Abstract
Mesenchymal stem cells (MSC) hold great potential for regenerative medicine because of their ability for self‐renewal and differentiation into tissue‐specific cells such as osteoblasts, chondrocytes, and adipocytes. MSCs orchestrate tissue development, maintenance and repair, and are useful for musculoskeletal regenerative therapies to treat age‐related orthopedic degenerative diseases and other clinical conditions. Importantly, MSCs produce secretory factors that play critical roles in tissue repair that support both engraftment and trophic functions (autocrine and paracrine). The development of uniform protocols for both preparation and characterization of MSCs, including standardized functional assays for evaluation of their biological potential, are critical factors contributing to their clinical utility. Quality control and release criteria for MSCs should include cell surface markers, differentiation potential, and other essential cell parameters. For example, cell surface marker profiles (surfactome), bone‐forming capacities in ectopic and orthotopic models, as well as cell size and granularity, telomere length, senescence status, trophic factor secretion (secretome), and immunomodulation, should be thoroughly assessed to predict MSC utility for regenerative medicine. We propose that these and other functionalities of MSCs should be characterized prior to use in clinical applications as part of comprehensive and uniform guidelines and release criteria for their clinical‐grade production to achieve predictably favorable treatment outcomes for stem cell therapy. Stem Cells Translational Medicine 2017;6:2173–2185
Keywords: Mesenchymal stem/stromal cells, Bone marrow, Characterization, Release criteria, Regenerative medicine
Significance Statement.
There is a pressing need for more wide‐ranging characterization metrics for mesenchymal stem cells (MSCs) that better and more accurately predict treatment outcomes of MSC‐based therapies. This Review provides a detailed account of what are currently thought to be defining characteristics of MSCs and further considers recent advances that may prove to be important criteria when considering clinical applications. The relationship between in vitro characteristics and in vivo potency and strategies to improve the efficacy of MSC therapy is also addressed.
Introduction
Mesenchymal stem cells (MSC) constitute a heterogeneous subset of stromal regenerative cells which can be harvested from several adult tissues. Other descriptive names for MSC populations in the literature include mesenchymal stromal cells, mesenchymal progenitor cells, multipotent mesenchymal stromal cells, bone marrow stromal cells, bone marrow‐derived MSC, multipotent stromal cells, mesenchymal precursor cells, skeletal stem cells, as well as medicinal signaling cells. They are multipotent cells capable of differentiating into various types of specialized cells including osteoblasts, chondrocytes, and adipocytes 1. Recent studies indicate that MSCs resemble pericytes and emerge from the peripheral stromal region surrounding blood vessels, thus clarifying their broad regenerative potential in adult tissues, although there are also other sources for MSCs 2, 3, 4. Their relative ease of isolation, combined with their capacities for self‐renewal 5 and multipotentiality make MSCs a promising treatment option for a variety of clinical conditions. Yet, administration of MSCs (either intravenously or by direct injection in tissue) has not yielded consistent clinical results, because injected cells exhibit limited survival in host tissue. The fact that clinical improvement may be seen even despite the apparent short survival times of MSCs has led to alternative ideas about trophic effects 6. Several wide‐ranging investigations have attempted to address this issue of unpredictable outcomes by seeking to establish standard practices for the isolation, characterization, and maintenance of cells in culture. In this Review, we discuss human adult bone marrow‐derived MSCs, their various characterization methods, including an assessment of trophic factors secreted by isolated and culture‐expanded cells. Our group has recently proposed benchmarks for MSC functionality that require an improvement in MSC selection criteria 7. This Review considers several functional aspects of MSCs (Fig. 1) as they pertain to potency, and of the need to adopt multiple‐parameter analyses for useful stem cell selection.
Figure 1.
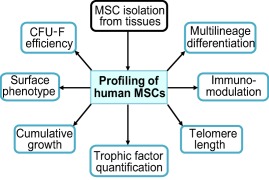
Profiling of MSCs. The diagram depicts the key parameters for the characterization of adult stem cells from different sources. Three of these parameters are linked to cell growth, survival, quiescence and/or senescence (i.e., viability and growth, CFU‐Fs, telomere length), two are associated with cell identity (i.e., multilineage differentiation and surface marker expression), and the remaining two refer to the ability of MSCs to communicate with their microenvironment (i.e., immunomodulation and paracrine effects of trophic factors). Immunomodulation is important for regulating macrophage function during tissue repair (e.g., M1 to M2 macrophage transition) and for anticipating graft rejection (e.g., mixed lymphocyte reaction). Collectively, these parameters should be considered for the development of release criteria that validate the quality of GMP‐grade MSCs for stem cell therapy. Abbreviations: MSCs, mesenchymal stem cells; CFU‐Fs, colony‐forming units‐fibroblastic.
Identification and Tissue Sources of MSCs
The first identified multipotent stromal precursor cell populations from the bone marrow were described as nonphagocytic, fibroblast‐like in appearance and able to form adherent colonies that were henceforth termed “colony‐forming units‐fibroblastic” (CFU‐F) for this population 8, 9. Other studies revealed that bone marrow‐derived MSCs represent precursor cells for mesenchymal tissues. Some investigations have reported conversion of multipotent stem cells into cells from another lineage through a process termed trans‐differentiation, although there are varying opinions on this phenomenon 10, 11, 12, 13, 14. While epigenetic transcriptional mechanisms control neuronal versus mesenchymal cell fates 15, MSCs can be induced experimentally to express neural markers 16.
Although MSCs were initially identified in bone marrow, MSC‐like populations have since been harvested from autologous and allogeneic sources, including adipose tissue 17, peripheral blood 18, 19, lung 20, marrow spaces of long bone 21, synovial fluids 22, periodontal ligament 23, and muscle 24. In addition, MSCs are also obtained from placenta 25, 26, umbilical cord 27, and cord blood 28, 29 as well as dental pulp 30, 31. Investigations into the lineage of these cells strongly suggest that progenitor cells of cultured MSCs arise from around the blood vessels (capillaries, arteries and veins) in vivo, and are thus of perivascular origin 3, 31, 32. Notably, MSCs obtained from various sources differ in their biological characteristics 33, 34, 35, 36. A recent comprehensive report on the proteome and transcriptome profiles of MSCs revealed source specific markers 37. In addition, differences that exist in CFU‐F efficiency, surfactome profiles, multi‐lineage differentiation as well as paracrine functions 35, 36, 38, 39, 40, 41 may determine their different clinical applications.
Recent reports have indicated that MSCs from allogeneic sources are more commonly used in trials than autologous MSCs 42, 43, even though both sources of cells have demonstrated comparable clinical effects 42, 44, 45, 46. Generally referred to as “universal donor cells” 44, 47, owing to their immune tolerance property, these cells possess several clinical advantages 48. Nevertheless, as with any cell‐based therapy, it is of utmost importance to fully evaluate the safety and efficacy of allogeneic strategies before clinical use 49, 50. MSCs from bone marrow are the most commonly investigated candidates that are providing most of the cells being used to create functional clinical therapies. In this Review, the isolation and functional characteristics described pertain to human bone marrow‐derived MSCs.
Isolation of MSCs
MSCs are obtained after bone marrow aspiration and then isolated by sieving for plastic adherence in vitro. They readily form colonies capable of clonal expansion and differentiation. Other isolation methods with different degrees of sophistication have been investigated, including density gradient cell separation 51, as well as fluorescence‐ or magnetic‐activated cell sorting 52, 53. The latter two flow cytometric methods rely on expression of cell surface markers displaying relatively high specificity for MSCs. Clearly, standard isolation procedures and generic molecular characterization of MSCs are vital for any consistent cell isolations 54, 55. The key characteristics that have hitherto defined MSCs have been based on their capacity for colony formation, potential for self‐renewal, expression of surface markers, and subsequent capacity for multilineage differentiation 56.
Multifaceted Characterization of MSCs
Colony Formation
In vitro, plastic‐adherent clonogenic cells, denoted as CFU‐Fs, can be obtained from bone marrow and give rise to colonies during their initial growth 31, 57. CFU‐Fs are thought to be mostly composed of primary bone marrow‐derived MSCs that upon further proliferative expansion in culture, constitute mesenchymal stem/stromal cells. Colonies of MSCs display heterogeneous morphological characteristics ranging from fibroblastoid to spindle‐shaped or from large‐flattened to small‐round cells. Passaged cells are usually seeded at 100 to 150 cells per 10‐cm dish and allowed to adhere and form colonies over a period of 14 days that are visualized by staining with crystal violet or toluidine blue 58. Evaluation of the CFU‐F potential is usually being done by seeding bone marrow cells at densities of 0.5–3 million in 50–75 cm2 culture vessels 7. Bone marrow mononuclear cells are typically seeded at 50,000–200,000 cells per cm2 to yield colonies of MSCs. Although this approach provides a relatively crude estimate of MSC titers in bone marrow cells 59, CFU‐F efficiency remains a routinely used and accepted standard to identify and characterize MSCs 56.
Surface Phenotype
To acquire a more complete understanding of MSC biology and to generate a reliable stem cell product for clinical trials and routine patient care in the future, it is necessary to isolate homogenous population of MSCs. The principal approach for improving homogeneity of MSC populations uses antibodies that target specific cell surface markers. This homogeneity is a relative term, because MSC populations have natural variation in the expression of cell surface markers around a common mean. The identification of MSCs in vivo is far from straightforward, owing to extremely low frequencies in tissues 59, 60. Furthermore, isolation methods are impeded because MSCs are dynamic and exhibit phenotypic variation over time (“plasticity”). Also, there is only a limited number of useful MSC markers, but none of these is definitively specific for MSCs in a strict sense (i.e., absolutely required and sufficient to establish MSC identity). Nevertheless, it is well established that cultured colonies of MSCs express CD105, CD73, and CD90, but do not express CD45, CD34, CD14 or CD11b, CD19, and HLA‐DR 56. Some labeling strategies have also been used to successfully isolate MSCs enriched for markers such as STRO‐1 52, 61, 62, 63, 64, CD146 5, SSEA‐4 65, CD271 (NGFR) 66, 67, 68, and MSC antigen 1 (MSCA‐1) 69, 70, although there is no absolute agreement yet on the markers that could prospectively assist in the isolation of MSCs from either fresh bone marrow or other tissues. Initial studies by Pittenger and coworkers have identified markers such as SH2 and SH3, which correspond to CD105 and CD73, respectively. These markers, together with CD90, have been considered by the International Society for Cellular Therapy (ICST) as the primary markers expressed on greater than 95% of MSCs in a given culture. However, it should be noted that the expression of CD105, CD90, and CD73 may not be absolutely specific to undifferentiated multipotent MSCs, as some of these markers are also expressed by vascular populations 71, 72, smooth muscle cells 73, and mature stromal cells such as fibroblasts 71, 74. Consequently, there is a critical need to develop highly sensitive cell sorting and immunohistochemical assays and reagents to distinguish immature/undifferentiated MSCs from committed stromal cell populations.
Currently not included in the ISCT panel is STRO‐1, a particularly important marker, with relatively high specificity for early‐passage bone marrow‐derived MSCs. STRO‐1 facilitates the identification, isolation, and functional characterization of clonogenic stromal cell progenitors 52, 61, 62, 63, 75. The absolute selectivity of STRO‐1 for naïve MSCs is yet to be resolved and the presence of STRO‐1 antigen on MSCs is progressively downregulated following culture expansion. Notwithstanding these limitations, the putative STRO‐1 antigen remains very useful because STRO‐1 positive cells have favorable stem cell properties for translational applications 76.
Other strategies using cell‐sorting have exploited the expression of CD49a (integrin α1; ITGA1) 39, PDGFR‐α/β (platelet‐derived growth factor receptors PDGFA and PDGFB), EGF receptor (EGFR), insulin‐like growth factor receptor (IGFR), and STRO‐3 52, 77 to enable isolation of MSC populations enriched for multi‐lineage differentiation potential (see Table 1). Andersen and colleagues have isolated antibodies against Collagen VI (COL6A1), CD44 and HLA‐DR, and that have proven useful for identifying subpopulations in MSC cultures 79. CD146 (melanoma cell adhesion molecule, MCAM) has attracted major interest following reports on its expression being linked to pericytes 3, 5. It has also been shown that expression of CD146 on MSCs expressing CD271 (nerve growth factor receptor, NGFR) is associated with their in situ localization 80, 81. However, it was also shown, particularly for CD146, that its expression is variable during in vitro culture and its cell surface presence fluctuates depending on the type of culture media 4. Bone marrow‐derived CFU‐Fs express surface markers such as STRO‐1, CD271 68, CD49a 39, 82, stage‐specific embryonic antigen‐4 (SSEA‐4) 65, and CD146 83. CD271+ cells display multipotentiality and is considered a suitable marker of bone marrow‐derived MSCs 84. Other reports have also detailed the use of D7‐FIB (a fibroblast or epithelial surface antigen) 85 and CD56 (neural cell adhesion molecule, NCAM) 86 for multipotent MSC isolation. Nestin, a neural stem cell marker, has characterized as a selective marker for bone marrow‐derived MSCs 87, 88. On a parallel note, the use of mouse models to study MSC biology has yielded novel information, highlighting the similarities in the expression of some of the surface markers, including CD140a 89, 90 and CD295 (leptin receptor) 91, between mouse and human MSCs 92. However, species‐specific heterogeneity in phenotype and in vivo residence of MSCs must be taken into account when extrapolating information derived from other species.
Table 1.
Surface antigen expression on human bone marrow‐derived mesenchymal stem cells (MSCs)
| CD (cluster of differentiation) | Gene Symbol | Protein description | MSC specificity |
|---|---|---|---|
| CD11a | ITGAL | Integrin alpha L chain | – |
| CD11b | ITGAM | Integrin alpha M chain | – |
| CD13 | ANPEP | Aminopeptidase N | – |
| CD14 | CD14 | Myeloid cell‐specific leucine‐rich glycoprotein | – |
| CD19 | CD19 | B‐lymphocyte surface antigen B4 | – |
| CD29 | ITGB1 | Integrin β1 chain | + |
| CD31 | PECAM1 | Platelet endothelial cell adhesion molecule | + |
| CD34 | CD34 | Hematopoietic progenitor cell antigen CD34, transmembrane phosphoglycoprotein | +/– |
| CD36# | CD36 | Collagen Type I Receptor, Thrombospondin Receptor | +/– |
| CD44# | CD44 | Hyaluronan receptor | + |
| CD45 | PTPRC | Lymphocyte common antigen; protein tyrosine phosphatase, receptor type, C | – |
| CD49a | ITGA1 | Integrin subunit alpha 1 chain | + |
| CD49b | ITGA2 | Integrin subunit alpha 2 chain | + |
| CD49c | ITGA3 | Integrin subunit alpha 3 chain | + |
| CD49d | ITGA4 | Integrin subunit alpha 4 chain | + |
| CD49e | ITGA5 | Integrin subunit alpha 5 chain | + |
| CD51 | ITGAV | Integrin subunit alpha V chain | + |
| CD54 | ICAM1 | Intracellular adhesion molecule | + |
| CD58 | CD58 | Lymphocyte function‐associated antigen | + |
| CD61 | ITGB3 | Integrin β3 chain | + |
| CD71 | TFRC | Transferrin receptor | + |
| CD73* | NT5E | Ecto‐5'‐nucleotidase | + |
| CD90* | THY1 | Thy‐1 | + |
| CD102 | ICAM2 | Intracellular adhesion molecule | + |
| CD104 | ITGB4 | Integrin β4 chain | + |
| CD105* | ENG | Endoglin, TGFβ R III | + |
| CD106 | VCAM1 | Vascular cell adhesion molecule | + |
| CD120a | TNFRSF1A | Tumor necrosis factor receptor 1A, TNF IR | + |
| CD120b | TNFRSF1B | Tumor necrosis factor receptor type II, TNF IIR | + |
| CD121a | IL1R1 | Interleukin‐1 receptor | + |
| CD124 | IL4R | Interleukin‐4 receptor | + |
| CD133 | PROM1 | Prominin‐1 transmembrane glycoprotein | – |
| CD140a | PDGFRA | Platelet‐derived growth factor receptor alpha | + |
| CD140b | PDGFRB | Platelet‐derived growth factor receptor beta | + |
| CD146 | MCAM | Melanoma cell adhesion molecule | + |
| CD166 | ALCAM | Activated leukocyte cell adhesion molecule | + |
| CD200 | CD200 | OX‐2 membrane glycoprotein | + |
| CD221 | IGF1R | Insulin‐like growth factor 1 receptor, IGF‐R | + |
| CD271 | NGFR | Nerve growth factor receptor, NGF‐R | + |
| SSEA‐4 | SSEA4 | Stage specific embryonic antigen‐4 | + |
| STRO‐1 | N.A. | Stromal antigen 1 | + |
| W8‐B2/MSCA‐1 | N.A. | MSC antigen 1 | + |
The signs (+) or (–) indicate the presence or absence of markers respectively. * refers to antigens that have been proposed by the International Society for Cellular Therapy (ISCT) to define human MSCs 56; # refers to markers specifically retained by adipose stem cells, according to the recently revised ISCT and International Federation for Adipose Therapeutics and Science joint statement 78. Abbreviation: N.A., not available.
Lastly, it is important to understand that the innate levels of expression of a set of surface markers are not a guarantee of MSC homogeneity. Since labs around the world use different sets of antigens for characterization, comparisons reveal that there is no consistency in the use of cell surface antigens for the isolation of MSCs and there is no marker that uniquely identifies MSCs that could be used reliably for their isolation. In our previous study comparing in vitro and in vivo functions of bone marrow‐derived MSCs from multiple donors, we showed that STRO‐1 and PDGFRα (CD140a) were able to identify MSCs that were more potent at forming bone in vivo 7. We showed that MSCs with high‐growth capacity had higher levels of expression of these markers and promoted increased bone‐formation compared with low‐growth capacity cells, thus highlighting the possible utility of STRO‐1 and PDGFRα markers to aid in selection of efficacious MSCs for bone regenerative applications. Also, it is important to note that MSCs from different tissue origins have different surface marker expression 93, 94. Consequently, investigators are now performing characterization to determine the genomic and proteomic profiles of MSCs to establish mechanisms that mediate self‐renewal and maintenance of homogenous cell populations. In keeping with the trophic functions of MSCs (see below), their secretory and exosomal profiles may reveal unique biomarkers that reflect their biological properties and could potentially aid in their selection.
Multi‐Lineage Differentiation
While surface markers are easily assessable, a proper definition of what constitutes an MSC can be completed by their ability to differentiate into classic mesodermal lineages of bone, fat, and cartilage. When late passage cultures are left in maintenance media for longer periods (weeks) and cells become confluent, at least a subset is capable of spontaneously mineralizing, indicating that bone‐marrow MSCs are predisposed to differentiation into the bone lineage 95. This property is not exclusive of bone marrow MSCs and is also exhibited by human umbilical cord perivascular cells 96. Factors such as ascorbic acid and dexamethasone, at defined concentrations, are able to direct the MSCs toward osteogenic differentiation. Similarly, BMPs, WNTs, FGFs and other heparan sulfate‐sensitive morphogens, and growth factors are able to stimulate osteogenic differentiation 97, 98, 99, 100, 101, 102. To stimulate adipogenesis, dexamethasone, indomethacin, insulin, and isobutylmethylxanthine are usually added to the cultures 103. Ascorbate, insulin, transferrin, selenic acid, and TGF‐β are well‐established inducers of chondrogenesis 104, 105, 106. It is broadly appreciated that differentiation protocols followed by laboratories around the world are not necessarily the same. Factors such as antibiotics and growth supplements such as serum and platelet lysate can influence the phenotypic properties of MSCs and their multi‐lineage potential 107, 108, 109.
We note that when cultured under defined conditions with specific inducing factors, MSCs can be directed to differentiate into neural, myocyte, and epithelial cells, thereby demonstrating their endodermic and neuroectodermic differentiation potential 14, 95, 110, 111. It is not clear whether this process represents culture‐induced aberrant trans‐differentiation or perhaps reflects the inherent natural ability of adult stromal cells to reprogram under specific conditions. Forced trans‐differentiation in culture may perhaps be analogous to established developmental events (e.g., neural crest formation, epithelial‐to‐mesenchymal, and endothelial‐to‐mesenchymal transitions). In several studies using in vivo transdifferentiation models, MSCs have been reported to engraft and differentiate resulting in functional improvement of endogenous tissues 112, 113, 114, 115, 116, 117, as well as integrate via cell fusion mechanisms 118, 119, 120.
Differences exist in MSC differentiation properties. Culture‐expanded colonies display progressively limited differentiation potential. Reports from various groups have suggested that only a fraction of the total number of clones can differentiate into all the three lineages. The majority of the clones appear to be bi‐potent and only able to commit to osteogenic and chondrogenic lineages. Clones that showed increased adipogenic potential had decreased chondrogenic potential, and vice versa. From our previous work, we observed a bimodal differentiation pattern in MSCs that alternate between osteogenesis and adipogenesis 7. Furthermore, monopotent MSCs have also been reported 121, 122, 123. Thus, tri‐, bi‐, and mono‐potent colonies have been identified.
The reasons for heterogeneity in differentiation potential are not fully understood, but most likely reflect epigenetic adaptations that predispose cells to different cell fates depending on the source tissue. The latter concept remains conjecture at present, but is a logical implication from current concepts of the physiological micro‐environment of stromal cells. For example, stromal cells surrounding blood vessels in fat tissue or the bone marrow cavity are exposed to different growth factors, morphogens, cytokines and chemokines. Ultimately, such extracellular signals are sensed by cell surface receptors and transduced to the nucleus to mediate epigenetic chromatin changes. The latter changes are more likely to ensure that stromal cells in fat tissue differentiate into pre‐adipocytes, while analogous stromal cells in bone marrow may more easily convert into skeletal progenitor cells.
Considering the heterogeneity in lineage‐predisposition of different MSC preparations, characterization of the lineage‐differentiation potential is important, albeit that it is not prudent to base MSC selection solely on this biological property. Differentiation properties of MSCs are important for tissue maintenance and repair, as well as engineering strategies, and cell‐based therapies that require engraftment and differentiation into host tissues. However, the clinical potential of the trophic functions of MSCs is recently gaining significant traction as the basis for new stem cell therapies.
Trophic Functions of MSCs
The trophic function of MSCs refers to their functional capacity to generate a reparative milieu through cell‐to‐cell contact concomitant with paracrine secretion of a broad array of bioactive macromolecules that promote immunomodulation of inflammatory cells that participate in tissue repair (e.g., T cells, macrophages, and mast cells) and differentiation of endogenous progenitor cells (e.g., osteo‐ and chondroprogenitors). The current catalogue of trophic factors includes growth factors, morphogens, chemokines, cytokines, extracellular vesicles ([EVs] e.g., exosomes), and glycosaminoglycans (GAGs) 6, 124, 125, 126, 127, 128 (Fig. 2). The immunomodulatory properties of MSCs support suppression of local immune responses and fibrotic tissue formation, while modulating angiogenesis, apoptosis, and cell proliferation. These properties collectively generate a microenvironment that enables injured tissues to mount a self‐regulated regenerative response 129, 130.
Figure 2.
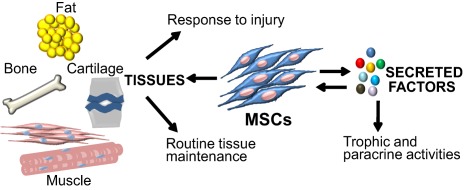
Dual functions of MSCs in tissue regeneration and repair. MSCs play a central role during regeneration and repair of musculoskeletal tissues (i.e., bone, cartilage, ligament, tendon, muscle and synovium). In addition, MSCs provide a microenvironment for hematopoietic stem cells, including cells of the myeloid and lymphoid lineages. Effects of MSCs on their microenvironment are mediated by secretion of trophic factors that have both autocrine and paracrine functions. Abbreviation: MSCs, mesenchymal stem cells.
Historically, MSCs originally attracted attention because of their “stemness” and potential use as therapeutic agents through engraftment to replace cells in damaged tissues. However, in many experimental settings, transplanted cells restore tissue functions with no detectable engraftment within host tissue or differentiation 131, 132, 133. Reports on the trophic functions of MSCs date back to studies by Dexter and colleagues, that showed the ability of MSCs to support HSCs 134, to be able to suppress the local immune system by secretion of cytokines 131, 135, 136, to aid in regeneration of the meniscus 137, to promote neurotrophic and functional recovery after stroke 138, and promote cardiac repair 139. The mechanisms governing these functions imply that MSCs facilitate normal tissue healing by cell‐to‐cell contact and/or secretion of bioactive factors.
Several published findings reinforce the proposition that persistent engraftment of the cells at a skeletal defect site is not mandatory for tissue healing or repair. Horwitz and colleagues showed that OI (osteogenesis imperfecta) in babies was improved with transplantation of allogenic bone marrow cells, and resulted in increased bone mineral density and reduced bone fractures, even though less than 2% of the donor MSCs were found to be engrafted 140. Other studies have shown that few implanted MSCs survive 6 weeks post‐implantation in a rat ectopic model 141 and 3 to 7 weeks in an orthotopic femoral defect model 100. The observed bone regeneration is attributable to a burst of active trophic factors secreted by the implanted MSCs. Similarly, MSC transplants in other disease models have resulted in improved cardiac function, neurogenesis, pancreatic islet survival and functionality, as well as modulation of the immune system in graft‐versus‐host‐disease 142. These findings are generally consistent with the now prevalent idea that MSCs do not promote tissue repair only through engraftment, but also by delivery of bioactive factors.
Given the therapeutic potential of EVs secreted by MSCs 143, 144, 145, 146, it would be useful to include characterization of EVs while assessing MSC potency. EVs have been shown to possess anti‐inflammatory properties 147, rescue radiation damage to bone marrow MSCs 148, as well as mitigate airway hyper‐reactivity and lung inflammation in preclinical disease models 144. Assaying for their reported immunomodulatory, cytoprotective, and regenerative properties may be important for advancing MSC‐based EV‐mediated therapies 147, 149, 150.
Despite the fact that MSCs isolated from separate donors show no major differences in their in vitro differentiation potential or in their surface markers expression 151, differences in their secretion profile may be the key to the observed variability in their in vivo healing capacity. The in vitro secretome of MSCs has been well documented and several secretory molecules relevant to MSC potency have been investigated 7. Although recombinant bioactive factors are essential for the future of regenerative medicine, the use of most of them remains experimental, mainly due to difficulties in optimizing the clinical dose of the factors based on in vitro results and preclinical models. Understanding the secretory activity of MSCs, in conjunction with their in vivo behavior and paracrine effects, is thus of paramount importance for the exploitation of their clinical potential.
Immunomodulatory Properties of MSCs
The mechanisms underlying immunoregulation by MSCs are not fully understood, but involve cell‐to‐cell contact and secretory mechanisms. Typical in vitro modulatory functions of MSCs are inhibition of T cell 152 and B cell proliferation, as well as dendritic cell differentiation 153. MSCs also regulate immune responses by upregulating the numbers of regulatory T cells (Tregs) which actively suppress effector T cell functions 154. MSC‐immune cell contact involves adhesion molecules 155. In addition, factors including IL‐10, indoleamine 2, 3‐dioxygenase (IDO), VEGF, CCL‐5 or RANTES, prostaglandin E2, and nitric oxide (NO) are secreted by MSCs (either constitutively or by interaction with target cells). Interleukin‐6 (IL‐6), TGFβ1, hepatocyte growth factor, CCL‐1 or MCP‐1 (monocyte chemoattractant protein), and leukemia inhibitory factor (LIF) are other notable immunoregulatory factors secreted by MSCs 156, 157. MSC‐to‐T cell contact induces IL‐10 secretion, which attenuates T cell proliferation, and stimulates HLA‐G5 secretion which in turn inhibits activated T cells and NK‐cell cytotoxicity 158 (Fig. 3).
Figure 3.
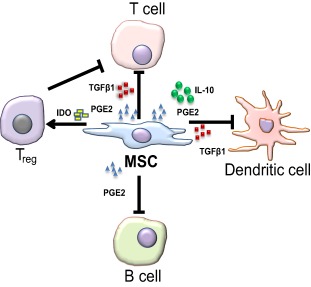
Interactions of MSC with immune cells. MSCs secrete soluble molecules, such as nitric oxide, PGE2, IDO, IL‐10 and TGFβ1. The secretion of these factors suppresses the proliferation and/or activity of a variety of immune cells, including T cells, B cells, Natural Killer cells, and dendritic cells, as well as activated Tregs. Abbreviations: IDO, indoleamine 2, 3‐dioxygenase; IL‐10, interleukin‐10; MSCs, mesenchymal stem cells; PGE2, prostaglandin; TGFβ1, transforming growth factor‐beta 1; Tregs, regulatory T cells.
In vivo, systemic administration of MSCs facilitates immunosuppression in graft‐versus‐host‐disease models 159, multiple sclerosis, inflammatory bowel disease, diabetes 160 as well as cardiomyopathies 161, 162. Following successful outcomes from animal models, clinical trials for Crohn's disease (e.g., Mayo Clinic), acute graft‐versus‐host‐disease (e.g., Osiris Therapeutics), and severe osteogenesis imperfecta by allogenic BMT (e.g., St. Jude Children's Research Hospital) have been conducted. Trials are ongoing for acute myocardial infarction, aplastic anemia, osteoarthritis, SLE, diabetes, and other conditions 142, 163. Because MSC therapy appears to be promising for treating immunological disorders, characterization of MSC immunosuppressive functions will provide an important functional indicator for in vivo efficacy of MSCs, even though they may not be specific to multipotent MSCs, since stromal fibroblasts also exhibit immunosuppressive functions 164, 165. Furthermore, it is also important to note that MSCs from different sources may differ in their mechanisms and capacities for immunomodulation 166.
Because of their trophic and immunomodulatory functions, MSCs are generally considered to possess greater advantages in cell‐based regenerative medicine. However, it is important to note that MSCs can either support or suppress tumorigenesis (reviewed in 167, 168). In contrast to their anti‐apoptotic and anti‐inflammatory functions, MSCs have been shown to interact with tumor cells via paracrine signaling and possibly increase the risk for metastasis by mediating epithelial‐to‐mesenchymal transition in addition to augmenting angiogenesis 169, 170, 171, 172. This less‐desirable effect imparted by MSC immunomodulatory activities at tumor microenvironments warrants some caution in their use in circumstances of pre‐existing tumor conditions.
Telomere Length Analysis
Cell preparations of MSCs have variable and limited proliferative potential. The variability depends on differences in sources and methods of isolation, as well as the age and health conditions of the donors 173, 174. For clinical use, extensive subculturing is performed to attain the required cell numbers for therapy. As a result, cells rapidly reach a stage of growth arrest and replicative senescence as their telomeres progressively shorten with repeated cell replications in vitro. Obtaining both quality and quantity of MSCs for an efficacious therapy is a major bottleneck in translational medicine. Telomere maintenance is carried out via telomerase reverse transcriptase (hTERT) which functions to lengthen telomeres by adding repetitive TTAGGG sequences to chromosome termini. Overexpression of TERT in MSCs restores telomerase activity, preserves telomere length and increases MSC life span. The status of telomeres is a key parameter for MSC quality that should be routinely monitored; however, reports on telomerase functions in MSCs are incompatible 111, 175, 176. Differences in results could be due to different sensitivities in measurement and the nonestablished reference levels of telomerase to define cells as either telomerase‐positive or negative 177. Therefore, assaying for telomere lengths, as well as overall telomere status in MSCs should assist in the benchmarking process and in quality control decisions required before MSC transplantation 7, 178.
Standardization of Strategies for Improving MSC Growth and Regenerative Efficacy
Uniform standards for MSC preparation are essential for fundamental characterization and clinical translation of MSCs. Standard operating procedures avoid variability in cell preparation that may arise for technical reasons. Yet, currently most laboratories use their own optimized protocols, and cell preparations between labs clearly vary. Therefore, it would be beneficial for laboratories to agree upon standard operating procedures and to improve comparison of results.
The illustration provided in Figure 4 is a schematic of a BM aspirate sample processing. The aspirate is layered over Ficoll‐Paque to obtain mononuclear cell fractions by density gradient method, from which MSCs are isolated. Between 1 and 3 million MNCs are seeded in 50–75 cm2 dishes or flasks to obtain colonies that provide an estimate of MSC numbers per 105 cells seeded. Flow cytometry is performed to determine the MSC percentages in the original sample before they are plated for isolation to get the baseline MSC levels in a given patient/donor sample. Also, growth capacities of cells (fast or slow growing) are assayed starting at P0 by taking a fraction of the MNCs and allowing MSC colonies to form and proliferate so that cumulative growth can be plotted. Cells that have undergone three or four passages are typically evaluated for matching the ISCT criteria 56. In addition, other criteria such as population doubling time, amounts of growth factor/cytokine secretions, levels of STRO‐1, PDGFR‐α/β, and telomere length are some measures to assess efficacy.
Figure 4.
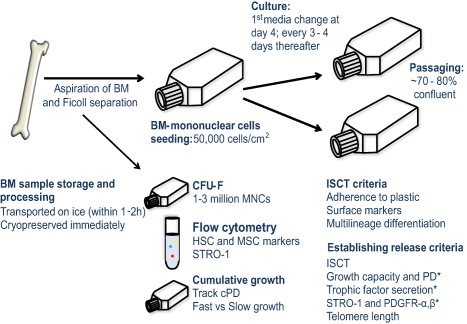
Standard operating procedures for isolating mesenchymal stem cells (MSCs). The diagram shows the basic steps for isolating and validating MSCs from bone marrow aspirates derived from either human donors or patients, including evaluation of key potency parameters of these cells before release to the clinic. Abbreviations: BM, bone marrow; cPD, cumulative population doubling; HSC, hematopoietic stem cells; ISCT, International Society for Cellular Therapy; MNC, mononuclear cells; PD, population doubling; PDGFR‐α, platelet‐derived growth factor receptor‐alpha; STRO‐1, stromal antigen‐1. Asterisk (*) indicates proposition of additional criteria that could potentially facilitate better selection of MSCs.
Strategies to clear the hurdle of achieving clinically relevant numbers of MSCs include the use of growth media supplements like serum, platelet lysates, growth factors, and so forth. Importantly however, the use of such supplements is currently hindered by their cost, degradation in culture and thus their limited bioactivity. It is to be noted that the transfer of retained non‐human antigens from serum may elicit an inappropriate immune response upon transplantation 179, 180, and therefore necessitates the use of human‐derived components such as plasma or serum platelet lysate as a suitable alternative 181, 182. Sustaining the bioactivity of growth factors can be achieved by harnessing their interaction with GAGs such as heparan sulfate (HS) 100. HS GAGs that bind to growth factors with high affinity can be purified using well‐established chromatographic techniques 100, 183. Introducing GAGs to the culture at the time of isolation, and pre‐conditioning cells in HS appears to be a promising approach to improve MSC numbers while maintaining their characteristics. On the contrary, heparin has been shown to alter biological properties of MSCs and is not a recommended additive 184. HS‐GAGs could interact and protect growth factors from extracellular proteases, as well as from pH and thermal changes, so enhancing growth factor activity and downstream signaling, and ultimately stimulating MSCs to proliferate and be useful for tissue repair and regeneration. More recently, pre‐conditioning strategies using BMP‐2 and Wnt5a has proven useful for cartilage repair 185.
Economic Potential and Market Impact of MSC Research and Therapies
Stem cells are central components of regenerative medicine holding huge market potential that is projected to reach $170 billion by 2020, as per recent reports by Grand View Research, Inc. published in 2015. Several unmet medical needs drive the stem cell research economy. The consumers of this market are usually hospitals, clinical laboratories, stem cell banks, and academic institutes. Adult stem cells dominate the market as they do not raise the ethical controversy that surrounds embryonic stem cells, as well as due to relatively low production labor and maintenance costs, lower risk of tumors, and better immunocompatibility 186, 187, 188, 189.
Human MSCs are currently administered for several clinical conditions, including bone, heart, neurodegenerative, and immunological disorders, and have reached phase I and II clinical trials 190. We performed a search using the keyword “mesenchymal stem cell” in ClinicalTrials.gov in order to find the number of studies conducted worldwide. The potential of MSCs for clinical applications is supported by the fact that the clinical trials database currently lists nearly 650 clinical trials globally, excluding studies of unknown status (Fig. 5A) [Source: https://ClinicalTrials.gov]. Most of the trials in phase II are for conditions such as osteoarthritis, neurological diseases, pulmonary disorders, spinal cord injury, myocardial infarction, severe coronary ischemia, Crohn's disease, and diabetes mellitus. There is a strong correlation between global economic burden due to health disorders and the potential for stem cells to treat such ailments. We performed a Scopus search using strings “hematopoietic stem cells,” “embryonic stem cells,” “mesenchymal stem cells,” “neuronal stem cells,” “induced pluripotent stem cells,” and “umbilical cord stem cells” to assess the number of research articles published between 1995 and 2015. Clearly, research trends keep pace with market trends alongside clinical trials (Fig. 5B), and it is anticipated that this industry will continue to open up, with products for cardiovascular, diabetes, and nerve repair becoming commercially available. To accelerate this, it is important that the new 3Rs (regulation, reimbursement, and realization of value) recently proposed by Caplan and colleagues are taken into consideration 191. As MSC research continues to increase (Fig. 5B), the overall revenue for adult stem cell products is estimated at $10.9 billion by the end of this decade (Source: http://www.grandviewresearch.com).
Figure 5.
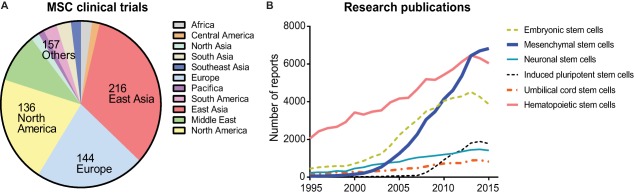
Mesenchymal stem cells (MSCs) in clinical trials and stem cell market forecast. (A): MSC‐based clinical trials were charted by region based on search results sourced from https://ClinicalTrials.gov (retrieved July 1, 2017). (B): Scopus search results show the number of stem cell research articles published between 1995 and 2015, indicating the rising number of published studies on MSCs (retrieved July 1, 2017; https//:www.scopus.com).
In recent years, as cell preparations of MSCs become commercially available, several stem cell companies have formulated their own criteria for the selection of clinical grade cells: for example, the enrichment for STRO‐1+ and STRO‐3+ mesenchymal precursor cells by Mesoblast 64, 192, and the selection of MSCs secreting TNF‐α receptor Type I at a minimum of 13 pg/10 million cells defined by Osiris Therapeutics 193. Another important development was the identification of a subpopulation of MSCs by Smith and colleagues, which are characterized by their smaller size and rapid self‐renewal potency. These cells are enriched for precursor cells that could be efficacious for therapy 194. Stempeutics Research's specifications for their allogeneic BMMSC product, Stempeucel, includes parameters such as morphology (fibroblastic and spindle‐shaped), cell counts of 180–220 million cells per bag, viability of >85%, ISCT‐defined surface marker levels >80% along with CD166 > 80% 71 and CD133 < 5% as their release criteria for administration 195, 196. As more strategies evolve and new criteria are published, the selection panel is continuously being developed. Therefore, it is essential to adopt broader characterization schemes if we seek to better understand MSC function and utility for commercial and clinical applications.
Conclusion
There is a compelling need to broaden the characterization landscape by identifying novel stable markers and refining selection criteria for establishing optimal classes of MSCs. Yet, current definitions of MSCs based on surface markers and/or differentiation parameters have so far been incomplete. Differences in the cellular phenotypes of MSCs can be attributed to the methods by which MSCs are isolated and expanded, ways of handling the cells, particularly seeding densities and media supplements, as well as other components of the culture conditions. The technical discrepancies in methods for defining MSC characteristics prevents general interpretations of results from stem cell laboratories or any beneficial effects of stem cell therapies observed in clinical trials with a range of stem cell preparations. Therefore, it is essential to obtain uniformity of methods for isolating and characterizing MSCs. To address ambiguities related to MSC identification and function, the ISCT criteria aimed to standardize isolation methods by serving as the basis for characterization of these cells, and to enable comparison of investigations among laboratories. This initiative is a key step in the right direction, but many more steps remain to be taken. Definition of novel biomarkers using genomic, epigenomic, transcriptomic, proteomic and metabolomic approaches, beyond the classical techniques that measure colony‐forming ability, CD marker expression, telomere length and cellular morphology (among a myriad of other tests) may collectively provide for a new generation of highly sophisticated standardized tests as necessary quality control parameters for characterization of MSC preparations in clinical practice.
Author Contributions
R.M.S: manuscript writing, figure artwork and illustrations, final approval of manuscript; M.R.: manuscript review, writing, final approval of manuscript; V.N., J.H.H., A.J.W., and S.M.C.: financial support, manuscript review, writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
This study was supported by funding from NUS Research Scholarship (to R.M.S.), NMRC (to S.M.C., J.H.H., M.R., and V.N.), A*STAR (to S.M.C. and V.N.), NIH (AR049069 to AJ.v.W.), the Mayo Clinic Center of Regenerative Medicine (to R.M.S.), and our generous benefactors William and Karen Eby. We appreciate our institutional colleagues in Singapore and Minnesota for stimulating discussions, including Profs. Hee Kit Wong (NUH), James Goh (NUS), and Eng Hin Lee (NUH), as well as Allan Dietz and Amel Dudakovic (Mayo Clinic).
Contributor Information
Andre J. van Wijnen, Email: vanwijnen.andre@mayo.edu.
Simon M. Cool, Email: Simon.Cool@imb.a-star.edu.sg.
References
- 1. Caplan AI. Mesenchymal stem cells. J Orthop Res 1991;9:641–650. [DOI] [PubMed] [Google Scholar]
- 2. Corselli M, Chin CJ, Parekh C et al. Perivascular support of human hematopoietic stem/progenitor cells. Blood 2013;121:2891–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crisan M, Yap S, Casteilla L et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–313. [DOI] [PubMed] [Google Scholar]
- 4. Blocki A, Wang Y, Koch M et al. Not all MSCs can act as pericytes: Functional in vitro assays to distinguish pericytes from other mesenchymal stem cells in angiogenesis. Stem Cells Dev 2013;22:2347–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sacchetti B, Funari A, Michienzi S et al. Self‐renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007;131:324–336. [DOI] [PubMed] [Google Scholar]
- 6. Caplan AI, Correa D. The MSC: An injury drugstore. Cell Stem Cell 2011;9:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samsonraj RM, Rai B, Sathiyanathan P et al. Establishing criteria for human mesenchymal stem cell potency. Stem Cells 2015;33:1878–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea‐pig bone marrow and spleen cells. Cell Tissue Kinet 1970;3:393–403. [DOI] [PubMed] [Google Scholar]
- 9. Castro‐Malaspina H, Gay RE, Resnick G et al. Characterization of human bone marrow fibroblast colony‐forming cells (CFU‐F) and their progeny. Blood 1980;56:289–301. [PubMed] [Google Scholar]
- 10. Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J 2004;18:980–982. [DOI] [PubMed] [Google Scholar]
- 11. Crain BJ, Tran SD, Mezey E. Transplanted human bone marrow cells generate new brain cells. J Neurol Sci 2005;233:121–123. [DOI] [PubMed] [Google Scholar]
- 12. Krabbe C, Zimmer J, Meyer M. Neural transdifferentiation of mesenchymal stem cells–a critical review. APMIS 2005;113:831–844. [DOI] [PubMed] [Google Scholar]
- 13. Ries C, Egea V. Human mesenchymal stem cell transdifferentiation to neural cells: Role of tumor necrosis factor alpha In: Hayat MA, ed. Stem Cells and Cancer Stem Cells, Volume 8: Therapeutic Applications in Disease and Injury. Dordrecht: Springer, 2012:71–78. [Google Scholar]
- 14. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA 1999;96:10711–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aguilar R, Bustos FJ, Saez M et al. Polycomb PRC2 complex mediates epigenetic silencing of a critical osteogenic master regulator in the hippocampus. Biochim Biophys Acta 2016;1859:1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okolicsanyi RK, Camilleri ET, Oikari LE et al. Human mesenchymal stem cells retain multilineage differentiation capacity including neural marker expression after extended in vitro expansion. PLoS One 2015;10:e0137255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aust L, Devlin B, Foster SJ et al. Yield of human adipose‐derived adult stem cells from liposuction aspirates. Cytotherapy 2004;6:7–14. [DOI] [PubMed] [Google Scholar]
- 18. Smiler D, Soltan M, Albitar M. Toward the identification of mesenchymal stem cells in bone marrow and peripheral blood for bone regeneration. Implant Dent 2008;17:236–244. [DOI] [PubMed] [Google Scholar]
- 19. He Q, Wan C, Li G. Concise review: Multipotent mesenchymal stromal cells in blood. Stem Cells 2007;25:69–77. [DOI] [PubMed] [Google Scholar]
- 20. Griffiths MJD, Bonnet D, Janes SM. Stem cells of the alveolar epithelium. Lancet 2005;366:249–260. [DOI] [PubMed] [Google Scholar]
- 21. Tuli R, Li WJ, Tuan RS. Current state of cartilage tissue engineering. Arthritis Res Ther 2003;5:235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan J, Varshney RR, Ren L et al. Synovium‐derived mesenchymal stem cells: A new cell source for musculoskeletal regeneration. Tissue Eng Part B Rev 2009;15:75–86. [DOI] [PubMed] [Google Scholar]
- 23. Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res 2007;10:149–160. [DOI] [PubMed] [Google Scholar]
- 24. Jackson WM, Nesti LJ, Tuan RS. Potential therapeutic applications of muscle‐derived mesenchymal stem and progenitor cells. Expert Opin Biol Ther 2010;10:505–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. In't Anker PS, Scherjon SA, Kleijburg‐van der Keur C et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004;22:1338–1345. [DOI] [PubMed] [Google Scholar]
- 26. Miao Z, Jin J, Chen L et al. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol Int 2006;30:681–687. [DOI] [PubMed] [Google Scholar]
- 27. Corrao S, La Rocca G, Lo Iacono M et al. Umbilical cord revisited: From Wharton's jelly myofibroblasts to mesenchymal stem cells. Histol Histopathol 2013;28:1235–1244. [DOI] [PubMed] [Google Scholar]
- 28. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 2000;109:235–242. [DOI] [PubMed] [Google Scholar]
- 29. Mareschi K, Biasin E, Piacibello W et al. Isolation of human mesenchymal stem cells: Bone marrow versus umbilical cord blood. Haematologica 2001;86:1099–1100. [PubMed] [Google Scholar]
- 30. Seo BM, Miura M, Gronthos S et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004;364:149–155. [DOI] [PubMed] [Google Scholar]
- 31. Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 2003;18:696–704. [DOI] [PubMed] [Google Scholar]
- 32. Corselli M, Chen CW, Crisan M et al. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol 2010;30:1104–1109. [DOI] [PubMed] [Google Scholar]
- 33. Elahi KC, Klein G, Avci‐Adali M et al. Human mesenchymal stromal cells from different sources diverge in their expression of cell surface proteins and display distinct differentiation patterns. Stem Cells Int 2016;2016:5646384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kwon A, Kim Y, Kim M et al. Tissue‐specific differentiation potency of mesenchymal stromal cells from perinatal tissues. Sci Rep 2016;6:23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davies JE, Walker JT, Keating A. Concise review: Wharton's Jelly: The rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Translational Medicine 2017;6:1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen J‐Y, Mou X‐Z, Du X‐C et al. Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins. Asian Pac J Trop Med 2015;8:739–746. [DOI] [PubMed] [Google Scholar]
- 37. Billing AM, Ben Hamidane H, Dib SS et al. Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci Rep 2016;6:21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sakaguchi Y, Sekiya I, Yagishita K et al. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum 2005;52:2521–2529. [DOI] [PubMed] [Google Scholar]
- 39. Rider DA, Nalathamby T, Nurcombe V et al. Selection using the alpha‐1 integrin (CD49a) enhances the multipotentiality of the mesenchymal stem cell population from heterogeneous bone marrow stromal cells. J Mol Histol 2007;38:449–458. [DOI] [PubMed] [Google Scholar]
- 40. Hass R, Kasper C, Böhm S et al. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue‐derived MSC. Cell Commun Signal CCS 2011;9:12–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maleki M, Ghanbarvand F, Reza Behvarz M et al. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells 2014;7:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trounson A, McDonald C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 2015;17:11–22. [DOI] [PubMed] [Google Scholar]
- 43. Monsarrat P, Vergnes JN, Planat‐Bénard V et al. An innovative, comprehensive mapping and multiscale analysis of registered trials for stem cell‐based regenerative medicine. Stem Cells Translational Medicine 2016;5:826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Atoui R Chiu RCJ. Concise review: Immunomodulatory properties of mesenchymal stem cells in cellular transplantation: Update, controversies, and unknowns. Stem Cells Translational Medicine 2012;1:200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steinert AF, Rackwitz L, Gilbert F et al. Concise review: The clinical application of mesenchymal stem cells for musculoskeletal regeneration: Current status and perspectives. Stem Cells Translational Medicine 2012;1:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hare JM, Fishman JE, Gerstenblith G et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kinkaid HY, Huang XP, Li RK et al. What's new in cardiac cell therapy? Allogeneic bone marrow stromal cells as “universal donor cells”. J Card Surg 2010;25:359–366. [DOI] [PubMed] [Google Scholar]
- 48. Zhang J, Huang X, Wang H et al. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell‐based therapy. Stem Cell Res Ther 2015;6:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Griffin MD, Elliman SJ, Cahill E et al. Concise review: Adult mesenchymal stromal cell therapy for inflammatory diseases: how well are we joining the dots? Stem Cells 2013;31:2033–2041. [DOI] [PubMed] [Google Scholar]
- 50. Alagesan S, Griffin MD. Autologous and allogeneic mesenchymal stem cells in organ transplantation: What do we know about their safety and efficacy? Curr Opin Organ Transplant 2014;19:65–72. [DOI] [PubMed] [Google Scholar]
- 51. Grisendi G, Annerén C, Cafarelli L et al. GMP‐manufactured density gradient media for optimized mesenchymal stromal/stem cell isolation and expansion. Cytotherapy 2010;12:466–477. [DOI] [PubMed] [Google Scholar]
- 52. Gronthos S, Zannettino AC, Hay S et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 2003;116:1827–1835. [DOI] [PubMed] [Google Scholar]
- 53. Miltenyi S, Müller W, Weichel W et al. High gradient magnetic cell separation with MACS. Cytometry 1990;11:231–238. [DOI] [PubMed] [Google Scholar]
- 54. Wagner J, Kean T, Young R et al. Optimizing mesenchymal stem cell‐based therapeutics. Curr Opin Biotechnol 2009;20:531–536. [DOI] [PubMed] [Google Scholar]
- 55. Wagner W, Saffrich R, Ho AD. The stromal activity of mesenchymal stromal cells. Transfus Med Hemother 2008;35:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 57. Friedenstein AJ, Deriglasova UF, Kulagina NN et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 1974;2:83–92. [PubMed] [Google Scholar]
- 58. Pochampally R. Colony Forming Unit Assays for MSCs, In: Prockop DJ, Bunnell BA, Phinney DG, eds. Mesenchymal Stem Cells: Methods and Protocols. Totowa, NJ: Humana Press; 2008:83–91. [DOI] [PubMed] [Google Scholar]
- 59. Caplan AI. Why are MSCs therapeutic? New data: New insight. J Pathol 2009;217:318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Haynesworth SE, Goldberg VM, Caplan AI. Diminution of the number of mesenchymal stem cells as a cause for skeletal aging. In: Musculoskeletal Soft‐tissue Aging: Impact on Mobility. Rosemont, IL. 1994:79–87.
- 61. Gronthos S, Graves SE, Ohta S et al. The STRO‐1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood 1994;84:4164–4173. [PubMed] [Google Scholar]
- 62. Gronthos S, Simmons PJ. The growth factor requirements of STRO‐1‐positive human bone marrow stromal precursors under serum‐deprived conditions in vitro. Blood 1995;85:929–940. [PubMed] [Google Scholar]
- 63. Gronthos S, Zannettino AC, Graves SE et al. Differential cell surface expression of the STRO‐1 and alkaline phosphatase antigens on discrete developmental stages in primary cultures of human bone cells. J Bone Miner Res 1999;14:47–56. [DOI] [PubMed] [Google Scholar]
- 64. Simmons PJ, Torok‐Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO‐1. Blood 1991;78:55–62. [PubMed] [Google Scholar]
- 65. Gang EJ, Bosnakovski D, Figueiredo CA et al. SSEA‐4 identifies mesenchymal stem cells from bone marrow. Blood 2007;109:1743–1751. [DOI] [PubMed] [Google Scholar]
- 66. Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 2008;47:126–131. [DOI] [PubMed] [Google Scholar]
- 67. Buhring HJ, Battula VL, Treml S et al. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci 2007;1106:262–271. [DOI] [PubMed] [Google Scholar]
- 68. Quirici N, Soligo D, Bossolasco P et al. Isolation of bone marrow mesenchymal stem cells by anti‐nerve growth factor receptor antibodies. Exp Hematol 2002;30:783–791. [DOI] [PubMed] [Google Scholar]
- 69. Buhring HJ, Treml S, Cerabona F et al. Phenotypic characterization of distinct human bone marrow‐derived MSC subsets. Ann N Y Acad Sci 2009;1176:124–134. [DOI] [PubMed] [Google Scholar]
- 70. Sobiesiak M, Sivasubramaniyan K, Hermann C et al. The mesenchymal stem cell antigen MSCA‐1 is identical to tissue non‐specific alkaline phosphatase. Stem Cells Dev 2010;19:669–677. [DOI] [PubMed] [Google Scholar]
- 71. Halfon S, Abramov N, Grinblat B et al. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev 2011;20:53–66. [DOI] [PubMed] [Google Scholar]
- 72. Jurisic G, Iolyeva M, Proulx ST et al. Thymus cell antigen 1 (Thy1, CD90) is expressed by lymphatic vessels and mediates cell adhesion to lymphatic endothelium. Exp Cell Res 2010;316:2982–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kisselbach L, Merges M, Bossie A et al. CD90 Expression on human primary cells and elimination of contaminating fibroblasts from cell cultures. Cytotechnology 2009;59:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ishii M, Koike C, Igarashi A et al. Molecular markers distinguish bone marrow mesenchymal stem cells from fibroblasts. Biochem Biophys Res Commun 2005;332:297–303. [DOI] [PubMed] [Google Scholar]
- 75. Fitter S, Gronthos S, Ooi SS et al. The mesenchymal precursor cell marker antibody STRO‐1 binds to cell surface heat shock cognate 70. Stem Cells 2017;35:940–951. [DOI] [PubMed] [Google Scholar]
- 76. Bianco P, Cao X, Frenette PS et al. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat Med 2013;19:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Arthur A, Zannettino A, Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J Cell Physiol 2009;218:237–245. [DOI] [PubMed] [Google Scholar]
- 78. Bourin P, Bunnell BA, Casteilla L et al. Stromal cells from the adipose tissue‐derived stromal vascular fraction and culture expanded adipose tissue‐derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013;15:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Andersen DC, Kortesidis A, Zannettino AC et al. Development of novel monoclonal antibodies that define differentiation stages of human stromal (mesenchymal) stem cells. Mol Cells 2011;32:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tormin A, Li O, Brune JC et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood 2011;117:5067–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Boxall SA, Jones E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int 2012;2012:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Deschaseaux F, Gindraux F, Saadi R et al. Direct selection of human bone marrow mesenchymal stem cells using an anti‐CD49a antibody reveals their CD45(med,low) phenotype. Br J Haematol 2003;122:506–517. [DOI] [PubMed] [Google Scholar]
- 83. Tormin A, Li O, Walsh S et al. CD146 expression in primary bone marrow MSC progenitor/stem cells is dependent on their in vivo location. Blood 2009;114:107–107. [Google Scholar]
- 84. Jones EA, English A, Kinsey SE et al. Optimization of a flow cytometry‐based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom 2006;70B:391–399. [DOI] [PubMed] [Google Scholar]
- 85. Jones EA, Kinsey SE, English A et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum 2002;46:3349–3360. [DOI] [PubMed] [Google Scholar]
- 86. Battula VL, Treml S, Bareiss PM et al. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen‐1. Haematologica 2009;94:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nombela‐Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol 2011;12:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mendez‐Ferrer S, Michurina TV, Ferraro F et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466:829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Morikawa S, Mabuchi Y, Kubota Y et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med 2009;206:2483–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pinho S, Julie Lacombe, Hanoun Maher et al. PDGFRα and CD51 mark human Nestin(+) sphere‐forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med 2013;210:1351–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhou BO, Yue R, Murphy MM et al. Leptin‐receptor‐expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014;15:154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jones E, Schäfer R. Where is the common ground between bone marrow mesenchymal stem/stromal cells from different donors and species?. Stem Cell Res Ther 2015;6:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Camilleri ET, Gustafson MP, Dudakovic A et al. Identification and validation of multiple cell surface markers of clinical‐grade adipose‐derived mesenchymal stromal cells as novel release criteria for good manufacturing practice‐compliant production. Stem Cell Res Ther 2016;7:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ong WK, Tan CS, Chan KL et al. Identification of specific cell‐surface markers of adipose‐derived stem cells from subcutaneous and visceral fat depots. Stem Cell Reports 2014;2:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Reger RL, Tucker AH, Wolfe MR. Differentiation and characterization of human MSCs. Methods Mol Biol 2008;449:93–107. [DOI] [PubMed] [Google Scholar]
- 96. Sarugaser R, Hanoun L, Keating A et al. Human mesenchymal stem cells self‐renew and differentiate according to a deterministic hierarchy. PLoS One 2009;4:e6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bhakta G, Rai B, Lim ZX et al. Hyaluronic acid‐based hydrogels functionalized with heparin that support controlled release of bioactive BMP‐2. Biomaterials 2012;33:6113–6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bramono DS, Murali S, Rai B et al. Bone marrow‐derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein‐2 (BMP‐2). Bone 2012;50:954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dombrowski C, Helledie T, Ling L et al. FGFR1 signaling stimulates proliferation of human mesenchymal stem cells by inhibiting the cyclin‐dependent kinase inhibitors p21(Waf1) and p27(Kip1). Stem Cells 2013;31:2724–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Helledie T, Dombrowski C, Rai B et al. Heparan sulfate enhances the self‐renewal and therapeutic potential of mesenchymal stem cells from human adult bone marrow. Stem Cells Dev 2012;21:1897–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ling L, Dombrowski C, Foong KM et al. Synergism between Wnt3a and heparin enhances osteogenesis via a phosphoinositide 3‐kinase/Akt/RUNX2 pathway. J Biol Chem 2010;285:26233–26244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Teplyuk NM, Haupt LM, Ling L et al. The osteogenic transcription factor Runx2 regulates components of the fibroblast growth factor/proteoglycan signaling axis in osteoblasts. J Cell Biochem 2009;107:144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Scott MA, Nguyen VT, Levi B et al. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev 2011;20:1793–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Johnstone B, Hering TM, Caplan AI et al. In vitro chondrogenesis of bone marrow‐derived mesenchymal progenitor cells. Exp Cell Res 1998;238:265–272. [DOI] [PubMed] [Google Scholar]
- 105. Mackay AM, Beck SC, Murphy JM et al. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 1998;4:415–428. [DOI] [PubMed] [Google Scholar]
- 106. Barry F, Boynton RE, Liu B et al. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation‐dependent gene expression of matrix components. Exp Cell Res 2001;268:189–200. [DOI] [PubMed] [Google Scholar]
- 107. Hoch AI, Leach JK. Concise review: Optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Translational Medicine 2014;3:643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Riis S, Nielsen FM, Pennisi CP et al. Comparative analysis of media and supplements on initiation and expansion of adipose‐derived stem cells. Stem Cells Translational Medicine 2016;5:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pountos I, Georgouli T, Henshaw K et al. Mesenchymal stem cell physiology can be affected by antibiotics: An in vitro study. Cell Mol Biol (Noisy‐le‐grand) 2014;60:1–7. [PubMed] [Google Scholar]
- 110. Petersen BE, Bowen WC, Patrene KD et al. Bone marrow as a potential source of hepatic oval cells. Science 1999;284:1168–1170. [DOI] [PubMed] [Google Scholar]
- 111. Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 112. Quinn C, Flake AW. In vivo differentiation potential of mesenchymal stem cells: Prenatal and postnatal model systems. Transfus Med Hemother 2008;35:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li Q, Zhou X, Shi Y et al. In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PLoS One 2013;8:e62363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Munoz‐Elias G, Marcus AJ, Coyne TM et al. Adult bone marrow stromal cells in the embryonic brain: Engraftment, migration, differentiation, and long‐term survival. J Neurosci 2004;24:4585–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bae JS, Han HS, Youn DH et al. Bone marrow‐derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cells 2007;25:1307–1316. [DOI] [PubMed] [Google Scholar]
- 116. Liu WH, Song FQ, Ren LN et al. The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J Cell Mol Med 2015;19:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Meier RPH, Müller YD, Morel P et al. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence?. Stem Cell Res 2013;11:1348–1364. [DOI] [PubMed] [Google Scholar]
- 118. Terada N, Hamazaki T, Oka M et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002;416:542–545. [DOI] [PubMed] [Google Scholar]
- 119. Sottile F, Aulicino F, Theka I et al. Mesenchymal stem cells generate distinct functional hybrids in vitro via cell fusion or entosis. Sci Rep 2016;6:36863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nygren JM, Jovinge S, Breitbach M et al. Bone marrow‐derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med 2004;10:494–501. [DOI] [PubMed] [Google Scholar]
- 121. Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci 2000;113:1161–1166. [DOI] [PubMed] [Google Scholar]
- 122. Pevsner‐Fischer M, Levin S, Zipori D. The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev 2011;7:560–568. [DOI] [PubMed] [Google Scholar]
- 123. Russell KC, Phinney DG, Lacey MR et al. In vitro high‐capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells 2010;28:788–798. [DOI] [PubMed] [Google Scholar]
- 124. Kordelas L, Rebmann V, Ludwig AK et al. MSC‐derived exosomes: A novel tool to treat therapy‐refractory graft‐versus‐host disease. Leukemia 2014;28:970–973. [DOI] [PubMed] [Google Scholar]
- 125. Lai RC, Arslan F, Lee MM et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010;4:214–222. [DOI] [PubMed] [Google Scholar]
- 126. Amable PR, Teixeira MV, Carias RB et al. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton's jelly. Stem Cell Res Ther 2014;5:53‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Eirin A, Riester SM, Zhu XY et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue‐derived mesenchymal stem cells. Gene 2014;551:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Eirin A, Zhu XY, Puranik AS et al. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue‐derived mesenchymal stem/stromal cells. Sci Rep 2016;6:36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hong HS, Kim YH, Son Y. Perspectives on mesenchymal stem cells: Tissue repair, immune modulation, and tumor homing. Arch Pharm Res 2012;35:201–211. [DOI] [PubMed] [Google Scholar]
- 130. Marfy‐Smith SJ, Clarkin CE. Are mesenchymal stem cells so bloody great after all? Stem Cells Translational Medicine 2017;6:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Le Blanc K, Frassoni F, Ball L et al. Mesenchymal stem cells for treatment of steroid‐resistant, severe, acute graft‐versus‐host disease: A phase II study. Lancet 2008;371:1579–1586. [DOI] [PubMed] [Google Scholar]
- 132. Ortiz LA, Dutreil M, Fattman C et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 2007;104:11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shabbir A, Zisa D, Suzuki G et al. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: A noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol 2009;296:H1888–H1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dexter TM. Regulation of hemopoietic cell growth and development: Experimental and clinical studies. Leukemia 1989;3:469–474. [PubMed] [Google Scholar]
- 135. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815–1822. [DOI] [PubMed] [Google Scholar]
- 136. Di Nicola M, Carlo‐Stella C, Magni M et al. Human bone marrow stromal cells suppress T‐lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838–3843. [DOI] [PubMed] [Google Scholar]
- 137. Centeno CJ, Busse D, Kisiday J et al. Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells. Med Hypotheses 2008;71:900–908. [DOI] [PubMed] [Google Scholar]
- 138. Andrews EM, Tsai SY, Johnson SC et al. Human adult bone marrow‐derived somatic cell therapy results in functional recovery and axonal plasticity following stroke in the rat. Exp Neurol 2008;211:588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Shabbir A, Zisa D, Lin H et al. Activation of host tissue trophic factors through JAK‐STAT3 signaling: A mechanism of mesenchymal stem cell‐mediated cardiac repair. Am J Physiol Heart Circ Physiol 2010;299:H1428–H1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Horwitz EM, Prockop DJ, Fitzpatrick LA et al. Transplantability and therapeutic effects of bone marrow‐derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999;5:309–313. [DOI] [PubMed] [Google Scholar]
- 141. Luong‐Van E, Grøndahl L, Song S et al. The in vivo assessment of a novel scaffold containing heparan sulfate for tissue engineering with human mesenchymal stem cells. J Mol Histol 2007;38:459–468. [DOI] [PubMed] [Google Scholar]
- 142. Singer NG, Caplan AI. Mesenchymal stem cells: Mechanisms of inflammation. Annu Rev Pathol 2011;6:457–478. [DOI] [PubMed] [Google Scholar]
- 143. Lener T, Gimona M, Aigner L et al. Applying extracellular vesicles based therapeutics in clinical trials ‐ an ISEV position paper. J Extracell Vesicles 2015;4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Cruz FF, Borg ZD, Goodwin M et al. Systemic administration of human bone marrow‐derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract‐induced allergic airway inflammation in immunocompetent mice. Stem Cells Translational Medicine 2015;4:1302–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Reiner AT, Witwer KW, van Balkom BWM et al. Concise review: Developing best‐practice models for the therapeutic use of extracellular vesicles. Stem Cells Translational Medicine 2017;6:1730–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhang B, Yeo RW, Tan KH et al. Focus on extracellular vesicles: Therapeutic potential of stem cell‐derived extracellular vesicles. Int J Mol Sci 2016;17:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lo Sicco C, Reverberi D, Balbi C et al. Mesenchymal stem cell‐derived extracellular vesicles as mediators of anti‐inflammatory effects: Endorsement of macrophage polarization. Stem Cells Translational Medicine 2017;6:1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wen S, Dooner M, Cheng Y et al. Mesenchymal stromal cell‐derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016;30:2221–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Xie H, Wang Z, Zhang L et al. Extracellular vesicle‐functionalized decalcified bone matrix scaffolds with enhanced pro‐angiogenic and pro‐bone regeneration activities. Sci Rep 2017;7:45622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Pachler K, Lener T, Streif D et al. A Good Manufacturing Practice‐grade standard protocol for exclusively human mesenchymal stromal cell‐derived extracellular vesicles. Cytotherapy 2017;19:458–472. [DOI] [PubMed] [Google Scholar]
- 151. Rai B, Lin JL, Lim ZX et al. Differences between in vitro viability and differentiation and in vivo bone‐forming efficacy of human mesenchymal stem cells cultured on PCL‐TCP scaffolds. Biomaterials 2010;31:7960–7970. [DOI] [PubMed] [Google Scholar]
- 152. Glennie S, Soeiro I, Dyson PJ et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005;105:2821–2827. [DOI] [PubMed] [Google Scholar]
- 153. Corcione A, Benvenuto F, Ferretti E et al. Human mesenchymal stem cells modulate B‐cell functions. Blood 2006;107:367–372. [DOI] [PubMed] [Google Scholar]
- 154. Gonzalez MA, Gonzalez‐Rey E, Rico L et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose‐derived mesenchymal stem cells. Arthritis Rheum 2009;60:1006–1019. [DOI] [PubMed] [Google Scholar]
- 155. Ren G, Roberts AI, Shi Y. Adhesion molecules: Key players in mesenchymal stem cell‐mediated immunosuppression. Cell Adh Migr 2011;5:20–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–736. [DOI] [PubMed] [Google Scholar]
- 157. Kyurkchiev D, Bochev I, Ivanova‐Todorova E et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells 2014;6:552–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Selmani Z, Naji A, Zidi I et al. Human leukocyte antigen‐G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008;26:212–222. [DOI] [PubMed] [Google Scholar]
- 159. Ren G, Zhang L, Zhao X et al. Mesenchymal stem cell‐mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008;2:141–150. [DOI] [PubMed] [Google Scholar]
- 160. Lee RH, Seo MJ, Reger RL et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA 2006;103:17438–17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Corrao S, La Rocca G, Lo Iacono M et al. New frontiers in regenerative medicine in cardiology: The potential of Wharton's jelly mesenchymal stem cells. Curr Stem Cell Res Ther 2013;8:39–45. [DOI] [PubMed] [Google Scholar]
- 162. Anzalone R, Lo Iacono M, Corrao S et al. New emerging potentials for human Wharton's jelly mesenchymal stem cells: Immunological features and hepatocyte‐like differentiative capacity. Stem Cells Dev 2010;19:423–438. [DOI] [PubMed] [Google Scholar]
- 163. Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: An Update. Cell Transplant 2016;25:829–848. [DOI] [PubMed] [Google Scholar]
- 164. Miteva K, Van Linthout S, Pappritz K et al. Human endomyocardial biopsy specimen‐derived stromal cells modulate angiotensin ii‐induced cardiac remodeling. Stem Cells Translational Medicine 2016;5:1707–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016;16:582–598. [DOI] [PubMed] [Google Scholar]
- 166. Mattar P, Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth‐associated tissue mesenchymal stromal cells. Front Immunol 2015;6:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Rhee K‐J, Lee JI, Eom YW. Mesenchymal stem cell‐mediated effects of tumor support or suppression. Int J Mol Sci 2015;16:30015–30033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Klopp AH, Spaeth EL, Dembinski JL et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res 2007;67:11687–11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Costanza B, Umelo IA, Bellier J et al. Stromal modulators of TGF‐β in cancer. J Clin Med 2017;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Lacerda L, Debeb BG, Smith D et al. Mesenchymal stem cells mediate the clinical phenotype of inflammatory breast cancer in a preclinical model. Breast Cancer Res 2015;17:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: Key players in cancer progression. Mol Cancer 2017;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ye H, Cheng J, Tang Y et al. Human bone marrow‐derived mesenchymal stem cells produced TGFbeta contributes to progression and metastasis of prostate cancer. Cancer Invest 2012;30:513–518. [DOI] [PubMed] [Google Scholar]
- 173. Caimi PF, Reese J, Lee Z et al. Emerging therapeutic approaches for multipotent mesenchymal stromal cells. Curr Opin Hematol 2010;17:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Solchaga LA, Penick K, Goldberg VM et al. Fibroblast growth factor‐2 enhances proliferation and delays loss of chondrogenic potential in human adult bone‐marrow‐derived mesenchymal stem cells. Tissue Eng Part A 2010;16:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Parsch D, Fellenberg J, Brümmendorf TH et al. Telomere length and telomerase activity during expansion and differentiation of human mesenchymal stem cells and chondrocytes. J Mol Med (Berl) 2004;82:49–55. [DOI] [PubMed] [Google Scholar]
- 176. Zimmermann S, Voss M, Kaiser S et al. Lack of telomerase activity in human mesenchymal stem cells. Leukemia 2003;17:1146–1149. [DOI] [PubMed] [Google Scholar]
- 177. Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev 2006;5:91–116. [DOI] [PubMed] [Google Scholar]
- 178. Samsonraj RM, Raghunath M, Hui JH et al. Telomere length analysis of human mesenchymal stem cells by quantitative PCR. Gene 2013;519:348–355. [DOI] [PubMed] [Google Scholar]
- 179. Hemeda H, Giebel B, Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy 2014;16:170–180. [DOI] [PubMed] [Google Scholar]
- 180. Spees JL Gregory CA, Singh H et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther 2004;9:747–756. [DOI] [PubMed] [Google Scholar]
- 181. Bieback K. Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus Med Hemother 2013;40:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Bieback K, Hecker A, Kocaömer A et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells 2009;27:2331–2341. [DOI] [PubMed] [Google Scholar]
- 183. Wijesinghe SJ, Ling L, Murali S et al. Affinity selection of FGF2‐binding heparan sulfates for ex vivo expansion of human mesenchymal stem cells. J Cell Physiol 2017;232:566–575. [DOI] [PubMed] [Google Scholar]
- 184. Ling L, Camilleri ET, Helledie T et al. Effect of heparin on the biological properties and molecular signature of human mesenchymal stem cells. Gene 2016;576:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Gibson JD, O'Sullivan MB, Alaee F et al. Regeneration of articular cartilage by human esc‐derived mesenchymal progenitors treated sequentially with BMP‐2 and Wnt5a. Stem Cells Translational Medicine 2017;6:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Eaker S, Armant M, Brandwein H et al. Concise review: Guidance in developing commercializable autologous/patient‐specific cell therapy manufacturing. Stem Cells Translational Medicine 2013;2:871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Muller‐Cohn J, Diaz P, Muller R. Stem cell value chains In:Vertès AA, Qureshi N, Caplan AI. et al., eds. Stem Cells in Regenerative Medicine. Chichester, UK: John Wiley, 2015:341–354. [Google Scholar]
- 188. Schnitzler AC, Verma A, Kehoe DE et al. Bioprocessing of human mesenchymal stem/stromal cells for therapeutic use: Current technologies and challenges. Biochem Eng J 2016;108:3–13. [Google Scholar]
- 189. Research GV. Stem cells market size, global industry research report, 2014–2025. Report ID: 978‐1‐68038‐130‐6; Available at http://www.grandviewresearch.com/industry-analysis/stem-cells-market, 2017: p. 150. Last accessed date July 1, 2017.
- 190. Nery AA, Nascimento IC, Glaser T et al. Human mesenchymal stem cells: From immunophenotyping by flow cytometry to clinical applications. Cytometry Part A 2013;83A:48–61. [DOI] [PubMed] [Google Scholar]
- 191. Caplan AI, Mason C, Reeve B. The 3Rs of cell therapy. Stem Cells Translational Medicine 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Gronthos S, Fitter S, Diamond P et al. A novel monoclonal antibody (STRO‐3) identifies an isoform of tissue nonspecific alkaline phosphatase expressed by multipotent bone marrow stromal stem cells. Stem Cells Dev 2007;16:953–963. [DOI] [PubMed] [Google Scholar]
- 193. Bubnic S et al. Mesenchymal stem cells expressing TNF‐receptor. US Patent EP 1 971 679 A2, 2008.
- 194. Smith JR, Pochampally R, Perry A et al. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells 2004;22:823–831. [DOI] [PubMed] [Google Scholar]
- 195. Gupta PK, Chullikana A, Parakh R et al. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med 2013;11:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Gupta PK, Krishna M, Chullikana A et al. Administration of adult human bone marrow‐derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to Buerger's disease: Phase II study report suggests clinical efficacy. Stem Cells Translational Medicine 2017;6:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]


