Abstract
The possibility of using adipose tissue‐derived stromal cells (ATSC) as alternatives to bone marrow‐derived stromal cells (BMSC) for bone repair has garnered interest due to the accessibility, high cell yield, and rapid in vitro expansion of ATSC. For clinical relevance, their bone forming potential in comparison to BMSC must be proven. Distinct differences between ATSC and BMSC have been observed in vitro and comparison of osteogenic potential in vivo is not clear to date. The aim of the current study was to compare the osteogenesis of human xenofree‐expanded ATSC and BMSC in vitro and in an ectopic nude mouse model of bone formation. Human MSC were implanted with biphasic calcium phosphate biomaterials in subcutis pockets for 8 weeks. Implant groups were: BMSC, ATSC, BMSC and ATSC mixed together in different ratios, as well as MSC primed with either osteogenic supplements (250 μM ascorbic acid, 10 mM β‐glycerolphosphate, and 10 nM dexamethasone) or 50 ng/ml recombinant bone morphogenetic protein 4 prior to implantation. In vitro results show osteogenic gene expression and differentiation potentials of ATSC. Despite this, ATSC failed to form ectopic bone in vivo, in stark contrast to BMSC, although osteogenic priming did impart minor osteogenesis to ATSC. Neovascularization was enhanced by ATSC compared with BMSC; however, less ATSC engrafted into the implant compared with BMSC. Therefore, in the content of bone regeneration, the advantages of ATSC over BMSC including enhanced angiogenesis, may be negated by their lack of osteogenesis and prerequisite for osteogenic differentiation prior to transplantation. Stem Cells Translational Medicine 2017;6:2160–2172
Keywords: Adipose stem cells, Bone marrow stromal cells, Bone, Tissue regeneration, Vascular development
Significance Statement.
Adipose tissue‐derived stem cells (ATSC) as a potential bone regenerative therapy has garnered widespread interest. In this study, significantly inferior osteogenesis and engraftment of ATSC were demonstrated in ectopic sites in nude mice compared with human bone marrow stem cells (BMSC). Conversely, ATSC showed enhanced angiogenesis compared with BMSC. These findings have implications for the use of ATSC in bone repair since their enhanced angiogenesis, high accessibility, high cell yield, and rapid in vitro expansion may be negated by their lack of osteogenesis and prerequisite of osteogenic differentiation prior to transplantation.
Introduction
Autologous bone grafts are compromised by the ensuing pain and morbidity associated with the bone harvest site. Tissue engineering strategies comprising synthetic biomaterial scaffolds with mesenchymal stromal stem cells (MSC) offer alternatives to overcome the limitations of autologous bone grafts. MSC isolated from the bone marrow (BMSC) 1 successfully initiate bone formation when transplanted into animals 2, 3, 4 and are being investigated in clinical practice for bone tissue repair 5, 6. MSC derived from adipose tissue (ATSC) are receiving attention as a possible alternative to BMSC because they can be easily harvested by liposuction 7, the yield of ATSC from the same volume of adipose tissue is 500‐fold greater 8, and they expand rapidly in vitro 9. Many consider ATSC to have the same potential as BMSC since they bear all the hallmarks of stemness in vitro similar to BMSC, such as tri‐lineage differentiation potential 10 and expression profiles for many surface markers 11, 12. However, despite these similarities, further analysis reveals definite differences at the phenotypic, proteomic, and mRNA levels 13, and distinct surface antigen expression 14, according to their tissue origin.
Numerous studies have demonstrated that ATSC are capable of osteogenic differentiation in vitro 15, 16. However, since bone formation encompasses multi‐step coordinated participations from various cell types 4, a relevant assessment of bone forming capacity can only be gained by investigations in vivo. There are few clinical reports of bone reconstruction using ATSC. ATSC implanted ectopically with a ceramic scaffold coated with BMP‐2 formed bone in a patient 17; however, since BMP‐2 alone induces bone formation 18, the bone formed in 17 cannot simply be attributed to ATSC. A second case reported ATSC mediated repair of skull defects in a 7‐year‐old 19; however, it is not possible to attribute the bone healing to ATSC and not to the biomaterials alone, or indeed the rapid regenerative capacity of children. Finally, osteogenically differentiated ATSC showed healing in maxillary bone defects in patients 20.
Ectopic bone formation can be achieved by ATSC in ectopic sites when transplantation is preceded by either osteogenic pre‐differentiation/priming 21, 22, 23, 24, 25 or by genetic manipulation with bone inducing genes 26. However, while un‐primed BMSC routinely form ectopic bone 2, 4, whether un‐primed ATSC are capable of osteoinduction remains largely elusive, with only one study to date showing that unprimed ATSC can form bone in ectopic sites 21. Others however showed that unprimed mice 27 and human 28, 29 ATSC could repair critical sized animal defects. Differences in passage number of MSC may contribute to dissimilar findings of bone forming potential since low passage numbers are vital in retaining the bone forming capabilities of MSC 30.
The lack of rapid vascularization within an engineered construct is a major hurdle in the healing of large bone defects and their translation into clinical therapies 31. Therefore, strategies to enhance rapid angiogenesis within bone implants are crucial. Various studies investigated the potential of coculture systems, combining endothelial cells for their neovascularization potential with MSC for their osteogenic capacity 32. A single source of human cells, having both osteogenic and angiogenic capabilities for clinical translation would be indispensable. Interestingly, ATSC have the ability to differentiate into endothelial cells, and improve neovascularization in vivo 33, 34, 35 to a larger extent than BMSC 36. We therefore hypothesized that bone regeneration may be enhanced by the pro‐angiogenic properties of ATSC.
Prior to the clinical use of ATSC for bone repair, it is necessary to show that their bone formation abilities are equivalent to BMSC. In this study, we characterized the in vitro osteogenic differentiation of BMSC and ATSC prepared in xeno‐free conditions. The osteogenesis of expanded human BMSC and ATSC, in both passages 2 and 5, were then compared in an ectopic mouse model. Since no host osteogenic cells naturally reside in an ectopic site, the real osteoinductive capacity of implanted cells can be evaluated. This is the first study to evaluate bone formation from ATSC expanded in human platelet lysates (PL), which is used for the expansion of good manufacturing practice grade MSC for clinical use. We sought to quantify the degree of ectopic bone tissue formation, bone marrow presence, blood vessel density, and human cell engraftment. Finally, to evaluate the necessity of osteogenic priming of ATSC prior to implantation in order to achieve bone formation, we compared BMSC and ATSC that were primed with standard osteogenic supplements or bone morphogenetic protein 4 (BMP‐4) prior to implantation.
Materials and Methods
Biomaterial
Granules of biphasic calcium phosphate (BCP), composed of hydroxyapatite and beta‐tricalcium phosphate in a ratio of 20:80 in weight, ranging in size from 0.5 to 1 mm, were supplied by Biomatlante (Vigneux de Bretagne, France, http://biomatlante.com). The overall porosity (%vol) was 75% ± 5%, with a pore size distribution of 70% (0–10 μm), 20% (10–100 μm), and 10% (100 to 300 μm). Fifty milligrams aliquots of biomaterials were steam sterilized by autoclaving.
Isolation and Characterization of MSC
Bone marrow (BM) aspirates were obtained from the iliac crest of healthy donors, by standard puncture and aspiration in heparinized syringes. BMSC were isolated from mononuclear cells cultured at 1 × 104 cells per cm2. ATSC were obtained by culturing the stromal vascular fraction (4 × 103 cells per cm2) after collagenase digestion (NB4, ServaNordmark) of adipose tissue derived via liposuction as described previously 37. Informed consent was obtained according to the Declaration of Helsinki and Ethical approval was received from the Ethical Committee of Toulouse University. In order to compensate for donor variability, BM of three human donors and AT of four human donors were used. Cells were expanded ex vivo in basal culture medium Alpha Minimum Essential Medium (α‐MEM) supplemented with 100 U ml−1 penicillin and 100 μg ml−1 streptomycin, 8% pooled human PL, and 1 IU ml−1 medical grade heparin. PL from several blood donors was prepared by IKT Ulm, as previously described 38. PL was used as this permits a xenobiotic‐free culture system, and we have previously shown that it can successfully maintain MSC expansion in vitro 2, 39, 40.
For phenotypic characterization of MSC, flow cytometry analysis was performed as previously described 39. Briefly, cells were characterized by using the following antibodies: (a) CD105‐FITC (clone 43A3), (b) CD90‐FITC (clone 5E10), (c) CD73‐PE (clone AD2), (d) CD45, (e) CD34‐PE (clone 4H11); (f) CD3‐FITC (clone UCHT1). CD73 was sourced from BD Biosciences (Le Pont de Claix, France, http://www.bd.com), CD3 was sourced from Beckman Coulter (Paris, France, https://www.beckman.com), while all others were purchased from BioLegend through Ozyme (Paris, France, https://www.ozyme.fr).
Tri‐lineage differentiation capacity of expanded MSC was assessed. MSC differentiation was induced toward an osteogenic lineage by using standard osteogenic supplements (250 μM ascorbic acid, 10 mM β‐glycerolphosphate, and 100 nM dexamethasone), while adipogenic or chondrogenic lineages were induced by using StemPro Adipogenesis Differentiation Kit and Chondrogenesis Differentiation Kit, respectively, (Gibco, Life Technologies, France, https://www.thermofisher.com). Mineralization was detected by alizarin red staining 39; adipogenic differentiation was assessed by Oil Red O staining, while chondrogenic differentiation was detected by Alcian Blue staining.
In Vitro Cell Growth and Osteogenic Potential of BMSC and ATSC Cultured with Standard Osteogenic Supplements
MSC were plated at a seeding density of 5 × 103/cm2 in 24 well plates. BMSC from three human donors and ATSC from four human donors were used in the current study for each experiment. Each donor was plated in triplicate for each measured output. Seeded MSC were cultured for 24 hours in xeno‐free basal media, after which they were cultured with or without the addition of standard osteogenic supplements for up to 21 days.
Pico‐Green Assay for DNA Content
The quantification of cells was assessed at 1, 7, 14, and 21 days of culture. Cells were lysed by using a solution of 0.1% Triton X‐100, 5 mM Tris‐HCL, pH 8 and three freeze/thaw cycles. The quantity of double stranded DNA was measured in the supernatant of the solutions using a fluorescent Quant‐iT PicoGreen dsDNA reagent assay kit (P7589, Invitrogen by Life Technologies, Saint Aubin, France, https://www.thermofisher.com) according to the manufacturer's instructions. Fluorescent intensity was measured at 485 nm Excitation and 535 nm Emission on a microplate reader (Tristar LB 941; Berthold Technologies, Thoiry, France, https://www.berthold.com) and converted to ng of DNA by using a standard curve of known concentrations of a lambda DNA solution.
Alkaline Phosphatase Measurement
Extracellular alkaline phosphatase (ALP) was qualitatively evaluated by staining at days 7, 14, and 21 with a solution containing Fast Blue RR salt (Sigma Aldrich, Lyon, France, http://www.sigmaaldrich.com) and Naphthol AS‐MX phosphates alkaline solution (Sigma Aldrich). Intracellular ALP was quantified at 1, 7, 14, and 21 days by using an ALP Colorimetric Assay Kit (Abcam, Cambridge, UK, http://www.abcam.com), on the same cell lysates that were used for DNA quantification. ALP enzyme converts the p‐nitrophenyl phosphate (pNPP) substrate to an equal amount of colored p‐Nitrophenol (pNP). Colorimetric absorbance was measured at 405 nm using a microplate reader.
Alizarin Red Staining for Detection of Mineralized Matrix
Mineralization was quantified at 14 and 21 days by staining with 40 mM alizarin red solution (pH 4.1–4.3). The unbound stain was removed, and the bound stain was solubilized by using 10% (vol/vol) acetic acid. Ammonium hydroxide solution 10% (vol/vol) was added to neutralize the acid. Staining was enumerated by measuring samples in triplicate at an absorbance of 405 nm on a microplate reader and normalized to cell number using the measured DNA content per sample.
Quantitative RT‐PCR for Gene Expression of BMSC and ATSC Cultured with BMP‐4
P1‐P2 passage BMSC and ATSC were cultured in 6 well‐plates for 21 days in 50 ng/ml BMP‐4, 10 mM β‐glycerophosphate, and 50 mM ascorbic acid. Total RNA was extracted from MSC and purified by AllPrep DNA/RNA/Protein kit (Qiagen, Courtaboeuf, France, https://www.qiagen.com) and dosed by nanodrop (Thermo Fisher, Waltham, MA, https://www.thermofisher.com). RNA purity and integrity were checked using the Experion RNA Std Sens Reagents on the Experion automatized system (Biorad, Marnes‐la‐Coquette, France, http://www.bio-rad.com). All samples used for cDNA synthesis displayed a RNA integrity number (RIN) above 9.8. The reverse transcription (RT) was performed on 1 µg RNA by using high capacity cDNA reverse transcription kit (Applied Biosystem, https://www.thermofisher.com) and random hexamers in a final volume of 20 µl. The oligonucleotides for each target of interest, designed by using Primer Express software (PerkinElmer Life Sciences, Waltham, MA, http://www.perkinelmer.com) were used (forward and reverse) and listed in Supporting Information Table I. Quantitative PCR was performed on diluted cDNA (equivalent to 25 ng of starting purified RNA) using SSoFast EvaGreen Supermix (Biorad) with 500 nM of forward and reverse primers in a total volume of 20 µl on a CFX Real Time System (Biorad) following: 95°C, 3 minutes, and 40 cycles of denaturation (95°C, 10 seconds) and primers hybridization and amplification (60°C, 30 seconds). Each primer couple displayed interpretable PCR efficiencies (95%–105%). Melt curves and appropriate No RT control were used to validate amplification specificity. Data were analyzed on Bio‐Rad CFX manager (threshold = 0.2), exported to DataAssist Software v3.0 (Applied Biosystems). Gene expression was calculated using the 2−ΔCt (or 2−ΔΔCt) method using Peptidylprolyl Isomerase A (PPIA) as appropriate reference gene which had the lesser M score.
MSC/Biomaterial Implantation in the Subcutis of Nude Mice
All animal experiments were performed according to Directive 2010/63/UE and after approval of protocols from the local ethical committee (CEEA, Pays‐de‐la‐Loire, France). Cell groups, controls, and sample sizes are presented in Supporting Information Table II. In total, there were 6 to 8 implants per group, using cells from seven different human donors: three for BM and four for AT. These were the same donors as those used for in vitro experiments. Prior to in vivo implantations, BMSC and ATSC in passage 2 were cultured for 6 days in either basal media or media supplemented with osteogenic supplements (250 μM ascorbic acid, 10 mM β‐glycerolphosphate, and 10 nM dexamethasone) or 50 ng/ml rh BMP‐4 (R&D systems, Minneapolis, MN, https://www.rndsystems.com). BMSC and ATSC were suspended 100 μl of basal media and mixed with BCP granules (2 × 106 cells/50 mg BCP for each implant) for 1 hour to allow cells to attach to the biomaterial. This cell/biomaterial ratio was previously determined to be optimal 2. BMSC and ATSC were implanted separately or mixed together at the time of implantation in various ratios (9:1, 1:1, and 1:9). To evaluate the importance of passage number on bone formation and cell engraftment, a further group consisted of both BMSC and ATSC from the same donors as above, but in passage 5. Immunocompromised nude female mice (RjOrl: NMRI‐Foxn1nu/Foxn1nu), sourced from a professional breeder (Janvier Labs, Saint‐Berthevin, France, https://www.janvier-labs.com) were placed under general anesthesia by inhalation of isoflurane, and two subcutaneous implants were placed on the dorsal side of each mouse. Animals were sacrificed at 8 weeks and following dissection, explants were fixed in 4% formol buffered solution.
Histological Staining and Histomorphometry
Explants were decalcified in a pH 7.4 solution containing 4.13% EDTA/0.2% PFA in PBS for 96 hours at 50°C using an automated microwave decalcifying apparatus (KOS Histostation, Milestone Med. Corp). Samples were dehydrated in ascending series of ethanol followed by butanol in an automated dehydration station (MicromMicrotech, Lyon, France). Samples were embedded in paraffin (Histowax; Histolab, Gottenburg, Sweden). Thin histology sections (3 µm thick) were stained by Masson trichrome technique. Slides were scanned (NanoZoomer; Hamamatsu, Photonics, Hamamatsu City, Shizuoka, Japan, http://www.hamamatsu.com) and observed by using the virtual microscope (NDP view; Hamamatsu). Histomorphometry of images was performed by using ImageJ software (National Institute of Health, Bethesda, MD). Four sections through each implant were analyzed and the percentages of biomaterial, bone, and BM were calculated per total area of explants.
Immunohistochemistry
Immunohistochemistry was performed on deparaffinized and rehydrated sections with the CD146 antibody (Rabbit monoclonal to CD146, ab75769, Abcam) which identifies pericyte cells in order to observe blood vessels. The section was pre‐treated using heat mediated antigen retrieval with Tris‐EDTA (pH 9) overnight at 60°C, followed by quenching of endogenous peroxidase activity by 3% hydrogen peroxide. Nonspecific binding sites were blocked with 2% goat serum, 1% bovine serum albumin (BSA). Sections were incubated with CD146 primary antibody overnight at 4°C, followed by incubation with goat anti‐rabbit secondary antibody. The target antigen signal was amplified by using streptavidin peroxidase (DAKO, P0397). Diaminobenzidine (DAB) was used as the chromogen. All sections were dehydrated and counterstained using Gill's hematoxylin and mounted using permanent mounting medium. The total number of blood vessels per implant was measured by using an automatic macro written in Image J and this was then normalized to the total cross‐sectional area of the implants to give the number of blood vessels per mm2. The automatic macro also calculated the average diameter of each blood vessel.
Cellular Engraftment at Implant Site
In Situ Hybridization
In situ hybridization using the human‐specific repetitive Alu sequence, which encompasses approximately 5% of the total human genome was carried out to identify human cells. Sections were deparaffinized and rehydrated in graded series of ethanol and then treated with 20 μg/ml proteinase K (P2308, Sigma Aldrich, France), followed by tris buffered saline (TBS) tween 0.05% pH 7.6. Slides were treated with 3% H2O2 to block endogenous peroxidase activity. Sections were treated with 0.25% acetic acid in 0.1 M triethanolamine pH 8.0, followed by dehydration in graded series of ethanol. The Dioxigenin (DIG)‐labeled Dioxigenin human locked nucleic acid (LNA) Alu probe 5DigN/5′‐TCTCGATCTCCTGACCTCATGA‐3′/3DigN (Exiqon, Vedbaek, Denmark) was added at a concentration of 70 nM to hybridization buffer containing 4× SSC (S6639, Sigma Aldrich France), 50% deionized formamide, 1× Denhardt's solution, 5% dextran sulphate, and 100 μg/ml salmon sperm DNA, and molecular grade H2O. Hybridization was carried out for 16 hours at 56°C in a wet chamber. Nonspecific binding was blocked using 2% BSA in TBS/tween for 25 minutes at RT. The hybridized probe was detected by immunohistochemistry using biotin‐SP‐conjugated IgG fraction monoclonal mouse anti‐DIG (Jackson Immunoresearch, Baltimore, MD) diluted 1/200 for 1 hour at 37°C. Streptaperoxidase was added (1/800 in TBS tween 0.05%) for 1 hour at 37°C before DAB substrate addition (Dako). Sections were counterstained with Gill‐2 hematoxylin (Thermo Shandon Ltd, Runcorn, U.K.) dehydrated, and mounted using Pertex (HistoLab Products AB, Sweden).
Quantitative Real‐Time Polymerase Chain Reaction
Samples were lysed in DNA lysis buffer (Qiagen, France) and ground using the FastPrep‐24 tissue and cell homogenizer (MP Biomedicals, Santa Ana, CA). Total DNA was isolated using QIAamp DNA Investigator Kit as described by the manufacturer (Qiagen, France). Human DNA was quantified by using human TaqMan Copy Number Reference Assay, RNase P (Applied Biosystems, France), and monitored with a 7500HT Fast Real‐Time PCR System (Applied Biosystems). Results were normalized to that of time T0 (ΔCt = Cttime Tx–Cttime T0) and were expressed as the number of grafted hMSC per scaffold.
Statistical Analysis
MSC were plated in triplicate for each in vitro experiment and repeated for three different BM donors (n = 3) and four different AT donors (n = 4). A two‐way analysis of ANOVA, with Sidak's multiple comparison test was used to compare groups. For in vivo analysis, the same 3 BM donors and 4 AT donors were used. In vivo sample groups and sample sizes are presented in Supporting Information Table II. Kruskal‐Wallis test with Dunn's multiple comparison test was used to compare BM and AT groups. All statistical analysis was performed using GraphPad Prism 6 (GraphPad Software Inc; La Jolla, CA) with statistical significance set as p < .05.
Results
Phenoptypic Characterization and Tri‐Lineage Differentiation of ATSC and BMSC In Vitro
Characterization of BMSC and ATSC by flow cytometry is presented in Supporting Information Figure 1a. Both BMSC and ATSC displayed positive expression of stem cell surface markers CD 105, CD90, CD73, and negative for CD45, CD34, and CD3. Osteogenic, adipogenic and chondrogenic in vitro differentiation capacity of both BMSC and ATSC was confirmed by histochemical staining by alizarin red, Oil red O, or Alcian blue, respectively, (Supporting Information Figure 1b).
Superior Osteogenic Differentiation of ATSC Compared with BMSC In Vitro
As illustrated in Supporting Information Figure 2, proliferation of MSC progressed rapidly from days 1 to 7, and plateaued thereafter. It appeared that at all time points, the ATSC group had higher cell numbers compared with BMSC; however, this was not statistically significant.
Representative images of extracellular ALP production by BMSC and ATSC are shown in Figure 1A. In the BMSC group, ALP staining intensity increased from day 7 to 14 and it declined thereafter, and as expected there was also notably more staining in the osteogenic induced group compared with their undifferentiated control counterparts. ALP staining appears more intense and homogeneously spread in the ATSC compared with BMSC group. While osteogenic induction of ATSC did increase ALP staining, strikingly, the undifferentiated ATSC controls also showed significant ALP staining, and in particular more intense staining compared with their BMSC equivalents at all time points.
Figure 1.
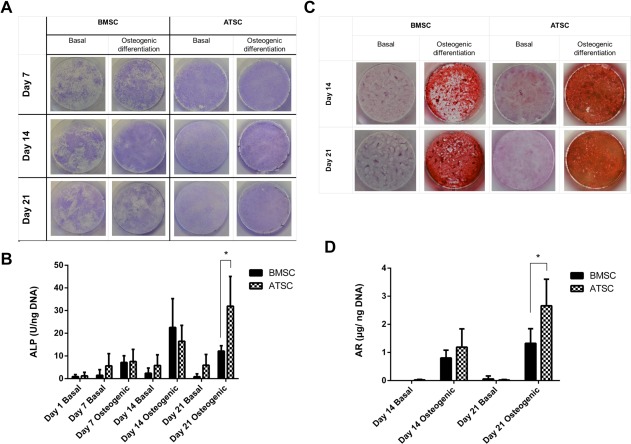
In vitro osteogenic differentiation of BMSC and ATSC when MSC were cultured with or without standard osteogenic induction media (250 μM ascorbic acid, 10 mM β‐glycerolphosphate, and 100 nM dexamethasone) for up to 21 days. BMSC of three different human donors (n = 3) and ATSC of four different human donors (n = 4) were plated in triplicate for each time point. (A): Extracellular ALP secretion is shown qualitatively by Fast Blue RR salt and Naphthol AS‐MX phosphates alkaline staining. (B): Intracellular ALP activity, data are presented as mean ± SD. (C): Mineralization was qualitatively assessed by alizarin red (AR) staining and representative images from each group are presented. (D): AR staining was solubilized and enumerated per well and normalized to DNA content, presented as mean ± SD. * indicates statistical differences (p < .05) between groups. Abbreviations: ALP, alkaline phosphatase; ATSC, adipose tissue‐derived stem cells; BMSC, bone marrow‐derived stem cells.
Intracellular ALP, quantified in cell lysates, and normalized to DNA quantity in the same sample, is presented in Figure 1B. As expected, osteogenic supplements enhanced ALP within cells. In keeping with the extracellular ALP results, intracellular ALP increased up to day 14 in the BMSC group and decreased thereafter. By day 21, intracellular ALP production by ATSC was significantly higher than BMSC, p < .05.
Representative images of alizarin red staining from each group are presented in Figure 1C, showing more intense mineralization in the ATSC group compared with the BMSC group. Quantitative data, obtained by solubilizing the stain, confirmed that ATSC had significantly more mineralization compared with BMSC at both days 14 (481 ± 143 vs. 181 ± 25 µg/well, p < .05) and 21 (739 ± 40 vs. 372 ± 24 µg per well, p < .05). Mineralization normalized to DNA quantity in each group is presented in Figure 1D and similarly shows that ATSC had significantly higher mineralization compared with BMSC after 21 days of osteogenic induction (2.66 ± 0.95 vs. 1.32 ± 0.52 µg/ng DNA, p < .05).
Regarding osteoblastic gene markers, both BMSC and ATSC significantly increased their expression of bone ALP, RUNX2, OSTERIX (OSX), parathyroid hormone‐receptor1 (PTHR1), Distal less homeobox 5 (DLX5), and Osteocalcin (OSC) when induced by BMP‐4 (Fig. 2). Gene expression by osteogenically induced BMSC were significantly higher than those of ATSC however.
Figure 2.
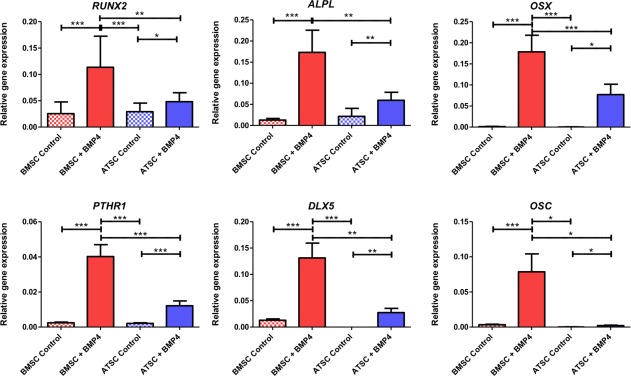
Osteogenic gene expression of BMSC and ATSC cultured in either basal media or induced toward the osteogenic lineage by supplementing media with 50 ng/ml BMP‐4, 10 mM β‐glycerophosphate, and 50 mM ascorbic acid. Data are represented as mean ± SE of the mean (*, p < .05; **, p < .01; ***, p < .001). Abbreviations: ATSC, adipose tissue‐derived stem cells; BMP‐4, bone morphogenetic protein 4; BMSC, bone marrow‐derived stem cells; DLX5, Distal less homeobox 5; OSC, Osteocalcin; OSX, OSTERIX; PTHR1, parathyroid hormone‐receptor 1.
Inferior Ectopic Bone Formation Potential of ATSC Compared with BMSC
Eight weeks after implantation of passage 2 MSC, isolated from BM or AT, cross‐sections through implants are shown in Figure 3A. BCP biomaterials alone showed no osteogenesis. In all BCP + BMSC implants (6/6 implants) there was abundant bone formation with mature BM present. Conversely, there was no bone formed in the BCP + ATSC group (0/8 implants). Figure 3B and 3C show that there was significantly more ectopic bone formation and BM presence in the BMSC group compared with BCP alone, BCP + ATSC, and the BCP + 1:9 BMSC/ATSC group (p < .05).
Figure 3.
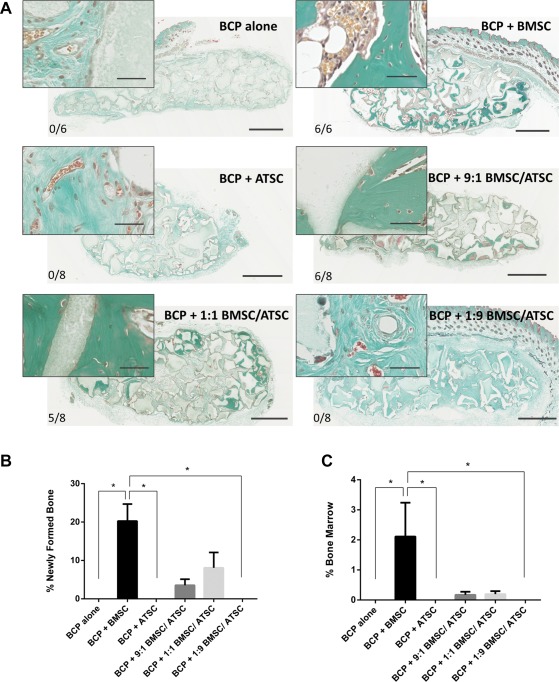
Ectopic bone formation by BCP biomaterial with MSC in the subcutis of nude mice after 8 weeks. (A): Masson trichrome staining on sections through implants with BCP (gray) and MSC from three bone marrow donors (BMSC) and four adipose tissue donors (ATSC), or a mixture of both. Newly formed bone is evidenced in green. Scale bars: 1 mm for images (40 μm for image inserts). The bone incidence score on each group represents the number of implants with newly formed ectopic bone over the total number of implants in that group. (B): Histomorphometry quantification of bone and (C) of mature bone marrow was performed on four sections of every implant. * indicates statistical differences between groups (p < .05). Data are presented as mean ± SE of the mean. Abbreviations: ATSC, adipose tissue‐derived stem cells; BCP, biphasic calcium phosphate; BMSC, bone marrow‐derived stem cells.
Osteogenic Priming Initiates Ectopic Bone Formation by ATSC but It Remains Inferior to BMSC
Figure 4A shows ectopic bone formation when BMSC and ATSC were either expanded in basal media or osteogenically primed prior to implantation and Figure 4B and 4C shows quantitative date of sections. Bone was formed in significantly higher quantities by BMSC compared with ATSC (p < .05) in undifferentiated, or those primed with osteogenic supplements or BMP‐4 (Fig. 4B). Priming of BMSC with osteogenic supplements or BMP‐4 showed no significant difference in bone quantity compared with undifferentiated BMSC. Priming ATSC with osteogenic supplements and BMP‐4 yielded minute bone quantities in 1/8 implants and 2/8 implants, respectively.
Figure 4.
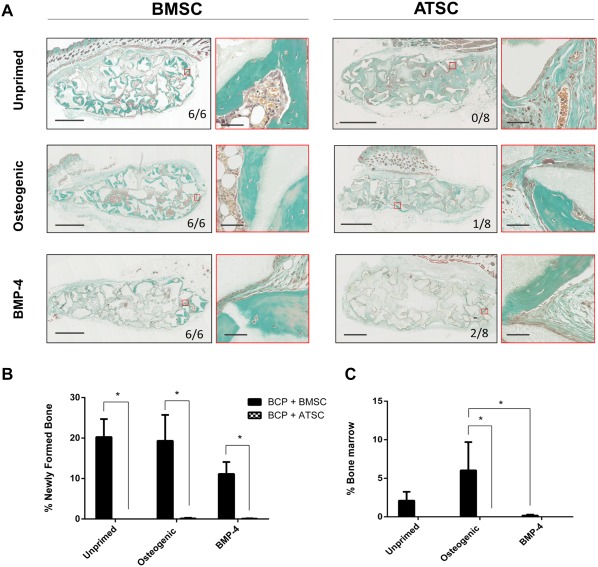
Ectopic bone formation by osteogenically primed MSC in nude mice after 8 weeks. (A): Masson trichrome staining on sections through implants with BCP biomaterial (gray) and MSC from three bone marrow donors (BMSC) and four adipose tissue donors (ATSC) with or without 6 days of priming prior to implantation with either basal media or the addition of osteogenic supplements (250 μM ascorbic acid, 10 mM β‐glycerolphosphate, and 10 nM dexamethasone) or 50 ng/ml rh BMP‐4. Newly formed bone is evidenced in green, while bone marrow is observed in brown. Highlighted regions in red are magnified and shown to the right of each image of whole implants. Scale bars represent 1 mm and 50 μm for magnified images. The bone incidence score on each group represents the number of implants with newly formed ectopic bone over the total number of implants in that group. (B): Histomorphometry quantification of bone and (C) of mature bone marrow were performed on four sections of every implant with * indicating statistical differences between groups (p < .05). Data are presented as mean ± SE of the mean. Abbreviations: ATSC, adipose tissue‐derived stem cells; BCP, biphasic calcium phosphate; BMP‐4, bone morphogenetic protein 4; BMSC, bone marrow‐derived stem cells.
ATSC Enhance Neovascularization In Vivo
All samples appeared well vascularized when analyzed after 8 weeks of implantation. Figure 5A shows CD146 immunostaining, showing blood vessels in brown. Figure 5B shows quantitative analysis of the number of blood vessels per mm2 of implants. The addition of ATSC to BMSC in the mixed‐cell type groups caused significantly increased neovascularization in these implants. Figure 6A shows CD146 immunostaining of groups with/without osteogenic priming. Figure 6B shows the quantitative analysis of this immunostaining, revealing that the unprimed ATSC group had significantly more (p < .05) blood vessels present compared with the BMSC group. The average size of the blood vessels between groups did not differ (data not shown). With osteogenic priming, there was no significant difference in the number of vessels/area between the ATSC or BMSC groups.
Figure 5.
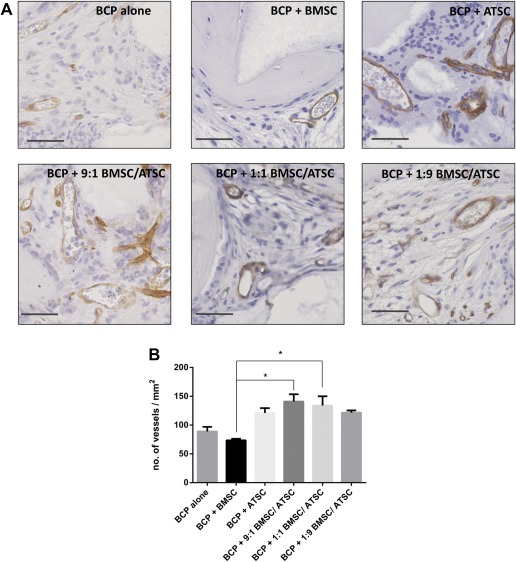
Neovascularization within implants of BCP with MSC in the subcutis of nude mice after 8 weeks. (A): CD146 immunostaining for pericytes on sections through implants with BCP biomaterial (gray) and MSC from three bone marrow donors (BMSC) and four adipose tissue donors (ATSC) or a mixture of both. Blood vessels are stained in brown. Scale bars represent 50 μm. (B): Histomorphometry quantification of the number of blood vessels/mm2 with * indicating statistical differences between groups (p < .05). Data are presented as mean ± SE of the mean. Abbreviations: ATSC, adipose tissue‐derived stem cells; BCP, biphasic calcium phosphate; BMSC, bone marrow‐derived stem cells.
Figure 6.
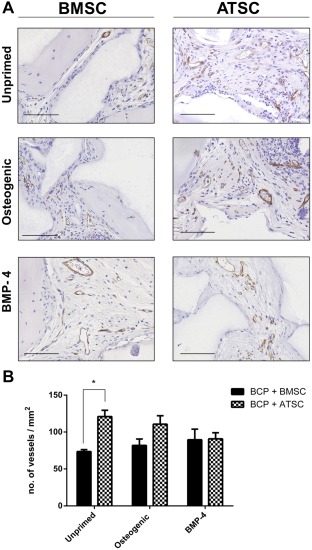
Neovascularization within implants of BCP with MSC in the subcutis of nude mice after 8 weeks. (A): CD146 immunostaining for pericytes on sections through implants with BCP biomaterial (gray) and MSC from three bone marrow donors (BMSC) and four adipose tissue donors (ATSC) which were either unprimed or underwent priming osteogenic supplements (250 μM ascorbic acid, 10 mM β‐glycerolphosphate, and 10 nM dexamethasone) or 50 ng/ml rh BMP‐4 prior to implantation. Blood vessels are stained in brown. Scale bars represent 100 μm. (B): Histomorphometry quantification of the number of blood vessels/mm2 with * indicating statistical differences between groups (p < .05). Data are presented as mean ± SE of the mean. Abbreviations: ATSC, adipose tissue‐derived stem cells; BCP, biphasic calcium phosphate; BMP‐4, bone morphogenetic protein 4; BMSC, bone marrow‐derived stem cells.
Impact of MSC Passage Number on Ectopic Bone Formation and Cell Engraftment
While passage 2 BMSC formed ample bone in vivo (Figs. 3, 4, we found that passage 5 cells from the exact same donors failed to form any ectopic bone in vivo (data not shown). There was no ectopic bone formed with either passage 2 or passage 5 ATSC. With regard to cellular survival and engraftment, when passage 2 BMSC and ATSC were implanted, in situ hybridization revealed that there were still human BMSC present at the implant site after 8 weeks, albeit a very minute portion compared with the quantity of host mouse cells; however, there were no human ATSC present after 8 weeks (Fig. 7A). With implanted passage 5 cells, rtPCR showed a major loss of human cells at the implant site, as soon as 24 hours, 70% of the cells had disappeared; however, no difference was seen between the BMSC and the ATSC. Then cells persisted on the biomaterial during the first week. At 4 weeks after implantation ATSC have all disappeared whereas a minute portion of BMSC are still present at the implant site (Fig. 7B).
Figure 7.
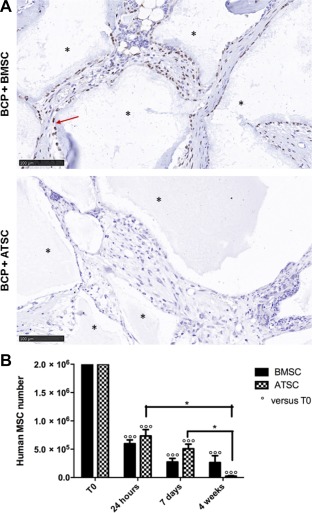
Engraftment of human cells. (A): In situ hybridization of the human Alu sequence showing some human cells (brown—highlighted with red arrow) present in BMSC implants after 8 weeks, and mouse cells (purple), whereas no human cells were found in the ATSC group. Biomaterial is shown with an asterisk. Scale bars represent 100 μm. (B): Evaluation of human BMSC and ATSC number persisting in vivo on the scaffold, at T0, 24 hours, 1, and 4 weeks post‐graft, using human‐specific quantitative real‐time polymerase chain reaction assay. Data are represented as mean ± SE of the mean. *, p < .05. Abbreviations: ATSC, adipose tissue‐derived stem cells; BCP, biphasic calcium phosphate; BMSC, bone marrow‐derived stem cells.
Discussion
Adipose tissue stromal cells are considered a favorable source of stem cells for tissue regeneration owing to their abundance and accessibility. This study shows that although ATSC achieve in vitro osteoblastic differentiation, and enhanced in vivo neovascularization compared with BMSC, passage 2 ATSC are significantly inferior in terms of their osteo‐induction abilities in an ectopic environment in vivo. These findings may have implications for the use of ATSC in bone repair strategies as alternatives to BMSC.
BMSC and ATSC showed similar but not identical phenotypic characteristics by flow cytometry analysis. Although osteogenic gene expression was elevated by BMP‐4 in BMSC compared with ATSC, ATSC showed increased ALP modulation and calcium deposition when induced by using standard osteogenic supplements. In spite of this no bone formation was observed with undifferentiated ATSC and it is important to note that the same human donors were used for both the in vitro and in vivo experiments. This supports the idea that in vitro osteogenic differentiation assays may not directly correlate with in vivo bone forming potential 41. Whether the passage number of MSC affected their phenotypic characteristics or osteogenic potential in vitro was not evaluated in the current study.
In keeping with our previous studies, BMSC achieved ample bone formation with mature BM territories 2, 4. This was cell passage dependent, with passage 2 cells forming abundant bone in vivo and passage 5 of the same donors completely losing this ability, which is in agreement with a previous study 42, but the cause is undetermined. The underlying exact mechanisms of bone formation by BMSC are unknown. However, based on our previous observations and those of others 2, 4, 43 we hypothesize that MSC release paracrine factors that recruit host cells to repair bone. The immune system and bone formation are inherently linked and we showed recently that implanted MSC recruit host monocytes to differentiate into macrophages and osteoclasts and then bone is formed by host MSC 4. The exact mechanisms leading to the stark lower ability of passage 2 ATSC compared with BMSC in ectopic bone forming ability has not yet been investigated; however, it is in agreement with previous studies using cells of human 11, 44 and animal origin 45, 46. There are differences in the secreted paracrine factors of BMSC and ATSC 47 and perhaps this plays a role.
To evaluate the bone‐forming potential of harnessing the osteogenic capabilities of BMSC with the pro‐angiogenic advantages of ATSC, BMSC, and ATSC were mixed together at the time of implantation in various different ratios. Although the addition of ATSC to BMSC gave rise to more vessels, no osteo‐inductive advantage was garnered from mixing MSC of these different tissue origins.
Priming cells with standard osteogenic supplements prior to implantation made no difference to the in vivo bone formation by BMSC. However, the duration of osteogenic priming is paramount 48; therefore, it is possible that the 6‐day duration in the present study has surpassed the timing whereby osteogenesis is enhanced. Alternatively, the 8‐week end time point may be too late to observe such a difference. Osteogenic priming of ATSC either with osteogenic supplements or BMP‐4 prior to implantation imparted a minor bone forming ability to ATSC, in agreement with previous studies 22, 49. The quantity of bone formed by osteogenic primed ATSC remained significantly lower than BMSC in all groups however. To the best of our knowledge there are only two other studies which compared the bone forming potential of unprimed and osteogenically primed ATSC and these produced conflicting findings 21, 50, whereby 21 found that unprimed hATSC were capable of osteoinduction in ectopic sites 21, in contrast to our findings here, and those of 50. Importantly, no study to date compared ATSC to their BMSC counterparts, with or without osteogenic induction, as was the case in the current study.
While not exhaustive, there have been several studies which have evaluated the capacity of ATSC for bone regeneration in critical sized defects; however, a clear lack of consensus regarding the osteoinductive potential of ATSC exists. Some showed bone healing by osteogenic primed animal ATSC 51, 52, 53 and BMP‐2 transfected ATSC 54. Studies that directly compare the potential of unprimed or osteogenically primed ATSC in skull bones are in agreement with the current study, whereby osteogenic priming is a prerequisite for achieving bone healing using either animal 55, 56 or human 57 ATSC. Furthermore, the need for osteogenic manipulation of ATSC prior to implantation seems to also extend to orthopedic sites, whereby osteogenically primed ATSC and genetically modified ATSC transfected with BMP‐2 and RunX2 healed critical‐sized defects in animals 60, 61, 62, and unprimed ATSC failed to heal critical‐sized tibia defects in rabbits and sheep 58, 59. Conversely, others show that unprimed animal 27 or human 28, 29 ATSC can induce bone formation in skull bones, and while the discrepancies between the latter studies and the current study remains undetermined, several factors may play a role. First, they are defect models, unlike ectopic sites, and perhaps the interplay of ATSC with resident cells in the defect somehow augmented their osteoinductive capacities; however, this does not explain the lack of osteoinduction by unprimed ATSC in other defect model studies 55, 56, 57. Furthermore, although these studies use unprimed ATSC, they were cultured on apatite coated polymers prior to implantation 27, 28. Apatite scaffolds have been shown to induce osteogenic differentiation of MSC without the addition of osteogenic supplements 63, 64, 65, 66, although the 24‐hour incubation 27, 28 may be too short to trigger any differentiation in this case. Finally, these studies culture ATSC in DMEM with FBS 27, 28, in contrast to our α‐MEM with PL media, and were lower passage number (from passage 1) 27, compared with the present study. It may be possible that ATSC lose their bone induction abilities with culture in different serums or after passage 1 and explain the lack of osteoinductivity in the current study. Indeed, it has been shown that adipose floating spheroids cultured in serum‐free medium contain cells that show high in vitro osteogenic differentiation and in vivo bone formation capacity which is lost by expanding plastically adhered ATSC 67. Whether any of these parameters are critical factors controlling the bone forming potential of ATSC warrants further investigation and there remains a shortage of studies using human ATSC. Very recently, a rare, long‐term patient follow‐up (6 years) of cranial defect reconstruction using autologous ATSC found all five patient outcomes unsatisfactory, and that no clear evidence that this technique induced bone healing, or that ATSC or their progeny formed new bone 68.
In this study, ATSC significantly increased neovascularization compared with BMSC. This is in agreement with findings from vascular ischemia studies 36 and may be due to their greater production of vascular endothelial cell growth factor compared with BMSC 69. Since lack of vascularization is an important limitation of tissue engineered constructs, we hypothesized that increased angiogenesis would enhance bone formation. However, the addition of ATSC to BMSC in the mixed‐cell groups increased angiogenesis compared with BMSC alone, but this enhanced angiogenesis did not translate to more bone formation. This indicates that ATSC may be more suitable as a cell therapy for ischemic diseases than for bone repair strategies.
The difference in the survival ability of implanted BMSC and ATSC found in the current study is interesting, and the cause is not known. Both ATSC and BMSC possess immunoregulatory properties 70, 71, 72, 73. The nude NMRI mice used in this study lack a thymus and therefore have a hugely reduced number of T cells, thereby permitting the evaluation of human cells in this model. However, the natural killer cells (NK) are still present, and it was demonstrated previously that only BMSC inhibit NK cells cytotoxicity, not ATSC 74, which may permit the more sustained survival and activity of BMSC compared with ATSC found in this study. Whether this contributes to the striking difference in bone formation is unlikely however due to the very small number of remaining MSC and also since we showed that passage 5 BMSC also persist at the implant site but do not initiate bone formation. This would suggest that BMSC lose their capacity to release key bone initiating paracrine factors with passaging, a hypothesis that is currently under investigation.
Conclusion
This study has shown consistent and robust osteogenic differentiation of ATSC in vitro; however, this did not translate to in vivo findings which showed that ATSC are not osteoinductive, in contrast to BMSC. Osteogenic priming is a prerequisite for achieving ectopic bone formation by ATSC. Together, this data suggests that ATSC are not as suitable for bone regeneration applications as BMSC, particularly in terms of the isolation and culture procedures used in the current study. Nevertheless, ATSC do still hold the advantage of ease of harvesting, high yield, and improved neovascularization over BMSC and may therefore be advantageous for alternative cell therapy applications.
Author Contributions
M.Á.B.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; A.R. and M.M: collection of data, final approval of manuscript; F.G. and L.S.: conception and design, provision of study material, final approval of manuscript; V.T.: collection and assembly of data, final approval of manuscript. F.D.: conception and design, administrative support, provision of study material, manuscript writing, final approval of manuscript; N.C.: collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; L.P.: conception and design, financial support, administrative support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supplement Figure 1
Supplement Figure 2
Supplement Table 1
Supplement Table 2
Acknowledgments
This work was supported by grants from the European Commission: REBORNE (HEALTH‐2009‐1.4.2–241879) and the Etablissement Francais du Sang: Appel d'offre Recherche 2014. We appreciate the excellent technical assistance of Jérôme Amiaud.
References
- 1. Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 2. Brennan MÁ, Renaud A, Amiaud J et al. Pre‐clinical studies of bone regeneration with human bone marrow stromal cells and biphasic calcium phosphate. Stem Cell Res Ther 2014;5:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mankani MH, Kuznetsov SA, Robey PG. Formation of hematopoietic territories and bone by transplanted human bone marrow stromal cells requires a critical cell density. Exp Hematol 2007;35:995–1004. [DOI] [PubMed] [Google Scholar]
- 4. Gamblin A‐L, Brennan MA, Renaud A et al. Bone tissue formation with human mesenchymal stem cells and biphasic calcium phosphate ceramics: The local implication of osteoclasts and macrophages. Biomaterials 2014;35:9660–9667. [DOI] [PubMed] [Google Scholar]
- 5. Watson L, Elliman SJ, Coleman CM. From isolation to implantation: A concise review of mesenchymal stem cell therapy in bone fracture repair. Stem Cell Res Ther 2014;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stanovici J, Le Nail L‐R, Brennan MA et al. Bone regeneration strategies with bone marrow stromal cells in orthopaedic surgery. Curr Res Transl Med 2016;64:83–90. [DOI] [PubMed] [Google Scholar]
- 7. Zuk PA, Zhu M, Mizuno H et al. Multilineage cells from human adipose tissue: Implications for cell‐based therapies. Tissue Eng 2001;7:211–228. [DOI] [PubMed] [Google Scholar]
- 8. Baer PC, Geiger H. Adipose‐derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int 2012;2012:812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bodle JC, Hanson AD, Loboa EG. Adipose‐derived stem cells in functional bone tissue engineering: Lessons from bone mechanobiology. Tissue Eng Part B Rev 2011;17:195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winter A, Breit S, Parsch D et al. Cartilage‐like gene expression in differentiated human stem cell spheroids: A comparison of bone marrow‐derived and adipose tissue‐derived stromal cells. Arthritis Rheum 2003;48:418–429. [DOI] [PubMed] [Google Scholar]
- 11. Brocher J, Janicki P, Voltz P et al. Inferior ectopic bone formation of mesenchymal stromal cells from adipose tissue compared to bone marrow: Rescue by chondrogenic pre‐induction. Stem Cell Res 2013;11:1393–1406. 24140198 [Google Scholar]
- 12. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 13. Noël D, Caton D, Roche S et al. Cell specific differences between human adipose‐derived and mesenchymal‐stromal cells despite similar differentiation potentials. Exp Cell Res 2008;314:1575–1584. [DOI] [PubMed] [Google Scholar]
- 14. Pachón‐Peña G, Yu G, Tucker A et al. Stromal stem cells from adipose tissue and bone marrow of age‐matched female donors display distinct immunophenotypic profiles. J Cell Physiol 2011;226:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. John K, Fraser MZ. Adipose‐derived stem cells. Methods Mol Biol 2008;449:59–67. [DOI] [PubMed] [Google Scholar]
- 16. Hattori H, Sato M, Masuoka K et al. Osteogenic potential of human adipose tissue‐derived stromal cells as an alternative stem cell source. Cells Tissues Organs 2004;178:2–12. [DOI] [PubMed] [Google Scholar]
- 17. Mesimäki K, Lindroos B, Törnwall J et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg 2009;38:201–209. [DOI] [PubMed] [Google Scholar]
- 18. van de Watering FCJ, van den Beucken JJJP, van der Woning SP et al. Non‐glycosylated BMP‐2 can induce ectopic bone formation at lower concentrations compared to glycosylated BMP‐2. J Control Release 2012;159:69–77. [DOI] [PubMed] [Google Scholar]
- 19. Lendeckel S, Jödicke A, Christophis P et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J Craniomaxillofac Surg 2004;32:370–373. [DOI] [PubMed] [Google Scholar]
- 20. Kulakov AA, Goldshtein DV, Grigoryan AS et al. Clinical study of the efficiency of combined cell transplant on the basis of multipotent mesenchymal stromal adipose tissue cells in patients with pronounced deficit of the maxillary and mandibulary bone tissue. Bull Exp Biol Med 2008;146:522–525. [DOI] [PubMed] [Google Scholar]
- 21. Liu Y, Zhao Y, Zhang X et al. Flow cytometric cell sorting and in vitro pre‐osteoinduction are not requirements for in vivo bone formation by human adipose‐derived stromal cells. PLoS One 2013;8:e56002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hattori H, Masuoka K, Sato M et al. Bone formation using human adipose tissue‐derived stromal cells and a biodegradable scaffold. J Biomed Mater Res Part B Appl Biomater 2006;76:230–239. [DOI] [PubMed] [Google Scholar]
- 23. Hao W, Hu Y‐Y, Wei Y‐Y et al. Collagen I gel can facilitate homogenous bone formation of adipose‐derived stem cells in PLGA‐beta‐TCP scaffold. Cells Tissues Organs 2008;187:89–102. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Zhou Y, Feng H et al. Injectable tissue‐engineered bone composed of human adipose‐derived stromal cells and platelet‐rich plasma. Biomaterials 2008;29:3338–3345. [DOI] [PubMed] [Google Scholar]
- 25. Hicok KC, Du Laney TV, Zhou YS et al. Human adipose‐derived adult stem cells produce osteoid in vivo. Tissue Eng 2004;10:371–380. [DOI] [PubMed] [Google Scholar]
- 26. Jeon O, Rhie JW, Kwon I‐K et al. In vivo bone formation following transplantation of human adipose‐derived stromal cells that are not differentiated osteogenically. Tissue Eng Part A 2008;14:1285–1294. [DOI] [PubMed] [Google Scholar]
- 27. Cowan CM, Shi Y‐Y, Aalami OO et al. Adipose‐derived adult stromal cells heal critical‐size mouse calvarial defects. Nat Biotech 2004;22:560–567. [DOI] [PubMed] [Google Scholar]
- 28. Levi B, James AW, Nelson ER et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One 2010;5:e11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linero I, Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS One 2014;9:e107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Janicki P, Boeuf S, Steck E et al. Prediction of in vivo bone forming potency of bone marrow‐derived human mesenchymal stem cells. Eur Cell Mater 2011;21:488–507. [DOI] [PubMed] [Google Scholar]
- 31. Brennan MA, Davaine J‐M, Layrolle P. Pre‐vascularization of bone tissue‐engineered constructs. Stem Cell Res Ther 2013;4:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grellier M, Granja PL, Fricain J‐C et al. The effect of the co‐immobilization of human osteoprogenitors and endothelial cells within alginate microspheres on mineralization in a bone defect. Biomaterials 2009;30:3271–3278. [DOI] [PubMed] [Google Scholar]
- 33. Cao Y, Sun Z, Liao L et al. Human adipose tissue‐derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 2005;332:370–379. [DOI] [PubMed] [Google Scholar]
- 34. Miranville A, Heeschen C, Sengenès C et al. Improvement of postnatal neovascularization by human adipose tissue‐derived stem cells. Circulation 2004;110:349–355. [DOI] [PubMed] [Google Scholar]
- 35. Planat‐Benard V, Silvestre J‐S, Cousin B et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation 2004;109:656–663. [DOI] [PubMed] [Google Scholar]
- 36. Kim Y, Kim H, Cho H et al. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem 2007;20:867–876. [DOI] [PubMed] [Google Scholar]
- 37. Zuk PA, Zhu M, Ashjian P et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13:4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fekete N, Gadelorge M, Fürst D et al. Platelet lysate from whole blood‐derived pooled platelet concentrates and apheresis‐derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: Production process, content and identification of active components. Cytotherapy 2012;14:540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brennan MÁ, Renaud A, Gamblin A‐L et al. 3D cell culture and osteogenic differentiation of human bone marrow stromal cells plated onto jet‐sprayed or electrospun micro‐fiber scaffolds. Biomed Mater 2015;10:045019. [DOI] [PubMed] [Google Scholar]
- 40. Chevallier N, Anagnostou F, Zilber S et al. Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials 2010;31:270–278. [DOI] [PubMed] [Google Scholar]
- 41. Mendes SC, Tibbe JM, Veenhof M et al. Relation between in vitro and in vivo osteogenic potential of cultured human bone marrow stromal cells. J Mater Sci Mater Med 2004;15:1123–1128. [DOI] [PubMed] [Google Scholar]
- 42. Agata H, Asahina I, Watanabe N et al. Characteristic change and loss of in vivo osteogenic abilities of human bone marrow stromal cells during passage. Tissue Eng Part A 2010;16:663–673. [DOI] [PubMed] [Google Scholar]
- 43. Giannoni P, Scaglione S, Daga A et al. Short‐time survival and engraftment of bone marrow stromal cells in an ectopic model of bone regeneration. Tissue Eng Part A 2010;16:489–499. [DOI] [PubMed] [Google Scholar]
- 44. Fennema EM, Tchang LAH, Yuan H et al. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J Tissue Eng Regen Med 2017. (in press). [DOI] [PubMed] [Google Scholar]
- 45. Hayashi O, Katsube Y, Hirose M et al. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int 2008;82:238–247. [DOI] [PubMed] [Google Scholar]
- 46. Zhang W, Zhang X, Wang S et al. Comparison of the use of adipose tissue‐derived and bone marrow‐derived stem cells for rapid bone regeneration. J Dent Res 2013;92:1136–1141. [DOI] [PubMed] [Google Scholar]
- 47. Elman JS, Li M, Wang F et al. A comparison of adipose and bone marrow‐derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm (Lond) 2014;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Castano‐Izquierdo H, Alvarez‐Barreto J, van den Dolder J et al. Pre‐culture period of mesenchymal stem cells in osteogenic media influences their in vivo bone forming potential. J Biomed Mater Res A 2007;82:129–138. [DOI] [PubMed] [Google Scholar]
- 49. Elabd C, Chiellini C, Massoudi A et al. Human adipose tissue‐derived multipotent stem cells differentiate in vitro and in vivo into osteocyte‐like cells. Biochem Biophys Res Commun 2007;361:342–348. [DOI] [PubMed] [Google Scholar]
- 50. Lee JA, Parrett BM, Conejero JA et al. Biological alchemy: Engineering bone and fat from fat‐derived stem cells. Ann Plast Surg 2003;50:610–617. [DOI] [PubMed] [Google Scholar]
- 51. Cui L, Liu B, Liu G et al. Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials 2007;28:5477–5486. [DOI] [PubMed] [Google Scholar]
- 52. Liu G, Zhang Y, Liu B et al. Bone regeneration in a canine cranial model using allogeneic adipose derived stem cells and coral scaffold. Biomaterials 2013;34:2655–2664. [DOI] [PubMed] [Google Scholar]
- 53. Cheng S‐W, Lin Z‐Q, Wang W et al. Osteogenic capability of autologous rabbit adipose‐derived stromal cells in repairing calvarial defects. Chin J Traumatol 2011;14:288–292. [PubMed] [Google Scholar]
- 54. Lin C‐Y, Chang Y‐H, Li K‐C et al. The use of ASCs engineered to express BMP2 or TGF‐β3 within scaffold constructs to promote calvarial bone repair. Biomaterials 2013;34:9401–9412. [DOI] [PubMed] [Google Scholar]
- 55. Di Bella C, Farlie P, Penington AJ. Bone regeneration in a rabbit critical‐sized skull defect using autologous adipose‐derived cells. Tissue Eng Part A 2008;14:483–490. [DOI] [PubMed] [Google Scholar]
- 56. Conejero JA, Lee JA, Parrett BM et al. Repair of palatal bone defects using osteogenically differentiated fat‐derived stem cells. Plast Reconstr Surg 2006;117:857–863. [DOI] [PubMed] [Google Scholar]
- 57. Yoon E, Dhar S, Chun DE et al. In vivo osteogenic potential of human adipose‐derived stem cells/poly lactide‐co‐glycolic acid constructs for bone regeneration in a rat critical‐sized calvarial defect model. Tissue Eng 2007;13:619–627. [DOI] [PubMed] [Google Scholar]
- 58. Niemeyer P, Fechner K, Milz S et al. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet‐rich plasma. Biomaterials 2010;31:3572–3579. [DOI] [PubMed] [Google Scholar]
- 59. Arrigoni E, de Girolamo L, Di Giancamillo A et al. Adipose‐derived stem cells and rabbit bone regeneration: Histomorphometric, immunohistochemical and mechanical characterization. J Orthop Sci 2013;18:331–339. [DOI] [PubMed] [Google Scholar]
- 60. Peterson B, Zhang J, Iglesias R et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng 2005;11:120–129. [DOI] [PubMed] [Google Scholar]
- 61. Han D, Li J. Repair of bone defect by using vascular bundle implantation combined with Runx II gene‐transfected adipose‐derived stem cells and a biodegradable matrix. Cell Tissue Res 2013;352:561–571. [DOI] [PubMed] [Google Scholar]
- 62. Chen Q, Yang Z, Sun S et al. Adipose‐derived stem cells modified genetically in vivo promote reconstruction of bone defects. Cytotherapy 2010;12:831–840. [DOI] [PubMed] [Google Scholar]
- 63. Whited BM, Whitney JR, Hofmann MC et al. Pre‐osteoblast infiltration and differentiation in highly porous apatite‐coated PLLA electrospun scaffolds. Biomaterials 2011;32:2294–2304. [DOI] [PubMed] [Google Scholar]
- 64. Thibault RA, Scott Baggett L, Mikos AG et al. Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng Part A 2010;16:431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leong NL, Jiang J, Lu HH. Polymer‐ceramic composite scaffold induces osteogenic differentiation of human mesenchymal stem cells. Conf Proc IEEE Eng Med Biol Soc 2006;1:2651–2654. [DOI] [PubMed] [Google Scholar]
- 66. Cordonnier T, Langonné A, Corre P et al. Osteoblastic differentiation and potent osteogenicity of three‐dimensional hBMSC‐BCP particle constructs. J Tissue Eng Regen Med 2014;8:364–376. [DOI] [PubMed] [Google Scholar]
- 67. Giammona A, Di Stefano AB, Leto Barone AA et al. Identification and expansion of adipose stem cells with enhanced bone regeneration properties. J Regen Med 2016;4:1–11. [Google Scholar]
- 68. Thesleff T, Lehtimäki K, Niskakangas T et al. Cranioplasty with adipose‐derived stem cells, beta‐tricalcium phosphate granules and supporting mesh: Six‐year clinical follow‐up results. Stem Cells Translational Medicine 2017;6:1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ikegame Y, Yamashita K, Hayashi S‐I et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 2011;13:675–685. [DOI] [PubMed] [Google Scholar]
- 70. McIntosh K, Zvonic S, Garrett S et al. The immunogenicity of human adipose‐derived cells: Temporal changes in vitro. Stem Cells 2006;24:1246–1253. [DOI] [PubMed] [Google Scholar]
- 71. Le Blanc K, Tammik L, Sundberg B et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003;57:11–20. [DOI] [PubMed] [Google Scholar]
- 72. Djouad F, Plence P, Bony C et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003;102:3837–3844. [DOI] [PubMed] [Google Scholar]
- 73. Bartholomew A, Sturgeon C, Siatskas M et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002;30:42–48. [DOI] [PubMed] [Google Scholar]
- 74. Blanco B, Herrero‐Sánchez MDC, Rodríguez‐Serrano C et al. Immunomodulatory effects of bone marrow versus adipose tissue‐derived mesenchymal stromal cells on NK cells: Implications in the transplantation setting. Eur J Haematol 2016;97:528–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1
Supplement Figure 2
Supplement Table 1
Supplement Table 2


