC-UV/Vis analysis. The procedure described here has been successfully utilized for the analysis of c-di-GMP levels from P. aeruginosa cultures where the following growth variables have been altered: mid- to late-log and stationary phase planktonic cells; 3- to 6-day-old biofilm cells grown under flowing conditions; Lennox broth or Vogel-Bonner minimal medium (VBMM) (21); wild-type and mutant strains; strains harboring plasmids and subjected to antibiotics for plasmid maintenance. However, the culture conditions and cell numbers/optical density measurements used for c-di-GMP extraction may need to be optimized when other bacterial species and/or growth conditions are tested.
Both 190 and 200 proof ethanol can be utilized for the c-di-GMP extraction protocol, and the appropriate volumes for the respective concentrations are provided in the protocol. However, due to its hygroscopic nature, it is preferable to store the 200 proof ethanol in an airtight glass container.
The HPLC Solvents A and B should be remade at least every 5 days due to the volatile nature of ammonium acetate. Use of old solvents with reduced concentrations of ammonium acetate may negatively affect the reproducibility of the separations and c-di-GMP retention times.
While preparing the EDTA solution, it is important to note that the EDTA disodium salt will not go into solution until the pH is ~8.0. When adjusting the pH, solid NaOH pellets should be used, rather than a solution of NaOH, due to the high amount of NaOH required to reach pH 8.0.
If working with samples with significant aggregation and cell-cell adhesion (e.g.: biofilms), including a step to homogenize (10 sec on high) the cultures to disrupt cell aggregates prior to OD600 determination may be beneficial to ensure accurate OD measurements and subsequent c-di-GMP quantitation.
This biomass has been optimized for the analysis of c-di-GMP in P. aeruginosa strains PAO1 and PA14 planktonic and biofilm samples. Analysis of c-di-GMP levels in other strains or species may require the initial biomass harvested for extraction to be adjusted.
Following the incubation at 100°C and consequent cell lysis, the remaining cellular debris may become viscous and may aggregate. Make sure that the pellet is vortexed vigorously upon addition of ethanol.
Depending on the vacuum concentrator/centrifugal evaporator used, the drying time for the extracted samples can vary significantly. The drying time also depends on whether the instrument has separate settings for evaporation of aqueous or alcohol solvents. In our experience, the average evaporation time is 4 hr, with no less than 3 hr required. Do not apply heat to accelerate the evaporation process.
Analysis of c-di-GMP levels using a different reverse-phase column and/or HPLC system may require optimization of HPLC separation gradients.
Ensure that the selected HPLC separation procedure results in a distinct sharp peak for c-di-GMP that demonstrates reproducible retention times for all of the standard concentrations tested. Also, confirm that the peak retention time is >5 min, to ensure that c-di-GMP binds to the column and is not in the initial unbound flow-through.
The generated c-di-GMP standard curve should only be used with R2 > 0.98. Coefficients of correlation smaller than 0.98 indicate issues with c-di-GMP standard dilution preparation or with HPLC c-di-GMP separation and quantitation.
Small sample volume loss may occur during the filtration of the resuspended c-di-GMP samples, but will not interfere with downstream application, as only a limited sample volume (20 µl out of 200 µl) is subjected to HPLC analysis.
This procedure has been optimized for P. aeruginosa PAO1 and PA14. Analysis of c-di-GMP levels in other strains or species may require the adjustment of sample volumes to account for significant differences in levels of c-di-GMP detected in the samples. Additionally, presence of other compounds eluting at similar times as c-di-GMP may hinder analysis, if distinct c-di-GMP peaks are no longer detectable. In this case, the HPLC conditions, including the gradient and potentially solvents, will have to be altered to accomplish sharp separation of the c-di-GMP peaks. If separation conditions have to be adjusted, the pure c-di-GMP standards will have to be re-analyzed using the new conditions.
To prevent overheating of the protein samples during sonication, the microfuge tubes containing the samples can be suspended in an ice water bath using floating foam tube racks for the duration of the sonication procedure.
References
- 1.Römling U, Simm R. Prevailing concepts of c-di-GMP signaling. Contributions to Microbiology. 2009;16:161–181. doi: 10.1159/000219379. [DOI] [PubMed] [Google Scholar]
- 2.Sondermann H, Shikuma NJ, Yildiz FH. You’ve come a long way: C-di-GMP signaling. Current Opinion in Microbiology. 2012;15:140–146. doi: 10.1016/j.mib.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross P, Aloni Y, Weinhouse C, et al. An unusual guanyl oligonucleotide regulates cellulose synthesis in Acetobacter xylinum. FEBS Letters. 1985;186:191–196. doi: 10.1016/0014-5793(85)80706-7. [DOI] [PubMed] [Google Scholar]
- 4.Ross P, Weinhouse H, Aloni Y, et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature. 1987;325:279–281. doi: 10.1038/325279a0. [DOI] [PubMed] [Google Scholar]
- 5.Schirmer T. C-di-GMP Synthesis: Structural Aspects of Evolution, Catalysis and Regulation. Journal of Molecular Biology. 2016;428:3683–3701. doi: 10.1016/j.jmb.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Hengge R. Principles of c-di-GMP signalling in bacteria. Nature reviews. Microbiology. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 7.Ross P, Mayer R, Weinhouse H, et al. The cyclic diguanylic acid regulatory system of cellulose synthesis in Acetobacter xylinum. Chemical synthesis and biological activity of cyclic nucleotide dimer, trimer, and phosphothioate derivatives. Journal of Biological Chemistry. 1990;265:18933–18943. [PubMed] [Google Scholar]
- 8.Thormann KM, Duttler S, Saville RM, et al. Control of formation and cellular detachment from Shewanella oneidensis MR-1 biofilms by cyclic di-GMP. Journal of Bacteriology. 2006;188:2681–2691. doi: 10.1128/JB.188.7.2681-2691.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda A, Wood TK. Connecting quorum sensing, c-di-GMP, pel polysaccharide, and biofilm formation in Pseudomonas aeruginosa through tyrosine phosphatase TpbA (PA3885) PLoS pathogens. 2009;5:e1000483. doi: 10.1371/journal.ppat.1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Beyhan S, Lim B, et al. Identification and characterization of a phosphodiesterase that inversely regulates motility and biofilm formation in Vibrio cholerae. Journal of Bacteriology. 2010;192:4541–4552. doi: 10.1128/JB.00209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hickman JW, Harwood CS. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Molecular microbiology. 2008;69:376–89. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burhenne H, Kaever V. Quantification of cyclic dinucleotides by reversed-phase LC-MS/MS. Methods in Molecular Biology. 2013;1016:27–37. doi: 10.1007/978-1-62703-441-8_3. [DOI] [PubMed] [Google Scholar]
- 13.Spangler C, Böhm A, Jenal U, et al. A liquid chromatography-coupled tandem mass spectrometry method for quantitation of cyclic di-guanosine monophosphate. Journal of Microbiological Methods. 2010;81:226–231. doi: 10.1016/j.mimet.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Simm R, Morr M, Remminghorst U, et al. Quantitative determination of cyclic diguanosine monophosphate concentrations in nucleotide extracts of bacteria by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Analytical Biochemistry. 2009;386:53–58. doi: 10.1016/j.ab.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Ryjenkov DA, Tarutina M, Moskvin OV, et al. Cyclic Diguanylate Is a Ubiquitous Signaling Molecule in Bacteria: Insights into Biochemistry of the GGDEF Protein Domain. Journal of Bacteriology. 2005;187:1792–1798. doi: 10.1128/JB.187.5.1792-1798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt AJ, Ryjenkov DA, Gomelsky M. The Ubiquitous Protein Domain EAL Is a Cyclic Diguanylate-Specific Phosphodiesterase: Enzymatically Active and Inactive EAL Domains. Journal of Bacteriology. 2005;187:4774–4781. doi: 10.1128/JB.187.14.4774-4781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Heine S, Entian M, et al. NO-induced biofilm dispersion in Pseudomonas aeruginosa is mediated by an MHYT domain-coupled phosphodiesterase. Journal of Bacteriology. 2013;195:3531–3542. doi: 10.1128/JB.01156-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wojcik W, Olianas M, Parenti M, et al. A simple fluorometric method for cAMP: application to studies of brain adenylate cyclase activity. J Cyclic Nucleotide Res. 1981;7:27–35. [PubMed] [Google Scholar]
- 19.Van Lookeren Campagne MM, Van Haastert PJM. A sensitive cyclic nucleotide phosphodiesterase assay for transient enzyme kinetics. Analytical Biochemistry. 1983;135:146–150. doi: 10.1016/0003-2697(83)90743-1. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Valdez H, Kothari RM, Hershey HV, et al. Rapid and reliable method for the analysis of nucleotide pools by reversed-phase high-performance liquid chromatography. Journal of Chromatography A. 1982;247:307–314. doi: 10.1016/s0021-9673(00)85954-3. [DOI] [PubMed] [Google Scholar]
- 21.Vogel H, Bonner D. Acetylornithinase of Escherichia coli: partial purification and some properties. Journal of Biological Chemistry. 1956:97–106. [PubMed] [Google Scholar]
- 22.Petrova OE, Sauer K. PAS domain residues and prosthetic group involved in BdlA-dependent dispersion response by Pseudomonas aeruginosa biofilms. Journal of bacteriology. 2012;194:5817–28. doi: 10.1128/JB.00780-12. [DOI] [PMC free article] [PubMed] [Google Scholar]