Abstract
Dentin‐pulp complex regeneration is a promising alternative treatment for the irreversible pulpitis caused by tooth trauma or dental caries. This process mainly relies on the recruitment of endogenous or the transplanted dental pulp stem cells (DPSCs) to guide dentin‐pulp tissue formation. Platelet‐derived growth factor (PDGF), a well‐known potent mitogenic, angiogenic, and chemoattractive agent, has been widely used in tissue regeneration. However, the mechanisms underlying the therapeutic effects of PDGF on dentin‐pulp complex regeneration are still unclear. In this study, we tested the effect of PDGF‐BB on dentin‐pulp tissue regeneration by establishing PDGF‐BB gene‐modified human dental pulp stem cells (hDPSCs) using a lentivirus. Our results showed that PDGF‐BB can significantly enhance hDPSC proliferation and odontoblastic differentiation. Furthermore, PDGF‐BB and vascular endothelial growth factor (VEGF) secreted by hDPSCs enhanced angiogenesis. The chemoattractive effect of PDGF‐BB on hDPSCs was also confirmed using a Transwell chemotactic migration model. We further determined that PDGF‐BB facilitates hDPSCs migration via the activation of the phosphatidylinositol 3 kinase (PI3K)/Akt signaling pathway. In vivo, CM‐DiI‐labeled hDPSCs were injected subcutaneously into mice, and our results showed that more labeled cells were recruited to the sites implanted with calcium phosphate cement scaffolds containing PDGF‐BB gene‐modified hDPSCs. Finally, the tissue‐engineered complexes were implanted subcutaneously in mice for 12 weeks, the Lenti‐PDGF group generated more dentin‐like mineralized tissue which showed positive staining for the DSPP protein, similar to tooth dentin tissue, and was surrounded by highly vascularized dental pulp‐like connective tissue. Taken together, our data demonstrated that the PDGF‐BB possesses a powerful function in prompting stem cell‐based dentin‐pulp tissue regeneration. Stem Cells Translational Medicine 2017;6:2126–2134
Keywords: Cell migration, Cell transplantation, Stem cells, Tissue regeneration
Significance Statement.
This study demonstrated that over‐expressing platelet‐derived growth factor (PDGF)‐BB in human dental pulp stem cells could improve their proliferation and odontoblastic differentiation ability and also that PDGF‐BB can facilitate stem cell homing via the phosphatidylinositol 3 kinase/Akt pathway and improve human dental pulp stem cell‐mediated dentin‐pulp complex regeneration in vivo. These findings suggested that PDGF‐BB has a great application prospect in prompting stem cell‐based dentin‐pulp complex regeneration.
Introduction
Irreversible pulpitis is a common and severe oral disease that can be caused by long‐term exposure of dental pulp to the harmful external oral environment following dental caries or injury. Traditional root canal therapy is a classical therapeutic method in the clinic. The pulp is extirpated, followed by root canal enlargement and obturation with gutta‐percha 1. However, the maintenance of tooth homeostasis is essential for tooth longevity. Dentin‐pulp complex regeneration will be of great importance in keeping tooth homeostasis. Stem cell‐based dentin‐pulp complex regeneration has been suggested as a new and promising strategy for preserving teeth that are suffering from dental caries and pulpitis 2, 3. Human dental pulp stem cells (hDPSCs) isolated from the dental pulp have been well documented in stem cell and dental tissue regeneration studies 4, 5. These cells, originating from the cranial neural crest 6, 7, possess high proliferative ability and are multipotent 8, 9, and they have been recognized as ideal seed cells for dentin‐pulp complex regeneration 2, 10, 11. However, previous studies indicated that limited mineralized tissue can be formed when scaffolds with hDPSCs alone were implanted subcutaneously in nude mice 12, 13. More importantly, the narrow root foramen/canal limited tissue infiltration and the revascularization process, which is also adverse for the implanted DPSCs. Generally, there are still many limitations for the application of hDPSCs in dentin‐pulp complex regeneration, and thus, applying new and optimized strategies to protect and enhance the functions of DPSCs is necessary 14. A combination of stem cells with some powerful factors which possess proliferative, angiogenic, and odontogenic activities, and even chemotactic capacity will be useful to resolve the aforementioned problems.
Platelet‐derived growth factor (PDGF) was originally identified in platelets 15, and there are five polypeptides included in the family: PDGF‐AA, PDGF‐AB, PDGF‐BB, PDGF‐CC, and PDGF‐DD 16. Among these isoforms, PDGF‐BB is a unique ligand that can interact with all three PDGF receptors including PDGFR‐αα, PDGFR‐αβ, and PDGFR‐ββ 17. PDGF‐BB, as a potent mitogenic factor 15, has been recognized as a key mediator in wound healing and tissue repair 18. In addition, PDGF‐BB is well known for its indirect angiogenic effect through its promotion of vascular endothelial growth factor (VEGF) secretion 19, and it also plays an important role in maintaining the stabilization of newly formed blood vessels 20, 21. PDGF‐BB can facilitate the osteogenic differentiation of bone marrow stem cells (BMSCs) in a dose‐dependent manner and has been widely used in bone regeneration 22. More importantly, PDGF‐BB is also a powerful chemoattractive agent for mesenchymal stem cells (MSCs) and many other cell types 23, 24, 25. A few studies have reported that PDGF‐BB can promote tissue regeneration via the recruitment of stem cells 26. However, the mechanisms and effects of PDGF‐BB contributes to hDPSCs mediated dentin‐pulp complex regeneration remain unclear.
In this study, we established a PDGF‐BB gene‐modified hDPSCs using lentivirus gene deliver vector and then thoroughly explored the influence of PDGF‐BB on hDPSCs proliferation and odontogenic differentiation. We further investigated the mechanism of PDGF‐BB‐induced hDPSC migration in vitro and the recruitment of hDPSCs in vivo for dentin‐pulp complex regeneration. Finally, PDGF‐BB‐modified hDPSCs were mixed with a porous calcium phosphate cement (CPC) scaffold and implanted subcutaneously in nude mice. Dentin‐pulp complex regeneration was evaluated using histological and immunohistochemical analyses.
Materials and Methods
Materials and Methods are shown in the Supporting Information (References: 27–33).
Results
Human DPSC Culture and Characterization
The teeth were dissected around their cervical part with a dental bur to exposure the dental pulp tissue (Fig. 1A, 1B). The isolated pulp tissue (Fig. 1C) was minced into 1–2 mm3 pieces, and these tissue pieces were then plated in a petri dish and cultured in Dulbecco's Modified Eagle's medium (DMEM) containing 20% fetal bovine serum (FBS). The primary hDPSCs (outgrowth cells) presented a fibroblast‐like spindle shape (Fig. 1D). At passage three, hDPSCs were harvested for flow cytometric analysis. Flow cytometric analysis revealed that these cells were negative for the hematopoietic markers CD31 and CD34 (3.6% and 3.1%, respectively), and that they expressed high levels of stem cell markers CD90 and CD105 (71.9% and 96.4%, respectively), (Fig. 1E). We also tested the multidirectional differentiation potential of hDPSCs. The results show that the isolated cells could differentiate into adipocytes positive staining for lipid droplets with oil Red O (Fig. 1F), chondrocytes positive staining for cartilage proteoglycan with Alcian blue (Fig. 1G) and osteoblasts positive staining for mineral nodules with alizarin red S (Fig. 1H), respectively.
Figure 1.
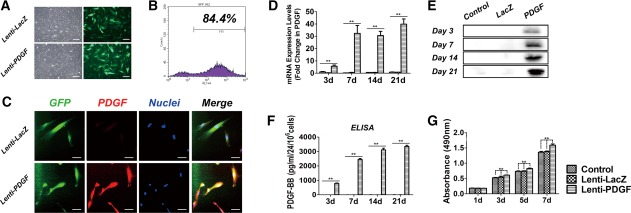
Detection of PDGF‐BB expression and human dental pulp stem cell proliferation. (A): At day 3 after gene transduction, both Lenti‐LacZ‐transfected and Lenti‐PDGF‐transfected hDPSCs grew well, and more than 80% of cells were positive for green fluorescent protein (GFP). (B): Flow cytometry showed 84.4% transfection efficiency. (C): Immunofluorescence detection of PDGF‐BB expression. (D): PDGF‐BB mRNA expression in the Control, Lenti‐LacZ, and Lenti‐PDGF groups on days 3, 7, 14, and 21. (E): Protein expression of PDGF‐BB in Control, Lenti‐LacZ, and Lenti‐PDGF groups on days 3, 7, 14, and 21. (F): Secretion of PDGF‐BB from each group for 24 hours at days 3, 7, 14, and 21. (G): MTT assay for cell viability and proliferation (**, p < .01). Scale bar, 100 μm. Abbreviations: PDGF, platelet‐derived growth factor; ELISA, enzyme‐linked immunoabsorbent assay.
Cell Transduction and Proliferation Analysis
Three days after gene transduction, the inverted fluorescence microscopy observation and flow cytometry results showed that the transfection efficiency of the target gene PDGF‐BB was greater than 80% (Fig. 1A, 1B). The expression of PDGF‐BB was evaluated in the Lenti‐PDGF and Lenti‐LacZ groups by immunofluorescence. The red fluorescent staining of PDGF‐BB in cells from the Lenti‐PDGF group was significantly enhanced compared with that of the Lenti‐LacZ group (Fig. 1C). Furthermore, both real‐time quantitative PCR (RT‐qPCR)and Western blot assays indicated that the expression of PDGF‐BB in hDPSCs was significantly upregulated after gene transduction (Fig. 1D, 1E). More importantly, the enzyme‐linked immunoabsorbent assay (ELISA) results demonstrated that the Lenti‐PDGF‐BB‐transduced hDPSCs could stably and continuously express PDGF‐BB (Fig. 1F). To investigate the effects of PDGF‐BB on hDPSC proliferation, an MTT assay was conducted after gene transduction. The hDPSC proliferation of each group (Control, Lenti‐LacZ, and Lenti‐PDGF) at different time points is shown in Figure 1G. The results showed enhanced cell proliferation in the Lenti‐PDGF group compared with both the Lenti‐LacZ and Control groups.
Cell Differentiation Analysis
The odontogenic vital marker genes DMP‐1 and DSPP, which both play important roles in tooth development and dentin mineralization, were significantly increased on day 3 and continuously increased from days 7 to 21 compared with the Lenti‐LacZ and Control groups (Fig. 2A). OCN was also significantly increased on day 7, but there was less distinction among the three groups at 3, 14, and 21 days (Fig. 2A). The protein expression of DMP‐1 and DSPP was consistent with the RT‐qPCR results (Fig. 2A, 2B). ALP staining was performed on days 7 and 14 after gene transduction. Staining was more intense in the Lenti‐PDGF group than in the Control and Lenti‐LacZ groups. Similarly, the semiquantitative analysis showed the same result (Fig. 2C). Calcium precipitation was also evaluated by alizarin red S (ARS) staining. The result showed a significant increase in calcium deposition in Lenti‐PDGF group, and the quantitative analysis was consistent with ARS staining findings (Fig. 2D).
Figure 2.

Detection of odontogenic differentiation. (A): mRNA expression levels of DMP‐1, OCN, and DSPP at days 3, 7, 14, and 21. (B): Protein expression levels of DMP‐1 and DSPP at days 3, 7, 14, and 21. (C): ALP staining and semiquantitative analysis of ALP activity at days 7 and 14. (D): ARS staining and semiquantitative analysis. (**, p < .01). Abbreviations: ALP, alkaline phosphatase; ARS, alizarin red S.
Cell Attachment and Viability
The attachment and growth of each group of cells (Lenti‐LacZ, Lenti‐PDGF) seeded on the porous CPC scaffolds were examined by scanning electron microscopy (SEM) (Fig. 3). After culturing for 1 day, hDPSCs in each group were attached and spread well on the surface of the CPC scaffolds (Fig. 3A1–3A3, 3B1–3B3). When cultured for 3 days in vitro, the cells grew well and connected with each other to form cellular connections (Fig. 3C1–3C3, 3D1–3D3). After transfected with Lenti‐LacZ and Lenti‐PDGF‐BB virus, hDPSCs highly expressed green fluorescent protein (GFP) (Fig. 1). The cell viability was also investigated with GFP observation. The fluorescence microscope images showed that the GFP positive cells pervaded the scaffold both in Lenti‐LacZ and Lenti‐PDGF group after culture 12 hours in vitro (Fig. 3E1, 3F1). After culture for 3 days in vitro, confocal laser scanning microscope (CLSM) observation was performed and the cell nuclei were stained with DAPI before observation. CLSM images still showed high levels of GFP expression and the cells elongated to form cellular connections (Fig. 3E2, 3E3, 3F2, 3F3). These results demonstrated that a porous CPC scaffold has good biocompatibility, making them suitable for the following in vivo study.
Figure 3.

SEM analysis and Fluorescence microscopy images. (A1–A3, B1–B3): Human dental pulp stem cells (hDPSCs) from each group were attached and spread well on the calcium phosphate cement (CPC) scaffolds on day 1. (C1–C3, D1–D3): By day 3, hDPSCs from each group had grown well and the cells elongated to find each other to form cellular connections. (E1, F1): Fluorescence microscopy images of Lenti‐LacZ‐transfected hDPSCs and Lenti‐PDGF‐BB transfected hDPSCs on CPC scaffold after culture 12 hours in vitro. (E2–E3, F2–F3): 3D reconstructed Laser confocal scanning microscopy images of Lenti‐LacZ‐transfected hDPSCs and Lenti‐PDGF‐BB transfected hDPSCs on CPC scaffold after culture 3 days in vitro. Cell nuclei were stained with DAPI. Scale bar, 100 μm. Abbreviations: PDGF, platelet‐derived growth factor; DAPI, 4′,6‐diamidino‐2‐phenylindole.
Chemotactic Activity of PDGF‐BB on hDPSCs In Vitro
After 20 hours of culture, the migrated cells on the underside of the filter were observed and quantified. As shown in Figure 4A, more migrated cells were detected in the supernatant of both the Lenti‐PDGF and DMEM + rhPDGF groups. PDGF‐BB significantly stimulated the chemotactic migration of hDPSCs. These results further confirmed that the secreted PDGF‐BB protein from PDGF‐BB gene modified hDPSCs possessed chemotactic activity for hDPSCs. A fourfold stimulatory effect was obtained in the supernatant Lenti‐PDGF group and the DMEM + rhPDGF group when compared with the Lenti‐LacZ and DMEM groups (Fig. 4B). The phosphatidylinositol 3 kinase (PI3K)/AKT pathway has been reported to be involved in PDGF‐induced migration in various cell types. To investigate whether this pathway was responsible for the migration of the hDPSCs, the cells were pretreated with LY294002 to block the PI3K/AKT pathway. As shown in Figure 4A, the inhibitor significantly reduced the chemotactic effect of PDGF‐BB on the hDPSCs, which demonstrated that the PI3K/AKT pathway was also required for PDGF‐BB‐induced hDPSC migration. To further demonstrate that the intracellular PI3K/AKT pathway was activated, the cells were pretreated with supernatant from the Lenti‐PDGF cells or PDGF‐BB (5 ng/ml). We observed that the expression of phosphorylated AKT was significantly increased in Lenti‐PDGF group compared with the Control group and that its activation was inhibited by LY294002 (Fig. 4C). rhPDGF‐BB protein showed the same results as the supernatant collected from Lenti‐PDGF group. The amount of phosphorylated AKT was strongly induced by short (5 minutes) incubation with rhPDGF‐BB. The high expression of phosphorylated AKT can sustain for 1 hour, and after 90 minutes the expression of phosphorylated AKT decreased (Fig. 4D).
Figure 4.
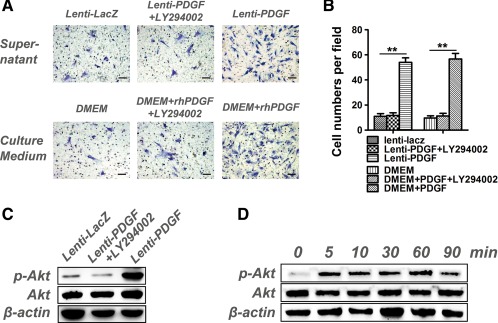
PDGF‐BB stimulates human dental pulp stem cells (hDPSC) migration via PI3K/AKT signaling. (A): hDPSCs on chemotaxis membranes stained with hematoxylin. (B): The cell numbers in each field. (C): PDGF‐BB secreted by PDGF‐BB transfected hDPSCs induced AKT activation. (D): rhPDGF‐BB protein continuously induced AKT activation in hDPSCs. (**, p < .01) Scale bar, 100 μm. Abbreviations: DMEM, Dulbecco's Modified Eagle's medium; PDGF, platelet‐derived growth factor.
In Vitro Angiogenesis
As we know, both PDGF‐BB and VEGF play an important role in regulating angiogenesis during tissue regeneration. Previous study has demonstrated that hDPSCs can secrete VEGF which are necessary for complete pulp healing 34. Furthermore, several researches have showed that PDGF‐BB can increase the expression of several angiogenic factors including VEGF in some types of cell such as fibroblasts and vascular endothelial cells 35, 36, 37. So in this study, we also detected the expression of VEGF in hDPSCs after PDGF‐BB gene transfection. Our results showed that VEGF expression increased both in RNA level and protein level (Figs. 2C, 5A, 5B, ). PDGF‐BB and VEGF secreted by hDPSCs may enhance the angiogenic potential, and then we performed angiogenesis assay. Human umbilical vein endothelial cells (HUVECs) were seeded into 96‐well culture plates, which were coated with matrigel, and cultured with following media: (a) supernatant collected from control group, (b) supernatant collected from Lenti‐LacZ group, and (c) supernatant collected from Lenti‐PDGF group for 12 hours. The results revealed that significantly increased number of tube‐like structure was observed in the Lenti‐PDGF group compared with the Control group or Lenti‐LacZ group after 12 hours culture (Fig. 5C, 5D).
Figure 5.

VEGF expression and in vitro angiogenesis assay of endothelial cells. (A): mRNA expression levels of VEGF at days 3, 7, 14, and 21. (B): Protein expression levels of VEGF at days 3, 7, 14, and 21. (C): Optical images of endothelial cells cultured on matrigel in the presence of the supernatant from Control, Lenti‐LacZ, and Lenti‐PDGF group for 12 hours. (D): The tube‐like numbers of each group (**, p < .01). Scale bar, 100 μm. Abbreviations: PDGF, platelet‐derived growth factor; VEGF, vascular endothelial growth factor.
Effects of PDGF‐BB on hDPSC Recruitment In Vivo
In order to investigate the effects of PDGF‐BB on hDPSC recruitment in vivo, hDPSCs were labeled with the cell tracker dye CM‐DiI prior to in vivo administration. DiI‐labeled hDPSCs showed red fluorescence (Fig. 3). One week after the subcutaneous injection of the labeled cells (Fig. 6A), the implants from each group were harvested. Labeled cells that migrated into the subcutaneous implants were observed (Fig. 6B). Interestingly, more labeled cells migrated into the subcutaneous implants in the Lenti‐PDGF group compared with the DPSC and Lenti‐LacZ groups (Fig. 6C). The PDGF‐BB protein secreted by PDGF‐BB‐modified hDPSCs might play an important role in recruiting more labeled cells into the subcutaneous implants. However, few labeled cells were observed in the CPC‐only group.
Figure 6.
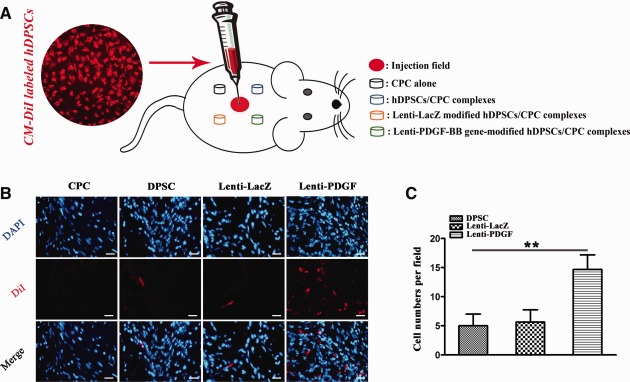
Chemotactic activity of PDGF‐BB on hDPSCs in vivo. (A): Schematic representation of CM‐DiI‐labeled hDPSCs subcutaneous injection. (B): PDGF‐BB secreted by PDGF‐BB gene‐modified hDPSCs increases hDPSC (labeled with CM‐DiI) migration to subcutaneous implants. (C): The statistics of CM‐DiI labeled cell numbers of each field (**, p < .01). Scale bar, 100 μm. Abbreviations: CPC, calcium phosphate cement; DAPI, 4′,6‐diamidino‐2‐phenylindole; hDPSC, human dental pulp stem cell; PDGF, platelet‐derived growth factor.
In Vivo Dentin‐Pulp Complex Formation and Immunohistochemical Analysis
The tissue regenerative capacity of PDGF‐BB gene‐modified hDPSCs was determined using a subcutaneous model of immunocompromised mice. Cells from each group were mixed with the porous CPC scaffolds, and the implants were subcutaneously transplanted into the mice for 12 weeks. The histological results showed that newly formed mineralized tissue was observed in all groups except for the CPC‐only group (Fig. 7A1‐7D1). The mineralized tissue possessed dentin‐like features: odontoblast‐like cells lined the surface of the newly formed predentin; ordered collagen fibers within predentin perpendicular to the odontoblast‐like cell layer; and the predentin‐like tissue was surrounded by pulp‐like connective tissue, which was infiltrated with blood vessels, and the outermost layer was mineralized dentin‐like tissue (Fig. 7B2‐7D3). However, the amount of newly formed mineralized tissue varied among the groups, and more mineralized tissue formation was observed in the Lenti‐PDGF group. Histomorphometric analysis showed that the areas of newly formed tissue (the percentage of mineralized tissue area among the whole implant) were 9.88% ± 1.58%, 10.16% ± 1.78%, and 19.92% ±2.71% for groups DPSC, Lenti‐LacZ, and Lenti‐PDGF, respectively, (Fig. 7E). The number of blood vessels number counted on the histological sections also showed the same result (Fig. 7F).
Figure 7.

Histological and immunohistochemical analysis. (A1–D3): Histological analysis. The images in (A1–A3), (B1–B3), (C1–C3), and (D1–D3) represent the CPC, DPSC, Lenti‐LacZ, and Lenti‐PDGF groups, respectively. MD‐like tissue and OD‐like cells lining the surface of the newly formed PD, and pulp‐like connective tissue, which was infiltrated with BV were observed in the DPSC, Lenti‐LacZ, and Lenti‐PDGF groups. (E): Analysis of the amount of dentin‐like mineralized tissues formed by hDPSCs among the different groups (n = 6 for each group, *, p < .05). (F): The statistics of the blood vessels number (n = 6 for each group, *, p < .05). (H–L): Immunohistochemical analysis. Immunohistochemistry assay for DSPP protein on the samples from DSPC group (H), Lenti‐LacZ group (I), and Lenti‐PDGF group (J). The samples of human tooth (dentin layer) (K), and alveolar bone tissue (L) were treated as control group. Scale bar, 100 μm. Abbreviations: BV, blood vessels; CPC, calcium phosphate cement; DPSC, dental pulp stem cell; MD, mineralized dentin; OD, odontoblast; PD, predentin; PDGF, platelet‐derived growth factor.
The DSPP immunohistochemical analysis further demonstrated our histological results. The outmost layer mineralized dentin‐like tissue and odontoblast‐like cells distributed in the pulp‐like connective tissue showed positive staining for the DSPP protein, the middle layer predentin showed negative staining for DSPP (Fig. 7H–7J). The sample of human tooth tissue (dentin layer) showed similar positive staining for the DSPP protein (Fig. 7K). However, DSPP was not detectable or was almost negative in the human alveolar bone sample (Fig. 7L).
Discussion
Stem cell‐based dentin‐pulp complex regeneration is a promising therapy for irreversible pulpitis 10. DPSCs, as an ideal seed cell source for dentin‐pulp tissue regeneration, have been widely used for dentin‐pulp regeneration 38. However, the application of DPSCs alone in vivo showed insufficient dentin tissue formation in previous studies 12, 13. To address that limitation, a combination of stem cells and special growth factors with the ability to enhance odontoblastic differentiation and induce cell homing have been reported to improve dentin‐pulp complex regeneration 1, 14, 39. In our study, we investigated the effects of PDGF‐BB on hDPSCs mediated dentin‐pulp complex regeneration. PDGF‐BB not only promoted the proliferation, angiogenesis and odontoblastic differentiation of hDPSCs but also facilitated stem cell homing via the PI3K/Akt pathway. Together, PDGF‐BB significantly improved hDPSC‐mediated dentin‐pulp complex regeneration in vivo.
PDGF‐BB has been recognized as a potent mitogen 15. In this study, the MTT assay results showed that the overexpression of PDGF‐BB enhanced hDPSC proliferation, which is favorable for providing sufficient amounts of cells for tissue regeneration. Recently, an increasing number of studies have shown that PDGF‐BB gene‐modified MSCs are equipped with enhanced osteogenic differentiation ability 40. However, the influence of PDGF‐BB on the odontoblastic differentiation of hDPSCs is still largely unknown. Therefore, the odontoblastic differentiation of PDGF‐BB‐ transfected hDPSCs was systematically evaluated in the present study. DMP‐1, a noncollagenous protein, regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation 41. DSPP is considered a terminal phenotypic marker of mature odontoblasts and plays a crucial role in dentinogenesis 42, 43. ALP, as an early differentiation marker, plays an important role in calcium–phosphate mineral formation in both bone and dentin 44. OCN, as a late marker of cell differentiation, is synthesized by mature osteoblasts and odontoblasts 45. In the present study, we selected these four key markers to evaluate the efficacy of PDGF‐BB in promoting hDPSC odontoblastic differentiation. Our results showed that all of these markers were significantly upregulated in PDGF‐BB‐transfected hDPSCs. These findings indicated that PDGF‐BB could promote odontoblastic differentiation of hDPSCs.
Recently, stem cell homing strategies have been widely used in tissue regeneration 31, 46. The recruitment of stem/progenitor cells and their subsequent differentiation is necessary for tissue development, remodeling, and regeneration. Fortunately, PDGF‐BB has also been recognized as a potent chemoattractant 24, 47. After lentiviral transfection with PDGF‐BB, both RT‐qPCR and Western blot assays indicated that the expression of PDGF‐BB in hDPSCs was significantly upregulated. More importantly, the ELISA results indicated that the overexpressed PDGF‐BB protein was secreted into the medium. We further confirmed that the secreted PDGF‐BB protein from PDGF‐BB‐modified hDPSCs possessed chemotactic activity using a Transwell migration assay. Upon further investigation, we found that PDGF‐BB, as a chemoattractant, stimulated the migration of hDPSCs through the activation of the PI3K/Akt signaling pathway. Inhibition of the PI3K/AKT pathway by the addition of LY294002 could suppress PDGF‐BB‐mediated hDPSC migration in vitro. We further investigated whether the PDGF‐BB protein secreted by PDGF‐BB‐modified hDPSCs could induce hDPSC homing in vivo. Our results showed that more CM‐DiI‐labeled hDPSCs, which were injected subcutaneously, were recruited to the sites containing PDGF‐BB‐modified hDPSCs. Therefore, the enhanced secretion of PDGF‐BB might promote dental‐pulp complex regeneration in situ in part by inducing endogenous stem/progenitor cell homing.
One of the key mechanisms for stem cells to promote tissue regeneration is by secretion of soluble growth factors. As we know, both PDGF‐BB and VEGF play an important role in regulating angiogenesis during tissue regeneration. Previous study has demonstrated that hDPSCs can secrete VEGF which are necessary for complete pulp healing 34. Furthermore, several researches have showed that PDGF‐BB can increase the expression of several angiogenic factors including VEGF in some types of cell such as fibroblasts and vascular endothelial cells 35, 36, 37 Based on our results, gene modified hDPSCs could secrete PDGF‐BB stably and continuously, and also VEGF expression increased in hDPSCs after PDGF‐BB transfection. In vitro angiogenesis assay showed that PDGF‐BB and VEGF secreted by hDPSCs stimulated the angiogenic potential of HUVECs, which may further facilitate hDPSCs based tissue regeneration.
Suitable scaffolds are also very important for dental tissue regeneration. CPC scaffolds, used in our study, possess potential resorbability and highly biocompatible properties, and they have been widely applied in the clinic for bone regeneration 48, 49. CPC scaffolds are also good carriers for hDPSCs. SEM observations showed that hDPSCs attached, spread, and grew well on the scaffold surface and also Fluorescence images showed the same result. Moreover, CPC scaffolds carrying hDPSCs were implanted subcutaneously in nude mice to evaluate the efficacy of in vivo dentin‐pulp complex regeneration. According to the histological results, newly formed mineralized tissue was observed in all groups except for the CPC‐only group. The regenerated mineralized tissues displayed typical dentin‐like features, with odontoblast‐like cells lining the surface of the newly formed predentin and with ordered collagen fibers within predentin perpendicular to the odontoblast‐like cell layer. In addition, the newly formed hard tissues were surrounded by highly vascularized dental pulp‐like connective tissue. Immunohistochemical analysis further confirmed that the newly formed mineralized dentin‐like tissue expressed the dentin‐specific protein DSPP. Indeed, hDPSCs are ideal seed cells for dentin‐pulp complex regeneration. By comparison, the amount of newly formed dentin‐like tissue and blood vessels were significantly enhanced when using the hDPSCs transfected with PDGF‐BB. Therefore, based on our research, PDGF‐BB has a powerful effect on promoting hDPSCs mediated dentin‐pulp complex regeneration.
Conclusion
In summary, our results show that PDGF‐BB gene‐modified hDPSCs can continuously secrete the PDGF‐BB protein and that the overexpression of PDGF‐BB can significantly enhance hDPSC proliferation, angiogenesis, and odontogenic differentiation. Furthermore, PDGF‐BB secreted by PDGF‐BB‐modified hDPSCs can facilitate stem cell homing via the PI3K/Akt pathway and improve hDPSC‐mediated dentin‐pulp complex regeneration in vivo. These findings represent an important step toward the optimal application of PDGF‐BB for improving hDPSCs mediated dentin‐pulp complex regeneration.
Author Contributions
M.Z. and F.J.: collection of data, data analysis and interpretation, manuscript writing, final approval of manuscript; X.Z., S.W., and Y.J.: collection of data, data analysis and interpretation, final approval of manuscript; W.Z. and X.J.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supporting Information
Supplemental Online References
Acknowledgments
This work was jointly supported by The National Key Research and Development Program of China (2016YFC1102900), the National Natural Science Foundation of China (81430012 and 81271114), the Shanghai Sailing Program (17YF1410800), and the Young Elite Scientist Sponsorship Program by CAST (2016QNRC001).
Contributor Information
Wenjie Zhang, Email: zhangwenjie586@126.com.
Xinquan Jiang, Email: xinquanj@aliyun.com.
References
- 1. Suzuki T, Lee CH, Chen M et al. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J Dent Res 2011;90:1013–1018. [DOI] [PubMed] [Google Scholar]
- 2. Murray PE, Garcia‐Godoy F, Hargreaves KM. Regenerative endodontics: A review of current status and a call for action. J Endod 2007;33:377–390. [DOI] [PubMed] [Google Scholar]
- 3. Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod 2005;31:711–718. [DOI] [PubMed] [Google Scholar]
- 4. Mao JJ, Prockop DJ. Stem cells in the face: Tooth regeneration and beyond. Cell Stem Cell 2012;11:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gronthos S, Mankani M, Brahim J et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 2000;97:13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chai Y, Jiang X, Ito Y et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 2000;127:1671–1679. [DOI] [PubMed] [Google Scholar]
- 7. Kawanabe N, Murata S, Fukushima H et al. Stage‐specific embryonic antigen‐4 identifies human dental pulp stem cells. Exp Cell Res 2012;318:453–463. [DOI] [PubMed] [Google Scholar]
- 8. Graziano A, D'aquino R, Laino G et al. Dental pulp stem cells: A promising tool for bone regeneration. Stem Cell Rev 2008;4:21–26. [DOI] [PubMed] [Google Scholar]
- 9. Arthur A, Rychkov G, Shi S et al. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 2008;26:1787–1795. [DOI] [PubMed] [Google Scholar]
- 10. Sloan AJ, Smith AJ. Stem cells and the dental pulp: Potential roles in dentine regeneration and repair. Oral Dis 2007;13:151–157. [DOI] [PubMed] [Google Scholar]
- 11. Huang GT, Yamaza T, Shea LD et al. Stem/progenitor cell‐mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A 2010;16:605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang W, Walboomers XF, van Osch GJ et al. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng Part A 2008;14:285–294. [DOI] [PubMed] [Google Scholar]
- 13. Zhang W, Walboomers XF, van Kuppevelt TH et al. The performance of human dental pulp stem cells on different three‐dimensional scaffold materials. Biomaterials 2006;27:5658–5668. [DOI] [PubMed] [Google Scholar]
- 14. Yang X, van der Kraan PM, Dolder J et al. STRO‐1 selected rat dental pulp stem cells transfected with adenoviral‐mediated human bone morphogenetic protein 2 gene show enhanced odontogenic differentiation. Tissue Eng 2007;13:2803–2812. [DOI] [PubMed] [Google Scholar]
- 15. Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev 2004;15:205–213. [DOI] [PubMed] [Google Scholar]
- 16. Fredriksson L, Li H Eriksson U. The PDGF family: Four gene products form five dimeric isoforms. Cytokine Growth Factor Rev 2004;15:197–204. [DOI] [PubMed] [Google Scholar]
- 17. Hollinger JO, Hart CE, Hirsch SN et al. Recombinant human platelet‐derived growth factor: Biology and clinical applications. J Bone Joint Surg Am 2008;90:48–54. [DOI] [PubMed] [Google Scholar]
- 18. Greenhalgh DG, Sprugel KH, Murray MJ et al. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol 1990;136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- 19. Stavri GT, Hong Y, Zachary IC et al. Hypoxia and platelet‐derived growth factor‐BB synergistically upregulate the expression of vascular endothelial growth factor in vascular smooth muscle cells. FEBS Lett 1995;358:311–315. [DOI] [PubMed] [Google Scholar]
- 20. Yancopoulos GD, Davis S, Gale NW et al. Vascular‐specific growth factors and blood vessel formation. Nature 2000;407:242–248. [DOI] [PubMed] [Google Scholar]
- 21. Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer 2002;2:795–803. [DOI] [PubMed] [Google Scholar]
- 22. Xu L, Lv K, Zhang W et al. The healing of critical‐size calvarial bone defects in rat with rhPDGF‐BB, BMSCs, and beta‐TCP scaffolds. J Mater Sci Mater Med 2012;23:1073–1084. [DOI] [PubMed] [Google Scholar]
- 23. Sun X, Gao X, Zhou L et al. PDGF‐BB‐induced MT1‐MMP expression regulates proliferation and invasion of mesenchymal stem cells in 3‐dimensional collagen via MEK/ERK1/2 and PI3K/AKT signaling. Cell Signal 2013;25:1279–1287. [DOI] [PubMed] [Google Scholar]
- 24. Ozaki Y, Nishimura M, Sekiya K et al. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev 2007;16:119–129. [DOI] [PubMed] [Google Scholar]
- 25. Mishima Y Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res 2008;26:1407–1412. [DOI] [PubMed] [Google Scholar]
- 26. Fiedler JR, Etzel N, Brenner RE. To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J Cell Biochem 2004;93:990–998. [DOI] [PubMed] [Google Scholar]
- 27. Peng W, Liu W, Zhai W et al. Effect of tricalcium silicate on the proliferation and odontogenic differentiation of human dental pulp cells. J Endod 2011;37:1240–1246. [DOI] [PubMed] [Google Scholar]
- 28. Zhang W, Li Z, Liu Y et al. Biofunctionalization of a titanium surface with a nano‐sawtooth structure regulates the behavior of rat bone marrow mesenchymal stem cells. Int J Nanomed 2012;7:4459–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dai J, Wang J, Lu J et al. The effect of co‐culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials 2012;33:7699–7711. [DOI] [PubMed] [Google Scholar]
- 30. Chen B, Sun HH, Wang HG et al. The effects of human platelet lysate on dental pulp stem cells derived from impacted human third molars. Biomaterials 2012;33:5023–5035. [DOI] [PubMed] [Google Scholar]
- 31. Zhang W, Zhu C, Wu Y et al. VEGF and BMP‐2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. Eur Cell Mater 2014;27:1–11. discussion 11‐2. [DOI] [PubMed] [Google Scholar]
- 32. Cesselli D, Beltrami AP, Rigo S et al. Multipotent progenitor cells are present in human peripheral blood. Circ Res 2009;104:1225–1234. [DOI] [PubMed] [Google Scholar]
- 33. Xia L, Zhang M, Chang Q et al. Enhanced dentin‐like mineralized tissue formation by AdShh‐transfected human dental pulp cells and porous calcium phosphate cement. PLoS One 2013;8:e62645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tran‐Hung L, Mathieu S, About I. Role of human pulp fibroblasts in angiogenesis. J Dent Res 2006;85:819–823. [DOI] [PubMed] [Google Scholar]
- 35. Guo P, Hu B, Gu W et al. Platelet‐derived growth factor‐B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol 2003;162:1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nauck M, Roth M, Tamm M et al. Induction of vascular endothelial growth factor by platelet‐activating factor and platelet‐derived growth factor is downregulated by corticosteroids. Am J Respir Cell Mol Biol 1997;16:398–406. [DOI] [PubMed] [Google Scholar]
- 37. Wang D, Huang HJS, Kazlauskas A et al. Induction of vascular endothelial growth factor expression in endothelial cells by platelet‐derived growth factor through the activation of phosphatidylinositol 3‐kinase. Cancer Res 1999;59:1464–1472. [PubMed] [Google Scholar]
- 38. Iohara K, Imabayashi K, Ishizaka R et al. Complete pulp regeneration after pulpectomy by transplantation of CD105+stem cells with stromal cell‐derived factor‐1. Tissue Eng Part A 2011;17:1911–1920. [DOI] [PubMed] [Google Scholar]
- 39. Kim JY, Xin X, Moioli EK et al. Regeneration of dental‐pulp‐like tissue by chemotaxis‐induced cell homing. Tissue Eng Part A 2010;16:3023–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fierro FA, Kalomoiris S, Sondergaard CS et al. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells 2011;29:1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Narayanan K, Gajjeraman S, Ramachandran A et al. Dentin matrix protein 1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation. J Biol Chem 2006;281:19064–19071. [DOI] [PubMed] [Google Scholar]
- 42. Sreenath T, Thyagarajan T, Hall B et al. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem 2003;278:24874–24880. [DOI] [PubMed] [Google Scholar]
- 43. Zhang X, Zhao J, Li C et al. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet 2001;27:151. [DOI] [PubMed] [Google Scholar]
- 44. Garimella R, Bi X, Anderson HC et al. Nature of phosphate substrate as a major determinant of mineral type formed in matrix vesicle‐mediated in vitro mineralization: An FTIR imaging study. Bone 2006;38:811–817. [DOI] [PubMed] [Google Scholar]
- 45. Du R, Wu T, Liu W et al. Role of the extracellular signal‐regulated kinase 1/2 pathway in driving tricalcium silicate‐induced proliferation and biomineralization of human dental pulp cells in vitro. J Endod 2013;39:1023–1029. [DOI] [PubMed] [Google Scholar]
- 46. Nakamura Y, Ishikawa H, Kawai K et al. Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell‐derived factor‐1. Biomaterials 2013;34:9393–9400. [DOI] [PubMed] [Google Scholar]
- 47. Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet‐derived growth factor. Physiol Rev 1999;79:1283–1316. [DOI] [PubMed] [Google Scholar]
- 48. Zhu C, Chang Q, Zou D et al. LvBMP‐2 gene‐modified BMSCs combined with calcium phosphate cement scaffolds for the repair of calvarial defects in rats. J Mater Sci Mater Med 2011;22:1965–1973. [DOI] [PubMed] [Google Scholar]
- 49. Ambard AJ, Mueninghoff L. Calcium phosphate cement: Review of mechanical and biological properties. J Prosthodont 2006;15:321–328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supplemental Online References


